Abstract
Understanding how structural features determine specific biological activities has often proved elusive. With over 161 000 steroid structures described, an algorithm able to predict activity from structural attributes would provide manifest benefits. Molecular simulations of a range of 35 corticosteroids show striking correlations between conformational mobility and biological specificity. Thus steroid ring A is important for glucocorticoid action, and is rigid in the most specific (and potent) examples, such as dexamethasone. By contrast, ring C conformation is important for the mineralocorticoids, and is rigid in aldosterone. Other steroids that are less specific, or have mixed functions, or none at all, are more flexible. One unexpected example is 11-deoxycorticosterone, which the methods predict (and our activity studies confirm) is not only a specific mineralocorticoid, but also has significant glucocorticoid activity. These methods may guide the design of new corticosteroid agonists and antagonists. They will also have application in other examples of ligand–receptor interactions.
Keywords: glucocorticoid, mineralocorticoid, cortisol, aldosterone, deoxycorticosterone, conformational mobility
1. Introduction
Over 161 000 compounds have been described that have the characteristic steroid structure [1]. Doubtless there will be many with interesting, potentially useful, biological actions that will remain unknown until algorithms can be developed that predict activity from structural attributes. Such algorithms largely remain elusive, particularly for the corticosteroids. The family of steroid hormones regulates a huge range of physiological functions. Although all are closely related in composition, the different groups have highly distinct biological activities. The two classes of the steroid hormones of the adrenal cortex, the corticosteroids, show this especially clearly, having different targets and often very different actions. Thus, mineralocorticoids regulate electrolyte exchange and cardiovascular function, and glucocorticoids are named for their effects on carbohydrate metabolism, but also have a wide range of other functions [2].
Despite these differences in activity, mineralocorticoids and glucocorticoids are very similar in chemical composition. Thus, aldosterone and corticosterone differ by two hydrogens and a single oxygen atom. How then is the discrimination between their different actions achieved?
Several known mechanisms may contribute to these distinct effects. First, the two corticosteroid receptor subtypes, designated mineralocorticoid and glucocorticoid receptors (MR and GR), have somewhat different tissue distributions [3]. The nomenclature is misleading, however, since both classes of steroid will bind (to a greater or lesser degree) to both receptor classes [4]. Furthermore, activated MR and GR bind to the same corticosteroid response elements in the promoter region of target genes [5]. Indeed, GR and MR may even form heterodimers [6]. Naturally, binding does not necessarily imply activation, and different ligands can evoke different conformational changes in receptors, or attract different co-activators [7]. Nevertheless, cortisol, usually considered to be a glucocorticoid, can behave as a mineralocorticoid under some circumstances [7]. It is, therefore, not obvious that specific hydrogen bond contacts can alone explain specificity of action [8].
An apparent solution to this problem comes from the discovery that some mineralocorticoid targets, notably the kidney, abundantly contain the enzyme 11β-hydroxysteroid dehydrogenase type II (11β-HSD2) [9]. This enzyme may act as a ‘gatekeeper’ to MR [10], catalysing the transformation of 11β-hydroxysteroids, such as the glucocorticoid cortisol, to inactive 11-ketosteroids, such as cortisone. However, in man, total free cortisol and transcortin-bound cortisol are present in circulating blood at about 1000 times (and free cortisol about 100 times) the concentration of aldosterone, the main mineralocorticoid [11,12]. Given that 11β-HSD2 may not always be exactly co-located with MR [13], clearance of active glucocorticoid by 11β-HSD2 is unlikely to be complete [14]. Even in tissues with plentiful 11β-HSD2, MR may be chronically occupied by glucocorticoid, though seemingly only activated under special conditions [12].
Steroid structure–function relationships have been studied in this context, most recently by Galigniana et al. [15], who concluded that the only clear structural relationship with mineralocorticoid activity was the planarity of the steroid molecule. However, though useful, planarity alone does not explain different activities, for example, the contrast between cortisol and aldosterone.
Though there are good crystal structure data for the ligand-bound receptors [16,17], we know little of the dynamic processes by which the receptor is activated. We can only speculate on the origin of the energy required to change the receptor conformation. Clearly, a good dynamic model of the ligand–receptor interaction might throw light both on these points [18], and on the physical basis for glucocorticoid versus mineralocorticoid effects.
An important contribution from Duax et al. [19] showed that, perhaps contrary to expectation, steroids may be remarkably flexible, and individual compounds can present different crystal conformations. In general, this feature is not shown in structural studies of steroid ligand-binding sites [16,17]. However, our recent studies [20] of another large group of receptor ligands, the olfactants, showed the importance of conformational mobility in determining whether the odours of left- and right-handed chiral molecules differed. Structural (and sometimes even functional) similarities of steroids and olfactants [21] suggested it might be fruitful to apply molecular dynamics (MD) simulation methods to the steroids. Calculating corticosteroid MD, to simulate various degrees of a molecule's propensity for flexibility, we now find a correlation between specificity and conformational mobility. In particular, an important factor is the ease with which the three six-carbon atom rings switch between chair-, twist- and boat-like configurations.
2. Methods
2.1. Molecular dynamics and dynamic flexibility
We used the molecular dynamics (MD) methodology that was successfully applied in earlier studies on odorants [20] and which was validated by comparison with cyclohexane, for which experimental data were available. In the present study, further validation was obtained by comparison of selected data with that obtained using Langevin dynamics (LD). The main difference between the two methods MD and LD is that the latter introduced a stochastic ‘noise’ term, and thus for large systems, it is useful to probe conformational space. However, the results obtained are essentially the same for the two methods (data not shown). We also considered the use of accelerated dynamics, but this is not the most suitable choice in this first investigation, given our aim is to compare a set of 36 compounds in equal time frames and context, so as to determine how rare or frequent changes in conformation might be. We did, however, carry out some simulations at elevated temperatures to capture rarer events; this is a technique to accelerate events in order to minimize computer time while retaining a consistent but hastened approximation to real time.
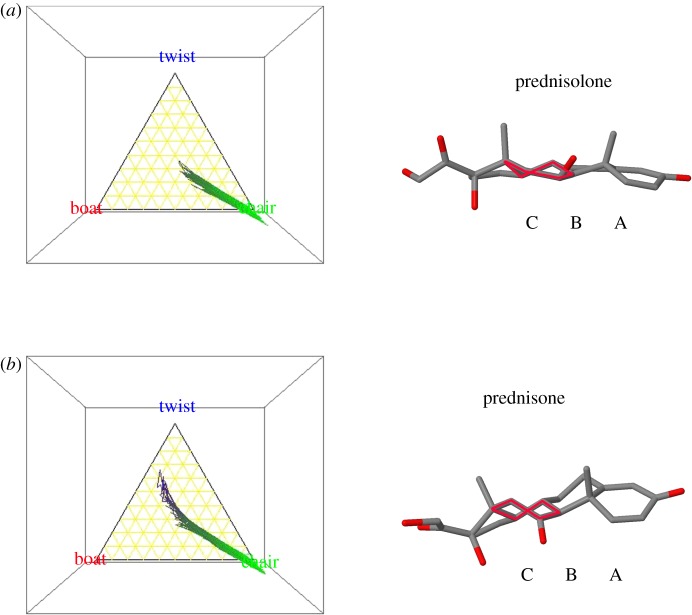
In these studies, we use the term ‘flexibility’ to reflect the probability that six-carbon rings (in steroids these are rings A, B and C) adopt chair-, boat- or twist-like conformations, see, for example, figure 5 for a literal ‘twist’ in ring C of prednisone.
Figure 5.
Three-dimensional-ternary diagrams for (a) the dynamics of ring C in prednisolone versus (b) prednisone from set 3. The birds-eye view orientation is shown to illustrate the ability for the C11-ketone version (here prednisone) to reach twist-like states. This type of contrast is shown in all the 11β-hydroxy versus 11-keto steroids studied here. Also shown are the geometries taken from the simulation in which ring C is most twist-like. Note in prednisolone, a flat pseudo-rotating-like state is reached, whereas the prednisone meets a more defined twist-like state (the conformation of ring C is highlighted in red for each case)—this contrasts with the differences in orientation of the C11 substituent.
The starting geometry for each simulation was a global minimum energy determined using either the semi-empirical Austin Model 1 (AM1; ArgusLab v. 4.0.1, http://www.arguslab.com) [22] or the Becke Three Parameter Hybrid Functionals (B3LYP; Gaussian '03; http://www.Gaussian.com) [23] density functional, with a 6–31G** basis set. The B3LYP method was implemented for steroids containing fluorine (most of the 35 tested do not contain fluorine). MD simulations were performed using DL_POLY v. 2.16 (www.ccp5.ac.uk/DL_POLY/). We used an NVE ensemble, with constant number of particles (N), volume (V) and energy (E) in a gas-phase environment [24]. Temperatures were set to 300 K or at 600 K, with 300 K data enabling comparisons with previous studies [20] and the well-characterized and experimentally verified MD of cyclohexane; the 600 K runs allow the acceleration of rare events. The two sets agree quite well. The time length of the simulations was determined based on transition state estimates and successful previous conformational searches on structurally related odorants, which were in turn parametrized in accordance with experimental and well-established results for cyclohexane. Details are given in Brookes et al. [20]. Time steps were 0.2 fs long, for a 1 000 000 calculation steps, resulting in a simulation length of 200 ps: 100 ps equilibrating and 100 ps production phase. Trajectories were examined at intervals of 100 steps resulting in an effective time step of 0.02 ps. The many short steps retain high accuracy, while the analysis at every 100th step is sufficiently short in order not to miss any conformational event. Three sets of simulations were performed, the first two implementing the Dreiding force field [25], at 300 K and 600 K (sets 1 and 2, respectively). The third set implements the General (AMBER) Force Field for organic molecules (gaff, set 3) [26].
The trajectories let us compare the varied conformational changes steroids undergo under identical conditions. For each output geometry, the root mean square deviation from the cyclohexane ‘boat’ ‘twist’ or ‘chair’ form was calculated, and the value for the closest geometry is the output. Each six-membered ring (A, B, C) was examined independently. Three-dimensional ternary diagrams indicating chair-like, boat-like and twist-like points over time provide a qualitative analysis of the conformational space (figure 1). As a quantitative measure, we evaluated the area of the convex polygon (CP) that encloses all the points of the ternary diagram (figures 2–4) as a fraction of the area of triangle base. Despite some experimental evidence for the puckering of cyclopentane rings [27], it is difficult to establish the ‘planar’, ‘envelope’ and ‘half-chair’ forms of ring D because the five atoms in the ring pseudorotate rapidly between these positions, with little change in internal energy [28]. However, there could be an interesting future study of considered substitutions that alter the stability of one of these forms, combined with experimental determinations of the stable conformations. In the present work, it is assumed that any deviation from the global envelope minima in ring D is either very fast and/or very modest.
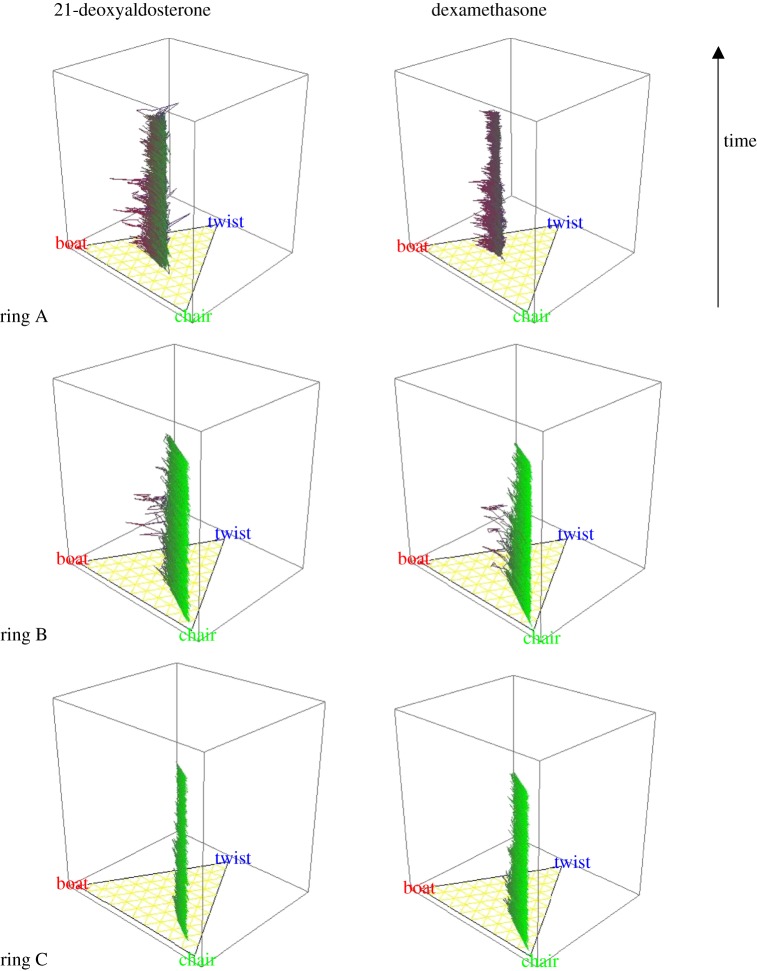
Figure 1.
The MD trajectory is depicted in ternary diagrams to show the changes in geometry for each six-membered ring (independently A, B and C). Here, the plots show a line drawn between configurations of the ring that are coloured according to green for chair-like, blue for twist-like, and red for boat-like. The vertical axis is time, giving a trajectory between the points in configurational space. The examples given here are for (upper figure) 21-deoxyaldosterone and (lower figure) dexamethasone, potent mineralocorticoid and glucocorticoid, respectively. See the electronic supplementary material, tables S1 and S2, figures S1 and S2 and movies (a), (b) and (c) for steroid activities, structures and exemplary steroid mobility simulations.
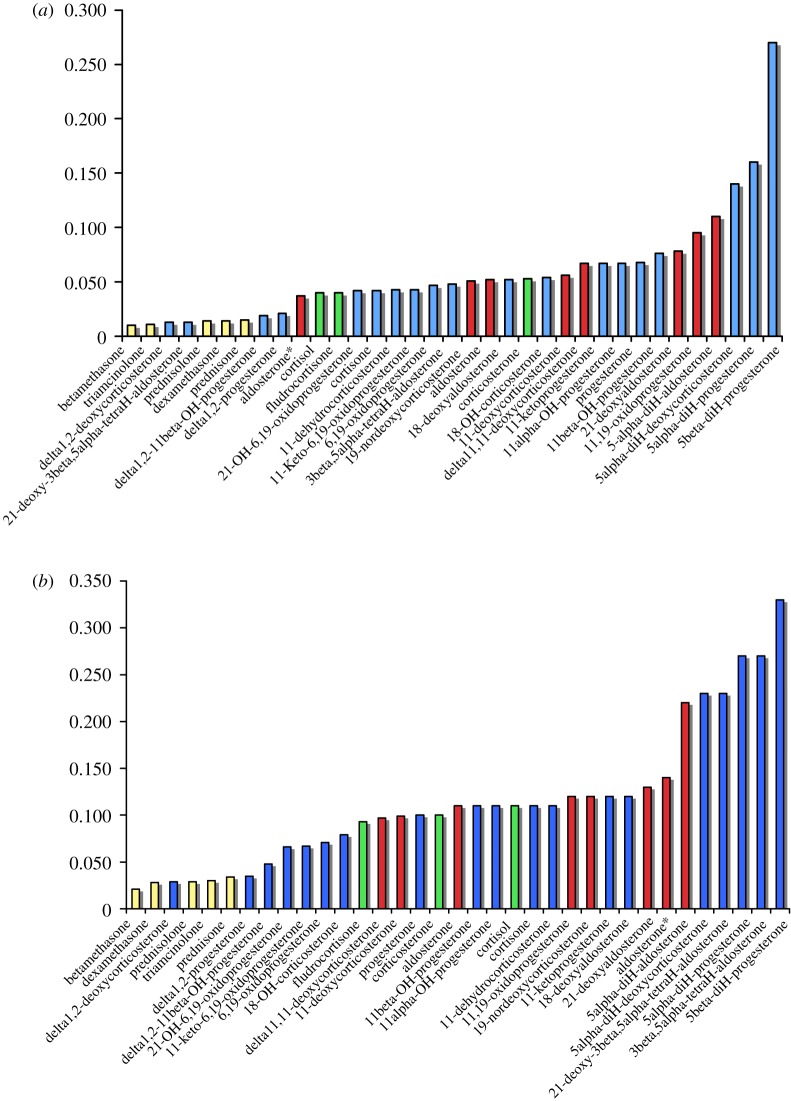
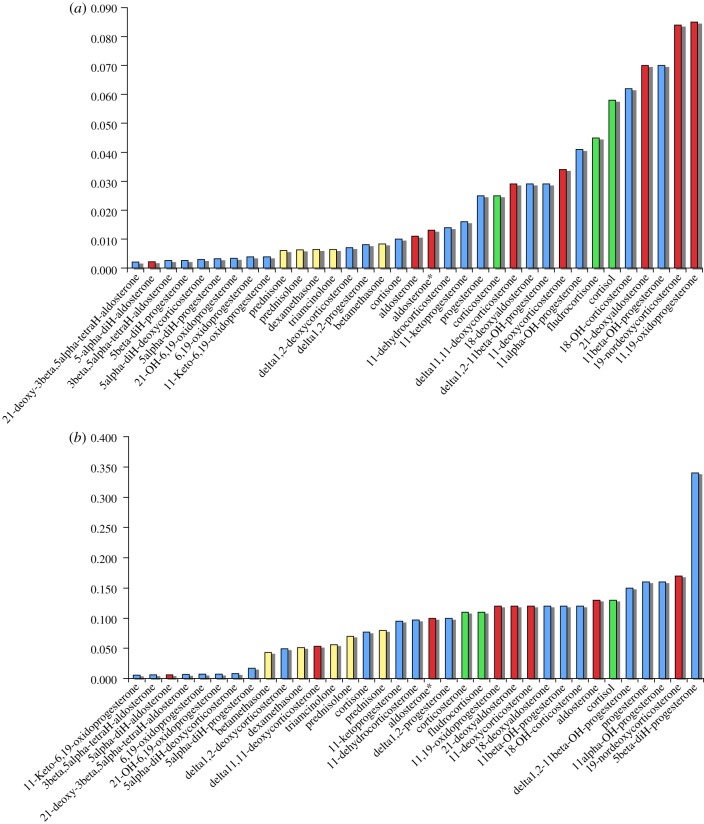
Figure 2.
Ring A flexibility (y-axis), CP measures the convex polygon of the points within the three-dimensional-ternary diagram of conformational space. Exemplary glucocorticoids are shown in yellow, and mineralocorticoids in red. Examples of compounds that may show either function are shown in green and inactive or low activity compounds are shown in blue. (a) Simulations at 300 K, (b) at 600 K (sets 1 and 2, respectively). See the electronic supplementary material, tables S1 and S2, figures S1 and S2 and movies (a), (b) and (c) for steroid activities, structures and exemplary steroid mobility simulations. *Aldosterone full acetal (see supplementary material).
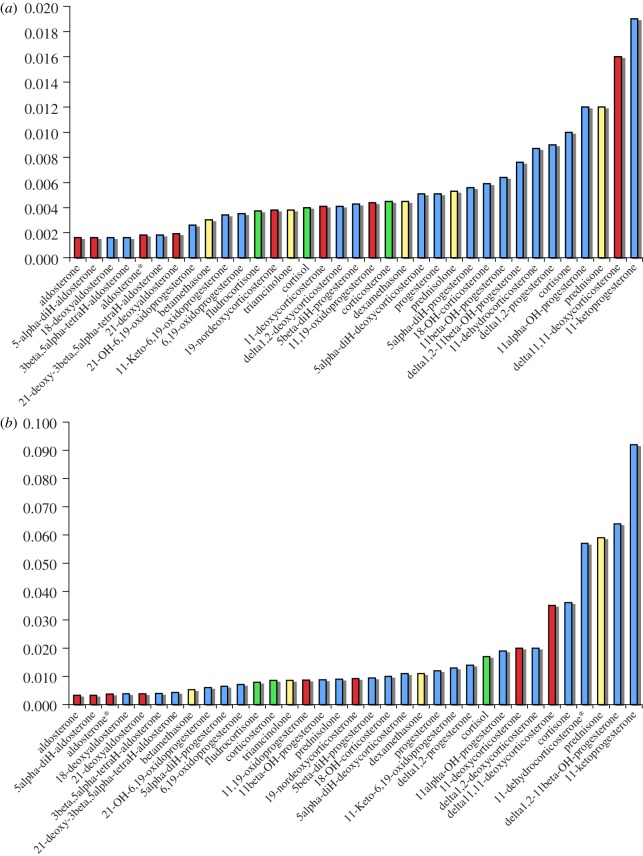
Figure 4.
Ring C flexibility (y-axis), CP measures the convex polygon of the points within the three-dimensional-ternary diagram of conformational space. Exemplary glucocorticoids are shown in yellow, and mineralocorticoids in red. Examples of compounds that may show either function are shown in green and inactive or low activity compounds are shown in blue. (a) Simulations at 300 K, (b) at 600 K (sets 1 and 2, respectively). See the electronic supplementary material, tables S1 and S2, figures S1 and S2 and movies (a), (b) and (c) for steroid activities, structures and exemplary steroid mobility simulations. *Aldosterone full acetal (see supplementary material).
2.2. Steroids
We examined steroids representing the different classes for which biological activity data are available [15,29,30]. Dexamethasone, betamethasone, prednisolone, prednisone and triamcinolone are glucocorticoids. Cortisol, corticosterone and fludrocortisone have mixed functions. Aldosterone (either full acetal or hemiacetal configuration), 5α-diH-aldosterone, 21-deoxyaldosterone, 11,19 oxidoprogesterone, 11-deoxycorticosterone and delta11,11-deoxycorticosterone are all highly potent or specific mineralocorticoids. In this study, we have not included the inhibitors, which are fewer in number and have widely different structures and specificities, making systematic analysis inappropriate. These steroids are specifically indicated in figures 2–4. The identification of 11-deoxycorticosterone as a specific mineralocorticoid follows the received view in numerous reviews and texts over the past 50 years [31–33]. Biological activity data and steroid structures are given in the electronic supplementary material, tables S1 and S2 and figure S1.
2.3. Glucocorticoid activity assays
2.3.1. Relative binding affinity for glucocorticoid receptor
L929 fibroblasts grown in DMEM/10 per cent bovine calf serum were homogenized in HEM buffer (10 mM Hepes, pH 7.4, 1 mM EDTA, 20 mM sodium molybdate) and centrifuged for 30 min at 67 000 × g at 0°C. One hundred microlitres of the supernatant was incubated with 5 nM [3H]-corticosterone (SA = 80 Ci mmol−1) and increasing amounts of non-radioactive steroid. Free steroid was separated from bound steroid by adsorption with charcoal/dextran. The relative binding affinity (RBA) was determined as the concentration of ligand that displaced 50 per cent of maximal [3H]-steroid binding.
2.3.2. Liver glycogen deposition
Liver glycogen deposition was measured as described [34]. Briefly, adrenalectomized male Sprague–Dawley rats (approx. 200 g) were injected (IM) on the evening before study with 100 µg steroid/100 g body weight dissolved in ethanol:propylene glycol:0.9 per cent NaCl (3:3:34). Controls received vehicle alone. On the morning of the experiment, the dose was repeated (IP) and 3 h after the animals were sacrificed and livers removed immediately. Glycogen purification and quantification were carried out according to Krisman [35]. All animals were handled in strict accordance with good animal practice as defined by the relevant national and/or local animal welfare bodies, and all animal work was approved by the appropriate committee of the Universidad de Buenos Aires.
2.4. Tyrosine aminotransferase activity
Tyrosine aminotransferase (TAT) induction was achieved in rat hepatocytes as previously described [36].
2.5. Luciferase activity
293T cells were used for transactivation assays by cotransfection of 100 ng of pSV2rec-mGR, 0.5 µg of mouse mammary tumour virus-luciferase (MMTV-Luc), and 100 ng of Rous sarcoma virus (RSV)-β-galactosidase according to the calcium phosphate precipitation standard method as described [37]. After 20 h in a medium-containing charcoal-stripped serum, cells were stimulated with 100 nM steroid for 12 h. Both luciferase and β-galactosidase activities were measured, and the luciferase activity was normalized to the β-galactosidase expression.
3. Results
Movies of exemplary 600K MD simulations are provided in the electronic supplementary material, see (a) 11-deoxycorticosterone, (b) aldosterone and (c) dexamethasone.
Exemplary data for 600 K simulations, using Set 3 potentials, for the mineralocorticoid 21-deoxyaldosterone, and for the glucocorticoid dexamethasone, are shown in figure 1. These ternary diagrams show the propensity for each ring to adopt chair, boat or twist configurations, displayed within a triangle. Each plot contains 10 000 points: 10 000 geometries visited in conformational space within 200 ps. In 21-deoxyaldosterone, ring A behaviour shows a high population of conformational states in the middle of the triangle, whereas for dexamethasone, the states cover a much smaller fraction of the conformational space. On average, dexamethasone has a more planar ring A (grouped in a smaller region), whereas 21-deoxyaldosterone's ring A has the ability to access more boat- and twist-like states. In contrast, the two steroids exhibit quite similar ring B behaviour, and although there is a high population of chair-like states, both have the ability to reach non-global, but higher energy, local energy minima. Finally, in their ring C behaviour, although both 21-deoxyaldosterone and dexamethasone are both dominated by chair-like states, 21-deoxyaldosterone explores a lower fraction of the conformational space: 21-deoxyaldosterone is less flexible.
Figures 2, 3 and 4 show overall flexibility data (CP, the complex polygon area) for steroid rings A, B and C, respectively, for all 35 steroids studied. For each ring, the two datasets at 300 and 600 K (sets 1 and 2) are broadly similar, with a few interesting exceptions discussed later. The use of 600 K simulations, while not physiologically reflective, allow us to scope for low-frequency events in order to examine what conformations the ligands may be able to take given time and/or environmental influences. The mineralocorticoids and glucocorticoids are similarly segregated at both temperatures.
Figure 3.
Ring B flexibility (y-axis), CP measures the convex polygon of the points within the three-dimensional-ternary diagram of conformational space. Exemplary glucocorticoids are shown in yellow, and mineralocorticoids in red. Examples of compounds that may show either function are shown in green and inactive or low activity compounds are shown in blue. (a) Simulations at 300 K, (b) at 600 K (sets 1 and 2, respectively). See the electronic supplementary material, tables S1 and S2, figures S1 and S2 and movies (a), (b) and (c) for steroid activities, structures and exemplary steroid mobility simulations.*Aldosterone full acetal (see supplementary material).
Some striking differences emerge consistently between the values obtained in the three rings. The ‘pure’ glucocorticoids, including synthetic Δ1 compounds like dexamethasone, all have comparatively inflexible configurations for ring A (figure 2). Mineralocorticoids, including aldosterone and 11-deoxycorticosterone, have more flexible A rings. The ‘pure’ glucocorticoids also have relatively more rigid B rings (figure 3). The position is reversed in ring C (figure 4), where the more specific mineralocorticoids such as aldosterone have a constrained ring structure, whereas the pure glucocorticoids are relatively flexible. Exceptions to the general rule, however, are 11-deoxycorticosterone and its synthetic derivative delta11,12-deoxycorticosterone, which are mineralocorticoids that nevertheless have flexible C rings. Figure 4 also shows clear differences in dynamic flexibility between 11β-hydroxyl compounds and their inactive 11-ketone companions. The 11β-hydroxyl group includes cortisol, corticosterone and 11β-hydroxyprogesterone; their corresponding 11-ketones are cortisone, 11-dehydrocorticosterone and 11-ketoprogesterone. In each case, the ketones have more flexible C rings than the 11β-hydroxysteroids. The greater relative rigidity of the hydroxyl stems from steric repulsion conflicting with the rotation of C19. This causes ring C to be comparatively more rigid, as shown in a comparison of prednisolone and prednisone (figure 5). The ketone group in prednisone enables the ring C to reach twist-like states, where the hydroxyl group in prednisolone hinders this flexibility.
These results show how molecular dynamic simulations can reveal quite large differences in molecular behaviour which are not evident from a two-dimensional structural depiction of the molecule's most stable geometry. The ‘anomalies’ across the 300 and 600 K sets further support the use of MD simulations for observations of non-obvious conformational mobility. For example, 5β-diH-progesterone exhibits low flexibility in ring B (figure 3) at 300 K but at 600 K exhibits the most flexibility. This dramatic jump illustrates a surprising influence of the orientation of a single carbon-hydrogen bond. At 600 K, the energy barrier for ring B conversion is more easily surmounted reflecting 5β-diH-progesterone's ability to be very flexible in contrast to 5α-diH-progesterone or the other Δ4 steroids by comparison. A similar comparison is found between odorants nootkatone and tetrahydronootkatone [20]. For similar reasons, 5α-diH-aldosterone also stands out from the set: because, even at 600 K, it is not able to reach certain conformational states as the ring B is locked by the α hydrogen. Note the flexibility of ring B does not seem to clearly segregate these mineralocorticoid and glucocorticoid sets, but the various dynamics of this ring may be important in other ways.
Some further examples in the set of 35 do not fit the correlation perfectly, for example, aldosterone full acetal (see supplementary material); at 300 K has quite an inflexible ring A. Though not physiological, the 600 K set is more revealing, exhibiting a complete range of conformational mobility where sterically possible. The sets allow a comparison across related steroids with very different activities yet in the same environment. Now knowing what conformations these steroids are able to take, it is appropriate to consider how these geometries may be interpreted by the environment and affect signal transduction.
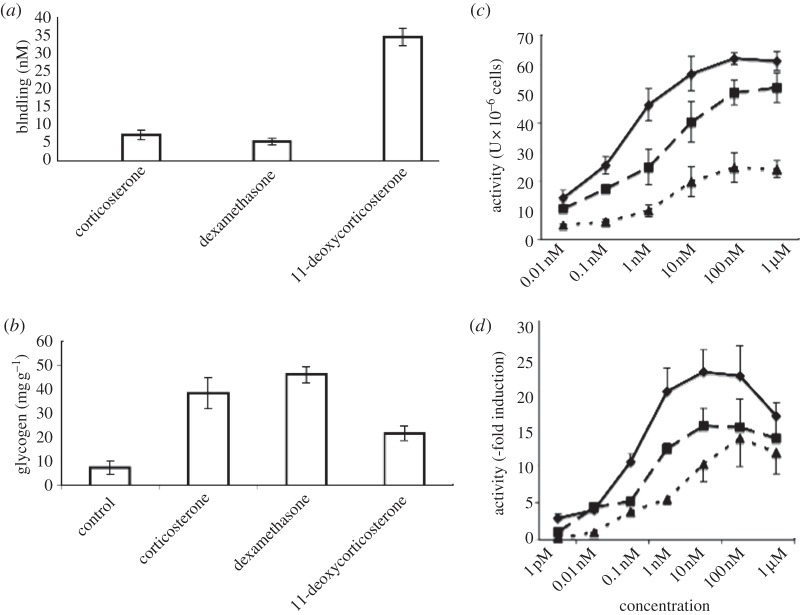
Figure 6 shows that 11-deoxycorticosterone, though binding to GR with lower affinity than corticosterone or dexamethasone, nevertheless has significant glucocorticoid activity by all three of the assays used here.
Figure 6.
Glucocorticoid attributes of 11-deoxycorticosterone. (Statistical analyses: one-way non-parametric analysis of variance and Kruskal–Wallis test.) (a) Corticosterone, dexamethasone and 11-deoxycorticosterone specific binding to GR, y-axis: relative binding activities, mean ± s.e.m., n = 4 throughout. (b) Induction of liver glycogen deposition in adrenalectomized rats by corticosterone, dexamethasone and 11-deoxycorticosterone, 100 µg per 100 g body weight: controls received vehicle alone. Values are means ± s.e.m., n = 8. All three steroids significantly induced glycogen deposition, p < 0.05 or better. (c) Induction of tyrosine aminotransferase (TAT) activity in rat hepatocytes by dexamethasone, corticosterone and 11-deoxycorticosterone (DOC). Values are means ± s.e.m., n = 3. TAT was significantly stimulated by 11-deoxycorticosterone at concentrations above 1 nM, compared with 0.01 nM values (p < 0.05 or better), but significantly less than corticosterone-stimulated values at concentrations of 0.1 nM and above (p < 0.05 or better). (d) Luciferase activity induction in the GR reporter system by dexamethasone, corticosterone and 11-deoxycorticosterone (DOC) Values are means ± s.e.m., n = 3. Luciferase was significantly stimulated by 11-deoxycorticosterone at concentrations above 1 nM, compared with 0.01 nM values (p < 0.05 or better), but significantly less than corticosterone-stimulated values at concentrations of 1 nM (p < 0.05) and 10 nM (p < 0.005). (c,d) Diamonds, dexamethasone; squares, corticosterone and triangles, DOC.
4. Discussion
Structure–function relationships in biologically active molecules frequently present problems of interpretation. Partly this may be because aspects of activity, such as specificity, potency and affinity, are independently determined by different structural features. For a family of compounds such as the corticosteroids, several factors must play a part in determining the nature of their biological actions, including size of the molecule, degree of unsaturation and the disposition and nature of substituents. Our work now follows others [19] in suggesting that the conformational mobility could also play a key role. For steroids, the dynamic flexibility of the three six-carbon atom rings A, B and C varies substantially from molecule to molecule. These variations correlate with specificity.
The characterization of corticosteroid activity comes from in vivo assays using established methods [15,29,30]. Since the time of Selye, the primary assayable attribute of glucocorticoids has been considered to be the stimulation of gluconeogenesis and glycogen deposition in the liver of adrenalectomised rats, and for mineralocorticoids, the stimulation of sodium retention [38]. These remain the most widely understood criteria, and it is these that we have used in designating specific compounds as either mineralocorticoids or glucocorticoids. They have the advantage that they are indeed physiological and the assays are carried out in vivo. Today, however we know that these properties by no means summarize all that is physiologically important about these hormones, and it may well be that other measures of activity (e.g. vascular smooth muscle cell/fibroblast proliferation for mineralocorticoids or induction of insulin resistance for glucocorticoids [7,39]) might produce different results, however systematic data for these activities are not available for a wide range of compounds such as studied here. Even in assaying what appear to be similar actions, different methods and different tissues may yield varying results, and some of the effects of a steroid may be indirect. For example, while the 11-ketocorticosteroids are generally far less potent than the 11β-hydroxycorticosteroids, they may have a physiological role, perhaps in modulating responses to aldosterone [40]. Cortisol blocks aldosterone action in the cardiomyocyte, yet it is an aldosterone agonist in vascular smooth muscle or kidney [29]. Comparisons of biological activity thus depend very much on the system that is being studied, and present a challenge.
One way forward may be through systematic analysis of trends, such as those we discuss here between steroid flexibility and function. It should be emphasized that in these calculations, we seek a measure of conformational mobility of the free molecule and, in our present study, we are not seeking the mechanism by which receptor activation occurs, for which LD or similar dynamics might have been more appropriate. In this application, we find that data given by LD are in no way more illuminating than those given by MD, which more efficiently finds results (see the electronic supplementary material). We chose our particular method of simulation because we knew it successfully described cyclohexane (for which the dynamics were verified by experiment as well as by other theory) as well as a number of odorants that have some similarities to steroid ligand.
Here, we highlight those active steroids for which the biological data are sound, and reasonably comparable between laboratories. Doubtless, future studies would benefit from a more extensive analysis to identify why compounds deviate from the trend, and the reliability of the trends could be tested by including more examples from both steroid classes. But until substantially, more biological information is available in the public sector, this is not really feasible.
What distinguishes a steroid that is primarily glucocorticoid from one that is mineralocorticoid? While several mechanisms may contribute, our data imply that critical features are located in different parts of the molecule. Pure glucocorticoid activity is associated with a relatively rigid ring A (figure 2), and to some extent Ring B (figure 3) but more flexible ring C. High mineralocorticoid potency, in contrast, is often (though not exclusively) reflected in a rigid ring C (figure 4). Furthermore, unlike the glucocorticoids, the mineralocorticoids examined here show significant variability in ring A conformational mobility (figure 2).
Molecular shape is surely important in binding to a receptor, but the distinct glucocorticoid and mineralocorticoid activities cannot be interpreted solely in terms of the lowest energy, static and molecular geometry. For the most specific glucocorticoids, the optimal shape seems rigidly maintained by the delta-1,4 diene, whereas, for the most specific mineralocorticoids, it is (for example) the hemi-acetal of aldosterone that rigidly maintains their optimal shape. When steroids such as cortisol can show either of these activities, one can argue that their flexibilities that allow them to adopt similar effective shapes: they may also be less potent if the more effective conformations are not rigidly maintained.
One steroid, 11-deoxycorticosterone clearly demonstrates this pluripotentiality. Deoxycorticosterone has been frequently described as a weak mineralocorticoid, devoid of glucocorticoid activity, almost since Selye first introduced the classifications 50 years ago [31,41]. Although the ring C flexibility of 11-deoxycorticosterone (figure 4) argues that it should not be classed with aldosterone, in fact, 11-deoxycorticosterone is a very potent mineralocorticoid, with an activity similar to that of aldosterone in some assays [15]. Further, using a definitive range of assays, we now show that it also exhibits glucocorticoid activity (figure 6). Quite how 11-deoxycorticosterone has come to be so misdescribed over the years deserves a special study of its own. In relation to the present results, its characteristic flexibility in both rings A and C, together with their clear glucocorticoid activity (figure 6), place it squarely in the group of mixed function steroids, with cortisol, cortisone and fludrocortisone (figures 2 and 4).
In such steroids that can show either activity, the interchanges of boat, chair and twist may conceivably optimize separate steps in some sequence of reactions. The activities of the more flexible compounds would then show more ambiguity or versatility. Such complex behaviour would depend on the range of conformations that flexibility allows. This is illustrated by comparisons of active 11β-hydroxysteroids, such as prednisolone or cortisol, and their ‘inactive’ (in the sense of binding only weakly to GR) 11-ketosteroid companions, such as prednisone or cortisone. The detailed trajectories of 11β-hydroxysteroids show more rigid rings C than the inactive 11-ketosteroids. Despite this, the 11-ketone compounds may not achieve appropriate configurations for activity, because their C ring flexibility tends towards the twist configuration (figure 5). In other words, while A ring rigidity is associated with glucocorticoid activity, and C ring rigidity with mineralocorticoid activity, the role of C ring flexibility in glucocorticoids and A ring flexibility in glucocorticoids is ambiguous. Thus, C ring flexibility does not assure GR activation and the range of conformations that such flexibility affords may also be critical.
Conformational mobility may alter the affinity or the efficacy of the steroid independently, for example, if receptor activation involves some rare conformations of the steroid that are only possible for a flexible molecule. In the case of a compound such as cortisol, which sometimes displays mineralocorticoid activity [7], but may also bind but not activate the MR [12], such flexibility might work in combination with receptor flexibility in binding appropriately to hormone response elements, or in the recruitment of co-activators or repressors. Certainly, there is evidence for different activated receptor conformations associated with different ligands, for both MR and GR [37]. Hence, it is entirely pertinent to ask whether steroids that can adopt varying conformations may evoke a range of active or inactive states of the receptor. Similar suggestions have recently been made in other contexts [20].
The flexibility we describe occurs on the picosecond time scale, a process that is fast compared with likely docking and reaction times, or the time spent in a receptor. A plethora of experimental and theoretical techniques has recently established that, in bacterial Δ5,3-ketosteroid isomerase, differences at the enzyme-binding site of just picometres can make large changes to enzyme activity [42]. The dynamical flexibility reported here, while seemingly subtle, certainly corresponds to differences much greater than picometres between flexible and rigid steroids. Thus, it may be the amplitude, as well asthe frequency, of changes of conformation that may matter. This argues against phenomena based on stochastic resonance, although it does leave open the influence of the wide range of vibrational frequencies associated with protein motions. We propose that conformational mobility of the ligand and protein combined may both initiate and affect signal transduction, consistent with the type of ‘long-range transmission’ across steroids that was reported decades ago by Barton [43].
This analysis of 35 corticosteroids of wide-ranging character brings together two strands: systematic and strictly comparable activity data, some new here, and MD simulations that examine the extent to which the molecular six-membered rings move between chair, boat and twist configurations. The major conclusion of this work is the striking correlation between the predicted dynamical properties of steroids and their observed biological actions. That the correlation is found from calculations on free molecules is especially valuable.
Acknowledgements
We would like to acknowledge and thank William Motherwell for enlightening discussions and Carlos Lantos for his enthusiastic and thorough review of pre-submitted manuscripts. We also thank Bill Smith for advice towards dl_poly calculations, and Dorothy Duffy, Sarah Harris, John Harding and Colin Freeman for guidance with AMBER potentials and Joel Franklin and Jan Lipfert for their patient attention and advice.
This work was supported in part by grants from ANPCyT (PICT-2010-1170) and UBACYT-X085 (to M.D.G.).
References
- 1.Lipkus A. H., Yuan Q., Lucas K. A., Funk S. A., Bartelt W. F., III, Schenck R. J., Trippe A. J. 2008. Structural diversity of organic chemistry. A scaffold analysis of the CAS Registry. J. Org. Chem. 73, 4443–4451 10.1021/jo8001276 (doi:10.1021/jo8001276) [DOI] [PubMed] [Google Scholar]
- 2.Vinson G. P. 2009. The adrenal cortex and life. Mol. Cell. Endocrinol. 300, 2–6 10.1016/j.mce.2008.09.008 (doi:10.1016/j.mce.2008.09.008) [DOI] [PubMed] [Google Scholar]
- 3.Han F., Ozawa H., Matsuda K., Nishi M., Kawata M. 2005. Colocalization of mineralocorticoid receptor and glucocorticoid receptor in the hippocampus and hypothalamus. Neurosci. Res. 51, 371–381 10.1016/j.neures.2004.12.013 (doi:10.1016/j.neures.2004.12.013) [DOI] [PubMed] [Google Scholar]
- 4.Bureik M., Bruck N., Hubel K., Bernhardt R. 2005. The human mineralocorticoid receptor only partially differentiates between different ligands after expression in fission yeast. FEMS Yeast Res. 5, 627–633 10.1016/j.femsyr.2004.12.007 (doi:10.1016/j.femsyr.2004.12.007) [DOI] [PubMed] [Google Scholar]
- 5.Fuller P. J., Lim-Tio S. S., Brennan F. E. 2000. Specificity in mineralocorticoid versus glucocorticoid action. Kidney Int. 57, 1256–1264 10.1046/j.1523-1755.2000.00959.x (doi:10.1046/j.1523-1755.2000.00959.x) [DOI] [PubMed] [Google Scholar]
- 6.Nishi M., Kawata M. 2007. Dynamics of glucocorticoid receptor and mineralocorticoid receptor: implications from live cell imaging studies. Neuroendocrinology 85, 186–192 10.1159/000101917 (doi:10.1159/000101917) [DOI] [PubMed] [Google Scholar]
- 7.Baxter J. D., Funder J. W., Apriletti J. W., Webb P. 2004. Towards selectively modulating mineralocorticoid receptor function: lessons from other systems. Mol. Cell. Endocrinol. 217, 151–165 10.1016/j.mce.2003.10.044 (doi:10.1016/j.mce.2003.10.044) [DOI] [PubMed] [Google Scholar]
- 8.Hellal-Levy C., Couette B., Fagart J., Souque A., Gomez-Sanchez C., Rafestin-Oblin M. 1999. Specific hydroxylations determine selective corticosteroid recognition by human glucocorticoid and mineralocorticoid receptors. FEBS Lett. 464, 9–13 10.1016/S0014-5793(99)01667-1 (doi:10.1016/S0014-5793(99)01667-1) [DOI] [PubMed] [Google Scholar]
- 9.Krozowski Z., et al. 1999. The type I and type II 11 beta-hydroxysteroid dehydrogenase enzymes. J. Steroid Biochem. Mol. Biol. 69, 391–401 10.1016/S0960-0760(99)00074-6 (doi:10.1016/S0960-0760(99)00074-6) [DOI] [PubMed] [Google Scholar]
- 10.Ferrari P. 2003. Cortisol and the renal handling of electrolytes: role in glucocorticoid-induced hypertension and bone disease. Best Pract. Res. Clin. Endocrinol. Metab. 17, 575–589 10.1016/S1521-690X(03)00053-8 (doi:10.1016/S1521-690X(03)00053-8) [DOI] [PubMed] [Google Scholar]
- 11.Funder J. W. 1993. Aldosterone action. Annu. Rev. Physiol. 55, 115–130 10.1146/annurev.ph.55.030193.000555 (doi:10.1146/annurev.ph.55.030193.000555) [DOI] [PubMed] [Google Scholar]
- 12.Funder J. W. 2005. Mineralocorticoid receptors: distribution and activation. Heart Fail. Rev. 10, 15–22 10.1007/s10741-005-2344-2 (doi:10.1007/s10741-005-2344-2) [DOI] [PubMed] [Google Scholar]
- 13.Roland B. L., Krozowski Z. S., Funder J. W. 1995. Glucocorticoid receptor, mineralocorticoid receptors, 11 beta-hydroxysteroid dehydrogenase-1 and -2 expression in rat brain and kidney: in situ studies. Mol. Cell. Endocrinol. 111, R1–R7 10.1016/0303-7207(95)03559-P (doi:10.1016/0303-7207(95)03559-P) [DOI] [PubMed] [Google Scholar]
- 14.Usa K., Singh R. J., Netzel B. C., Liu Y., Raff H., Liang M. 2007. Renal interstitial corticosterone and 11-dehydrocorticosterone in conscious rats. Am. J. Physiol. Renal Physiol. 293, F186–F192 10.1152/ajprenal.00484.2006 (doi:10.1152/ajprenal.00484.2006) [DOI] [PubMed] [Google Scholar]
- 15.Galigniana M. D., Piwien Pilipuk G., Kanelakis K. C., Burton G., Lantos C. P. 2004. Molecular mechanism of activation and nuclear translocation of the mineralocorticoid receptor upon binding of pregnanesteroids. Mol. Cell. Endocrinol. 217, 167–179 10.1016/j.mce.2003.10.041 (doi:10.1016/j.mce.2003.10.041) [DOI] [PubMed] [Google Scholar]
- 16.Bledsoe R. K., et al. 2005. A ligand-mediated hydrogen bond network required for the activation of the mineralocorticoid receptor. J. Biol. Chem. 280, 31 283–31 293 10.1074/jbc.M504098200 (doi:10.1074/jbc.M504098200) [DOI] [PubMed] [Google Scholar]
- 17.Bledsoe R. K., et al. 2002. Crystal structure of the glucocorticoid receptor ligand binding domain reveals a novel mode of receptor dimerization and coactivator recognition. Cell 110, 93–105 10.1016/S0092-8674(02)00817-6 (doi:10.1016/S0092-8674(02)00817-6) [DOI] [PubMed] [Google Scholar]
- 18.Nabuurs S. B., Wagener M., de Vlieg J. 2007. A flexible approach to induced fit docking. J. Med. Chem. 50, 6507–6518 10.1021/jm070593p (doi:10.1021/jm070593p) [DOI] [PubMed] [Google Scholar]
- 19.Duax W. L., Weeks C. M., Rohrer D. C. 1976. Crystal structure of steroids: molecular conformation and biological function. Recent Progr. Hormone Res. 32, 81–116 [DOI] [PubMed] [Google Scholar]
- 20.Brookes J. C., Horsfield A. P., Stoneham A. M. 2009. Odour character differences for enantiomers correlate with molecular flexibility. J. R. Soc. Interface 6, 75–86 10.1098/rsif.2008.0165 (doi:10.1098/rsif.2008.0165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohloff G., Maurer B., Winter B., Giersch W. 1983. Structural and configurational dependence of the sensory process. Helvetica Chim. Acta 66, 192–217 [Google Scholar]
- 22.Dewar M. J., Zoebisch E. G., Healy E. F., Stewart J. J. 1985. A new general quantum mechanical molecular model. J. Am. Chem. Soc. 107, 3902 [Google Scholar]
- 23.Becke A. D. 1993. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 10.1063/1.464913 (doi:10.1063/1.464913) [DOI] [Google Scholar]
- 24.Allen M. P., Tildesley D. J. 1989. Computer simulation of liquids. Oxford, UK: Oxford Science Publications [Google Scholar]
- 25.Mayo S. L., Olafson B. D., Goddard W. A., III 1990. DREIDING: a generic force field for molecular simulations. J. Phys. Chem. 94, 8897–8909 10.1021/j100389a010 (doi:10.1021/j100389a010) [DOI] [Google Scholar]
- 26.Wang J., Wolf R. M., Caldwell J. W., Kollman P. A., Case D. A. 2004. Development and testing of a general amber force field. J. Comput. Chem. 25, 1157–1174 10.1002/jcc.20035 (doi:10.1002/jcc.20035) [DOI] [PubMed] [Google Scholar]
- 27.Almenningen A., Bastiansen O., Skancke P. N. 1961. Preliminary results of an electron diffraction reinvestigation of cyclobutane and cyclopentane. Acta Chem. Scand. 15, 711 [Google Scholar]
- 28.Eliel E. L., Allinger N. L., Angyal S. J., Morrison G. A. 1965. Conformational analysis. London, UK: John Wiley & Sons [Google Scholar]
- 29.Baxter J. D., Rousseau G. G. 1979. Glucocorticoid hormone action. Berlin, Germany: Springer; [DOI] [PubMed] [Google Scholar]
- 30.Haynes R. C., Murad F. 1985. Adrenocorticotrophic hormone; adrenocortical steroids and their synthetic analogues; inhibitors of adrenocortical steroid biosynthesis. In The pharmacological basis of therapeutics (eds Gilman A. G., Goodman L. S., Rall T. W., Murad F.). New York, NY: Macmillan [Google Scholar]
- 31.Genest J. 1955. The present status of aldosterone in clinical medicine. Can. Med. Assoc. J. 73, 876–883 [PMC free article] [PubMed] [Google Scholar]
- 32.Orth D. N., Kovacs W. J. 1998. The adrenal cortex. In Williams' textbook of endocrinology (ed. Wilson J. D.), pp. 517–664 Philadelphia, PA: W.B. Saunders [Google Scholar]
- 33.Steele R. 1975. Influence of corticosteroids on protein and carbohydrate metabolism. In Handbook of physiology (eds Blaschko H., Smith A. D., Sayers G.), pp. 135–167 Washington, DC: American Physiological Society [Google Scholar]
- 34.Vicent G. P., Pecci A., Ghini A. A., Piwien-Pilipuk G., Veleiro A. S., Burton G., Lantos C. P., Galigniana M. D. 1999. The glucocorticoid properties of the synthetic steroid pregna-1,4-diene-11beta-ol-3,20-dione (deltaHOP) are not entirely correlated with the steroid binding to the glucocorticoid receptor. Mol. Cell. Endocrinol. 149, 207–219 10.1016/S0303-7207(98)00205-6 (doi:10.1016/S0303-7207(98)00205-6) [DOI] [PubMed] [Google Scholar]
- 35.Krisman C. R. 1962. A method for the colorimetric estimation of glycogen with iodine. Anal. Biochem. 4, 17–23 [DOI] [PubMed] [Google Scholar]
- 36.Galigniana M. D., Vicent G. P., Burton G., Lantos C. P. 1997. Features of the shuttle pair 11 beta-hydroxyprogesterone-11-ketoprogesterone. Steroids 62, 358–364 [DOI] [PubMed] [Google Scholar]
- 37.Gallo L. I., Ghini A. A., Pilipuk G. P., Galigniana M. D. 2007. Differential recruitment of tetratricorpeptide repeat domain immunophilins to the mineralocorticoid receptor influences both heat-shock protein 90-dependent retrotransport and hormone-dependent transcriptional activity. Biochemistry 46, 14 044–14 057 10.1021/bi701372c (doi:10.1021/bi701372c) [DOI] [PubMed] [Google Scholar]
- 38.Selye H., Jensen H. 1946. The chemistry of the hormones. Ann. Rev. Biochem. 15, 347–360 [DOI] [PubMed] [Google Scholar]
- 39.Purnell J. Q., Kahn S. E., Samuels M. H., Brandon D., Loriaux D. L., Brunzell J. D. 2009. Enhanced cortisol production rates, free cortisol, and 11beta-HSD-1 expression correlate with visceral fat and insulin resistance in men: effect of weight loss. Am J Physiol Endocrinol Metab 296, E351–E357 10.1152/ajpendo.90769.2008 (doi:10.1152/ajpendo.90769.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morris D. J., Souness G. W., Brem A. S., Oblin M. E. 2000. Interactions of mineralocorticoids and glucocorticoids in epithelial target tissues. Kidney Int. 57, 1370–1373 10.1046/j.1523-1755.2000.00977.x (doi:10.1046/j.1523-1755.2000.00977.x) [DOI] [PubMed] [Google Scholar]
- 41.Selye H. 1946. The general adaptation syndrome and the diseases of adaptation. J. Clin. Endocrinol. Metab. 6, 117–230 [DOI] [PubMed] [Google Scholar]
- 42.Sigala P. A., Kraut D. A., Caaveiro J. M., Pybus B., Ruben E. A., Ringe D., Petsko G. A., Herschlag D. 2008. Testing geometrical discrimination within an enzyme active site: constrained hydrogen bonding in the ketosteroid isomerase oxyanion hole. J. Am. Chem. Soc. 130, 13 696–13 708 10.1021/ja803928m (doi:10.1021/ja803928m) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barton D. H. 1972. The principles of conformational analysis. In Nobel Lectures, 1963–1970, pp. 298–311 Amsterdam, The Netherlands: Elsevier [Google Scholar]