Abstract
Conditions that compromise the blood-brain barrier (BBB) have been increasingly implicated in the pathogenesis of Alzheimer disease (AD). AGRIN is a heparan sulfate proteoglycan (HSPG) found abundantly in basement membranes of the cerebral vasculature, where it has been proposed to serve a functional role in the BBB. Furthermore, AGRIN is the major HSPG associated with amyloid plaques in AD brains. To examine the relationship of AGRIN, the BBB and AD-related pathologies, we generated mice in which the Agrn gene was deleted from either endothelial cells or neurons using gene-targeting or was overexpressed using a genomic transgene construct. These mice were combined with a transgenic model of AD that overexpresses disease-associated forms of amyloid precursor protein and presenilin1. In mice lacking endothelial cell expression of Agrn, the BBB remained intact but aquaporin 4 levels were reduced, indicating that the loss of AGRIN affects BBB-associated components. This change in Agrn resulted in an increase in β-amyloid (Aβ) in the brain. Conversely, overexpression of Agrn decreased Aβ deposition, whereas elimination of Agrn from neurons did not change Aβ levels. These results indicate that AGRIN is important for maintaining BBB composition and that changes in Agrn expression (particularly vessel-associated AGRIN) influence Aβ homeostasis in mouse models of AD.
Keywords: Alzheimer disease, Aquaporin 4, Blood-brain barrier, Conditional knockout, Fibrillogenesis, Isoform-specific knockout, Orthogonal arrays, Transmembrane agrin
INTRODUCTION
Alzheimer disease (AD) pathology is characterized by the accumulation of intracellular tangles containing tau, and extracellular plaques containing β-amyloid (Aβ) (1). The relationship between these pathological features and the progression of neuronal loss and dementia is unclear (2), although several molecular and physiological pathways that contribute to the neurodegeneration are emerging. A longstanding observation is that heparan sulfate proteoglycans (HSPGs) are associated with plaques in AD brains (3). There is a direct interaction between Aβ and heparan sulfate moieties that hastens Aβ fibril formation, suggesting that the presence of HSPGs in plaques may result in a positive feedback mechanism and increased plaque pathology (4–6).
HSPGs may affect AD progression in other ways as well. HSPGs are key components of the basement membranes that surround cerebral vessels and may, therefore, be required for the integrity of the blood-brain barrier (BBB). Compromised function of the BBB has been increasingly implicated in AD pathogenesis (7–9). The BBB is comprised of cerebral endothelial cells, astrocytes, and pericytes. It physically consists of specialized tight junctions between endothelial cells, a basement membrane of extracellular matrix (ECM) surrounding the endothelium, and extensive abluminal basement membrane contact, primarily with endfeet of pericytes and astrocytes (10). Changes in the function of the BBB may result in altered permeability and active transport across the barrier, with resulting defects in cerebral extracellular fluid Aβ homeostasis (11–13). Accumulation of soluble forms of Aβ in cerebral extracellular fluid has been implicated in early synaptic dysfunction (10, 14). Furthermore, pathology involving cerebral microvasculature often parallels or even precedes AD pathology (15). In light of these findings, impaired clearance of Aβ across the BBB is increasingly a focus of possible early AD pathology (8, 13).
A primary HSPG core protein associated with both AD plaques and basement membranes of the BBB is AGRIN (16–18). AGRIN may contribute to Aβ deposition and AD pathology through multiple mechanisms. Like other HSPGs, the in vitro binding of Aβ and the heparan sulfate moieties of AGRIN promotes fibril formation (17). The direct interaction of AGRIN and Aβ may lead to a positive-feedback cycle of Aβ fibril formation causing amyloid to accumulate along with additional HS, thereby further promoting fibrillogenesis (19). Alternatively, the presence of AGRIN in the cerebral vasculature coincides with the formation of the BBB (20). AGRIN may influence AD pathology through its role in BBB integrity and/or function (21).
Testing the in vivo functional consequences of AGRIN/Aβ association has been hampered (even in animal models) because the disruption of the Agrin gene (mouse gene symbol Agrn) causes perinatal lethality due to its critical function in neuromuscular junction (NMJ) formation (22). We have generated mouse models that allow us to address roles of AGRIN in Aβ deposition by reducing AGRIN selectively in the vasculature or on neurons or by overexpressing Agrn throughout the brain. We then asked whether altered Agrn expression affected accumulation of insoluble Aβ in a transgenic mouse model of AD expressing mutant forms of amyloid precursor protein (APP) and presenilin1 (PS1) [APP(Swe)/PS1(Dex9)line85] (23).
MATERIALS AND METHODS
Study Animals
All mice used in these experiments were housed under standard conditions in the Research Animal Facility at The Jackson Laboratory and provided food and water ad libitum. The Institutional Animal Care and Use Committee of The Jackson Laboratory approved all animal procedures.
The AGRIN-cyan fluorescent protein (CFP) bacterial artificial chromosome (BAC) transgenic mice (C57BL/6J-Tg[MGS1-19375/CFP]2R9Rwb, abbreviated AGRIN-CFP) express all AGRIN isoforms under the control of the endogenous regulatory elements and splicing (24). The Agrn-loxP conditional allele allows the deletion of exons 7 through 33 (B6;129-Agrntm1Rwb abbreviated Agrnfl) in response to Cre expression and has been previously described (25). The APP (Swe)/PS1(Dex9)line85 co-integrated transgenic mice (abbreviated APP/PS1) were generously provided by Dr. David Borchelt (23). Transgenic mice expressing Cre in endothelial cells driven by the Tie2-promoter (B6.Cg-Tg[Tek-Cre]1Ywa/J, abbreviated Tie2-Cre) were obtained from The Jackson Laboratory (stock 8863) (26).
Deletion of Short Isoform-Agrn
The short isoform (SN)-specific exon of Agrn and approximately 2 kb of upstream sequence were deleted from the mouse genome by homologous recombination. A targeting vector consisting of an upstream EcoRI/BglII genomic fragment and a second downstream BglII fragment was used to replace the SN exon with a loxP-flanked Neor cassette that is in the opposite orientation relative to the Agrn gene. A thymidine kinase-negative selection cassette was also included in the vector outside of the regions of homology. Mouse RI embryonic stem cells were targeted by electroporation and G418 and FIAU selection using standard techniques. Homologous recombinant embryonic stem cell clones were identified by polymerase chain reaction (PCR) and confirmed by Southern blotting. Proper targeting resulted in the introduction of an XmaI site and the loss of an SphI site, causing a decrease from 14 kb to 6kb in the XmaI fragment detected by a downstream probe, and an increase from 8.2kb to 16.3 kb in the SphI fragment. The second, smaller SphI fragment detected by the probe was unaffected, as anticipated. Chimeric mice were generated from recombinant embryonic stem cell clones by standard blastocyst injection and were bred for germline transmission of the mutation. To insure that expression of LN forms of AGRIN were unaffected, the floxed Neo cassette was deleted by breeding to b-Actn-Cre transgenic mice (27). These mice were subsequently bred to pass the allele, which lacks the SN exon and carries a LoxP site in its place, with no deleterious effects on long isoform (LN)-AGRIN expression or any common exons.
Acetylcholine Receptor Clustering Assay of AGRIN Activity
The acetylcholine receptor clustering (AChR) clustering activity of brain homogenates from control and SN-Agrn knockout animals (n = 3 for each genotype) was tested as previously described(28). In brief, cultured C2C12 myotubes were treated with homogenates prepared in DMEM media and standardized for tissue weight/volume. Extracts were then diluted and applied to the cultures and clusters/myotubes were counted after 24 hours. Treatment with an inactive recombinant AGRIN construct was used to establish background levels of AChR clustering, and a saturating dose of recombinant Z8 AGRIN was used to define 100% activity.
Filter Trap Assay for Insoluble Aβ
Study mice were killed by CO2 inhalation at 186 ± 7 days. The brain was immediately removed and divided sagittally with half placed in 4% paraformaldehyde (PFA) for sectioning and immunocytochemistry and the other half processed for use in an enzyme-linked immunosorbent assay (ELISA) or filter trap assay (29). Half brains were either immediately homogenized in TBS with 1% protease inhibitors (Complete, Roche), TBS-PI, using a mechanical homogenizer and placed at −80°C, or flash frozen in liquid N2, stored at −80°C and homogenized by a Dounce homogenizer in TBS-PI at the time of performing the assays. Samples were initially centrifuged at slow speed (3000 RPM, 5 min in a microfuge) to remove incompletely disrupted tissue, which interferes with the filter trap assay (29). This initial low speed spin changed absolute values by reducing total protein but did not affect relative levels of soluble or insoluble total Aβ, Aβ-40, or Aβ-42, as determined by ELISA performed on the same samples prepared with and without the initial spin (data not shown).
For the filter trap assay, total protein was measured with a BCA Assay kit (Pierce, Catalog # 23227, Rockford, IL) and equal amounts of protein from each study brain were further solubilized by addition of SDS (1% final concentration), loaded in a dilution series on a 96-well dot-blot apparatus, and vacuum-filtered through a 0.2-μm cellulose acetate membrane (OE 66, Schleicher and Schuell, Keene, NH). For detection of insoluble Aβ, a rabbit polyclonal anti-Aβ antibody was used to probe the membrane after filtration (1:500 dilution in block of 5% nonfat dry milk and 0.1% Tween in phosphate-buffered saline (PBS; Zymed, South San Francisco, CA), followed by an HRP-conjugated goat anti-rabbit IgG secondary antibody (1:10,000 Perkin Elmer, Boston, MA). Signals were developed using enhanced chemiluminescence (Western Lighting, Perkin Elmer) to expose the film, which was then scanned. The densities of the resulting dots were measured using Image J software (www.nih.gov). Background was determined by scanning an equivalent area adjacent to the spots and background values were subtracted from signal intensities.
ELISA Assay for Aβ
ELISAs measuring Aβ-40 and Aβ-42 were performed using commercial kits according to the manufacturer's instructions (KHB3442 and KHB3482, Invitrogen, Camarillo, CA), with the following modifications: Homogenates were further separated into soluble and insoluble fractions by treatment with diethylamine (DEA) to a final concentration of 2%, sonication (30 seconds at 10 watts in a 500-μl sample volume), and then centrifugation at 100,000 g for 30 minutes. The supernatant was used to determine soluble Aβ. The pellet was resuspended in 20 μl 5M guanidine in TBS-PI and used to measure insoluble Aβ.
ELISA Assay for Aquaporin 4
Aquaporin 4 (AQP4) protein concentrations in mouse hemi-brain samples were measured using a solid-phase sandwich ELISA method (wild type, n = 6; Agrnfl/fl;+/+ [no Cre], n = 3; and Agrnfl/fl;Tie2-Cre/+, n = 6). Samples of brain tissue were snap-frozen in liquid N2 and then ground over a bed of dry ice. 100 mg of each sample were homogenized thoroughly in 8 volumes of a cold 5 M guanidine HCl/50 mM Tris-HCl, pH 8.0, buffer. The resultant homogenates were mixed for 3.5 hours at room temperature and then diluted 10-fold with 1× protease inhibitor cocktail (Sigma-Aldrich, Catalog # P-2714, St. Louis, MO) in cold Dulbecco's PBS. Samples were centrifuged at 16,000 × g for 20 minutes at 4°C, and the supernatant was stored on ice until use with the assays.
Following protein extraction, AQP4 concentrations were measured using an ELISA kit (USCN Life, Catalog # E0582m, Wuhan, China), in accordance with the manufacturer's instructions. Samples and standards were added in duplicate to microtiter plate wells pre-coated with an antibody directed against AQP4. After a 2-hour incubation at 37°C, wells were aspirated and then incubated for 1 hour at 37°C with a biotin-conjugated polyclonal antibody preparation specific for AQP4. Wells were washed 4 times prior to the addition of avidin-conjugated horseradish peroxidase for 1 hour at 37°C. After a final series of 4 washes, the color was developed by adding a 3,3',5,5'-tetramethylbenzidine (TMB) solution to each well. The reaction was terminated 30 minutes later using the stop solution from the kit. The plate was read on a Multiskan EX spectrophotometer (Thermo Electron Corporation, Catalog # 1507300, Vantaa, Finland) at 450 nm; data were analyzed using Ascent Software (Thermo Electron Corporation). Immunoassay results were corrected for tissue weight and the total protein content of each sample was determined using a BCA Protein Assay kit, with absorbance read at 562 nm.
Immunocytochemistry
The half brains were immersed in PFA for 24 hours, placed in 30% sucrose for 24 hours, embedded in OCT media, and frozen using liquid N2for sectioning in a cryostat. Sections (14 mm) were cut coronally through hippocampus and cortex and placed on slides. For amyloid plaque identification, sections were immersed in 70% formic acid for 30 minutes then rinsed in TBS, blocked using 5% normal goat serum or 1% bovine serum albumen and 0.5% Tween-20 in TBS, and stained with antibody in the same blocking solution. Primary antibody was mouse monoclonal anti-Aβ (4G8, Covance Research, Dedham, MA), and secondary antibody was fluorescent-tagged goat anti-mouse Ig2b (AlexaFluor 568, Molecular Probes, Eugene, OR). A Nikon E600 fluorescent microscope equipped with SPOT camera and software was used for individual plaque area measurement and morphology examination.
For evaluation of BBB immunocytochemistry, sections from older mice (including homozygous Agrnfl/fl with and without the Tie2-Cre transgene) were prepared as above but without formic acid treatment. The sections were evaluated using polyclonal rabbit primary antibodies to mouse occludin (1:100) and claudin-5 (1:100) (both from Zymed). For LRP -1 detection, primary antibody was goat anti-LRP-1 (N20 1:100, Santa Cruz Biotechnology, Santa Cruz, CA). Secondary antibody was a fluorescently tagged goat anti-rabbit IgG (1:200, AlexaFluor 500, Molecular Probes), except for LRP-1, which was a fluorescently tagged chicken anti-goat IgG (AlexaFluor 594, Molecular Probes).
AQP4 and AGRIN protein in the brain vasculature was also evaluated. After quick rinses in 95% ethanol, 70% ethanol and distilled water, sections were treated for antigen retrieval with hot (85°C) 10 mM citrate buffer (pH 6.0) for 15 minutes. Sections were then washed with distilled water and then quenched with a peroxidase-blocking reagent (DAKO, Carpinteria, CA; S2001) for 10 minutes at room temperature to eliminate endogenous peroxidase activity. Non-specific binding sites were blocked by incubation with 5% normal goat or rabbit serum for 24 hours at 4°C prior to incubation with one of the following primary antibodies: polyclonal rabbit anti-rat AQP4 antibody (Alpha Diagnostic International, San Antonio, TX; AQP41-A; diluted 1:250), polyclonal rabbit anti-AGRIN C95-Rb-204 antibody (courtesy of Dr. M. Ruegg, University of Basel, Basel, Switzerland; diluted 1:2000), or polyclonal sheep anti-rat AGRIN GR-14 antibody (courtesy of Dr. J. Berden, University Medical Center Nijmegen, Nijmegen, The Netherlands; diluted 1:800). Following an overnight incubation at 4°C, the tissue sections were subjected to a modified ABC technique using the Vectastain Elite ABC rabbit or sheep peroxidase system (Vector Laboratories, Burlingame, CA). 3,3-diaminobenzidine (DAKO; K3468) was used as the chromogen, and the slides were coverslipped and sealed using Cytoseal, a xylene-based mounting medium (Stevens Scientific, Riverdale, NJ). Antibody specificities have been previously demonstrated (30, 31). Primary antibody omission controls were run alongside the other samples to check for nonspecific binding caused by the secondary antibody, along with positive control tissue (kidney, thymus gland and lung). Images of mouse brain sections were obtained using an Olympus BH2-RFCA microscope (Olympus America, Inc., Melville, NY), and acquired with a CoolSNAP cf camera (Roper Scientific, Tucson, AZ).
Electron Microscopy and Elemental Analysis
Brain samples of 1-year-old wild type and of Agrnfl/fl;Tie2-Cre mice were prepared for electron microscopy using standard techniques. Tissue was fixed in 2% PFA/2% glutaraldehyde in 0.1M cacodylate buffer. A JEOL JEM-1230 electron microscope with AMT digital camera and an attached Genesis 2000 EDAX unit were used, which also allowed spectroscopic analysis of elemental content within a given EM field. Measurements of the brain vascular basement membrane sulfur content relative to phosphate were made at 200,000× magnification to maximize the area represented by the basement membrane. Eight independent measurements were made from 2 mice of each genotype.
Quantitative PCR
Adult mice were euthanized under CO2 and the brain immediately removed and processed for RNA by Trizol extraction and DNAse treatment to remove contaminating genomic DNA. Equal amounts (5 μg) of total RNA were reverse transcribed following manufacturer directions using Superscript III reverse transcriptase and a mix of random and oligo dT priming (Invitrogen). Equal volumes of the resulting cDNA were used for quantitative PCR using an ABI Prism 7000 unit (Applied Biosystems, Foster City, CA). Samples were run minimally in triplicate and SYBR green (SYBR green PCR Master Mix, Applied Biosystems) was used for quantification of amplification products. β-actin mRNA was used as the standard for comparison by the DDCT method. β-actin primers were F: CAT TGC TGA CAG GAT GCA GAA and R: GCC ACC GAT CCA CAC AGA GT. Pan-Agrn primers were F: GGT GCT GTG GAT TGG AAA GGT and R: TCA CAG TGG AGC GCA GCA. Z+ isoform specific primers were F: TCC CAG CCC CCG AAA CT and R: GTA GTC TGC ACG TTC TCC AAC CTT. Primers used for APP were F: CGA CAT GAC TCA GGA TAT GAA GTT CA and R: ACC ATG ATG AAT GGA TGT GTA CTG TT.
Sulforhodamine B and Fluorescently Conjugated Dextran
Sulforhodamine B (approximately 1 kD) and 4kD fluorescein isothiocyanate (FITC)-Dextran (both from Sigma-Aldrich) were injected i.p. The mice were killed 3 hours later and blood was drained via right atrial cut and replaced with PBS then 4% PFA by transcardiac perfusion. The brains and livers were removed and processed as above for fluorescent microscopic examination without antibody treatment and using appropriate excitation and emission wavelengths.
Data Analysis and Statistics
Values are presented as means ± SD or percent of control values ± SE. Statistical significance was determined using a t test pair-wise comparison of mutant vs. control values. Significance was not different when comparing absolute empirical quantities or percent of control values. A threshold of significance of 0.05 was used.
RESULTS
Deletion of Agrn from Endothelial Cells
To examine the role of AGRIN in the BBB, and to determine if AGRIN-related changes in BBB integrity or composition may result in altered amyloid deposition in mouse models of AD, we selectively deleted Agrn from endothelial cells using a loxP-flanked conditional allele of the mouse gene (Agrnfl) (25). The Agrnfl allele was combined with transgenic Cre driven in endothelial cells by the Tie2 promoter (26).
Deletion of Agrn from endothelial cells resulted in a reduction in vessel-associated AGRIN. Studies sing a primary antibody against the C-terminus of AGRIN (31) and indirect immunofluorescence showed a reduction of AGRIN staining intensity in cerebral blood vessels, but not in the pia, in Agrnfl/fl;Tie2-Cre mice compared to controls (Fig. 1A, B). In additional tests using a polyclonal antibody specifically recognizing the LN isoform of AGRIN (30), immunoreactivity was again observed in the cerebral microvasculature, with more robust LN-AGRIN staining in control mice (Fig. 1C, E) than in Agrnfl/fl;Tie2-Cre mice (Fig. 1D, F). Extracellular matrix-associated LN-AGRIN is the major AGRIN isoform in the cerebral microvasculature, consistent with the reduced staining with the LN-specific antibody. Deletion of the Agrnfl allele results in an N-terminal protein product that can be detected by the antibody used in Figure 1C–F, but not the C-terminal antibody used in Figure 1A, B (25). Because the staining intensity of AGRIN immunocytochemistry depended on both the primary and secondary antibodies used, and because we were unable to specifically determine the levels in blood vessels apart from the remainder of the brain by Western blotting or ELISAs, we did not quantify the loss of AGRIN in cerebral vessels.
Figure 1.
AGRIN deletion from endothelial cells. Combining the Agrinfl/fl allele with transgenic Tie2-Cre expression results in a reduction in blood vessel-associated AGRIN in the brain, as detected by immunostaining in adult mice (6 months of age and greater). (A) By indirect immunofluorescence, cerebral blood vessels and the pia separating the cortex and midbrain in a control mouse are strongly immunoreactive for carboxy-terminal anti-AGRIN. (B) The anti-AGRIN staining intensity is reduced to near the threshold of detection in Agrnfl/fl; Tie2-Cre mice, although the pia remains intensely labeled. (C, D) Using more sensitive enhanced peroxidase immunohistochemistry and a long isoform (LN-AGRIN)-specific amino-terminal antibody, anti-AGRIN labeling is reduced but not eliminated from the cerebral vasculature in Agrnfl/fl; Tie2-Cre samples. (E, F) Vessel morphology and the reduction in staining intensity at higher magnification.
Effects on the BBB and AQP4 Mediated by Loss of Endothelial cell AGRIN
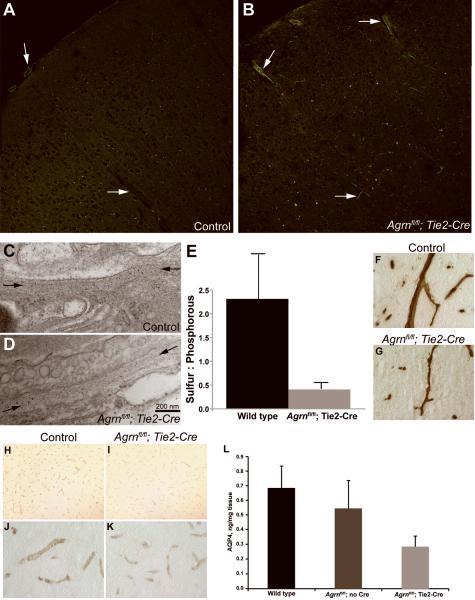
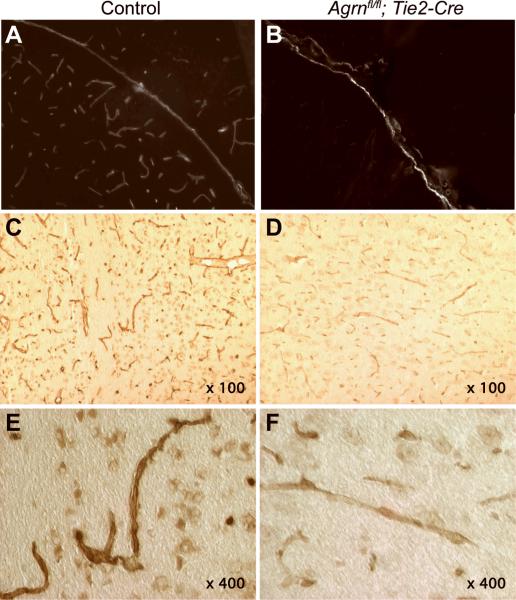
To assess BBB integrity, tracers were used to examine permeability; no leakage of sulforhodamine B (<1 kD) or FITC-labeled dextran (4 kD) from the circulation into the brain was observed following the loss of endothelial cell AGRIN (Fig. 2A, B). In some cases, the vessels retained fluorescent tracers following perfusion, but this was not a consistent result and entry of the tracers into the brain parenchyma causing diffuse halos around vessels was not observed in either genotype. Clarified tissue homogenates were also analyzed by fluorescence photospectroscopy for the presence of either rhodamine or FITC, but these were below detection in both wild type and Agrnfl/fl;Tie2-Cre mice (not shown). The basement membranes surrounding cerebral vessels were examined by transmission electron microscopy (Fig. 2C, D). No thinning or obvious gaps were observed, suggesting that the loss of AGRIN does not result in a dramatic change in integrity, or that there is compensation by other matrix molecules such as perlecan or laminin. However, elemental analysis spectroscopy performed using transmission electron microscopy did detect a decrease in the sulfur content (expressed as a ratio relative to phosphorous) in the basement membranes of mice lacking endothelial cell AGRIN (Fig. 2E). The majority of the sulfur in the basement membrane is presumably contributed by sulfated glycans, and the decrease suggests that the reduction of AGRIN is not compensated for by another HSPG such as perlecan. The reduction also suggests that there is probably a reduction in the negative charge associated with the basement membrane, which might influence the permeability of the barrier to some solutes.
Figure 2.
Effects of endothelial cell Agrn deletion on blood-brain barrier composition. (A, B) FITC dextran and sulforhodamine injected intravenously did not enter the brain parenchyma in either control (A) or Agrnfl/fl; Tie2-Cre (B) samples from mice ages 6 months and greater. Arrows denote blood vessels. (C, D) Transmission electron microscopy of cerebral basement membrane on the abluminal surface of endothelial cells (between arrows) in control (C) and Agrnfl/fl; Tie2-Cre (D) mice does not reveal any thinning or breaches in the structural integrity of the matrix. (E) Elemental spectroscopy analysis of the basement membrane revealed a decrease in the ratio of sulfur to phosphorus following endothelial cell Agrn deletion, suggesting that the decrease in AGRIN is not compensated for by an increase in other sulfated proteoglycans. Results are from 8 independent fields examined in each of 2 mice of each genotype. (F, G) Cerebral blood vessels labeled with anti-AGRIN in control (F) and Agrnfl/fl; Tie2-Cre (G) mice demonstrate a reduction in labeling surrounding the vessels, smaller vessel diameters, and more ragged profiles in the Agrnfl/fl; Tie2-Cre samples, consistent with changes in the neurovascular unit in response to endothelial cell Agrn deletion. (H–K) Immunostaining for Aquaporin4 (AQP4) in wild type (H, J) and Agrnfl/fl; Tie2-Cre (I, K) mice demonstrates a marked decrease in immunoreactivity following endothelial cell Agrn deletion. Higher magnification panels (J, K) indicate that the localization of the AQP4 was not changed. (L) An enzyme-linked immunosorbent assay (ELISA) was used to quantify AQP4 in brain homogenates from age and strain matched control mice (n = 6), Agrnfl/fl mice with no transgenic Cre expression (n = 3), and Agrnfl/fl; Tie2-Cre mice lacking endothelial cell Agrin expression (n = 6). Consistent with the reduced staining intensity in immunocytochemistry, AQP4 levels were reduced from 0.68 ± 0.15 ng AQP4/mg of tissue in wild type controls vs. 0.28 ± 0.07 in Agrnfl/fl; Tie2-Cre mice (mean ± SD, t test. p = 0.0002).
Despite retaining sufficient integrity to be impermeable to the tracers tested, samples stained with the C-terminal anti-AGRIN antibody using the enhanced peroxidase detection method demonstrated that immunopositive microvessels in Agrnfl/fl;Tie2-Cre mice have attenuated diameters and ragged profiles when compared to controls (Fig. 2F, G), consistent with the contribution of AGRIN to the structure of the microvessels.
Other protein components of the BBB that may depend on basement membrane anchoring for their localization and stabilization were also examined. No changes in occludin, claudin-5, or LRP-1 staining were seen following the loss of endothelial cell AGRIN (not shown). However, AQP4, which is arranged in orthogonal arrays that are reported to interact with AGRIN and dystroglycan (7, 32–35), was reduced in staining intensity in mice lacking endothelial cell AGRIN, although its distribution was not changed (Fig. 2H–K). Using an ELISA, levels of AQP4 were reduced to 41% (p < 0.001) relative to strain and age-matched control samples (Fig. 2L). Therefore, the cerebral vessels have reduced but not eliminated AGRIN. This reduction does not result in obvious leakiness or failure of integrity of the BBB, but does impact AQP4, and presumably other proteins that interact with the vascular basement membrane.
Deletion of SN-Agrn
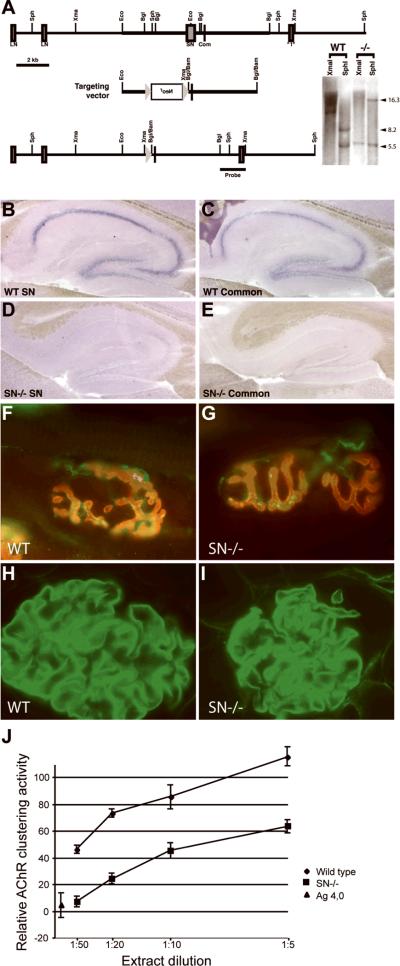
Agrn has 2 alternative transcriptional and translational start sites, generating a secreted, matrix-associated LN-AGRIN, which is found extensively around cerebral blood vessels and at the neuromuscular junction, and a type II transmembrane SN-AGRIN that is expressed primarily by CNS neurons (28, 36–38). We took advantage of this differential distribution to decrease levels of AGRIN in neurons without affecting the BBB. To this end, we generated an isoform-specific knockout of Agrn that lacks expression of the SN form of the protein. This was accomplished by targeting the SN-specific first exon and approximately 2 kb of upstream sequence, while leaving the upstream LN-specific exons, as well as all common downstream exons, intact (Fig. 3A). In situ hybridization with a probe that recognizes both SN- and LN-Agrn isoforms revealed that expression in brain regions such as the adult hippocampus was effectively eliminated in the SN-Agrn knockout samples (Fig. 3B–E). In situ hybridization with probes recognizing just the SN-isoform gave hybridization patterns similar to those of the pan-Agrn probe in wild-type mice, but no signal in the SN-Agrn mutant. In younger mice (postnatal day 7), the reduction in expression by in situ hybridization using pan-Agrn probes was less dramatic, suggesting that at early postnatal ages, some neurons may express LN-Agrn as well as SN (not shown). Sites of LN-Agrn expression, such as the NMJ and basement membranes in the kidney glomeruli, were unaffected by the loss of SN-AGRIN, and were still immunoreactive for anti-AGRIN antibodies (Fig. 3F–I). QPCR demonstrated that Agrn mRNA in the brain was reduced by approximately 70% in the SN-Agrn mutant (data not shown).
Figure 3.
Deletion of short isoform (SN)-Agrn. (A) The single exon encoding the SN amino terminus of AGRIN and approximately 2 kb of upstream sequence were targeted by homologous recombination; upstream exons encoding the long isoform (LN)-N-terminus and downstream exons encoding common sequences were left intact. The loxP-flanked selectable marker, Neo, for embryonic stem cells was removed from the genome by mating to Cre transgenic mice and a single loxP site and flanking restriction sites were left in the Agrn gene. Southern blotting DNA samples from control and homozygous SN-deletion mice confirmed the homologous recombination event with the anticipated shifts in restriction fragment sizes. (B) In situ hybridization with a probe specific to the SN-isoform of Agrn revealed strong expression in the adult hippocampus. (C) Hybridization with a probe recognizing all Agrn isoforms showed a similar pattern of expression. D, E: Hybridization with SN-specific (D) or pan-Agrn (E) probes indicated a lack of Agrn expression in the hippocampus of adult SN-Agrn knockout mice, indicating that SN-Agrn is the predominant isoform expressed in the hippocampus. (F, G) Adult neuromuscular junctions stained with α-bungarotoxin to label AChRs (red) and an antibody against the C-terminus of AGRIN (green) revealed no changed in AGRIN localization or NMJ morphology in the SN-Agrn knockout mice. (H, I) Kidney glomeruli stained with an antibody against the C-terminus of AGRIN revealed abundant basement membrane staining in both control (H) and SN-Agrn knockout (I) kidneys. Data in panels F–I are consistent with preserved expression of LN- AGRIN. (J) SN-AGRIN is the predominant isoform in brain. In AChR clustering activity assays, homogenates from SN-Agrn knockout brains were reduced in clustering activity by 4- to 5-fold compared to homogenates from control brains (n = 3 mice of each genotype), indicating SN-AGRIN is the predominant Z+ isoform of AGRIN expressed by CNS neurons. Background AChR clustering activity was determined by treating myotube cultures with recombinant inactive AGRIN and 100% activity was determined using saturating doses of recombinant Z8 AGRIN.
As a second test of the efficacy of the SN-Agrn allele, we used an AGRIN activity assay in which cultured C2C12 myotubes were treated with brain homogenates and the acetylcholine esterase (AChE) clustering activity was quantified (Fig. 3J). This activity depends on the inclusion of “Z” exons at a C-terminal alternative splice site, and these Z+ isoforms of AGRIN are only made by neurons. An 80% reduction in Z+ AGRIN in the brain was demonstrated by a 1:50 dilution of a wild type brain extract containing roughly the same AChR clustering activity as a 1:10 dilution of an SN-AGRIN mutant extract.
Surprisingly, mice lacking SN-AGRIN expression appear normal and have no overt neurological phenotypes such as ataxia, seizures, or spasticity. These mice breed as homozygotes and have no reduction in lifespan to the extent it has been tested (>18 months). Based on these results, we used SN-Agrn knockout mice to eliminate the predominant neuron-associated form of AGRIN in the brain, while leaving intact blood vessels and NMJs, which rely on LN-AGRIN (28).
Amyloid Plaque-Forming Transgenic Mice
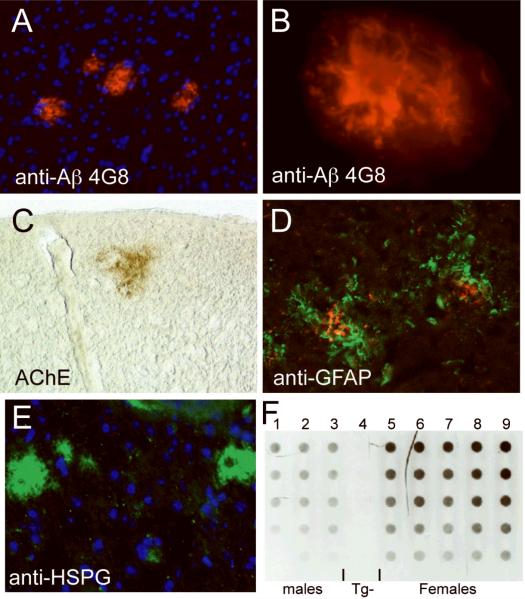
To examine the in vivo relationship between AGRIN and Aβ deposition, we used the animal models above that allowed us to manipulate Agrn expression in the presence of transgenes that promote the formation of amyloid deposits in the mouse brain. Experiments below were performed in the background of APP(Swe)/PS1(Dex9)line85, a transgenic model (hereafter referred to as APP/PS1) (23). These mice express the Swedish (K594N, M595L) form of APP, as well as the Presenilin1 gene with a deletion of exon 9. Both of these alleles are associated with human early-onset familial AD (39, 40). In the mice, both transgenes are driven by the prion promoter and are co-integrated in the genome so that they segregate as a single locus. These mice begin to show amyloid deposits detectable by immunocytochemistry at 5.5 to 6 months of age (Fig. 4A, B). The composition of plaques closely resembles human amyloid plaques with associated-AChE and astrocytosis in the cortex and hippocampus of transgenic animals (Fig. 4C, D). Immunoreactivity for HSPGs was detected in the Aβ deposits in the transgenic mouse brain (Fig. 4E), but (consistent with other studies) not all deposits labeled with HSPG (41). In these mice, Aβ levels were quantified using a filter trap assay (Fig. 4F) and by quantitative ELISA to examine 40 vs. 42 amino acid forms of Aβ. As reported for other similar models, female mice accumulate 2- to 3-fold higher levels of insoluble Aβ than age-matched male mice (42–45).
Figure 4.
Composition of APP/PS1 transgenic mouse plaques. Plaques are detectable in the brains of APP/PS1 mice beginning between 5 and 6 months of age. (A) Low magnification of plaques stained with 4G8 (red), a monoclonal antibody specific for human β-Amyloid (Aβ); nuclei are counterstained in blue (DAPI). (B) High magnification of a plaque stained with 4G8 shows a dense core of Aβ surrounded by fibrillar protein. (C) Plaques are also reactive for acetylcholine esterase (AChE, brown). (D) Activated astrocytes detected with anti-glial fibrillary acidic protein (GFAP, green) surround plaques labeled with 4G8 (red). (E) Plaques in the transgenic mice are also positive for heparan sulfate proteoglycans (HSPG, green). (F) Filter trap analysis of SDS-insoluble Aβ. Nine mice were examined at 6 most of age; they consistent of 3 male APP/PS1 transgenics (columns 1–3), a non-transgenic wild type mouse (column 4), and 5 female APP/PS1 transgenics (columns 5–9). Each column represents a dilution series for each sample, amounts of total protein loaded per well are 100 μg in the top row, 80, 60, 40 and 20 μg in the bottom row. Note the consistency of the technique and the relative quantification (20–40 μg of protein in females is equivalent in intensity to 80 to 100 μg of protein in males, consistent with other results indicating that females express approximately 3-fold higher levels of Aβ). The male/female difference has been reported by others and provided an internal control for subsequent assays. Note the total lack of signal in column 4, the non-transgenic mouse.
Endothelial Cell Deletion of Agrn, but not Deletion of SN-Agrn, Increases Aβ Accumulation
To assess the functional relationship between AGRIN and Aβ, we first combined the plaque-forming APP/PS1 mice with the Agrnfl allele and the Tie2-Cre transgene to delete Agrn from endothelial cells. Mice were examined at 6 months of age, when amyloid accumulation is underway and easily documented but not yet highly variable between animals. In this way, we sought to determine if the age of onset of amyloid deposition was markedly changed (hastened or delayed) as an indicator of the severity of the eventual pathology and of changes in Aβ biochemistry.
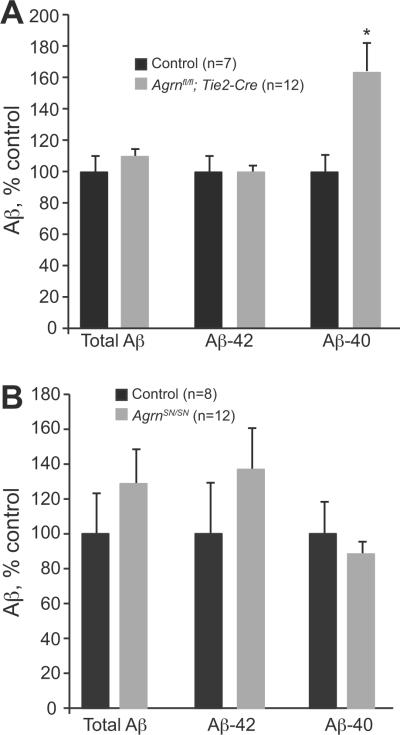
Reduction in vessel-associated AGRIN resulted in a significant increase in the levels of Aβ-40 in female mice (Fig. 5A, p = 0.02). In all mice, we found levels of Aβ-42 to be consistently 3 to 5 times higher than levels of Aβ-40, consistent with previously published studies using these mice (23), and reflecting the aggressive plaque-forming nature of the model. Results in Figure 5 are presented as percentage of control values. Because there was little change in Aβ-42, the predominant form of Aβ found in this transgenic model, there was no significant decrease in total Aβ levels. No significant changes were seen in male mice (not shown). The increase in Aβ-40 following endothelial cell deletion of Agrn indicates that Aβ deposition is influenced by AGRIN associated with the BBB, possibly through direct interactions or through indirect mechanisms such as altered localization of BBB components such as transporters of Aβ.
Figure 5.
β-Amyloid (Aβ) levels in mice lacking endothelial cell or short isoform (SN)-Agrn. (A) Female mice 6 months of age and lacking endothelial cell AGRIN (Agrnfl/fl; Tie2-Cre) showed significantly higher levels of Aβ-40 vs. control mice (t test p value = 0.018); levels of Aβ-42 and total Aβ were not different. Resul ts are reported as mean percent of control values ± SEM; animal numbers are indicated. (B) Aβ in SN-Agrn KO mice. Deletion of SN-Agrn did not result in significant differences from controls in Aβ levels at 6 months of age, as determined by ELISA analysis. Data shown are for male mice; values are mean percent of control ± SEM; animal numbers are indicated.
We next examined the impact of deleting neuron-associated SN-Agrn on Aβ levels in the brain. Despite the large reduction in AGRIN levels in the brain, and the almost complete elimination of Agrn expression from regions such as the adult hippocampus, no significant effect was seen on Aβ levels in mice lacking SN-AGRIN (Fig. 5B). Neither males nor females showed statistically significant differences in soluble or insoluble total Aβ, Aβ-40, or Aβ-42 at 6 months of age. Male mice (shown) trended towards an increase in Aβ levels, whereas female mice had no differences in Aβ levels between wild type and SN-deleted animals or trended towards a decrease in Aβ. Other studies have suggested that AGRIN is only associated with 10% to 30% of plaques in APP/PS1 mice, and this may decrease the impact of the loss of SN-AGRIN (41). However, the finding that Aβ levels were changed by endothelial cell deletion of Agrn indicates that AGRIN levels do have a functional consequence on Aβ homeostasis and that this effect appears to be primarily mediated by vessel-associated AGRIN isoforms.
Transgenic Overexpression of Agrn Reduces Aβ Accumulation
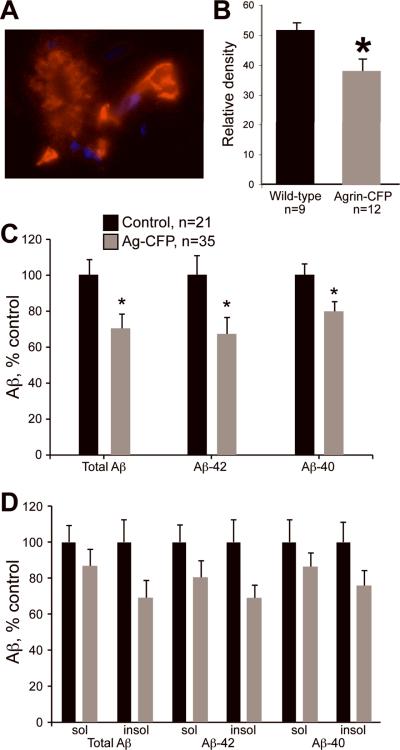
Having determined that Aβ increases as a consequence of Agrn loss-of-function in endothelial cells, we also wanted to address the converse question of whether increasing Agrn expression could have a protective effect and reduce Aβ deposition in the brain. To this end, we used Agrn transgenic mice that carry extra copies of the entire Agrn gene on a BAC incorporated into chromosome 8. CFP cDNA was fused to the 3' end of the Agrn coding sequence in the BAC to mark AGRIN. This strain (2R9) overexpresses Agrn 2- to 4-fold depending on the age and tissue examined, and the transgene is fully functional based upon its ability to rescue the NMJ and perinatal lethality phenotypes of Agrn knockout mice (24). When bred to APP/PS1 mice, transgenic AGRIN protein was detected strongly in blood vessels and also in plaques using anti-green fluorescence protein antibodies (which cross-react with CFP) (Fig. 6A).
Figure 6.
Effect of transgenic Agrn overexpression on β-Amyloid (Aβ) levels. (A) Staining with an anti-GFP antibody detects transgenic AGRIN-CFP in both plaques and blood vessels, indicating that the transgenic protein localizes to plaques and the blood-brain barrier. Note the staining intensity is much higher in the blood vessel (arrow) than in the plaque. (B) In filter trap assays, 6-month-old Agrn-CFP transgenic mice had significantly reduced SDS-insoluble Aβ levels vs. age-matched control littermates. Data shown are for male mice only; mean relative signal intensity based on densitometry ± SD, t test p = 0.02; animal numbers are shown. (C) Levels of Aβ were further examined by ELISA in 6-month-old mice. Transgenic expression of Agrn-CFP significantly reduced total amyloid, Aβ-42, and Aβ-40 levels compared to littermate controls. Asterisks indicate t test p values <0.05, animal numbers are given. Results shown are for male mice, values are mean percent of control values ± SEM. (D) Further subdividing Aβ species into DEA-soluble and insoluble fractions, indicated that the greatest contribution of the reduction seen with Agrn-CFP overexpression came from reduced Aβ-42 insoluble amyloid, although differences did not reach statistical significance (p values 0.06–0.08).
The effect of Agrn overexpression on the accumulation of insoluble Aβ was tested using both the filter trap assay and ELISA. A decrease in SDS-insoluble Aβ was observed by filter trap assay in the Agrn transgenic samples compared to mice that were wild type for Agrn at 6 months of age (Fig. 6B, p = 0.02). The antibody used for detection in this assay recognizes human amyloid, but does not distinguish between Aβ-40 and Aβ-42; only SDS-insoluble aggregates are bound in the filter. To examine these effects in more detail, brain tissue was solubilized in DEA and used in a quantitative ELISA. Extracts were fractionated into soluble and insoluble fractions by centrifugation and independent assays were performed to quantify both 40 and 42 amino acid forms of Aβ. Agrn transgenic mice had significantly lower levels of both Aβ-40 and Aβ-42, contributing to decreased total Aβ (Fig. 6C), consistent with the filter trap results. The bulk of this decrease came from a decrease in the Aβ insoluble forms,, suggesting that less Aβ is deposited into insoluble plaques in these mice (Fig. 6D). The differences related to Agrn overexpression were significant in male mice (shown), and while female mice continued to show higher Aβ levels than age-matched males, no significant changes in Aβ levels were seen in response to Agrn overexpression in females.
Plaques were also qualitatively examined by immunofluorescence using the 4G8 antibody against human Aβ (not shown). This analysis did not reveal a change in the number, size, or distribution (hippocampal and cortical) of the plaques. This may reflect lack of sensitivity in the technique.
DISCUSSION
We have demonstrated that genetically altering Agrn expression results in differences in Aβ accumulation in the brains of transgenic and knockout mice. Deletion of Agrn from endothelial cells resulted in an increase in Aβ-40 in female mice, whereas increasing Agrn expression resulted in decreased insoluble Aβ in male mice. In contrast, deletion of the predominant neuronal isoform of Agrn (SN-AGRIN) did not result in a change in Aβ levels in either sex. Whether the sex-specific differences observed in the response to altered Agrn expression reflect real differences in physiology and biochemistry between males and females or are secondary to differences in Aβ deposition resulting from differences in prion promoter expression between the sexes remains unclear. Nevertheless, these results indicate that AGRIN levels do indeed influence amyloid accumulation in vivo, and that AGRIN associated with the cerebral vasculature is largely responsible for these effects.
The inverse correlation between Agrn expression and Aβ accumulation does not support a model in which the interaction of Aβ with the heparan sulfate side chains of AGRIN promotes fibril formation and plaque accumulation in vivo. In that scenario, increased AGRIN would be expected to result in increased Aβ interaction and therefore increased fibrillogenesis and insoluble Aβ accumulation, whereas the opposite was seen. Instead, the data support an interpretation in which the presence of AGRIN presence around the vasculature influences Aβ levels. This is indicated most directly by the changes in Aβ seen with endothelial cell-specific deletion of Agrn, but not with deletion of the neuronal SN-isoform.
The effects of altered Agrn expression on Aβ levels may in part be indirect and result from changes in the BBB and associated cellular proteins or changes in the microvasculature itself in response to reduced AGRIN levels. AD-associated changes in the cerebral microvasculature include a hypercontractile state of vessel smooth muscle, altered cerebral blood flow, and plaque-associated diseased blood vessels (46, 47). Furthermore, co-morbidities such as cerebral amyloid angiopathy (CAA), which is found in 40% of AD patients (48), suggest a common link between vascular/BBB dysfunction and AD. However, we did not find evidence that BBB integrity is severely compromised by the removal of endothelial cell AGRIN. AGRIN levels were reduced but not eliminated in the vasculature. This may be explained by a combination of several factors, including some AGRIN being contributed by astrocytes, inefficient deletion of the Agrn gene in endothelial cells by the Tie2-Cre, and by the stability of AGRIN protein in the ECM even after the gene is deleted. The inability to completely eliminate vessel-associated AGRIN may have reduced the magnitude of the effects observed, we were nonetheless able to document a significant reduction in sulfur content in the basement membrane of the cerebral vasculature by elemental analysis spectroscopy that was presumably from a decrease in heparan sulfate content after Agrn deletion. This change should also result in a decrease in negative charge in the ECM. Despite these effects, the BBB remained impermeable to tracers such as sulforhodamine B and fluorescein-conjugated dextrans, which did not leak from the circulation into the brain in mice lacking endothelial cell Agrn. These results are consistent with a lack of apparent effect on the tight junction proteins claudin 5 and occludin with the reduction of endothelial cell AGRIN.
Instead of overt compromise of BBB integrity, our results indicate that reduced AGRIN levels affect other cell surface proteins that depend on ECM interactions for their localization or stability. We noted a decrease in AQP4 levels in mice lacking endothelial cell Agrn expression. The exact mechanisms governing the complex relationship between AGRIN and AQP4 remain to be determined, but basement membrane AGRIN depletion may lead to the loss of glial AQP4 along the basal lamina and a breakdown of the BBB in human patients with high-grade gliomas (49). Our data in mice indicate that the reduction of basement membrane AGRIN in cerebral capillaries leads to a decrease in AQP4 immunoreactivity along the basal lamina. This is the first demonstration of a functional consequence of the interaction between AGRIN and AQP4 in situ. A decrease in AQP4 has also been described in other mouse models of AD, as well as in AD patients (50). It will be of great interest to determine if the loss of perivascular AQP4 immunoreactivity in Agrnfl/fl;Tie2-Cre mice leads to a physiological derangement of the BBB that may contribute to AD-like pathology. Thus far, we have only tested for leakage of the tracers sulforhodamine B and fluorescein-conjugated dextrans in the Agrnfl/fl;Tie2-Cre mice; whether BBB permeability to small peptides like Aβ is altered will require further investigation.
The impact of changes in AGRIN levels on amyloid deposition in the cerebral vessels themselves remains unclear. Although Aβ deposition in capillaries (dyshoric angiopathy) has been recognized for many years, the effects of Aβ on capillary structure and function remain poorly understood. We previously reported a decrease in basal lamina AGRIN content in AD patients that was most pronounced in patients with the APOE 4,4 genotype (51). Capillary Aβ correlates significantly with recognized CERAD, Braak, and NIA-Reagan-Institute criteria for diagnosing AD (52). Two distinct types of sporadic CAA have been reported: in type I CAA, Aβ deposits are detectable in cortical capillaries, whereas in type II CAA, they are not. (53). The APOE4 allele is over 4 times more common in type I CAA than in type II CAA (53) and we previously found that reduced AGRIN within the capillary basement membrane correlated strongly with the presence of 1 or more APOE4 alleles (51). Accordingly, APOE4, which directly interacts with HSPGs, may cause a reduction in basement membrane AGRIN content that may in turn be a predisposing factor for Aβ deposition. However, our ability to address the role of AGRIN in CAA may be limited by differences in expression between mice and humans, and even more severely by limitations of the APP/PS1 plaque-forming mice. The vast majority of these models, including the strain studied here, use the prion protein promoter to drive transgene expression primarily in neurons. A different model with more targeted amyloid accumulation in the vasculature (54) may be required to address the relationship of AGRIN in the microvascular basement membrane and changes in amyloid deposition in the brain parenchyma vs. in the vasculature itself. Such differences in expression of both Agrn and APP between mice and humans may also explain the decreased association of AGRIN in transgenic mouse plaques compared to human samples (41).
Changes in Aβ receptor protein expression may have a more direct impact on Aβ accumulation. The decrease in AQP4 suggests that such changes may be downstream consequences of the decrease in vessel-associated AGRIN. For example, the proteins LRP-1 and RAGE are involved in Aβ transport, with LRP-1 removing Aβ from the brain into the circulation, and RAGE functioning in the opposite direction (55). Imbalance in the activity of these proteins could, therefore, directly impact cerebral Aβ levels. The distribution of LRP-1 did not appear to be altered by the removal of endothelial cell Agrn, but subtle changes or changes in other proteins with similar properties could have been missed. Furthermore, the treatment of pericytes in culture with Aβ results in an upregulation of AGRIN, LRP-1, glypican (another HSPG), and the low-density lipoprotein receptor, suggesting a complex co-regulated relationship among these proteins (56). In such a system, changes in the composition of the BBB, such as reduced AGRIN, could result in alterations in other proteins that are directly involved in Aβ homeostasis. Our results using genetic manipulation of Agrn in mice suggest that such changes do indeed influence Aβ levels. It will be important to determine whether similar changes occur with age or following injury in humans, and if such changes correlate with increased risk or severity of AD. Additional studies in mice will also be required to determine if the changes in Aβ observed in response to altered Agrn expression also correlate with changes in other cellular pathological responses such as reactive astrogliosis, or with changes in cognitive performance.
Previous studies have confirmed that AGRIN is a major component of amyloid plaques in AD brains and is an important basement membrane-associated HSPG in cerebral capillaries, as well as in other organs with blood-tissue barriers (e.g. thymus, testis etc.). Our studies in human brain have indicated that fragmentation of the basal lamina is an early event in the pathogenesis of AD, as well as in aging-related microvascular injury (16, 21). Because AGRIN is an integral component of the basement membrane, and its reduction has a significant effect on proteins such as AQP4, the complex interactions of AGRIN with Aβ and other BBB-associated proteins may provide important new insights into the pathogenesis of AD and lead to the development of novel treatment regimens.
ACKNOWLEDGMENTS
We would like to thank the scientific services at The Jackson Laboratory for their assistance, particularly the Histology and Electron Microscopy service and the Cell Biology and Microinjection service. We would also like to thank Dr. Kevin Seburn for his comments on the manuscript, and Renee and Scott Relf for their generous support of this work.
This work was supported by The Alzheimer's Association (R.W.B and E.G.S.), the NIH (5R01AG027910-04 to CEJ and 5R37NS019195-29 and 5R01AG032322-03 to JRS), and the philanthropic generosity of Renee and Scott Relf (R.W.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–66. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 2.Nelson PT, Braak H, Markesbery WR. Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J Neuropathol Exp Neurol. 2009;68:1–14. doi: 10.1097/NEN.0b013e3181919a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snow AD, Mar H, Nochlin D, et al. The presence of heparan sulfate proteoglycans in the neuritic plaques and congophilic angiopathy in Alzheimer's disease. Am J Pathol. 1988;133:456–63. [PMC free article] [PubMed] [Google Scholar]
- 4.Yang DS, Serpell LC, Yip CM, et al. Assembly of Alzheimer's amyloid-β fibrils and approaches for therapeutic intervention. Amyloid. 2001;8(Suppl 1):10–9. [PubMed] [Google Scholar]
- 5.Castillo GM, Ngo C, Cummings J, et al. Perlecan binds to the β-amyloid proteins (Aβ) of Alzheimer's disease, accelerates Aβ fibril formation, and maintains Aβ fibril stability. J Neurochem. 1997;69:2452–65. doi: 10.1046/j.1471-4159.1997.69062452.x. [DOI] [PubMed] [Google Scholar]
- 6.Castillo GM, Lukito W, Wight TN, et al. The sulfate moieties of glycosaminoglycans are critical for the enhancement of β-amyloid protein fibril formation. J Neurochem. 1999;72:1681–7. doi: 10.1046/j.1471-4159.1999.721681.x. [DOI] [PubMed] [Google Scholar]
- 7.Wolburg H, Noell S, Wolburg-Buchholz K, et al. Agrin, aquaporin-4, and astrocyte polarity as an important feature of the blood-brain barrier. Neuroscientist. 2009;15:180–93. doi: 10.1177/1073858408329509. [DOI] [PubMed] [Google Scholar]
- 8.Weller RO, Subash M, Preston SD, et al. Perivascular drainage of amyloid-β peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer's disease. Brain Pathol. 2008;18:253–66. doi: 10.1111/j.1750-3639.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Yankner BA, Lu T. Amyloid β-protein toxicity and the pathogenesis of Alzheimer disease. J Biol Chem. 2009;284:4755–9. doi: 10.1074/jbc.R800018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanzi RE, Moir RD, Wagner SL. Clearance of Alzheimer's Aβ peptide: the many roads to perdition. Neuron. 2004;43:605–8. doi: 10.1016/j.neuron.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 12.Bell RD, Sagare AP, Friedman AE, et al. Transport pathways for clearance of human Alzheimer's amyloid β-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab. 2007;27:909–18. doi: 10.1038/sj.jcbfm.9600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mawuenyega KG, Sigurdson W, Ovod V, et al. Decreased clearance of CNS β-amyloid in alzheimer's disease. Science. 2010;24(330):1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walsh DM, Selkoe DJ. A-β oligomers - a decade of discovery. J Neurochem. 2007;101:1172–84. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 15.Bell RD, Zlokovic BV. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer's disease. Acta Neuropathologica. 2009;118:103–13. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donahue JE, Berzin TM, Rafii MS, et al. Agrin in Alzheimer's disease: altered solubility and abnormal distribution within microvasculature and brain parenchyma. Proc Nat Acad Sci USA. 1999;96:6468–72. doi: 10.1073/pnas.96.11.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cotman SL, Halfter W, Cole GJ. Agrin binds to β-amyloid (Aβ), accelerates Aβ fibril formation, and is localized to Aβ deposits in Alzheimer's disease brain. Molec Cell Neurosci. 2000;15:183–98. doi: 10.1006/mcne.1999.0816. [DOI] [PubMed] [Google Scholar]
- 18.Verbeek MM, Otte-Höller I, van den Born J, et al. Agrin is a major heparan sulfate proteoglycan accumulating in Alzheimer's disease brain. Am J Pathol. 1999;155:2115–25. doi: 10.1016/S0002-9440(10)65529-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ancsin JB. Amyloidogenesis: historical and modern observations point to heparan sulfate proteoglycans as a major culprit. Amyloid. 2003;10:67–79. doi: 10.3109/13506120309041728. [DOI] [PubMed] [Google Scholar]
- 20.Barber AJ, Lieth E. Agrin accumulates in the brain microvascular basal lamina during development of the blood-brain barrier. Dev Dyn. 1997;208:62–74. doi: 10.1002/(SICI)1097-0177(199701)208:1<62::AID-AJA6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 21.Berzin TM, Zipser BD, Rafii MS, et al. Agrin and microvascular damage in Alzheimer's disease. Neurobiol Aging. 2000;21:349–55. doi: 10.1016/s0197-4580(00)00121-4. [DOI] [PubMed] [Google Scholar]
- 22.Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annual Rev Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- 23.Jankowsky JL, Fadale DJ, Anderson J, et al. Mutant presenilins specifically elevate the levels of the 42 residue β-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Molec Gen. 2004;13:159–70. doi: 10.1093/hmg/ddh019. [DOI] [PubMed] [Google Scholar]
- 24.Fuerst PG, Rauch SM, Burgess RW. Defects in eye development in transgenic mice overexpressing the heparan sulfate proteoglycan agrin. Devel Biol. 2007;303:165–80. doi: 10.1016/j.ydbio.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harvey SJ, Jarad G, Cunningham J, et al. Disruption of glomerular basement membrane charge through podocyte-specific mutation of agrin does not alter glomerular permselectivity. Am J Pathol. 2007;171:139–52. doi: 10.2353/ajpath.2007.061116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kisanuki YY, Hammer RE, Miyazaki J, et al. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Devel Biol. 2001;230:230–42. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 27.Lewandoski M, Martin GR. Cre-mediated chromosome loss in mice. Nature Gen. 1997;17:223–5. doi: 10.1038/ng1097-223. [DOI] [PubMed] [Google Scholar]
- 28.Burgess RW, Skarnes WC, Sanes JR. Agrin isoforms with distinct amino termini: differential expression, localization, and function. J Cell Biol. 2000;151:41–52. doi: 10.1083/jcb.151.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu G, Gonzales V, Borchelt DR. Rapid detection of protein aggregates in the brains of Alzheimer patients and transgenic mouse models of amyloidosis. Alzheimer Dis Assoc Dis. 2002;16:191–5. doi: 10.1097/00002093-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Raats CJ, Bakker MA, Hoch W, et al. Differential expression of agrin in renal basement membranes as revealed by domain-specific antibodies. J Biol Chem. 1998;273:17832–8. doi: 10.1074/jbc.273.28.17832. [DOI] [PubMed] [Google Scholar]
- 31.Eusebio A, Oliveri F, Barzaghi P, et al. Expression of mouse agrin in normal, denervated and dystrophic muscle. Neuromuscul Disord. 2003;13:408–15. doi: 10.1016/s0960-8966(03)00036-1. [DOI] [PubMed] [Google Scholar]
- 32.Noell S, Fallier-Becker P, Deutsch U, et al. Agrin defines polarized distribution of orthogonal arrays of particles in astrocytes. Cell and tissue research. 2009;337:185–95. doi: 10.1007/s00441-009-0812-z. [DOI] [PubMed] [Google Scholar]
- 33.Noell S, Fallier-Becker P, Beyer C, et al. Effects of agrin on the expression and distribution of the water channel protein aquaporin-4 and volume regulation in cultured astrocytes. The European journal of neuroscience. 2007;26:2109–18. doi: 10.1111/j.1460-9568.2007.05850.x. [DOI] [PubMed] [Google Scholar]
- 34.Fallier-Becker P, Sperveslage J, Wolburg H, et al. The impact of agrin on the formation of orthogonal arrays of particles in cultured astrocytes from wild-type and agrin-null mice. Brain Res. 2010;1367:2–12. doi: 10.1016/j.brainres.2010.09.092. [DOI] [PubMed] [Google Scholar]
- 35.Noell S, Wolburg-Buchholz K, Mack AF, et al. Evidence for a role of dystroglycan regulating the membrane architecture of astroglial endfeet. Eur J Neurosci. 2011;33:2179–86. doi: 10.1111/j.1460-9568.2011.07688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobayashi H. Identification and function of agrin expressed in rat brain microvessels. J of UOEH. 2009;31:219–30. doi: 10.7888/juoeh.31.219. [DOI] [PubMed] [Google Scholar]
- 37.Neumann FR, Bittcher G, Annies M, et al. An alternative amino-terminus expressed in the central nervous system converts agrin to a type II transmembrane protein. Molec Cell Neurosci. 2001;17:208–25. doi: 10.1006/mcne.2000.0932. [DOI] [PubMed] [Google Scholar]
- 38.Burgess RW, Dickman DK, Nunez L, et al. Mapping sites responsible for interactions of agrin with neurons. J Neurochem. 2002;83:271–84. doi: 10.1046/j.1471-4159.2002.01102.x. [DOI] [PubMed] [Google Scholar]
- 39.Perez-Tur J, Froelich S, Prihar G, et al. A mutation in Alzheimer's disease destroying a splice acceptor site in the presenilin-1 gene. Neuroreport. 1995;7:297–301. [PubMed] [Google Scholar]
- 40.Crook R, Verkkoniemi A, Perez-Tur J, et al. A variant of Alzheimer's disease with spastic paraparesis and unusual plaques due to deletion of exon 9 of presenilin 1. Nature medicine. 1998;4:452–5. doi: 10.1038/nm0498-452. [DOI] [PubMed] [Google Scholar]
- 41.Timmer NM, Herbert MK, Kleinovink JW, et al. Limited expression of heparan sulphate proteoglycans associated with Aβ deposits in the APPswe/PS1dE9 mouse model for Alzheimer's disease. Neuropathol Appl Neurobiol. 2010;36:478–86. doi: 10.1111/j.1365-2990.2010.01091.x. [DOI] [PubMed] [Google Scholar]
- 42.Wang J, Tanila H, Puolivali J, et al. Gender differences in the amount and deposition of amyloid-β in APPswe and PS1 double transgenic mice. Neurobiol Dis. 2003;14:318–27. doi: 10.1016/j.nbd.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 43.Howlett DR, Richardson JC, Austin A, et al. Cognitive correlates of Aβ deposition in male and female mice bearing amyloid precursor protein and presenilin-1 mutant transgenes. Brain Res. 2004;1017:130–6. doi: 10.1016/j.brainres.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 44.Hirata-Fukae C, Li HF, Hoe HS, et al. Females exhibit more extensive amyloid, but not tau, pathology in an Alzheimer transgenic model. Brain Res. 2008;1216:92–103. doi: 10.1016/j.brainres.2008.03.079. [DOI] [PubMed] [Google Scholar]
- 45.Callahan MJ, Lipinski WJ, Bian F, et al. Augmented senile plaque load in aged female β-amyloid precursor protein-transgenic mice. Am J Pathol. 2001;158:1173–7. doi: 10.1016/s0002-9440(10)64064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dai W, Lopez OL, Carmichael OT, et al. Mild cognitive impairment and alzheimer disease: patterns of altered cerebral blood flow at MR imaging. Radiology. 2009;250:856–66. doi: 10.1148/radiol.2503080751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chow N, Bell RD, Deane R, et al. Serum response factor and myocardin mediate arterial hypercontractility and cerebral blood flow dysregulation in Alzheimer's phenotype. Proc Nat Acad Sci USA. 2007;104:823–8. doi: 10.1073/pnas.0608251104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ellis RJ, Olichney JM, Thal LJ, et al. Cerebral amyloid angiopathy in the brains of patients with Alzheimer's disease: the CERAD experience, Part XV. Neurology. 1996;46:1592–6. doi: 10.1212/wnl.46.6.1592. [DOI] [PubMed] [Google Scholar]
- 49.Warth A, Kroger S, Wolburg H. Redistribution of aquaporin-4 in human glioblastoma correlates with loss of agrin immunoreactivity from brain capillary basal laminae. Acta Neuropathologica. 2004;107:311–8. doi: 10.1007/s00401-003-0812-0. [DOI] [PubMed] [Google Scholar]
- 50.Wilcock DM, Vitek MP, Colton CA. Vascular amyloid alters astrocytic water and potassium channels in mouse models and humans with Alzheimer's disease. Neuroscience. 2009;159:1055–69. doi: 10.1016/j.neuroscience.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salloway S, Gur T, Berzin T, et al. Effect of APOE genotype on microvascular basement membrane in Alzheimer's disease. J Neurol Sci. 2002;203-204:183–7. doi: 10.1016/s0022-510x(02)00288-5. [DOI] [PubMed] [Google Scholar]
- 52.Attems J, Jellinger KA. Only cerebral capillary amyloid angiopathy correlates with Alzheimer pathology--a pilot study. Acta Neuropathologica. 2004;107:83–90. doi: 10.1007/s00401-003-0796-9. [DOI] [PubMed] [Google Scholar]
- 53.Thal DR, Ghebremedhin E, Rub U, et al. Two types of sporadic cerebral amyloid angiopathy. J Neuropathol Exp Neurol. 2002;61:282–93. doi: 10.1093/jnen/61.3.282. [DOI] [PubMed] [Google Scholar]
- 54.Davis J, Xu F, Deane R, et al. Early-onset and robust cerebral microvascular accumulation of amyloid β-protein in transgenic mice expressing low levels of a vasculotropic Dutch/Iowa mutant form of amyloid β-protein precursor. J Biol Chem. 2004;279:20296–306. doi: 10.1074/jbc.M312946200. [DOI] [PubMed] [Google Scholar]
- 55.Donahue JE, Flaherty SL, Johanson CE, et al. RAGE, LRP-1, and amyloid-β protein in Alzheimer's disease. Acta Neuropathologica. 2006;112:405–15. doi: 10.1007/s00401-006-0115-3. [DOI] [PubMed] [Google Scholar]
- 56.Timmer NM, van Horssen J, Otte-Holler I, et al. Amyloid β induces cellular relocalization and production of agrin and glypican-1. Brain Res. 2009;1260:38–46. doi: 10.1016/j.brainres.2008.12.063. [DOI] [PubMed] [Google Scholar]