Abstract
Dispersal plays a key role in the response of populations to climate change and habitat fragmentation. Here, we use data from a long-term metapopulation study of a non-migratory bird, the house sparrow (Passer domesticus), to examine the influence of increasing spring temperature and density-dependence on natal dispersal rates and how these relationships depend on spatial variation in habitat quality. The effects of spring temperature and population size on dispersal rate depended on the habitat quality. Dispersal rate increased with temperature and population size on poor-quality islands without farms, where house sparrows were more exposed to temporal fluctuations in weather conditions and food availability. By contrast, dispersal rate was independent of spring temperature and population size on high-quality islands with farms, where house sparrows had access to food and shelter all the year around. This illustrates large spatial heterogeneity within the metapopulation in how population density and environmental fluctuations affect the dispersal process.
Keywords: climate change, density-dependence, habitat quality, intraspecific competition, natal dispersal, phenology
1. Introduction
Dispersing individuals have important consequences for the distribution of genetic variation, population dynamics and shifts in species ranges [1]. Past and present human-induced loss and fragmentation of habitats in combination with climate change pose a serious threat for the persistence of populations and species [2,3]. Under such circumstances, dispersal may be critical in allowing individuals to track the climate-induced changes in distribution of suitable habitat in a fragmented landscape. In addition, dispersal affects gene flow and thereby contributes to the genetic variation necessary to adapt to a changing environment. Thus, dispersal plays a key role for the response of populations, and ultimately persistence of species, subject to environmental change, which has been shown in theoretical studies [4,5] and gained support from empirical studies in a wide range of taxa (e.g. [6–9]).
There is a vast body of empirical work demonstrating that dispersal may vary considerably within species [1]. One reason for this is that dispersal behaviour often is sensitive to external environmental cues experienced by individuals during emigration from a patch and during transfer between patches and during immigration [10]. A large number of proximate factors may be involved, e.g. patch size and patch configuration [11], social structure [12,13] and food availability [14]. In many species, several aspects of dispersal are influenced by population size. A high density of conspecifics may provide information on different local conditions with opposing influence on an individual's propensity to disperse. Negative density-dependent dispersal may result if individuals are attracted to sites with many conspecifics [15]. Such conspecific attraction may occur if anti-predator behaviour and foraging are more efficient at high densities. Similarly, a high number of conspecifics may be used as a cue to identify high-quality habitats in terms of mate availability or food [10,16]. On the other hand, in the majority of species studied, the probability of emigration away from the area of birth often increases with population size, e.g. in insects [17], birds and mammals [18]. Positive density-dependent dispersal may result from high population density itself, mediated through social interactions, such as interference and harassment. A large number of conspecifics also causes pressure on resources. Thus, the underlying factor that causes emigration may not be population size per se, but rather the population size in relation to the resource availability of the habitat [19]. Thus, it is possible that population size interacts with habitat quality (e.g. food availability) or temporal habitat quality variation to determine dispersal rates [16]. Fluctuations in environmental conditions may therefore be responsible for the observed temporal variation in dispersal [20–23]. However, as pointed out by Matthysen [18], many of the studies that failed to find a relationship between dispersal propensity and population density [18,24] have only considered local movements within a restricted study area. Obviously, this points to the importance of a large study area to obtain reliable, unbiased estimates of dispersal rates [25].
Despite the mounting theoretical and empirical interest in how the effects of climate change are influenced by dispersal (see above), there are few studies, especially in vertebrates, which have addressed how dispersal itself is influenced by climate change. For example, in the common lizard (Lacerta vivipara), natal dispersal has declined with increasing temperature [26]. In birds, the influence of temperature on demographic traits such as timing of breeding, survival and fecundity is now rather well-documented (reviewed in [27,28]). However, the relationship between temporal variation in avian dispersal and climate has received much less attention [29–33]. An additional complication is that the effects of the same climate factor on demographic processes and population dynamics may differ between populations, even within regions experiencing similar temperature change [28]. Together with the fact that dispersal rates often are sensitive to population size, this implies that in order to understand the effect of climate variation on dispersal, one must consider fluctuations in population size and how the effects of density-dependence and environmental variation differ between populations, for example, owing to differences in habitat quality. In order to determine the ecological and demographic properties of the natal population, we must therefore know the origin of dispersing individuals. However, this is often not the case in studies of dispersal in natural populations (e.g. [31–33]). In particular, how the interaction between the climate factors and properties of the natal habitat affects dispersal remains more or less unstudied (but see [26]).
The aim of this study is to investigate how spring temperature and population size affect dispersal rate in an insular metapopulation of the house sparrow (Passer domesticus) and how these relationships depend on natal habitat quality. The study area is large in relation to the dispersal range [34] and a large proportion of the sparrows is individually marked [35]. This implies major advantages when studying dispersal: it allows unambiguous identification of dispersal events between islands and bias in estimates of dispersal rates caused by emigration out of the study area is low [25,35]. As in most other passerines in temperate areas, warm weather reduces nestling mortality in the house sparrow and increases fledgling size [36]. Furthermore, high spring temperature may advance the onset of breeding [37], which may influence individual dispersal probability [38]. In addition, early onset breeding may increase the incidence of additional broods and increase production of young, as found in many bird species (e.g. [39]). In years with high spring temperature, early breeding and increased survival may lead to a high number of conspecifics in the natal habitat, which in turn could influence the dispersal decision of individuals. Thus, we hypothesize that one possible proximate link between temperature and dispersal in our study system is temperature-dependent production of juveniles and density-dependent dispersal. Population size per se may induce emigration as a consequence of social interactions. However, if the effect of population size on dispersal is mediated through its pressure on resources, the dispersal response to population size needs to be considered in relation to spatial variation in habitat quality [16]. Thus, we expect that factors influencing production of juveniles and population size, e.g. temperature and onset of breeding, affect dispersal differently depending on habitat quality. Accordingly, to evaluate how the effects of spring temperature and population size on dispersal are influenced by habitat quality, we compared dispersal from two types of islands. On the first set of islands, the house sparrows mainly breed in close association with dairy farms, with access to cattle-food and shelter all year round. On the second set of islands, there are no farms and sparrows are much more exposed to variation in food availability and weather conditions. During the winter, the ground is regularly snow covered, which reduces access to grass seeds and other natural food resources on the non-farm islands. Therefore, we assume that farm islands represent superior habitat, whereas the non-farm islands represent inferior habitat for the house sparrows.
2. Material and methods
(a). General field procedures
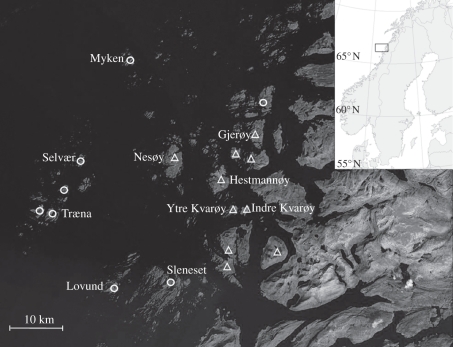
The study was conducted from 1993 to 2008 in a house sparrow metapopulation in northern Norway (66° N, 13° E; figure 1). During the study period, individuals were observed and captured annually on 18 islands covering an area of more than 1600 km2 [36]. Owing to differences between islands in the accessibility to the nests, breeding was monitored more closely on nine of the islands than on the remaining islands. These nine islands were included as natal islands in this study. All the 18 islands were included as potential targets for dispersal. The natal islands were divided into two categories that differed in quality in terms of food availability and shelter: five farm islands and four non-farm islands (see §1).
Figure 1.
The house sparrow metapopulation study system on the coast of Norway (66° N, 13° E). The 18 study islands are marked with either a triangle (island with cattle farms) or a circle (islands without farms). The farm islands are assumed to represent superior habitat for the house sparrows, whereas the non-farm islands represent inferior habitat. The nine natal populations included in this study are labelled with the island name. Temperature data were collected from a weather station on the study island Myken (not included as natal population, but indicated with name).
Fieldwork was performed from early May to mid August each year during this period and for approximately one month from the end of September to the beginning of November. The birds reach sexual maturity in their second calendar year, and can lay up to three clutches per season, each containing on average five eggs. Each island was searched thoroughly for any active nests at least once a week during the summer. Nests were usually visited two to three times during the incubation period. The onset of breeding was estimated as day of first egg. If nests were found during egg-laying, laying date could be determined directly. If nests were found during incubation or during the nestling stage, a hatching day was calculated, based on an estimate of nestling age on the first visit after hatching. Laying day was then calculated from an assumed incubation period of 11 days (median incubation time in this study area) and the minimum clutch size, which was estimated from the sum of number of eggs and living and dead offspring. We used data from 854 first clutches to determine the onset of breeding. When nestlings were between age 8 and 12 days, ca 25 µl of blood was collected from their brachial vein and they were banded with a numbered metal ring and unique combinations of plastic colour leg rings. Nestling data are available from 1993 on the five farm islands and from 1999 on the four non-farm islands. The last cohort included in this study was born in 2007 and thus 2008 was the last year of study. The sum of number of adults and number of juveniles was used as an index of total population size. The adult population size was estimated differently on the two categories of islands: on the non-farm islands, adult population size was censused by counting individuals just before the onset of breeding in May. On the farm-islands, the proportion of ringed birds was very high (greater than 90%) so we assume that the number of different adult birds, which were observed or captured during the breeding season, provided a better estimate of population size than the census data [34]. The annual number of fledged juveniles was estimated as the number of nestlings per nest alive at day 8–13 [40], averaged across nests, multiplied by the number of adult females.
The house sparrow is a highly sedentary species, and has limited natal dispersal (ca 10%) and negligible breeding dispersal (less than 2‰) in the study population [34,35]. Most natal dispersal takes place in the autumn [41]. Recaptures and observations of marked birds were used to determine whether fledglings survived until their second calendar year, i.e. recruited to the breeding population, and whether inter-island dispersal had occurred. Recapture rates do not differ between residents and immigrants [35]. A dispersing individual was defined as an individual which was marked as a nestling at one of the nine main study islands and then performed inter-island dispersal and was recorded as a recruit in their second calendar year on any of the other islands in the whole study system. Similarly, a resident was defined as an individual which was marked as a nestling on any of the nine main study islands and then recorded as a recruit on its natal island.
(b). Weather data
We used mean temperature for April–May collected from Myken weather station, located within the study system (66°45′52″ N, 12°28′37″ E; figure 1). The justification for the use of this weather variable is that house sparrows and the ecologically similar tree sparrow (Passer montanus) breeding in the UK adjust their breeding time to spring air temperature [37]. Furthermore, the temperature during this period is critical for nestling and juvenile survival [36] and may therefore influence the local abundance of conspecifics. The weather showed high spatial correlation in the study area [36]. Thus, temperature is considered to be the same across islands but to vary between years.
(c). Statistical analyses
The relationship between onset of breeding and spring temperature was analysed with linear mixed models with natal island as a random factor. In the first set of dispersal models, overall annual dispersal rate was analysed with logistic regression models with a binomial error and a logit link function, using the number of recruits that had performed natal dispersal versus the number of philopatric recruits as response variable. In the second set of models, we tested if the relationship between dispersal rate and temperature, onset of breeding and population size, respectively, depended on habitat quality. This was achieved by testing if the relationships differed between the two categories of islands that differed in quality (farm islands versus non-farm islands), by including the interaction term between island category and the various predictor variables. For all models in this study, the coefficients for the interaction with island category were calculated relative to an intercept representing the farm islands. Thus, for example, a positive interaction term implies a more positive slope on non-farm islands compared with farm-islands. To quantify the predictive ability of the logistic regression models, we used the squared Pearson's correlation (r2) between the observed dispersal rates and the corresponding predicted dispersal rates derived from the models [42]. Coefficients within each of the two island categories were always calculated relative to an intercept representing the same island category. For all analyses, the software R v. 2.11.1 [43] was used.
3. Results
(a). Spring temperature and onset of breeding
The spring temperature in the study area showed a positive trend between 1954 and 2009 (β = 0.015 ± 0.0078, t = 2.00, p = 0.051; model r2 = 0.08, d.f. = 48) and the mean spring temperature has increased by 1°C, from 4.5°C to 5.5°C, during this half-century period. The spring temperature increased also during the study period (β = 0.11 ± 0.042, t = 2.51, p = 0.025; model r2 = 0.31, d.f. = 13). High spring temperatures were associated with early onset of breeding (β = −2.09 ± 0.84, t = −2.49, n = 854 clutches, p = 0.017).
(b). Dispersal rate
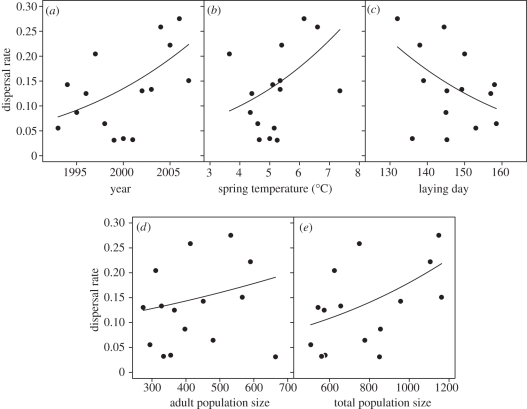
We first analysed annual dispersal rate (i.e. proportion of recruits that had performed natal dispersal) without accounting for habitat type, using logistic regression models. In the models, we used data on 558 individuals that we had followed from nestling stage and that we had registered in their second calendar year: 474 of these individuals remained on their natal island and 84 had successfully performed natal dispersal to a new island (electronic supplementary material, table S1). The dispersal rate increased with year (β = 0.087 ± 0.028, z = 3.13, p = 0.002; model r2 = 0.26, d.f. = 13; figure 2a), and increasing spring temperature (β = 0.33 ± 0.13, z = 2.49, p = 0.013; model r2 = 0.13, d.f. = 13; figure 2b), and was higher in years with early onset of breeding (β = −0.037 ± 0.015, z = −2.49, p = 0.013; model r2 = 0.10, d.f. = 13; figure 2c). The proportion of dispersing recruits was not significantly related to adult population size on the natal islands (β = 0.0013 ± 0.0010, z = 1.27, p = 0.203; model r2 = 0.03, d.f. = 13; figure 2d). However, the proportion of dispersing recruits was positively related to total population size (adults plus juveniles) on the natal islands (β = 0.0015 ± 0.00051, z = 2.93, p = 0.003; model r2 = 0.25, d.f. = 13; figure 2e).
Figure 2.
The relationship between mean annual dispersal rate in a house sparrow metapopulation in northern Norway and: (a) year, (b) spring temperature, (c) egg laying day for the clutch from which the individual hatched, (d) adult population size and (e) total population size (adults plus juveniles). The dispersal rates were estimated as the proportion of second-year individuals (i.e. recruits) that dispersed from their natal island. Data on 558 individuals were used, 474 philopatric and 84 dispersers. The regression lines are predicted values from generalized linear models with binomial error and logit link function. For further information about the pattern of interchange of individuals among islands, see the electronic supplementary material, table S1.
(c). Dispersal rate and habitat quality
We analysed if the temporal variation in dispersal rate differed between the two types of habitat (i.e. farm-islands versus non-farm islands), which differed in resource availability in terms of food and shelter, by testing for an interaction between habitat type and the different predictor variables in logistic regression models. We followed the fate of 407 individuals born on farm islands: 371 of these individuals remained on their natal island and 36 had successfully performed natal dispersal to a new island. Of the 151 individuals born on non-farm islands and that were registered in their second calendar year, 103 recruited on their natal island and 48 had dispersed (electronic supplementary material, table S1).
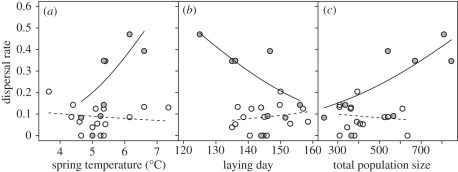
The relationship between dispersal rate and spring temperature differed between the two habitat types, as indicated by the significant interaction between temperature and habitat (β = 0.96 ± 0.36, z = 2.65, p = 0.008). On the non-farm islands, the dispersal rate increased with temperature (β = 0.83 ± 0.36, z = 2.71, p = 0.007). On the farm islands, the dispersal rate did not change with temperature (β = −0.13 ± 0.19, z = −0.68, p = 0.494; overall model: p < 0.001, r2 = 0.55, d.f. = 19; figure 3a). The relationship between dispersal rate and onset of breeding differed between the two habitat types (interaction date of egg-laying × habitat type: β = −0.070 ± 0.029, z = −2.39, p = 0.017). On the non-farm islands, the dispersal rate was lower in years with late onset of breeding (β = −0.050 ± 0.018, z = −2.72, p = 0.007). On the farm islands, there was no significant relationship between dispersal rate and onset of breeding (β = 0.021 ± 0.023, z = 0.89, p = 0.372; overall model: p < 0.001, r2 = 0.51, d.f. = 19; figure 3b). The relationship between dispersal rate and total population size on the natal islands tended to differ between the two habitat types (interaction total population size × habitat type: β = 0.0037 ± 0.0020, z = 1.90, p = 0.058). On the non-farm islands, dispersal rate increased with total population size on the natal islands (β = 0.0028 ± 0.0010, z = 2.73, p = 0.006). On the farm islands, there was no significant relationship between dispersal rate and total population size (β = −0.0098 ± 0.0017, z = −0.58, p = 0.563; overall model: p < 0.001, r2 = 0.60, d.f. = 19; figure 3c).
Figure 3.
The relationship between annual dispersal rate (i.e. the proportion of recruits that dispersed from their natal island) in a house sparrow metapopulation in northern Norway and (a) spring temperature, (b) onset of breeding estimated as mean day of first egg and (c) total population size (adults plus juveniles) on the natal islands. Islands were categorized as farm islands (open circles and dashed lines; in total 371 philopatric and 36 dispersing individuals) or non-farm islands (grey circles and solid lines; in total 103 philopatric and 48 dispersing individuals). The dispersal rates were estimated as the proportion of recruits that performed inter-island natal dispersal. The regression lines are predicted values from generalized linear models with binomial error and logit link function. For further information about the pattern of interchange of individuals among islands, see the electronic supplementary material, table S1.
In a model including both spring temperature and total population size on the natal islands, and their interaction with habitat type, there was still a significant positive relationship between dispersal rate and spring temperature (β = 0.73 ± 0.33, z = 2.20, p = 0.028) and total population size (β = 0.0025 ± 0.0011, z = 2.28, p = 0.023; overall model: p < 0.001, r2 = 0.74, d.f. = 17) on the non-farm islands. On the farm-islands, the same model revealed no significant relationship between dispersal rate and spring temperature or between dispersal rate and total population size (both p > 0.5). The analyses indicated that dispersal rate increased with both spring temperature and total population size at the natal island on the non-farm islands. By contrast, no such significant relationships were found for the farm islands.
4. Discussion
This study demonstrates that increasing spring temperature affects both onset of breeding and annual dispersal rate (figure 2b) of house sparrows. Furthermore, the relationships between dispersal rates and both spring temperature and population size differ among populations, dependent on their habitat quality. Dispersal rates increased with temperature only on islands without farms where house sparrows lacked the food resources and shelter provided by farms (figure 3a). Furthermore, dispersal rates increased with natal population size on islands without farms, but not on islands where farms were present (figure 3c). Thus, spatial variation in habitat quality generates spatial variation in temperature- and density-dependent dispersal behaviour.
(a). Dispersal, spring temperature and population size
The overall increase in annual dispersal rate with temperature in house sparrows is consistent with other studies on birds. Dispersal distances increased with spring temperature in the arctic tern (Sterna paradisaea) [29] and the common buzzard (Buteo buteo) [30]. In great tits (Parus major), the number of immigrant females was positively related to the spring temperature of the previous year [31]. Furthermore, in dippers (Cinclus cinclus), the number of immigrants was positively related to winter temperature [32]. A similar pattern is also described in other taxa. For instance, in three species of Daphnia sp., colonization rates of empty rock pools by resting eggs were higher following warmer and drier summers [44]. In insects, a positive relationship between flight activity and temperature is well known [45]. Thus, the winter pine processionary moth (Thaumetopoea pityocampa) rapidly expanded its range following a heat wave [46]. In common lizards, the annual proportion of dispersing juveniles decreased with June temperature, but increased with August temperature [26]. These results suggest that temporal variation in climate may influence the dispersal process, often with an increase in dispersal propensity with increasing temperature.
In addition, within a limited geographical region, there may be ecological differences between populations of the same species that may modify the response to climate change [28]. Thus, in the present study, dispersal rate was positively related to temperature in the ‘low-quality’ habitats without farms, whereas no such relationship was found in the ‘high-quality’ habitats with farms. In general, little is known about how variation in natal habitat quality may modify the relationship between dispersal rate and climate factors, often for the obvious reason that, unlike in our study system, the origin of dispersers is unknown and hence the ecological and demographical properties of the natal population cannot be assessed. One exception is a long-term experiment on common lizards [26], in which the interaction between climate and resource availability was investigated by manipulating maternal dietary conditions. A decrease in natal dispersal rate with June temperature was unaffected by maternal food availability, whereas an increase in dispersal rate with August temperature was not present under poor food conditions.
A possible mechanism that may explain why the relationship between dispersal rate and spring temperature differed between the two set of islands is related to temperature-dependent production of young and how habitat quality may influence the degree of density-dependence in dispersal. In warm years, fledging success and juvenile survival were higher than in years with cold weather [36]. Furthermore, the advance in timing of breeding with warmer spring temperature, as found in the current study, may increase the probability of raising additional broods, as found in many bird species (e.g. [39]). Thus, a warm spring will lead to a larger number of conspecifics during late summer and autumn, i.e. during the period when dispersal commonly occurs in the house sparrow [35,41]. In fact, there was a positive correlation between spring temperature and number of juveniles produced (r = 0.21, p = 0.05). The correlation between total population size and spring temperature was also positive, albeit weaker (r = 0.17, p = 0.12). If competition over resources is the proximate factor inducing emigration in warm years, the interaction between population size and habitat quality in this study suggests that the strength of competition depends not only on population size itself, but rather population size in relation to habitat quality, e.g. in terms of availability and predictability of critical food resources. A limiting resource that often seems to trigger dispersal in birds is food [14]. For example, an experiment with food supplementation reduced dispersal distances in the song sparrow (Melospiza melodia) [47]. Although the house sparrow is a highly social species, which prefers to feed in flocks rather than alone [41], increased competition over food may lead to aggression among flock members and reduced efficiency of food acquisition. On the non-farm islands, the sparrows often have to rely on bird feeders in human settlements, which leads to much higher densities of foraging sparrows at such spatially concentrated food sources and also a much more variable supply of food compared with the farm islands. In fact, the area of a high-density food patch predicts flock size in the house sparrow [41], which may contribute to the differences between the two sets of islands in addition to the amount of food itself. Thus, aggression between sparrows may be higher on islands without farms than on the islands with farms. This may incite juveniles to disperse to a greater extent, in order to reach a less crowded island or an island of higher quality. Accordingly, dispersal rate was positively related to population size on non-farm islands. Positive density-dependent dispersal, in particular more emigration from high densities, is expected from theory under a wide range of conditions [16] and has broad empirical support [12]. On the other hand, on the farm islands, where the availability and predictability of the food supply are high, there was no relationship between emigration rate and natal population size. Thus, densities in relation to habitat quality may be lower on the farm islands than on the non-farm islands. This seems to mitigate the competition over resources and explain why there is no density-dependence at the range of population sizes recorded on the high-quality islands during the present study.
In a parametric model of dispersal patterns, using data from the same house sparrow metapopulation as in this study, Tufto et al. [34] suggested that the probability of staying resident increased with increasing local density, which contrasts with our findings. One possible explanation for these seemingly contradictory results is that Tufto et al. [34] assumed that the probability of staying resident and the probability of immigrating into a new population depended on density in the same way, which is not necessarily the case [14]. For example, high density in the natal habitat may increase emigration propensity, whereas high density in the recipient habitat may inhibit immigration.
The fact that dispersal rate is determined by an interaction between population size and habitat quality, suggests that dispersal decision of juvenile house sparrows is based on a combination of cues related to local population size as well as habitat quality, such as food abundance [10,16]. A long-term study of pike (Esox lucius) [48] revealed a similar pattern when comparing density-dependence of dispersal in two lake basins. Large pike from the most productive south basin showed a low degree of density-dependence in dispersal, whereas the opposite was found for the north basin. Habitat-specific influence of density on dispersal, such as in our study, has also been documented in insects [49–51], but has only rarely been investigated in birds. One exception is a study on great tits (Parus major), where the sign of density-dependence of immigration differed between populations in The Netherlands [31]. The type of density-dependence is not only influenced by habitat characteristics, but may also vary across the range of densities that a species encounter. For example, in an experimental study on a butterfly, Glanville fritillary (Melitaea cinxia), positive density-dependent dispersal was demonstrated [50], whereas another experiment in the same species showed that individuals left low-density patches at a higher rate [52]. Thus, Enfjäll & Leimar [50] suggested that the relationship between dispersal and density in M. cinxia might follow a U-shaped curve.
As described above, temperature may be an environmental driver for density-dependent emigration in our study system. In addition, temperature may also influence density-independent emigration, via the effect of temperature on onset of breeding. We found that dispersal rate was higher in years of early breeding. There is some evidence that individuals from early clutches are of low phenotypic quality [40], which potentially could affect their dispersal propensity. We have no strong a priori reasons why early breeding by itself should cause an increase in dispersal in the low-quality habitat but not in the high-quality habitat. However, we cannot exclude a differential effect of early breeding in the two habitat types that are related to habitat quality and differences in phenology.
(b). Onset of breeding and temperature
There was a negative relationship between laying date and spring temperature in this study. The direction of response in laying date to temperature was similar to that in British house sparrows and the closely related tree sparrow [37], as well as in many other bird species [27]. On the other hand, in a population of Italian sparrows (Passer italiae) in northern Italy, the onset of breeding was not related to temperature, but instead advanced with increasing spring rainfall [53]. Such a regional difference in the response to temperature between species within the genus Passer suggests that different environmental factors are important to initiate breeding in northern and southern ranges of Passer populations, possibly through their effect on food resources necessary for adults (shoots, buds and seeds) and nestlings (i.e. insects) [41].
(c). Implications
Climate change forces species to move, adapt or die. Thus, dispersal has a central role in this context: by allowing individuals to shift their distribution, and through gene flow that contributes to the genetic variation necessary to adapt to climatic stress. Furthermore, relatively small changes in climate together with temperature-dependent immigration may have a large impact on population dynamics [31,32]. In addition, density-dependent emigration may strongly affect the local population size and hence the probability of extinction of a metapopulation [54].
In this study, we have shown that the effect of temperature on dispersal may differ between populations, also within a limited geographical area. It adds to the complexity when exploring the consequences of climate change—not only are dispersal characteristics and density-dependence important for population dynamics and the response to climate change [4,28], but the dispersal process itself is a plastic trait that may respond to climate in a habitat-specific way. This is likely to have important consequences for dynamics and viability of populations and needs to be accounted for when exploring how populations respond to climate change. For instance, climate-induced dispersal influenced by spatial heterogeneity in habitat quality may result in large shifts of the abundance of the species within its geographical distribution range [55]. Similarly, in a metapopulation model of the European mountain hare (Lepus timidus), the response of range limits to climate change was sensitive to the degree of dispersal [9]. Thus, knowledge about the causes of dispersal is of great importance in order to predict the long-term consequences of climate change on the viability of populations both at local and regional scales [4,9,56,57].
Acknowledgements
This study was approved by the Norwegian Directorate for Nature Management.
We want to thank: two anonymous referees for valuable comments, our dedicated fieldworkers and the inhabitants in our study area for their hospitality. This study was funded by the Norwegian Research Council's PREDCLIM grant and the Norwegian University of Science and Technology (NTNU) through core funding of the Center of Conservation Biology.
References
- 1.Clobert J., Danchin E., Dhondt A. A., Nichols J. D. 2001. Dispersal. New York, NY: Oxford University Press [Google Scholar]
- 2.Travis J. M. J. 2003. Climate change and habitat destruction: a deadly anthropogenic cocktail. Proc. R. Soc. Lond. B 270, 467–473 10.1098/rspb.2002.2246 (doi:10.1098/rspb.2002.2246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Ann. Rev. Ecol. Evol. Syst. 37, 637–669 10.1146/annurev.ecolsys.37.091305.110100 (doi:10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 4.Best A. S., Johst K., Münkemüller T., Travis J. M. J. 2007. Which species will succesfully track climate change? The influence of intraspecific competition and density dependent dispersal on range shifting dynamics. Oikos 116, 1531–1539 10.1111/j.2007.0030-1299.16047.x (doi:10.1111/j.2007.0030-1299.16047.x) [DOI] [Google Scholar]
- 5.Brooker R. W., Travis J. M. J., Clark E. J., Dytham C. 2007. Modelling species' range shifts in a changing climate: the impacts of biotic interactions, dispersal distance and the rate of climate change. J. Theor. Biol. 245, 59–65 10.1016/j.jtbi.2006.09.033 (doi:10.1016/j.jtbi.2006.09.033) [DOI] [PubMed] [Google Scholar]
- 6.Jump A. S., Peñuelas J. 2005. Running to stand still: adaptation and the response of plants to rapid climate change. Ecol. Lett. 8, 1010–1020 10.1111/j.1461-0248.2005.00796.x (doi:10.1111/j.1461-0248.2005.00796.x) [DOI] [PubMed] [Google Scholar]
- 7.Hughes C. L., Dytham C., Hill J. K. 2007. Modelling and analysing evolution of dispersal in populations at expanding range boundaries. Ecol. Entomol. 32, 437–445 10.1111/j.1365-2311.2007.00890.x (doi:10.1111/j.1365-2311.2007.00890.x) [DOI] [Google Scholar]
- 8.Jiguet F., Gadot A. S., Julliard R., Newson S. E., Couvet D. 2007. Climate envelope, life history traits and the resilience of birds facing global change. Glob. Change Biol. 13, 1672–1684 10.1111/j.1365-2486.2007.01386.x (doi:10.1111/j.1365-2486.2007.01386.x) [DOI] [Google Scholar]
- 9.Anderson B. J., Akçakaya H. R., Araujo M. B., Fordham D. A., Martinez-Meyer E., Thuiller W., Brook B. W. 2009. Dynamics of range margins for metapopulations under climate change. Proc. R. Soc. B 276, 1415–1420 10.1098/rspb.2008.1681 (doi:10.1098/rspb.2008.1681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clobert J., Le Galliard J. F., Cote J., Meylan S., Massot M. 2009. Informed dispersal, heterogeneity in animal dispersal syndromes and the dynamics of spatially structured populations. Ecol. Lett. 12, 197–209 10.1111/j.1461-0248.2008.01267.x (doi:10.1111/j.1461-0248.2008.01267.x) [DOI] [PubMed] [Google Scholar]
- 11.Andreassen H. P., Ims R. A. 2001. Dispersal in patchy vole populations: role of patch configuration, density dependence, and demography. Ecology 82, 2911–2926 10.1890/0012-9658(2001)082[2911:DIPVPR]2.0.CO;2 (doi:10.1890/0012-9658(2001)082[2911:DIPVPR]2.0.CO;2) [DOI] [Google Scholar]
- 12.Lambin X., Aars J., Piertney S. B. 2001. Interspecific competition, kin competition and kin facilitation: a review of empirical evidence. In Dispersal (eds Clobert J., Danchin E., Dhondt A. A., Nichols J. D.), pp. 110–122 New York, NY: Oxford University Press [Google Scholar]
- 13.Le Galliard J. F., Ferriere R., Clobert J. 2003. Mother-offspring interactions affect natal dispersal in a lizard. Proc. R. Soc. Lond. B 270, 1163–1169 10.1098/rspb.2003.2360 (doi:10.1098/rspb.2003.2360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ims R. A., Hjermann D. Ø. 2001. Condition-dependent dispersal. In Dispersal (eds Clobert J., Danchin E., Dhondt A. A., Nichols J. D.), pp. 203–216 Oxford, UK: Oxford University Press [Google Scholar]
- 15.Stamps J. A. 1988. Conspecific attraction and aggregation in territorial species. Am. Nat. 131, 329–347 [Google Scholar]
- 16.Enfjäll K., Leimar O. 2009. The evolution of dispersal: the importance of information about population density and habitat characteristics. Oikos 118, 291–299 10.1111/j.1600-0706.2008.16863.x (doi:10.1111/j.1600-0706.2008.16863.x) [DOI] [Google Scholar]
- 17.Denno R. F., Peterson M. A. 1995. Density-dependent dispersal and its consequences for population dynamics. In Population dynamics: new approaches and synthesis (eds Cappucino N., Price P. W.), pp. 113–130 San Diego, CA: Academic Press [Google Scholar]
- 18.Matthysen E. 2005. Density-dependent dispersal in birds and mammals. Ecography 28, 403–416 10.1111/j.0906-7590.2005.04073.x (doi:10.1111/j.0906-7590.2005.04073.x) [DOI] [Google Scholar]
- 19.McPeek M. A., Holt R. D. 1992. The evolution of dispersal in spatially and temporally varying environments. Am. Nat. 140, 1010–1027 [Google Scholar]
- 20.Hansson B., Bensch S., Hasselquist D. 2003. Heritability of dispersal in the great reed warbler. Ecol. Lett. 6, 290–294 10.1046/j.1461-0248.2003.00436.x (doi:10.1046/j.1461-0248.2003.00436.x) [DOI] [Google Scholar]
- 21.Doncaster C. P., Clobert J., Doligez B., Gustafsson L., Danchin E. 1997. Balanced dispersal between spatially varying local populations: an alternative to the source-sink model. Am. Nat. 150, 425–445 10.1086/286074 (doi:10.1086/286074) [DOI] [PubMed] [Google Scholar]
- 22.Altermatt F., Ebert D. 2010. Populations in small, ephemeral habitat patches may drive dynamics in a Daphnia magna metapopulation. Ecology 91, 2975–2982 10.1890/09-2016.1 (doi:10.1890/09-2016.1) [DOI] [PubMed] [Google Scholar]
- 23.Szulkin M., Sheldon B. 2008. Dispersal as a means of inbreeding avoidance in a wild bird population. Proc. R. Soc. B 275, 703–711 10.1098/rspb.2007.0989 (doi:10.1098/rspb.2007.0989) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massot M., Clobert J., Pilorge T., Lecomte J., Barbault R. 1992. Density dependence in the common lizard: demographic consequences of a density manipulation. Ecology 73, 1742–1756 10.2307/1940026 (doi:10.2307/1940026) [DOI] [Google Scholar]
- 25.Doligez B., Pärt T. 2008. Estimating fitness consequences of dispersal: a road to ‘know-where’? Non-random dispersal and the underestimation of dispersers' fitness. J. Anim. Ecol. 77, 1199–1211 10.1111/j.1365-2656.2008.01446.x (doi:10.1111/j.1365-2656.2008.01446.x) [DOI] [PubMed] [Google Scholar]
- 26.Massot M., Clobert J., Ferriere R. 2008. Climate warming, dispersal inhibition and extinction risk. Glob. Change Biol. 14, 461–469 10.1111/j.1365-2486.2007.01514.x (doi:10.1111/j.1365-2486.2007.01514.x) [DOI] [Google Scholar]
- 27.Dunn P., Winkler D. W. 2010. Effects of climate change on timing of breeding and reproductive success in birds. In Effects of climate change on birds (eds Møller A. P., Fiedler W., Berthold P.), pp. 113–128 Oxford, UK: Oxford University Press [Google Scholar]
- 28.Sæther B.-E., Engen S. 2010. Population consequences of climate change. In Effects of climate change on birds (eds Møller A. P., Fiedler W., Berthold P.), pp. 191–212 Oxford, UK: Oxford University Press [Google Scholar]
- 29.Møller A. P., Flensted-Jensen E., Mardal W. 2006. Dispersal and climate change: a case study of the Arctic tern Sterna paradisaea. Glob. Change Biol. 12, 2005–2013 10.1111/j.1365-2486.2006.01216.x (doi:10.1111/j.1365-2486.2006.01216.x) [DOI] [Google Scholar]
- 30.Walls S. S., Kenward R. E., Holloway G. J. 2005. Weather to disperse? Evidence that climatic conditions influence vertebrate dispersal. J. Anim. Ecol. 74, 190–197 10.1111/j.1365-2656.2005.00914.x (doi:10.1111/j.1365-2656.2005.00914.x) [DOI] [Google Scholar]
- 31.Grøtan V., Sæther B.-E., Engen S., Van Balen J. H., Perdeck A. C., Visser M. E. 2009. Spatial and temporal variation in the relative contribution of density dependence, climate variation and migration to fluctuations in the size of great tit populations. J. Anim. Ecol. 78, 447–459 10.1111/j.1365-2656.2008.01488.x (doi:10.1111/j.1365-2656.2008.01488.x) [DOI] [PubMed] [Google Scholar]
- 32.Sæther B.-E., Tufto J., Engen S., Jerstad K., Røstad O. W., Skåtan J. E. 2000. Population dynamical consequences of climate change for a small temperate songbird. Science 287, 854–856 10.1126/science.287.5454.854 (doi:10.1126/science.287.5454.854) [DOI] [PubMed] [Google Scholar]
- 33.Balbontín J., Møller A. P., Hermosell I. G., Marzal A., Reviriego M., de Lope F. 2009. Divergent patterns of impact of environmental conditions on life history traits in two populations of a long-distance migratory bird. Oecologia 159, 859–872 10.1007/s00442-008-1267-8 (doi:10.1007/s00442-008-1267-8) [DOI] [PubMed] [Google Scholar]
- 34.Tufto J., Ringsby T. H., Dhondt A. A., Adriaensen F., Matthysen E. 2005. A parametric model for estimation of dispersal patterns applied to five passerine spatially structured populations. Am. Nat. 165, E13–E26 10.1086/426698 (doi:10.1086/426698) [DOI] [PubMed] [Google Scholar]
- 35.Pärn H., Jensen H., Ringsby T. H., Sæther B.-E. 2009. Sex-specific fitness correlates of dispersal in a house sparrow metapopulation. J. Anim. Ecol. 78, 1216–1225 10.1111/j.1365-2656.2009.01597.x (doi:10.1111/j.1365-2656.2009.01597.x) [DOI] [PubMed] [Google Scholar]
- 36.Ringsby T. H., Sæther B.-E., Tufto J., Jensen H., Solberg E. J. 2002. Asynchronous spatiotemporal demography of a house sparrow metapopulation in a correlated environment. Ecology 83, 561–569 10.1890/0012-9658(2002)083[0561:ASDOAH]2.0.CO;2 (doi:10.1890/0012-9658(2002)083[0561:ASDOAH]2.0.CO;2) [DOI] [Google Scholar]
- 37.Seel D. C. 1968. Breeding seasons of house sparrow and tree sparrow Passer spp. at Oxford. Ibis 110, 129–144 [Google Scholar]
- 38.Altwegg R., Ringsby T. H., Sæther B.-E. 2000. Phenotypic correlates and consequences of dispersal in a metapopulation of house sparrows Passer domesticus. J. Anim. Ecol. 69, 762–770 10.1046/j.1365-2656.2000.00431.x (doi:10.1046/j.1365-2656.2000.00431.x) [DOI] [PubMed] [Google Scholar]
- 39.Brinkhof M. W. G., Cave A. J., Daan S., Perdeck A. C. 2002. Timing of current reproduction directly affects future reproductive output in European coots. Evolution 56, 400–411 10.1111/j.0014-3820.2002.tb01349.x (doi:10.1111/j.0014-3820.2002.tb01349.x) [DOI] [PubMed] [Google Scholar]
- 40.Ringsby T. H., Sæther B.-E., Solberg E. J. 1998. Factors affecting juvenile survival in house sparrow Passer domesticus. J. Avian Biol. 29, 241–247 [Google Scholar]
- 41.Anderson T. R. 2006. Biology of the ubiquitous house sparrow: from genes to populations. New York, NY: Oxford University Press [Google Scholar]
- 42.Zheng B. Y., Agresti A. 2000. Summarizing the predictive power of a generalized linear model. Stat. Med. 19, 1771–1781 (doi:10.1002/1097-0258(20000715)19:13<1771::AID-SIM485>3.0.CO;2-P) [DOI] [PubMed] [Google Scholar]
- 43.R Development Core Team 2010. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 44.Altermatt F., Pajunen V. I., Ebert D. 2008. Climate change affects colonization dynamics in a metacommunity of three Daphnia species. Glob. Change Biol. 14, 1209–1220 10.1111/j.1365-2486.2008.01588.x (doi:10.1111/j.1365-2486.2008.01588.x) [DOI] [Google Scholar]
- 45.Parmesan C. 2001. Coping with modern times? Insect movement and climate change. In Insect movement: mechanisms and consequences (eds Woiwood I., Reynolds D. R., Thomas C. D.), pp. 387–413 Wallingford, UK: CABI Publishing [Google Scholar]
- 46.Battisti A., Stastny M., Buffo E., Larsson S. 2006. A rapid altitudinal range expansion in the pine processionary moth produced by the 2003 climatic anomaly. Glob. Change Biol. 12, 662–671 10.1111/j.1365-2486.2006.01124.x (doi:10.1111/j.1365-2486.2006.01124.x) [DOI] [Google Scholar]
- 47.Arcese P. 1989. Intrasexual competition, mating system and natal dispersal in song sparrows. Anim. Behav. 38, 958–979 [Google Scholar]
- 48.Haugen T. O., Winfield I. J., Vøllestad L. A., Fletcher J. M., Ben James J., Stenseth N. C. 2007. Density dependence and density independence in the demography and dispersal of pike over four decades. Ecol. Monogr. 77, 483–502 10.1890/06-0163.1 (doi:10.1890/06-0163.1) [DOI] [Google Scholar]
- 49.Simmons A. D., Thomas C. D. 2004. Changes in dispersal during species' range expansions. Am. Nat. 164, 378–395 10.1086/423430 (doi:10.1086/423430) [DOI] [PubMed] [Google Scholar]
- 50.Enfjäll K., Leimar O. 2005. Density-dependent dispersal in the Glanville fritillary, Melitaea cinxia. Oikos 108, 465–472 10.1111/j.0030-1299.2005.13261.x (doi:10.1111/j.0030-1299.2005.13261.x) [DOI] [Google Scholar]
- 51.Bengtsson G., Hedlund K., Rundgren S. 1994. Food- and density-dependent dispersal: evidence from a soil collembolan. J. Anim. Ecol. 63, 513–520 [Google Scholar]
- 52.Kuussaari M., Nieminen M., Hanski I. 1996. An experimental study of migration in the Glanville fritillary butterfly Melitaea cinxia. J. Anim. Ecol. 65, 791–801 [Google Scholar]
- 53.Rubolini D., Ambrosini R., Caffi M., Brichetti P., Armiraglio S., Saino N. 2007. Long-term trends in first arrival and first egg laying dates of some migrant and resident bird species in northern Italy. Int. J. Biometeorol. 51, 553–563 10.1007/s00484-007-0094-7 (doi:10.1007/s00484-007-0094-7) [DOI] [PubMed] [Google Scholar]
- 54.Sæther B.-E., Engen S., Lande R. 1999. Finite metapopulation models with density-dependent migration and stochastic local dynamics. Proc. R. Soc. Lond. B 266, 113–118 10.1098/rspb.1999.0610 (doi:10.1098/rspb.1999.0610) [DOI] [Google Scholar]
- 55.Holt R. D., Keitt T. H., Lewis M. A., Maurer B. A., Taper M. L. 2005. Theoretical models of species' borders: single species approaches. Oikos 108, 18–27 10.1111/j.0030-1299.2005.13147.x (doi:10.1111/j.0030-1299.2005.13147.x) [DOI] [Google Scholar]
- 56.Hovestadt T., Poethke H. J. 2006. The control of emigration and its consequences for the survival of populations. Ecol. Model. 190, 443–453 10.1016/j.ecolmodel.2005.03.023 (doi:10.1016/j.ecolmodel.2005.03.023) [DOI] [Google Scholar]
- 57.Münkemüller T., Johst K. 2007. How does intraspecific density regulation influence metapopulation synchrony and persistence? J. Theor. Biol. 245, 553–563 10.1016/j.jtbi.2006.10.020 (doi:10.1016/j.jtbi.2006.10.020) [DOI] [PubMed] [Google Scholar]