Abstract
Functional specialization, or division of labour (DOL), of parts within organisms and colonies is common in most multi-cellular, colonial and social organisms, but it is far from ubiquitous. Several mechanisms have been proposed to explain the evolutionary origins of DOL; the basic feature common to all of them is that functional differences can arise easily. These mechanisms cannot explain the many groups of colonial and social animals that exhibit no DOL despite up to 500 million years of evolution. Here, I propose a new hypothesis, based on a multi-level selection theory, which predicts that a reproductive DOL is required to evolve prior to subsequent functional specialization. I test this hypothesis using a dataset consisting of the type of DOL for living and extinct colonial and social animals. The frequency distribution of DOL and the sequence of its acquisition confirm that reproductive specialization evolves prior to functional specialization. A corollary of this hypothesis is observed in colonial, social and also within multi-cellular organisms; those species without a reproductive DOL have a smaller range of internal variation, in terms of the number of polymorphs or cell types, than species with a reproductive DOL.
Keywords: division of labour, germ-soma evolution, coloniality, transitions in individuality, levels of selection, multi-cellularity
1. Introduction
Division of labour (DOL) within organisms is not universal but it is a derived feature. DOL evolved at every level of individuality from some organelles of single-celled eukaryotes, through different cell types, tissues and organs within multi-cellular organisms, to polymorphic zooids and castes in colonial and social animals. DOL occurs in at least 10 colonial and social animal phyla [1–4]. We generally think that the benefits of DOL are profound, allowing an organism to simultaneously accomplish several physiological processes [1,5,6]. Without it, the ecology of organisms must remain simple or their physiology must constantly adjust to any changes in their environments.
All multi-cellular organisms consist of cells that are homologous to ancestral single-celled organisms. Likewise, colonial and social organisms are derived from solitary ancestors. The origin of a new hierarchical level of organization, where a new whole emerges from the aggregation and integration of previously independent wholes, is called as evolutionary transition in individuality [7–13]. With the exception of the origin of eukaryotes and an unknown number of transitions more ecological than organismal in nature [12,14], which have been called egalitarian transitions [15,16], all examples of transitions in individuality originate from aggregates of closely related organisms. The members within these new aggregates are therefore initially similar but potentially vary morphologically and genetically.
Despite the variability in the expression of DOL across various types of organisms, two basic types can be recognized. I differentiate between reproductive and ‘other’ types of DOL. Reproductive DOL occurs when some members of group do not reproduce. ‘Other’ DOL is defined as non-reproductive functional differences between members (for example, between feeding and defence). Four states of DOL are possible by distinguishing between the presence and absence of reproductive and ‘other’ DOL.
Two basic mechanisms have been previously proposed that jointly explain the origin of DOL within organisms, colonies and societies. In the first, functional variants arise initially through heterochrony or plastic differentiation along a reaction norm that is subsequently fixed [3,17,18]. Because such variation is common, the new functional specialists are expected to constantly arise over evolutionary time. In the second mechanism, DOL arises as a solution to a functional optimization problem [19]. For example, feeding must occur, but if defensive needs arise, a DOL between feeding and defence can arise to maximize both simultaneously, if the benefits of having multiple types are larger than the benefits of having only a single type. The number of members in each functional type is determined by the relationship of tasks to castes. These two mechanisms together constitute a single hypothesis for the origin of DOL where functional optimization is readily achieved by heterochrony. This hypothesis predicts that DOL can arise as needed. As a consequence, there is no tendency for a particular type of DOL, such as defensive or reproductive specialists, to be evolutionarily primitive. Nor does this hypothesis predict specific patterns to occur in the extent of DOL or the number of co-occurring specialists within an organism. For example, an organism with extensive DOL could have feeding, defensive, reproductive and structural specialists, whereas an organism without extensive DOL may only have defensive specialists.
If this hypothesis is true, then all organisms can evolve DOL from whatever phenotype they currently have. Since the advantage of DOL is presumably high, why then do whole lineages never evolve DOL? For example, nearly all colonial hexacorals have monomorphic corallites, even after over 500 million years of evolution [20]. Clearly, something is missing in our evolutionary understanding of DOL.
Here, I develop a new hypothesis. When individual members of a species first aggregate together, they naturally vary, genetically and phenotypically, and some variants are detrimental to life in the aggregate. These deleterious members can easily be purged from the population if fragmentation occurs and new aggregates form only from small fragments containing cooperative members [8,21,22]. As an illustration of this process, consider the phenotype of a descendent aggregate to be produced by random sampling of the parent. Consider an aggregate that has N members. If j is the number of members that are detrimental to the group and N − j members that are beneficial to the aggregate, what is the probability of producing offspring with only k beneficial members (such that k = n)? Using the hypergeometric distribution, the probability of a forming an offspring with k beneficial members is given by:
 |
The probability of producing offspring where k = n is highest when n is equal to one and it equals the frequency of beneficial members in the parent.
However, when reproduction is similar to the random sampling of this sort, a small propagule, which is advantageous for eliminating detrimental members, also works against the inheritance of multiple types of beneficial members that otherwise would require multiple members to be present in a propagule. If more members are included in a propagule, the probability of a daughter also inheriting a detrimental morph will be high. This single-cell or single-organism bottleneck, whatever its cause, is also empirically common (e.g. [23]), so that DOL must be re-established each generation.
Consider a specific example of two aggregates, both with 50 members. In one aggregate, 40 members are beneficial but come in two morphs of 20 each; the 10 additional members are detrimental. In the second aggregate, the 40 beneficial members are monomorphic and there are also 10 detrimental members present. The probability of producing an offspring with at least one detrimental member is the same for both aggregates and approaches unity as the number of members in a propagule (propagule size) increases. The probability that offspring will contain no detrimental member increases as propagule size becomes smaller, but at a faster rate in monomorphic aggregates than dimorphic ones (figure 1). Importantly, the probability of forming a small monomorphic propagules becomes higher than the probability of containing at least one detrimental member.
Figure 1.
The influence of propagule size—the number of members that found a new colony—on the probability of forming a descendent colony of a particular phenotype. Monomorphic colonies have one beneficial morph whereas dimorphic colonies have an equal number of two morphs. In this figure, there are initially 50 members, 10 of which are detrimental to the colony. The probability of producing an offspring with at least one detrimental member is the same for both aggregates and approaches unity as the number of members in a propagule (propagule size) increases.
This trade-off between purging detrimental morphs and inheriting beneficial ones limits the number of morphs that can occur in aggregates with random reproduction. As a side-effect of a small propagule, groups will tend to be monomorphic. Monomorphic groups will have an advantage if detrimental morphs have a strong negative effect on the group and so are expected to predominate when group cohesion is important, as, for example, in colonial corals.
When there is a small propagule bottleneck in a group life cycle, any DOL accumulated during the expansion of the group will probably be lost when daughter groups are formed along with accumulated detrimental members. There are two possible (but not mutually exclusive) pathways to overcome the constraint. The first occurs if every member can revert to a totipotent state. In this scenario, any accumulated DOL of members must be able to revert to a totipotent form prior to group reproduction in order for the variation within a group as a whole to occur if it is to be inherited. There are reasons to believe that this pathway to DOL is more difficult in colonial and social organisms than it is in multi-cellular organisms. Organisms as a whole are more complex than cells, and so their ability to revert to a totipotent state from any of the range of forms they can take will be limited. Cells within a multi-cellular whole may well have a greater range of totipotent forms than the more complex multi-cellular organisms within a colonial whole. If this first pathway is taken, ‘other’ DOL may occur before reproductive DOL but may nevertheless be limited in extent. Often this pathway, along with a structural constraint that produces alternative ‘other’ and reproductive morphs automatically, is invoked to explain DOL (e.g. the ‘flagellation constraint’ [11,24,25]). Structural trade-offs are common, but nowhere near universal. Consider metazoans: cell division and cell function can co-occur along with the reproduction of the animal as a whole.
The second pathway to DOL occurs when reproductive members do not differentiate in the first place. Only with this reproductive DOL—where a whole group is consistently founded by a non-random undifferentiated totipotent subset of the group—will internal variation among members become heritable and unconstrained. A non-random subset of totipotent members could still be a large proportion of the group, but if these totipotent members become less common, the unconstrained somatic members will make up a larger proportion of the group.
I propose that two steps are required for successful transitions in individuality to occur. The first is that groups require small propagules in their life cycles. Monomorphic and often highly related group members are produced from this step. The second step occurs when a reproductive DOL evolves. Functional DOL, while possible prior to a reproductive DOL, will be minimal or absent until a reproductive DOL evolves. This hypothesis predicts that (i) a reproductive DOL will evolve first in a majority of cases resulting in an uneven distribution of taxa with different types of DOL, and (ii) that the total extent of functional differentiation will be larger if there is a reproductive DOL.
I test the first prediction with a dataset derived from fossil and living colonial and social organisms consisting of the presence and absence of reproductive and other functional DOL. The relative timing of the two types of DOL is estimated on a subset of these data for which published molecular phylogenies are available. The second prediction is tested at two levels: first, with a subset of the colonial dataset, and also with previously published data on cell types and germ-line determination in multi-cellular organisms.
2. Material and methods
Reproductive DOL occurs when some members of a colony or society do not contribute to reproduction. Non-sterile castes are considered non-reproductive if their offspring are unlikely to produce fully reproductive members or disperse and found a new colony. In vertebrates and some social insects, juveniles are present in a group for an extended period of time so that multiple litters co-occur. When this occurs, the oldest juveniles are counted as non-reproductive members of the group but the youngest generation is not. Species are considered to possess ‘other’ DOL when members have specialized tasks represented morphologically. For example, in bryozoans, a kenozooid fills space whereas an avicularium defends. Often, reproductive and ‘other’ DOL will coincide, as it does when queen bees reproduce but do not forage, while the workers in the colony forage but do not reproduce.
The presence or absence of the two types of DOL was recorded at the species level where possible and is presented in the electronic supplementary material; however, all analysis presented in this paper was done at the genus level to allow for the inclusion of fossil groups, many of which have more uncertain species-level taxonomy. The trait values of species within each genus were averaged together. Even though each genus may not be phylogenetically independent, and nor does the frequency distribution of DOL directly estimate their relative origination times, inclusion of both fossil and living examples provides a larger range of colonies and societies than would be possible with total phylogenetic control.
To identify the sequence of DOL evolution at the colonial/social level, I use published phylogenies of the major colonial and social groups [26–36]. At the multi-cellular level, I use volovocine algae [37] as an example.
All the molecular phylogenies have branch lengths calibrated to time or proportional to molecular (nucleotide or amino acid) substitution rate. For tree files that were not provided directly, the phylogenies with branch lengths were digitized from the publication figures. I measured branch lengths using ImageJ from a jpeg snapshot of each tree. Each phylogeny was then converted into chronograms using a non-parametric rate scaling [38], with the time of the base of the tree set to 1, which allowed me to use a few trees that were plotted with branch lengths proportional to substituting rate but without a scale bar. To measure the relative timing of the acquisition of DOL, the ancestral state for each node in the phylogeny was reconstructed using an equal rates model of discrete character evolution (using the R package ape [39]). Nodes where the probability of possessing DOL was greater than or equal to 0.9 were identified, and their distance relative to the base of the tree was measured. For lineages that have not evolved one or both types of DOL, the relative time from the base of the tree was left undefined, but these lineages count towards three of the four types of DOL.
For a subset of genera, counts of the number of polymorph types and estimates of the proportion of non-reproductive members were collected. A polymorph type in colonial organisms is a named structure that is homologous to a solitary organism. In bryozoans, these would be autozooids, avicularia, gonozoids and so on. The proportion of non-reproductive members is estimated by counting the numbers of polymorph types in illustrations of each species. Polymorph types in social animals are defined as morphologically distinct forms (e.g. castes in ants). Named phases of temporal castes in termites are considered separate polymorph types. In social vertebrates, only morphologically distinct organisms are considered polymorph types. In mammals, this criterion is rarely met; the best example is in the mole-rat Heterocephalus glaber, where queens are morphologically distinct from other members of the colony [40]. Reproductive and non-reproductive members of a dominance hierarchy are considered to be of the same polymorph type (even if they show reproductive differences) if the variation among members in a dominance hierarchy is not discontinuous. For social organisms, the proportion of non-reproductive members is estimated at a single point in time after the group as a whole has entered a reproductive phase. Sampling at a single time slice is done because caste and polymorph membership in social organisms are often dynamic, so that one individual member may change caste or rank as it ages. Sampling in this way captures population structure of the group as a whole at that moment in time.
At the multi-cellular level, I compare a proxy for the proportion of non-reproductive cells given by the mechanism of germ-cell formation in an organism to the number of distinct cell types, using previously published compilations [7,41–43]. The proportion of non-reproductive cells within multi-cellular organisms is approximated by the mechanism of germ-line determination. Three distinct mechanisms are recognized. Somatic determination occurs when any cell can form germ cells. Species with this form of germ-line determination have a very low proportion of non-reproductive members. The second mechanism, epigenetic determination, occurs when differentiated cells can be induced developmentally to form germ cells and have an intermediate proportion of non-reproductive members. Finally, species with a high proportion of non-reproductive member germ cells have a preformed germ line. The estimates for the number of cell types used in this comparison are from Bell & Mooers [43] and include species of colonial amoebae and ciliates, brown, green and red algae, plants, fungi, and animals.
3. Results and discussion
(a). Evolutionary sequence
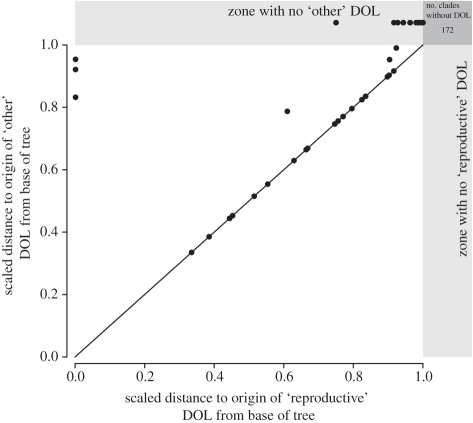
Only one out of 2788 genera—the Palaeozoic tabulate coral Striatopora—has polymorphic zooids that may all be reproductive (table 1). The hypothesis that genera are evenly distributed across the four types of DOL is thus easily rejected (χ2 = 2186.465, d.f. = 3, p < 0.0001). The majority of sampled genera possess either no DOL at all or both types. The relative sequence of DOL evolution is inferred from comparing the relative distance from the base of the phylogeny for each separate origin of DOL (figure 2a). Reproductive DOL evolves first in those points that fall above the one-to-one line, and both types of DOL evolve simultaneously if the points fall directly on the one-to-one line. For all groups sampled, reproductive DOL either evolves first, concurrently with ‘other’ DOL, or no DOL occurs at all. The majority of lineages that evolve coloniality do not evolve DOL of any sort (table 2) and the number of phylogenetically independent origins of DOL is not uniformly distributed (χ2 = 469.511, d.f. = 4, p < 0.0001).
Table 1.
The number of fossil and recent colonial and social genera that show one of four types of division of labour (DOL).
| other DOL |
|||
|---|---|---|---|
| no | yes | ||
| reproductive DOL | yes | 76 | 753 |
| no | 1949 | 1 | |
Figure 2.
A comparison of the relative timing of reproductive division of labour (DOL) and ‘other’ DOL derived from molecular phylogenies of extant colonial and social organisms and multi-cellular algae. Each point represents the time relative to the base of the tree for a phylogenetically independent origin of DOL. Groups that have evolved one type of DOL but not the other are plotted within the grey bars, but the relative time of the DOL these groups do posses is plotted. Groups with reproductive but not ‘other’ DOL plot along the top of the figure and groups with ‘other’ but not reproductive DOL would plot along the right margin. The number of independently evolved groups that are colonial or social, but without DOL of any kind is also shown in the top right dark grey box. The organisms included are hydrozoans, octocoralls, scleractinian corals, bryozoans, entoprocts, phoronids, hemichordates and tunicates, the hymenopteran groups of the Vespoidea, termites, mammals and green algae.
Table 2.
The number of phylogenetically independent origins of DOL derived from extant species of colonial and social organisms that show one of four types of DOL.
| other DOL |
|||
|---|---|---|---|
| no | yes | ||
| reproductive DOL | yes | 15 | 39 |
| no | 172 | 0 | |
(b). Range of polymorphism
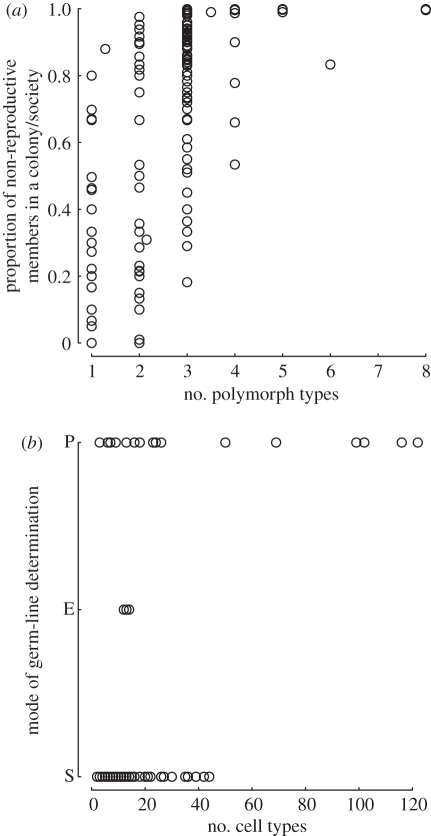
To test whether the groups with only reproductive members have a limited range of polymorph types, I plotted the proportion of non-reproductive members against the number of polymorph types (figure 3a). I find the smallest range in the number of polymorph types in groups where the proportion of non-reproductive members equals zero. The maximum observed number of polymorph types in these groups is two, observed in Striatopora. The mean number of polymorph types in genera with no reproductive DOL is significantly smaller than genera with reproductive DOL (Wilcoxon W = 42 947.5, p < 0.0001). The mean number of polymorph types is 1.00 among genera without reproductive DOL and 2.66 in those with reproductive DOL.
Figure 3.
Patterns of DOL for multi-cellular and colonial organisms. (a) The proportion of non-reproductive members plotted against polymorph types for colonial and social animals. (b) The mechanism of germ-cell formation (which estimates the proportion of non-reproductive members) plotted against the number of cell types in multi-cellular animals, plants, fungi and algae, as well as colonial algae, ciliates and bacteria. S, somatically derived; E, epigenetically derived; P, preformed.
Multi-cellular organisms show a similar pattern, with one important difference (figure 3b). For organisms with somatic germ-line determination in this sample, the maximum number of cell types observed is much larger than what is observed in colonial organisms (maximum = 44, mean = 13.51, s.d. = 10.76). Organisms with preformed or epigenetically determined germ lines show the widest range in the number of cell types they possess (maximum = 122, mean = 31.48, s.d. = 38.03). The difference between the mean number of cell types in genera without reproductive DOL (somatic germ-line determination) is significantly smaller than those for which reproductive DOL is also present (Wicoxon W = 612.5, p = 0.038).
4. Conclusions
The observed patterns of DOL in nature support the hypothesis that reproductive specialization is a prerequisite for further functional specialization. Additionally, if all members of a group are reproductive, the internal variation in functional types is observed to be limited, although to different degrees at each hierarchical level. The second pattern is observed consistently across two levels of organization: multi-cellular colonies of single-celled eukaryotes along with multi-cellular organisms at one level of organization, and colonial/social animals at another (higher) level of organization.
The emergence of a new level of reproduction, which is indicated by a reproductive DOL, does coincide with high relatedness within colonies and societies. But high relatedness may be just a by-product of the single member bottleneck during the life cycle of a group and nothing more. In terms of the number of independently derived groups, colonial and social life is dominantly simple, with no DOL between members despite the often high degree of relatedness within colonies and societies. When DOL does occur, reproductive DOL is a prerequisite for further DOL. These observations provide evidence that the evolution of DOL is not linked to relatedness but rather to the emergence of a new level of reproduction. If high relatedness is just a by-product and not an evolutionary explanation for eusociality and complex colonial organisms, then the explanatory domain of kin selection and inclusive fitness is indeed limited [44]. It is the emergence of a new level of reproduction that is not fully accounted for in the traditional theories of kin selection or multi-level selection [45] (but see the extensions of Keller & Reeve [46] and Nowak et al. [47] for attempts to incorporate something like group reproduction into existing theories).
It is worth drawing a parallel between colonial invertebrates such as bryozoans and social animals such as bees, to understand the non-equivalence between inclusive fitness and a new level of reproductive fitness. Inclusive fitness has never been much discussed with respect to brozoans; after all, the zooids within a colony are clonal and so perfectly related. As the colony grows, the number of zooids does too. From the perspective of a single zooid, its inclusive fitness increases while from the perspective of the colony it grows. In a social insect, the colony is more diffuse and so there is an understandable reluctance to read much into patterns at the colony level. But, similar to the bryozoan, colony growth and increasing inclusive fitness are two views of the same process. The component of fitness that is represented by both growth and the more diffuse increases in population size or inclusive fitness within a group has been called expansion, and supplements the reproductive and persistence components of fitness normally recognized [12,48,49]. Expansion is explicitly hierarchical; expansion at one level is traditional fitness at the level below [12].
Significant colony-level evolution, represented by ‘other’ DOL, only occurs when the colonies as a whole reproduce (that is, after reproductive DOL evolves). At this point, colony-level expansion—growth and inclusive fitness at the organismal level—is joined by the reproductive component of fitness at the colony level. Each transition in individuality results in the emergence of a new level of demographic fitness in addition to the previously existing expansive components of fitness; and does so without turning off the lower level.
Acknowledgements
Thanks to Leonore Fleming for help with data collection, to Wolfgang Kiessling, Daniel McShea, David McCandlish and Lauren McCall for discussion and comments, and to two anonymous reviewers for helpful comments and critiques. I am grateful to P. Cartwright, C. Dunn, M. Kitahara, M. Herron and C. Rasmussen for providing me with their molecular phylogenies, and to R. Hechinger for providing data on a eusocial flatworm. The Cambridge-Templeton Foundation grant no. 038 funded this work.
References
- 1.Beklemeshev W. N. 1969. Principles of comparative anatomy of invertebrates. Chicago, IL: University of Chicago Press [Google Scholar]
- 2.Costa J. T. 2006. The other insect societies. Cambridge, MA: Belknap Press of Harvard University Press [Google Scholar]
- 3.Harvell C. D. 1994. The evolution of polymorphism in colonial invertebrates and social insects. Q. Rev. Biol. 69, 155–185 10.1086/418538 (doi:10.1086/418538) [DOI] [Google Scholar]
- 4.Wilson E. O. 1975. Sociobiology: the new synthesis. Cambridge, MA: Belknap Press of Harvard University Press [Google Scholar]
- 5.Huxley J. 1912. The individual in the animal kingdom. New York, NY: Cambridge University Press [Google Scholar]
- 6.Weismann A. 1893. The germ-plasm: a theory of heredity. New York, NY: Scribner [Google Scholar]
- 7.Buss L. 1987. The evolution of individuality. Princeton, NJ: Princeton University Press [Google Scholar]
- 8.Maynard Smith J. 1988. Evolutionary progress and levels of selection. In Evolutionary progress (ed. Nitecki M. H.), pp. 219–230 Chicago, IL: University of Chicago Press [Google Scholar]
- 9.Maynard Smith J., Szathmáry E. 1995. The major transitions in evolution. New York, NY: Oxford University Press [Google Scholar]
- 10.McShea D. W. 2001. The minor transitions in hierarchical evolution and the question of a directional bias. J. Evol. Biol. 14, 502–518 10.1046/j.1420-9101.2001.00283.x (doi:10.1046/j.1420-9101.2001.00283.x) [DOI] [Google Scholar]
- 11.Michod R. E. 1999. Darwinian dynamics: evolutionary transitions in fitness and individuality. Princeton, NJ: Princeton University Press [Google Scholar]
- 12.Simpson C. 2011. How many levels are there? How insights from evolutionary transitions in individuality help measure the hierarchical complexity of life. In The major transitions in evolution revisited (eds Sterelney K., Calcott B.), pp. 199–226 Cambridge, MA: MIT Press [Google Scholar]
- 13.McShea D. W., Simpson C. 2011. The miscellaneous transitions in evolution. In The major transitions in evolution revisited (eds Sterelney K., Calcott B.), pp. 19–34 Cambridge, MA: MIT Press [Google Scholar]
- 14.Queller D. C., Strassmann J. E. 2009. Beyond society: the evolution of organismality. Phil. Trans. R. Soc. B 364, 3143–3155 10.1098/rstb.2009.0095 (doi:10.1098/rstb.2009.0095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Queller D. C. 1997. Cooperators since life began. Q. Rev. Biol. 72, 184–188 10.1086/419766 (doi:10.1086/419766) [DOI] [Google Scholar]
- 16.Queller D. C. 2000. Relatedness and the fraternal major transitions. Phil. Trans. R. Soc. Lond. B 355, 1647–1655 10.1098/rstb.2000.0727 (doi:10.1098/rstb.2000.0727) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonner J. T. 2001. First signals: the evolution of multicellular development. Princeton, NJ: Princeton University Press [Google Scholar]
- 18.Schlichting C. D. 2003. Origins of differentiation via phenotypic plasticity. Evol. Dev. 5, 98–105 10.1046/j.1525-142X.2003.03015.x (doi:10.1046/j.1525-142X.2003.03015.x) [DOI] [PubMed] [Google Scholar]
- 19.Oster G., Wilson E. O. 1979. Caste and ecology in the social insects. Princeton, NJ: Princeton University Press; [PubMed] [Google Scholar]
- 20.Coates A. G., Oliver W. A., Jr 1973. Coloniality in zoantharian corals. In Animal colonies: development and function through time (eds Boardman R. S., Cheetham A. H., Oliver W. A.), pp. 3–27 Stroudsburg, PA: Downden, Hutchinson, and Ross [Google Scholar]
- 21.Rice S. H. 2004. Evolutionary theory: mathematical and conceptual foundations. Sunderland, MA: Sinauer Associates [Google Scholar]
- 22.Wade M. J. 1978. A critical review of the models of group selection. Q. Rev. Biol. 53, 101–114 10.1086/410450 (doi:10.1086/410450) [DOI] [Google Scholar]
- 23.Grosberg R. K., Strathmann R. R. 1998. One cell, two cell, red cell, blue cell: the persistence of a unicellular stage in multicellular life histories. Trends Ecol. Evol. 13, 112–116 10.1016/S0169-5347(97)01313-X (doi:10.1016/S0169-5347(97)01313-X) [DOI] [PubMed] [Google Scholar]
- 24.Koufopanou V. 1994. The evolution of soma in the Volvocales. Am. Nat. 143, 907–931 10.1086/285639 (doi:10.1086/285639) [DOI] [Google Scholar]
- 25.Michod R. E., Viossat Y., Solari C. A., Hurand M., Nedelcu A. M. 2006. Life-history evolution and the origin of multicellularity. J. Theor. Biol. 239, 257–272 10.1016/j.jtbi.2005.08.043 (doi:10.1016/j.jtbi.2005.08.043) [DOI] [PubMed] [Google Scholar]
- 26.Bininda-Emonds O. R. P., et al. 2007. The delayed rise of present-day mammals. Nature 446, 507–512 10.1038/nature05634 (doi:10.1038/nature05634) [DOI] [PubMed] [Google Scholar]
- 27.Cannon J. T., Rychel A. L., Eccleston H., Halanych K. M., Swalla B. J. 2009. Molecular phylogeny of hemichordata, with updated status of deep-sea enteropneusts. Mol. Phylogenet. Evol. 52, 17–24 10.1016/j.ympev.2009.03.027 (doi:10.1016/j.ympev.2009.03.027) [DOI] [PubMed] [Google Scholar]
- 28.Cartwright P., Nawrocki A. 2010. Character evolution in Hydrozoa (phylum Cnidaria). Integr. Comp. Biol. 50, 456–472 10.1093/icb/icq089 (doi:10.1093/icb/icq089) [DOI] [PubMed] [Google Scholar]
- 29.Duffy J. E., Macdonald K. S. 2010. Kin structure, ecology and the evolution of social organization in shrimp: a comparative analysis. Proc. R. Soc. B 277, 575–584 10.1098/rspb.2009.1483 (doi:10.1098/rspb.2009.1483) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuchs J., Iseto T., Hirose M., Sundberg P., Obst M. 2010. The first internal molecular phylogeny of the animal phylum Entoprocta (Kamptozoa). Mol. Phylogenet. Evol. 56, 370–379 10.1016/j.ympev.2010.04.009 (doi:10.1016/j.ympev.2010.04.009) [DOI] [PubMed] [Google Scholar]
- 31.Fuchs J., Obst M., Sundberg P. 2009. The first comprehensive molecular phylogeny of Bryozoa (Ectoprocta) based on combined analyses of nuclear and mitochondrial genes. Mol. Phylogenet. Evol. 52, 225–233 10.1016/j.ympev.2009.01.021 (doi:10.1016/j.ympev.2009.01.021) [DOI] [PubMed] [Google Scholar]
- 32.Kitahara M. V., Cairns S. D., Stolarski J., Blair D., Miller D. J. 2010. A comprehensive phylogenetic analysis of the Scleractinia (Cnidaria, Anthozoa) based on mitochondrial CO1 Sequence data. PLoS ONE 5, e11490. 10.1371/journal.pone.0011490 (doi:10.1371/journal.pone.0011490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Legendre F., Whiting M. F., Bordereau C., Cancello E. M., Evans T. A., Grandcolas P. 2008. The phylogeny of termites (Dictyoptera: Isoptera) based on mitochondrial and nuclear markers: implications for the evolution of the worker and pseudergate castes, and foraging behaviors. Mol. Phylogenet. Evol. 48, 615–627 10.1016/j.ympev.2008.04.017 (doi:10.1016/j.ympev.2008.04.017) [DOI] [PubMed] [Google Scholar]
- 34.McFadden C. S., France S. C., Sánchez J. A., Alderslade P. 2006. A molecular phylogenetic analysis of the Octocorallia (Cnidaria: Anthozoa) based on mitochondrial protein-coding sequences. Mol. Phylogenet. Evol. 41, 513–527 10.1016/j.ympev.2006.06.010 (doi:10.1016/j.ympev.2006.06.010) [DOI] [PubMed] [Google Scholar]
- 35.Pilgrim E., von Dohlen C., Pitts J. 2008. Molecular phylogenetics of Vespoidea indicate paraphyly of the superfamily and novel relationships of its component families and subfamilies. Zool. Scr. 37, 539–560 10.1111/j.1463-6409.2008.00340.x (doi:10.1111/j.1463-6409.2008.00340.x) [DOI] [Google Scholar]
- 36.Tsagkogeorga G., et al. 2009. An updated 18S rRNA phylogeny of tunicates based on mixture and secondary structure models. BMC Evol. Biol. 9, 187. 10.1186/1471-2148-9-187 (doi:10.1186/1471-2148-9-187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herron M. D., Michod R. E. 2008. Evolution of complexity in the volvocine algae: transitions in individuality through Darwin's eye. Evolution 62, 436–451 10.1111/j.1558-5646.2007.00304.x (doi:10.1111/j.1558-5646.2007.00304.x) [DOI] [PubMed] [Google Scholar]
- 38.Sanderson M. J. 1997. A nonparametric approach to estimating divergence times in the absence of rate constancy. Mol. Biol. Evol. 14, 1218 [Google Scholar]
- 39.Paradis E., Claude J., Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290 10.1093/bioinformatics/btg412 (doi:10.1093/bioinformatics/btg412) [DOI] [PubMed] [Google Scholar]
- 40.O'Riain M., Jarvis J., Alexander R., Buffenstein R., Peeters C. 2000. Morphological castes in a vertebrate. Proc. Natl Acad. Sci. USA 97, 13 194–13 197 10.1073/pnas.97.24.13194 (doi:10.1073/pnas.97.24.13194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buss L. 1983. Evolution, development, and the units of selection. Proc. Natl Acad. Sci. USA 80, 1387–1391 10.1073/pnas.80.5.1387 (doi:10.1073/pnas.80.5.1387) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nieuwkoop P. D., Sutasurya L. A. 1981. Primordial germ cells in the invertebrates: from epigenesis to preformation. Cambridge, UK: Cambridge University Press [Google Scholar]
- 43.Bell G., Mooers A. Ø. 1997. Size and complexity among multicellular organisms. Biol. J. Linn. Soc. 60, 345–363 10.1111/j.1095-8312.1997.tb01500.x (doi:10.1111/j.1095-8312.1997.tb01500.x) [DOI] [Google Scholar]
- 44.Wilson D. S., Wilson E. O. 2007. Rethinking the theoretical foundation of sociobiology. Q. Rev. Biol. 82, 327–348 10.1086/522809 (doi:10.1086/522809) [DOI] [PubMed] [Google Scholar]
- 45.Damuth J., Heisler I. 1988. Alternative formulations of multilevel selection. Biol Philos. 3, 407–430 10.1007/BF00647962 (doi:10.1007/BF00647962) [DOI] [Google Scholar]
- 46.Keller L., Reeve H. K. 1994. Partitioning of reproduction in animal societies. Trends. Ecol. Evol. 9, 98–102 10.1016/0169-5347(94)90204-6 (doi:10.1016/0169-5347(94)90204-6) [DOI] [PubMed] [Google Scholar]
- 47.Nowak M. A., Tarnita C. E., Wilson E. O. 2010. The evolution of eusociality. Nature 466, 1057–1062 10.1038/nature09205 (doi:10.1038/nature09205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Valen L. M. 1976. Energy and evolution. Evol. Theory 1, 179–229 [Google Scholar]
- 49.Van Valen L. M. 1989. Three paradigms of evolution. Evol. Theory 9, 1–17 [Google Scholar]