Abstract
Clinical resistance to chemotherapy is a frequent event in cancer treatment and is closely linked to poor outcome. High-grade serous (HGS) ovarian cancer is characterized by p53 mutation and high levels of genomic instability. Treatment includes platinum-based chemotherapy and initial response rates are high; however, resistance is frequently acquired, at which point treatment options are largely palliative. Recent data indicate that platinum-resistant clones exist within the sensitive primary tumor at presentation, implying resistant cell selection after treatment with platinum chemotherapy. The AKT pathway is central to cell survival and has been implicated in platinum resistance. Here, we show that platinum exposure induces an AKT-dependent, prosurvival, DNA damage response in clinically platinum-resistant but not platinum-sensitive cells. AKT relocates to the nucleus of resistant cells where it is phosphorylated specifically on S473 by DNA-dependent protein kinase (DNA-PK), and this activation inhibits cisplatin-mediated apoptosis. Inhibition of DNA-PK or AKT, but not mTORC2, restores platinum sensitivity in a panel of clinically resistant HGS ovarian cancer cell lines: we also demonstrate these effects in other tumor types. Resensitization is associated with prevention of AKT-mediated BAD phosphorylation. Strikingly, in patient-matched sensitive cells, we do not see enhanced apoptosis on combining cisplatin with AKT or DNA-PK inhibition. Insulin-mediated activation of AKT is unaffected by DNA-PK inhibitor treatment, suggesting that this effect is restricted to DNA damage-mediated activation of AKT and that, clinically, DNA-PK inhibition might prevent platinum-induced AKT activation without interfering with normal glucose homeostasis, an unwanted toxicity of direct AKT inhibitors.
Introduction
AKT (v-akt murine thymoma viral oncogene homologue/protein kinase B [PKB]) is a serine/threonine kinase controlling physiological processes such as cell growth, proliferation, survival and motility. Dysregulation of the AKT pathway is well described in cancer (reviewed in Vivanco and Sawyers [1]) and has been implicated in tumorigenesis and resistance to chemotherapy [2–6]. The canonical pathway leading to AKT activation involves receptor tyrosine kinase recruitment of phosphatidylinositol 3-kinase (PI3K) leading to the conversion of phosphatidyl-inositol 4,5-diphosphate (PIP2) to phosphatidyl-inositol 3,4,5-triphosphate (PIP3) at the cell membrane. Subsequently AKT is recruited to the cell surface through interaction with phosphatidyl-inositol 3, 4, 5-triphosphate. AKT is activated after phosphorylation on two key residues: serine 473 (S473) and threonine 308 (T308) [1]. Phosphorylation of T308 is performed by 3-phosphoinositide-dependent kinase 1 (PDK1) [1]. The identity of the kinase responsible for phosphorylation of S473 has been more elusive; however, it has now been shown that mammalian target of rapamycin complex 2 (mTORC2) can catalyze this reaction [7] as can DNA-dependent protein kinase (DNA-PK) [8,9], integrin-linked kinase 1, mitogen-activated protein kinase-activated protein kinase 2, protein kinase CβII (PKCβII), ataxia-telangiectasia mutant, and ataxia-telangiectasia and Rad3 related, which are thought to reflect the numerous cellular contexts in which AKT plays a role [10].
Cisplatin and carboplatin are widely used agents in the treatment in of cancers including ovarian, testicular, head and neck, and non-small cell lung cancer where they act by forming covalent adducts with the cellular DNA, leading to replicative and transcriptional blockage and ultimately growth arrest and apoptosis. The clinical use of platinum agents is, however, limited by the frequent development of resistance, which is thought to occur through a variety of mechanisms (reviewed in Agarwal and Kaye [11]). One of the key mediators of platinum resistance is the AKT pathway. Hyperactivation of the PI3K/AKT can occur by mutations involving p110/p85 PI3K subunits, AKT isoforms, or the negative regulator of AKT, PTEN (phosphatase and tensin homolog deleted on chromosome 10) (reviewed in Stronach et al. [12]). Numerous additional components of the AKT pathway have been implicated in chemoresistance. Recently, a positive feedback loop in which AKT activates FOXO3a, which in turn enhances the expression of PI3K p110α, has been associated with doxorubicin resistance in leukemic cells [13]. AKT negatively regulates apoptosis-initiating factor in cisplatin-resistant ovarian cancer cells to prevent caspase-independent cisplatin-induced apoptosis [14]. In malignant melanoma cells, knockdown of PRAS40 (a downstream target of AKT) or AKT3 enhanced the apoptotic response to staurosporine [15]. In addition, AKT prevents mitochondrial accumulation of p53 and release of cytochrome c and Smac/DIABLO, conferring cisplatin resistance to ovarian cancer cells [3]. The mechanism by which cisplatin activates the AKT survival response in clinical platinum resistance is currently undetermined.
We used a series of matched clinically platinum-sensitive/resistant paired cell lines [16–18] to assess the role of DNA-PK in the activation of AKT in response to cisplatin and show that DNA-PKcs is expressed in high-grade serous (HGS) ovarian cancer cells and phosphorylates AKT at S473 in response to cisplatin-induced DNA damage in cells with clinically acquired resistance to cisplatin but not in matched sensitive cells. Furthermore, we show colocalization and binding of DNA-PKcs and AKT in the nuclei of resistant but not sensitive tumor cells and that inhibition of DNA-PK prevents AKT activation and enhances sensitivity to cisplatin in platinum-resistant ovarian cancer cells. We also show that activation of AKT by DNA-PK occurs in response to cisplatin, but not insulin, across a range of tumor types, suggesting a nuclear, DNA damage-mediated pathway distinct from canonical cell surface PI3K/AKT activation. These findings have implications for the clinical management of ovarian and other cancers.
Materials and Methods
Cell Lines and Reagents
The paired HGS ovarian carcinoma cell lines PEO1, PEO4, PEO6, PEA1, PEA2, PEO14, and PEO23 were obtained from Dr Simon Langdon (Edinburgh, UK) and have been described [16–19]. Cell lines were verified by STR DNA fingerprinting. In the matched pairs PEO1 versus PEO4/PEO6, PEA1 versus PEA2, and PEO14 versus PEO23, the first set of cell lines (PEO1, PEA1, and PEO14) was derived before and the second set (PEO4/PEO6, PEA2, PEO23) was derived after the onset of acquired clinical platinum resistance. Paired cell lines PEO1/PEO4, PEA1/PEA2, and PEO14/PEO23 were sequenced for COSMIC mutations as described previously [20].
Clear cell ovarian cancer cell line, HCH1, was a gift from Dr Kigawa Tottori University, Japan. SKOV3, PANC-1, A549, HCC95, and PC3 cells were obtained from European Collection of Cell Cultures. Cisplatin response in vitro was reported elsewhere [18], confirming maintained clinical platinum resistance in vitro. IC50 (inhibitory concentration 50%) values for ovarian lines are summarized in Table W1. Cells were maintained in RPMI 1640 media (10% fetal calf serum) at 37°C/5% CO2. Antibodies and suppliers were as follows: AKT1, AKT2, AKT3, panAKT, pAKT-S473, pAKT-T308, pBAD-S136, pPRAS40, integrin-linked kinase 1, and Rictor (Cell Signaling Technology, Danvers, MA); DNA-PKcs (Thermo Scientific, Waltham, MA); γH2AX (Abcam, Cambridge, United Kingdom); Lamin A/C (Millipore, Billerica, MA); and β-tubulin (Santa Cruz, Santa Cruz, CA).
Cell Proliferation and Apoptosis Assays
Cells were seeded in triplicate in 96-well trays and allowed to adhere for 24 hours. Treatments were as described. Apoptotic assessment was by detection of active caspase 3/7 using caspase Glo 3/7 assay (Promega, Madison, WI) following the manufacturer's protocol. Cell proliferation was by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as described elsewhere [18]. Caspase activity was normalized to cell density (MTT) data for each treatment. For isobologram analyses, cells were seeded into 96-well plates and allowed to adhere. The medium was replaced with serially diluted AKT inhibitor (API-2; Berry & Associates, Dexter, MI) and left for 1 hour. Cisplatin was then added in serial dilutions, from 50 to 0.391 µM in a matrix format with inhibitor-treated cells. MTT assays were performed after three doubling times. The IC50 values were calculated for each drug alone and plotted onto an IC50-(cisplatin)-versus-IC50 (API-2) graph to generate the isobole. Combination values that achieved IC50 growth inhibition ±10% were plotted, and superadditivity was indicated by points below the isobole.
Western Blot and Immunoprecipitation
Western blots were preformed as described previously [18]. For immunoprecipitation (IP), cells were treated with 25 µM cisplatin or control for 24 hours as appropriate before lysis (IP lysis buffer: 1% Triton X, 150 mM NaCl, 50 mM Tris-HCl, 0.2 mM Na3VO4, 50 mM NaF, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride (PMSF), 25 µg/ml aprotinin, 25 µg/ml leupeptin). One hundred microliters of protein G sepharose (PGS) beads was washed in phosphate-buffered saline (PBS) and then IP lysis buffer. To address nonspecific protein binding to PGS, 1 mg of sample lysate was incubated with 30 µl of PGS rotating at 4°C for 1 hour. Precleared lysates were incubated overnight at 4°C with 2 µg of primary antibody. Thirty microliters of PGS was added to each sample, including whole-cell extract control, and incubated rotating at 4°C before centrifuging at 10,000 rpm for 2 minutes. Collected beads were washed three times with IP lysis buffer and then dissolved in 50 µl of 2x sample buffer (1% Triton X, 150 mM NaCl, 50 mM Tris-HCl, 2 mM EDTA, 1 mM PMSF) at 95°C for 10 minutes. Equal volumes of the IP sample, extract-only, and controls were separated and visualized by Western blot as described previously.
Small Interfering RNA Transfection and Apoptosis Assay
Cells grown to 60% confluence in six-well plates were transfected at 100 nM final small interfering RNA (siRNA) concentration (Dharmacon, Lafayette, CO). Cells were retransfected after 48 hours. SiRNAs in 1x siRNA buffer were mixed with 2 µl of transfection reagent no. 1 (Dharmacon) per transfection in a total volume of 400 µl with Opti-MEM (Invitrogen, Paisley, United Kingdom). After 30 minutes of incubation, siRNAs were added to 1600 µl of antibiotic-free RPMI 1640/10% fetal calf serum on cells. Twenty-four hours after the second transfection, cells were reseeded. Cells in six-well trays were incubated for 48 hours, and protein samples were prepared. Cells in clear and opaque 96-well trays were treated identically: for each transfection condition, 24 hours after seeding, three replicate wells were treated with 25 µM cisplatin and three wells were left untreated. After 24 hours, cells' caspase activation was measured by caspase Glo 3/7, and viable cell numbers were inferred by MTT assay.
Immunofluorescent Microscopy
Coverslips (VWR International, Lutterworth, United Kingdom) were treated with 1 M HCl before cell seeding and incubation for 24 hours. After serum starvation and indicated treatments, cells were washed with PBS and then fixed/permeabilized at 37°C for 30 minutes with 4% paraformaldehyde/1.8% Triton X-100/PBS. Coverslips were blocked in 10% goat serum/2% bovine serum albumin/PBS for 30 minutes, washed with PBS, and incubated with primary antibodies overnight at 4°C. Coverslips were washed in PBS and incubated with fluorochrome-conjugated secondary antibodies (fluorescein isothiocyanate anti-goat IgG, fluorescein isothiocyanate anti-rabbit IgG, Alexa- Fluor 555 anti-mouse IgG, and Alexa-Fluor 488 goat anti-rabbit IgG, 1:500; Invitrogen) and directly labeled actin stain (Alexa-Fluor 633 phalloidin; Invitrogen) in blocking buffer for 1 hour. Cells were rinsed in PBS and mounted onto slides using VECTASHIELD media containing 4′,6-diamidino-2-phenylindole (Vector Laboratories, Peterborough, United Kingdom). Slides were visualized on an inverted confocal microscopy system (TCS SP5; Leica, Wetzlar, Germany).
Subcellular Fractionation
Cells were serum-starved overnight and then treated with 25 µM cisplatin for the indicated time points. Cells were washed with cold PBS, and pellets were collected by trypsinization. Fractionation was by nuclear/cytosolic or mitochondrial/cytosolic fractionation kits according to the manufacturer's protocols (BioVision, Mountain View, CA).
Results
AKT Is Activated in Response to Cisplatin Treatment in Clinically Platinum-Resistant Cells Only and AKT Inhibition Restores Platinum Sensitivity
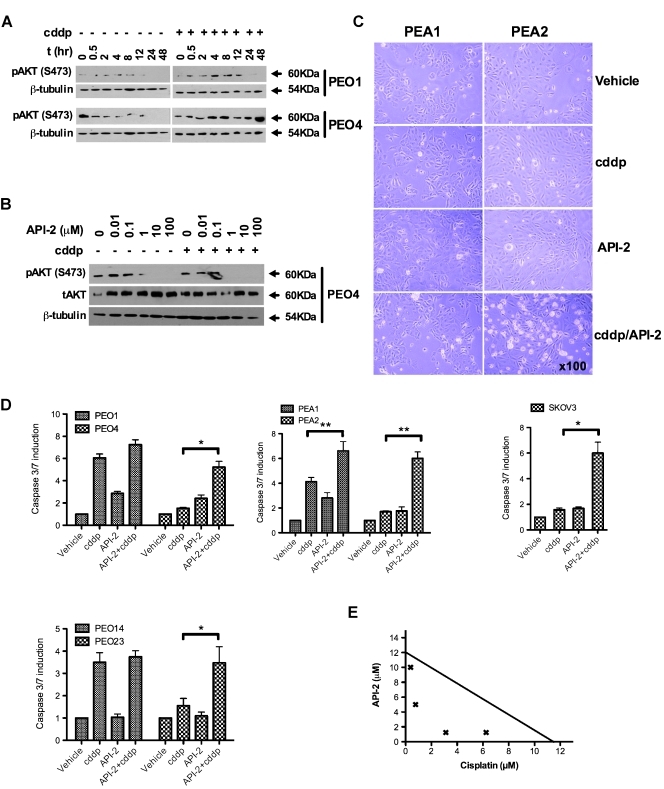
Previously, we reported upregulation of PIK3R1, the p85α subunit of PI3K, in clinically platinum-resistant ovarian cancer cells [18] and showed that knockdown of PIK3R1 enhanced sensitivity to cisplatin. We therefore examined activation of AKT in response to cisplatin in clinically derived platinum-sensitive and -resistant ovarian cancer cells. Sensitive cells (PEO1) showed minimal platinum-induced phosphorylation of AKT-S473 during a 48-hour period. Conversely, clinically platinum-resistant cells cultured from the same patient (PEO4) after relapse, S473 phosphorylation induction is evident from 4 hours after cisplatin (Figure 1A). Densitometry indicates three- to four-fold induction of S473 8 hours after cisplatin treatment maintained at 48 hours (Figures 1A and W1A). Interestingly, previous analysis of these matched cell line pairs indicated that platinum-resistant cells existed clinically at presentation and were selected for by platinum therapy [16]. Our data suggest activation of AKT after cisplatin treatment is a specific molecular feature of the resistant tumor, emerging after clearance of sensitive cells by chemotherapy, implicating AKT-mediated prosurvival signaling as a resistance mechanism. Hence, we examined the effect of AKT inhibition on platinum sensitivity using the small-molecule AKT inhibitor API-2 [21], which binds the PH domain of AKT preventing its activation [22]. Figure 1B demonstrates a dose-dependent, API-2-mediated reduction in pAKT-S473 in the presence and absence of cisplatin (see Figure W1B for densitometry). We hypothesized that prevention of cisplatin-induced activation of AKT may restore apoptotic potential, and we therefore compared caspase 3/7 activation in response to cisplatin in the presence and absence of API-2. Figure 1, C and D, demonstrates enhancement of apoptotic induction in platinum-resistant ovarian cancer cells (PEO4, PEA2, PEO23, and SKOV3) after inhibition of AKT, suggesting that AKT inhibition primes the resistant cells for apoptosis, after which a cytotoxic insult from cisplatin provokes caspase 3/7 activation. This has implications for AKT inhibitor strategies, suggesting that AKT inhibitor monotherapy may be inactive in this setting compared with combination with platinum. Strikingly, AKT inhibition seems to have little effect on platinum-induced caspase activity in the platinum-sensitive lines PEO1, PEA1, and PEO14 derived from the same patients as the resistant lines (Figure 1, C and D). This is in keeping with data from Figure 1A, indicating that AKT is not activated after cisplatin treatment in sensitive cells, suggesting that this is a truly acquired molecular mechanism underlying platinum resistance in HGS ovarian cancer. In addition, AKT inhibition was also effective in clear cell ovarian cancer cells (HCH-1), pancreatic (PANC-1), and prostate (PC3) cancer cells (Figure W2). To further assess the combinatorial effect of cisplatin and API-2, we performed isobologram analyses [23], which indicated synergistic interaction between cisplatin and API-2 in resistant PEO4 cells (Figure 1E).
Figure 1.
AKT inhibition reverses resistance to platinum treatment in ovarian cancer cells. Western blot indicates differential AKT-S473 response to platinum treatment between platinum-sensitive and -resistant cells from the same patient (A) and shows inhibition of pAKT by AKT inhibitor API-2 (B). Visual appearance of platinum-sensitive (PEA1) and matched resistant (PEA2) cells illustrates enhancement of cytotoxicity in resistant cells 8 hours after combination treatment with 20 µM API-2 and equitoxic cisplatin (5 µM PEA1; 25 µM PEA2) (C). Caspase 3/7 assays 24 hours after treatment with cisplatin and/or API-2 reveal enhanced apoptosis when combining API-2 with cisplatin compared to platinum alone in platinum-resistant cell lines: PEO4 (P = .019), PEA2 (P = .003), PEO23 (P = .042), and SKOV3 (P = .02) (paired t tests). Conversely, platinum-induced apoptosis is more modestly enhanced in the matched platinum-sensitive cells lines PEO1, PEA1, and PEO14, with only PEA1 achieving statistical significance (P = .01) (D). Isobologram analysis of acquired platinum-resistant PEO4 cells supports the synergistic interaction between platinum and API-2 (E). n ≥ 3. *P < .05. **P < .01.
Cisplatin Resistance Is Not Determined by a Single, Common AKT Isoform
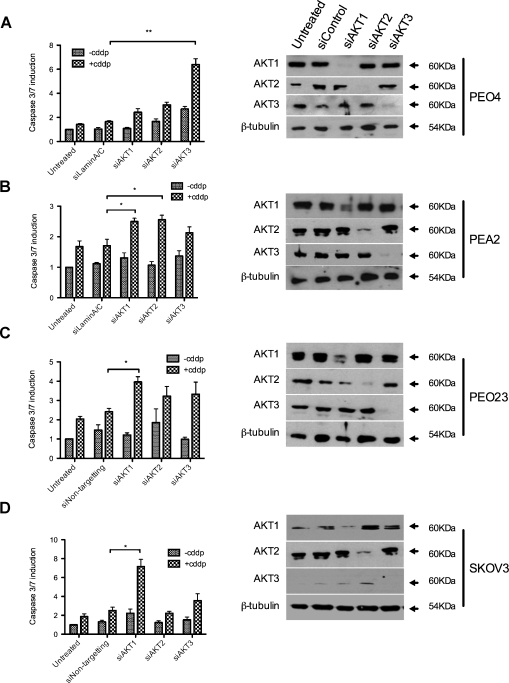
A drawback to targeting AKT therapeutically is its fundamental role in biological processes such as insulin signaling and normal growth control [24]. Studies of AKT1, 2, and 3 knockout mouse models indicate nonredundancy in AKT isoform function [25]. We therefore considered the potential of single-isoform effects in platinum resistance. SiRNAs to each of the three isoforms of AKT, namely, AKT1(PKBα), AKT2(PKBβ), and AKT3(PKBγ), in platinum-resistant cell lines showed that each cell line tested seems to have an isoform dependency: PEO23 and SKOV3 require AKT1 for cisplatin resistance, PEA2 requires AKT2, whereas PEO4 requires AKT3 (Figure 2, A–D). To determine whether known activating mutations in PI3K and AKT were responsible for the drug-resistant phenotype, we sequenced DNA from each of the paired cell lines. No mutations were found at tested sites in any AKT isoform or in PIK3CA or PIK3R1. Furthermore, 118 additional common variants were screened in 29 cancer associated genes, which identified a heterozygous G2677A variant in ABCB1 in PEA2 and a heterozygous G1154A variant in VEGFA in PEA1 as the only alterations that differed between sensitive and resistant pairs. These changes are not thought to relate to platinum resistance (for further details and discussion, see Table W2). It seems that no single AKT isoform is specifically selected in platinum resistance; hence, pan-AKT inhibition is more rational in this setting.
Figure 2.
The functional effect of AKT in platinum resistance is not determined by a single common AKT isoform. After knockdown of individual AKT isoforms 1, 2, and 3, cells were treated with cisplatin, and caspase 3/7 activation was assayed for platinum-resistant cell lines PEO4 (A), PEA2 (B), PEO23 (C), and SKOV3 (D). Isoform-specific knockdowns are indicated by Western blot (right panels). Statistically significant differences, compared with control transfections, are indicated: *P < .05. **P < .01.
mTORC2 Does Not Phosphorylate AKT-S473 in Response to Cisplatin in Platinum-Resistant Cells
We hypothesized that the identification of the kinase responsible for activation of AKT in response to cisplatin treatment might suggest a therapeutic target with better phenotypic specificity than targeting AKT itself.
The best-characterized kinase phosphorylating AKT-S473 is mTORC2, a protein complex composed of mTOR, mLST8, and Rictor [7]. We performed siRNA to the Rictor subunit of mTORC2 and show that knockdown had no significant effect on platinum response (Figure 3A). Furthermore, Rictor knockdown has no effect on platinum-mediated phosphorylation of AKT-S473 in resistant SKOV3 cells (Figure 3A). Rapamycin treatment also fails to prevent cisplatin-mediated induction of pAKT-S473 and actually seems to inhibit the apoptotic response to cisplatin (Figure 3B). Finally, IP in the presence and absence of platinum failed to reveal any interaction between Rictor and AKT (pS473; Figure 3C). We conclude that mTORC2 is not involved in cisplatin-mediated activation of AKT and that mTOR in general is probably not involved in the downstream prosurvival effects of activated AKT in platinum-resistant cells.
Figure 3.
AKT is not activated by mTOR-Rictor in response to platinum, and its inhibition does not enhance platinum-mediated apoptosis. Caspase 3/7 activation was measured 24 hours after cisplatin treatment in control and Rictor siRNA-transfected cells, which indicated no enhancement of apoptosis or effect on pAKT-S473 (A). Treatment of sensitive (PEO1) and resistant (PEO4) cells with the mTOR inhibitor rapamycin (200 nM) and/or cisplatin (25 µM) indicated no enhancement of platinum sensitivity on combination with rapamycin and no effect on AKT phosphorylation (B). No interaction between Rictor and pAKT was detected by IP in the presence or absence of 25 µM cisplatin (2-hour exposure) in platinum-resistant PEO4 cells (C).
DNA-PK Phosphorylates AKT-S473 in Response to Cisplatin in the Nucleus of Platinum-Resistant, But Not Sensitive, Cells and Enhances Cisplatin Response in Clinically Resistant Cells without Affecting Insulin-Mediated AKT Activation
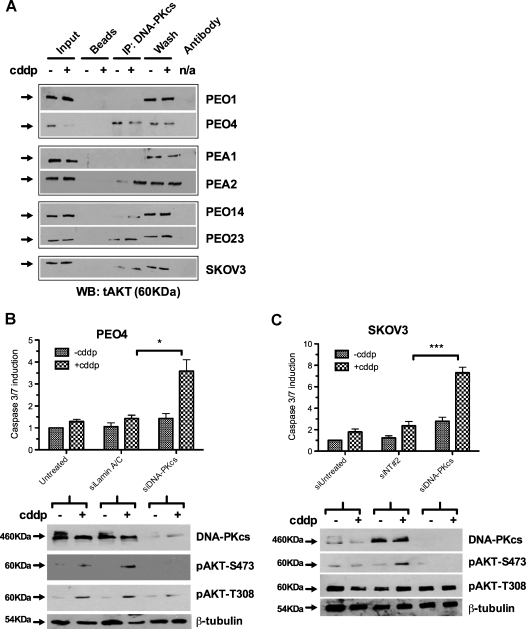
We next considered if DNA-PK was responsible for platinum-mediated prosurvival activation of AKT seen on acquisition of clinical platinum resistance in ovarian cancer. Interaction between AKT and DNA-PK was detected by IP in platinum-resistant cells (Figures 4A and W3A). By contrast, this interaction was either not seen or was less readily detectable in intrapatient-matched sensitive cells (Figure 4A).
Figure 4.
DNA-PKcs phosphorylates AKT in response to cisplatin treatment and mediates platinum resistance. IP of DNA-PKcs in a panel of platinum-sensitive and -resistant cells indicates interaction with AKT in resistant cells, in the presence and absence of 25 µM cisplatin (2-hour exposure), which is not seen in sensitive PEO1 and PEA1 cells and is only detectable on platinum treatment in sensitive PEO14 cells (A). Knockdown of DNA-PKcs in platinum-resistant PEO4 (B) and SKOV3 (C) cells increases the apoptotic induction on platinum treatment compared with control transfections (P = .01, PEO4: P = .0003, SKOV3). Western blots indicate specific loss of cisplatin-induced phosphorylation of AKT-S473 but not T308. n = 3. *P < .05. ***P < .001.
Knockdown of DNA-PKcs significantly enhanced apoptotic response to cisplatin in PEO4-, SKOV3-, PEA2-, and PEO23-resistant ovarian cancer cells (P < .05; Student's t test) (Figures 4, B and C, and W3, B or C). Western blot analysis showed that, in the absence of DNA-PKcs, platinum-induced activation of AKT by phosphorylation at S473 was ablated. Phosphorylation of AKT at T308, known to be catalyzed by PDK1, was unaffected by DNA-PKcs knockdown confirming site specific activity and indicating that T308 phosphorylation alone is insufficient for the platinum-resistant phenotype (Figure 4, B and C).
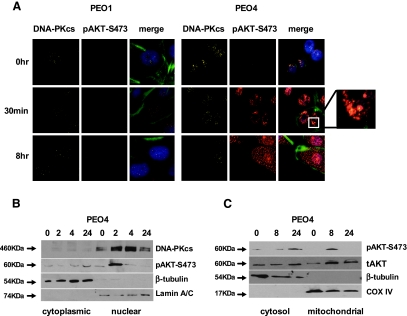
Given platinum's mode of action, damaging DNA, and the role of DNA-PK in DNA repair, we performed immunofluorescent confocal microscopy, which revealed nuclear accumulation of pAKT in resistant cells within 30 minutes of platinum treatment with apparent cytoplasmic redistribution by 8 hours (Figures 5A and W4). By contrast, platinum-sensitive cells do not accumulate nuclear pAKT. Nuclear pAKT was confirmed by subcellular fractionation experiments (Figure 5B), which also indicated mitochondrial redistribution of pAKT at 8 hours (Figure 5C). Together with the IP and siRNA data (Figure 4), this suggests AKT is activated in the nucleus by DNA-PKcs after cisplatin-induced DNA damage in platinum-resistant, but not platinum-sensitive, cells and subsequently redistributes to mitochondria.
Figure 5.
After platinum treatment, AKT is translocated to the nucleus of cisplatin-resistant but not matched cisplatin-sensitive cells. Immunofluorescent microscopy of platinum-sensitive (PEO1) and -resistant (PEO4) cells after treatment with 25 µM cisplatin reveals induction and nuclear accumulation of pAKT-S473 in PEO4 but not PEO1. The enlarged box indicates nuclear colocalization of pAKT and DNA-PKcs in PEO4 cells 30 minutes after platinum treatment. After an 8-hour exposure to cisplatin, pAKT appears redistributed to the cytoplasmic compartment in PEO4. Counterstaining of nuclei is indicated in blue in the merged images (secondary antibody-only controls are shown in Figure W4; A). Western blot of fractionated PEO4 cells confirms early nuclear location of pAKT after 25 µM cisplatin with delayed cytoplasmic accumulation. Purity of fractions is indicated by β-tubulin (cytoplasmic) and Lamin A/C (nuclear) markers (B). Mitochondrial fractionation indicates that punctate staining seen 8 hours after cisplatin treatment in A corresponds with a mitochondrial relocalization of pAKT. Purity of fractions is indicated by β-tubulin (cytoplasmic) and COX IV (mitochondrial) markers (C).
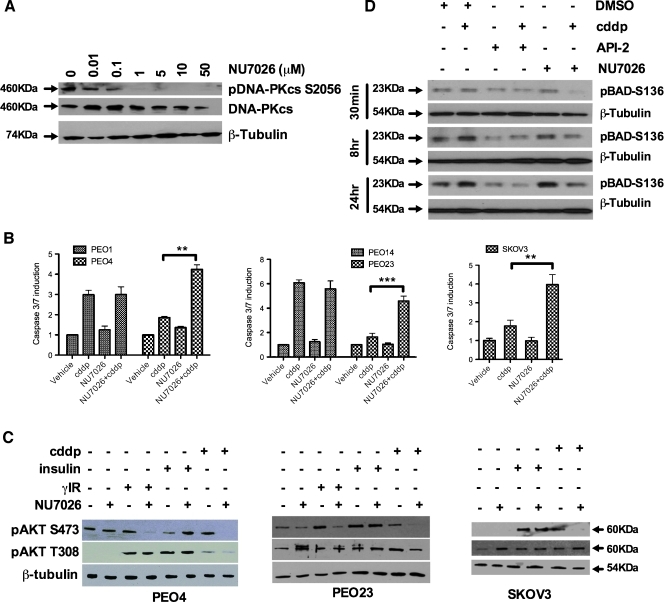
Next we considered the broader effects of these initial observations using the DNA-PK inhibitor, NU7026 [26]. Figure 6A demonstrates a dose-dependent inhibition of DNA-PKcs phosphorylation at serine 2056 by NU7026 in resistant PEO4 cells, consistent with inhibition of catalytic activity and hence autophosphorylation of DNA-PKcs at this site [27]. NU7026 significantly sensitized platinum-resistant SKOV3 cells and the intrapatient-matched platinum-resistant cells (PEO4, PEO23, and PEA2) to platinum-induced caspase 3/7 activity with little effect on their platinum-sensitive counterparts (PEO1, PEO14, and PEA1; Figures 6B and W5). As with DNA-PKcs siRNA, enhancement of apoptosis was associated with loss of platinum-induced pAKT-S473 but not T308 (Figure 6C). We examined the cellular levels of phosphorylated BAD (S136), an AKT-mediated phosphorylation event that inhibits this proapoptotic BCL-2 family member [28]. Figure 6D shows that the AKT inhibitor API-2 decreases pBAD-S136 in the presence and absence of cisplatin treatment, consistent with a direct effect on AKT. NU7026 also prevents pBAD accumulation in the presence of cisplatin; however, it has no effect on pBAD levels in the absence of platinum, consistent with the role of DNA-PK as a DNA damage-specific activator of AKT and consistent with the reversal of cisplatin resistance observed in Figures 4 and 6.
Figure 6.
DNA-PK inhibition enhances cisplatin-induced apoptosis in platinum-resistant but not platinum-sensitive cells through inhibition of AKT-S473 phosphorylation. Inhibition of DNA-PKcs phosphorylation at serine 2056 was observed from 1 to 50 µM DNA-PK inhibitor NU7026, consistent with DNA-PKcs autophosphorylation at this residue (A). Forty-eight hours of cisplatin treatment of intrapatient paired platinum-sensitive (PEO1 and PEO14; 5 µM cisplatin) and -resistant (PEO4 and PEO23; 25 µM cisplatin) after serum starvation indicates clear and significant resensitization to platinum-mediated apoptosis after 10 µM NU7026 treatment in resistant cells (P = .0017, PEO4; P = .0004, PEO23) but no enhancement of apoptosis in sensitive cells (P = n.s.). The unmatched platinum-resistant SKOV3 cell line behaves similarly to the other resistant lines (P = .001) (B). Western blot indicates a consistent inhibition of pAKT-S473 in platinum- or IR-treated cells when cotreated with NU7026 for 24 hours. Conversely, AKT T308 phosphorylation is unaffected by NU7026 treatment. DNA-PK inhibition failed to prevent insulin-mediated AKT-S473 phosphorylation (C). Western blot indicates inhibition of pBAD-S136 at 30 minutes, 8 hours, and 24 hours after combined treatment with cisplatin (25 µM) and the AKT inhibitor (API-2; 10 µM) or DNA-PK inhibitor (NU7026; 10 µM). (D) **P < .01. ***P < .001.
We also looked at the effect of DNA-PK inhibition on platinum response in a broader panel of cell lines: HCH-1 ovarian clear cell, A549 and HCC95 lung cells, and PANC-1 pancreatic cells (Figure W5). Each showed significant enhancement of platinum-mediated caspase 3/7 induction on DNA-PK inhibition.
Clinical use of AKT inhibitors has been associated with hyperglycemia/hyperinsulinemia reflecting the central role of AKT in insulin signaling [24]. We sought to determine whether platinum-induced, DNA-PK-mediated AKT activation occurred independently of insulin-induced AKT activation in cancer, as has been indicated for γ-irradiation-induced damage in HUVEC cells [8]. Figures 6C and W5 show that DNA damage induced by either γIR or cisplatin activates AKT through a DNA-PK-dependent phosphorylation at AKT-S473. However, insulin stimulation induces pAKT-S473 in a DNA-PK-independent manner in PEO4, PEO23, SKOV3, PANC-1, and A549 cells. These data have implications for clinical inhibition of AKT in combination with DNA-damaging chemotherapeutics, suggesting that DNA-PK inhibition may circumvent the effects on glucose homeostasis seen with direct AKT inhibitors while maintaining the proapoptotic effect associated with preventing DNA damage-induced AKT activation-mediated survival.
Discussion
HGS ovarian cancer is the most common subtype of the ovarian neoplasms and is associated with poor outcome. High TP53 mutation rate and defects in homologous recombination repair create the genomic instability that underlies cellular heterogeneity in this tumor type (reviewed in Bowtell [29]). Interestingly, DNA damage response defects in HGS ovarian cancer render the cells typically sensitive to the initial treatment with cytotoxic chemotherapy. However, this feature also generates the cellular heterogeneity that has been postulated to account for the high frequency of acquired resistance to platinum-based chemotherapy. Cooke et al. [16] reported, using the same cell line models studied here, that resistant and sensitive cells from a single patient contain mutually exclusive genomic features, indicating that acquired resistance does not develop by mutation to the sensitive tumor on platinum exposure but by selection of preexisting platinum-resistant subclones within the heterogeneous tumor mass. These observations have significance in understanding and managing clinical platinum resistance. By implication, if resistant cells are present in the presenting tumor, targeting of resistant cells could be applied to the front-line setting to delay resistant relapse.
Here, we demonstrate that AKT activation in response to platinum is an important mechanism underlying platinum-resistant clinical relapse: the impact of AKT inhibition on both cisplatin-induced apoptosis and cisplatin-mediated phosphorylation of AKT are minimal in platinum-sensitive tumor cells, whereas in resistant cells from the same patient, S473 phosphorylation of AKT mediates platinum resistance.
Previously, constitutive activation of AKT2 has been shown to cause cisplatin resistance in ovarian cancer models and its expression in platinum-sensitive cells prevents cisplatin-induced down-regulation of XIAP and represses proapoptotic BAX [2,30]. In addition, constitutively active PI3K induces taxol resistance in xenograft models of ovarian cancer; a phenotype reversed by PI3K inhibition [31]. Cisplatin treatment of sensitive, but not resistant, cells was reported to cause caspase-mediated cleavage and inactivation of AKT and reduced intracellular levels of XIAP, resulting in cisplatin-induced apoptosis. Conversely, overexpression of XIAP, a direct inhibitor of caspase 3/7, promotes AKT phosphorylation and decreases cisplatin-induced apoptosis (reviewed in Stronach et al. [12]). Pei et al. [32] showed that FKBP51, which promotes the dephosphorylation of AKT-S473, is associated with sensitivity to chemotherapy, although not specifically platinum agents. Platinum-treated ovarian cancer patients with complete responses and patients with more than 6 months of progression-free survival (platinum-sensitive) were reported to be less likely to have PIK3CA genomic alterations at presentation than those who relapsed within 6 months. PTEN expression has been observed to correlate with chemosensitivity in ovarian cancer cell lines and PTEN modulation can alter sensitivity to cisplatin (reviewed in Stronach et al. [12]). However, the studies discussed here used in vitro generated models of resistance that do not arise by the same processes as the in vivo derived lines described here [16,18], and these studies did not address the direct link between platinum-induced DNA damage and AKT activation that suggest a nuclear AKT phosphorylation event that is distinct from the canonical activation pathway at the cell surface.
Data presented here indicate that prolonged activation of AKT in response to cisplatin exposure is a feature acquired on the development of clinical resistance to cisplatin within an individual patient. Enhancement of apoptosis and accumulation of nuclear AKT are only seen in clinically resistant cells and not in their sensitive matched counterparts, further indicating that AKT activation prevents cisplatin-induced apoptosis as a mechanism of clinically acquired resistance.
Numerous AKT inhibitors are currently in development with a number in phase 1/2 trials [33,34], and so combining AKT inhibition with conventional platinum therapy is a feasible strategy for managing clinically acquired platinum resistance. Interestingly, however, inhibition of AKT, or indeed IGF-1R or mTOR, has been associated with hyperglycemia and diabetes [24,35–37]. AKT is an essential component of the insulin signaling pathway being activated in response to insulin stimulation through phosphorylation by PDK1/mTORC2. Activated AKT causes translocation of GLUT4 to the plasma-membrane facilitating glucose uptake while also inactivating GSK-3, thereby enhancing glycogen synthesis (reviewed in Asano et al. [38]). Furthermore, AKT phosphorylates and inhibits the transcription factor FOXO1, which can suppress glucose production in the liver and kidney by downregulation of phosphoenolpyruvate carboxykinase and glucose-6-phosphatase. In addition, active AKT phosphorylates the TSC1-TSC2 complex, leading to mTOR activation, which regulates protein synthesis/cell growth in response to insulin (reviewed in Avruch et al. [39]). Studies of knockout mice lacking AKT1, AKT2, or AKT3 identified specific phenotypes relating to each isoform with AKT2 knockout mice demonstrating insulin resistance, hyperinsulinemia, and glucose intolerance [25]. Our data do not support a single AKT isoform as being responsible for the acquired resistance to cisplatin-induced apoptosis, suggesting that implementation of isoform-specific inhibitors may not be beneficial in this indication.
We were therefore interested in the mechanism of AKT activation after platinum-induced DNA damage. DNA-PK is a nuclear serine/threonine kinase composed of a 470-kDa catalytic subunit, DNA-PKcs, and two DNA binding proteins, Ku70 and Ku80. After DNA damage, Ku70/Ku80 detect dsDNA damage and bind DNA double-strand breaks (DSBs) as heterodimers, subsequently attracting the DNA-PKcs subunit and initiating nonhomologous end-joining (NHEJ) repair. Together with ataxia-telangiectasia mutant and ataxia-telangiectasia and Rad3 related, DNA-PK forms a critical early component of the DNA damage response [40]. In addition to initiating NHEJ repair, DNA-PK can activate DNA damage response signaling cascades after activation at DSBs, for example, by regulating the p53 and AKT pathways: Feng et al. [9] demonstrated that DNA-PK had in vitro kinase activity for S473 of AKT. Subsequently, Bozulic et al. [8] showed that DNA-PK phosphorylates AKT on S473 in the nucleus of HUVEC cells and is required for activation of AKT in response to IR or doxorubicin-induced DNA damage. Our findings here indicate that depletion of Rictor, a unique component of the known AKT-S473 kinase mTORC2, is ineffective at preventing cisplatin-mediated activation of AKT or in restoring platinum sensitivity to resistant cells, indicating that cisplatin-mediated AKT activation is mTORC2 independent. In contrast, disruption ofDNA-PK in our studies prevented cisplatin-induced AKT phosphorylation at S473 and reversed the attenuated apoptotic response to cisplatin in acquired platinum-resistant cells while not interfering with insulin-mediated AKT activation. We also showed that this reversal of cisplatin resistance was associated with abrogation of AKT-mediated BAD phosphorylation, a phosphomodification known to inhibit the proapoptotic function of BAD [28]. Conversely, platinum-sensitive cells were not further sensitized to platinum by these treatments, indicating an acquired mechanism specific to the platinum resistant state. In other studies, DNA-PKcs-/- mice were used to address the physiological relevance of DNA-PK in activation of AKT, and these showed that DNA-PKcs was required for γIR DNA damage-induced activation but not growth factor- or insulin-induced AKT activation and demonstrated no differences between blood glucose response between DNA-PKcs-null mice and wild-type controls when treated with either insulin or glucose [41].
Nuclear translocation of AKT after DNA damage induced by doxorubicin has been reported, and these studies indicated that such DNA damage can give rise to DNA-PK-mediated phosphorylation of AKT-S473. However, the authors argue that the phosphorylation of T308, which they prevent by using a PI3K inhibitor, is the critical step and that, without this, the DNA-PK-mediated S473 phosphorylation will not allow sufficient AKT activity [42]. In contrast, our findings suggest that phosphorylation of the T308 site is insufficient to produce the AKT-mediated, platinum-resistant phenotype because our data demonstrate that loss of DNA-PK-mediated S473 phosphorylation in the presence of strong T308 phosphorylation by targeting DNA-PK restores the apoptotic response to cisplatin treatment in clinically resistant ovarian (and other) cancer cells. We would further emphasize that targeting the DNA damage-specific activator, DNA-PKcs, rather than the generic upstream activator, PI3K, would logically produce a more phenotype-specific effect with a mechanism that is different from the canonical PI3K/AKT pathway.
Recently, it was reported that PARP inhibition can result in phosphorylation of DNA-PKcs T2609 and γH2AX (S139) (known substrates of DNA-PKcs) and can stimulate NHEJ in a BRCA2 mutant background [43]. DNA-PK inhibition rescued the lethality of PARP inhibition specifically in HR-deficient cells, suggesting that genomic instability produced by NHEJ may underlie PARP inhibitor synthetic lethality. This implies that DNA-PK inhibitors may be better suited to HR-proficient tumors, entirely consistent with our hypothesis of selective prosurvival activation of AKT in clinically acquired platinum-resistant tumors. HR-deficient tumors tend to be highly sensitive to cisplatin, becoming less so after selective evolution associated with numerous molecular alterations, including reversion of BRCA-inactivating mutations where present in the sensitive tumor [18,19].
Conversely, a combinatorial selection process to identify synthetic peptides that bind and inhibit DNA repair proteins was recently reported and demonstrated that a peptide with DNA-PKcs inhibitory properties enhanced radiation-induced DSB formation and cell killing in BRCA1- and BRCA2-deficient cells, suggesting that, in certain circumstances, DNA-PK inhibition is compatible with a homologous recombination-deficient background [44].
In summary, we have presented evidence that the clinically platinum-resistant phenotype in ovarian cancer uses AKT activation by phosphorylation at S473 selectively. This AKT activation in response to cisplatin is mediated through DNA-PK using a mechanism apparently separate from the canonical cell surface-mediated AKT activation pathway. We therefore propose DNA-PK inhibition as a therapeutic strategy to specifically reverse clinically acquired platinum-resistant ovarian cancer while avoiding the growth factor/insulin effects that may problematically accompany pan-AKT inhibition.
Supplementary Material
Supplementary References
- 1.Stronach EA, Alfraidi A, Rama N, Datler C, Studd JB, Agarwal R, Guney TG, Gourley C, Hennessy BT, Mills GB, et al. HDAC4-regulated STAT1 activation mediates platinum resistance in ovarian cancer. Cancer Res. 2011;71(13):4412–4422. doi: 10.1158/0008-5472.CAN-10-4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goh LB, Spears KJ, Yao D, Ayrton A, Morgan P, Roland Wolf C, Friedberg T. Endogenous drug transporters in in vitro and in vivo models for the prediction of drug disposition in man. Biochem Pharmacol. 2002;64(11):1569–1578. doi: 10.1016/s0006-2952(02)01355-2. [DOI] [PubMed] [Google Scholar]
- 3.Shahbazi M, Fryer AA, Pravica V, Brogan IJ, Ramsay HM, Hutchinson IV, Harden PN. Vascular endothelial growth factor gene polymorphisms are associated with acute renal allograft rejection. J Am Soc Nephrol. 2002;13(1):260–264. doi: 10.1681/ASN.V131260. [DOI] [PubMed] [Google Scholar]
Footnotes
The authors thank the Ovarian Cancer Action and Hammersmith Clinical Cancer Research and Treatment Fund-Imperial Trustees for funding and the National Institute for Health Research Biomedical Research Centre, the Cancer Research UK Clinical Centre and the Experimental Cancer Medicine Centre at Imperial College London for infrastructural support. Mutational analysis was kindly performed by Katherine Stemke-Hale at the Cancer Center Support grant-funded Characterized Cell Line Core at MD Anderson Cancer Center (National Cancer Institute no. CA16672).
This article refers to supplementary materials, which are designated by Tables W1 and W2 and Figures W1 to W5 and are available online at www.neoplasia.com.
References
- 1.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 2.Fraser M, Leung BM, Yan X, Dan HC, Cheng JQ, Tsang BK. p53 is a determinant of X-linked inhibitor of apoptosis protein/Akt-mediated chemoresistance in human ovarian cancer cells. Cancer Res. 2003;63:7081–7088. [PubMed] [Google Scholar]
- 3.Yang X, Fraser M, Moll UM, Basak A, Tsang BK. Akt-mediated cisplatin resistance in ovarian cancer: modulation of p53 action on caspase-dependent mitochondrial death pathway. Cancer Res. 2006;66:3126–3136. doi: 10.1158/0008-5472.CAN-05-0425. [DOI] [PubMed] [Google Scholar]
- 4.Molina JR, Hayashi Y, Stephens C, Georgescu MM. Invasive glioblastoma cells acquire stemness and increased Akt activation. Neoplasia. 2010;12:453–463. doi: 10.1593/neo.10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blando JM, Carbajal S, Abel E, Beltran L, Conti C, Fischer S, DiGiovanni J. Cooperation between Stat3 and Akt signaling leads to prostate tumor development in transgenic mice. Neoplasia. 2011;13:254–265. doi: 10.1593/neo.101388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wittig-Blaich SM, Kacprzyk LA, Eismann T, Bewerunge-Hudler M, Kruse P, Winkler E, Strauss WS, Hibst R, Steiner R, Schrader M, et al. Matrix-dependent regulation of AKT in hepsin-overexpressing PC3 prostate cancer cells. Neoplasia. 2011;13:579–589. doi: 10.1593/neo.11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the Rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 8.Bozulic L, Surucu B, Hynx D, Hemmings BA. PKBα/Akt1 acts downstream of DNA-PK in the DNA double-strand break response and promotes survival. Mol Cell. 2008;30:203–213. doi: 10.1016/j.molcel.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 9.Feng J, Park J, Cron P, Hess D, Hemmings BA. Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J Biol Chem. 2004;279:41189–41196. doi: 10.1074/jbc.M406731200. [DOI] [PubMed] [Google Scholar]
- 10.Fayard E, Tintignac LA, Baudry A, Hemmings BA. Protein kinase B/Akt at a glance. J Cell Sci. 2005;118:5675–5678. doi: 10.1242/jcs.02724. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 2003;3:502–516. doi: 10.1038/nrc1123. [DOI] [PubMed] [Google Scholar]
- 12.Stronach EA, Cheraghchi-Bashi A, Chen M, Gabra H. Targeting the AKT Pathway in Ovarian Cancer. New York, NY: Springer; 2011. [Google Scholar]
- 13.Hui RC, Francis RE, Guest SK, Costa JR, Gomes AR, Myatt SS, Brosens JJ, Lam EW. Doxorubicin activates FOXO3a to induce the expression of multidrug resistance gene ABCB1 (MDR1) in K562 leukemic cells. Mol Cancer Ther. 2008;7:670–678. doi: 10.1158/1535-7163.MCT-07-0397. [DOI] [PubMed] [Google Scholar]
- 14.Yang X, Fraser M, Abedini MR, Bai T, Tsang BK. Regulation of apoptosis-inducing factor-mediated, cisplatin-induced apoptosis by Akt. Br J Cancer. 2008;98:803–808. doi: 10.1038/sj.bjc.6604223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madhunapantula SV, Sharma A, Robertson GP. PRAS40 deregulates apoptosis in malignant melanoma. Cancer Res. 2007;67:3626–3636. doi: 10.1158/0008-5472.CAN-06-4234. [DOI] [PubMed] [Google Scholar]
- 16.Cooke SL, Ng CKY, Melnyk N, Garcia MJ, Hardcastle T, Temple J, Langdon S, Huntsman D, Brenton JD. Genomic analysis of genetic heterogeneity and evolution in high-grade serous ovarian carcinoma. Oncogene. 2010;29:4905–4913. doi: 10.1038/onc.2010.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langdon SP, Lawrie SS, Hay FG, Hawkes MM, McDonald A, Hayward IP, Schol DJ, Hilgers J, Leonard RC, Smyth JF. Characterization and properties of nine human ovarian adenocarcinoma cell lines. Cancer Res. 1988;48:6166–6172. [PubMed] [Google Scholar]
- 18.Stronach EA, Alfraidi A, Rama N, Datler C, Studd JB, Agarwal R, Guney TG, Gourley C, Hennessy BT, Mills GB, et al. HDAC4-regulated STAT1 activation mediates platinum resistance in ovarian cancer. Cancer Res. 2011;71:4412–4422. doi: 10.1158/0008-5472.CAN-10-4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakai W, Swisher EM, Jacquemont C, Chandramohan KV, Couch FJ, Langdon SP, Wurz K, Higgins J, Villegas E, Taniguchi T. Functional restoration of BRCA2 protein by secondary BRCA2 mutations in BRCA2-mutated ovarian carcinoma. Cancer Res. 2009;69:6381–6386. doi: 10.1158/0008-5472.CAN-09-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, Neve RM, Kuo WL, Davies M, Carey M, Hu Z, Guan Y, Sahin A, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68:6084–6091. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang L, Dan HC, Sun M, Liu Q, Sun XM, Feldman RI, Hamilton AD, Polokoff M, Nicosia SV, Herlyn M, et al. Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res. 2004;64:4394–4399. doi: 10.1158/0008-5472.CAN-04-0343. [DOI] [PubMed] [Google Scholar]
- 22.Berndt N, Yang H, Trinczek B, Betzi S, Zhang Z, Wu B, Lawrence NJ, Pellecchia M, Schonbrunn E, Cheng JQ, et al. The Akt activation inhibitor TCN-P inhibits Akt phosphorylation by binding to the PH domain of Akt and blocking its recruitment to the plasma membrane. Cell Death Differ. 2010;17:1795–1804. doi: 10.1038/cdd.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 24.Crouthamel M-C, Kahana JA, Korenchuk S, Zhang S-Y, Sundaresan G, Eberwein DJ, Brown KK, Kumar R. Mechanism and management of AKT inhibitor-induced hyperglycemia. Clin Cancer Res. 2009;15:217–225. doi: 10.1158/1078-0432.CCR-08-1253. [DOI] [PubMed] [Google Scholar]
- 25.Dummler B, Hemmings BA. Physiological roles of PKB/Akt isoforms in development and disease. Biochem Soc Trans. 2007;35:231–235. doi: 10.1042/BST0350231. [DOI] [PubMed] [Google Scholar]
- 26.Nutley BP, Smith NF, Hayes A, Kelland LR, Brunton L, Golding BT, Smith GC, Martin NM, Workman P, Raynaud FI. Preclinical pharmacokinetics and metabolism of a novel prototype DNA-PK inhibitor NU7026. Br J Cancer. 2005;93:1011–1018. doi: 10.1038/sj.bjc.6602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cui X, Yu Y, Gupta S, Cho YM, Lees-Miller SP, Meek K. Autophosphorylation of DNA-dependent protein kinase regulates DNA end processing and may also alter double-strand break repair pathway choice. Mol Cell Biol. 2005;25:10842–10852. doi: 10.1128/MCB.25.24.10842-10852.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 29.Bowtell DD. The genesis and evolution of high-grade serous ovarian cancer. Nat Rev Cancer. 2010;10:803–808. doi: 10.1038/nrc2946. [DOI] [PubMed] [Google Scholar]
- 30.Yuan Z-Q, Feldman RI, Sussman GE, Coppola D, Nicosia SV, Cheng JQ. AKT2 inhibition of cisplatin-induced JNK/p38 and Bax activation by phosphorylation of ASK1: implication of AKT2 in chemoresistance. J Biol Chem. 2003;278:23432–23440. doi: 10.1074/jbc.M302674200. [DOI] [PubMed] [Google Scholar]
- 31.Shahbazi M, Fryer AA, Pravica V, Brogan IJ, Ramsay HM, Hutchinson IV, Harden PN. Vascular endothelial growth factor gene polymorphisms are associated with acute renal allograft rejection. J Am Soc Nephrol. 2002;13:260–264. doi: 10.1681/ASN.V131260. [DOI] [PubMed] [Google Scholar]
- 32.Pei H, Li L, Fridley BL, Jenkins GD, Kalari KR, Lingle W, Petersen G, Lou Z, Wang L. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell. 2009;16:259–266. doi: 10.1016/j.ccr.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gungor H, Saleem A, Agarwal R, Blagden S, Michael A, Stronach E, Chen M, Pickford E, Rama N, Lewis Y, et al. Pharmacokinetic (PK)/pharmacodynamic (PD) analysis of escalating repeat doses of the AKT inhibitor GSK2141795 (GSK795) in patients (pts) with ovarian cancer. J Clin Oncol. 2011;29(suppl) Abstract 5064. [Google Scholar]
- 34.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 35.Atkins MB, Hidalgo M, Stadler WM, Logan TF, Dutcher JP, Hudes GR, Park Y, Liou SH, Marshall B, Boni JP, et al. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol. 2004;22:909–918. doi: 10.1200/JCO.2004.08.185. [DOI] [PubMed] [Google Scholar]
- 36.Haluska P, Carboni JM, Loegering DA, Lee FY, Wittman M, Saulnier MG, Frennesson DB, Kalli KR, Conover CA, Attar RM, et al. In vitro and in vivo antitumor effects of the dual insulin-like growth factor-I/insulin receptor inhibitor, BMS-554417. Cancer Res. 2006;66:362–371. doi: 10.1158/0008-5472.CAN-05-1107. [DOI] [PubMed] [Google Scholar]
- 37.Ihle NT, Paine-Murrieta G, Berggren MI, Baker A, Tate WR, Wipf P, Abraham RT, Kirkpatrick DL, Powis G. The phosphatidylinositol-3-kinase inhibitor PX-866 overcomes resistance to the epidermal growth factor receptor inhibitor gefitinib in A-549 human non-small cell lung cancer xenografts. Mol Cancer Ther. 2005;4:1349–1357. doi: 10.1158/1535-7163.MCT-05-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asano T, Fujishiro M, Kushiyama A, Nakatsu Y, Yoneda M, Kamata H, Sakoda H. Role of phosphatidylinositol 3-kinase activation on insulin action and its alteration in diabetic conditions. Biol Pharm Bull. 2007;30:1610–1616. doi: 10.1248/bpb.30.1610. [DOI] [PubMed] [Google Scholar]
- 39.Avruch J, Hara K, Lin Y, Liu M, Long X, Ortiz-Vega S, Yonezawa K. Insulin and amino-acid regulation of mTOR signaling and kinase activity through the Rheb GTPase. Oncogene. 2006;25:6361–6372. doi: 10.1038/sj.onc.1209882. [DOI] [PubMed] [Google Scholar]
- 40.Yang J, Yu Y, Hamrick HE, Duerksen-Hughes PJ. ATM, ATR and DNA-PK: initiators of the cellular genotoxic stress responses. Carcinogenesis. 2003;24:1571–1580. doi: 10.1093/carcin/bgg137. [DOI] [PubMed] [Google Scholar]
- 41.Surucu B, Bozulic L, Hynx D, Parcellier A, Hemmings BA. In vivo analysis of protein kinase B (PKB)/Akt regulation in DNA-PKcs-null mice reveals a role for PKB/Akt in DNA damage response and tumorigenesis. J Biol Chem. 2008;283:30025–30033. doi: 10.1074/jbc.M803053200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wallin JJ, Guan J, Prior WW, Edgar KA, Kassees R, Sampath D, Belvin M, Friedman LS. Nuclear phospho-Akt increase predicts synergy of PI3K inhibition and doxorubicin in breast and ovarian cancer. Sci Transl Med. 2010;2:48ra66. doi: 10.1126/scitranslmed.3000630. [DOI] [PubMed] [Google Scholar]
- 43.Patel AG, Sarkaria JN, Kaufmann SH. Nonhomologous end joining drives poly(ADP-ribose) polymerase (PARP) inhibitor lethality in homologous recombination-deficient cells. Proc Natl Acad Sci USA. 2011;108:3406–3411. doi: 10.1073/pnas.1013715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moeller BJ, Sidman RL, Pasqualini R, Arap W. Discovery of DNA repair inhibitors by combinatorial library profiling. Cancer Res. 2011;71:1816–1824. doi: 10.1158/0008-5472.CAN-10-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.