Abstract
Accurate chromosome segregation during meiosis relies on homology between the maternal and paternal chromosomes. Yet by definition, sex chromosomes of the heterogametic sex lack a homologous partner. Recent studies in a number of systems have shed light on the unique meiotic behavior of heteromorphic sex chromosomes, and highlight both the commonalities and differences in divergent species. During meiotic prophase, the homology-dependent processes of pairing, synapsis, and recombination have been modified in many different ways to ensure segregation of heteromorphic sex chromosomes at the first meiotic division. Additionally, an almost universal feature of heteromorphic sex chromosomes during meiosis is transcriptional silencing, or meiotic sex chromosome inactivation, an essential process proposed to prevent expression of genes deleterious to meiosis in the heterogametic sex as well as to shield unpaired sex chromosomes from recognition by meiotic checkpoints. Comparative analyses of the meiotic behavior of sex chromosomes in nematodes, mammals, and birds reveal important conserved features as well as provide insight into sex chromosome evolution.
Keywords: checkpoints, meiosis, meiotic sex chromosome inactivation (MSCI), synapsis, recombination
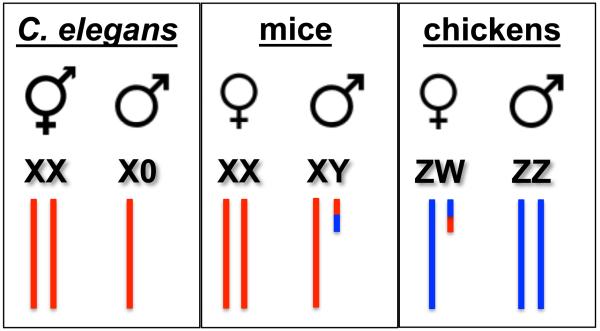
Sexual reproduction has been proposed to generate novel genotypes, purge deleterious mutations, and combat disease, offering a selective advantage over asexual reproduction (Bell, 1982; Crow, 1994; Hamilton et al., 1990; Horandl, 2009). These advantages must overcome the two-fold cost of sex, as only one parent bears progeny and only half of the genome is inherited from each parent (Smith, 1979). The reduction in ploidy is accomplished by meiosis, where in many organisms pairing, synapsis, and recombination between homologous chromosomes mediate chromosome segregation. In the heterogametic sex (i.e., the sex of the species in which the sex chromosomes are not the same), however, sex chromosomes are largely non-homologous. Consequently, multiple strategies have evolved to ensure that heteromorphic sex chromosomes are segregated faithfully. Here we focus on the meiotic behavior of heteromorphic sex chromosomes in three species: nematodes (Caenorhabditis elegans), where males have a single X chromosome that completely lacks a pairing partner, mammals (Mus musculus), where males have heterologous X and Y chromosomes with only discrete regions of homology, and birds (Gallus gallus), where females are the heterogametic sex with Z and W chromosomes, which are also largely non-homologous (Figure 1). These distinct sex chromosome karyotypes illustrate both the similarities and differences underlying meiotic sex chromosome behavior in species that rely on homologous recombination for the segregation of chromosomes.
Figure 1.
Sex chromosomes in C. elegans, mice and chickens. Schematic of sex chromosome karyotypes in the two sexes of worms (hermaphrodites and males), mice (females and males) and chickens (females and males). Note that females are the heterogametic sex in chickens.
Double strand break formation, synapsis, and recombination on sex chromosomes
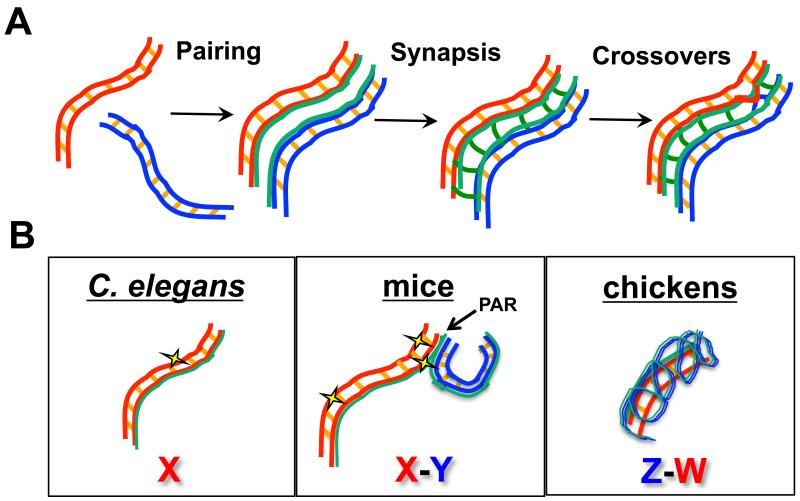
Meiosis is the specialized cellular division program that results in the precise halving of the genome to generate haploid gametes for sexual reproduction. To ensure that each gamete receives the correct complement of DNA, a highly orchestrated program of nuclear events occurs (Figure 2A). The genome must first be faithfully replicated; this is followed by a lengthy prophase where homologous chromosomes pair, synapse, and undergo genetic exchange (Kleckner, 1996; Page and Hawley, 2003; Roeder, 1997; Zickler and Kleckner, 1999). Pairing assesses homology between DNA sequences, and culminates in the close alignment of the maternal and paternal homologous chromosomes. Chromosome alignment is stabilized by synapsis, which is mediated by a large proteinaceous structure called the synaptonemal complex. The synaptonemal complex is made up of axial elements that form along and between sister chromatids during early meiotic prophase and is the central element that connects the two axial elements, now termed lateral elements, of the homologous chromosomes. Within the context of the synaptonemal complex, crossover recombination between homologous chromosomes forms the connections (chiasmata) that, in conjunction with regulated sister chromatid cohesion release, enable segregation of homologous chromosomes to opposite poles at the meiosis I division. During meiosis II, which occurs without an intervening S-phase, sister chromatids separate from each other. The meiotic divisions are coupled to a program of cellular differentiation that results in the production of highly differentiated gametes, e.g. sperm and egg, competent for sexual reproduction. Errors in meiotic chromosome segregation result in sterility or the production of inviable or defective progeny. Given the heterologous nature of sex chromosomes, it is not surprising that the nondisjunction rate for sex chromosomes is higher than for autosomes in human males (Shi et al., 2001).
Figure 2.
Autosome and sex chromosome behavior in meiotic prophase. A) Major events in meiotic prophase are shown for homologous autosomes (red and blue). Duplicated sister chromatids are held together by sister chromatid cohesion (orange cross lines between sisters) and elaborate chromosomal axes (green) during the process of pairing, where homologous chromosomes align. During synapsis, the synaptonemal complex (green cross lines) stabilizes the aligned homologous chromosomes. In this context, crossover recombination occurs to allow for segregation of homologous chromosomes at the first meiotic division. B) Configuration of heteromorphic sex chromosomes in C. elegans, mice, and chickens in meiotic prophase. In worms, a single X chromosome lacks a partner but elaborates axial components and incurs DSBs (yellow star). In mice, the X and Y pair and synapse at the pseudoautosomal region (PAR), and DSBs are found along the length of the XY chromosomes but occur at a higher frequency at the PAR. In chickens, the larger Z chromosome synapses with the smaller, non-homologous W chromosome; at this stage no markers of DSBs are observed.
Meiotic recombination is initiated by the formation of double strand breaks (DSBs), mediated by the conserved topoisomerase-like protein Spo11 and associated proteins (Cole et al., 2010). Unlike mitosis, where the sister chromatid is the preferred template for repair, during meiosis, the homologous chromosome is used as the repair template to generate inter-homolog crossovers that serve to link the homologous chromosomes together for metaphase alignment and segregation at the first meiotic division (Kleckner, 1996; Page and Hawley, 2003; Roeder, 1997; Zickler and Kleckner, 1999). In most male placental mammals, homology between the X and Y sex chromosomes is limited to the small pseudo-autosomal region(s) (PAR). Consequently, synapsis and recombination between the X and Y occurs exclusively at the PAR(s) (Handel, 2004) (Figure 2B). Interestingly, meiotic recombination in the PAR is approximately ten-fold greater in male mice compared with female mice; this higher rate of recombination is presumed to ensure disjunction of the largely non-homologous XY pair (Rouyer et al., 1986; Soriano et al., 1987). A recent study found that both the timing and rates of meiotic DSB formation are different at the mouse PAR compared to the autosomes in males, and provides evidence that this is achieved by both altered chromosome axis structure and dedicated recombination machinery (Kauppi et al., 2011). Cytological analyses revealed that XY pairing occurs later in meiotic prophase than autosomal pairing. Further, higher-order chromosome structure is distinct in the PAR such that PAR chromosomal axes are longer than corresponding autosomal chromosomal segments. Fluorescence in situ hybrisization (FISH) analyses revealed that longer chromosomal axes correspond to shorter chromatin loops extending from the axis. As DSBs are proposed to occur in the loops, the authors suggest that this chromosome configuration is conducive for break formation, providing an explanation for the higher frequency of DSBs and consequently recombination at the PAR. In addition, the recombination machinery appears to have evolved to ensure that DSBs occur at the PAR; an isoform of Spo11 is expressed later in meiotic prophase and appears to be responsible for the differential timing and levels of DSBs at the PAR in male mice (Kauppi et al., 2011).
In contrast to the XY in male mice, where homology is present, but limited, there is a single X chromosome but no corresponding Y in C. elegans males (Figure 1), thus no homologous sequences are present to promote pairing, synapsis and recombination. On the other hand, XX worms are hermaphrodites, producing sperm during the initial wave of gametogenesis and then switching to oocyte production as adults, and are thus functionally female; the homologous Xs pair, synapse and recombine similarly to the autosomes. Analogous to the non-PAR regions of mammalian X and Y chromosomes, the C. elegans single X chromosome of males does not participate in pairing and synapsis, although axial components are loaded along the length of the X (Henzel et al., 2011; Jaramillo-Lambert and Engebrecht, 2010; She et al., 2009) (Figure 2B). Surprisingly, both the single X in C. elegans males and the regions of mammalian sex chromosomes that do not participate in pairing and synapsis are substrates for the meiotic recombination machinery (Ashley et al., 1995; Jaramillo-Lambert and Engebrecht, 2010; Moens et al., 1997). DSBs generated on the asynapsed regions of unpaired sex chromosomes must use either the sister chromatid as the repair template or alternatively, a repair pathway that does not rely on homology such as non-homologous end joining (NHEJ). While NHEJ is repressed during early prophase in mammalian meiosis, NHEJ components become enriched on the X-Y body at the pachytene stage (Goedecke et al., 1999), suggesting NHEJ could play a role in the repair of DSBs generated on non-homologous regions of sex chromosomes. Additionally, studies in both mouse and C. elegans indicate that NHEJ plays a role in late prophase in response to DNA damage or chromosomal asynapsis (Ahmed et al., 2010; Smolikov et al., 2007). Thus, mechanisms must exist to promote sister chromatid recombination and/or NHEJ (and perhaps other repair pathways) when breaks are induced on regions of sex chromosomes lacking a pairing partner.
Analysis of a chromosomal fusion between the X and an autosome in C. elegans provides insight into how differences in recombination rates and synaptic behavior of sex chromosomes may have evolved (Henzel et al., 2011). Worms carrying a single copy of the X-autosome fusion are males and have a similar chromosome configuration as the XY in mammals, where there are both homologous (PAR) and non-homologous sequences between the chromosomes. Despite sharing an extensive domain of homology (corresponding to the autosomal sequences), most of the recombination that occurs between these two chromosomes is restricted to a limited region of the distal end of the chromosome pair furthest away from the fusion. Over time, this altered pattern of recombination would allow sequence divergence, except at the distal-most end, resulting in establishment of a PAR. In addition to altered recombination, cytological analyses revealed that the axis of the fusion chromosome has a unique coiled morphology. As prophase proceeds, the much larger fusion chromosome and corresponding autosome achieve almost complete synapsis (Henzel et al., 2011). These results suggest that there is synaptic adjustment between the fusion and partner chromosome, similar to what is observed between the heteromorphic sex chromosomes of chickens.
In birds, females are the heterogametic sex containing Z and W chromosomes, while males are homogametic (ZZ) (Figure 1). Similar to the XY in mice, Z and W share a small PAR (Solovei et al., 1993); like XY, pairing of ZW is also delayed relative to the pairing of the autosomes (Schoenmakers et al., 2009). Unlike the behavior of the XY in male mammals, the entire ZW pair achieves complete synapsis during chicken female oogenesis. This is due to synaptic adjustment where the longer Z thickens and shortens, and appears to wrap itself around the smaller W (Solari, 1992) (Figure 2B). Thus, as was observed on the X-autosome fusion chromosome in the study described above in C. elegans, the chicken female ZW chromosomes undergo synapsis of non-homologous sequences. As prophase progresses, the Z and W chromosomes desynapse but remain attached at their tips, presumably at the PAR.
As with both mammals and worms, the chicken Z and W chromosomes are substrates for the meiotic recombination machinery (Schoenmakers et al., 2009). Similar to recombination in the XY PAR, there appears to be differential regulation of recombination on the Z and W chromosomes. Using histone H2AX phosphorylated at serine 139 (γH2AX) and the recombinase RAD51 as markers for DSB formation and processing, Schoenmakers and colleagues found that there are two waves of γH2AX and RAD51 accumulation on the Z chromosome (Schoenmakers et al., 2009). The first wave occurs early in prophase, when γH2AX and RAD51 are observed on all chromosome pairs; these markers disappear once the Z and W achieve complete synapsis. Upon desynapsis, a second wave of γH2AX and RAD51 appears specifically on the Z chromosome. Whether this represents new breaks (by an isoform of Spo11, as in mice) or a delay in the repair of breaks to prevent recombination between non-homologous Z and W sequences has not been determined. While the specific mechanisms underlying the regulation of recombination on the chicken ZW chromosomes are currently unknown, it is likely that sister chromatid recombination and/or NHEJ is used in this context as well. Taken together, multiple strategies have evolved to regulate pairing, synapsis and recombination between heteromorphic sex chromosomes.
Meiotic Sex Chromosome Inactivation
Meiotic sex chromosome inactivation (MSCI) is a repressive mechanism that occurs during meiotic prophase I, and involves elaboration of a specialized heterochromatin domain and transcriptional silencing of heteromorphic sex chromosomes (Turner, 2007). MSCI has been proposed to be a genomic defense mechanism against selfish genetic elements (Kelly and Aramayo, 2007), a consequence of sexual antagonism, where expression of a gene is beneficial to one sex but harmful to the other (Meiklejohn and Tao, 2010; Wu and Xu, 2003) and/or a mechanism to avoid recombination between non-homologous regions of sex chromosomes (Inagaki et al., 2010; McKee and Handel, 1993) (see below). MSCI is required for efficient meiotic progression in heterogametic germ lines, as failure to inactivate partnerless or non-homologous regions of sex chromosomes results in elevated germline apoptosis in both worms and mice (Checchi and Engebrecht, 2011; Turner et al., 2006), and may contribute to male infertility in humans (Royo et al., 2010; Turner, 2007). In mammals, MSCI occurs exclusively within the primary spermatocytes and is coincident with formation of the XY (sex) body, a specialized sub-nuclear domain whose proposed role is to prevent illegitimate recombination events outside of the PAR (Handel, 2004; Hoyer-Fender, 2003; Solari, 1974). Similar to the inactive/heterochromatinized X chromosome (Barr body) present in female somatic cells, a prominent feature of the XY body is that it recruits heterochromatin-associated proteins and exhibits a condensed morphology that is distinct from the transcriptionally active autosomes (Chadwick and Willard, 2003; Handel, 2004; Peters et al., 2001; Solari, 1974). Additionally, both the Barr body X and the XY body accumulate Xist RNA; while this is required for X inactivation in females, the absence of Xist does not disrupt MSCI, indicating that the two mechanisms of X-specific silencing are regulated independently (Ayoub et al., 1997; Turner et al., 2002).
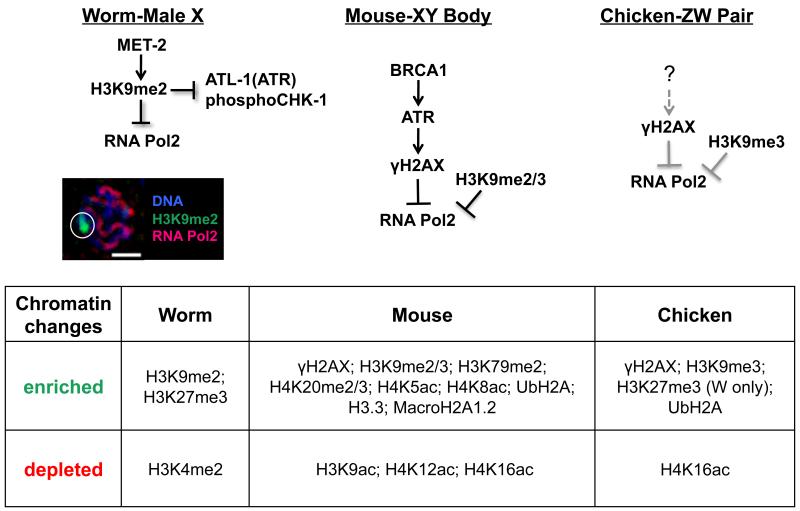
A hallmark of MSCI is the deposition of histone modifications corresponding to transcriptional inactivation (Zamudio et al., 2008) (Figure 3). The amino-terminal tails of histones are subject to a number of post-translational modifications that account for many of the dynamic changes in chromatin organization that occur throughout meiotic progression (Kota and Feil, 2010; Ooi and Henikoff, 2007). Histone modifications associated with MSCI have been studied most extensively in mouse spermatogenesis (Baarends and Grootegoed, 2003; Zamudio et al., 2008), during which nucleosomes within the XY body are subject to widespread replacement of core histones with histone variants including macroH2A.1 (Hoyer-Fender et al., 2000) and de novo incorporation of histone variant H3.3 (van der Heijden et al., 2007) (Figure 3B). Beginning at the early pachytene stage, both di- and tri-methylation of lysine 9 on histone H3 (H3K9me2/3), modifications corresponding to transcriptional inactivation and heterochromatin domains (Grewal and Jia, 2007; Peters et al., 2001; Wang et al., 2008), become enriched within the XY body (Cowell et al., 2002; Khalil et al., 2004; van der Heijden et al., 2007) coinciding with significant deacetylation of H3 and H4 (Khalil et al., 2004). MSCI in mice also involves a transient increase in the repressive histone marks H4K20me2/3 and H3K79me2, which are detected in early pachytene spermatocytes yet disappear by mid-pachytene stage (van der Heijden et al., 2007) (Figure 3B).
Figure 3.
Regulation of MSCI. A) Schematic of proposed molecular events targeting MSCI activation in worm, mouse, and chicken. Left: In C. elegans, the heterochromatinized male X accumulates met-2-dependent H3K9me2, a repressive mark required to block accumulation of the activated form of RNA polymerase as well as components of the recombination repair pathway, including kinases ATL-1 (ATR) and phosphorylated CHK-1 (pCHK-1). Bottom: Fluorescent micrograph of a single, C. elegans male pachytene nucleus demonstrating accumulation of H3K9me2 (green) and absence of activated RNA polymerase II (red) on the male X (identified by white circle); Scale bar=2 μM. Middle: In mouse, MSCI is activated by BRCA1-dependent ATR recruitment, which in turn phosphorylates H2AX and subsequently blocks RNA polymerase II activation, coincident with accumulation of H3K9me2/3 to the XY body (Fernandez-Capetillo et al., 2003; Turner et al., 2004). Right: In chicken, cytological analyses reveal that γH2AX and H3K9me3 are recruited to the ZW chromosomes, and RNA polymerase is excluded (Schoenmakers et al. 2009); however, whether or not this is dependent on BRCA1/ATR, as in mouse, is unknown (?). B) During MSCI, heteromorphic sex chromosomes are characterized by a number of epigenetic modifications, including the incorporation of histone variants, as well as both the acquisition and loss of sex-chromosome-specific histone modifications [see also (Zamudio et al. 2008) and (Maine 2010) for a more extensive discussion of these processes in mouse and worm].
While less is known about MSCI in worms and birds, both species also accrue repressive histone marks on heteromorphic sex chromosomes: in C. elegans, the lone X chromosome of males is characterized by the specific enrichment of H3K9me2 and the corresponding depletion of the activating mark H3K4me2 (Bean et al., 2004; Jaramillo-Lambert and Engebrecht, 2010), while in chicken oocytes, the ZW pair becomes enriched for H3K9me3 (Schoenmakers et al., 2009) (Figure 3B). Similar to the XY body in mouse, the ZW pair is also depleted for H4K16ac, which is coincident with an increase in H3K27me3 on the W chromosome (Schoenmakers et al., 2009). As in chicken, the male X in C. elegans is enriched for H3K27me3; however, it is unclear whether or not this mark directly contributes to MSCI as H3K27me3 is also enriched on paired X chromosomes (Bender et al., 2006).
In mouse, worm and chicken, accumulation of H3K9me2/3 on sex chromosomes corresponds to the concurrent exclusion of activated RNA polymerase II (Checchi and Engebrecht, 2011; Fernandez-Capetillo et al., 2003; Schoenmakers et al., 2009), which is consistent with transcriptional repression being a primary output of MSCI (Figure 3A). These data are supported by both in situ hybridization analyses, comparing X-linked transcripts in homogametic (XX) versus heterogametic (X0) worms (Bean et al., 2004; Jaramillo-Lambert and Engebrecht, 2010), as well as real-time RT-PCR assays, comparing stage-specific expression of ZW-linked transcripts during chicken oogenesis (Schoenmakers et al., 2009). Additionally, microarray data and analyses of expression sequence tag libraries reveal a significant under-representation of male-biased, X-linked genes expressed during meiosis in worms and mammals (Kaiser and Ellegren, 2006; Khil et al., 2004; Reinke et al., 2000; Storchova and Divina, 2006). The paucity of male-biased meiotic X-linked genes is presumably a result of MSCI, indicating that regulation of heteromorphic sex chromosome gene expression may be a potential driving force of sex chromosome evolution (Ellegren, 2011; Khil et al., 2004). In contrast to mice and worms, transcriptional silencing in chickens does not appear to be limited to meiotic genes as there is an under-representation of both somatic and germline, female-biased genes on the Z chromosome (Kaiser and Ellegren, 2006; Khil et al., 2004; Reinke et al., 2000; Storchova and Divina, 2006). Furthermore, whereas MSCI in worms and mammals persists into the post-meiotic period, transcriptional silencing in chicken oocytes is limited to the first meiotic prophase (Schoenmakers et al., 2009).
A number of additional chromatin remodeling events correspond with transcriptional repression during MSCI. In mouse, this includes the recruitment of phosphorylated H2AX (γH2AX) and ubiquitination of histone H2A (UbH2A) (Baarends et al., 1999; Fernandez-Capetillo et al., 2003; Turner et al., 2004). Studies in H2AX−/− mice have underscored the importance of this modification for mammalian spermatogenesis, as males lacking γH2AX are infertile and display a severe prophase phenotype corresponding to increased X-Y asynapsis and a failure to form an XY body or undergo transcriptional inactivation (Celeste et al., 2002; Fernandez-Capetillo et al. 2003). Additional work by Turner and colleagues demonstrated that H2AX is phosphorylated by the checkpoint sensor kinase ATR, and that this is dependent upon the tumor suppressor protein BRCA1; during MSCI, BRCA1 recruits ATR and localizes to the XY body, and its activity corresponds to the dynamics of γH2AX localization (Bellani et al., 2005; Mahadevaiah et al., 2001; Turner et al., 2004). Interestingly, while γH2AX is recruited in response to the initiation of meiotic DSBs, γH2AX is still detected in Spo11−/− spermatocytes, suggesting a Spo11-independent role for γH2AX localization to the sex body (Mahadevaiah et al., 2001). However, the γH2AX-containing domain formed in Spo11 mutants is not specific to XY chromatin, arguing that SPO11 is required for elaboration of the XY body during MSCI (Barchi et al., 2005; Bellani et al., 2005). Subsequent studies distinguished two distinct classes of γH2AX foci, which were characterized as either Spo11-dependent (corresponding to meiotic DSB repair events) or Spo11-independent (later DSBs/chromatin remodeling events), raising the possibility that in the absence of SPO11, additional γH2AX-dependent chromatin remodeling occurs within the pseudo-sex body (Chicheportiche et al., 2007). More recently, studies from Mahadevaiah and colleagues have demonstrated that while the γH2AX-containing pseudo sex body in Spo11−/− spermatocytes displays a number of features characteristic of MSCI, including transcriptional inactivation and ATR and BRCA1 localization, these components were not recruited to all unsynapsed chromatin (Mahadevaiah et al., 2008). Thus, while components of meiotic silencing are recruited to the pseudo-sex body in Spo11−/− spermatocytes, their activity is compromised due to the absence of SPO11, as evidenced by elevated pachytene apoptosis, characteristic of MSCI failure (Mahadevaiah et al., 2008).
Ubiquinated histone H2A (UbH2A) is also enriched on the XY body during meiotic prophase (Baarends et al., 1999; Baarends et al., 2005). The ubiquitin ligase RNF8, which has been shown to be responsible for the bulk of ubiquitinated H2A on the XY body, is not required for MSCI and is instead required for nucleosomal removal during spermatogenesis (Lu et al., 2010). Additional enzymes that mediate ubiquitination have been implicated in MSCI, including UBR2 and HR6B (Baarends et al., 1999; Baarends et al., 2005; Kwon et al., 2003), although the specific targets of these enzymes are not clear. While analysis of UBR2 knockout mice revealed that UbH2A is absent and transcriptional silencing on the XY is perturbed (An et al. 2010), it has been suggested that this is a consequence of an early block in the UBR2 knockout and not a direct affect of the absence of this ubiquitin ligase (Mulugeta Achame et al., 2010). On the other hand, the absence of HRB6 affects a number of histone modifications, but not UbH2A; prevailing evidence indicates that HRB6 plays a role in the maintenance of MSCI (An et al., 2010; Baarends et al., 2007).
The ZW pair in chicken also accumulates both UbH2A and γH2AX; however, appearance of these marks is transient (Schoenmakers et al., 2009). Although it is not yet known if the histone variant H3.3 plays a role during MSCI in chicken, data from the Henikoff lab indicate that H3.3 is depleted from the X chromosome during worm spermatogenesis (Ooi et al., 2006), highlighting a potential disconnect between H3.3’s contribution to heteromorphic sex chromosome regulation in mammals versus worms. Additionally, there is no worm homolog of H2AX, and instead, MSCI appears to be solely dependent upon a repressive chromatin architecture that blocks RNA polymerase II recruitment (Maine, 2010). Further, unlike in mice, BRC-1 (the worm homolog of BRCA1) does not appear to be required for MSCI in worms; during the pachytene stage, the male X is transcriptionally silenced and accumulates robust H3K9me2 in brc-1 mutants (Checchi and Engebrecht, unpublished observations). Thus, it appears that while repressive chromatin structure is a common feature of MSCI, there are differences in the molecular machinery that orchestrate its establishment (Figure 3A).
The relationship between MSCI and meiotic checkpoints
Errors in meiosis are sensed by meiotic checkpoints, conserved surveillance mechanisms that safeguard the integrity of the genome and limit the formation of aneuploid gametes. In response to asynapsis or unrepaired recombination intermediates, meiotic checkpoints respond by either activating repair mechanisms or culling the faulty germ cells by apoptosis (Borner, 2006; Jordan, 2006; Longhese et al., 2009; Macqueen and Hochwagen, 2011; Roeder and Bailis, 2000). An outstanding question, however, is how heteromorphic sex chromosomes, which by definition lack a homologous pairing partner, are barred from meiotic checkpoint activation. In mice, transcriptional silencing of the sex chromosomes appears to supersede the need to block checkpoint signaling. In fact, the checkpoint sensor ATR and mediator of DNA damage checkpoint 1 (MDC1) accumulate on the sex chromosomes and are essential for MSCI, yet in this context do not activate checkpoint-dependent apoptosis (Ichijima et al., 2011; Turner et al. 2004, 2005). Prevailing evidence suggests that when MSCI is perturbed by mutations in genes required for meiotic recombination, leading to chromosome asynapsis (Mahadevaiah et al., 2008) or unusual sex chromosome karyotypes that result in changes in synapsis, it is the expression of meiotic lethal genes that leads to apoptosis and not checkpoint activation per se (Royo et al., 2010).
In contrast to mice, the transcriptionally silenced X chromosome of C. elegans males does not recruit ATL-1 (the worm ortholog of ATR) or other checkpoint components and specifically evades checkpoint activation (Checchi and Engebrecht, 2011; Jaramillo-Lambert et al., 2010). In C. elegans, two distinct checkpoint pathways have been uncovered that detect unrepaired DSBs and asynapsed chromosomes, respectively (Bhalla and Dernburg, 2005; Gartner et al., 2000), and while the male X loads axial components and accrues DSBs, it does not activate either the synapsis nor recombination checkpoints (Jaramillo-Lambert and Engebrecht, 2010). As in X0 mice, the partnerless X in worms can load synaptonemal complex components and is capable of achieving non-homologous self-synapsis; however, there is no difference in checkpoint-activation between C. elegans X0 nuclei containing a self-synapsed X and those that do not achieve self-synapsis, indicating that the capability to self-synapse is independent from checkpoint activation in heterogametic worms (Jaramillo-Lambert and Engebrecht, 2010).
As in mouse and chicken, the single X in the worm heterogametic germ line is transcriptionally silent and adopts a heterochromatinized architecture (Bean et al., 2004; Jaramillo-Lambert and Engebrecht, 2010), suggesting that repressive chromatin modifying proteins may directly regulate checkpoint evasion on the male X. Indeed, C. elegans heterogametic germ lines lacking the histone methyltransferase MET-2, which is necessary for H3K9me2 deposition on the partnerless X (Bessler et al., 2010), exhibit MSCIs defects including recruitment of meiotic checkpoint proteins and ectopic accumulation of activated RNA polymerase (Checchi and Engebrecht, 2011). The defects in MSCI observed in C. elegans met-2 heterogametic mutant germ lines are dependent upon the recombination checkpoint; met-2-dependent checkpoint activation involves recruitment of ATL-1 (ATR) and is fully suppressed in the absence of CEP-1 (the worm homolog of p53) (Checchi and Engebrecht, 2011). In contrast, met-2-dependent checkpoint activation was unaffected in heterogametic germ lines lacking PCH-2 (a component of the synapsis checkpoint), suggesting that the recombination checkpoint is solely responsible for detecting the lone X in germ lines lacking MET-2 (Checchi and Engebrecht, 2011).
Conclusions
Heteromorphic sex chromosomes have evolved multiple strategies to navigate meiosis without a homologous partner. This includes alterations in the timing and extent of pairing, synapsis and recombination, as well as the molecular machinery, to ensure proper segregation of non-homologous sex chromosomes. Regardless of how different organisms have modified these homology-dependent processes, heterogametic sex chromosomes of worms, mice and chicken all undergo MSCI, a specialized form of meiotic silencing (Kelly and Aramayo, 2007; Maine, 2010; Turner, 2009). Studies from a large number of organisms indicate that aspects of MSCI have common features, including acquisition of repressive chromatin and transcriptional inactivity. Moreover, genetic analyses in both C. elegans and mice have demonstrated that inappropriate RNA polymerase II recruitment to either the single X (worm) or XY body (mouse) corresponds to MSCI failure (Checchi and Engebrecht, 2011; Fernandez-Capetillo et al., 2003).
A partnerless, unpaired sex chromosome (e.g. C. elegans) or transiently/incompletely paired set of sex chromosomes (e.g. chicken and mouse) must also evade detection from meiotic checkpoints. As discussed above, a shared feature among heteromorphic sex chromosomes is that they are subject to transcriptional silencing via MSCI, and a plausible scenario supported by recent studies in C. elegans (Checchi and Engebrecht, 2011) suggest that the molecular machinery controlling MSCI may play a dual role in directly blocking checkpoint proteins from accessing the heteromorphic sex chromosomes. Interestingly, in Drosophila males (XY), meiosis occurs in the absence of recombination and hence the need to block checkpoint activation should be alleviated. Indeed, while the X is deplete of male-biased genes (Parisi et al., 2003), a recent study provided evidence that MSCI does not occur in Drosophila (Mikhaylova and Nurminsky, 2011; but see Vibranovski et al., 2009), suggesting that MSCI is intimately connected to blocking checkpoint signaling. An exciting prospect for the future will be to tease out the functional relationship(s) between MSCI and meiotic checkpoint regulation, and to ultimately elucidate how these processes have shaped sex chromosome evolution.
Acknowledgments
We thank Sean Burgess, Kate Lawrence, Carly Stevens and reviewers for input and critical reading of the manuscript. We apologize to those colleagues whose primary work could not be cited or is cited only through reviews because of space limitations.
Grant Sponsor: National Institute of Health, grant number: GM086505 (JE) and T32 CA10849 (PMC).
Abbreviations
- DSB
double strand breaks
- MSCI
meiotic sex chromosome inactivation
- NHEJ
non-homologous end joining
- PAR
pseudo-autosomal region
Footnotes
Quote: “[M]ultiple strategies have evolved to ensure that heteromorphic sex chromosomes are segregated faithfully.”
References
- Ahmed EA, Philippens ME, Kal HB, de Rooij DG, de Boer P. Genetic probing of homologous recombination and non-homologous end joining during meiotic prophase in irradiated mouse spermatocytes. Mutat Res. 2010;688(1-2):12–18. doi: 10.1016/j.mrfmmm.2010.02.004. [DOI] [PubMed] [Google Scholar]
- An JY, Kim EA, Jiang Y, Zakrzewska A, Kim DE, Lee MJ, Mook-Jung I, Zhang Y, Kwon YT. UBR2 mediates transcriptional silencing during spermatogenesis via histone ubiquitination. Proc Natl Acad Sci U S A. 2010;107(5):1912–1917. doi: 10.1073/pnas.0910267107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley T, Plug AW, Xu J, Solari AJ, Reddy G, Golub EI, Ward DC. Dynamic changes in Rad51 distribution on chromatin during meiosis in male and female vertebrates. Chromosoma. 1995;104(1):19–28. doi: 10.1007/BF00352222. [DOI] [PubMed] [Google Scholar]
- Ayoub N, Richler C, Wahrman J. Xist RNA is associated with the transcriptionally inactive XY body in mammalian male meiosis. Chromosoma. 1997;106(1):1–10. doi: 10.1007/s004120050218. [DOI] [PubMed] [Google Scholar]
- Baarends WM, Grootegoed JA. Chromatin dynamics in the male meiotic prophase. Cytogenet Genome Res. 2003;103(3-4):225–234. doi: 10.1159/000076808. [DOI] [PubMed] [Google Scholar]
- Baarends WM, Hoogerbrugge JW, Roest HP, Ooms M, Vreeburg J, Hoeijmakers JH, Grootegoed JA. Histone ubiquitination and chromatin remodeling in mouse spermatogenesis. Dev Biol. 1999;207(2):322–333. doi: 10.1006/dbio.1998.9155. [DOI] [PubMed] [Google Scholar]
- Baarends WM, Wassenaar E, Hoogerbrugge JW, Schoenmakers S, Sun ZW, Grootegoed JA. Increased phosphorylation and dimethylation of XY body histones in the Hr6b-knockout mouse is associated with derepression of the X chromosome. J Cell Sci. 2007;120(Pt 11):1841–1851. doi: 10.1242/jcs.03451. [DOI] [PubMed] [Google Scholar]
- Baarends WM, Wassenaar E, van der Laan R, Hoogerbrugge J, Sleddens-Linkels E, Hoeijmakers JH, de Boer P, Grootegoed JA. Silencing of unpaired chromatin and histone H2A ubiquitination in mammalian meiosis. Mol Cell Biol. 2005;25(3):1041–1053. doi: 10.1128/MCB.25.3.1041-1053.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean CJ, Schaner CE, Kelly WG. Meiotic pairing and imprinted X chromatin assembly in Caenorhabditis elegans. Nat Genet. 2004;36(1):100–105. doi: 10.1038/ng1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. The masterpiece of nature: the evolution and genetics of sexuality. Kluwer Academic Publishers; 1982. [Google Scholar]
- Bellani MA, Romanienko PJ, Cairatti DA, Camerini-Otero RD. SPO11 is required for sex-body formation, and Spo11 heterozygosity rescues the prophase arrest of Atm−/− spermatocytes. J Cell Sci. 2005;118(Pt 15):3233–3245. doi: 10.1242/jcs.02466. [DOI] [PubMed] [Google Scholar]
- Bender LB, Suh J, Carroll CR, Fong Y, Fingerman IM, Briggs SD, Cao R, Zhang Y, Reinke V, Strome S. MES-4: an autosome-associated histone methyltransferase that participates in silencing the X chromosomes in the C. elegans germ line. Development. 2006;133(19):3907–3917. doi: 10.1242/dev.02584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessler JB, Andersen EC, Villeneuve AM. Differential localization and independent acquisition of the H3K9me2 and H3K9me3 chromatin modifications in the Caenorhabditis elegans adult germ line. PLoS Genet. 2010;6(1):e1000830. doi: 10.1371/journal.pgen.1000830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla N, Dernburg AF. A conserved checkpoint monitors meiotic chromosome synapsis in Caenorhabditis elegans. Science. 2005;310(5754):1683–1686. doi: 10.1126/science.1117468. [DOI] [PubMed] [Google Scholar]
- Borner GV. Balancing the checks: surveillance of chromosomal exchange during meiosis. Biochem Soc Trans. 2006;34(Pt 4):554–556. doi: 10.1042/BST0340554. [DOI] [PubMed] [Google Scholar]
- Burgoyne PS, Mahadevaiah SK, Turner JM. The consequences of asynapsis for mammalian meiosis. Nat Rev Genet. 2009;10(3):207–216. doi: 10.1038/nrg2505. [DOI] [PubMed] [Google Scholar]
- Celeste A, Petersen S, Romanienko PJ, Fernandez-Capetillo O, Chen HT, Sedelnikova OA, Reina-San-Martin B, Coppola V, Meffre E, Difilippantonio MJ. Genomic instability in mice lacking histone H2AX. Science. 2002;296(5569):922. doi: 10.1126/science.1069398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick BP, Willard HF. Chromatin of the Barr body: histone and non-histone proteins associated with or excluded from the inactive X chromosome. Hum Mol Genet. 2003;12(17):2167–2178. doi: 10.1093/hmg/ddg229. [DOI] [PubMed] [Google Scholar]
- Checchi P, Engebrecht J. C. elegans Histone Methyltransferase MET-2 Shields the Male X Chromosome from Checkpoint Machinery and Mediates Meiotic Sex Chromosome Inactivation. PLoS Genet. 2011 doi: 10.1371/journal.pgen.1002267. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicheportiche A, Bernardino-Sgherri J, de Massy B, Dutrillaux B. Characterization of Spo11-dependent and independent phospho-H2AX foci during meiotic prophase I in the male mouse. J Cell Sci. 2007;120(Pt 10):1733–1742. doi: 10.1242/jcs.004945. [DOI] [PubMed] [Google Scholar]
- Cole F, Keeney S, Jasin M. Evolutionary conservation of meiotic DSB proteins: more than just Spo11. Genes Dev. 2010;24:1201–1207. doi: 10.1101/gad.1944710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell IG, Aucott R, Mahadevaiah SK, Burgoyne PS, Huskisson N, Bongiorni S, Prantera G, Fanti L, Pimpinelli S, Wu R, Gilbert DM, Shi W, Fundele R, Morrison H, Jeppesen P, Singh PB. Heterochromatin, HP1 and methylation at lysine 9 of histone H3 in animals. Chromosoma. 2002;111(1):22–36. doi: 10.1007/s00412-002-0182-8. [DOI] [PubMed] [Google Scholar]
- Crow JF. Advantages of sexual reproduction. Dev Genet. 1994;15(3):205–213. doi: 10.1002/dvg.1020150303. [DOI] [PubMed] [Google Scholar]
- Ellegren H. Sex-chromosome evolution: recent progress and the influence of male and female heterogamety. Nat Rev Genet. 2011;12(3):157–166. doi: 10.1038/nrg2948. [DOI] [PubMed] [Google Scholar]
- Fernandez-Capetillo O, Mahadevaiah SK, Celeste A, Romanienko PJ, Camerini-Otero RD, Bonner WM, Manova K, Burgoyne P, Nussenzweig A. H2AX is required for chromatin remodeling and inactivation of sex chromosomes in male mouse meiosis. Dev Cell. 2003;4(4):497–508. doi: 10.1016/s1534-5807(03)00093-5. [DOI] [PubMed] [Google Scholar]
- Gartner A, Milstein S, Ahmed S, Hodgkin J, Hengartner MO. A conserved checkpoint pathway mediates DNA damage--induced apoptosis and cell cycle arrest in C. elegans. Mol Cell. 2000;5(3):435–443. doi: 10.1016/s1097-2765(00)80438-4. [DOI] [PubMed] [Google Scholar]
- Goedecke W, Eijpe M, Offenberg HH, van Aalderen M, Heyting C. Mre11 and Ku70 interact in somatic cells, but are differentially expressed in early meiosis. Nat Genet. 1999;23(2):194–198. doi: 10.1038/13821. [DOI] [PubMed] [Google Scholar]
- Goldstein P. The synaptonemal complexes of Caenorhabditis elegans: pachytene karyotype analysis of male and hermaphrodite wild-type and him mutants. Chromosoma. 1982;86(4):577–593. doi: 10.1007/BF00330128. [DOI] [PubMed] [Google Scholar]
- Grewal SIS, Jia S. Heterochromatin revisited. Nat Rev Genet. 2007;8(1):35–46. doi: 10.1038/nrg2008. [DOI] [PubMed] [Google Scholar]
- Hamilton WD, Axelrod R, Tanese R. Sexual reproduction as an adaptation to resist parasites (a review) Proc Natl Acad Sci U S A. 1990;87(9):3566–3573. doi: 10.1073/pnas.87.9.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handel MA. The XY body: a specialized meiotic chromatin domain. Exp Cell Res. 2004;296(1):57–63. doi: 10.1016/j.yexcr.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Henzel JV, Nabeshima K, Schvarzstein M, Turner BE, Villeneuve AM, Hillers KJ. An asymmetric chromosome pair undergoes synaptic adjustment and crossover redistribution during Caenorhabditis elegans meiosis: Implications for sex chromosome evolution. Genetics. 2011;187(3):685–699. doi: 10.1534/genetics.110.124958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horandl E. A combinational theory for maintenance of sex. Heredity. 2009;103(6):445–457. doi: 10.1038/hdy.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer-Fender S. Molecular aspects of XY body formation. Cytogenet Genome Res. 2003;103(3-4):245–255. doi: 10.1159/000076810. [DOI] [PubMed] [Google Scholar]
- Hoyer-Fender S, Costanzi C, Pehrson JR. Histone macroH2A1. 2 is concentrated in the XY-body by the early pachytene stage of spermatogenesis. Exp Cell Res. 2000;258(2):254–260. doi: 10.1006/excr.2000.4951. [DOI] [PubMed] [Google Scholar]
- Inagaki A, Schoenmakers S, Baarends WM. DNA double strand break repair, chromosome synapsis and transcriptional silencing in meiosis. Epigenetics. 2010;5(4):255–266. doi: 10.4161/epi.5.4.11518. [DOI] [PubMed] [Google Scholar]
- Ichijima Y, Ichijima M, Lou Z, Nussenzweig A, Camerini-Otero RD, Chen J, Andreassen PR, Namekawa SH. MDC1 directs chromosome-wide silencing of the sex chromosomes in male germ cells. Genes Dev. 2011;25(9):959–971. doi: 10.1101/gad.2030811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo-Lambert A, Engebrecht J. A single unpaired and transcriptionally silenced X chromosome locally precludes checkpoint signaling in the Caenorhabditis elegans germ line. Genetics. 2010;184(3):613–628. doi: 10.1534/genetics.109.110338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P. Initiation of homologous chromosome pairing during meiosis. Biochem Soc Trans. 2006;34(Pt 4):545–549. doi: 10.1042/BST0340545. [DOI] [PubMed] [Google Scholar]
- Kaiser VB, Ellegren H. Nonrandom distribution of genes with sex-biased expression in the chicken genome. Evolution. 2006;60(9):1945–1951. [PubMed] [Google Scholar]
- Kauppi L, Barchi M, Baudat F, Romanienko PJ, Keeney S, Jasin M. Distinct properties of the XY pseudoautosomal region crucial for male meiosis. Science. 2011;331(6019):916–920. doi: 10.1126/science.1195774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly WG, Aramayo R. Meiotic silencing and the epigenetics of sex. Chromosome Res. 2007;15(5):633–651. doi: 10.1007/s10577-007-1143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil AM, Boyar FZ, Driscoll DJ. Dynamic histone modifications mark sex chromosome inactivation and reactivation during mammalian spermatogenesis. Proc Natl Acad Sci U S A. 2004;101(47):16583–16587. doi: 10.1073/pnas.0406325101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khil PP, Smirnova NA, Romanienko PJ, Camerini-Otero RD. The mouse X chromosome is enriched for sex-biased genes not subject to selection by meiotic sex chromosome inactivation. Nat Genet. 2004;36(6):642–646. doi: 10.1038/ng1368. [DOI] [PubMed] [Google Scholar]
- Kleckner N. Meiosis: how could it work? Proc Natl Acad Sci U S A. 1996;93(16):8167–8174. doi: 10.1073/pnas.93.16.8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota SK, Feil R. Epigenetic transitions in germ cell development and meiosis. Dev Cell. 2010;19(5):675–686. doi: 10.1016/j.devcel.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Kwon YT, Xia Z, An JY, Tasaki T, Davydov IV, Seo JW, Sheng J, Xie Y, Varshavsky A. Female lethality and apoptosis of spermatocytes in mice lacking the UBR2 ubiquitin ligase of the N-end rule pathway. Mol Cell Biol. 2003;23(22):8255–8271. doi: 10.1128/MCB.23.22.8255-8271.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longhese MP, Bonetti D, Guerini I, Manfrini N, Clerici M. DNA double-strand breaks in meiosis: checking their formation, processing and repair. DNA Repair (Amst) 2009;8(9):1127–1138. doi: 10.1016/j.dnarep.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Lu LY, Wu J, Ye L, Gavrilina GB, Saunders TL, Yu X. RNF8-dependent histone modifications regulate nucleosome removal during spermatogenesis. Dev Cell. 2010;18(3):371–384. doi: 10.1016/j.devcel.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevaiah SK, Bourc’his D, de Rooij DG, Bestor TH, Turner JM, Burgoyne PS. Extensive meiotic asynapsis in mice antagonises meiotic silencing of unsynapsed chromatin and consequently disrupts meiotic sex chromosome inactivation. J Cell Biol. 2008;182(2):263–276. doi: 10.1083/jcb.200710195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevaiah SK, Turner JM, Baudat F, Rogakou EP, de Boer P, Blanco-Rodriguez J, Jasin M, Keeney S, Bonner WM, Burgoyne PS. Recombinational DNA double-strand breaks in mice precede synapsis. Nat Genet. 2001;27(3):271–276. doi: 10.1038/85830. [DOI] [PubMed] [Google Scholar]
- Maine EM. Meiotic silencing in Caenorhabditis elegans. Int Rev Cell Mol Biol. 2010;282:91–134. doi: 10.1016/S1937-6448(10)82002-7. [DOI] [PubMed] [Google Scholar]
- McKee BD, Handel MA. Sex chromosomes, recombination, and chromatin conformation. Chromosoma. 1993;102(2):71–80. doi: 10.1007/BF00356023. [DOI] [PubMed] [Google Scholar]
- Meiklejohn CD, Tao Y. Genetic conflict and sex chromosome evolution. Trends Ecol Evol. 2010;25(4):215–223. doi: 10.1016/j.tree.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhaylova lM, Nurminsky DI. Lack of global meiotic sex chromosome inactivation, and paucity of tissue-specific gene expression on the Drosophila X chromosome. EMC Biol. 2011;9:29. doi: 10.1186/1741-7007-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens PB, Chen DJ, Shen Z, Kolas N, Tarsounas M, Heng HH, Spyropoulos B. Rad51 immunocytology in rat and mouse spermatocytes and oocytes. Chromosoma. 1997;106(4):207–215. doi: 10.1007/s004120050241. [DOI] [PubMed] [Google Scholar]
- Mulugeta Achame E, Wassenaar E, Hoogerbrugge JW, Sleddens-Linkels E, Ooms M, Sun ZW, van IJcken WF, Grootegoed JA, Baarends WM. The ubiquitin-conjugating enzyme HR6B is required for maintenance of X chromosome silencing in mouse spermatocytes and spermatids. BMC Genomics. 2010;11:367. doi: 10.1186/1471-2164-11-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu H, Wan L, Baumgartner B, Schaefer D, Loidl J, Hollingsworth NM. Partner choice during meiosis is regulated by Hop1-promoted dimerization of Mek1. Mol Biol Cell. 2005;16(12):5804–5818. doi: 10.1091/mbc.E05-05-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi SL, Henikoff S. Germline histone dynamics and epigenetics. Curr Opin Cell Biol. 2007;19(3):257–265. doi: 10.1016/j.ceb.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Ooi SL, Priess JR, Henikoff S. Histone H3.3 variant dynamics in the germline of Caenorhabditis elegans. PLoS Genet. 2006;2(6):e97. doi: 10.1371/journal.pgen.0020097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page SL, Hawley RS. Chromosome choreography: the meiotic ballet. Science. 2003;301(5634):785–789. doi: 10.1126/science.1086605. [DOI] [PubMed] [Google Scholar]
- Parisi M, Nuttall R, Naiman D, Bouffard G, Malley J, Andrews J, Eastman S, Oliver B. Paucity of genes on the Drosophila X chromosome showing male-biased expression. Science. 2003;299(5607):697–700. doi: 10.1126/science.1079190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters AHFM, Mermoud JE, O’Carroll D, Pagani M, Schweizer D, Brockdorff N, Jenuwein T. Histone H3 lysine 9 methylation is an epigenetic imprint of facultative heterochromatin. Nat Genet. 2001;30(1):77–80. doi: 10.1038/ng789. [DOI] [PubMed] [Google Scholar]
- Reinke V, Smith HE, Nance J, Wang J, Van Doren C, Begley R, Jones SJ, Davis EB, Scherer S, Ward S, Kim SK. A global profile of germline gene expression in C. elegans. Mol Cell. 2000;6(3):605–616. doi: 10.1016/s1097-2765(00)00059-9. [DOI] [PubMed] [Google Scholar]
- Rodriguez T, Burgoyne P. Evidence that sex chromosome asynapsis, rather than excess Y gene dosage, is responsible for the meiotic impairment of XYY mice. Cytogenetic and Genome Res. 2000;89(1-2):38–43. doi: 10.1159/000015559. [DOI] [PubMed] [Google Scholar]
- Roeder GS. Meiotic chromosomes: it takes two to tango. Genes Dev. 1997;11(20):2600–2621. doi: 10.1101/gad.11.20.2600. [DOI] [PubMed] [Google Scholar]
- Roeder GS, Bailis JM. The pachytene checkpoint. Trends Genet. 2000;16(9):395–403. doi: 10.1016/s0168-9525(00)02080-1. [DOI] [PubMed] [Google Scholar]
- Rouyer F, Simmler MC, Johnsson C, Vergnaud G, Cooke HJ, Weissenbach J. A gradient of sex linkage in the pseudoautosomal region of the human sex chromosomes. Nature. 1986;319(6051):291–295. doi: 10.1038/319291a0. [DOI] [PubMed] [Google Scholar]
- Royo H, Polikiewicz G, Mahadevaiah SK, Prosser H, Mitchell M, Bradley A, de Rooij DG, Burgoyne PS, Turner JM. Evidence that meiotic sex chromosome inactivation is essential for male fertility. Curr Biol. 2010;20(23):2117–2123. doi: 10.1016/j.cub.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Schoenmakers S, Wassenaar E, Hoogerbrugge JW, Laven JS, Grootegoed JA, Baarends WM. Female meiotic sex chromosome inactivation in chicken. PLoS Genet. 2009;5(5):e1000466. doi: 10.1371/journal.pgen.1000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She X, Xu X, Fedotov A, Kelly WG, Maine EM. Regulation of heterochromatin assembly on unpaired chromosomes during Caenorhabditis elegans meiosis by components of a small RNA-mediated pathway. PLoS Genet. 2009;5(8):e1000624. doi: 10.1371/journal.pgen.1000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Spriggs E, Field LL, Ko E, Barclay L, Martin RH. Single sperm typing demonstrates that reduced recombination is associated with the production of aneuploid 24,XY human sperm. Am J Med Genet. 2001;99(1):34–38. doi: 10.1002/1096-8628(20010215)99:1<34::aid-ajmg1106>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Smith JM. The evolution of sex. Cambridge University Press; 1979. [Google Scholar]
- Smolikov S, Eizinger A, Hurlburt A, Rogers E, Villeneuve AM, Colaiacovo MP. Synapsis-defective mutants reveal a correlation between chromosome conformation and the mode of double-strand break repair during Caenorhabditis elegans meiosis. Genetics. 2007;176(4):2027–2033. doi: 10.1534/genetics.107.076968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solari AJ. The behavior of the XY pair in mammals. Int Rev Cytol. 1974;38(0):273–317. doi: 10.1016/s0074-7696(08)60928-6. [DOI] [PubMed] [Google Scholar]
- Solari AJ. Equalization of Z and W axes in chicken and quail oocytes. Cytogenet Cell Genet. 1992;59(1):52–56. doi: 10.1159/000133199. [DOI] [PubMed] [Google Scholar]
- Solovei I, Gaginskaya E, Hutchison N, Macgregor H. Avian sex chromosomes in the lampbrush form: the ZW lampbrush bivalents from six species of bird. Chromosome Res. 1993;1(3):153–166. doi: 10.1007/BF00710769. [DOI] [PubMed] [Google Scholar]
- Soriano P, Keitges EA, Schorderet DF, Harbers K, Gartler SM, Jaenisch R. High rate of recombination and double crossovers in the mouse pseudoautosomal region during male meiosis. Proc Natl Acad Sci U S A. 1987;84(20):7218–7220. doi: 10.1073/pnas.84.20.7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storchova R, Divina P. Nonrandom representation of sex-biased genes on chicken Z chromosome. J Mol Evol. 2006;63(5):676–681. doi: 10.1007/s00239-006-0022-1. [DOI] [PubMed] [Google Scholar]
- Turner J, Aprelikova O, Xu X, Wang R, Kim S, Chandramouli GVR, Barrett JC, Burgoyne PS, Deng CX. BRCA1, histone H2AX phosphorylation, and male meiotic sex chromosome inactivation. Curr Biol. 2004;14(23):2135–2142. doi: 10.1016/j.cub.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Turner J, Mahadevaiah SK, Elliott DJ, Garchon HJ, Pehrson JR, Jaenisch R, Burgoyne PS. Meiotic sex chromosome inactivation in male mice with targeted disruptions of Xist. J Cell Science. 2002;115(Pt 21):4097. doi: 10.1242/jcs.00111. [DOI] [PubMed] [Google Scholar]
- Turner J, Mahadevaiah SK, Ellis PJI, Mitchell MJ, Burgoyne PS. Pachytene asynapsis drives meiotic sex chromosome inactivation and leads to substantial postmeiotic repression in spermatids. Dev Cell. 2006;10(4):521–529. doi: 10.1016/j.devcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Turner JM. Meiotic sex chromosome inactivation. Development. 2007;134(10):1823–1831. doi: 10.1242/dev.000018. [DOI] [PubMed] [Google Scholar]
- Turner JMA. In: Meiotic Silencing, Infertility and X Chromosome Evolution. Ferguson-Smith AC, Greally JM, Martienssen RA, editors. Epigenomics; Springer Netherlands: 2009. pp. 301–318. [Google Scholar]
- van der Heijden GW, Derijck AAHA, PÛsfai E, Giele M, Pelczar P, Ramos L, Wansink DG, van der Vlag J, Peters AHFM, de Boer P. Chromosome-wide nucleosome replacement and H3. 3 incorporation during mammalian meiotic sex chromosome inactivation. Nat Genet. 2007;39(2):251–258. doi: 10.1038/ng1949. [DOI] [PubMed] [Google Scholar]
- Vibranovski MD, Lopes HF, Karr TL, Long M. Stage-specific expression profiling of Drosophila spermatogenesis suggests that meiotic sex chromosome inactivation drives genomic relocation of testis-expressed genes. PLoS Genet. 5(11):e1000731. doi: 10.1371/journal.pgen.1000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zang C, Rosenfeld JA, Schones DE, Barski A, Cuddapah S, Cui K, Roh TY, Peng W, Zhang MQ, Zhao K. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat Genet. 2008;40(7):897–903. doi: 10.1038/ng.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CI, Xu EY. Sexual antagonism and X inactivation--the SAXI hypothesis. Trends Genet. 2003;19(5):243–247. doi: 10.1016/s0168-9525(03)00058-1. [DOI] [PubMed] [Google Scholar]
- Zamudio NM, Chong S, O’Bryan MK. Epigenetic regulation in male germ cells. Reproduction. 2008;136(2):131–146. doi: 10.1530/REP-07-0576. [DOI] [PubMed] [Google Scholar]
- Zickler D, Kleckner N. Meiotic chromosomes: integrating structure and function. Annu Rev Genet. 1999;33:603–754. doi: 10.1146/annurev.genet.33.1.603. [DOI] [PubMed] [Google Scholar]