Abstract
Polychlorinated biphenyls (PCBs), persistent chemicals widely used for industrial purposes, have been banned in most parts of the world for decades. Owing to their bioaccumulative nature, PCBs are still found in high concentrations in marine mammals, particularly those that occupy upper trophic positions. While PCB-related health effects have been well-documented in some mammals, studies among dolphins and whales are limited. We conducted health evaluations of bottlenose dolphins (Tursiops truncatus) near a site on the Georgia, United States coast heavily contaminated by Aroclor 1268, an uncommon PCB mixture primarily comprised of octa- through deca-chlorobiphenyl congeners. A high proportion (26%) of sampled dolphins suffered anaemia, a finding previously reported from primate laboratory studies using high doses of a more common PCB mixture, Aroclor 1254. In addition, the dolphins showed reduced thyroid hormone levels and total thyroxine, free thyroxine and triiodothyronine negatively correlated with PCB concentration measured in blubber (p = 0.039, < 0.001, 0.009, respectively). Similarly, T-lymphocyte proliferation and indices of innate immunity decreased with blubber PCB concentration, suggesting an increased susceptibility to infectious disease. Other persistent contaminants such as DDT which could potentially confound results were similar in the Georgia dolphins when compared with previously sampled reference sites, and therefore probably did not contribute to the observed correlations. Our results clearly demonstrate that dolphins are vulnerable to PCB-related toxic effects, at least partially mediated through the endocrine system. The severity of the effects suggests that the PCB mixture to which the Georgia dolphins were exposed has substantial toxic potential and further studies are warranted to elucidate mechanisms and potential impacts on other top-level predators, including humans, who regularly consume fish from the same marine waters.
Keywords: endocrine disruptors, polychlorinated biphenyls, immune suppression, thyroid, marine mammals
1. Introduction
Polychlorinated biphenyls (PCBs), persistent chemicals widely used for a variety of industrial purposes until banned in the late 1970s, are ubiquitous environmental contaminants associated with a broad range of toxic effects. Experimental as well as epidemiological studies have provided evidence that PCBs can adversely impact immune, endocrine and reproductive systems [1–3]. Exposure to environmental PCBs has been linked to detrimental impacts on wildlife and monitoring of sentinel populations has been proposed to warn of potential human exposure risk as well as to provide insight into potential human health effects [4].
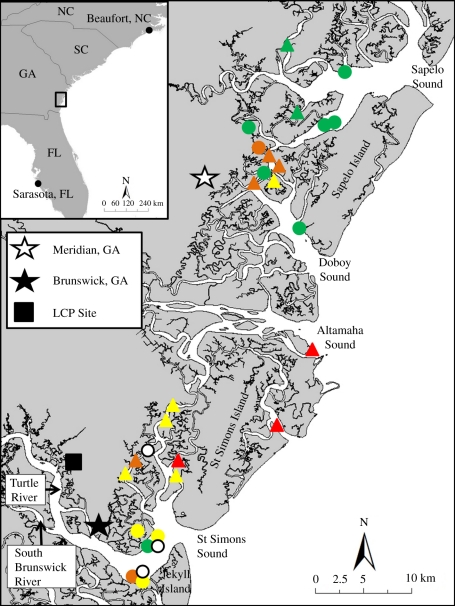
Extreme concentrations, up to 2900 mg kg−1 lipid, of PCBs were documented in bottlenose dolphins along the southern coast of Georgia (GA), United States of America (USA) [5,6]. The PCBs exhibit a distinct pattern of highly chlorinated octa- through deca-chlorobiphenyl congeners, characteristic of Aroclor 1268, an uncommon technical PCB mixture. Aroclor 1268 was used at a former chlor-alkali facility, Linden Chemicals and Plastics (LCP) located on the banks of the Turtle/Brunswick River Estuary (TBRE), near Brunswick, GA, USA (figure 1). High concentrations of PCBs characteristic of Aroclor 1268 were also reported in soil, marsh sediments and marine biota sampled in close proximity to the LCP site [7,8]. However, the PCB concentrations reported in bottlenose dolphins, an apex predator in the TBRE, are among the highest ever reported in wildlife [6]. Even more concerning is that the high PCB concentrations are not localized to the TBRE. Dolphins sampled approximately 40 km northeast near the Sapelo Island National Estuarine Research Reserve (NERR; figure 1) were also found to have PCB concentrations at or above the highest levels reported for dolphins near urban centres such as Biscayne Bay, Florida and Charleston, South Carolina [9,10].
Figure 1.
Map of area with dolphin capture locations and PCB concentration (µg g−1 lipid weight) measured in blubber. Circles represent females, triangles represent males. Dolphins sampled in Altamaha Sound and north were categorized as ‘Sapelo’; dolphins sampled south of the Altamaha were categorized as ‘Brunswick’. PCB µg g−1, white symbols, no data; green symbols, 0–100; yellow symbols, 101–200; orange symbols, 201–300; red symbols, >301.
Several infectious disease epidemics resulting in large-scale mortalities have occurred in dolphin populations with high PCB and other persistent organic pollutants (POP) levels [11,12], suggesting, but not demonstrating, a causal link between POP exposure, immune function and susceptibility to disease. While suppressed immune function has been well documented in some marine mammals through experimental feeding [13], health impacts in cetacean species have been more difficult to elucidate owing to ethical and logistical considerations. An early study found decreased immune responses associated with blood concentration of PCBs and DDT in a wild dolphin population near Sarasota, Florida, but the sample size was small (n = 5) and the study did not control for confounding factors such as age [14]. A more recent and robust study of harbour porpoises (Phoecoena phocoena) quantified the increased risk of infectious disease mortality in association with PCB exposure [15]. However, because the study relied on stranded and by-caught (i.e. dead) porpoises, the mechanisms of toxicity associated with the infection and mortality risk could not be determined.
The highly exposed dolphins from the Georgia coast provide an opportunity to elucidate potential toxic mechanisms and effects of chronic PCB exposure in cetaceans. The potential adverse health effects in the dolphins could have further significance for understanding potential human health impacts related to exposure to an uncommon, highly chlorinated Aroclor mixture. This study details the results of a capture-release health assessment of dolphins from the Georgia coast to examine potential detrimental health effects associated with the extreme PCB exposure.
2. Methods
(a). Dolphin capture and processing
Dolphin sampling was conducted over a two week period, 3–14 August 2009. For the first week, operations were based out of Meridian, Georgia; sampling was concentrated in waters near Sapelo Island NERR (figure 1). The base of operations was moved to Brunswick, Georgia for the second week of the fieldwork and sampling was concentrated around the TBRE.
Methods for dolphin capture-release have been previously described [16,17]. Briefly, dolphins were encircled with a net (366 m long, 7 m deep and 22 cm stretch mesh seine net) and then restrained by handlers. Female dolphins greater than or equal to 220 cm were held in the water until an ultrasound could be conducted to assess pregnancy status. Only non-pregnant females and males were brought aboard a specially designed processing boat for physical examination, weighing and morphometric measurement and diagnostic sampling. Pregnant females were not taken on board the boat and instead an abbreviated in-water examination and sampling was conducted. Standardized data collection was conducted as previously described [16,17].
Blood was drawn from ventral fluke vasculature as described in Schwacke et al. [16]. Whole blood was kept cool, wrapped and shipped overnight on ice packs to the University of Connecticut for immunological testing. Whole blood for haematology was immediately stored in an onboard cooler until the end of each day when samples were shipped overnight to the Animal Health Diagnostic Laboratory (AHDL) at Cornell University, Ithaca, NY, USA.
For serum biochemistry and hormone analysis, blood samples were allowed to sit at room temperature until clot formation (approx. 30 min) prior to being centrifuged. Serum was then transferred into cryovials and kept cool until the end of each day when biochemistry samples were shipped overnight on ice packs to the AHDL at Cornell University. Serum for hormone analysis was frozen at −80°C and shipped to AHDL at the end of the two week field period.
Surgical biopsies of skin and blubber were taken for contaminant analysis as described by Wells et al. [18]. A single tooth was extracted under local anaesthesia from some of the dolphins to determine age. Teeth were not taken from dolphins that were apparently very young (less than or equal to 220 cm) or pregnant. In addition, when the lead veterinarian felt that a dolphin was becoming overly stressed, as evidenced by rapid respirations (greater than 8 breaths min−1) or arching, the dolphin was released and a tooth was not taken. Two dolphins were also released early owing to approaching thunderstorms. Teeth were prepared for sectioning using standard procedures [19].
(b). Laboratory analyses
(i). Chemical analysis
POP including 55 PCB congeners, seven brominated diphenyl ether (BDE) congeners and a suite of organochlorine pesticides were measured in blubber. Methods are described in detail elsewhere [10] and are summarized in the electronic supplementary material.
(ii). Functional immune assays
Isolation of leukocytes was performed prior to the evaluation of all immune functions and is described in the electronic supplementary material. Neutrophil and monocyte phagocytosis were evaluated in vitro as previously described [20]. Phagocytosis was reported as the percentage of cells that had phagocytized one or more (1+), two or more (2+) and three or more beads (3+). The percentage of cells that phagocytized 3+ beads was used as the endpoint for statistical analysis.
Mitogen-induced lymphocyte proliferation was evaluated in vitro as previously described [21]; Concanavalin A (Con A) and lipopolysaccharide (LPS) were used as T-cell and B-cell mitogens, respectively. A stimulation index was calculated as the per cent increase in proliferation in the mitogen-stimulated cells when compared with the unstimulated cells. Further details of immunological assays can be found in the electronic supplementary material.
For quality control purposes, functional immune assays were performed on B6C3F1 mice in parallel with dolphin samples on each day assays were performed. Mouse data were then analysed for outliers to detect and eliminate experiments for which the variability was greater than expected for any technical reason. This quality control programme ensured that technical errors on one given day would not translate in misinterpretation of the data for several dolphins run on that day. Mouse data were also used to facilitate comparisons of samples analysed over time using different reagent batches.
(c). Data analysis
Dolphins were categorized into age classes by applying criteria as previously described [16].
(i). Comparison with baseline values
Haematological and serum biochemical values were compared with reference intervals for the appropriate age class established from previously sampled wild dolphin populations [22]. Appropriate reference thresholds were not available for endocrine parameters. Therefore, data from two independent dolphin health assessment studies were obtained and used for comparison. Total thyroxine (TT4), free thyroxine (FT4) and total triiodothyronine (TT3) measures were obtained from dolphin health assessment studies near Beaufort, NC, USA (n = 14) conducted in April 2006 and Sarasota Bay, FL, USA (n = 63), conducted in June 2005, June 2006, May 2008 and May 2009. Protocols for data and sample collection were standardized across the studies, and hormone analysis was performed by the same laboratory at Cornell University. However, the laboratory changed reagents for the measurement of TT3 in August 2006, because the production of the previously used radioimmunoassay was discontinued (see the electronic supplementary material). This may have introduced bias for comparisons of TT3 measurements before and after this date. Inter-assay comparison data collected by the laboratory using reference samples were used to calculate a conversion equation to allow comparison of values analysed using the two different assays. The equation and regression statistics for the inter-assay comparison are given in the electronic supplementary material. The conversion equation was used to adjust dolphin TT3 measurements that were obtained prior to August 2006.
An analysis of variance (ANOVA) was applied to test for differences in TT4, FT4 and adjusted TT3 among sites. Age class was also included as a factor in the model. When a significant difference was determined among sites, a follow-up analysis was conducted to specifically test for differences between the two reference sites and the two Georgia sites (Sarasota and Beaufort versus Sapelo Island and Brunswick) using an a priori linear contrast. A critical value α = 0.05 was used to assess statistical significance.
(ii). Relationship of endocrine, immune parameters and PCB concentration
An analysis of covariance (ANCOVA) was conducted to examine correlation of serum thyroid hormones (TT4, FT4 and TT3) and functional immune indices (Con A stimulation, LPS stimulation, neutrophil phagocytosis and monocyte phagocytosis) with measured blubber PCB concentration. PCBs are lipid-soluble compounds that are readily passed from a lactating female to her nursing offspring [23]. Thus, PCB concentrations measured in adult female dolphins are generally not meaningful indicators of cumulative exposure. For this reason, adult females was excluded from the ANCOVA.
Endocrine data from the reference sites for which concurrent blubber PCB measurements were available were also included in the regression analysis. This included seven and 11 samples from Beaufort and Sarasota, respectively. The seven Beaufort dolphins also had matching functional immune data, which were included in the regression analysis for immune indices. Chemical analysis for the blubber samples from Sarasota and Beaufort was conducted by the same laboratory and the same suite of congeners was summed to derive total PCBs. Age class and sampling site were included as categorical covariates in the ANCOVA; total PCBs (sum of 55 congeners) expressed on a lipid weight basis was included as a continuous covariate.
Data were examined for outlying values and extreme outliers were removed prior to analysis. Details are described in the electronic supplementary material.
Prior to combining the functional immune data from Beaufort collected in 2006 with the data from Brunswick and Sapelo collected in 2009, a one-way ANOVA was performed to compare the mouse control data between the 2 years. We found that mouse proliferation measured in 2006 in conjunction with the Beaufort dolphin samples were significantly higher than the mouse proliferation measures that were made in conjunction with the 2009 Georgia dolphin samples. This is probably owing to slight differences in lots of reagents, kits and supplies across years. Therefore, to avoid the introduction of potential laboratory bias, dolphin data were normalized to the concurrent proliferation measures in mice.
Recent research has indicated a relationship between T-lymphocyte proliferation and TT3 levels [24] so an ANCOVA was also conducted to examine the association of TT3 with the Con A stimulation index.
3. Results
During the 10 day capture-release health assessment, 29 dolphins were captured, examined and sampled (figure 1). The number of dolphins sampled, stratified by site and age-class are provided in the electronic supplementary material. A tooth was obtained from 13 dolphins for age determination, and six females were determined to be pregnant.
Several of the captured dolphins were found to be unusually small for their estimated age. One male whose age was later estimated to be 11 years was only 224 cm. The median length for the adult males (estimated to be at least 10 years of age by dentinal analysis) was only 242 cm. In comparison, the asymptotic length for male dolphins sampled from South Carolina, only 100–500 km to the north of the Georgia study site, is 255.5 cm (95% credible interval = 250.3–261.8) [19]. The median length for adult females from Georgia was 236 cm when compared with 241.5 cm (95% credible interval = 238.5–244.7) reported as the asymptotic length for females from South Carolina [19].
(a). Haematology and serum chemistry
Blood samples for haematology and serum chemistry were obtained for 27 and 29 dolphins, respectively. A number of abnormal haematological and serum biochemical parameters were determined for dolphins sampled near both sites (table 1). Most notable, 26 per cent of the sampled dolphins were found to be anaemic, diagnosed as having a haemoglobin value below the 2.5th percentile reference threshold. Of the seven anaemia cases, three were normocytic and showed signs of depressed erythropoiesis; two were microcytic concurrent with reduced mean corpuscular haemoglobin, low iron and elevated lactate dehydrogenase; and one was macrocytic with depressed erythropoiesis and indication of mild rouleaux formation with elevation of serum protein. The remaining anaemia case had no other abnormal haematological or biochemical parameters. The three normocytic cases were adult males, and the two microcytic cases were females (one adult and one juvenile, neither pregnant). In addition, two adult males who were within the normal range for haemoglobin demonstrated elevated mean corpuscular volume and mild polychromasia, potentially indicative of recent regenerative anaemia.
Table 1.
Abnormal haematological and serum biochemical conditions observed in dolphins sampled near Sapelo Island and Brunswick, GA, USA. (Parameter values were compared with reference intervals for appropriate age- and sex-class as described by Schwacke et al. [22]. Prevalence calculation based on n = 27 samples for haematology (haemoglobin) and n = 29 for serum chemistry (all other parameters). For comparison, prevalence was also calculated using samples from Sarasota (n = 63) and Beaufort (n = 12 and n = 14 for haematology and serum chemistry, respectively). GGT, gamma-glutamyl transferase; ALT, alanine aminotransferase; AST, aspartate transaminase.)
| condition | defining parameters | no. cases Sapelo | no. cases Brunswick | total cases | prevalence | 95% CI | Sarasota prevalence | Beaufort prevalence |
|---|---|---|---|---|---|---|---|---|
| anaemia | haemoglobin below lower threshold | 3 | 4 | 7 | 0.26 | 0.11–0.46 | 0.03 | 0 |
| elevated hepatic enzymes | one or more above upper threshold: GGT, ALT, AST | 4 | 0 | 4 | 0.14 | 0.04–0.32 | 0.02 | 0.07 |
| elevated electrolytes | one or more above upper threshold: sodium, chloride, potassium | 1 | 4 | 5 | 0.17 | 0.06–0.36 | 0.03 | 0 |
| elevated lactate dehydrogenase | lactate dehydrogenase above upper threshold | 3 | 6 | 9 | 0.31 | 0.15–0.51 | 0.10 | 0.07 |
| hypermagnesemia | magnesium above upper threshold | 5 | 4 | 9 | 0.31 | 0.15–0.51 | 0 | 0 |
Elevated liver enzymes and elevated electrolytes, suggestive of potential hepatic and/or renal pathologies, were noted in 14 and 17 per cent of dolphins, respectively. Elevated LDH, a non-specific enzyme indicative of cell damage, was seen in 31 per cent of dolphins. In addition, hypermagnesemia (magnesium value > 1.7 mEq l−1 for adults, >1.8 mEq l−1 for calves and juveniles) was found in 31 per cent of the sampled dolphins. The clinical significance of the hypermagnesemia is unclear, but seven of the nine hypermagnesemia dolphins had other indications of health issues, including renal (elevated sodium, potassium, calcium, blood urea nitrogen and/or uric acid) and/or hepatic (elevated alanine amino transferase and/or gamma-glutamyl transferase) concerns.
(b). Endocrine assessment
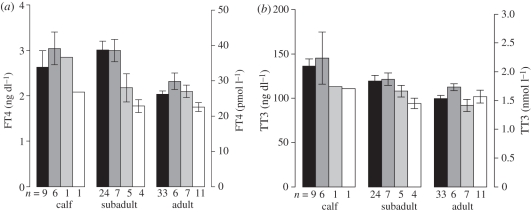
While TT4 did not vary significantly among sites (not shown), TT3 and FT4 concentrations were considerably lower for dolphins sampled in Georgia with the differences most apparent in subadults (figure 2). The ANOVAs supported the conclusion of differences in TT3 and FT4 among sites (p < 0.001 for both) and found no difference among sites for TT4 (p = 0.525). The age-class covariate term was significant for all three parameters (p < 0.001). The linear contrast indicated a significant difference between the two control sites (Sarasota and Beaufort) and the two Georgia sites (Brunswick and Sapelo Island) for FT4 (p = 0.002). The p-value for TT3 linear contrast (p = 0.053) was only slightly above the established significance threshold.
Figure 2.
(a) FT4 and (b) TT3 measured from serum of dolphins sampled near Sarasota, FL; Beaufort, NC; Sapelo Island, GA and Brunswick, GA. Bars represent mean and whiskers represent 95% CI. Sample size (n) is indicated below x-axis. FT4 differed (p = 0.002) between the Georgia sites (Sapelo and Brunswick) and the two reference sites (Sarasota and Beaufort); TT3 was slightly above the critical value (p = 0.053). (a,b) Black bars, Sarasota; dark grey bars, Beaufort; light grey bars, Sapelo; white bars, Brunswick.
(c). Contaminant analysis
Blubber samples for chemical analysis were stratified into two classes: (i) adult females (n = 5), (ii) males and non-adult females (n = 21). Measured PCB concentrations ranged from 10.3 to 761 µg g−1 lipid in males and non-adult females and 43.7 to 173 µg g−1 lipid in adult females (figure 1). Further description of results of chemical analysis can be found in the electronic supplementary material.
(d). Relationship of endocrine, immune parameters and PCB concentration
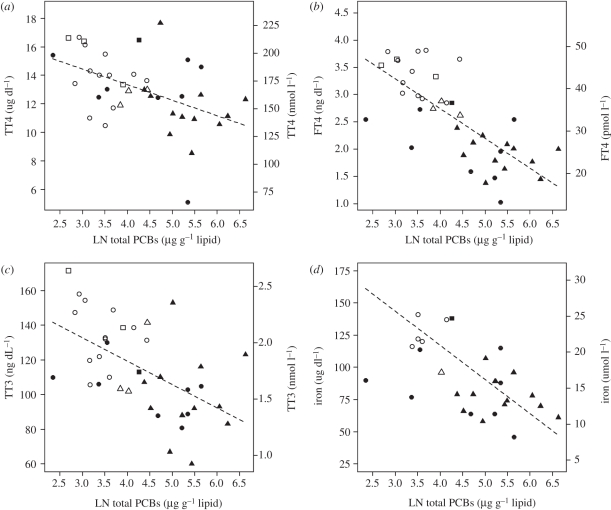
Each of the endocrine parameters showed a significant negative relationship with blubber PCB concentration (table 2 and figure 3), although the association between PCBs and TT4 was weaker than for TT3 and FT4. As anticipated, age class also significantly influenced endocrine parameters and was retained in all of the models as a covariate. Site was not indicated as a significant factor for TT4 and TT3; however, site was retained as a significant covariate for FT4.
Table 2.
p-values from ANCOVA examining association of serum endocrine values, iron and functional immune indices with natural logarithm of total PCBs measured in blubber. (Model included all males as well as calf, juvenile and subadult females. Length class and site were included as covariate terms. n.s. indicates a non-significant p-value. Covariate factors determined to be non-significant were removed from the model and the analysis was repeated to obtain final significance levels.)
| n | model r2 | LN(PCB) | length class | site | |
|---|---|---|---|---|---|
| endocrine parameters | |||||
| TT3 | 37 | 0.35 | 0.009 | 0.009 | n.s. |
| TT4 | 38 | 0.29 | 0.039 | 0.012 | n.s. |
| FT4 | 38 | 0.79 | <0.001 | <0.001 | <0.001 |
| functional immune parameters | |||||
| Con A stimulation index | 29 | 0.56 | 0.001 | n.s. | 0.001 |
| LPS stimulation index | 31 | 0.47 | n.s. | n.s. | <0.001 |
| neutrophil phagocytosis | 30 | 0.57 | 0.003 | n.s. | <0.001 |
| monocyte phagocytosis | 31 | 0.67 | <0.001 | n.s. | <0.001 |
| other | |||||
| iron | 28 | 0.76 | 0.018 | <0.001 | 0.01 |
Figure 3.
Results from linear model (ANCOVA) examining association of serum (a) TT4; (b) FT4; (c) TT3; and (d) iron with natural logarithm (LN) of total PCBs measured in blubber. The model included all males as well as calf and juvenile females sampled near Brunswick, GA, Sapelo Island, GA, Sarasota, FL and Beaufort, NC. Age class and site were included as covariate terms. Circles, squares and triangles represent calves, juveniles and adults, respectively. Solid filled symbols represent samples from the Georgia sites (Sapelo and Brunswick), and hollow symbols represent samples from the reference sites (Sarasota and Beaufort).
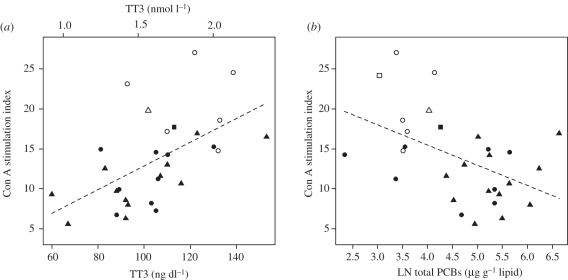
Con A stimulation index, an indicator of T-lymphocyte proliferative response, showed a strong positive correlation with TT3 (p < 0.001, figure 4) and a negative correlation with PCB concentration (table 2 and figure 4b). The two indicators of innate immunity, neutrophil and monocyte phagocytosis, also showed significant negative associations with PCB concentration (table 2). By contrast, LPS stimulation index, an indicator of B-lymphocyte proliferation, did not show a significant association with PCB concentration (p = 0.377, table 2).
Figure 4.
Results from linear model (ANCOVA) examining association of T-lymphocyte stimulation index with (a) TT3 and (b) natural logarithm (LN) of total PCBs measured in blubber. The model included all males as well as calf and juvenile females sampled near Brunswick, GA, Sapelo Island, GA, Sarasota, FL and Beaufort, NC. Age class and site were included as covariate terms. Circles, squares and triangles represent calves, juveniles and adults, respectively. Solid filled symbols represent samples from the Georgia sites (Sapelo and Brunswick), and hollow symbols represent samples from the reference site (Beaufort).
4. Discussion
Our study clearly demonstrates a multitude of health concerns in dolphins exposed to high concentrations of PCBs, and the observed effects are consistent with adverse effects reported from experimental studies of other mammal species. For example, we found a high prevalence of anaemia in the dolphins sampled along the Georgia coast (table 2), similar to findings in primates after chronic exposure to Aroclor 1254 or 1248 [25,26]. Depressed erythropoiesis with normocytic anaemia was reported for cynomolgus monkeys (Macaca fascicularis) after being fed relatively high doses of Aroclor 1254 and both macrocytic and normocytic anaemia were reported after dosing with Aroclor 1248 [26]. We noted similar cases (n = 3) of depressed erythropoiesis with normocytic anaemia as well as a macrocytic anaemia in the Georgia dolphins. We also noted two cases of microcytic anaemia with associated iron deficiency. Iron deficiency is often associated with hypothyroidism [27] and the latter two dolphin cases had very low levels of TT3 (0.80 ng ml−1 and 0.88 ng ml−1) and FT4 (1.28 ng dl−1 and 1.59 ng dl−1). In general, iron levels negatively correlated with blubber PCB concentrations (figure 3).
The PCB concentrations measured from the Georgia dolphins' blubber (maximum = 761 µg g−1 lipid) far exceed the concentrations from oil extract of adipose tissue (maximum = 27.5 µg g−1) taken from the laboratory primates at autopsy [26]. The higher concentrations could be in part owing to greater accumulation of the higher chlorinated congeners, which are characteristic of Aroclor 1268. Based on their primate study, Tryphonas et al. [26] suggest that the higher chlorinated Aroclor 1254 may accumulate to greater concentration yet have a slightly lower toxic potential when compared with the lesser chlorinated congeners comprising Aroclor 1248. This difference in accumulation and toxicity among PCB mixtures, along with interspecies differences in sensitivity and lipid storage, could explain the dolphins' ability to tolerate the higher PCB tissue concentrations without becoming moribund, which eventually occurred in the laboratory primates after extended dosing.
In addition to the haematological effects, we found severely decreased TT3 and FT4 levels in dolphins sampled from the Georgia coast (figure 2) and the highly significant negative correlation of these hormones with measured blubber PCB concentration (figure 3). This is consistent with the strong evidence of PCB-related thyroid hormone disruption from studies of other marine mammals [28,29] as well as laboratory studies of rats dosed with Aroclor 1254 [30]. The mechanism(s) by which PCBs disrupt thyroid hormones are not completely understood, but experimental evidence suggests that there are potentially multiple toxic pathways. Aside from directly interfering with hormone production in the thyroid gland, at least some PCBs can interact with metabolic enzymes to accelerate clearance of thyroid hormones [2]. Specifically, increased glucuronidation and elimination of thyroxine (T4) through induction of hepatic uridine diphosphate glucuronosyltransferase (UDP-GT) was found in rats fed an Aroclor 1254 mixture [31]. An additional study using specific coplanar PCB congeners suggests that the increased UDP-GT activity could be mediated through the aryl hydrocarbon receptor signal transduction pathway [32]. The PCBs generally associated with this pathway are similar in structure to the dioxins and having one or no chlorine substitutions at the ortho positions on the benzene rings. Although not primary constituents of Arcolor 1268, some of these dioxin-like congeners, particularly PCB 189, were measured in the Georgia dolphins (see the electronic supplementary material).
Alternatively, PCBs could interfere with thyroid hormone transport to peripheral tissues. Some PCBs, as well hydroxylated PCB metabolites, are structurally similar to T4 and may competitively bind to a thyroid hormone transport protein, transthyretin [2]. However, once again the experimental studies to date have examined the role of dioxin-like congeners [33] or the hydroxylated metabolites [34] which are more readily transformed from lower chlorinated PCBs [35]. Thus, competitive binding to transthyretin is not an obvious mechanistic pathway for the primary congeners in the Aroclor 1268 mixture. Furthermore, if there was a high degree of competition for binding to transport proteins, we might expect to see higher levels of unbound T4; we observed lower levels of FT4 in association with PCB concentration.
Regardless of the toxic mechanism, the hypothyroidism observed in the Georgia dolphins could certainly produce a variety of adverse health outcomes as thyroid hormones play a critical role in metabolism and growth. In fact, the dolphins sampled in Georgia were smaller than expected based on measurements of dolphins from other populations. This suggests a potential for reduced growth in the Georgia dolphins, although our sample size (nine males and four females) was too small for meaningful statistical comparisons.
It is unclear whether decreased thyroid hormones also mediated the reduction in innate and acquired immune response. T-lymphocyte mitogen-induced proliferation showed a strong positive correlation with TT3 (figure 4a), consistent with a study which found that hypothyroid mice displayed lower T-cell mitogen-induced proliferation but that the administration of triiodothyronine (T3) recovered the proliferative response [24]. The same mouse study provided evidence that T3 has a role in regulating cytokine production which could in turn influence lymphocyte response as well as innate immune response. Although this scenario of thyroid-mediated immune suppression is certainly plausible, we cannot rule out simple covariation owing to the negative association of PCBs with both thyroid and immune endpoints. Innate and adaptive immune functions may also be directly modulated by PCBs. Dolphin T-lymphocyte proliferation and phagocytosis were shown to be significantly reduced upon in vitro exposure to simple mixtures of individual PCB congeners [20,21]. Importantly, one of the individual PCB congeners that reduced immune functions upon in vitro exposure, PCB 180, is a major component of Aroclor 1268 [7] and was detected in the Georgia dolphin blubber (geometric mean = 5.1 µg g−1 lipid in males and non-adult females). No matter the mechanism, a lowered T-cell response could increase susceptibility to infectious disease, particularly of viral origin while the suppressed innate immunity could leave animals vulnerable to invasion by bacterial, fungal and protozoan infections. A good correlation was demonstrated between changes in immune function and increased susceptibility to infectious and non-infectious disease in mice in that there were no instances where host resistance was altered without affecting an immune test [36].
While an extensive body of research exists addressing the health effects of PCBs (reviewed in [37]), most studies to date have focused on the widely used PCB mixtures with low to moderate chlorine content such as Aroclors 1248, 1254 and 1260. Less than 1 per cent of the PCBs sold in the USA from 1957 to 1975 were Aroclor 1268 [38,39], and thus toxicological investigations have been limited for this highly chlorinated mixture. While the concentrations measured in the dolphins were dominated by highly chlorinated octa- through decachlorobiphenyl congeners, mono-ortho congeners such as PCB 189 were also detected (see the electronic supplementary material) and strongly correlated with the summed 1268 congeners (r = 0.92). Both congener groups were significantly higher in the Georgia dolphins when compared with the two reference sites (see the electronic supplementary material), and it is likely that both congener groups contribute to the overall toxicity of the mixture.
Other persistent organic contaminants (POCs) have potential for endocrine and immune disruption and must be considered as possible confounding factors. However, concentrations of other POCs measured in the Georgia dolphins were similar or even lower than those measured from other areas of the US coast where such severe health impacts have not been observed. Concentrations of DDT and chlordane compounds in the Georgia dolphins were significantly lower than concentrations measured in one or both reference sites (see the electronic supplementary material). BDEs were higher for Georgia dolphins when compared with both reference sites, but were still lower than those measured in dolphins from many other US Atlantic coast sites [40]. Furthermore, total PCB concentrations were on average 43-fold higher than BDE concentrations in the Georgia dolphins, and the toxic mono-ortho PCBs alone were 1.6-fold higher than BDEs (see the electronic supplementary material). Therefore, while potential additive effects of the BDEs cannot be ruled out, with such large differences in concentrations, it is unlikely that the BDEs significantly influenced the correlations between PCBs and health endpoints.
5. Conclusions
The impact of PCBs on the health and sustainability of cetacean populations has been of concern for decades, prompted initially by a series of die-offs of dolphin and other marine mammal populations near industrialized coastlines (see [41] for review). Concern is particularly warranted for odontecetes (toothed whales and dolphins), which generally occupy high trophic positions. While a number of studies have documented high PCB exposures in these species, studies to examine associated health impacts have been limited owing to the ethical and logistical constraints of studying such large aquatic mammals, which are protected under federal laws of the USA as well as many other nations (see [41] for discussion). The results of our research clearly demonstrate that bottlenose dolphins are vulnerable to PCB-related health effects, at least partially mediated through the endocrine system, and that while PCBs are no longer used in the USA they remain in marine food webs where they still pose a significant risk to upper trophic marine wildlife. By confirming a strong exposure–response relationship between PCB burden and endocrine and immune endpoints in a cetacean species, our results have important implications for risk assessment, and ultimately the conservation and management, of cetacean populations worldwide.
These findings also have significance beyond conservation of dolphins and whales. To date, the majority of PCB toxicity studies have focused on the dioxin-like congeners or more common Aroclor mixtures. Higher chlorinated congeners are generally assumed to be more inert, resistant to dechlorination and metabolism, and the highly chlorinated mixtures are, therefore, considered to have lower toxic potential. This study provides evidence that although a potentially lower toxicity, Aroclor 1268 may accumulate to sufficiently high concentrations to produce significant health impacts. This suggests a need for further study to elucidate mechanisms and potential impacts on other top-level predators, as well as human populations who regularly consume fish from the same marine waters.
Acknowledgements
This work was conducted under NMFS Permit no. 932-1905/MA-009526 issued to Dr Teresa Rowles and protocols were reviewed and approved by a NOAA/NMFS ad hoc Institutional Animal Care and Use Committee (IACUC).
We thank the many researchers and veterinarians who supported the dolphin health assessment fieldwork, including those from the Navy Marine Mammal Programme, the University of Florida, the Sapelo Island National Estuarine Research Reserve, and the Georgia Aquarium, without whom this work would not have been possible. Special thanks to Rob Yordy (SeaWorld and Busch Gardens); Suzanne Lane, Todd Speakman and Leslie Burdett (NOAA); and Larry Fulford. Funding was provided by the NOAA Marine Mammal Health and Stranding Response Programme and NOAA's Oceans and Human Health Initiative.
This publication does not constitute an endorsement of any commercial product or intend to be an opinion beyond scientific or other results obtained by the NOAA.
References
- 1.Arnold D. L., et al. 1995. Toxicological consequences of Aroclor 1254 ingestion by female rhesus (Macaca mulatta) monkeys. II. Reproduction and infant findings. Food Chem. Toxicol. 33, 457–474 [DOI] [PubMed] [Google Scholar]
- 2.Brouwer A., Morse D., Lans M., Schuur A., Murk A., Klasson-Wehler E., Bergman A., Visser T. 1998. Interactions of persistent environmental organohalogens with the thyroid hormone system: mechansims and possible consequences for animal and human health. Toxicol. Ind. Health 14, 59–84 [DOI] [PubMed] [Google Scholar]
- 3.Tryphonas H., et al. 1991. Effect of chronic exposure of PCB (Aroclor 1254) on specific and nonspecific immune parameters in the rhesus (Macaca mulatta) monkey. Fundam. Appl. Toxicol. 16, 773–786 10.1016/0272-0590(91)90163-X (doi:10.1016/0272-0590(91)90163-X) [DOI] [PubMed] [Google Scholar]
- 4.Fox G. A. 2001. Wildlife as sentinels of human health effects in the Great Lakes–St. Lawrence basin. Environ. Health Perspect. 109(Suppl. 6), 853–861 10.2307/3454647 (doi:10.2307/3454647) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pulster E. L., Maruya K. A. 2008. Geographic specificity of Aroclor 1268 in bottlenose dolphins (Tursiops truncatus) frequenting the Turtle/Brunswick River Estuary, Georgia (USA). Sci. Total Environ. 393, 367–375 [DOI] [PubMed] [Google Scholar]
- 6.Balmer B., et al. 2011. Relationship between persistent organic pollutants (POPs) and ranging patterns in common bottlenose dolphins (Tursiops truncatus) from coastal Georgia, USA. Sci. Total Environ. 409, 2094–2101 10.1016/j.scitotenv.2011.01.052 (doi:10.1016/j.scitotenv.2011.01.052) [DOI] [PubMed] [Google Scholar]
- 7.Kannan K., Maruya K. A., Tanabe S. 1997. Distribution and characterization of polychorinated biphenyl congeners in soil and sediments from a Superfund site contaminated with Aroclor 1268. Environ. Sci. Technol. 31, 1483–1488 10.1021/es960721r (doi:10.1021/es960721r) [DOI] [Google Scholar]
- 8.Kannan K., Nakata H., Stafford R., Masson G., Tanabe S., Giesy J. P. 1998. Bioaccumulation and toxic potential of extremely hydrophobic polychlorinated biphenyl congeners in biota collected at a Superfund site contaminated with Aroclor 1268. Environ. Sci. Technol. 32, 1214–1221 10.1021/es9709435 (doi:10.1021/es9709435) [DOI] [Google Scholar]
- 9.Hansen L. J., Schwacke L. H., Mitchum G. B., Hohn A. A., Wells R. S., Zolman E. S., Fair P. A. 2004. Geographic variation in polychlorinated biphenyl and organochlorine pesticide concentrations in the blubber of bottlenose dolphins from the US Atlantic coast. Sci. Total Environ. 319, 147–172 [DOI] [PubMed] [Google Scholar]
- 10.Litz J., Garrison L., Feiber L., Martinez A., Contillo J., Kucklick J. 2007. Fine-scale spatial variation of persistent organic pollutants in bottlenose dolphins (Tursiops truncatus) in Biscayne Bay, Florida. Environ. Sci. Technol. 41, 7222–7228 10.1021/es070440r (doi:10.1021/es070440r) [DOI] [PubMed] [Google Scholar]
- 11.Aguilar A., Borrell A. 1994. Abnormally high polychlorinated biphenyl levels in striped dolphins (Stenella coeruleoalba) affected by the 1990–92 Mediterranean epizootic. Sci. Total Environ. 154, 237–247 10.1016/0048-9697(94)90091-4 (doi:10.1016/0048-9697(94)90091-4) [DOI] [PubMed] [Google Scholar]
- 12.Geraci J. 1989. Clinical investigation of the 1987–88 mass mortality of bottlenose dolphins along the U.S. central and south Atlantic coast: final report to the National Marine Fisheries Service, U.S. Navy Office of Naval Research and the Marine Mammal Commission, Ontario Veterinary College, Guelph, Canada.
- 13.Ross P., Swart R. D., Addison R., Loveren H. V., Vos J., Osterhaus A. 1996. Contaminant-induced immunotoxicity in harbour seals: wildlife at risk? Toxicology 112, 157–169 10.1016/0300-483X(96)03396-3 (doi:10.1016/0300-483X(96)03396-3) [DOI] [PubMed] [Google Scholar]
- 14.Lahvis G., Wells R., Kuehl D., Stewart J., Rhinehart H., Via C. 1995. Decreased lymphocyte responses in free-ranging bottlenose dolphins (Tursiops truncatus) are associated with increased concentrations of PCBs and DDT in peripheral blood. Environ. Health Perspect. 103(Suppl. 4), 67–72 10.2307/3432414 (doi:10.2307/3432414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall A. J., Hugunin K., Deaville R., Law R. J., Allchin C. R., Jepson P. D. 2006. The risk of infection from polychlorinated biphenyl exposure in the harbor porpoise (Phocoena phocoena): a case-control approach. Environ. Health Perspect. 114, 704–711 10.1289/ehp.8222 (doi:10.1289/ehp.8222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwacke L. H., et al. 2010. Eosinophilia and biotoxin exposure in bottlenose dolphins (Tursiops truncatus) from a coastal area impacted by repeated mortality events. Environ. Res. 110, 548–555 10.1016/j.envres.2010.05.003 (doi:10.1016/j.envres.2010.05.003) [DOI] [PubMed] [Google Scholar]
- 17.Wells R. S., et al. 2004. Bottlenose dolphins as marine ecosystem sentinels: developing a health monitoring system. EcoHealth 1, 246–254 10.1007/s10393-004-0094-6 (doi:10.1007/s10393-004-0094-6) [DOI] [Google Scholar]
- 18.Wells R., et al. 2005. Integrating life-history and reproductive success data to examine potential relationships with organochlorine compounds for bottlenose dolphins (Tursiops truncatus) in Sarasota Bay, Florida. Sci. Total Environ. 349, 106–119 10.1016/j.scitotenv.2005.01.010 (doi:10.1016/j.scitotenv.2005.01.010) [DOI] [PubMed] [Google Scholar]
- 19.McFee W. E., Schwacke J. H., Stolen M. K., Mullin K. D., Schwacke L. H. 2010. Investigation of growth phases for bottlenose dolphins using a Bayesian modeling approach. Mar. Mamm. Sci. 26, 67–85 [Google Scholar]
- 20.Levin M., Morsey B., Mori C., Nambiar P. R., De Guise S. 2005. PCBs and TCDD, alone and in mixtures, modulate marine mammal but not B6C3F1 mouse leukocyte phagocytosis. J. Toxicol. Environ. Health A 68, 635–656 10.1080/15287390590921766 (doi:10.1080/15287390590921766) [DOI] [PubMed] [Google Scholar]
- 21.Mori C., Morsey B., Levin M., Nambiar P. R., De Guise S. 2006. Immunomodulatory effects of in vitro exposure to organochlorines on T-cell proliferation in marine mammals and mice. J. Toxicol. Environ. Health A 69, 283–302 10.1080/15287390500227472 (doi:10.1080/15287390500227472) [DOI] [PubMed] [Google Scholar]
- 22.Schwacke L., Hall A., Townsend F., Wells R., Hansen L., Hohn A., Bossart G., Fair P., Rowles T. 2009. Hematologic and serum biochemical reference intervals for free-ranging common bottlenose dolphins (Tursiops truncatus) and variation in the distributions of clinicopathologic values related to geographic sampling site. Am. J. Vet. Res. 70, 973–985 10.2460/ajvr.70.8.973 (doi:10.2460/ajvr.70.8.973) [DOI] [PubMed] [Google Scholar]
- 23.Yordy J. E., Wells R. S., Balmer B. C., Schwacke L. H., Rowles T. K., Kucklick J. R. 2010. Life history as a source of variation for persistent organic pollutant (POP) patterns in a community of common bottlenose dolphins (Tursiops truncatus) resident to Sarasota Bay, FL. Sci. Total Environ. 408, 2163–2172 10.1016/j.scitotenv.2010.01.032 (doi:10.1016/j.scitotenv.2010.01.032) [DOI] [PubMed] [Google Scholar]
- 24.Klecha A. J., Genaro A. M., Gorelik G., Barreiro Arcos M. L., Silberman D. M., Schuman M., Garcia S. I., Pirola C., Cremaschi G. A. 2006. Integrative study of hypothalamus-pituitary-thyroid-immune system interaction: thyroid hormone-mediated modulation of lymphocyte activity through the protein kinase C signaling pathway. J. Endocrinol. 189, 45–55 10.1677/joe.1.06137 (doi:10.1677/joe.1.06137) [DOI] [PubMed] [Google Scholar]
- 25.Arnold D. L., et al. 1993. Toxicological consequences of Aroclor 1254 ingestion by female rhesus (Macaca mulatta) monkeys. Part 1B. Prebreeding phase: clinical and analytical laboratory findings. Food Chem. Toxicol. 31, 811–824 [DOI] [PubMed] [Google Scholar]
- 26.Tryphonas L., Truelove J., Zawidzka Z., Wong J., Mes J., Charbonneau S., Grant D. L., Campbell J. S. 1984. Polychlorinated biphenyl (PCB) toxicity in adult cynomolgus monkeys (M. fascicularis): a pilot study. Toxicol. Pathol. 12, 10–25 10.1177/019262338401200103 (doi:10.1177/019262338401200103) [DOI] [PubMed] [Google Scholar]
- 27.Horton L., Coburn R. J., England J. M., Himsworth R. L. 1976. The haematology of hypothyroidism. Q. J. Med. 45, 101–123 [PubMed] [Google Scholar]
- 28.Debier C., Ylitalo G. M., Weise M., Gulland F., Costa D. P., Le Boeuf B. J., de Tillesse T., Larondelle Y. 2005. PCBs and DDT in the serum of juvenile California sea lions: associations with vitamins A and E and thyroid hormones. Environ. Pollut. 134, 323–332 10.1016/j.envpol.2004.07.012 (doi:10.1016/j.envpol.2004.07.012) [DOI] [PubMed] [Google Scholar]
- 29.Tabuchi M., Veldhoen N., Dangerfield N., Jeffries S., Helbing C. C., Ross P. S. 2006. PCB-related alteration of thyroid hormones and thyroid hormone receptor gene expression in free-ranging harbor seals (Phoca vitulina). Environ. Health Perspect. 114, 1024–1031 10.1289/ehp.8661 (doi:10.1289/ehp.8661) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Byrne J. J., Carbone J. P., Hanson E. A. 1987. Hypothyroidism and abnormalities in the kinetics of thyroid hormone metabolism in rats treated chronically with polychlorinated biphenyl and polybrominated biphenyl. Endocrinology 121, 520–527 10.1210/endo-121-2-520 (doi:10.1210/endo-121-2-520) [DOI] [PubMed] [Google Scholar]
- 31.Barter R. A., Klaassen C. D. 1994. Reduction of thyroid hormone levels and alteration of thyroid function by four representative UDP-glucuronosyltransferase inducers in rats. Toxicol. Appl. Pharmacol. 128, 9–17 10.1006/taap.1994.1174 (doi:10.1006/taap.1994.1174) [DOI] [PubMed] [Google Scholar]
- 32.Van Birgelen A. P., et al. 1995. Subchronic effects of 2,3,7,8-TCDD or PCBs on thyroid hormone metabolism: use in risk assessment. Eur. J. Pharmacol. 293, 77–85 10.1016/0926-6917(95)90021-7 (doi:10.1016/0926-6917(95)90021-7) [DOI] [PubMed] [Google Scholar]
- 33.Han D. Y., Kang S. R., Park O. S., Cho J. H., Won C. K., Park H. S., Park K. I., Kim E. H., Kim G. S. 2010. Hypothyroidism induced by polychlorinated biphenyls and up-regulation of transthyretin. Bull Environ. Contam. Toxicol. 84, 66–70 10.1007/s00128-009-9890-6 (doi:10.1007/s00128-009-9890-6) [DOI] [PubMed] [Google Scholar]
- 34.Lans M. C., Spiertz C., Brouwer A., Koeman J. H. 1994. Different competition of thyroxine binding to transthyretin and thyroxine-binding globulin by hydroxy-PCBs, PCDDs and PCDFs. Eur. J. Pharmacol. 270, 129–136 [DOI] [PubMed] [Google Scholar]
- 35.Houde M., et al. 2006. Polychlorinated biphenyls and hydroxylated polychlorinated biphenyls in plasma of bottlenose dolphins (Tursiops truncatus) from the Western Atlantic and the Gulf of Mexico. Environ. Sci. Technol. 40, 5860–5866 10.1021/es060629n (doi:10.1021/es060629n) [DOI] [PubMed] [Google Scholar]
- 36.Luster M. I., et al. 1993. Risk assessment in immunotoxicology. II. Relationships between immune and host resistance tests. Fundam. Appl. Toxicol. 21, 71–82 10.1006/faat.1993.1074 (doi:10.1006/faat.1993.1074) [DOI] [PubMed] [Google Scholar]
- 37.ATSDR 2000. Toxicological profile for polychlorinated biphenyls (PCBs). Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service [Google Scholar]
- 38.Lloyd R., Moore R., Woolf B., Stein H. 1975. Current intelligence bulletin 7, polychlorinated biphenyls (PCBs). Cincinnati, OH: National Institute for Occupational Safety and Health; (updated 1997; cited 2010 10/4/2010); See http://www.cdc.gov/niosh/78127_7.html#references [Google Scholar]
- 39.Cantwell M. G., King J., Burgess R. M. 2006. Temporal trends of Aroclor 1268 in the Taunton River estuary: evidence of early production, use and release to the environment. Mar. Pollut. Bull 52, 1105–1111 10.1016/j.marpolbul.2006.05.019 (doi:10.1016/j.marpolbul.2006.05.019) [DOI] [PubMed] [Google Scholar]
- 40.Kucklick J., et al. 2011. Bottlenose dolphins as indicators of persistent organic pollutants in the Western North Atlantic Ocean and Northern Gulf of Mexico. Environ. Sci. Technol. 45, 4270–4277 10.1021/es1042244 (doi:10.1021/es1042244) [DOI] [PubMed] [Google Scholar]
- 41.Houde M., Hoekstra P. F., Solomon K. R., Muir D. C. 2005. Organohalogen contaminants in delphinoid cetaceans. Rev. Environ. Contam. Toxicol. 184, 1–57 10.1007/0-387-27565-7_1 (doi:10.1007/0-387-27565-7_1) [DOI] [PubMed] [Google Scholar]