Abstract
The venom gland of predatory cone snails (Conus spp.), which secretes neurotoxic peptides that rapidly immobilize prey, is a proposed key innovation for facilitating the extraordinary feeding behaviour of these gastropod molluscs. Nevertheless, the unusual morphology of this gland has generated controversy about its evolutionary origin and possible homologues in other gastropods. I cultured feeding larvae of Conus lividus and cut serial histological sections through the developing foregut during larval and metamorphic stages to examine the development of the venom gland. Results support the hypothesis of homology between the venom gland and the mid-oesophageal gland of other gastropods. They also suggest that the mid-region of the gastropod foregut, like the anterior region, is divisible into dorsal and ventral developmental modules that have different morphological, functional and ontogenetic fates. In larvae of C. lividus, the ventral module of the middle foregut transformed into the anatomically novel venom gland of the post-metamorphic stage by rapidly pinching-off from the main dorsal channel of the mid-oesophagus, an epithelial remodelling process that may be similar to other cases where epithelial tubes and vesicles arise from a pre-existing epithelial sheet. The developmental remodelling mechanism could have facilitated an abrupt evolutionary transition to the derived morphology of this important gastropod feeding innovation.
Keywords: developmental modularity, evolvability, venom gland, metamorphosis, gastropod
1. Introduction
Cone snails, members of the genus Conus, are a remarkable group of predatory gastropod molluscs that have undergone spectacular speciation since the Upper Pliocene to produce over 500 extant species [1]. Predatory feeding is a derived feeding mode for gastropods, which has involved diverse types of complex modifications to the ancestral gastropod feeding system for herbivorous grazing [2–4]. Nevertheless, the feeding specializations of Conus and other conoideans extend well beyond those of most predatory gastropods [5]. Cone snails use a highly modified radular tooth, shaped like a hollow harpoon [6], and a ballistic mechanism [7,8] to inoculate their prey with a cocktail of small peptide neurotoxins that rapidly immobilize the prey (reviewed in [9]). The neurotoxins, which typically block ion channels and neurotransmitter receptors in neuronal cell membranes, and have been extensively studied for potential medical applications [9–11], are secreted by a long, narrow venom gland extending from the foregut. Although the neurotoxin-secreting venom gland has been targeted as the key innovation that facilitated the conoidean mode of feeding [12], the gland's highly unusual anatomical characteristics have generated controversy about its evolutionary derivation [2,3,13].
Feeding systems that are both complex and diverse, like the feeding systems of gastropod molluscs and many other animal groups, bring into focus a core issue for evolutionary biology: how can any component of a complex system change during evolution without disrupting the functional integrity of the whole [14–16]? This puzzle deepens when feeding systems evolve within the context of a biphasic life cycle, where post-metamorphic structures are built on the template of larval structures. In this situation, which is typical for most gastropods, functional requirements of the larval system could potentially limit evolutionary options for the post-metamorphic system [17]. An emerging paradigm for explaining evolutionary change within complex systems is developmental modularity [16,18–21], wherein components (modules) of functionally integrated systems develop relatively independently, so that individual modules can change without major disruption to the development of other modules within the whole. Modularity may be the essential link between development and evolvability of phenotype.
Previous studies on developing gastropod feeding systems identified a dorsal and ventral module for the anterior foregut, which each have distinct morphological, functional and ontogenetic fates [22–25]. The dorsal module forms first as the larval oesophagus, which in feeding larvae transports ingested algal cells to the stomach and becomes the dorsal food channel after metamorphosis. The ventral module begins development as a thickening and then out-pocketing of the ventral wall of the anterior larval oesophagus. The out-pocketing has no function in larvae, but its differentiation during the larval phase eventually forms the post-metamorphic radular apparatus. In the primitive condition, the radular apparatus is a ribbon of recurved teeth that rasps algae off substrates. Initial studies on the developing foregut of predatory gastropods with a feeding larva, such as the buccinid neogastropod Nassarius mendicus [25], have suggested that many derived features of the post-metamorphic foregut result from extensively modified development of the ventral module. Modifications of the ventral module can occur without interfering with transport of larval food via the ciliated channel of the dorsal module.
The present study focuses on the development of the venom gland in cone snails, which is a derived feature of the foregut's middle region, the mid-oesophagus, in the juvenile and adult stages. The gland arises immediately behind the radular apparatus as a long, narrow and convoluted tube of venom-secreting glandular epithelium, which terminates in a muscular bulb [26]. Past speculation has interpreted the cone snail venom gland as a highly derived salivary gland [2,27] or as a totally new structure [28], but it is most often regarded as a homologue of the mid-oesophageal gland of other gastropods [29–31]. In the least derived state, the gastropod mid-oesophageal gland consists of glandular epithelium forming the ventro-lateral walls of the mid-oesophagus [32]. However, the mid-oesophageal gland of most neogastropods, which is the larger group that includes the conoideans, typically has the form of a large ‘gland of Leiblein’ that arises further posteriorly along the mid-oesophagus. Ponder [3,13] suggested that the gland of Leiblein may be homologous with the muscular bulb only of the cone snail venom apparatus.
Over a century ago, Amaudrut [29] speculated that the cone snail venom gland evolved when the mid-oesophageal gland in an ancestor stripped away from the non-glandular dorsal zone, except for a retained connection a short distance behind the radular sac. Nevertheless, the only existing developmental study on the morphogenesis of the venom gland provided little support for this seemingly fanciful ‘stripping away’ hypothesis, in that the venom gland of Conus anemone appeared to originate as an out-pocketing of the larval oesophageal wall [33]. However, only the initial events of venom gland development were documented for this species, which has a derived life history that lacks a feeding larval stage [34].
I examined development through metamorphosis of the venom gland in Conus lividus HWASS IN BRUGUIÈRE, 1792, a species that has retained feeding larvae. Results corroborate the previously proposed homology between the venom gland and the mid-oesophageal gland of other gastropods and are consistent with an interpretation of dorsal and ventral developmental modules for the middle foregut region. They also demonstrate how a conventional process for morphogenetic modelling of epithelia can profoundly alter the morphological phenotype of the mid-oesophageal gland in adult cone snails.
2. Material and methods
(a). Source and culture of larvae
A cluster of egg capsules laid on the underside of a limestone boulder in shallow, near shore water off Oahu, Hawaii provided larvae for this study. The location is given as ‘station 9’ in Kohn's [35] survey of Hawaiian cone snails. Species identification as C. lividus HWASS IN BRUGUIÈRE, 1792, was confirmed by amplification of a portion of mitochondrial 16S rRNA extracted from larvae (T. F. Duda 2007, personal communication). Hatched larvae were cultured at 24–27°C in 1.0 or 2.0 l glass beakers of coarse-filtered sea water (Millipore pre-filter) at an initial density not exceeding 0.2 larvae ml−1. After two weeks of culture, density was reduced to not more than 50 larvae in 2 l. Larvae were fed a mix of Isochrysis galbana and Pavlova lutheri at 3 × 104 cells ml−1, increased to 5 × 104 cells ml−1 after two weeks. Algae were cultured under continuous illumination in Guillard's f/2 enrichment medium without silicates [36]. Larvae were transferred to clean culture beakers with freshly filtered sea water and algal food every second day by gentle sieving and hand pipetting. Streptomycin sulphate at 50 µg ml−1 was added once every one to two weeks.
(b). Histology and three-dimensional reconstructions
Larvae and metamorphic stages were anaesthetized, chemically fixed in phosphate-buffered glutaraldehyde followed by bicarbonate-buffered osmium tetroxide, and embedded in epoxy resin according to methods described previously [24]. Serial transverse sections were cut through the foregut at the following developmental stages: larvae at hatching and at 8, 13, 24, 31, 42 and 49 days post-hatching (one to four specimens each) and post-larvae at 6, 12, 24 and 48 h post velum loss and 3, 4 and 5 days post velum loss (two specimens each). Sections were cut at a thickness of 0.75–1.0 µm using a Diatome histoknife and were stained with a mix of methylene blue and azure II [37]. Digital images were acquired with a Retiga 2000R digital camera (QImaging) mounted on a Zeiss Axioskop compound microscope.
Three-dimensional reconstructions of serial sections through the foregut of a late stage larva at 49 days post-hatching and a post-larva at 48 h after the onset of metamorphosis were prepared using Reconstruct v. 1.1.00 [38]. Score marks on the specimen blocks assisted with alignment of sequential sections. Profiles of every second section (section thickness 1 µm) were manually traced from section images with a digitizing tablet, and the stack of traces for each specimen was reconstructed and surface rendered using a Boissant surfacing algorithm. Models of three-dimensional reconstructions at desired orientations were imported into Adobe Photoshop CS4 for surface smoothing and colouring the region of presumptive venom gland.
3. Results
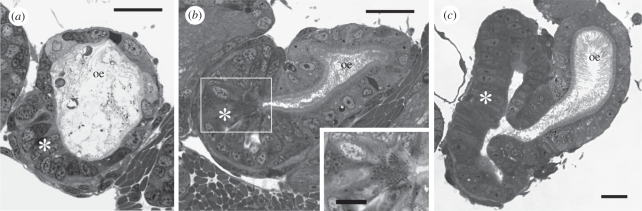
The rate of larval development varied, but the shortest period between hatching and metamorphosis was 42 days. The onset of metamorphosis was indicated by the sloughing of ciliated cells from the velum, the larval structure for swimming and capture of microalgal food. Histological sections showed the foregut of newly hatched larvae as a simple tube of ciliated cuboidal epithelium extending from mouth to stomach. At approximately four weeks after hatching, the presumptive radular apparatus was initiated as a thickening and then out-pocketing of the ventral wall of the anterior foregut (not shown). By contrast, the region of larval foregut posterior to the developing radular apparatus showed no obvious morphogenetic changes, other than growth, until the final days of larval development. During this late larval stage, epithelial cells running down the entire ventral zone of the foregut's mid-region began to enlarge and to stain more intensely (figure 1a). Continued hypertrophy of ventral zone cells gave the mid-region of the foregut (mid-oesophagus) a somewhat bilobed outline in sectioned profile; the two lobes corresponding to the dorsal and ventral zones (figure 1b). Some of the enlarged cells within the ventral zone accumulated many small, spherical granules (figure 1b, inset), a characteristic of venom gland cells in adult Conus californicus [26].
Figure 1.
Transverse sections through the mid-oesophagus (oe) of gastropod larvae. (a) Conus lividus larva 42 days after hatching showing the presumptive venom gland (asterisk) as enlarged cells extending down the ventral wall of the mid-oesophagus. (b) Conus lividus larva at or near metamorphic competence (49 days after hatching) showing further enlargement of presumptive venom gland cells (asterisk); boxed area enlarged in inset. Inset, detail of venom gland cells showing many small granules. (c) Marsenina stearnsii larva at or near metamorphic competence showing the ventral zone of enlarged cells (asterisk) that will become the mid-oesophageal gland at metamorphosis. Scale bars: (a) 20 µm; (b) 20 µm (inset 10 µm); (c) 20 µm.
Hypertrophy of a ventral zone of epithelial cells within the mid-foregut region was also observed in feeding larvae of several other species of gastropods. One example is shown in figure 1c, which is a section through the mid-foregut of a feeding larva of Marsenina stearnsii (Velutinidae). This feature was also seen in late stage feeding larvae of Euspira lewisii (Naticidae) and Crepipatella dorsalis (Calyptraeidae; not shown). In these three species, the hypertrophied ventral tissue became the mid-oesophageal gland following metamorphosis and the glandular epithelium remained confluent with the epithelium of the dorsal ciliated zone of the mid-oesophagus. The axial twist of the mid-region of the foregut, as evident in the three-dimensional reconstruction of a late stage larva of C. lividus shown in figure 2a and also seen in M. stearnsii, E. lewisii and C. dorsalis, is a characteristic of the mid-oesophageal region of gastropods and is attributed to ‘torsion’, a defining feature of the gastropod body plan.
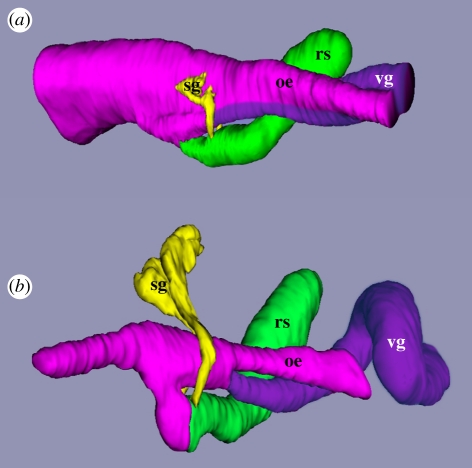
Figure 2.
Three dimensional reconstructions of transverse sections through the foregut of (a) a late-stage larva of Conus lividus and (b) a post-larva at 48 h after the onset of metamorphosis showing the separation of the venom gland (vg) from the mid-oesophagus (oe). Anterior is towards the left for both. The posterior extent of the reconstructions ends where the mid-oesophagus began to coil around the columella of the shell. Other abbreviations: rs, radular sac; sg, salivary glands.
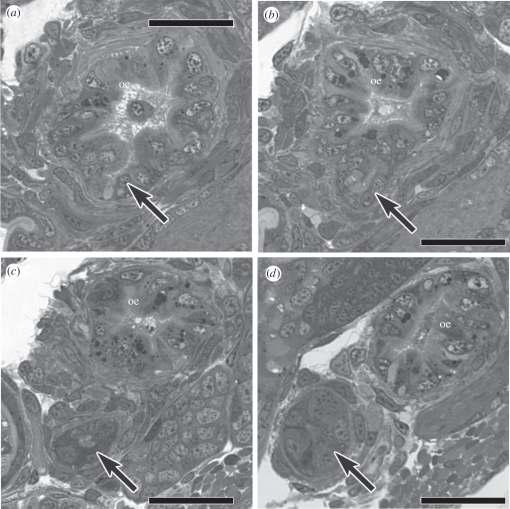
During metamorphosis of C. lividus, the ventral zone of hypertrophied epithelial cells pinched off from the dorsal zone so that two parallel epithelial tubes were formed (compare figure 2a,b). The tube of dorsal zone epithelium became the main channel of the internally ciliated mid-oesophagus of the post-metamorphic stage, whereas the tube of ventral zone epithelium became the blind-ending venom gland. The two channels retained a very narrow connection, a short distance posterior to the radular sac. The series of cross sections in figure 3a–d bracket the point of bifurcation between the dorsal (mid-oesophagus) and ventral channels (venom gland). The separation between the dorsal channel and venom gland had not occurred in specimens sectioned at 7 and 12 h after the onset of metamorphosis, but was evident in one of two specimens sectioned at 24 h and in all specimens sectioned at 2, 3, 4 and 5 days after the onset of metamorphosis.
Figure 3.
(a–d) Transverse sections through the mid-oesophagus (oe) of a post-larva of Conus lividus at 4 days after the onset of metamorphosis that bracket the small remaining connection between the dorsal channel of the mid-oesophagus and the venom gland (arrow), which has otherwise fully separated from the dorsal channel of the mid-oesophagus. Scale bars: (a–d) 25 µm.
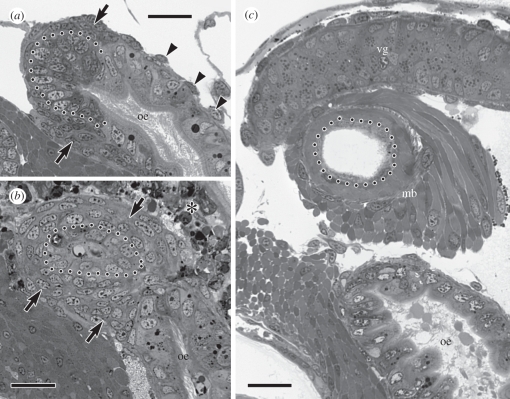
The muscular bulb that caps the terminal end of the venom gland is a conspicuous anatomical feature in post-metamorphic cone snails. In late stage larvae of C. lividus, myoblasts for the future muscular bulb were evident as an accumulation of small, undifferentiated cells at the posterior extremity of the prospective venom gland that formed the ventral wall of the mid-foregut (figure 4a). Immediately after metamorphic separation of the venom gland (at approx. 24 h after velum loss), the myoblasts had enlarged and had organized as a multi-layered sheath around the terminal end of the venom gland (figure 4b). Myofilaments were evident within these cells by 48 h after velum loss and specimens sectioned at 4 days after velum loss had what appeared to be a fully differentiated muscular bulb. The bulb consisted of at least two layers of muscle fibres, each having different fibre orientations, and the wall of the epithelial tube within the bulb had become squamous (figure 4c).
Figure 4.
Development of the muscular bulb of the venom apparatus; dotted line in all images demarcates the basal epithelial surface of the venom gland. (a) Oblique section through the mid-oesophagus (oe) as it begins to coil around the columella showing accumulation of myoblasts (arrows) around the posterior extremity of the future venom gland; arrowheads indicate nuclei of sparse oesophageal muscle cells. (b) Section through the same area of the mid-oesophagus at 24 h after velum loss showing recent separation of the venom gland from the mid-oesophagus and multi-layered myoblast sheath (arrows) around the terminus of the venom gland. The asterisk indicates degenerating cells of the velar lobes that have involuted into the head. (c) Section through the same area of the mid-oesophagus at 4 days after velum loss showing a secretory portion of the venom gland (vg) and the fully differentiated muscular bulb (mb). Scale bars: (a–c) 20 µm.
4. Discussion
This, to my knowledge, first histological study of venom gland development in a cone snail that retains the ancestral life history for the genus [34] supports the hypothesis of homology with the mid-oesophageal gland of other gastropods. Developmental correspondence has been traditionally viewed as an important criterion for recognizing homologues, together with correspondence in position and structure and consistency within a transformational series (reviewed in [39–41]). However, evidence that development can change during evolution without necessarily modifying final morphology has tempered the requirement for developmental correspondence when identifying homologues [16,19,40]. Nevertheless, although lack of developmental similarity does not disprove homology, developmental correspondence can certainly strengthen a homology hypothesis [40]. Developmental studies on two species of Conus, one with a feeding larval stage (present study) and one without [33] have now shown developmental correspondence between larval tissue that gives rise to the venom gland, and larval tissue in other gastropods that gives rise to the mid-oesophageal gland. Nevertheless, two features of metamorphic morphogenesis of the venom gland in C. lividus were extraordinary, when compared with metamorphosis of the mid-oesophageal gland in other gastropods: (i) the glandular epithelium pinched off from the dorsal oesophageal channel to form the blind-ending venom gland, and (ii) the terminal end of the separated venom gland became encapsulated with a thick muscle sheath.
Separation of the venom gland from the dorsal channel of the mid-foregut during metamorphosis of C. lividus appears similar to other cases where epithelial tubes or vesicles are generated from a pre-existing epithelial sheet during development. Familiar examples include formation of the hollow neural tube from the neural plate during primary neurulation of vertebrates and the generation of coelomic vesicles from the archenteron during echinoderm embryogenesis. All these occurrences may recruit a conserved battery of cellular and molecular mechanisms to accomplish the epithelial remodelling process. In the case of primary neurulation, which has received most research attention, the pinching-off of the neural tube is a multi-stage process that includes selective adhesion between the apices of epithelial cells and subsequent resorting of epithelial cell neighbours [42]. If separation of a tube of venom gland epithelium from the mid-oesophageal epithelium recruits a standard metazoan tool for morphogenetic remodelling of epithelia, then venom gland development in C. lividus illustrates how a simple change to a developmental module can have major consequences for final phenotype. A zone of glandular epithelium embedded within the wall of the mid-oesophagus becomes a tube of glandular epithelium that is almost completely separated from the lumen of the mid-oesophagus.
The only previous study on venom gland development in a species of cone snail was done on C. anemone, a species with a larval stage that does not feed [33]. Results suggested that the venom gland of C. anemone may be generated by out-pocketing of the ventral glandular region of the larval foregut, rather than by pinching-off of this epithelium. However, the developmental stages of C. anemone that were studied did not include metamorphosis. Results reported here on C. lividus showed that pinching-off of the glandular epithelium was initiated only after the onset of metamorphosis, suggesting that the metamorphic phase of C. anemone should be examined before concluding that these two species employ different developmental mechanisms to generate the post-metamorphic venom gland. It is clear, however, that the larvae of both C. anemone and C. lividus accumulate secretion granules within the presumptive venom gland prior to larval metamorphosis. If these granules are venom, then its manufacture within the late larval stage may facilitate rapid onset of predatory feeding after metamorphosis, which is known to occur at 4 days after metamorphic velum loss in Conus textile [43].
Information on foregut development is available for only a few species of gastropods and these meagre comparative data provide no clues about the evolutionary origin of the muscular bulb of the cone snail venom apparatus. On the basis of current information, the muscular bulb must be regarded as a new structure, possibly derived from muscle fibres investing the wall of the mid-oesophagus (figure 4a). Although the muscular bulb has been previously interpreted as a homologue of the gland of Leiblein in other neogastropods [3,13], there is currently no developmental support for this interpretation. Unlike the cells of the future venom gland, some of which displayed an advanced stage of cytodifferentiation prior to metamorphosis as indicated by the accumulation of secretion granules, cells of the prospective muscular bulb did not advance beyond a nest of undifferentiated myoblasts prior to metamorphosis. As a result, a rapid rate of myofilament synthesis during metamorphosis was required to produce a fully differentatiated muscular bulb by 4 days after the loss of ciliated cells of the velum.
Like the anterior portion of the gastropod foregut [23], the middle region may also be subdivided into dorsal and ventral modules that have distinct developmental programmes and phenotypic fates. The dorsal compartment is delegated to the essential but unremarkable task of conveying food to the stomach in both larval and post-metamorphic stages. By contrast, the ventral region, by way of different avenues of development, has assumed a variety of specialized post-metamorphic morphologies and functions related to different types of adult food and feeding methods. In many gastropods, the mid-oesophageal gland may simply secrete mucus to consolidate ingested food particles. In many neogastropods, the mid-oesophageal gland is elaborated as the gland of Leiblein, which may manufacture digestive enzymes and even phagocytize food material [32]. The venom gland of cone snails is a pinnacle of specialization for the mid-oesophageal gland, where the ventral module of the developing foregut has become a hollow, spaghetti-shaped organ for synthesizing hyperdiverse neurotoxic peptides that are delivered to prey by a mechanism that is still only partially understood [8]. The developmental process that generates this long, narrow gland in C. lividus corresponds remarkably well with the ‘stripping away’ hypothesis for evolutionary derivation of the Conus venom gland that Amaudrut [29] proposed over 100 years ago.
Acknowledgements
I thank Dr Michael Hadfield for his hospitality and generous provision of laboratory facilities at the Kewalo Marine Laboratory, University of Hawaii, where larvae were cultured. Dr Thomas Duda (Department of Ecology and Evolutionary Biology, University of Michigan) kindly sequenced a segment of mitochondrial 16S rRNA from larvae to confirm the species identification. Research funding from NSERC of Canada is gratefully acknowledged.
References
- 1.Kohn A. J. 1990. Tempo and mode of evolution in Conidae. Malacologia 32, 55–67 [Google Scholar]
- 2.Fretter V., Graham A. 1962. British prosobranch gastropods. London, UK: Ray Society [Google Scholar]
- 3.Ponder W. F. 1973. The origin and evolution of the Neogastropoda. Malacologia 12, 295–338 [PubMed] [Google Scholar]
- 4.Golding R. E., Ponder W. F., Byrne M. 2009. The evolutionary and biomechanical implications of snout and proboscis morphology in Caenogastropoda (Mollusca: Gastropoda). J. Nat. Hist. 43, 2723–2763 10.1080/00222930903219954 (doi:10.1080/00222930903219954) [DOI] [Google Scholar]
- 5.Taylor J. D., Kantor Y. I., Sysoev A. V. 1993. Foregut anatomy, feeding mechanisms, relationships and classification of the Conoidea (=Toxoglossa) (Gastropoda). Bull. Nat. Hist. Mus. Lond. 59, 125–170 [Google Scholar]
- 6.Kohn A. J., Nybakken J. W., Van Mol J. 1972. Radula tooth structure of the gastropod Conus imperialis elucidated by scanning electron microscopy. Science 176, 49–51 10.1126/science.176.4030.49 (doi:10.1126/science.176.4030.49) [DOI] [PubMed] [Google Scholar]
- 7.Schulz J. R., Norton A. G., Gilly W. F. 2004. The projectile tooth of a fish-hunting cone snail: Conus catus injects venom into fish prey using a high-speed ballistic mechanism. Biol. Bull. 207, 77–79 10.2307/1543581 (doi:10.2307/1543581) [DOI] [PubMed] [Google Scholar]
- 8.Salisbury S. M., Martin G. G., Kier W. M., Schulz J. R. 2010. Venom kinematics during prey capture in Conus: the biomechanics of a rapid injection system. J. Exp. Biol. 213, 673–682 10.1242/jeb.035550 (doi:10.1242/jeb.035550) [DOI] [PubMed] [Google Scholar]
- 9.Olivera B. M. 2006. Conus peptides: biodiversity-based discovery and exogenomics. J. Biol. Chem. 281, 31 173–31 177 10.1074/jbc.R600020200 (doi:10.1074/jbc.R600020200) [DOI] [PubMed] [Google Scholar]
- 10.Norton R. S., Olivera B. M. 2006. Conotoxins down under. Toxicon 48, 780–798 10.1016/j.toxicon.2006.07.022 (doi:10.1016/j.toxicon.2006.07.022) [DOI] [PubMed] [Google Scholar]
- 11.Han T. S., Teichert R. W., Olivera B. M., Bulaj G. 2008. Conus venoms: a rich source of peptide-based therapeutics. Curr. Pharm. Design 14, 2462–2479 10.2174/138161208785777469 (doi:10.2174/138161208785777469) [DOI] [PubMed] [Google Scholar]
- 12.Kantor Y. I. 1990. Anatomical basis for the origin and evolution of the toxoglossan mode of feeding. Malacologia 32, 3–18 [Google Scholar]
- 13.Ponder W. F. 1970. Some aspects of the morphology of four species of the neogastropod family Marginellidae with a discussion on the evolution of the toxoglossan poison gland. J. Malacol. Soc. Aust. 2, 55–81 [Google Scholar]
- 14.Lauder G. V., et al. 1989. Group report: how are feeding systems integrated and how have evolutionary innovations been introduced? In Complex organismal functions: integration and evolution in vertebrates (eds Wake D. B., Roth G.), pp. 97–115 Chichester, UK: Wiley & Sons [Google Scholar]
- 15.Wagner G. P., Altenberg L. 1996. Complex adaptations and the evolution of evolvability. Evolution 50, 967–976 10.2307/2410639 (doi:10.2307/2410639) [DOI] [PubMed] [Google Scholar]
- 16.Raff R. A. 1996. The shape of life: genes, development, and the evolution of animal form. Chicago, IL: University of Chicago Press [Google Scholar]
- 17.Wake D. B., Larsen A. 1987. Multidimensional analysis of an evolving lineage. Science 238, 42–48 10.1126/science.238.4823.42 (doi:10.1126/science.238.4823.42) [DOI] [PubMed] [Google Scholar]
- 18.Bolker J. A. 1999. Modularity in development and why it matters to evo-devo. Am. Zool 40, 770–776 10.1668/0003-1569(2000)040[0770:MIDAWI]2.0.CO;2 (doi:10.1668/0003-1569(2000)040[0770:MIDAWI]2.0.CO;2) [DOI] [Google Scholar]
- 19.Von Dassow G., Munro E. 1999. Modularity in animal development and evolution: elements of a conceptual framework for EvoDevo. J. Exp. Zool. (Mol. Dev. Evol.) 285, 307–325 (doi:10.1002/(SICI)1097-010X(19991215)285:4<307::AID-JEZ2>3.0.CO;2-V) [DOI] [PubMed] [Google Scholar]
- 20.Wagner G. P., Paclicev M., Cheverud J. M. 2007. The road to modularity. Nat. Rev. Genet. 8, 921–931 10.1038/nrg2267 (doi:10.1038/nrg2267) [DOI] [PubMed] [Google Scholar]
- 21.Yang A. 2001. Modularity, evolvability, and adaptive radiations: a comparison of the hemi- and holometabolous insects. Evol. Dev. 3, 59–72 10.1046/j.1525-142x.2001.003002059.x (doi:10.1046/j.1525-142x.2001.003002059.x) [DOI] [PubMed] [Google Scholar]
- 22.Fretter V. 1969. Aspects of metamorphosis in prosobranch gastropods. Proc. Malacol. Soc. Lond. 38, 375–385 [Google Scholar]
- 23.Page L. R. 2000. Development and evolution of the foregut in caenogastropods: overcoming larval functional constraints. Evol. Dev. 2, 25–34 10.1046/j.1525-142x.2000.00017.x (doi:10.1046/j.1525-142x.2000.00017.x) [DOI] [PubMed] [Google Scholar]
- 24.Page L. R. 2002. Larval and metamorphic development of the foregut and proboscis in the caenogastropod Marsenina (Lamellaria) stearnsii. J. Morphol. 252, 202–217 10.1002/jmor.1099 (doi:10.1002/jmor.1099) [DOI] [PubMed] [Google Scholar]
- 25.Page L. R. 2005. Development of foregut and proboscis in the buccinid neogastropod Nassarius mendicus: evolutionary opportunity exploited by a developmental module. J. Morphol. 264, 327–338 10.1002/jmor.10335 (doi:10.1002/jmor.10335) [DOI] [PubMed] [Google Scholar]
- 26.Marshall J., Kelley W. P., Rubakhin S. S., Bingham J.-P., Sweedler J. V., Gilly W. F. 2002. Anatomical correlates of venom production in Conus californicus. Biol. Bull. 203, 27–41 10.2307/1543455 (doi:10.2307/1543455) [DOI] [PubMed] [Google Scholar]
- 27.Alpers F. 1931. Zur Kenntnis der Anatomie von Conus lividus BRUG., besonders des Darmkanals. Jenaische Z. Nat. 65, 587–658 [Google Scholar]
- 28.Smith E. H. 1967. The proboscis and oesophagus of some British turrids. Trans. R. Soc. Edin. 67, 1–22 [Google Scholar]
- 29.Amaudrut A. 1898. La partie anteriéure du tube digestif et la torsion cheq les mollusques gastéropodes. Annal. Sci. Nat. Zool. 8, 1–291 [Google Scholar]
- 30.Shaw H. O. N. 1915. On the anatomy of Conus tulipa Linn. and Conus textile Linn. Q. J. Microsc. Sci. 60, 1–60 [Google Scholar]
- 31.Ponder W. F., Lindberg D. R. 1997. Towards a phylogeny of gastropod mollusks: an analysis using morphological characters. Zool. J. Linn. Soc. 119, 83–265 10.1111/j.1096-3642.1997.tb00137.x (doi:10.1111/j.1096-3642.1997.tb00137.x) [DOI] [Google Scholar]
- 32.Andrews E. B., Thorogood K. E. 2005. An ultrastructural study of the gland of Leiblein of muricid and nassariid neogastropods in relation to function, with a discussion on its homologies in other caenogastropods. J. Mollus. Stud. 71, 269–300 10.1093/mollus/eyi036 (doi:10.1093/mollus/eyi036) [DOI] [Google Scholar]
- 33.Ball A. D. 2002. Foregut ontogeny of the Neogastropoda: comparison of development in Nucella lapillus and Conus anemone. Boll. Malacologico Supplemento4, 51–78 [Google Scholar]
- 34.Duda T. F., Palumbi S. R. 1999. Developmental shifts and species selection in gastropods. Proc. Natl Acad. Sci. USA 96, 10 272–10 277 10.1073/pnas.96.18.10272 (doi:10.1073/pnas.96.18.10272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kohn A. J. 1959. The ecology of Conus in Hawaii. Ecol. Monogr. 29, 47–90 10.2307/1948541 (doi:10.2307/1948541) [DOI] [Google Scholar]
- 36.Guillard R. R. L. 1975. Culture of phytoplankton for feeding marine invertebrates. In Culture of marine invertebrate animals (eds Smith W. L., Chanley M. H.), pp. 26–60 New York, NY: Plenum [Google Scholar]
- 37.Richardson K. C., Jarrett L., Finke E. H. 1960. Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol. 35, 313–323 [DOI] [PubMed] [Google Scholar]
- 38.Fiala J. C. 2005. Reconstruct: a free editor for serial section microscopy. J. Microsc. 218, 52–61 10.1111/j.1365-2818.2005.01466.x (doi:10.1111/j.1365-2818.2005.01466.x) [DOI] [PubMed] [Google Scholar]
- 39.Roth V. L. 1984. On homology. Biol. J. Linn. Soc. 22, 13–29 10.1111/j.1095-8312.1984.tb00796.x (doi:10.1111/j.1095-8312.1984.tb00796.x) [DOI] [Google Scholar]
- 40.Hall B. K. 1999. Evolutionary developmental biology, 2nd edn. Dordrecht, The Netherlands: Kluwer Academic Publishers [Google Scholar]
- 41.Wagner G. P. 1989. The biological homology concept. Ann Rev. Ecol. Syst. 20, 51–69 10.1146/annurev.es.20.110189.000411 (doi:10.1146/annurev.es.20.110189.000411) [DOI] [Google Scholar]
- 42.Copp A. J., Greene N. D. E., Murdoch J. N. 2003. The genetic basis of mammalian neurulation. Nat. Rev. Genet. 4, 784–793 10.1038/nrg1181 (doi:10.1038/nrg1181) [DOI] [PubMed] [Google Scholar]
- 43.Perron F. E. 1980. Laboratory culture of the larvae of Conus textile Linne (Gastropoda: Toxoglossa). J. Exp. Mar. Biol. Ecol. 42, 27–38 10.1016/0022-0981(80)90164-1 (doi:10.1016/0022-0981(80)90164-1) [DOI] [Google Scholar]