Abstract
Chronic cannabis (marijuana, hashish) smoking can result in dependence. Rodent studies show reversible downregulation of brain cannabinoid CB1 (cannabinoid receptor type 1) receptors after chronic exposure to cannabis. However, whether downregulation occurs in humans who chronically smoke cannabis is unknown. Here we show, using positron emission tomography imaging, reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in human subjects who chronically smoke cannabis. Downregulation correlated with years of cannabis smoking and was selective to cortical brain regions. After ~4 weeks of continuously monitored abstinence from cannabis on a secure research unit, CB1 receptor density returned to normal levels. This is the first direct demonstration of cortical cannabinoid CB1 receptor downregulation as a neuroadaptation that may promote cannabis dependence in human brain.
Keywords: addiction, cannabis, CB1 receptor, positron emission tomography, receptor imaging
Introduction
Cannabis is the most widely used illicit drug in the world1 and causes multiple health problems.2 Chronic cannabis smoking can lead to tolerance3-5 and withdrawal symptoms,6,7 the two hallmarks of dependence. In human brain, the actions of the main psychoactive component of cannabis, Δ9-tetrahydrocannabinol (Δ9-THC), are mediated via cannabinoid CB1 (cannabinoid receptor type 1) receptors.8,9 CB1 receptors are widely distributed in human brain, and highest receptor densities are found in basal ganglia, hippocampus, cingulate cortex and the molecular layer of cerebellum.10,11 CB1 receptors are primarily located presynaptically, where they inhibit release of other neurotransmitters, such as glutamate and γ-amino butyric acid.12,13 However, the exact role of CB1 receptors in the development of tolerance and withdrawal in humans is unclear.
In rodent brain, downregulation of CB1 receptor signaling is thought to underlie tolerance.14 That is, chronic exposure to Δ9-THC and other cannabinoid agonists causes a reduction in the number and signaling efficiency of CB1 receptors as a homeostatic response.15-21 These changes closely parallel development of tolerance to typical cannabinoid effects, such as decreased motility and impaired memory.15 The magnitude and rate of downregulation are region dependent. The downregulation is larger and occurs more rapidly in cortical regions, such as hippocampus and cerebellum, than in subcortical regions, such as basal ganglia and midbrain.15 In addition, downregulation is reversible upon abstinence: this reversal is more rapid in striatum and midbrain than in cortical regions.20
Whether chronic cannabinoid exposure downregulates CB1 receptor signaling in human brain is unknown, although such downregulation has been hypothesized to underlie the development of tolerance to some effects of cannabis in heavy smokers.4 Lack of methods to accurately quantify CB1 receptors in human brain in vivo has hindered progress in answering this question. We recently developed a method to quantify cannabinoid CB1 receptors in human brain with positron emission tomography (PET) using a novel inverse agonist radioligand, [18F]FMPEP-d2.22,23 This radioligand has high affinity and selectivity for the CB1 receptor and, in monkey brain, the vast majority of the signal represents specific binding to CB1 receptors.22 In human brain, [18F]FMPEP-d2 has high brain uptake, and binding can be reliably measured as the distribution volume (VT), which is proportional to receptor density.22
To determine whether heavy cannabis smoking downregulates cannabinoid CB1 receptors in human brain, we measured CB1 receptors using PET and [18F]FMPEP-d2 in chronic daily cannabis smokers (N = 30 subjects) and in control subjects with minimal lifetime exposure to cannabis (N = 28 subjects). Cannabis smokers were scanned twice: immediately after chronic daily cannabis smoking, and after ~4 weeks of abstinence on a monitored unit. Based on animal experiments, we hypothesized that CB1 receptor binding is decreased in chronic daily cannabis smokers at baseline but recovers to normal levels after abstinence.
Subjects and methods
The National Institutes of Health (NIH) Central Nervous System Institutional Review Board approved the protocol and the consent forms. Written informed consent was obtained from all subjects.
Study design
We admitted male chronic daily cannabis smokers to a closed and monitored in-patient research unit for ~4 weeks. We imaged cannabis smokers with PET and [18F]FMPEP-d2 at two time points—namely, on the day following an evening admission and after ~4 weeks of abstinence. The time of first PET scan was chosen to maximize the effect size of the hypothesized downregulation while avoiding both acute intoxication and withdrawal symptoms. Healthy control male subjects underwent a single PET scan. To examine downregulation in cannabis smokers at baseline, we compared VT of [18F]FMPEP-d2 at baseline between cannabis smokers and control subjects (between-subject comparison). To examine whether downregulation is reversible upon abstinence, we compared VT before and after abstinence in cannabis smokers (within-subject comparison).
Subjects
A total of 30 male subjects who smoked cannabis daily but who were not seeking treatment were recruited by the National Institute on Drug Abuse (Table 1). Participants smoked an average of 10 ± 6 joints or blunts per day (range 1–30) for 12 ± 7 years (range 4–37). Age of first cannabis smoking was 15 ± 3 years (range 6–22). The participants underwent history and physical examination as well as laboratory tests of blood and urine to ensure that they were free of current somatic and psychiatric illness and did not currently abuse drugs other than cannabis. Urine drug tests were positive for cannabinoids in these subjects at admission. In addition, 24 cannabis smokers (80%) also smoked tobacco.
Table 1.
Demographic, clinical and radiochemical information of the study participants
| Cannabis smokers | Control subjects | P-value | |
|---|---|---|---|
| Number of subjects | 30 | 28 | |
| Age (years) | 28 ± 8 | 32 ± 10 | 0.096 |
| BMI (kg m−2) | 24 ± 4 | 27 ± 5 | 0.022 |
| Tobacco smokers/nonsmokers (N) | 24/6 | 1/27 | 0.001 |
| Amount of alcohol use (drinks per week) | 5 ± 7 | 2 ± 2 | 0.041 |
| Amount of cannabis smoking (joints per day) | 10 ± 6 | NA | NA |
| Duration of cannabis smoking (years) | 12 ± 7 | NA | NA |
| Age at first cannabis smoking (years) | 15 ± 3 | NA | NA |
| Injected activity of [18F]FMPEP-d2 (MBq) | 175 ± 17 | 181 ± 4 | 0.051 |
| Injected mass of [18F]FMPEP-d2 (μg) | 0.84 ± 0.45 | 0.74 ± 0.24 | 0.273 |
| Fraction of free [18F]FMPEP-d2in plasma (%) | 0.42 ± 0.2 | 0.40 ± 0.2 | 0.733 |
Abbreviations: BMI, body mass index; NA, not applicable.
Values are number, mean ± s.d. or range.
Control male subjects (N = 28) were recruited by the National Institute of Mental Health in Bethesda, MD. All subjects were free of somatic and psychiatric illness as confirmed by history, physical examination, electrocardiogram and blood and urine tests. Urine samples were negative for cannabinoids, opiates, amphetamines, cocaine metabolites and benzodiazepines. Control subjects had < 10 lifetime exposures to cannabis and no use in the preceding 3 months. One control subject (4%) smoked tobacco. Control subjects used slightly less alcohol than cannabis smokers (Table 1).
Residential stay and assessments in cannabis smokers
Cannabis smokers were admitted to the Johns Hopkins Behavioral Pharmacology Research Unit (BPRU) in Baltimore, MD. BPRU has a secure, continuously monitored research unit with nursing personnel present 24 h a day. Subjects were not allowed to leave the unit or receive visitors during their stay to exclude illicit drug use. Subjects were transported to the NIH Clinical Center for PET imaging on the day following an evening admission and again after ~4 weeks, as well as once for magnetic resonance imaging of the brain. Urine samples were tested before leaving the unit, at arrival to NIH and after returning to the unit to ensure abstinence from drugs. Psychological and physical symptoms of cannabis withdrawal were measured daily with 24 five-point Likert scale items.6 Cannabis craving was measured daily with the 12- point Marijuana Craving Questionnaire.24
PET and measurement of parent radioligand in arterial plasma
[18F]FMPEP-d2 was prepared as described previously23 and in detail in our Investigational New Drug Application 105 198, submitted to the US Food and Drug Administration (available at http://pdsp.med.unc.edu/snidd/). The radioligand was obtained in high radiochemical purity (> 99%) and had a specific radioactivity of 110 ± 43 MBq nmol−1 at the time of injection.
After intravenous injection of [18F]FMPEP-d2 (Table 1), images were acquired for 120 min using an Advance camera (GE Healthcare,Milwaukee,WI, USA) as previously described.22 Blood samples were drawn from the radial artery at 15 s intervals until 2 min, then at 3 and 5 min, followed by 3 to 6 ml samples at 10, 15, 20, 30, 45, 60, 75, 90, 105 and 120min. Plasma time-activity curve was corrected for the fraction of unchanged radioligand by radio-high-performance liquid chromatography separation,25 and the plasma free fraction was measured using ultrafiltration.26
PET images were analyzed by applying a template of volumes of interest27 as implemented in PMOD, version 3.0 (PMOD Technologies, Zurich, Switzerland),28 in the standard stereotactic space29 (see Supplementary Material for details). Distribution volume (VT) was estimated according to the two-tissue compartmental model30 with concentration of parent radioligand in plasma as input function, using PMOD, as previously described.22
Statistical analysis of VT data
Data were analyzed using SPSS Statistics 17.0 for Windows (Release 17.0.0, copyright SPSS, 1993–2007, Chicago, IL, USA). VT had normally distribution (Shapiro–Wilk test) and homogeneous variance across groups (Levene’s test). Behavioral data were non-normally distributed. To test whether CB1 receptors are decreased in cannabis smokers at baseline, we applied a repeated measures analysis of variance with group status as a between-subject factor and brain region as a within-subject factor. Body mass index (BMI) entered the model as a covariate as it was different between the groups (Table 1). Correlations with clinical variables were assessed with the non-parametric Spearman’s correlation coefficients (ρ) and BMI-adjusted VT. For correlations and visualizations, VT was adjusted for each subject to the average BMI in the whole sample as follows: VT adjusted = VT observed + b × (BMIaverage−BMIobserved), where b is the slope of the regression line between VT and BMI in control subjects. To test whether CB1 receptors increased after abstinence from cannabis, we applied a repeated measures analysis of variance with repetition (before and after abstinence) and brain region as within-subject factors. The P-values < 0.05 were considered statistically significant.
Voxel-wise analysis of VT
To confirm results from volume-of-interest analysis and to explore regional specificity of findings, we compared baseline parametric VT maps between groups at voxel level using SPM5 (Wellcome Trust Centre for Neuroimaging, University College London, London, UK) (see Supplementary Material for details).
Results
At baseline, VT of [18F]FMPEP-d2 was lower in cannabis smokers than in control subjects in a region-specific manner (group × region interaction: F = 6.5, P = 0.0001). BMI-adjusted VT was ~20% lower in neocortex and limbic cortex, but not in other brain regions (basal ganglia, midbrain, thalamus, pons, or cerebellum; Figures 1 and 2). This finding was confirmed by an independent statistical parametric mapping analysis of voxel-wise VT values, showing significantly lower cortical VT in cannabis smokers (Figure 3). Among the cannabis smokers, years of cannabis smoking correlated negatively with VT in cortical regions: subjects who had smoked cannabis for longer had smaller VT than subjects who had smoked cannabis for a shorter time (Supplementary Figure 1). This correlation was not confounded by effects of age on VT, because VT did not correlate with age in control subjects. Current daily amounts of cannabis smoking or age of first cannabis smoking did not correlate with VT.
Figure 1.
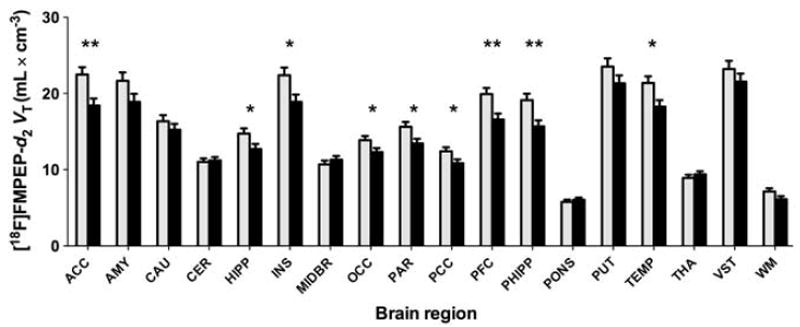
VT of [18F]FMPEP-d2 in cortical regions is lower at baseline in chronic daily cannabis smokers (black bars, n = 30) than in control subjects (gray bars, n = 28). Values are estimated marginal means from the repeated measures analysis variance that controls for BMI. Values are adjusted to an average BMI of 24.8 kg m−2. Error bars are s.e.m. Abbreviations: ACC, anterior cingulate cortex; AMY, amygdala; CAU, caudate nucleus; CER, cerebellum; HIPP, hippocampus; INS, insula; MIDBR, midbrain; OCC, occipital cortex; PAR, parietal cortex; PCC, posterior cingulate cortex; PFC, prefrontal cortex; PHIPP, parahippocampal gyrus; PUT, putamen; TEMP, lateral temporal cortex; THA, thalamus; VST, ventral striatum; WM, white matter; *P < 0.05; **P < 0.005, two-tailed t-test.
Figure 2.
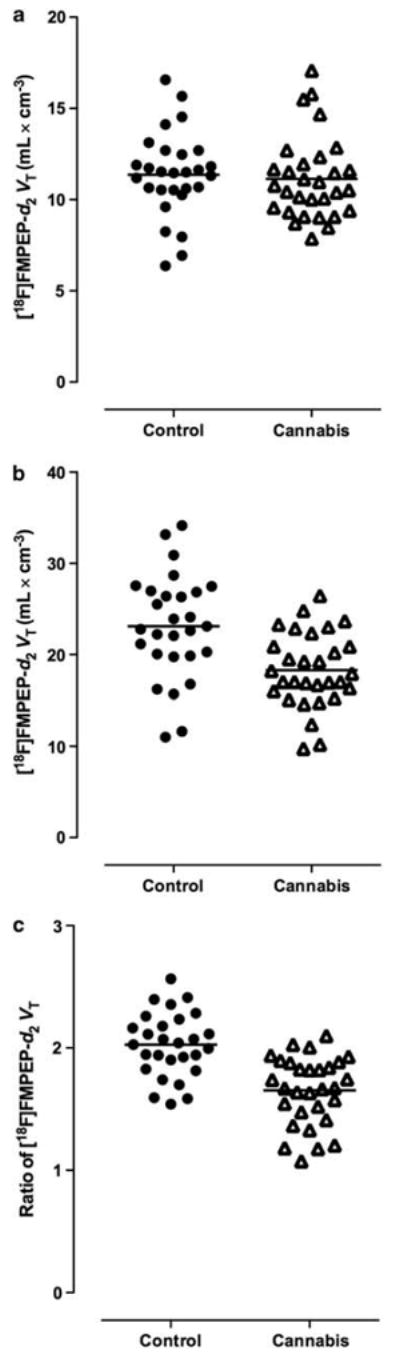
BMI-adjusted VT of [18F]FMPEP-d2 at baseline was unchanged in cerebellum (−2%, P = 0.703, two-tailed t-test; a), but decreased in anterior cingulate cortex (−21%, P = 0.0005, two-tailed t-test; b) in chronic daily cannabis smokers (n = 30) compared with control subjects (n = 28). Ratio of VT in anterior cingulate cortex to that in cerebellum decreased variance in both groups and showed a more significant reduction in cannabis smokers (−20%, P = 0.0000001, two-tailed t-test; c).
Figure 3.
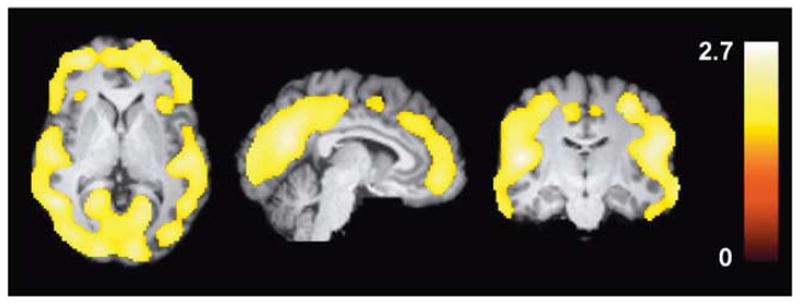
Statistical parametric mapping (SPM) analysis shows lower VT in chronic daily cannabis smokers (n = 30) than in control subjects (n = 28) at baseline as a large single cluster that includes only cortical regions. This cluster comprised 67 513 voxels, had a maximum t-value of 2.8 at [−34, −78, 16] and had a cluster-level corrected P-value of 0.043. Color bar represents t-value in each voxel within the significant cluster.
In addition, VT was not significantly correlated with withdrawal or craving measured on admission (for example, in the anterior cingulate cortex: withdrawal, ρ = 0.06, P = 0.762; craving, ρ = −0.07, P = 0.773) or as the mean value of these two scales for entire residential stay of ~4 weeks (for example, in the anterior cingulate cortex: withdrawal, ρ = −0.33, P = 0.077; craving, ρ = −0.05, P = 0.805). In addition, the change of VT during the residential stay was not significantly correlated with the change in withdrawal (for example, in the anterior cingulate cortex: ρ = 0.04, P = 0.904) or craving (for example, in the anterior cingulate cortex: ρ = 0.23, P = 0.431).
Ethnicity may affect VT of [18F]FMPEP-d2, as three control subjects of Indian descent had unusually low VT values, whereas no difference was found between African-American and Caucasian subjects of European descent (Supplementary Figure 2). There were no cannabis smokers of Indian descent. These three control subjects were not excluded from the analysis, but if they were excluded, the group × region interaction would become stronger (F = 9.4, P = 0.0000002).
BMI had a main effect on VT in all regions (F = 8.1, P = 0.006); in both groups, higher BMI was associated with lower VT. BMI did not correlate with the plasma free fraction of [18F]FMPEP-d2 in either group.
Only free (non-protein bound) radioligand in arterial plasma is able to enter the brain. However, smaller VT in cannabis smokers than in control subjects was not caused by smaller fraction of free radioligand in arterial plasma, as this fraction was similar between groups (Table 1).
Tobacco smoking was more prevalent among cannabis smokers than among control subjects. However, a repeated measures analysis of variance did not show a main effect of tobacco smoking (F < 0.01, P = 0.998) or a tobacco smoking × region interaction (F = 0.48, P = 0.735) on VT among cannabis smokers. In addition, when smoking was taken as a covariate in the overall model, the group × region interaction remained significant (F = 3.2, P = 0.017). Similarly, when the analysis was covaried for alcohol use, the group-region interaction remained significant (F = 6.6, P = 0.00009). Thus, neither tobacco nor alcohol use were likely to significantly confound the main finding.
As VT in cerebellum was similar between groups, we used cerebellum as a reference region to estimate binding in other regions of the brain. Although the majority of uptake in cerebellum represented specific binding, normalizing uptake in cortical regions to that in cerebellum for each subject was expected to reduce variability and to increase statistical power. For example, as BMI correlated with VT in all regions, such normalization would cancel the effects of BMI. We found that uptake normalized to cerebellum was ~20% lower in cannabis smokers than in control subjects in cortical regions, but not in caudate nucleus, midbrain, thalamus or pons (group × region interaction: F = 8.0, P = 0.0000002).
To determine whether decreased VT in cannabis smokers is reversible, we repeated PET measurements in 14 cannabis smokers after 26 ± 5 days of monitored abstinence (range 13–32 days). After monitored abstinence, VT increased specifically in those brain regions that had shown decreased VT at baseline (repetition × region interaction: F = 3.33, P = 0.037; Figure 4) except for hippocampus. At baseline, VT was not different between subjects who had two PET scans (N = 14) and those who had one (N = 16; main effect of completer status: F = 0.16, P = 0.691; completer status × region interaction: F = 0.43, P = 0.770), which argues against selection bias. Change in VT did not correlate with any of the clinical variables, such as current amount or years of cannabis smoking, or with the interval between the two PET scans. Fraction of free radioligand in arterial plasma did not change after abstinence (P = 0.971).
Figure 4.
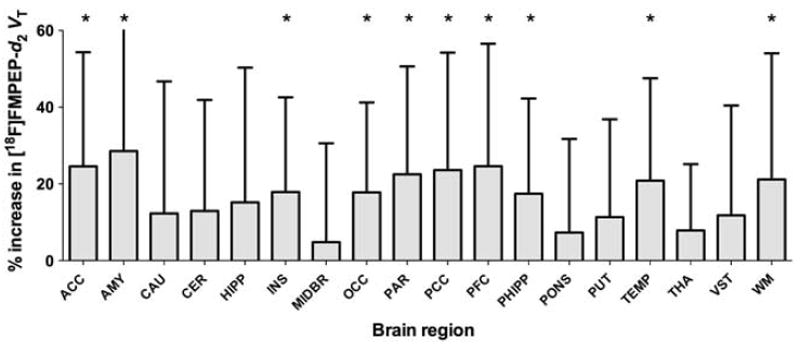
VT of [18F]FMPEP-d2 increased after abstinence in the 14 cannabis smokers who completed two PET scans before and after abstinence. Significant increases were mostly seen in regions with reduced VT at baseline. Error bars are s.d. of percent change. Abbreviations of brain regions are the same as those in Figure 1. *P < 0.05, two-tailed paired samples t-test.
Discussion
We found that VT of [18F]FMPEP-d2 at baseline was ~20% lower in chronic, daily cannabis smokers than in control subjects in cortical, but not in subcortical brain regions. VT was also negatively correlated with years of cannabis smoking: subjects who had smoked cannabis longer had lower VT than subjects who had smoked for a shorter time. Finally, VT increased in cannabis smokers after ~4 weeks of abstinence. We interpret these findings as reflecting changes in CB1 receptor density. Our findings are unaffected by group differences in peripheral distribution and metabolism of the radioligand, because the outcome measure VT corrects for these effects. These findings confirm that chronic cannabis exposure reversibly downregulates CB1 receptors in humans, similar to its effects in rodents. Region-specific decrease in CB1 receptor binding may be part of neuroadaptations promoting development of cannabis dependence in chronic daily smokers.
Decreased VT in cannabis smokers likely reflects a change in CB1 receptors (or affinity) rather than a confounding effect of an endogenous (for example, anandamide) or exogenous (for example, Δ9-THC) agonist. Daily cannabis smokers have prolonged clearance of cannabinoids from the body,31-33 and hence these subjects may have had cannabinoids present also in the brain during the first PET scan. Some PET radioligands are sensitive to changes in concentration of endogenous neurotransmitters, such that increased neurotransmitter concentration causes reduced binding of the radioligand.34 Whether Δ9-THC or other cannabinoid agonists compete with [18F]FMPEP-d2 in binding to CB1 receptors in human brain remains to be established, although such occupancy would not be expected to be restricted to cortical brain regions. In rat brain, binding of [11C]MePPEP, a close analog to [18F]FMPEP-d2, was not displaced by high doses of cannabinoid agonists, 35 consistent with large receptor reserve. Nevertheless, CB1 receptor occupancy by Δ9-THC or its metabolites remains a potential confound. Another potential confound is a change in G-protein coupling of the CB1 receptor (desensitization), which has been documented in rodent studies. The radioligand [18F]FMPEP-d2 is an inverse agonist and presumably labels receptors in both high- and low-affinity states, as binding of another structurally related inverse agonist, rimonabant, is not affected by guanyl nucleotide modulation in rat brain tissue in vitro.36 Therefore, [18F]FMPEP-d2 is unlikely to detect CB1 receptor desensitization that occurs after chronic agonist exposure in rodents.15
Although our results suggest changes in CB1 receptor density, our PET measurement cannot distinguish a change in receptor density and affinity. However, rodent studies suggest a change in receptor density rather than affinity. Our findings of reversible and regionally selective downregulation of CB1 receptors parallel those from a large number of rodents studies.15 These studies demonstrated substantial downregulation of CB1 receptors because of loss of CB1 receptor protein after chronic agonist exposure.15,20 One post-mortem study in humans also reported CB1 receptor downregulation in cannabis smokers that was greatest in hippocampus and least in globus pallidus and substantia nigra.37 The regional selectivity of downregulation is strikingly similar between rodents and humans in the current study: no significant downregulation was found in the basal ganglia and the midbrain in humans. Furthermore, we did not find downregulation in cerebellum, which is consistent with rodent studies showing less downregulation in this area compared with hippocampus. 16,21 The reasons for regional selectivity are unclear, but rodent studies suggest regional differences in distribution of markers of CB1 receptor signaling, such as G-protein subunits, subtypes of adenylyl cyclase, receptor kinases, arrestins and other proteins associated with receptor signaling.15,20,38
Could CB1 receptor downregulation be a mechanism for tolerance to cannabis in humans? That is, could regional differences in receptor downregulation predict differential development of tolerance toward various effects of cannabis? Interestingly, chronic daily cannabis smokers are tolerant to most, but not all, effects of cannabis. For example, tolerance develops for memory impairment,4,5 but not for feeling of high4,5 or motor impairment,5 induced by both intravenous and smoked Δ9-THC. Feelings of high and motor impairment might be driven by CB1 receptors in the basal ganglia, midbrain and cerebellum—regions that did not show receptor downregulation in the current study. Another interesting finding is that hippocampus did not show reversal of downregulation after abstinence: prolonged downregulation might contribute to long-term cognitive impairment in chronic daily cannabis smokers noted by some studies.39 This hypothesis, however, is simplistic because CB1 receptors are widespread in the human brain and complex psychological effects of cannabis are probably not limited to receptors in one particular brain region. In addition, CB1 receptor desensitization, which we cannot measure, may still occur in these regions despite unchanged receptor density. Finally, we did not objectively measure tolerance to cannabis. We only measured withdrawal and craving, neither of which correlated with CB1 receptor binding. Future studies should establish the relationship between tolerance to cannabis and regional CB1 receptor downregulation.
We found decreased CB1 receptor binding in subjects who had smoked large amounts of cannabis daily for years. Even in these heavy smokers, binding returned to normal levels in most regions after ~4 weeks of abstinence. We are unsure whether moderate and occasional cannabis smoking would produce similar downregulation. Considering that occasional cannabis smokers do not develop tolerance to the extent that chronic daily smokers do,40 downregulation of CB1 receptors may be smaller in occasional smokers, assuming that receptor downregulation contributes to tolerance. Nevertheless, future studies should specifically address that question by measuring CB1 receptor binding in occasional cannabis smokers.
Tobacco smoking was more prevalent among cannabis smokers than among control subjects. However, tobacco smoking is unlikely to explain the region-specific and reversible decrease in CB1 receptor binding in cannabis smokers, because tobacco smoking had no effects on VT in cannabis smokers or on the group × region interaction in the overall model. In addition, VT increased after ~4 weeks of abstinence from cannabis, even though subjects continued smoking tobacco during that time. Nevertheless, future studies should examine the effects of tobacco smoking on CB1 receptor binding in control subjects who do not smoke cannabis.
BMI correlated negatively with [18F]FMPEP-d2 VT in both groups, such that people with higher BMI tended to have lower VT than people with lower BMI. Although the cause of this association is unclear, we doubt that it represents an association with CB1 receptor density. Brain CB1 receptors are implicated in feeding behaviors.41 CB1 receptor stimulation in mouse brain can both promote and inhibit feeding behavior, depending on the dose of the agonist and the brain region involved.42 However, we found similar correlations in all brain regions, not restricted to those implicated in feeding behavior. Therefore, we suspect that this correlation is driven by some peripheral confound—potentially the fraction of free radioligand in plasma. For [18F]FMPEP-d2, this fraction is very small (< 1%) and imprecisely measured.22 Although we found no correlation between BMI and the measured plasma free fraction, a small but significant correlation cannot be excluded. The primary finding of decreased VT in cannabis smokers was region specific and therefore not likely confounded by the plasma free fraction that would affect VT globally. Of note, the outcome measure VT corrects for peripheral metabolism and disposition of the radioligand, which makes these factors unlikely confounders. The important implication of this finding is that BMI should be taken as a covariate in [18F]FMPEP-d2 studies, whatever the underlying mechanism.
In conclusion, we report for the first time decreased CB1 receptor binding in cortical, but not in subcortical, brain regions in chronic daily cannabis smokers, and that this downregulation is reversible after ~4 weeks of abstinence. Downregulation of CB1 receptors may underlie tolerance to effects of cannabis, and may provide insight into the role of CB1 receptors in diseases that are associated with chronic cannabis smoking, such as psychosis and depression. Future studies should determine whether occasional cannabis smokers or females show similar downregulation, and examine the time course of reversal of downregulation in greater detail.
Supplementary Material
Acknowledgments
We thank Kimberly Jenko, Kacey Anderson, and David Clark for measurements of radioligand in plasma; Maria D Ferraris Araneta, Yulin Chu, Denise Rallis-Frutos, Gerald Hodges, William C Kreisl, Christina Hines and Barbara Scepura as well as Kathleen Demuth and the NIDA and BPRU nursing staff for subject recruitment and care; the NIH PET Department for imaging; and PMOD Technologies for providing its image analysis and modeling software. This research was supported by the Intramural Programs of the NIMH (project no. Z01-MH-002852-04) and the NIDA (project no. Z01-DA000413-13). Jussi Hirvonen was supported by personal grants from The Academy of Finland; The Finnish Cultural Foundation; The Finnish Foundation for Alcohol Studies; The Finnish Medical Foundation; The Instrumentarium Foundation; The Jalmari and Rauha Ahokas Foundation; The Paulo Foundation; The Research Foundation of Orion Corporation; and The Yrjö Jahnsson Foundation.
Footnotes
Conflict of interest The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
References
- 1.Degenhardt L, Chiu WT, Sampson N, Kessler RC, Anthony JC, Angermeyer M, et al. Toward a global view of alcohol, tobacco, cannabis, and cocaine use: findings from the WHO World Mental Health Surveys. PLoS Med. 2008;5:e141. doi: 10.1371/journal.pmed.0050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet. 2009;374:1383–1391. doi: 10.1016/S0140-6736(09)61037-0. [DOI] [PubMed] [Google Scholar]
- 3.Jones RT, Benowitz NL, Herning RI. Clinical relevance of cannabis tolerance and dependence. J Clin Pharmacol. 1981;21:143S–152S. doi: 10.1002/j.1552-4604.1981.tb02589.x. [DOI] [PubMed] [Google Scholar]
- 4.D’Souza DC, Ranganathan M, Braley G, Gueorguieva R, Zimolo Z, Cooper T, et al. Blunted psychotomimetic and amnestic effects of Δ-9-tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology. 2008;33:2505–2516. doi: 10.1038/sj.npp.1301643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramaekers JG, Kauert G, Theunissen EL, Toennes SW, Moeller MR. Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. J Psychopharmacol. 2009;23:266–277. doi: 10.1177/0269881108092393. [DOI] [PubMed] [Google Scholar]
- 6.Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology (Berl) 1999;141:395–404. doi: 10.1007/s002130050849. [DOI] [PubMed] [Google Scholar]
- 7.Cooper ZD, Haney M. Actions of delta-9-tetrahydrocannabinol in cannabis: relation to use, abuse, dependence. Int Rev Psychiatry. 2009;21:104–112. doi: 10.1080/09540260902782752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huestis MA, Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, et al. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry. 2001;58:322–328. doi: 10.1001/archpsyc.58.4.322. [DOI] [PubMed] [Google Scholar]
- 9.Huestis MA, Boyd SJ, Heishman SJ, Preston KL, Bonnet D, Le Fur G, et al. Single and multiple doses of rimonabant antagonize acute effects of smoked cannabis in male cannabis users. Psychopharmacology (Berl) 2007;194:505–515. doi: 10.1007/s00213-007-0861-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- 12.Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 13.Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez S, Cebeira M, Fernandez-Ruiz J. Cannabinoid tolerance and dependence: a review of studies in laboratory animals. Pharmacol Biochem Behav. 2005;81:300–318. doi: 10.1016/j.pbb.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 15.Sim-Selley LJ. Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Crit Rev Neurobiol. 2003;15:91–119. doi: 10.1615/critrevneurobiol.v15.i2.10. [DOI] [PubMed] [Google Scholar]
- 16.Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Vogt LJ, Sim-Selley LJ. Chronic delta9-tetrahydrocannabinol treatment produces a time-dependent loss of cannabinoid receptors and cannabinoid receptor-activated G proteins in rat brain. J Neurochem. 1999;73:2447–2459. doi: 10.1046/j.1471-4159.1999.0732447.x. [DOI] [PubMed] [Google Scholar]
- 17.Oviedo A, Glowa J, Herkenham M. Chronic cannabinoid administration alters cannabinoid receptor binding in rat brain: a quantitative autoradiographic study. Brain Res. 1993;616:293–302. doi: 10.1016/0006-8993(93)90220-h. [DOI] [PubMed] [Google Scholar]
- 18.Sim-Selley LJ, Martin BR. Effect of chronic administration of R-(+)-[2,3-Dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl)methanone mesylate (WIN55,212-2) or delta 9-tetrahydrocannabinol on cannabinoid receptor adaptation in mice. J Pharmacol Exp Ther. 2002;303:36–44. doi: 10.1124/jpet.102.035618. [DOI] [PubMed] [Google Scholar]
- 19.Breivogel C, Scates SM, Beletskaya IO, Lowery OB, Aceto MD, Martin BR. The effects of Δ9-tetrahydrocannabinol physical dependence on brain cannabinoid receptors. Eur J Pharmacol. 2003;459:139–150. doi: 10.1016/s0014-2999(02)02854-6. [DOI] [PubMed] [Google Scholar]
- 20.Sim-Selley LJ, Schechter NS, Rorrer WK, Dalton GD, Hernandez J, Martin BR, et al. Prolonged recovery rate of CB1 receptor adaptation after cessation of long-term cannabinoid administration. Mol Pharmacol. 2006;70:986–996. doi: 10.1124/mol.105.019612. [DOI] [PubMed] [Google Scholar]
- 21.McKinney DL, Cassidy MP, Collier LM, Martin BR, Wiley JL, Selley DE, et al. Dose-related differences in the regional pattern of cannabinoid receptor adaptation and in vivo tolerance development to 9-tetrahydrocannabinol. J Pharmacol Exp Ther. 2008;324:664–673. doi: 10.1124/jpet.107.130328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terry GE, Hirvonen J, Liow JS, Zoghbi SS, Gladding R, Tauscher JT, et al. Imaging and quantitation of cannabinoid CB1 receptors in human and monkey brains using (18)F-labeled inverse agonist radioligands. J Nucl Med. 2010;51:112–120. doi: 10.2967/jnumed.109.067074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donohue SR, Krushinski JH, Pike VW, Chernet E, Phebus L, Chesterfield AK, et al. Synthesis, ex vivo evaluation, and radiolabeling of potent 1,5-diphenylpyrrolidin-2-one cannabinoid subtype-1 receptor ligands as candidates for in vivo imaging. J Med Chem. 2008;51:5833–5842. doi: 10.1021/jm800416m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heishman SJ, Singleton EG, Liguori A. Marijuana craving questionnaire: development and initial validation of a self-report instrument. Addiction. 2001;96:1023–1034. doi: 10.1046/j.1360-0443.2001.967102312.x. [DOI] [PubMed] [Google Scholar]
- 25.Zoghbi SS, Shetty HU, Ichise M, Fujita M, Imaizumi M, Liow JS, et al. PET imaging of the dopamine transporter with 18F-FECNT: a polar radiometabolite confounds brain radioligand measurements. J Nucl Med. 2006;47:520–527. [PubMed] [Google Scholar]
- 26.Gandelman MS, Baldwin RM, Zoghbi SS, Zea-Ponce Y, Innis RB. Evaluation of ultrafiltration for the free-fraction determination of single photon emission computed tomography (SPECT) radiotracers: beta-CIT, IBF, and iomazenil. J Pharm Sci. 1994;83:1014–1019. doi: 10.1002/jps.2600830718. [DOI] [PubMed] [Google Scholar]
- 27.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 28.Burger C, Mikolajczyk K, Grodzki M, Rudnicki P, Szabatin M, Buck A. Java tools for quantitative post-processing of brain PET data. J Nucl Med. 1998;39:277–278. [Google Scholar]
- 29.Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith C, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- 30.Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 31.Karschner EL, Schwilke EW, Lowe RH, Darwin WD, Herning RI, Cadet JL, et al. Implications of plasma Delta9-tetrahydrocannabinol, 11-hydroxy-THC, and 11-nor-9-carboxy-THC concentrations in chronic cannabis smokers. J Anal Toxicol. 2009;33:469–477. doi: 10.1093/jat/33.8.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karschner EL, Schwilke EW, Lowe RH, Darwin WD, Pope HG, Herning R, et al. Do Delta9-tetrahydrocannabinol concentrations indicate recent use in chronic cannabis users? Addiction. 2009;104:2041–2048. doi: 10.1111/j.1360-0443.2009.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lowe RH, Abraham TT, Darwin WD, Herning R, Cadet JL, Huestis MA. Extended urinary Delta9-tetrahydrocannabinol excretion in chronic cannabis users precludes use as a biomarker of new drug exposure. Drug Alcohol Depend. 2009;105:24–32. doi: 10.1016/j.drugalcdep.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Innis RB, Malison RT, al-Tikriti M, Hoffer PB, Sybirska EH, Seibyl JP, et al. Amphetamine-stimulated dopamine release competes in vivo for [123I]IBZM binding to the D2 receptor in nonhuman primates. Synapse. 1992;10:177–184. doi: 10.1002/syn.890100302. [DOI] [PubMed] [Google Scholar]
- 35.Terry G, Liow J, Chernet E, Zoghbi S, Phebus L, Felder C, et al. Positron emission tomography imaging using an inverse agonist radioligand to assess cannabinoid CB1 receptors in rodents. Neuroimage. 2008;41:690–698. doi: 10.1016/j.neuroimage.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rinaldi-Carmona M, Pialot F, Congy C, Redon E, Barth F, Bachy A, et al. Characterization and distribution of binding sites for [3H]-SR 141716A, a selective brain (CB1) cannabinoid receptor antagonist, in rodent brain. Life Sci. 1996;58:1239–1247. doi: 10.1016/0024-3205(96)00085-9. [DOI] [PubMed] [Google Scholar]
- 37.Villares J. Chronic use of marijuana decreases cannabinoid receptor binding and mRNA expression in the human brain. Neuroscience. 2007;145:323–334. doi: 10.1016/j.neuroscience.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 38.Martin BR, Sim-Selley LJ, Selley DE. Signaling pathways involved in the development of cannabinoid tolerance. Trends Pharmacol Sci. 2004;25:325–330. doi: 10.1016/j.tips.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 39.D’Souza DC. Cannabinoids and psychosis. Int Rev Neurobiol. 2007;78:289–326. doi: 10.1016/S0074-7742(06)78010-2. [DOI] [PubMed] [Google Scholar]
- 40.Cooper ZD, Haney M. Cannabis reinforcement and dependence: role of the cannabinoid CB1 receptor. Addict Biol. 2008;13:188–195. doi: 10.1111/j.1369-1600.2007.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cota D. CB1 receptors: emerging evidence for central and peripheral mechanisms that regulate energy balance, metabolism, and cardiovascular health. Diabetes Metab Res Rev. 2007;23:507–517. doi: 10.1002/dmrr.764. [DOI] [PubMed] [Google Scholar]
- 42.Bellocchio L, Lafenêtre P, Cannich A, Cota D, Puente N, Grandes P, et al. Bimodal control of stimulated food intake by the endocannabinoid system. Nat Neurosci. 2010;13:281–283. doi: 10.1038/nn.2494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


