Abstract
Plant surfaces covered with three-dimensional (3D) waxes are known to strongly reduce insect adhesion, leading to slippery surfaces. Besides 3D epicuticular waxes, cuticular folds are a common microstructure found on plant surfaces, which have not been quantitatively investigated with regard to their influence on insect adhesion. We performed traction experiments with Colorado potato beetles on five plant surfaces with cuticular folds of different magnitude. For comparison, we also tested (i) smooth plant surfaces and (ii) plant surfaces possessing 3D epicuticular waxes. Traction forces on surfaces with medium cuticular folds, of about 0.5 µm in both height and thickness and a spacing of 0.5–1.5 µm, were reduced by an average of 88 per cent in comparison to smooth plant surfaces. Traction forces were reduced by the same order of magnitude as on plant surfaces covered with 3D epicuticular waxes. For surface characterization, we performed static contact angle measurements, which proved a strong effect of cuticular folds also on surface wettability. Surfaces possessing cuticular folds of greater magnitude showed higher contact angles up to superhydrophobicity. We hypothesize that cuticular folds reduce insect adhesion mainly due to a critical roughness, reducing the real contact area between the surface and the insect's adhesive devices.
Keywords: adhesion, attachment, biomimetic surfaces, epicuticular waxes, insect–plant interaction, microstructure
1. Introduction
In the last few decades, the great impact of microstructuring of biological surfaces on the physical and biological environment came into focus in different fields of research. In plants, the cuticle constitutes the outermost layer of the plant body. Plant waxes are the obligatory final layer of the cuticle, but they differ in their chemical composition and structure [1]. The cuticle serves different functions, such as stabilizing the plant tissue, providing a transport barrier to reduce uncontrolled water loss, increasing or reducing surface wetting, allowing for self-cleaning by draining of water, providing a protection against harmful radiation and influencing the attachment of insects (reviewed in [2]). Plant surfaces show a wide range of structuring, both on the cellular level and on the level of superimposed microstructuring such as wax crystals (three-dimensional (3D) epicuticular waxes) and cuticular folds. A concise overview of the great variety of structuring with characterizations of shapes and dimensions is provided by Barthlott & Ehler [3] and in excerpts in the review of Koch et al. [2].
Many insects have evolved special adhesive organs and are able to adhere strongly to various surfaces [4]. These highly adapted attachment devices allow the insects to save energy by maintaining the chosen site for foraging and reproduction, partly withstanding forces of more than 100 times their own body weight on smooth surfaces [5] (see also [6]). However, on some plant surfaces attachment is impossible and the insects slip off. Plant surfaces with 3D epicuticular waxes have been shown to reduce strongly insect adhesion [7–14], and different mechanisms for their anti-adhesive properties have been proposed in past studies, outlined in Gorb & Gorb [15].
Besides 3D epicuticular waxes, cuticular folds are a common microstructure found on plant surfaces, but their influence on insect adhesion has scarcely been investigated. Cuticular folds can originate from different modifications of the epidermis, e.g. by folding of the cuticle itself and pectin accumulation beneath the cuticle or by a special shape of the cell wall [2]. Barthlott & Ehler [3] gave an overview on the morphology of cuticular folds with dimensions and orientation. In a few plant species, the influence of cuticular folds on the wettability of the plant surface has been investigated. Leaves of Fouquieria columnaris with convex epidermal cells covered with cuticular folds were found to be hydrophobic [16]. Both petals of a red rose with papillate epidermal cells possessing cuticular folds and replicas of Alocasia macrorrhiza (abaxial leaf; papillate epidermal cells) [17] and Rosa landora (petal; papillate epidermal cells with cuticular folds) showed superhydrophobic properties [18]. Furthermore, cuticular folds on petal surfaces were found to generate iridescence, thus changing the optical properties [19]. Koch et al. [2] pointed out that ‘Functional aspects of cuticular folding are rarely investigated, but their frequent occurrence on flower leaves led to the assumption that cuticular folding forms a favourable structure for insect pollinators to capture and walk on such leaves’. In contrast, Poppinga et al. [20] proposed that cuticular folds, occurring on surfaces of carnivorous plants and kettle trap flowers, reduce the contact area of adhesive pads due to the microroughness created. However, the influence of cuticular folds on insect attachment has not been quantitatively analysed so far.
The present study focuses on cuticular folds of different magnitude and on their impact on the attachment ability of a chrysomelid beetle. We determined the maximal traction forces of tethered beetles actively walking on surfaces of different structuring. For surface characterization the static contact angles were determined. The investigations on cuticular folds are compared to the effect of 3D epicuticular waxes and of smooth plant surfaces on adhesion of actively walking insects and wettability.
2. Materials and methods
2.1. Insect and plant species
The Colorado potato beetle Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae), with its adhesive pads of the hairy type, was used as a model insect species. Beetles were collected at organic potato fields in the Kaiserstuhl area near Freiburg and kept in a terrarium on their host plant, Solanum tuberosum, using a day–night regime of 16 L : 8 D (Osram Lumilux Daylight 865 lamp, 58 W). Beetles were individually marked with coding dots on the elytra. For experiments only male beetles were used, being 11–14 mm in length and with a body mass of 90–140 mg.
After an extensive screening process of over 40 plant species, six plant surfaces with tabular epidermal cells and differently structured cuticular folds were chosen (see §3). For comparison, we investigated Litchi chinensis (abaxial leaf surface) showing hierarchical structuring consisting of convex epidermal cells and cuticular folds. Additionally—as to the well described anti-adhesive effects of epicuticular waxes—an eighth surface with 3D epicuticular wax platelets on tabular epidermal cells was selected. All plants used (table 1) were grown in the Botanic Garden of the University of Freiburg, except for Cyclamen persicum, which was obtained from a garden shop.
Table 1.
Plant species tested.
| species | surface tested | accession no. FBGa |
|---|---|---|
| Magnolia grandiflora | adaxial leaf surface | 505–563 |
| Ilex aquifolium | adaxial leaf surface | 3409–320 |
| Litchi chinensis | adaxial and abaxial leaf surface | old accession |
| Cyclamen persicum | petal leaf surface | (Obi, Freiburg) |
| Hevea brasiliensis | adaxial and abaxial leaf surface | old accession |
| Diospyros kaki | fruit surface | 900–87 |
aAccession number of the University of Freiburg Botanic garden.
2.2. Scanning electron microscopy
Morphologies of plant surfaces and of attachment devices of the beetles were characterized using scanning electron microscopy (SEM). For SEM analysis, leaf samples with 3D epicuticular waxes were air-dried. Before investigation of the shape of epidermal cells and cuticular folds, leaf samples were dehydrated in methanol [21] and critical-point-dried (LPD 030, Bal-Tec). For cross-section analysis of plant surfaces, the critical-point-dried samples were cut with a razor blade. Tarsi of Colorado potato beetles were air-dried for SEM analysis. All samples were mounted on aluminium stubs using conductive double-sided adhesive tabs (Plano, Wetzlar). Samples were sputter-coated with gold (approximately 15 nm; Cressington Sputter Coater, 108 auto) and examined using SEM (Leo 435 vp, Leica, Wiesbaden, Germany).
2.3. Traction experiments
For traction experiments, plant samples were fixed to a glass slide using double-sided adhesive tape. To determine the maximum traction forces of beetles actively walking on the surfaces investigated, we used a highly sensitive force transducer (Fort 25, World Precision Instruments Inc., Sarasota, USA). The resolution of the measurements was ± 50 µN. Experiments were carried out at a steady room temperature of 25°C and a relative humidity above 35 per cent.
Individual beetles were tethered to the force transducer using a human hair (10–15 cm in length) glued to the beetle's elytra with beeswax. A small lamp was used to attract the beetles, and measurements were stopped if the beetle did not walk straight forward. The traction force of each beetle was recorded during at least 2 min of active walking on the respective test substrate (figure 1). Force was recorded at a sample rate of 100 data points per second using the software DataTrax2 (v. 2.056, WPI Instruments, Inc., Sarasota, USA). Data were processed and analysed using R (v. R 2.9.1).
Figure 1.
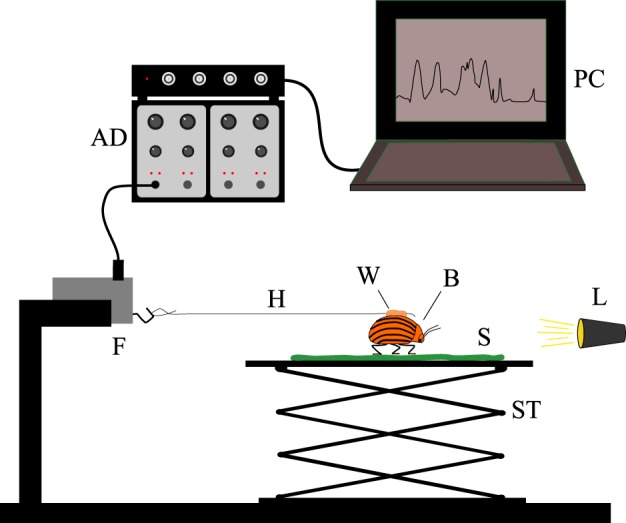
Experimental setup: the traction force of an actively walking beetle, being attracted by a small light, was recorded using a highly sensitive force transducer. AD, transducer amplifier and AD converter; PC, laptop for data acquisition; F, highly sensitive force transducer; H, hair; W, beeswax; B, beetle; S, plant surface; L, light source; ST, stage. (Online version in colour.)
Within each measurement the median of the 15 highest local maxima with a minimum time lag of three seconds between two neighbouring peaks was extracted. Before each measurement on the respective plant surface, traction force of the individual beetle was tested on a plain, cleaned glass slide, both to get a reference value and to test for individual differences in the attachment ability of beetles. After a pause of 5 min the measurement on the plant surface was performed. To compensate for individual differences in traction force, the traction forces on the plant surfaces were normalized to the forces measured on glass for each individual beetle, resulting in relative traction forces. Measurements were repeated with 6–8 different beetles per plant species. For each run a fresh piece of leaf was used.
2.4. Contact angle measurements
For further characterization of the plant surfaces investigated, the static contact angle between water and the surface was measured. A droplet of 5 µl of double-distilled water was applied to the surface and a picture taken within the first 2 min after applying the droplet using a horizontally aligned microscope (WILD MAKROSKOP M 420) with a camera attached (Colour View II, Olympus U-CMA D3, Japan). Pictures were analysed using the software ImageJ and the ‘ContactAngle’ plugin (ImageJ 1.42q, USA; plugin: v. 2006/12/07, author Marco Brugnara). Wetting behaviour was classified according to Koch et al. [2].
2.5. Statistics
Data of traction experiments were log-transformed and data of contact angle measurements were squared to achieve normal distribution (Shapiro test) and equality of variances (Levene test). Data were analysed using nested analysis of variance (ANOVA) and Tukey HSD post hoc tests.
3. Results
3.1. Morphology
Using SEM images the plant surfaces were classified according to their sculpturing both on the cellular level and on the level of superimposed microstructuring (figure 2). In the following, for Hevea brasiliensis and L. chinensis, results refer to the adaxial leaf surface, if not stated differently.
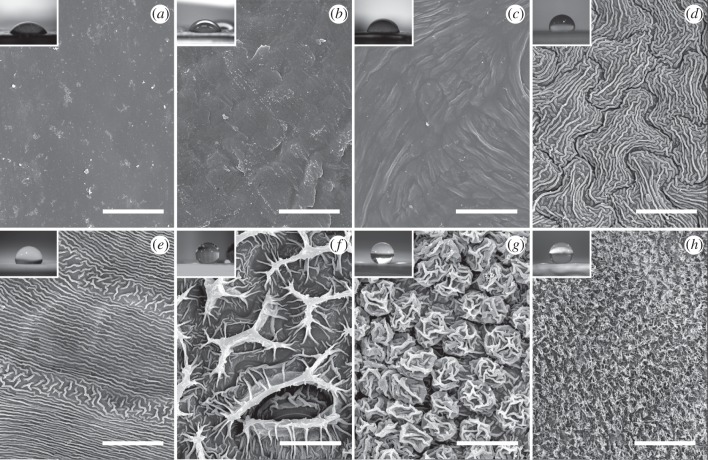
Figure 2.
SEM micrographs of plant surfaces with different types of structuring. (a,b) Smooth surfaces covered only with 2D layers or crusts of wax: (a) Magnolia grandiflora (adaxial leaf surface) and (b) Ilex aquifolium (adaxial leaf surface); (c) low cuticular folds: Litchi chinensis (adaxial leaf surface); (d,e) medium cuticular folds: (d) Hevea brasiliensis (adaxial leaf surface) and (e) Cyclamen persicum (petal leaf surface); (f) high cuticular folds: Hevea brasiliensis (abaxial leaf surface); (g) hierarchical surface with cuticular folds: Litchi chinensis (abaxial leaf surface); (h) 3D epicuticular waxes: Diospyros kaki (fruit surface). Scale bars, 20 µm. Insets show pictures of droplets of 5 µl of double-distilled water on the respective plant surface used for calculating the contact angles.
The epidermis of all plant surfaces investigated except for L. chinensis (abaxial leaf surface: convex cell shape) consists of tabular cells. Leaf surfaces of both Magnolia grandiflora (figure 2a) and Ilex aquifolium (figure 2b) are covered only with smooth two-dimensional (2D) layers of wax, in parts accumulated to form crusts of wax [1], but without any further microstructuring.
For describing plant surfaces with cuticular folds, we distinguished three types of cuticular folds according to the dimensions of the structuring. On L. chinensis (figure 2c) cuticular folds with a height between 0.1 and 0.4 µm and irregular spacing were found, indicated as ‘low cuticular folds’. Cuticular folds with both height and thickness of about 0.5 µm and a spacing of 0.5–1.5 µm, found on H. brasiliensis (figure 2d) and C. persicum (figure 2e), are labelled as ‘medium cuticular folds’. Cuticular folds considerably greater in height than medium cuticular folds, found on H. brasiliensis (abaxial leaf surface) (figure 2f), are indicated as ‘high cuticular folds’. Here, single folds are 8–14 µm in height, 2–5 µm in thickness and the spacing between the folds is 8–15 µm. L. chinensis (abaxial leaf surface) (figure 2g) shows hierarchical structuring, with convex cells being covered with cuticular folds of 0.5–1 µm in height, 0.5 µm in thickness and a spacing between the single folds of approximately 0.5 µm. The total peak to valley ratio over the two levels of hierarchy is 6–8 µm.
For comparison, we investigated Diospyros kaki fruits (figure 2h), possessing 3D epicuticular waxes in the shape of platelets (according to Barthlott et al. [1]). Single wax platelets are about 1 µm in height, 0.03 µm in thickness and 1–2 µm in length. Spacing between the platelets was between 0.5 and 1.5 µm.
Morphology of the attachment devices of male Colorado potato beetles was investigated using SEM (figure 3). The tarsus consists of five tarsomeres, with the fourth tarsomere being hidden, and a pair of curved claws on the pretarsus [22]. The diameter of the claw tips is 8–10 µm. Tarsi are of the hairy type, with tarsomeres 1–3 being covered with setae of four different types (according to Voigt et al. [22]).
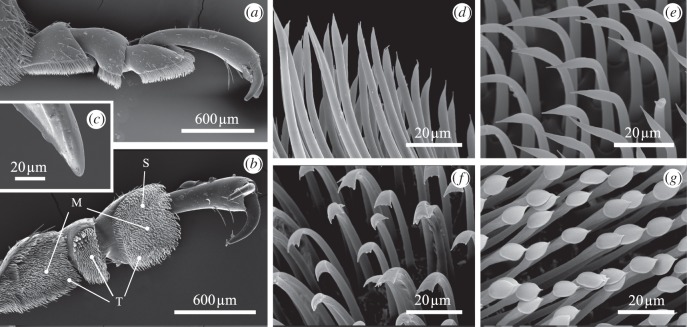
Figure 3.
SEM micrographs of the attachment devices in a male Leptinotarsa decemlineata. (a) Lateral view and (b) ventral view of a hind-leg. (c) Individual claw tip. Tarsal adhesive setae of the (d) filamentous, (e) lanceolate, (f) spatula-shaped and (g) discoidal type (latter found in males only). Setae are termed according to Voigt et al. [22], with setae of the filamentous and lanceolate type being grouped as tapered setae. M, setae of discoidal type; S, spatula-shaped setae; T, tapered setae.
3.2. Traction experiments
Comparing the relative traction forces of male L. decemlineata (figure 4) on plant surfaces of different structuring, we found significant differences between the four groups with cuticular folds of different magnitude, plant surfaces covered with 3D epicuticular wax crystals, and smooth plant surfaces without cuticular folds (nested ANOVA: F5,47 = 70.2; p < 0.001).
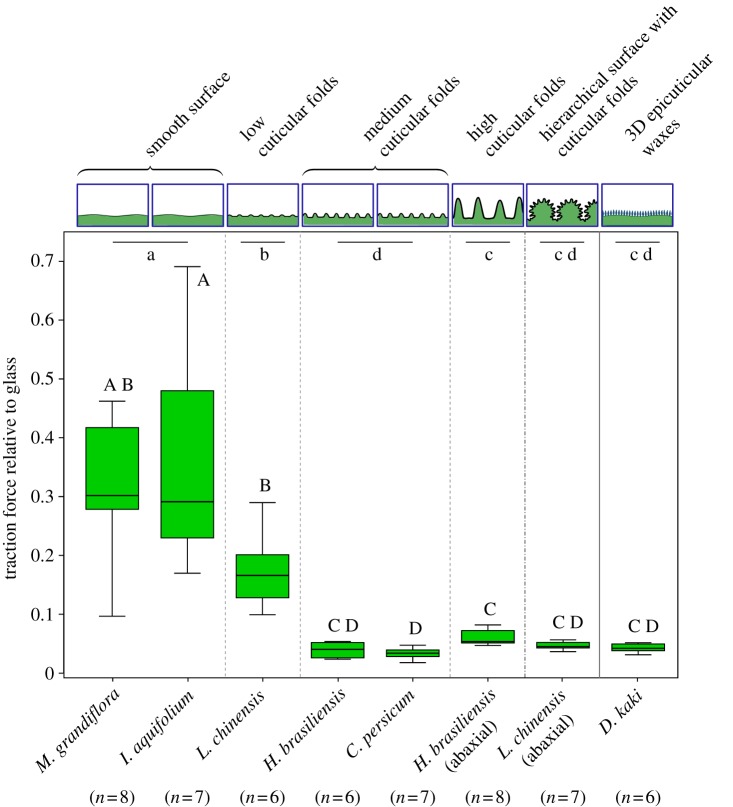
Figure 4.
Traction forces of actively walking male Leptinotarsa decemlineata beetles on plant surfaces relative to traction forces on glass (box–whisker plot). Comparison of different surface structuring with cuticular folds of different magnitude to plant surfaces showing 3D epicuticular waxes and smooth plant surfaces without cuticular folds. Results of statistical analysis (nested ANOVA and Tukey HSD post hoc tests) are based on log-transformed data. Significant differences between groups are indicated by lower case letters. Upper case letters indicate significant differences between individual plant species. (Online version in colour.)
We found a strong decrease in traction forces comparing smooth plant surfaces (M. grandiflora and I. aquifolium) to surfaces with medium cuticular folds (H. brasiliensis and C. persicum). The traction forces on the adaxial leaf surface of L. chinensis showing low cuticular folds were already significantly reduced in comparison to the smooth surfaces, but significantly higher than the traction forces on surfaces with medium cuticular folds. There was no further decrease in traction forces on high cuticular folds (H. brasiliensis, abaxial leaf surface) and a hierarchical surface with cuticular folds (L. chinensis, abaxial leaf surface) compared to medium cuticular folds. Comparing plant surfaces covered with 3D epicuticular waxes (D. kaki) to medium and high cuticular folds and the hierarchical surface, no significant differences in traction forces were found.
It could be shown that differences between the groups of structuring are greater than differences between the species within the groups (nested ANOVA: species [group] F2,47 = 0.5; p = 0.61; group F5,47 = 70.2; p < 0.001). Absolute traction forces measured for each surface are summarized in table 2.
Table 2.
Maximal traction forces of L. decemlineata on plant surfaces of different structuring—absolute data.
| surface tested | surface characteristics | max. traction force (mN) (mean ± s.d.) | n |
|---|---|---|---|
| Magnolia grandiflora | smooth surfaces with only 2D layers of wax | 13.54 ± 5.43 | 8 |
| Ilex aquifolium | smooth surfaces with only 2D layers of wax | 15.07 ± 7.75 | 7 |
| Litchi chinensis | low cuticular folds | 7.65 ± 4.05 | 6 |
| Hevea brasiliensis | medium cuticular folds | 1.75 ± 0.55 | 6 |
| Cyclamen persicum | medium cuticular folds | 1.6 ± 0.61 | 7 |
| Hevea brasiliensis (abaxial) | high cuticular folds | 2.41 ± 0.44 | 8 |
| Litchi chinensis (abaxial) | convex cells + cuticular folds | 1.91 ± 0.37 | 7 |
| Diospyros kaki | 3D epicuticular waxes | 1.73 ± 0.35 | 6 |
| glass | smooth technical surface | 43.38 ± 6.76 | 55 |
3.3. Contact angle measurements
Upon comparing the static contact angles of water on plant surfaces with cuticular folds of different magnitude to plant surfaces covered with epicuticular wax crystals and smooth plant surfaces, we found significant differences between the groups (figure 5) (nested ANOVA: species [group] F2,100 = 64.5; p < 0.001; group F5,100 = 1002.8; p < 0.001).
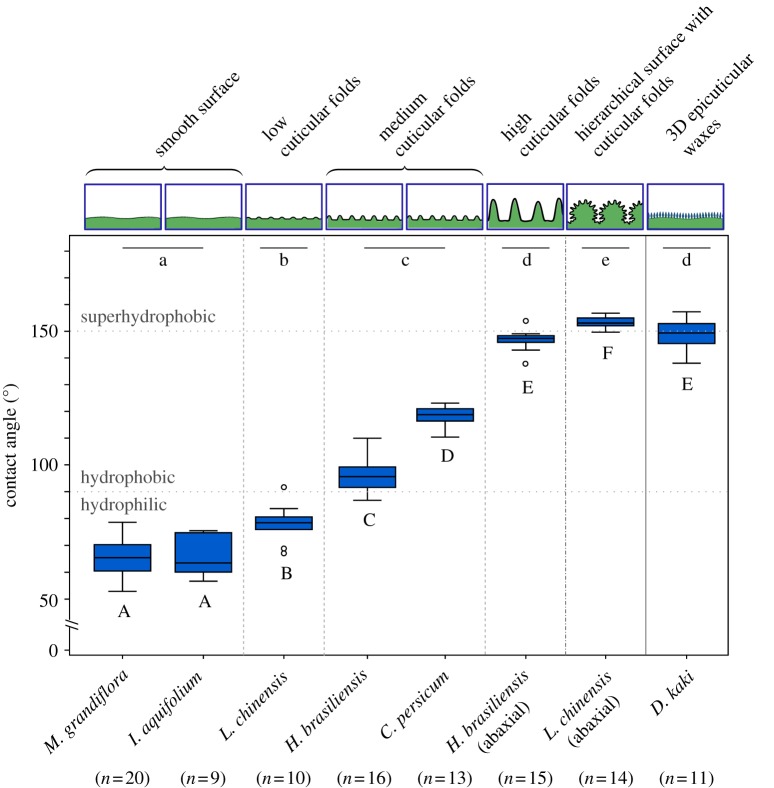
Figure 5.
Static contact angles of water droplets on plant surfaces with cuticular folds of different dimensions compared to plant surfaces showing 3D epicuticular waxes and smooth plant surfaces (box–whisker plot). The grey lines at 90° and 150° indicate the limit from hydrophilic to hydrophobic and from hydrophobic to superhydrophobic surfaces according to Koch et al. [2]. Results of statistical analysis (nested ANOVA and Tukey HSD post hoc tests) are based on square-transformed data. Significant differences between groups are indicated by lower case letters. Upper case letters indicate significant differences between individual plant species. (Online version in colour.)
The contact angles are significantly higher on surfaces possessing medium cuticular folds (97° ± 7° (mean ± s.d.) in H. brasiliensis and 118° ± 4° in C. persicum) than on smooth surfaces (65° ± 7° in M. grandiflora and 66° ± 8° in I. aquifolium) or surfaces possessing only low cuticular folds (78° ± 7° in L. chinensis). Plant surfaces with high cuticular folds (147° ± 4° in H. brasiliensis, abaxial leaf surface) show a further distinct increase in contact angle compared to surfaces with medium cuticular folds. Contact angles on a hierarchical surface with cuticular folds (153° ± 2° in L. chinensis, abaxial leaf surface) are even higher than on plant surfaces with high cuticular folds, leading to superhydrophobic properties.
In addition to examining cuticular folds we also conducted contact angle measurements on plant surfaces covered with 3D epicuticular waxes. The values for the wettability of plant surfaces with high cuticular folds (H. brasiliensis, abaxial leaf surface), convex cells with cuticular folds (L. chinensis, abaxial leaf surface) and tabular cells with 3D epicuticular waxes (D. kaki, fruit; 149° ± 6°) are all at the limit to superhydrophobicity at 150°. No significant difference exists between D. kaki and the abaxial leaf surface of H. brasiliensis. Static contact angles on L. chinensis (abaxial leaf surface) with its hierarchical surface (convex cells with cuticular folds) are still higher, leading to a superhydrophobic surface.
Upon dipping the plant surfaces into water, the surfaces of H. brasiliensis (abaxial leaf surface), L. chinensis (abaxial leaf surface) and D. kaki were covered with a silvery layer of air. After taking the leaves and the fruit out of the water again, the surfaces were not wetted. In all other plant surfaces tested we could not observe any layer of air under water, and surfaces were wetted after taking them out of the water.
4. Discussion
In the present study plant surfaces with cuticular folds were found to reduce strongly the adhesion of male L. decemlineata in comparison to smooth plant surfaces without cuticular folds. Traction forces on surfaces with medium cuticular folds of approximately 0.5 µm in both height and thickness and spacing between 0.5 and 1.5 µm are lowest, being of the same order of magnitude as traction forces on surfaces with 3D epicuticular waxes. In comparison to smooth plant surfaces, traction forces on medium cuticular folds are reduced on average by 88 per cent and in comparison to the reference substrate glass even by 96 per cent.
Our findings support the roughness hypothesis (proposed in [7,8,14,22,23]), assuming that a critical roughness reducing the real contact area between the insect's attachment devices and the substrate is a crucial parameter to minimize its attachment ability. According to the dimensions of the single setae of the Colorado potato beetle, the tip of a seta may come to lie on several folds. We hypothesize that the tips of single setae come in contact with the plant surface only at the peaks of the folds, without being able to fully adapt to the profile.
Several studies found surface roughness with dimensions similar to cuticular folds to strongly reduce insect attachment. Voigt et al. [22] found the strongest reduction in friction force of L. decemlineata on replicas of polishing paper with asperities of 0.3 and 1.0 µm in diameter. These asperity diameters are similar to the 0.5 µm thickness of medium cuticular folds showing the strongest reduction in adhesion in our study. In Scholz et al. [23] friction measurements were performed with juvenile stick insects on polishing paper of different roughness, finding a strong reduction of friction force on polishing paper of an approximate grain size of 3 µm, with a mean spacing of surface irregularities of 1.55 µm. Within the same study a similar spacing of 1.3 µm was recorded for 3D epicuticular waxes within the slippery zone in Nepenthes alata pitchers. Mean spacing of surfaces with the strongest anti-adhesive properties is similar to the 0.5–1.5 µm spacing found for medium cuticular folds in C. persicum and H. brasiliensis.
Plant surfaces with low cuticular folds proved to be less effective in reducing the attachment ability of the beetles, but traction forces were already significantly reduced in comparison to smooth plant surfaces without cuticular folds. Low cuticular folds were found to be irregularly distributed, with areas of several square micrometres without folds. Heights of the folds were lower than in medium cuticular folds. We assume the real contact area between setae and plant surfaces with low cuticular folds to be greater than on plant surfaces with medium cuticular folds, as single setae may come into contact with the plant surface in areas with larger spacing between the low cuticular folds. Furthermore, as individual folds are smaller in height, a slight flexibility would allow single setae to come in contact with areas between the folds thereby increasing the absolute contact area.
The individual attachment ability of beetles had a stronger influence on plant surfaces with high traction force results, whereas plant surfaces with cuticular folds appeared to be too slippery to bring out differences in individual attachment abilities leading to a reduced variability in the traction force measured.
There is a significant decrease in traction forces from smooth plant surfaces without cuticular folds, over low cuticular folds to medium cuticular folds. However, comparing high cuticular folds to medium cuticular folds, there exist no significant differences in traction forces. A similar trend is described by Gorb et al. [7]. Here, the length of wax crystals (between 0.4 and 2.6 µm) was found to affect the attachment of a coccinellid beetle, decreasing the attachment with increasing length of crystals. Besides spacing, we propose the height of structuring to influence the adhesion ability up to a critical height, with no further decrease in adhesion ability upon further exceeding the critical height.
Spacing between structures in high cuticular folds is larger than between medium cuticular folds. The slight differences in traction forces found between H. brasiliensis (abaxial leaf surface) and C. persicum might be explained by single setae getting into the space between the high folds and either being able to partly attach to the walls of single high folds or to interlock with them. Additionally, the beetle's claws might be able to grip the high folds, with spacing between the folds of 8–15 µm and a tip diameter of the claws of approximately 8–10 µm. Dai et al. [24] stated that insects with tarsi only equipped with claws are able to attach to a vertical surface only at surface asperities bigger than the diameter of the claw tips. On surfaces with particle diameters smaller than or comparable to the claw tip, the claws slip off the particle [24]. Spacing of folds in H. brasiliensis (abaxial leaf surface) might allow single claws to interlock, whereas in medium cuticular folds the smaller spacing should not allow claws to cling to the surface structures. However, differences in traction forces between H. brasiliensis (abaxial leaf surface) and C. persicum were small, questioning the success of claw attachment on high cuticular folds. For further investigation of the influence of claws on the beetles' attachment ability on plant surfaces with cuticular folds, experiments with beetles having clipped claw tips will be performed.
Comparing the surfaces of L. chinensis (abaxial leaf surface), showing hierarchical structuring with convex cells and superimposed cuticular folds, to both medium and high cuticular folds, we found no significant differences in traction forces. We could not find an influence of the hierarchical design on the attachment ability. Having a similar peak-to-valley ratio to H. brasiliensis (abaxial leaf surface), dimensions of single folds in L. chinensis (abaxial leaf surface) are similar compared to medium cuticular folds, presumably leading to a similar reduction of real contact area between plant surface and insect setae. According to Dai et al. [24], spacing between single folds should not allow claws to cling to the surface structures.
No significant differences in traction forces between plant surfaces with 3D cuticular waxes and all types of cuticular folds other than low cuticular folds could be found. Wax platelets on D. kaki were found to be much thinner (about 0.02 µm) than medium cuticular folds, and little greater in height (about 1 µm). Therefore, we hypothesize that both medium cuticular folds and 3D epicuticular waxes are within a range of critical roughness, both leading to slippery surfaces despite their differences in dimension.
Contamination of the insects' attachment devices with 3D epicuticular waxes has been proposed to reduce the attachment ability (e.g. [8]) and might be a possible reason for a reduction in insect adhesion, but this mechanism is not mandatory as already demonstrated for technical surfaces [22,23]. Cuticular folds are part of the cuticle [3] and are potentially less susceptible to abrasion than 3D epicuticular waxes. We propose cuticular folds to be an alternative to 3D epicuticular waxes for the plant to reduce adhesion of insects. Measuring the static contact angle, we found a relationship between contact angle and surface structuring. Contact angles increase with the height of cuticular folds, leading to a mean contact angle up to 147° in high cuticular folds, similar to D. kaki with 149°, covered with 3D epicuticular waxes. On plant surfaces with hierarchical structuring (L. chinensis, abaxial leaf surface), contact angles are even higher, leading to superhydrophobicity. We found cuticular folds to have a strong impact on surface wettability and thereby on surface free energy. The difference in height between medium cuticular folds and high cuticular folds has no impact on the traction forces of insects, but wettability is further reduced.
A silvery layer of air on both H. brasiliensis (abaxial leaf surface) with high cuticular folds and L. chinensis (abaxial leaf surface) of hierarchical structuring with cuticular folds was observed while dipping the plant surfaces into water. We hypothesize that both high cuticular folds and hierarchical structuring with cuticular folds enable plant surfaces to keep a thin film of air due to their dimensions, reducing the contact area between water and the surface and leading to a Cassie and Baxter air-trapping wetting regime [25]. Both adhesion ability of male L. decemlineata and wettability have been found to be strongly affected by cuticular folds of different structuring. We hypothesize that in both cases a reduction of the real contact area due to the surface microstructure is a critical parameter. However, dimension and complexity (hierarchy) of the surface structuring have a different influence on insect adhesion and contact angles. To better understand the influence of cuticular folds of different structuring on the wettability of a surface, further investigations are needed.
Cuticular folds being part of the cuticle should be mechanically more stable to physical stresses on the surface than the superimposed 3D epicuticular waxes. Being of slightly larger dimensions, they represent a promising role model alternative to wax-like coating, with a high potential for the design of biomimetic anti-adhesive surfaces.
Acknowledgements
We thank the gardeners of the Botanic Garden Freiburg for cultivating the plants investigated, and the engineers and technicians of the Technical Workshop of the Institute for Biology II/III for the construction of the traction force measurement device. We also thank Dr Stefan Stoll for help with the statistical analysis and Dr Randall Cassada for correcting the English version of the manuscript. Financial support from the Baden-Württemberg Stiftung within the scope of the research programme ‘Neue Materialien aus der Bionik’ is gratefully acknowledged.
References
- 1.Barthlott W., Neinhuis C., Cutler D., Ditsch F., Meusel I., Theisen I., Wilhelmi H. 1998. Classification and terminology of plant epicuticular waxes. Bot. J. Linnean Soc. 126, 237–260 10.1111/j.1095-8339.1998.tb02529.x (doi:10.1111/j.1095-8339.1998.tb02529.x) [DOI] [Google Scholar]
- 2.Koch K., Bhushan B., Barthlott W. 2008. Diversity of structure, morphology and wetting of plant surfaces. Soft Matter 4, 1943–1963 10.1039/b804854a (doi:10.1039/b804854a) [DOI] [Google Scholar]
- 3.Barthlott W., Ehler N. 1977. Raster-Elektronenmikroskopie der Epidermis-Oberflächen von Spermatophyten. Tropische und Subtropische Pflanzenwelt 19, 105 [Google Scholar]
- 4.Gorb S. 2001. Attachment devices of insect cuticle. Dordrecht, The Netherlands: Kluwer Academic Publishers [Google Scholar]
- 5.Federle W., Rohrseitz K., Holldobler B. 2000. Attachment forces of ants measured with a centrifuge: better ‘wax-runners’ have a poorer attachment to a smooth surface. J. Exp. Biol. 203, 505–512 [DOI] [PubMed] [Google Scholar]
- 6.Gorb E. V., Hosoda N., Miksch C., Gorb S. N. 2010. Slippery pores: anti-adhesive effect of nanoporous substrates on the beetle attachment system. Proc. R. Soc. Interface 7, 1571–1579 10.1098/rsif.2010.0081 (doi:10.1098/rsif.2010.0081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorb E., Voigt D., Eigenbrode S. D., Gorb S. 2008. Attachment force of the beetle Cryptolaemus montrouzieri (Coleoptera, Coccinellidae) on leaflet surfaces of mutants of the pea Pisum sativum (Fabaceae) with regular and reduced wax coverage. Arthropod Plant Interact. 2, 247–259 10.1007/s11829-008-9049-0 (doi:10.1007/s11829-008-9049-0) [DOI] [Google Scholar]
- 8.Stork N. E. 1980. Role of waxblooms in preventing attachment to brassicas by the mustard beetle, Phaedon-Cochleariae. Entomol. Exp. Appl. 28, 100–107 10.1111/j.1570-7458.1980.tb02992.x (doi:10.1111/j.1570-7458.1980.tb02992.x) [DOI] [Google Scholar]
- 9.Atkin D. S. J., Hamilton R. J. 1982. The effects of plant waxes on insects. J. Nat. Prod. 45, 694–696 10.1021/np50024a007 (doi:10.1021/np50024a007) [DOI] [Google Scholar]
- 10.Federle W., Maschwitz U., Fiala B., Riederer M., Holldobler B. 1997. Slippery ant-plants and skilful climbers: selection and protection of specific ant partners by epicuticular wax blooms in Macaranga (Euphorbiaceae). Oecologia 112, 217–224 10.1007/s004420050303 (doi:10.1007/s004420050303) [DOI] [PubMed] [Google Scholar]
- 11.Gaume L., Gorb S., Rowe N. 2002. Function of epidermal surfaces in the trapping efficiency of Nepenthes alata pitchers. New Phytol. 156, 479–489 10.1046/j.1469-8137.2002.00530.x (doi:10.1046/j.1469-8137.2002.00530.x) [DOI] [PubMed] [Google Scholar]
- 12.Gorb E., Kastner V., Peressadko A., Arzt E., Gaume L., Rowe N., Gorb S. 2004. Structure and properties of the glandular surface in the digestive zone of the pitcher in the carnivorous plant Nepenthes ventrata and its role in insect trapping and retention. J. Exp. Biol. 207, 2947–2963 10.1242/jeb.01128 (doi:10.1242/jeb.01128) [DOI] [PubMed] [Google Scholar]
- 13.Gorb E., Haas K., Henrich A., Enders S., Barbakadze N., Gorb S. 2005. Composite structure of the crystalline epicuticular wax layer of the slippery zone in the pitchers of the carnivorous plant Nepenthes alata and its effect on insect attachment. J. Exp. Biol. 208, 4651–4662 10.1242/jeb.01939 (doi:10.1242/jeb.01939) [DOI] [PubMed] [Google Scholar]
- 14.Gorb E., Gorb S. 2009. Effects of surface topography chemistry of Rumex obtusifolius leaves on the attachment of the beetle Gastrophysa viridula. Entomol. Exp. Appl. 130, 222–228 10.1111/j.1570-7458.2008.00806.x (doi:10.1111/j.1570-7458.2008.00806.x) [DOI] [Google Scholar]
- 15.Gorb E. V., Gorb S. N. 2002. Attachment ability of the beetle Chrysolina fastuosa on various plant surfaces. Entomol. Exp. Appl. 105, 13–28 10.1046/j.1570-7458.2002.01028.x (doi:10.1046/j.1570-7458.2002.01028.x) [DOI] [Google Scholar]
- 16.Neinhuis C., Barthlott W. 1997. Characterization and distribution of water-repellent, self-cleaning plant surfaces. Ann. Bot. 79, 667–677 10.1006/anbo.1997.0400 (doi:10.1006/anbo.1997.0400) [DOI] [Google Scholar]
- 17.Furstner R., Barthlott W., Neinhuis C., Walzel P. 2005. Wetting and self-cleaning properties of artificial superhydrophobic surfaces. Langmuir 21, 956–961 10.1021/la0401011 (doi:10.1021/la0401011) [DOI] [PubMed] [Google Scholar]
- 18.Feng L., Zhang Y. A., Xi J. M., Zhu Y., Wang N., Xia F., Jiang L. 2008. Petal effect: a superhydrophobic state with high adhesive force. Langmuir 24, 4114–4119 10.1021/la703821h (doi:10.1021/la703821h) [DOI] [PubMed] [Google Scholar]
- 19.Whitney H. M., Kolle M., Andrew P., Chittka L., Steiner U., Glover B. J. 2009. Floral iridescence, produced by diffractive optics, acts as a cue for animal pollinators. Science 323, 130–133 10.1126/science.1166256 (doi:10.1126/science.1166256) [DOI] [PubMed] [Google Scholar]
- 20.Poppinga S., Koch K., Bohn H. F., Barthlott W. 2010. Comparative and functional morphology of hierarchically structured anti-adhesive surfaces in carnivorous plants and kettle trap flowers. Funct. Plant Biol. 37, 952–961 10.1071/FP10061 (doi:10.1071/FP10061) [DOI] [Google Scholar]
- 21.Neinhuis C., Edelmann H. G. 1996. Methanol as a rapid fixative for the investigation of plant surfaces by SEM. J. Microsc. 184, 14–16 10.1046/j.1365-2818.1996.d01-110.x (doi:10.1046/j.1365-2818.1996.d01-110.x) [DOI] [Google Scholar]
- 22.Voigt D., Schuppert J. M., Dattinger S., Gorb S. N. 2008. Sexual dimorphism in the attachment ability of the Colorado potato beetle Leptinotarsa decemlineata (Coleoptera: Chrysomelidae) to rough substrates. J. Insect Physiol. 54, 765–776 10.1016/j.jinsphys.2008.02.006 (doi:10.1016/j.jinsphys.2008.02.006) [DOI] [PubMed] [Google Scholar]
- 23.Scholz I., et al. 2010. Slippery surfaces of pitcher plants: Nepenthes wax crystals minimize insect attachment via microscopic surface roughness. J. Exp. Biol. 213, 1115–1125 10.1242/jeb.035618 (doi:10.1242/jeb.035618) [DOI] [PubMed] [Google Scholar]
- 24.Dai Z. D., Gorb S. N., Schwarz U. 2002. Roughness-dependent friction force of the tarsal claw system in the beetle Pachnoda marginata (Coleoptera, Scarabaeidae). J. Exp. Biol. 205, 2479–2488 [DOI] [PubMed] [Google Scholar]
- 25.Koch K., Bohn H. F., Barthlott W. 2009. Hierarchically sculptured plant surfaces and superhydrophobicity. Langmuir 25, 14116–14120 10.1021/la9017322 (doi:10.1021/la9017322) [DOI] [PubMed] [Google Scholar]