Abstract
Life-history theory predicts that individuals should adjust their reproductive effort according to the expected fitness returns on investment. Because sexually selected male traits should provide honest information about male genetic or phenotypic quality, females may invest more when paired with attractive males. However, there is substantial disagreement in the literature whether such differential allocation is a general pattern. Using a comparative meta-regression approach, we show that female birds generally invest more into reproduction when paired with attractive males, both in terms of egg size and number as well as food provisioning. However, whereas females of species with bi-parental care tend to primarily increase the number of eggs when paired with attractive males, females of species with female-only care produce larger, but not more, eggs. These patterns may reflect adaptive differences in female allocation strategies arising from variation in the signal content of sexually selected male traits between systems of parental care. In contrast to reproductive effort, female allocation of immune-stimulants, anti-oxidants and androgens to the egg yolk was not consistently increased when mated to attractive males, which probably reflects the context-dependent costs and benefits of those yolk compounds to females and offspring.
Keywords: maternal effects, reproductive investment, differential allocation, parental care, birds, phenotypic plasticity
1. Introduction
Life-history theory predicts that species will evolve flexible reproductive strategies that allow individuals to adjust their reproductive effort in response to the expected returns on investment [1,2]. Of particular interest is the potential for females to invest more or less in relation to the phenotypic characters of her partner [3,4]. If females invest more into reproduction when paired with preferred males or males with highly expressed secondary sexual traits (both referred to as ‘attractive males’ hereafter), this pattern of allocation may increase offspring survival, growth or even reproductive performance (e.g. [5–12]). Such maternal effects not only confound estimates of direct or indirect benefits of mating with attractive males [13,14], but may also generate interesting evolutionary and ecological dynamics of both sexually and naturally selected phenotypes [15,16]. However, it is not clear when and how females should adjust their reproductive investment in response to sexually selected male traits [17–19]. Contrary to the prediction that females mated to attractive males should increase their investment, it has been argued that females may be favoured to increase investment when paired with unattractive males to compensate for poor offspring viability arising from, for example, inadequate paternal care or low paternal genetic quality or complementarity ([17,20] but see [18]).
Females may also adjust their reproductive effort in different ways, including behavioural adjustments (e.g. incubation effort and feeding rates) and resource allocation towards egg size or egg number. There is little theory to enable researchers to generate directional predictions with respect to aspects of reproductive investment that should show plasticity in response to male attractiveness [18,19]. However, patterns may differ across species according to the system of parental care. Because male ornamentation may reflect the ability to provide high-quality paternal care in species with male care (e.g. [21–24]) increased female investment in terms of clutch size may generate greater returns on investment than increasing egg size. On the contrary, in species with female-only care or where offspring are semi-independent at hatching, direct benefits of male attractiveness are less likely. The optimal returns on investment in the presence of indirect benefits may be better achieved via plasticity in egg size rather than egg number (e.g. to compensate for poor viability owing to genetic incompatibility [25]). Thus, both the aspect of reproductive effort that could be adjusted and the direction of the adjustment may vary across species according to mating or social systems [18,19].
More recent evidence also suggests that female birds not only adjust the size and number of their eggs across reproductive attempts, but also the content of those eggs (e.g. [26–28]). Yolk hormones and dietary compounds that are believed to have important immune or anti-oxidant functions, such as carotenoids, have received particular attention ([29–33]; reviewed in [34–36]). For example, Gil et al. [37] showed that female zebra finches increased the androgen content of their eggs when paired with males of artificially enhanced attractiveness (colour bands). Yolk androgens are now commonly measured in studies of differential allocation (e.g. [28,37,38]). However, it is not clear whether adjusting hormone or carotenoid content of eggs is subject to the same theoretical considerations as investment in terms of egg production or parental care. Firstly, there is little evidence that the small amounts of hormones and immune-stimulants (or anti-oxidants) typically found in egg yolk impose a cost on females that causes a trade-off between current and future reproduction (reviewed in [39]). Secondly, it is not obvious that ‘more is better’ [40]. On the contrary, the developmental effects of hormones are likely to be highly context- and sex-specific (e.g. [41–43]). Thus, the costs and benefits of increasing yolk hormone levels to offspring may vary more across environmental, social and developmental contexts than, for example, the costs and benefits of variation in egg size.
Here, we describe the results of a meta-analytical approach to address these issues. Our goals were fourfold. Firstly, we tested whether there was a general direction of female investment in response to male attractiveness across birds. Secondly, we examined whether the patterns differed depending on the stage of investment (egg production or feeding) and the nature of investment (egg size, egg number, yolk androgen content and immune-stimulants/anti-oxidants content of eggs), with the prediction that energetic investment should show a more consistent pattern of increased investment than would allocation of yolk micronutrients when females are paired with attractive males. Thirdly, we analysed whether the patterns differed depending on the system of parental care, with the expectation that species with bi-parental care should tend to increase the number of eggs to a greater extent than species with female-only care, where positive or negative differential allocation primarily should be manifested in terms of egg size. Finally, we looked at whether there was any evidence for a phylogenetic signal in patterns of differential allocation or differences owing to the experimental design.
2. Methods
We collected data from experimental studies that tested whether female allocation depends on the perceived attractiveness of the male. We searched for papers on ISI Web of Science using a series of key words such as ‘differential allocation’, ‘reproductive compensation’, ‘maternal investment’ and ‘male quality’ and by tracing citations to the landmark papers in the field (e.g. [3,4,17]). We subsequently searched all papers that were cited by those studies for additional papers. Finally, we also searched papers published online early in leading general journals (American Naturalist, Proc. R. Soc. Lond. B) and behavioural ecology journals (Animal Behaviour, Behavioural Ecology, Behavioural Ecology and Sociobiology) that tend to publish experimental studies of differential allocation. Studies typically measured egg size and clutch size, but some studies also investigated laying onset, post-hatching investment (e.g. feeding rate), yolk androgen content and proposed immune-stimulants or anti-oxidants (e.g. lysozymes, carotenoids and vitamins). We classified female investment as onset of laying, egg size (including egg mass), clutch size (number of eggs), yolk androgens, yolk immune-stimulants and post-hatching investment. The latter was quantified as feeding rate per chick or per brood. Studies that used the latter estimate typically experimentally or statistically controlled for clutch size (e.g. by using a single clutch size for all pairs) and we therefore pooled the two methods in our sample. We only included studies that experimentally manipulated male attractiveness or allocated males of different attractiveness randomly to females. This excluded some high-profile studies that did not control for non-random pairing with respect to male and female phenotypes, such as Burley's original study where the term differential allocation was coined ([3] see also [44]). However, this restriction on data collection is important as other designs do not allow one to disentangle effects of female reproductive plasticity and female quality owing to, for example, non-random pairing [4]. Furthermore, we only included studies that either assigned preferred or non-preferred males based on female preference trials (e.g. [25]) or for which the focal male trait had been shown (elsewhere or in the same paper) to be related to an estimate of male ‘quality’ (e.g. dominance and mating success) or experimentally demonstrated to be under sexual selection (e.g. via female choice). We classified the studies according to their experimental design in two ways; firstly, the experimental design by which females were exposed to males of different quality (i.e. randomized allocation of males, experimental manipulation after pairing, or within-female paired design where the same female was mated to attractive versus non-attractive males in a random order) and, secondly, whether the male trait was directly experimentally manipulated. We could not test for variation in female responses to different male traits (e.g. song and plumage colour) because of the low sample size per trait and strong covariance between the trait and species under study. Finally, we also collected data on the species to test whether reproductive investment varied according to the pattern of parental care (bi-parental versus female-only care; no species showed male-only care). The full dataset, consisting of 113 effect sizes, from 45 studies across 17 species, is provided as the electronic supplementary material.
Effect sizes were calculated from the available information in each paper, such as means and estimates of variance for treatment and controls, correlations, t-test or F-statistics, supplemented by additional information (e.g. direction of the effect) provided by authors whenever possible [45,46]. Where the design included experimental groups with means ± s.d. (or s.e.) reported for more than two levels (e.g. short tail, intermediate tail and long tail), we consistently calculated a single effect size based on the most extreme levels only as this is most consistent with theory (i.e. the strongest effect should be found when the difference in male quality is greatest). All effect sizes were converted into Fisher's Z (Zr) and its associated variance used for our meta-analytical procedures.
All statistical analyses were carried out in the R environment (v. 2.12.2) [47]. We adopted what is often referred to as a ‘meta-regression’ approach, which allows one to test the effects of multiple predictors, both categorical and continuous, in a single model (e.g. [48]). For meta-regression analysis, we used Bayesian generalized liner mixed-model, implemented in the R package, MCMCglmm [49]. We carried out meta-analytical models both without phylogeny (i.e. Bayesian mixed-effects meta-analysis, BMM) and with phylogeny (i.e. Bayesian phylogenetic mixed-effects meta-analysis, BPMM; see [50] for details). We employed mixed-effect models (BMM and BPMM) to deal with non-independence among data points originating from the same studies and the same species, and also BPMM to account for the lack of independence owing to the evolutionary history among 17 species in the dataset. In other words, for all BMM, we had study and species identities as random factors and for all BPMM, we had phylogeny in addition to study and species identities as random factors (estimating variance components of each of these random factors). A topology of a phylogenetic tree used for BPMM was constructed based on a collection of avian phylogenetic trees available at Bird Supertree Project (http://linnaeus.zoology.gla.ac.uk/~rpage/birdsupertree/) (see electronic supplementary material, figure S1). As an index of consistency (or heterogeneity), we assessed the proportion of the total variance (the total of all variance components in a model) accounted by a particular random factor (i.e. study, species and phylogeny). Such a proportion reflects heterogeneity at a particular level (e.g. study). Our four BMMs and four BPMMs were constructed according to our four goals outlined in §1 (table 1; see also the electronic supplementary material for details).
Table 1.
Meta-analytical models and their compositions (models 2, 4, 6 and 8 include phylogeny) along with corresponding deviance information criteria (DIC) and heterogeneity resulting from three random effects (the proportion of variance for a particular random factor in relation to the sum of all variance components); values for DIC and heterogeneity are the posterior modes (note that fixed effects estimates included in models 5 and 6 were omitted in models 7 and 8 as qualitative results remain the exactly the same in the corresponding models; for detailed results, see electronic supplementary material, tables S1–S4).
| model no. | moderators (fixed effects) | DIC | heterogeneity (%) (random effects) |
||
|---|---|---|---|---|---|
| study | species | phylogeny | |||
| model 1 | (intercept only) | −232.08 | 5.189 | 2.087 | — |
| model 2 | (intercept only) | −232.10 | 1.363 | 2.396 | 0.029 |
| model 3 | female traits | −207.71 | 5.227 | 1.916 | — |
| model 4 | female traits | −216.15 | 1.955 | 1.871 | 0.033 |
| model 5 | female traits, parental care types and their interactions | −258.01 | 3.337 | 1.963 | — |
| model 6 | female traits, parental care types and their interactions | −260.34 | 2.336 | 1.738 | 0.020 |
| model 7 | female traits, parental care types, their interactions, male traits and experimental designs | −254.72 | 4.952 | 1.832 | — |
| model 8 | female traits, parental care types, their interactions, male traits and experimental designs | −254.60 | 2.626 | 1.466 | 0.016 |
We also assessed signs of publication bias in our datasets by using funnel plots and Egger's regression [51]. The existence of publication bias (largely resulting from preferential publication of statistically significant results) can render meta-analytical results unreliable. More detailed statistical procedures are found in the electronic supplementary material.
3. Results
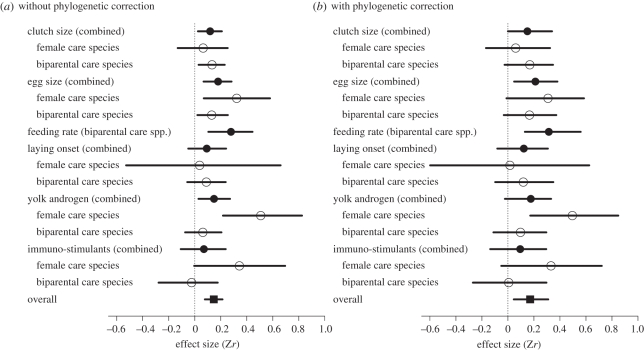
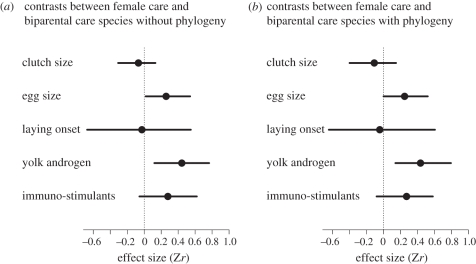
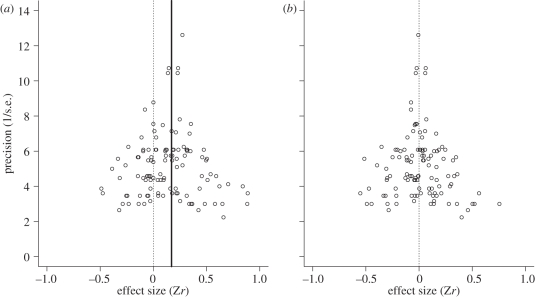
Regardless of whether or not phylogeny was taken into account, there was a significant positive effect of male attractiveness on female reproductive investment across studies (BMM: b(fixed effect estimate) = 0.146 (95% credible interval, CI: 0.078–0.213); BPMM: b = 0.171 (95% CI: 0.045–0.310); figure 1 and electronic supplementary material, table S1), although the effect can be considered small (the Zr values of approximately 0.10, 0.31 and 0.55 correspond with small, medium and large effect size, respectively, in Cohen's benchmarks; [52]). According to deviance information criterion, DIC, which is a Bayesian equivalent of more commonly used Akaike's information criterion, AIC [53], BPMM with female traits, parental care types and their interactions were the best model among the eight tested models (table 1). Highly energetically demanding allocation (i.e. clutch size, egg size and feeding rate) consistently showed a significant and expected overall effect regardless of phylogenetic corrections (BMM: b = 0.118 (95% CI: 0.025–0.208), b = 0.179 (95% CI: 0.068–0.283) and b = 0.278 (95% CI: 0.105–0.444), respectively; BPMM: b = 0.150 (95% CI: 0.0002–0.339), b = 0.211 (95% CI: 0.048–0.381) and b = 0.300 (95% CI: 0.131–0.559), respectively; figure 1 and electronic supplementary material, table S2), whereas yolk hormones and yolk immune-stimulants (including anti-oxidants) did not show a consistent significant overall effect (BMM: b = 0.148 (95% CI: 0.029–0.271) and b = 0.070 (95% CI: −0.108–0.238), respectively; BPMM, b = 0.176 (95% CI: −0.023–0.332) and b = 0.113 (95% CI: −0.136–0.295), respectively; figure 1 and electronic supplementary material, table S2). Furthermore, females of species with bi-parental care tended to respond to attractive males primarily by increasing the number of eggs rather than the size of eggs (BMM: b = 0.133 (95% CI: 0.030–0.230); BPMM: b = 0.168 (95% CI: −0.026–0.347); figure 1 and electronic supplementary material, table S3). Indeed, females of species with female-only care increased egg size to a significantly greater extent than did females of species with bi-parental care (estimated contrasts: BMM: b = 0.258 (95% CI: 0.018–0.541); BPMM: b = 0.246 (95% CI: 0.001–0.522); figure 2 and electronic supplementary material, table S3). There was also an accompanying significant contrast with respect to yolk androgens, with a significant positive effect of male attractiveness on androgen levels in species with female-only care, but not in species with bi-parental care (estimated contrasts: BMM: b = 0.442 (95% CI: 0.120–0.765); BPMM: b = 0.436 (95% CI: 0.141–0.796); figure 2 and electronic supplementary material, table S3). There was little evidence that experimental designs (how females were exposed to males and whether or not male traits were manipulated) significantly affected female investments (for estimates, see electronic supplementary material, table S4). Heterogeneity arising from studies, species and the phylogenetic relationship were low (all less than 6%; table 1 and electronic supplementary material, tables S1–S4), suggesting differences in effect sizes among studies and species, and from the phylogenetic relationship are relatively minor (cf. [54]). Also, there were little signs of publication bias after controlling for important modifiers, i.e. the types of female traits and the modes of parental care (figure 3 showing funnel plots) and the Egger's regression test supported the lack of publication bias in our dataset (b = −0.028 (95% CI: −0.119–0.072); the slope of the regression is not significantly different from zero, indicating little evidence for publication bias).
Figure 1.
A forest plot of meta-analytical results of models 1–6 (table 1; results from BMM shown in (a) and BPMM shown in (b)): models 1 and 2 (solid squares), models 3 and 4 (open circles) and models 5 and 6 (solid circles).
Figure 2.
A forest plot of contrast analysis from models 5 and 6 (table 1; results from BMM shown in (a) and BPMM shown in (b)).
Figure 3.
A funnel plot (effect sizes plotted against corresponding precision or the inverse of standard errors, s.e.) of (a) original data points with the meta-analytical mean from model 2 (table 1) and (b) residual points from model 6 (table 1).
4. Discussion
Our comparative meta-analysis showed small to moderate overall increases in costly female reproductive investment when exposed to (or mated with) attractive males across birds. Thus, female birds show substantial flexibility in their reproductive investment across contexts, both in terms of egg production and parental care. Despite some studies with results in the opposite direction [25,26,55], this overall result provides strong evidence in favour of the theoretical prediction that females generally should invest more into reproduction when mated with high-quality males, and that increased investment for low-quality or non-preferred males should only be favoured under restricted circumstances likely to be rare within and among species [4,18]. Importantly, greater investment in terms of egg size or feeding rate when paired with attractive males implies that studies that do not estimate or control for maternal investment will tend to overestimate the direct and indirect benefits of male attractiveness (e.g. ornament size) on offspring viability, perhaps even to the extent where ‘good genes’ effects are primarily driven by differential female allocation (reviewed in [14,56] see also [57]). Nevertheless, adaptive differential allocation requires that females gain direct or indirect benefits when mated to attractive males and that adjustment of reproductive effort in relation to those benefits maximizes lifetime reproductive success.
Burley's original formulation of differential allocation stressed that increased investment for high-quality males may be favoured if it encourages increased paternal care or maintains the pair bond with a high-quality male [3]. A positive relationship between male sexual ornamentation and paternal care has been found in some studies (e.g. [22,24]), including for species in our dataset (e.g. house sparrows [58]). However, empirical and theoretical studies show that this may not necessarily be a general pattern (e.g. [59–62]). If sexual traits honestly signal direct benefits in terms of care, this may suggest that female fitness would be best enhanced by producing larger clutches rather than larger eggs for more attractive males. The difference in allocation towards egg size versus number between species with bi-parental and female-only care is consistent with this hypothesis. However, interspecific variation in the relationship between male sexual traits and paternal care suggests that this explanation should apply only to some species. This warrants further exploration by empirically targeting additional species for which the relationship between male ornamentation and paternal care (positive or negative) is well established. Furthermore, since these adaptive scenarios invoke repeated behavioural interactions between males and females to determine parental care, which potentially is subject to sexual conflict, the predictions should be verified using models that incorporate the process by which decisions are made [19,63]. This approach would also help to assess whether the consistent positive effect of male attractiveness on female feeding rate in our dataset will be general or if it may depend on the signal content of male secondary sexual ornamentation.
The significant effect of male attractiveness on egg size (a costly investment; [64]) in species with female-only care is less easy to explain in adaptive terms unless there is significant additive genetic variance in fitness associated with male attractiveness (‘good genes’) or variation in offspring survival associated with genetic complementarity (that the average effect size of studies that directly manipulated male sexual traits did not differ from the effect sizes in studies that randomly allocated non-manipulated males to females suggests that male effects in birds are not the outcome of male manipulation analogous to the effect of seminal fluid in Drosophila [65]). Unfortunately, very few studies have estimated the effect of increased maternal investment on offspring fitness in birds (e.g. [66]), and disentangling genetic and maternal effects remains a challenge. Nevertheless, larger eggs tend to have positive effects on offspring survival and growth [11,67], although it should be noted that egg size may also vary with the amount of micro- and macro-nutrients that may be more important for variation in offspring development than egg size per se (e.g. [10]). Interestingly, despite the strong general direction of the effect in species with female care, one study of mallards found that females increased investment for non-preferred males [25], suggesting the fitness benefits (if any) of increasing investment when paired with attractive males may be context-dependent. Indeed, expected future reproductive returns will influence the cost-benefits of differential allocation and may cause, for example, younger females to invest more conservatively in response to male attractiveness than older females [18]. Unfortunately, age or context-dependencies on female investment in relation to male attractiveness have not yet been systematically addressed in experimental studies.
Although we have focused specifically on differences between species with bi-parental and female-only care, these could also be driven by differences between altricial and precocial life histories since they are largely overlapping in our dataset (and in birds in general; see [68,69]). However, we are not aware of any directional predictions in the differential allocation literature that specifically focus on whether the species is altricial or precocial (but see [70]). Furthermore, the scenarios remain speculations until the adaptive significance of the patterns of investment are explicitly tested; it is notable how few studies of differential allocation that estimate fitness of offspring [65,71,72]. Furthermore, to our knowledge, no study has attempted to estimate fitness of females that do versus that do not allocate resources differentially in relation to male attractiveness. We, therefore, suggest that research should move away from simply assessing patterns of female investment and towards studies that integrate manipulation of male attractiveness with estimation of offspring and female fitness (including the trade-off between current and future reproduction), preferably in ecologically relevant contexts (see e.g. [73,74], for a similar approach in the context of cooperative breeding).
Although our results support the prediction that costly resource investment would show a more consistent pattern than changes in yolk androgen, immune-stimulants and anti-oxidants, the strong effect of male attractiveness on yolk androgens in species with female-only care stands out. Despite that this result is based on only three species; houbara bustard (Chlamydotis undulata), grey partridge (Perdix perdix) and a particular strong effect in peacocks (Pavo cristatus), it may indicate a functional difference of yolk androgens between systems of parental care or, perhaps, along the altricial–precocial spectrum. For example, androgen levels in altricial species with bi-parental care typically vary substantially both within and between clutches. The within-clutch variation seems to be important for within-brood variation in begging behaviour [75,76] and may adaptively minimize the effect of hatching asynchrony or mediate parental feeding behaviour ([76,77]; review in [35]). Such within-brood effects are perhaps less likely in precocial species like peacocks, and may therefore enable evolution of strategies that involve more substantial between-brood variation in hormone levels. Importantly, the lack of a general effect in species with bi-parental care is opposite to that predicted by the theory that females manipulate paternal investment by adjusting the begging rates of their offspring [70]. Nevertheless, an increase in yolk androgens for females paired with attractive males has also been found in some species with bi-parental care, like the zebra finch [37], suggesting that adaptive allocation strategies may be quite complex or perhaps that the significance of yolk androgens for offspring fitness has been overestimated. For example, hormone levels may vary with egg size as a result of functional linkage between oocyte growth and accumulation of hormones [43,78], suggesting that high androgen levels in eggs produced by females exposed to high-quality males may largely be caused by changes in yolk size rather than ‘active’, adaptive, allocation of hormones per se (integration of oocyte growth and hormone accumulation may of course itself be adaptive). Finally, studies of house finches suggest that the accumulation of yolk androgens during oocyte growth may have causal effects on sex chromosome segregation ([43]; reviewed in [79]). If this is the case in any of the female-only care species, it raises the possibility that the adaptively significant trait in this context is sex ratio rather than yolk androgen. Studies of variation in yolk hormones and how such variation influences developmental trajectories across species with a wide range of life histories would be necessary to further our understanding of the causes and consequences of the comparative patterns revealed in this study.
In summary, female birds tend to increase reproductive investment when mated to attractive males. However, species differ with respect to whether they invest in terms of egg size or the number of eggs; species with female-only care tend to invest primarily in egg size. Adjustment of yolk androgens, immune-stimulants and anti-oxidants showed no consistent direction with respect to male attractiveness across birds, which probably reflect that they are not necessarily costly to females and that their benefits (and costs) are highly context-dependent. The patterns emerging from existing studies show that there is substantial flexibility in female reproductive investment in relation to male attractiveness. The challenge for behavioural and evolutionary ecologists is now to assess the adaptive significance of these patterns and their consequences for eco-evolutionary dynamics.
Acknowledgements
We are grateful to all researchers who took their time to provide additional information or unpublished data and an anonymous reviewer for helpful comments. S.N. is supported by the Marsden Fund and the University of Otago Research Fund.
References
- 1.Roff D. A. 1992. The evolution of life histories: theory and analysis, 2nd edn. New York, NY: Chapman & Hall [Google Scholar]
- 2.Roff D. A. 2002. Life history evolution. Sunderland, MA: Sinauer Associates Inc [Google Scholar]
- 3.Burley N. 1986. Sexual selection for aesthetic traits in species with biparental care. Am. Nat. 127, 415–445 10.1086/284493 (doi:10.1086/284493) [DOI] [Google Scholar]
- 4.Sheldon B. C. 2000. Differential allocation: test, mechanisms and implications. Trends Ecol. Evol. 15, 397–402 10.1016/S0169-5347(00)01953-4 (doi:10.1016/S0169-5347(00)01953-4) [DOI] [PubMed] [Google Scholar]
- 5.Forstmeier W., Leisler B. 2004. Repertoire size, sexual selection, and offspring viability in the great reed warbler: changing patterns in space and time. Behav. Ecol. 15, 555–563 10.1093/beheco/arh051 (doi:10.1093/beheco/arh051) [DOI] [Google Scholar]
- 6.Rubolini D., Romano M., Martinelli R., Saino N. 2006. Effects of elevated yolk testosterone levels on survival, growth and immunity of male and female yellow-legged gull chicks. Behav. Ecol. Sociobiol. 59, 344–352 10.1007/s00265-005-0057-0 (doi:10.1007/s00265-005-0057-0) [DOI] [Google Scholar]
- 7.Krist M. 2009. Short- and long-term effects of egg size and feeding frequency on offspring quality in the collared flycatcher (Ficedula albicollis). J. Anim. Ecol. 78, 907–918 10.1111/j.1365-2656.2009.01536.x (doi:10.1111/j.1365-2656.2009.01536.x) [DOI] [PubMed] [Google Scholar]
- 8.Partecke J., Schwabl H. 2008. Organizational effects of maternal testosterone on reproductive behavior of adult house sparrows. Dev. Neurobiol. 68, 1538–1548 10.1002/dneu.20676 (doi:10.1002/dneu.20676) [DOI] [PubMed] [Google Scholar]
- 9.Grüebler M. U., Naef-Daenzer B. 2010. Brood overlap and male ornamentation in the double-brooded barn swallow. Behav. Ecol. 21, 513–519 10.1093/beheco/arq017 (doi:10.1093/beheco/arq017) [DOI] [Google Scholar]
- 10.Reed W. L., Clark M. E., Vleck C. M. 2009. Maternal effects increase within-family variation in offspring survival. Am. Nat. 174, 685–695 10.1086/605962 (doi:10.1086/605962) [DOI] [PubMed] [Google Scholar]
- 11.Christians J. K. 2002. Avian egg size: variation within species and inflexibility within individuals. Biol. Rev. 77, 1–26 10.1017/S1464793101005784 (doi:10.1017/S1464793101005784) [DOI] [PubMed] [Google Scholar]
- 12.Clutton-Brock T. H. 1991. The evolution of parental care. Princeton, NJ: Princeton University Press [Google Scholar]
- 13.Gil D., Graves J. 2001. Differential allocation and ‘good genes’—comment from Gil & Graves. Trends Ecol. Evol. 16, 21–22 10.1016/S0169-5347(00)02017-6 (doi:10.1016/S0169-5347(00)02017-6) [DOI] [Google Scholar]
- 14.Qvarnström A., Price T. D. 2001. Maternal effects, paternal effects, and sexual selection. Trends Ecol. Evol. 16, 95–100 10.1016/S0169-5347(00)02063-2 (doi:10.1016/S0169-5347(00)02063-2) [DOI] [PubMed] [Google Scholar]
- 15.Moore A. J., Brodie E. D., III, Wolf J. B. 1997. Interacting phenotypes and the evolutionary process. I. Direct and indirect genetic effects of social interactions. Evolution 51, 1352–1362 10.2307/2411187 (doi:10.2307/2411187) [DOI] [PubMed] [Google Scholar]
- 16.Wolf J. B., Brodie E. D., III, Moore A. J. 1999. The role of maternal and paternal effects in the evolution of parental quality by sexual selection. J. Evol. Biol. 12, 1157–1167 10.1046/j.1420-9101.1999.00138.x (doi:10.1046/j.1420-9101.1999.00138.x) [DOI] [Google Scholar]
- 17.Gowaty P. A. 2008. Reproductive compensation. J. Evol. Biol. 21, 1189–1200 10.1111/j.1420-9101.2008.01559.x (doi:10.1111/j.1420-9101.2008.01559.x) [DOI] [PubMed] [Google Scholar]
- 18.Harris W. E., Uller T. 2009. Reproductive investment when mate quality varies: differential allocation versus reproductive compensation. Phil. Trans. R. Soc. B 364, 1039–1048 10.1098/rstb.2008.0299 (doi:10.1098/rstb.2008.0299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ratikainen I. I., Kokko H. 2010. Differential allocation and compensation: who deserves the silver spoon? Behav. Ecol. 21, 195–200 10.1093/beheco/arp168 (doi:10.1093/beheco/arp168) [DOI] [Google Scholar]
- 20.Gowaty P. A., Anderson W. W., Bluhm C. K., Drickamer L. C., Kim Y. K., Moore A. J. 2007. The hypothesis of reproductive compensation and its assumptions about mate preferences and offspring viability. Proc. Natl Acad. Sci. USA 104, 15 023–15 027 10.1073/pnas.0706622104 (doi:10.1073/pnas.0706622104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norris K. J. 1990. Female choice and the quality of parental care in the great tit Parus major. Behav. Ecol. Sociobiol. 27, 275–281 10.1007/BF00164900 (doi:10.1007/BF00164900) [DOI] [Google Scholar]
- 22.Hill G. E. 1991. Plumage coloration is a sexually selected indicator of male quality. Nature 350, 337–339 10.1038/350337a0 (doi:10.1038/350337a0) [DOI] [Google Scholar]
- 23.Palokangas P., Korpimäki E., Hakkarainen H., Huhta E., Tolonen P., Alatalo R. V. 1994. Female kestrels gain reproductive success by choosing brightly ornamented males. Anim. Behav. 47, 443–448 10.1006/anbe.1994.1058 (doi:10.1006/anbe.1994.1058) [DOI] [Google Scholar]
- 24.Buchanan K. L., Catchpole C. K. 2000. Song as an indicator of male parental effort in the sedge warbler. Proc. R. Soc. Lond. B 267, 321–326 10.1098/rspb.2000.1003 (doi:10.1098/rspb.2000.1003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bluhm C. K., Gowaty P. A. 2004. Reproductive compensation for offspring viability deficits by female mallards, Anas platyrhynchos. Anim. Behav. 68, 985–992 10.1016/j.anbehav.2004.01.012 (doi:10.1016/j.anbehav.2004.01.012) [DOI] [Google Scholar]
- 26.Saino N., Bertacche V., Ferrari R. P., Martinelli R., Møller A. P., Stradi R. 2002. Carotenoid concentration in barn swallow eggs is influenced by laying order, maternal infection and paternal ornamentation. Proc. R. Soc. Lond. B 269, 1729–1733 10.1098/rspb.2002.2088 (doi:10.1098/rspb.2002.2088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D'Alba L., Shawkey M. D., Korsten P., Vedder O., Kingma S. A., Komdeur J., Beissinger S. R. 2010. Differential deposition of antimicrobial proteins in blue tit (Cyanistes caeruleus) clutches by laying order and male attractiveness. Behav. Ecol. Sociobiol. 64, 1037–1045 10.1007/s00265-010-0919-y (doi:10.1007/s00265-010-0919-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kingma S. A., Komdeur J., Vedder O., von Engelhardt N., Korsten P., Groothuis T. G. G. 2009. Manipulation of male attractiveness induces rapid changes in avian maternal yolk androgen deposition. Behav. Ecol. 20, 172–179 10.1093/beheco/arn130 (doi:10.1093/beheco/arn130) [DOI] [Google Scholar]
- 29.Saino N., Dalĺara P., Møller A. P. 2002. Early maternal effects and antibacterial immune factors in the eggs, nestlings and adults of the barn swallow. J. Evol. Biol. 15, 735–743 10.1046/j.1420-9101.2002.00448.x (doi:10.1046/j.1420-9101.2002.00448.x) [DOI] [Google Scholar]
- 30.Isaksson C., Uller T., Andersson S. 2006. Parental effects on carotenoid-based plumage coloration in nestling great tits, Parus major. Behav. Ecol. Sociobiol. 60, 556–562 10.1007/s00265-006-0200-6 (doi:10.1007/s00265-006-0200-6) [DOI] [Google Scholar]
- 31.Hargitai R., Prechl J., Török J. 2006. Maternal immunoglobulin concentration in collared flycatcher (Ficedula albicollis) eggs in relation to parental quality and laying order. Funct. Ecol. 20, 829–838 10.1111/j.1365-2435.2006.01171.x (doi:10.1111/j.1365-2435.2006.01171.x) [DOI] [Google Scholar]
- 32.Romano M., Caprioli M., Ambrosini R., Rubolini D., Fasola M., Saino N. 2008. Maternal allocation strategies and differential effects of yolk carotenoids on the phenotype and viability of yellow-legged gull (Larus michahellis) chicks in relation to sex and laying order. J. Evol. Biol. 21, 1626–1640 10.1111/j.1420-9101.2008.01599.x (doi:10.1111/j.1420-9101.2008.01599.x) [DOI] [PubMed] [Google Scholar]
- 33.Bonisoli-Alquati A., Matteo A., Ambrosini R., Rubolini D., Romano M., Caprioli M., Dessì-Fulgheri F., Baratti M., Saino N. 2011. Effects of egg testosterone on female mate choice and male sexual behavior in the pheasant. Horm. Behav. 59, 75–82 10.1016/j.yhbeh.2010.10.013 (doi:10.1016/j.yhbeh.2010.10.013) [DOI] [PubMed] [Google Scholar]
- 34.Hasselquist D., Nilsson J. A. 2009. Maternal transfer of antibodies in vertebrates: trans-generational effects on offspring immunity. Phil. Trans. R. Soc. B 364, 51–60 10.1098/rstb.2008.0137 (doi:10.1098/rstb.2008.0137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Groothuis T. G. G., Eising C. M., Dijkstra C., Müller W. 2005. Balancing between costs and benefits of maternal hormone deposition in avian eggs. Biol. Lett. 1, 78–81 10.1098/rsbl.2004.0233 (doi:10.1098/rsbl.2004.0233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gil D. 2008. Hormones in avian eggs: physiology, ecology and behavior. Adv. Stud. Behav. 38, 337–398 10.1016/S0065-3454(08)00007-7 (doi:10.1016/S0065-3454(08)00007-7) [DOI] [Google Scholar]
- 37.Gil D., Graves J., Hazon N., Wells A. 1999. Male attractiveness and differential testosterone investment in zebra finch eggs. Science 286, 126–128 10.1126/science.286.5437.126 (doi:10.1126/science.286.5437.126) [DOI] [PubMed] [Google Scholar]
- 38.Loyau A., Saint Jalme M., Mauget R., Sorci G. 2007. Male sexual attractiveness affects the investment of maternal resources into the eggs in peafowl (Pavo cristatus). Behav. Ecol. Sociobiol. 61, 1043–1052 10.1007/s00265-006-0337-3 (doi:10.1007/s00265-006-0337-3) [DOI] [Google Scholar]
- 39.Groothuis T. G. G., Schwabl H. 2008. Hormone-mediated maternal effects in birds: mechanisms matter but what do we know of them? Phil. Trans. R. Soc. B 363, 1647–1661 10.1098/rstb.2007.0007 (doi:10.1098/rstb.2007.0007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carere C., Balthazart J. 2007. Sexual versus individual differentiation: the controversial role of avian maternal hormones. Trends Endocrinol. Metab. 18, 73–80 10.1016/j.tem.2007.01.003 (doi:10.1016/j.tem.2007.01.003) [DOI] [PubMed] [Google Scholar]
- 41.Saino N., Møller A. P., Bolzern A. M. 1995. Testosterone effects on the immune system and parasite infestations in the barn swallow (Hirundo rustica): an experimental test of the immunocompetence hypothesis. Behav. Ecol. 6, 397–404 10.1093/beheco/6.4.397 (doi:10.1093/beheco/6.4.397) [DOI] [Google Scholar]
- 42.Badyaev A. V., Duckworth R. A. 2003. Context-dependent sexual advertisement: plasticity in development of sexual ornamentation throughout the lifetime of a passerine bird. J. Evol. Biol. 16, 1065–1076 10.1046/j.1420-9101.2003.00628.x (doi:10.1046/j.1420-9101.2003.00628.x) [DOI] [PubMed] [Google Scholar]
- 43.Badyaev A. V., Young R. L., Hill G. E., Duckworth R. A. 2008. Evolution of sex-biased maternal effects in birds. IV. Intra-ovarian growth dynamics can link sex determination and sex-specific acquisition of resources. J. Evol. Biol. 21, 449–460 10.1111/j.1420-9101.2007.01498.x (doi:10.1111/j.1420-9101.2007.01498.x) [DOI] [PubMed] [Google Scholar]
- 44.Burley N. 1988. The differential allocation hypothesis: an experimental test. Am. Nat. 132, 612–628 10.1086/284877 (doi:10.1086/284877) [DOI] [Google Scholar]
- 45.Lipsey M. W., Wilson D. B. 2001. Practical meta-analysis. Thousand Oaks, CA: Sage [Google Scholar]
- 46.Nakagawa S., Cuthill I. C. 2007. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol. Rev. Camb. Phil. Soc. 82, 591–605 10.1111/j.1469-185X.2007.00027.x (doi:10.1111/j.1469-185X.2007.00027.x) [DOI] [PubMed] [Google Scholar]
- 47.R Development Core Team 2011. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 48.Jones K. S., Nakagawa S., Sheldon B. C. 2009. Environmental sensitivity in relation to size and sex in birds: meta-regression analysis. Am. Nat. 174, 122–133 10.1086/599299 (doi:10.1086/599299) [DOI] [PubMed] [Google Scholar]
- 49.Hadfield J. D. 2010. MCMC methods for multi-response generalised linear mixed models: the MCMCglmm R package. J. Stat. Softw. 33, 1–2220808728 [Google Scholar]
- 50.Hadfield J. D., Nakagawa S. 2010. General quantitative genetic methods for comparative biology: phylogenies, taxonomies and multi-trait models for continuous and categorical characters. J. Evol. Biol. 23, 494–508 10.1111/j.1420-9101.2009.01915.x (doi:10.1111/j.1420-9101.2009.01915.x) [DOI] [PubMed] [Google Scholar]
- 51.Egger M., Smith G. D., Schneider M., Minder C. 1997. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 315, 629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohen J. 1988. Statistical power analysis for the behavioral sciences, 2nd edn. Hillsdale, NJ: Lawrence Erlbaum Associates [Google Scholar]
- 53.Lunn D. J., Thomas A., Best N., Spiegelhalter D. 2000. WinBUGS—a Bayesian modelling framework: concepts, structure, and extensibility. Stat. Comput. 10, 325–337 10.1023/A:1008929526011 (doi:10.1023/A:1008929526011) [DOI] [Google Scholar]
- 54.Higgins J. P. T., Thompson S. G., Deeks J. J., Altman D. G. 2003. Measuring inconsistency in meta-analyses. BMJ 327, 557–560 10.1136/bmj.327.7414.557 (doi:10.1136/bmj.327.7414.557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bolund E., Schielzeth H., Forstmeier W. 2009. Compensatory investment in zebra finches: females lay larger eggs when paired to sexually unattractive males. Proc. R. Soc. B 276, 707–715 10.1098/rspb.2008.1251 (doi:10.1098/rspb.2008.1251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hettyey A., Hegyi G., Puurtinen M., Hoi H., Török J., Penn D. J. 2010. Mate choice for genetic benefits: time to put the pieces together. Ethology 116, 1–9 10.1111/j.1439-0310.2009.01704.x (doi:10.1111/j.1439-0310.2009.01704.x)21132114 [DOI] [Google Scholar]
- 57.Magrath M. J. L., Vedder O., van der Velde M., Komdeur J. 2009. Maternal effects contribute to the superior performance of extra-pair offspring. Curr. Biol. 19, 792–797 10.1016/j.cub.2009.03.068 (doi:10.1016/j.cub.2009.03.068) [DOI] [PubMed] [Google Scholar]
- 58.Voltura K. M., Schwagmeyer P. L., Mock D. W. 2002. Parental feeding rates in the house sparrow, Passer domesticus: are larger-badged males better fathers? Ethology 108, 1011–1022 10.1046/j.1439-0310.2002.00831.x (doi:10.1046/j.1439-0310.2002.00831.x) [DOI] [Google Scholar]
- 59.Kokko H. 1998. Should advertising parental care be honest? Proc. R. Soc. Lond. B 265, 1871–1878 10.1098/rspb.1998.0515 (doi:10.1098/rspb.1998.0515) [DOI] [Google Scholar]
- 60.Smiseth P. T., Örnborg J., Andersson S., Amundsen T. 2001. Is male plumage reflectance correlated with paternal care in bluethroats? Behav. Ecol. 12, 164–170 10.1093/beheco/12.2.164 (doi:10.1093/beheco/12.2.164) [DOI] [Google Scholar]
- 61.Badyaev A. V., Hill G. E. 2002. Paternal care as a conditional strategy: distinct reproductive tactics associated with elaboration of plumage ornamentation in the house finch. Behav. Ecol. 13, 591–597 10.1093/beheco/13.5.591 (doi:10.1093/beheco/13.5.591) [DOI] [Google Scholar]
- 62.Kelly N. B., Alonzo S. H. 2010. Does a trade-off between current reproductive success and survival affect the honesty of male signalling in species with male parental care? J. Evol. Biol. 23, 2461–2473 10.1111/j.1420-9101.2010.02111.x (doi:10.1111/j.1420-9101.2010.02111.x) [DOI] [PubMed] [Google Scholar]
- 63.Houston A. I., Székely T., McNamara J. M. 2005. Conflict between parents over care. Trends Ecol. Evol. 20, 33–38 10.1016/j.tree.2004.10.008 (doi:10.1016/j.tree.2004.10.008) [DOI] [PubMed] [Google Scholar]
- 64.Vézina F., Williams T. D. 2002. Metabolic costs of egg production in the European starling (Sturnus vulgaris). Physiol. Biochem. Zool. 75, 377–385 10.1086/343137 (doi:10.1086/343137) [DOI] [PubMed] [Google Scholar]
- 65.Chapman T., Liddle L. F., Kalb J. M., Wolfner M. F., Partridge L. 1995. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature 373, 241–244 10.1038/373241a0 (doi:10.1038/373241a0) [DOI] [PubMed] [Google Scholar]
- 66.Cunningham E. J. A., Russell A. F. 2000. Egg investment is influenced by male attractiveness in the mallard. Nature 404, 74–77 10.1038/35003565 (doi:10.1038/35003565) [DOI] [PubMed] [Google Scholar]
- 67.Krist M. In press Egg size and offspring quality: a meta-analysis in birds. Biol. Rev. (doi:10.1111/j.1469-185X.2010.00166.x) [DOI] [PubMed] [Google Scholar]
- 68.Starck J. M., Ricklefs R. E. 1998. Avian growth and development. Evolution within the altricial precocial spectrum. New York, NY: Oxford University Press [Google Scholar]
- 69.Cockburn A. 2006. Prevalence of different modes of parental care in birds. Proc. R. Soc. B 273, 1375–1383 10.1098/rspb.2005.3458 (doi:10.1098/rspb.2005.3458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Müller W., Lessells C. M., Korsten P., von Engelhardt N. 2007. Manipulative signals in family conflict? On the function of maternal yolk hormones in birds. Am. Nat. 169, 84–96 10.1086/511962 (doi:10.1086/511962) [DOI] [PubMed] [Google Scholar]
- 71.Gilbert L., Williamson K. A., Hazon N., Graves J. A. 2006. Maternal effects due to male attractiveness affect offspring development in the zebra finch. Proc. R. Soc. B 273, 1765–1771 10.1098/rspb.2006.3520 (doi:10.1098/rspb.2006.3520) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oksanen T. A., Alatalo R. V., Horne T. J., Koskela E., Mappes J., Mappes T. 1999. Maternal effort and male quality in the bank vole, Clethrionomys glareolus. Proc. R. Soc. Lond. B 266, 1495–1499 10.1098/rspb.1999.0806 (doi:10.1098/rspb.1999.0806) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Russell A. F., Langmore N. E., Cockburn A., Astheimer L. B., Kilner R. M. 2007. Reduced egg investment can conceal helper effects in cooperatively breeding birds. Science 317, 941–944 10.1126/science.1146037 (doi:10.1126/science.1146037) [DOI] [PubMed] [Google Scholar]
- 74.Russell A. F., Young A. J., Spong G., Jordan N. R., Clutton-Brock T. H. 2007. Helpers increase the reproductive potential of offspring in cooperative meerkats. Proc. R. Soc. B 274, 513–520 10.1098/rspb.2006.3698 (doi:10.1098/rspb.2006.3698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eising C. M., Eikenaar C., Schwabl H., Groothuis T. G. G. 2001. Maternal androgens in black-headed gull (Larus ridibundus) eggs: consequences for chick development. Proc. R. Soc. Lond. B 268, 839–846 10.1098/rspb.2001.1594 (doi:10.1098/rspb.2001.1594) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eising C. M., Groothuis T. G. G. 2003. Yolk androgens and begging behaviour in black-headed gull chicks: an experimental field study. Anim. Behav. 66, 1027–1034 10.1006/anbe.2003.2287 (doi:10.1006/anbe.2003.2287) [DOI] [Google Scholar]
- 77.Schwabl H. 1996. Maternal testosterone in the avian egg enhances postnatal growth. Comp. Biochem. Physiol. A Physiol. 114, 271–276 10.1016/0300-9629(96)00009-6 (doi:10.1016/0300-9629(96)00009-6) [DOI] [PubMed] [Google Scholar]
- 78.Badyaev A. V., Seaman D. A., Navara K. J., Hill G. E., Mendonca M. T. 2006. Evolution of sex-biased maternal effects in birds: III. Adjustment of ovulation order can enable sex-specific allocation of hormones, carotenoids, and vitamins. J. Evol. Biol. 19, 1044–1057 10.1111/j.1420-9101.2006.01106.x (doi:10.1111/j.1420-9101.2006.01106.x) [DOI] [PubMed] [Google Scholar]
- 79.Uller T., Badyaev A. V. 2009. Evolution of ‘determinants’ in sex determination: a novel hypothesis for the origin of environmental contingencies in avian sex bias. Semin. Cell Dev. Biol. 20, 304–312 10.1016/j.semcdb.2008.11.013 (doi:10.1016/j.semcdb.2008.11.013) [DOI] [PubMed] [Google Scholar]