Abstract
Testosterone (T) is thought to play a key role in male–male competition and courtship in many vertebrates, but its precise effects are unclear. We explored whether courtship behaviour in humans is modulated and preceded by changes in T. Pairs of healthy male students first competed in a non-physical contest in which their T levels became elevated. Each participant then had a short, informal interaction with either an unfamiliar man or woman. The sex of the stimulus person did not affect the participants' behaviour overall. However, in interactions with women, those men who had experienced a greater T increase during the contest subsequently showed more interest in the woman, engaged in more self-presentation, smiled more and made more eye contact. No such effects were seen in interactions with other men. This is the first study to provide direct evidence that elevating T during male–male competition is followed by increased affiliative behaviour towards women.
Keywords: courtship, testosterone, affiliative behaviour, male–male competition, humans, sexual selection
1. Introduction
Humans, like other animals, communicate their interest to potential mates through courtship [1], which may then be followed by negotiation to determine whether this interest is mutual. In many species, sexual behaviour is regulated by sex hormones, in particular testosterone (T). For example, T initiates sexual development, controls sperm production and supports sexual function [2]. Moreover, T seems to play a key role in courtship. In several species of birds, it has been shown that a wide variety of courtship behaviours increase when castrated males are implanted with T crystals in specific brain areas [3]. Additionally, appetitive sexual behaviour (e.g. courtship) of male rats is inhibited by castration but restored after treatment with exogenous T [4]. The administration of T in both mice and rats stimulates the expression of various copulatory behaviours [5,6].
The function of T in these contexts can be explained by the challenge hypothesis [7]. This hypothesis states that T levels rise during challenges that are relevant for reproduction and thereby prepare the body for an adaptive response. The challenge hypothesis was first applied to birds, but more recently has been extended to humans, by focusing on challenges faced by men such as disputes over social status and reactions to mating stimuli [8]. It is thought that, during these challenges, T functions to promote behaviour that facilitates mate acquisition or mating directly [9]. In support of this, it has been shown that a non-physical social interaction of 15 min (or even as little as 5 min) with an unfamiliar woman can provoke an increase in male T levels [9–12].
If T promotes behaviours that stimulate mate attraction, which specific behaviours might be affected? As in other species, human male courtship may involve specific display behaviours. In support of this, it has been shown that in social interactions those men who responded with an increase in their T levels were perceived by their female interaction partners as trying to impress [9], displaying extrovert behaviour and self-disclosing [10]. However, it remains unclear whether the T increases caused these behaviours or vice versa, as they both occurred at the same time. Furthermore, it has been shown that during courtship men reveal more about themselves than women do and consequently speak longer with a woman when they are interested in dating her, particularly if she seems interested too [13]. Additionally, men may attempt to display their dominance status: those men who successfully make social contact with women in bars exhibit more dominant behaviours such as glances that are short and direct, make more space-maximization movements, change location more often, and make more non-reciprocated touches to surrounding men and fewer closed movements (e.g. outward limb movements that cross over the main body torso) [14]. Such male–male competitive interactions may be important for reproductive success if they influence patterns of female choice, as emphasized by research on other animals [15]. For example, females may prefer dominant males if they are better able to provide food and protection for their offspring [16]. Evidence from the biological literature suggests that male T levels can affect both competitive behaviour and courtship of females [17].
We hypothesized that courtship behaviours in men are preceded and modulated by T. While no study to date has shown a causal link between T and courtship in humans, clinical research has shown that T can affect male sexual function directly. For example, when administering T to hypogonadal men, the libido and sexual function of the men increase [18]. Furthermore, a pharmacologically induced gonadal steroid deficiency in healthy men causes reductions in sexual desire and intercourse, which are restored shortly after stopping this treatment, over roughly the same time period as it takes serum T levels to recover [19].
In the study reported here we investigated whether endogenous T is linked to male affiliative behaviours (i.e. behaviours that promote social bonding) used in courtship. We focused on three types of affiliative behaviours in men: (i) interest in another person, such as giving them attention and asking questions; (ii) self-presentation, such as talking and revealing details about oneself; and (iii) positive facial cues, such as smiling and making eye contact. We first attempted to induce a change in T levels by pitting male participants against each other in a non-physical pairwise contest, in which the outcome was rigged [20]. After the contest, these men had a short informal interaction with either an unfamiliar woman (experimental condition) or an unfamiliar man (control condition). Salivary T was measured before and after the contest, and the behaviour of the participants during the subsequent interaction was unobtrusively filmed and later analysed by trained observers. We expected that an increase in T occurring during male–male competition would stimulate more affiliative behaviours when men then interacted with a woman, whereas there would be no such effect during interactions with another man.
2. Methods
(a). Participants
Eighty-four male students, aged 18–29 years (mean ± s.e.m.: 21.2 ± 0.32), participated in this study in exchange for €10. They were recruited by a male and a female research assistant, who approached men in the cafeterias of the University of Valencia and made announcements in the lecture halls. The participants had a mean body mass index (BMI) of 23.5 ± 0.46 and were all Caucasian. Average self-reported socio-economic status [21], on a scale from 1 (lowest) to 10 (highest), was 6.6 ± 0.09.
All participants were first interviewed by the research assistants and were asked to complete a questionnaire. We excluded individuals who were not heterosexual (open question: ‘what is your sexual orientation?’), who were currently in a relationship, who were enrolled in a psychology degree, who smoked more than five cigarettes a day or who reported a serious medical or psychological problem or drug abuse. Participants were also excluded if they were using any medication directly related to cardiac, emotional or cognitive function, or medication that was able to influence hormone levels, such as glucocorticoids or β-blockers. One participant was excluded from analyses because after participating he indicated he was bisexual, while another was excluded because he indicated he was in a relationship.
Up until one day before the experiment, the participants were asked to maintain their typical habits, including sleeping for as long as usual. Additionally, they were instructed to refrain from alcohol consumption and any heavy physical activity the day before the session. During the 2 h immediately prior to the session, participants were asked to drink only water and avoid any stimulants, such as coffee, cola, caffeine, tea or chocolate. All participants received some basic verbal information about the study and signed an informed consent form outlining the general procedure and the measurements taken.
(b). The competitive task
Each participant competed face-to-face with another participant on a computer task [20]. This task comprised 27 items similar to those used in intelligence tests. The participants were informed that they would win an item if they were the first to enter the correct answer. During the task, the participants were seated roughly 1 m apart on opposite sides of the same table, each behind their own computer screen and facing their opponent. The experimenter remained clearly visible to the participants and observed the progress of the task. Unknown to them, the outcome of the task was actually manipulated by the experimenter for a different purpose, investigated in a separate paper [20]. One participant was randomly assigned to the winner condition (20 items won and 7 items lost) and the other to the loser condition (7 items won and 20 items lost). At the end of the task, each participant was shown his final score on his computer screen, together with a statement of whether he had lost or won. Half of the participants in each condition (winner and loser) were then randomly assigned to have contact with another man, and the other half with a woman.
(c). Stimulus persons
Twelve men and six women acted as the stimulus persons. These were confederates of the experimenter chosen on the basis of similar age (23.33 ± 0.55 years) to the participants, and were of representative height and weight for the Spanish student population. All stimulus persons were thoroughly instructed before participating, to reduce differences between them in spontaneous verbal or non-verbal behaviour. They were told to act as if they were participants in the same study, to engage in friendly conversation with the participant in a natural manner and to allow long pauses if the participants elected not to talk. After the interaction, participants who had met a stimulus woman were asked to rate her physical attractiveness on a scale from 1 (not attractive) to 7 (highly attractive); the average rating given was 4.73 ± 0.18.
(d). Procedure
On arrival at the laboratory, the participants were greeted by the male experimenter (L.v.d.M.) and were briefed on the general procedure of the study (without telling them about the planned interaction with the stimulus person). To avoid confounds, the experimenter did not engage socially and kept contact to a minimum. Participants filled in an informed consent form, and their height and weight were measured. Next, participants provided a saliva sample (T1) for the measurement of their baseline T level.
To provoke a change in T, each participant first competed against another participant on the computer task, which took approximately 18 min to complete. Ten minutes after the completion of this task, the participants provided a second saliva sample (T2). Each participant was then taken to a separate room where they were instructed to work on a Sudoku puzzle. Already in this room was a stimulus person, pretending to be another participant completing a similar puzzle. Upon entering the room, the experimenter indicated that he did not have the correct version of the puzzle for the participant. The participant was asked to wait for the experimenter to return with the correct version. The experimenter then left the room and the participant and the stimulus person were left alone together for 5 min [22], during which the behaviour of the participant was discreetly filmed from a partly hidden camera. This camera could be spotted by participants but their attention was drawn away from it by the presence of the stimulus person. When the 5 min had elapsed, the experimenter returned with the correct version and the stimulus person then left the room with the experimenter.
After a further 15 min, the experimenter collected the puzzle, participants were debriefed about the true nature of the experiment and received €10. The whole procedure lasted 1.5 h and sessions were held from 16.00 to 20.00 h to control for the circadian rhythm of T [23].
(e). Behavioural measurements
Four female observers (22.00 ± 1.29 years old) were trained to interpret and reliably rate the participants' behaviour from the video recordings, on the basis of the behavioural scale shown in table 1. We chose to use only female observers to minimize the variation between their ratings and because we were specifically interested in male courtship behaviour. These observers were aware of the stimulus person's sex but blind to the participants' increases in salivary T. The scale consisted of nine items and the general frequency of each item could be rated from 1 (not at all) to 7 (all the time).
Table 1.
Three indices of participants' behaviour during their social interaction with the stimulus person.
| Indices | items | inter-observer reliability |
|---|---|---|
| interest in stimulus person | gave attention to stimulus person | 0.93 |
| showed interest in stimulus person | 0.93 | |
| interacted confidently with stimulus person | 0.95 | |
| asked questions | 0.76 | |
| was talkative | 0.96 | |
| self-presentation | talked about himself | 0.90 |
| revealed details about himself | 0.90 | |
| positive facial cues | was smiling | 0.92 |
| made eye contact | 0.92 |
Each observer rated the full set of videos on two separate occasions, with the second rating taking place one to three weeks after the first rating. To assess the repeatability of the ratings from a given observer, we calculated an intraclass correlation coefficient (ICC) for each item, based on the two separate ratings nested within each male subject. One item for one of the observers was removed from analyses as it had a repeatability score of ≤0.60. For each of the remaining items, the repeatability of the ratings ranged from 0.63 to 0.95 (0.82 ± 0.15) for the four observers.
We then averaged the observers' first and second ratings for each item, and used these average scores to assess the degree of agreement between the four observers. This was done by calculating an inter-observer ICC for each item (table 1) based on the four averages (one per observer) nested within each male subject.
Finally, for each item we took a grand average of that item's average scores from all four observers. We then created three composite indices on the basis of their theoretical relatedness, by averaging the final scores from each of the component items (table 1). These indices were: (i) interest in the stimulus person, ICC = 0.97; (ii) self-presentation, ICC = 0.98; (iii) positive facial cues, ICC = 0.75. The indices formed the dependent variables in our subsequent regression analyses (see below). Four participants were excluded from behavioural analysis as their interaction period was not correctly filmed owing to technical problems.
The three factors were correlated with each other (r between 0.506 and 0.799; all p ≤ 0.001). Confirmatory factor analysis showed that a three-factor model had a better fit than a one-factor model (Δ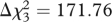 , p ≤ 0.001). The one-factor model did not provide an adequate fit to the data (
, p ≤ 0.001). The one-factor model did not provide an adequate fit to the data (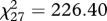 , p ≤ 0.001; comparative fit index, CFI = 0.87; standardized root mean square residual, SRMR = 0.072), whereas the three-factor model did provide an adequate fit (
, p ≤ 0.001; comparative fit index, CFI = 0.87; standardized root mean square residual, SRMR = 0.072), whereas the three-factor model did provide an adequate fit (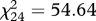 , p ≤ 0.001; CFI = 0.98; SRMR = 0.037). Note that an adequate fit requires both CFI ≥ 0.95 and SRMR ≤ 0.08 [24].
, p ≤ 0.001; CFI = 0.98; SRMR = 0.037). Note that an adequate fit requires both CFI ≥ 0.95 and SRMR ≤ 0.08 [24].
(f). Hormonal assays
Both saliva samples (T1 and T2) were collected by passive drooling. Participants deposited 5 ml of saliva in plastic vials which took approximately 5 min to fill. The samples were frozen at −20° C and shipped to the endocrinology laboratory at the University Medical Center Utrecht, the Netherlands. Depending on the starting time of the session, the first saliva sample (T1) was taken at approximately 16.10 or 18.10 h and the second saliva sample (T2) was taken at approximately 16.55 or 18.55 h, respectively. The T2 samples were taken 10 min after completing the competitive task as psychological stimulation needs some time to affect T levels [25]. There was no effect of starting time of the session on T1, T2 or the change in T levels (T2–T1; t-tests, all p ≥ 0.57). The saliva samples were analysed using radio-immunoassays. According to the modifications of Granger et al. [26], salivary T was determined with the double antibody T kit (DSL-4100) from Diagnostic Systems Laboratories Inc. (Webster, TX, USA). The detection limit was 4 pmol l−1. The mean inter-assay coefficient of variation was 7.5 ± 0.90 per cent and the mean intra-assay coefficient was 5.3 ± 1.30 per cent. One participant drank water while giving the saliva sample and was therefore excluded from the analysis.
(g). Statistical analysis
We first performed several independent t-tests to assess if there were any differences in the socio-demographic variables and baseline T levels between participants who interacted with a man or a woman. A multivariate analysis of variance (MANOVA) was then performed, including (i) interest in the stimulus person, (ii) self-presentation and (iii) positive facial cues as dependent variables, to investigate whether the behaviour of the participants was affected by the sex of the stimulus person (male or female), the outcome of the competition (winner or loser) and their interaction.
To test for an interaction between the sex of the stimulus person and the change in T during the competition on the behavioural indices, we used regression analyses following Aiken & West [27]. Using forward stepwise regression, three separate moderator regression analyses were performed with one of the behavioural indices as the dependent variable. The sex of the stimulus person was dummy-coded as 0 for a man and 1 for a woman. For calculating the change in T during the competition, we used the unstandardized residuals from regressing T1 on T2, because absolute changes in T levels are negatively correlated with baseline T and are sensitive to regression to the mean [28]. In step 1, the main effects of sex of the stimulus person and the change in T during the competition were entered. In step 2, the sex × change in T interaction was added and post hoc significance tests of the slopes were performed. For all behavioural indices, Bartlett's test [29] showed no violation of the assumption of homogeneity of error variance. For these final regression analyses, we excluded in total seven participants (one person who drank water during saliva sampling, one bisexual person, one person reporting to be in a relationship and four participants for whom we did not correctly film the contact period), leaving a final sample size of 77.
A value of p < 0.05 (two-tailed) was considered statistically significant. Statistical tests were performed with SPSS v. 16.0.
3. Results
(a). Preliminary analysis
As reported by van der Meij et al. [20], the change in salivary T between the first sample (before the contest) and the second sample (after the contest) did not differ between winners and losers (F1,80 = 0.70, p = 0.406). Overall, however, there was a significant increase in T of 16.4 per cent (±3.9), from 266.4 (±8.5) to 300.6 pmol l−1 (±10.5; F1,80 = 14.74, p < 0.001). For the remainder of our analysis, we focus on the subsequent interaction with a stimulus person. There were no differences between participants who had contact with a man or a woman for the following variables: change in T, baseline T, sex drive, age, height, weight, BMI, subjective socio-economic status, educational level, average weekly physical activity, alcohol use (t-tests, all p ≥ 0.15). Furthermore, the behaviour of participants as measured by the three behavioural indices was neither affected by the sex of the stimulus person (MANOVA: F3,71 = 1.56, p = 0.208) nor by the outcome of the competition (F3,71 = 0.41, p = 0.745), and there was no significant interaction effect between these variables (F3,71 = 0.87, p = 0.459). Behavioural ratings were equally variable for interactions with another man and with a woman (Levene's tests: interest in stimulus person, F = 1.52, p = 0.221; self-presentation, F = 0.01, p = 0.931; positive facial cues, F = 3.79, p = 0.055). The men's attractiveness ratings of the women were not correlated with the rated frequency of interest in the stimulus person (r39 = 0.079, p = 0.634), self-presentation (r39 = 0.205, p = 0.212) or positive facial cues (r39 = 0.035, p = 0.831).
(b). Testosterone and behaviour
For the main analyses, we considered how the subjects' behaviour was affected by their change in T, the stimulus person's sex and the interaction between these two factors. The outcome of the competition did not moderate any of these effects (see electronic supplementary material, appendix A), so we do not discuss it further. In supplementary analyses, we also found that baseline T neither predicted affiliative behaviours nor interacted with any of the experimental conditions (see electronic supplementary material, appendix B). Our results did not change substantially when controlling for the psychological state of the opponent [20], except that for positive facial cues the interaction term dropped to non-significance (see electronic supplementary material, appendix C).
(i). Interest in the stimulus person
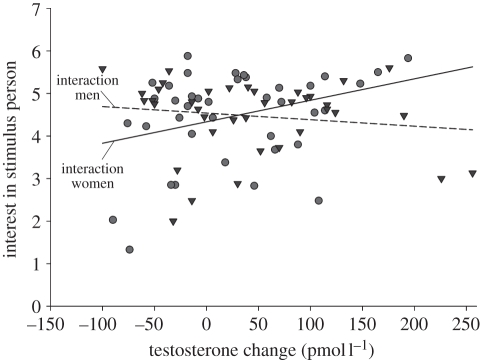
The model in step 1, including sex of the stimulus person and the change in T during the competition as predictor variables, did not explain a significant amount of variance in the index of interest in the stimulus person (F2,74 = 0.40, p = 0.670). However, entering the interaction term in step 2 significantly increased the amount of variance explained (ΔF1,73 = 5.13, p = 0.027, adjusted r2 = 3.8%, Δr2 = 6.5%). In this step, there were no main effects of sex of the stimulus person (p = 0.958) nor the change in T (p = 0.420), but there was a significant two-way interaction between the change in T and sex (β = 0.341, p = 0.027). Significance tests of the slopes revealed that the change in T did not affect the display of interest in the male stimulus person (t73 = −0.81, β = −0.12, p = 0.420), but a greater change in T was associated with the display of more interest in the female stimulus person (t73 = 2.31, β = 0.39, p = 0.024; figure 1).
Figure 1.
The relationship between the participant's change in testosterone and the extent to which he showed interest in a stimulus man (triangles) or woman (circles). Separate regression lines are plotted for each sex of the stimulus person.
(ii). Self-presentation
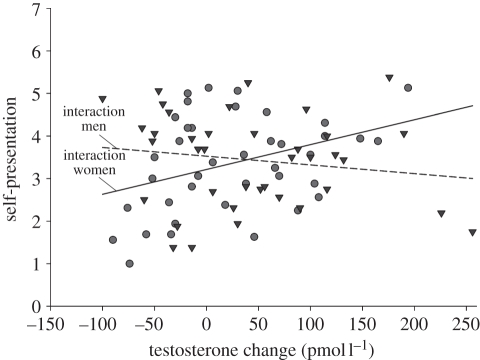
The model in step 1, including sex of the stimulus person and the change in T during the competition as predictor variables, did not explain a significant amount of variance in the index of self-presentation (F2,74 = 0.24, p = 0.789). However, entering the interaction term in step 2 significantly increased the amount of variance explained (ΔF1,73 = 5.44, p = 0.022, adjusted r2 = 3.7%, Δr2 = 6.9%). In this step, there were no main effects of sex of the stimulus person (p = 0.820) nor change in T (p = 0.293), but there was a significant two-way interaction between the change in T and sex (β = 0.351, p = 0.022). Significance tests of the slopes revealed that while the change in T did not affect self-presentation when interacting with a man (t73 = −1.06, β = −0.16, p = 0.293), a greater change in T was associated with more self-presentation when interacting with a woman (t73 = 2.18, β = 0.372, p = 0.033; figure 2).
Figure 2.
The relationship between a participant's change in testosterone and the extent to which he self-presented when interacting with a man (triangles) or woman (circles). Separate regression lines are plotted for each sex of the stimulus person.
(iii). Positive facial cues
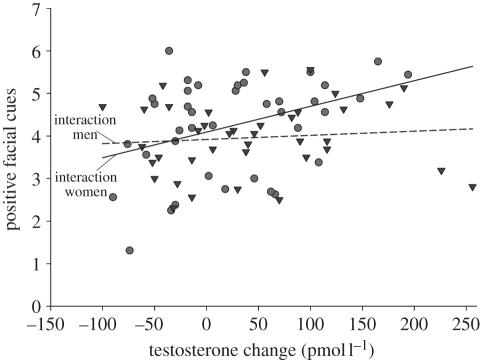
The model in step 1, including sex of the stimulus person and the change in T during the competition as predictor variables, explained a significant amount of variance in positive facial cues (F2,74 = 3.41, p = 0.038). In this step, the sex of the stimulus person did not affect the expression of positive facial cues (p = 0.126), but the change in T did (β = 0.253, p = 0.027). However, entering the interaction term in step 2 marginally increased the amount of variance explained (ΔF1,73 = 3.34, p = 0.072, adjusted r2 = 8.9%, Δr2 = 4.0%). In this step, there were no main effects of sex of the stimulus person (p = 0.124) nor change in T (p = 0.616), but there was a marginally non-significant two-way interaction between the change in T and sex (β = 0.268, p = 0.072). Significance tests of the slopes revealed that while the change in T did not affect the display of positive facial cues when interacting with a man (t73 = 0.50, β = 0.074, p = 0.616), a greater change in T was associated with more positive facial cues when interacting with a woman (t73 = 2.89, β = 0.480, p = 0.005; figure 3).
Figure 3.
The relationship between a participant's change in testosterone and his expression of positive facial cues when interacting with a man (triangles) or woman (circles). Separate regression lines are plotted for each sex of the stimulus person.
(iv). Overall affiliative behaviour
To check that our results were not driven by the particular grouping of the items into our three composite indices, we ran a similar analysis with all nine items grouped into a single variable: overall affiliative behaviour (electronic supplementary material, appendix D). This confirmed the previously reported effects: change in T did not affect overall affiliative behaviour when interacting with a man (t73 = −0.639, β = −0.096, p = 0.525), whereas a greater change in T was associated with more affiliative behaviour when interacting with a woman (t73 = 2.617, β = 0.443, p = 0.011).
4. Discussion
This is the first study to show that men experiencing elevated T levels during intrasexual competition subsequently show more affiliative behaviour during interactions with women. Those men with greater rises in T levels subsequently showed more interest in a woman, engaged in more self-presentation, smiled more and made more eye contact with her, but did not show a similar increase in these behaviours when interacting with a man. Although the behavioural ratings could have been affected by knowledge of the stimulus person's sex, this cannot account for the reported effect of the change in T on participants' behaviour as the raters were completely blind to this change. Our findings complement previous studies showing that contact with an unfamiliar woman may provoke an increase in T levels [9–12].
In addition, we report the novel finding that an endogenous T increase prior to social contact has an effect on subsequent behaviours, while previous work has shown the simultaneous occurrence of a T increase and an increase in display behaviours [9,10]. Taken together, the evidence suggests that during encounters with the opposite sex, T may function to promote the display of affiliative behaviours that increase a man's mating prospects. Although we did not specifically measure romantic or sexual interest, our findings suggest that the release of T in men during social contact with women is linked to the initiation of courtship behaviours.
The detection of these behavioural changes in men very soon after the competitive interaction suggests that T may have dynamically regulated their behaviour through non-genomic mechanisms [30]. Genomic mechanisms involve the activation of intracellular androgen receptors that modulate nuclear transcription after translocation of steroid–receptor complexes into the nucleus, typically taking days or hours to exert an influence [30]. By contrast, the non-genomic route can affect an organism rapidly through T binding to extracellular androgen receptors that can raise the intracellular calcium concentration, change membrane fluidity and activate second messenger pathways [31]. Through these changes, the activity of the central nervous system is modulated, thereby leading to a change in behaviour. The main findings of our study are consistent with the fast non-genomic actions of androgens, suggesting that transient changes in T levels can promote certain forms of social behaviour.
Our findings are also in line with the non-human animal literature, which shows that in a variety of species T causes more appetitive and consumptive sexual behaviours [3,4]. Interestingly, our study suggests that courtship in humans involves subtle verbal and non-verbal cues that are perhaps not specific to courtship. There was no overall difference in the frequency of affiliative behaviours between those men who interacted with a woman and those who interacted with another man. This was probably owing to the short contact period (5 min), as in the first few minutes of contact it may be quite normal to establish a rapport by smiling, making eye contact, speaking about one's experiences and showing interest in the other person. What our findings suggest is that when men meet an unfamiliar woman, a first step to show their romantic interest might be to display various forms of general affiliative behaviour.
Our findings are also consistent with the challenge hypothesis [8]. Those men who experienced a greater rise in T during the competitive task may have perceived that they were challenged [20] as, according to the challenge hypothesis, T should increase in contexts such as disputes over social status [8]. After this challenge, their elevated T levels not only prepared them for competing with other men but may also have had a generalized preparatory effect on mate acquisition by stimulating affiliative behaviours directed specifically towards women. However, the effect of intrasexual competition on our main results should be treated with caution as we cannot rule out that men with increased T levels owing to other circumstances also show more affiliative behaviours towards women. Despite this possibility, it seems plausible that intrasexual competition is associated with T and mating effort in humans, just as it has been shown in many non-human species that male–male competition is closely linked to mate acquisition [32]. Dominant males may have access to more or better females because they compete directly for those females (female-defence polygyny), or because they compete for resources to which females are attracted (resource-defence polygyny) [15]. This is one probable reason why high- and middle-ranking males typically have a lifetime reproductive advantage over the lowest-ranking males [16].
Instead of a direct, stimulatory effect of T on courtship behaviour, an alternative possibility is that those men whose T levels responded most to the competitive situation were also more focused on mating. In other words, those men who are more physiologically sensitive to challenges to their social status might be the same men who frequently engage in courtship. Following this reasoning, their high responsiveness to challenges relevant for reproductive success might have also have induced them to display more specific affiliative behaviours directed at women. To unravel these interesting possibilities, follow-up studies could pharmacologically administer T to men prior to social contact with a woman, to clarify its stimulating effects on affiliative and courtship behaviours.
Acknowledgements
This study was approved by the ethical committee of the Faculty of Psychology (University of Valencia) and conforms to the Declaration of Helsinki.
This study was supported by the Santiago Grisolía grant of the Generalitat Valenciana and by grants from the Royal Netherlands Academy of Arts and Sciences, and was developed in the framework of the Consolider Eje C Project SEJ2006-1408. T.W.F. was partly supported by a Marie Curie International Outgoing Fellowship from the European Commission (FP7-PEOPLE-2009-IOF, grant number 252618). We thank Nigel Bennett, Rosemary Knapp and three anonymous referees for constructive feedback on the manuscript.
References
- 1.Grammer K., Honda M., Juette A., Schmitt A. 1999. Fuzziness of nonverbal courtship communication unblurred by motion energy detection. J. Pers. Soc. Psychol. 77, 487–508 10.1037/0022-3514.77.3.487 (doi:10.1037/0022-3514.77.3.487) [DOI] [PubMed] [Google Scholar]
- 2.Nelson R. J. 2005. An introduction to behavioral endocrinology, 3rd edn. Sunderland, MA: Sinauer Associates [Google Scholar]
- 3.Fusani L. 2008. Testosterone control of male courtship in birds. Horm. Behav. 54, 227–233 10.1016/j.yhbeh.2008.04.004 (doi:10.1016/j.yhbeh.2008.04.004) [DOI] [PubMed] [Google Scholar]
- 4.Everitt B. J. 1990. Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci. Biobehav. Rev. 14, 217–232 10.1016/S0149-7634(05)80222-2 (doi:10.1016/S0149-7634(05)80222-2) [DOI] [PubMed] [Google Scholar]
- 5.James P. J., Nyby J. G. 2002. Testosterone rapidly affects the expression of copulatory behavior in house mice (Mus musculus). Physiol. Behav. 75, 287–294 10.1016/S0031-9384(01)00666-7 (doi:10.1016/S0031-9384(01)00666-7) [DOI] [PubMed] [Google Scholar]
- 6.Malmnäs C. O. 1977. Short-latency effect of testosterone on copulatory behaviour and ejaculation in sexually experienced intact male rats. J. Reprod. Fertil. 51, 351–354 10.1530/jrf.0.0510351 (doi:10.1530/jrf.0.0510351) [DOI] [PubMed] [Google Scholar]
- 7.Wingfield J. C., Hegner R. E., Dufty J., Alfred M., Ball G. F. 1990. The ‘challenge hypothesis’: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am. Nat. 136, 829–846 10.1086/285134 (doi:10.1086/285134) [DOI] [Google Scholar]
- 8.Archer J. 2006. Testosterone and human aggression: an evaluation of the challenge hypothesis. Neurosci. Biobehav. Rev. 30, 319–345 10.1016/j.neubiorev.2004.12.007 (doi:10.1016/j.neubiorev.2004.12.007) [DOI] [PubMed] [Google Scholar]
- 9.Roney J. R., Mahler S. V., Maestripieri D. 2003. Behavioral and hormonal responses of men to brief interactions with women. Evol. Hum. Behav. 24, 365–375 10.1016/S1090-5138(03)00053-9 (doi:10.1016/S1090-5138(03)00053-9) [DOI] [Google Scholar]
- 10.Roney J. R., Lukaszewski A. W., Simmons Z. L. 2007. Rapid endocrine responses of young men to social interactions with young women. Horm. Behav. 52, 326–333 10.1016/j.yhbeh.2007.05.008 (doi:10.1016/j.yhbeh.2007.05.008) [DOI] [PubMed] [Google Scholar]
- 11.van der Meij L., Buunk A. P., van de Sande J. P., Salvador A. 2008. The presence of a woman increases testosterone in aggressive dominant men. Horm. Behav. 54, 640–644 10.1016/j.yhbeh.2008.07.001 (doi:10.1016/j.yhbeh.2008.07.001) [DOI] [PubMed] [Google Scholar]
- 12.Roney J. R., Simmons Z. L., Lukaszewski A. W. 2010. Androgen receptor gene sequence and basal cortisol concentrations predict men's hormonal responses to potential mates. Proc. R. Soc. B 277, 57–63 10.1098/rspb.2009.1538 (doi:10.1098/rspb.2009.1538) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grammer K., Kruck K., Juette A., Fink B. 2000. Non-verbal behavior as courtship signals: the role of control and choice in selecting partners. Evol. Hum. Behav. 21, 371–390 10.1016/S1090-5138(00)00053-2 (doi:10.1016/S1090-5138(00)00053-2) [DOI] [PubMed] [Google Scholar]
- 14.Renninger L. A., Wade T. J., Grammer K. 2004. Getting that female glance: patterns and consequences of male nonverbal behavior in courtship contexts. Evol. Hum. Behav. 25, 416–431 10.1016/j.evolhumbehav.2004.08.006 (doi:10.1016/j.evolhumbehav.2004.08.006) [DOI] [Google Scholar]
- 15.Emlen S. T., Oring L. W. 1977. Ecology, sexual selection, and the evolution of mating systems. Science 197, 215–223 10.1126/science.327542 (doi:10.1126/science.327542) [DOI] [PubMed] [Google Scholar]
- 16.Ellis L. 1995. Dominance and reproductive success among nonhuman animals: a cross-species comparison. Ethol. Sociobiol. 16, 257–333 10.1016/0162-3095(95)00050-U (doi:10.1016/0162-3095(95)00050-U) [DOI] [Google Scholar]
- 17.Adkins-Regan E. 2005. Hormones and animal social behavior. Princeton, NJ: Princeton University Press [Google Scholar]
- 18.Shah K., Montoya C. 2007. Do testosterone injections increase libido for elderly hypogonadal patients? J. Fam. Pract. 56, 301–305 [PubMed] [Google Scholar]
- 19.Bagatell C. J., Heiman J. R., Rivier J. E., Bremner W. J. 1994. Effects of endogenous testosterone and estradiol on sexual behavior in normal young men. J. Clin. Endocrinol. Metab. 78, 711–716 10.1210/jc.78.3.711 (doi:10.1210/jc.78.3.711) [DOI] [PubMed] [Google Scholar]
- 20.van der Meij L., Buunk A. P., Almela M., Salvador A. 2010. Testosterone responses to competition: the opponent's psychological state makes it challenging. Biol. Psychol. 84, 330–335 10.1016/j.biopsycho.2010.03.017 (doi:10.1016/j.biopsycho.2010.03.017) [DOI] [PubMed] [Google Scholar]
- 21.Adler N. E., Epel E. S., Castellazzo G., Ickovics J. R. 2000. Relationship of subjective and objective social status with psychological and physiological functioning: preliminary data in healthy, white women. Health Psychol. 19, 586–592 10.1037/0278-6133.19.6.586 (doi:10.1037/0278-6133.19.6.586) [DOI] [PubMed] [Google Scholar]
- 22.van der Meij L., Buunk A. P., Salvador A. 2010. Contact with attractive women affects the release of cortisol in men. Horm. Behav. 58, 501–505 10.1016/j.yhbeh.2010.04.009 (doi:10.1016/j.yhbeh.2010.04.009) [DOI] [PubMed] [Google Scholar]
- 23.Gupta S. K., Lindemulder E. A., Sathyan G. 2000. Modeling of circadian testosterone in healthy men and hypogonadal men. J. Clin. Pharmacol. 40, 731–738 10.1177/00912700022009486 (doi:10.1177/00912700022009486) [DOI] [PubMed] [Google Scholar]
- 24.Brown T. A. 2006. Confirmatory factor analysis for applied research. New York, NY: Guilford Press [Google Scholar]
- 25.Hellhammer D. H., Hubert W., Schürmeyer T. 1985. Changes in saliva testosterone after psychological stimulation in men. Psychoneuroendocrinology 10, 77–81 10.1016/0306-4530(85)90041-1 (doi:10.1016/0306-4530(85)90041-1) [DOI] [PubMed] [Google Scholar]
- 26.Granger D. A., Schwartz E. B., Booth A., Arentz M. 1999. Salivary testosterone determination in studies of child health and development. Horm. Behav. 35, 18–27 10.1006/hbeh.1998.1492 (doi:10.1006/hbeh.1998.1492) [DOI] [PubMed] [Google Scholar]
- 27.Aiken L. S., West S. G. 1991. Multiple regression: testing and interpreting interactions. Thousand Oaks, CA: Sage Publications, Inc [Google Scholar]
- 28.Mehta P. H., Jones A. C., Josephs R. A. 2008. The social endocrinology of dominance: basal testosterone predicts cortisol changes and behavior following victory and defeat. J. Pers. Soc. Psychol. 94, 1078–1093 10.1037/0022-3514.94.6.1078 (doi:10.1037/0022-3514.94.6.1078) [DOI] [PubMed] [Google Scholar]
- 29.Aguinis H., Petersen S. A., Pierce C. A. 1999. Appraisal of the homogeneity of error variance assumption and alternatives to multiple regression for estimating moderating effects of categorical variables. Organ. Res. Methods 2, 315–339 10.1177/109442819924001 (doi:10.1177/109442819924001) [DOI] [Google Scholar]
- 30.Schmidt B. M., Gerdes D., Feuring M., Falkenstein E., Christ M., Wehling M. 2000. Rapid, nongenomic steroid actions: a new age? Front. Neuroendocrinol. 21, 57–94 10.1006/frne.1999.0189 (doi:10.1006/frne.1999.0189) [DOI] [PubMed] [Google Scholar]
- 31.Foradori C. D., Weiser M. J., Handa R. J. 2008. Non-genomic actions of androgens. Front. Neuroendocrinol. 29, 169–181 10.1016/j.yfrne.2007.10.005 (doi:10.1016/j.yfrne.2007.10.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andersson M. B. 1994. Sexual selection. Princeton, NJ: Princeton University Press [Google Scholar]