Abstract
Co-infecting parasite genotypes typically compete for host resources limiting their fitness. The intensity of such competition depends on whether parasites are reproducing in a host, or using it primarily as a transmission vehicle while not multiplying in host tissues (referred to as ‘competition hypothesis’). Alternatively, simultaneous attack and co-infection by several parasite genotypes might facilitate parasite infection because such a diverse attack could present an additional challenge to host immune defence (referred to as ‘facilitation hypothesis’). We tested the competition hypothesis by comparing the production of transmission stages (cercariae) from snails infected with one or two genotypes of the trematode Diplostomum pseudospathaceum. We found that cercarial production did not differ between the two groups of snails, suggesting lower per genotype production in double infections, and competition for host resources. Second, we tested the facilitation hypothesis by comparing parasite infection success on fishes (proportion of parasites establishing in the host) using cercariae originating from single-infected snails, double-infected snails and artificial mixtures of the single genotypes. In both cases, we found higher infection success when fishes were challenged with two parasite genotypes instead of one, supporting the facilitation hypothesis. Our results suggest that constraints defining the success of multiple genotype infections in parasites with multiple host life cycles include both between-genotype resource competition in the host and performance of host immune defences against a diverse parasite challenge.
Keywords: host–parasite interaction, multiple genotype infection, competition, facilitation, complex life cycle
1. Introduction
Co-infection, the infection of a host individual by two or more parasite genotypes or strains, is common in naturally infected hosts [1,2]. This within-host diversity can lead to competitive interactions among co-infecting parasites and have significant implications for epidemiology and evolution of host–parasite interactions [1]. Competitive interactions are typically strong during parasite life stages that multiply rapidly, show high host exploitation rate and compete directly for host resources [3,4]. Under such circumstances, competition may result in decreased rate of reproduction per genotype if sharing the host means fewer available resources for each co-infecting genotype. We refer to this as ‘competition hypothesis’ below. Alternatively, competition could also lead to increased rate of host exploitation and parasite multiplication when compared with a single-genotype infection [5]. Direct competition for resources, however, may be less severe in life stages that do not multiply within hosts. Such life stages are common in trophically transmitted parasites that encapsulate in intermediate host individuals and use them as vehicles to predatory definitive hosts.
Simultaneous attack and subsequent co-infection may also facilitate parasite infection, because simultaneous exposure to several parasite strains may compromise the immune system of the host [6]. Such a simultaneous exposure may be the result of a multiple infection in the previous host, but also of a synchronous release of genetically distinct transmission stages by several host individuals. If synchronous co-exposure is advantageous, it may lead to facilitation among parasite strains during transmission and have significant evolutionary consequences for parasite transmission strategies. This is referred to as ‘facilitation hypothesis’ below. To our knowledge, however, the relative importance of within-host competition and genetic heterogeneity of exposure for parasite transmission to the next host has not been addressed in a single study system.
We tested the competition and facilitation hypotheses by determining the rate of production of the infective stages (cercariae), and their infection success on fishes (proportion of parasites establishing in the host), in single- and double-genotype infections of the trematode eye fluke Diplostomum pseudospathaceum. This parasitic worm has a complex life cycle involving snail, fish and bird hosts. Adult worms live in the gut of the bird and reproduce sexually releasing eggs to the environment. Larvae that hatch from these eggs (miracidia) actively search for snail hosts and infect their gonad tissue. This is followed by exploitation of host resources, which ultimately leads to castration of the snail. All miracidia are genetically different, but one snail can be infected with more than one miracidia, which leads to multiple-genotype infection. Parasites in the infected gonad tissue produce cercariae larvae by multiplying asexually, which effectively clones the original genotype of the miracidia. In other words, cercariae emerging from one single-infected snail are genetically identical. Cercariae are released from snails in water where they infect fishes and develop to metacercariae in the eye lenses. These long-lived metacercariae do not multiply in the fishes and do not consume fish resources to a great extent, but settle in the lens until the fish is consumed by a bird. The range of immune responses against the parasite that are activated in fishes [7] can only take place during cercarial migration towards the eye, within 24 h from exposure. Afterwards, parasites are protected from host responses in the eye lenses which lack blood veins and circulating antibodies.
In this study, we first measured the rate of cercarial production from single- and double-genotype infected snail hosts to test the competition hypothesis. Second, we exposed fishes to cercariae originating from natural single- and double-genotype infections in the snails to address the facilitation hypothesis on infection success in the fish. We also tested the facilitation hypothesis using artificial mixtures of genotypes from the single-genotype infected snails in varying proportions. This set-up removed the possible confounding effect of between-genotype competition in the snail on the parasite transmission success on fishes. Our results were consistent with the competition hypothesis in the snail, where co-infection decreased the overall rate of transmission of the two genotypes, but also consistent with the facilitation hypothesis during the parasite establishment in the fish, where co-exposure of hosts by two parasite genotypes resulted in higher average infection success.
2. Material and methods
(a). Sampling and microsatellite analysis
Lymnaea stagnalis snails were collected from Lake Vuojärvi (62° N, 25° E), Central Finland, in July 2009. Snails were brought to the laboratory, where 15 D. pseudospathaceum cercaria larvae were randomly picked from each infected snail. Cercariae were stored individually in 1.5 ml Eppendorf tubes in 15 µl of water and frozen at −20°C for subsequent genetic analysis. In addition, a bulk sample of 15 cercariae was picked from each snail and stored in Eppendorf tubes as described above, resulting in a total of 30 cercariae that were collected and analysed from each snail. Individually stored cercariae were analysed to determine the relative proportions of the two parasite genotypes in the double-infected snails. The bulk samples of 15 cercariae from each snail were analysed separately and used to corroborate the number of parasite genotypes (one or two) in each snail. Infected snails were stored individually in 1 l of water at <5°C immediately after sampling and fed ad libitum with lettuce for one week until the beginning of the experiment. Cold temperature effectively stops parasite development, reproduction and cercarial release in the snails [8,9], which is why the genotype proportions in the double-infected snails were likely to remain unaltered during this time.
DNA from the individual cercariae and bulk samples was extracted with Chelex 100 resin [10]. Three microsatellite markers (Diplo06, Diplo09, Diplo23) designed for D. pseudospathaceum [11] were used to determine the number of parasite genotypes within the snails as described in Louhi et al. [12]. All snails were infected by different parasite multi-locus genotypes (appendix A in the electronic supplementary material), which was expected as these microsatellites are highly polymorphic (in a total of 32 parasite multi-locus genotypes we found 17 (Diplo06), 27 (Diplo09) and 11 (Diplo23) alleles per loci)), miracidia are produced sexually in the definitive host and these microsatellite loci have proved to be sufficient to separate parasite multi-locus genotypes [12]. We calculated the probability of a double infection in a snail by two genotypes exhibiting the same multi-locus genotype in relation to the allele frequencies of the lake population (estimated from a larger sample of infected snails from the same lake) and found this probability to be very low (<10−5; appendix A in the electronic supplementary material).
The probability of detecting a genotype in a double-infected snail based on the cercarial numbers can be calculated as 1 − (1 − p)n, where p is the frequency of the genotype and n the number of cercariae analysed. For example, in our case, by analysing 30 cercariae from each snail, the probability of detecting a rare parasite genotype would be 96 per cent if the rare genotype accounted for 10 per cent of the total cercarial production in a double-infected snail. Moreover, we calculated relatedness [13] between the co-infecting parasite genotypes using the software SPAGeDi [14] to analyse whether it had an effect on parasite infection success in natural double infections or in the artificial mixtures of the single genotypes (appendix B in the electronic supplementary material).
(b). Experimental exposures
Cercariae from 12 snails naturally infected with one parasite genotype and 10 snails infected with two parasite genotypes were used in the fish exposures. Prior to the exposures, all snails were taken out of the cold room, placed individually in 2.5 dl of water (17°C), and allowed to produce cercariae for 3 h. Cercarial density in the suspension, as well as the total number of cercariae produced, was estimated by taking five 1 ml samples from each snail. In the first experiment, 10 previously unexposed rainbow trout (Oncorhynchus mykiss, mean length ± s.e. = 102.83 ± 0.66 mm) were exposed individually to 100 cercariae from each of the 22 snails harbouring either one or two parasite genotypes, totalling 220 fish. In the second experiment, artificial mixtures of two parasite genotypes were produced by randomly assigning the 12 single-infected snails in six pairs (named snails A and B), and combining cercariae of each snail in a pair in seven different proportions while keeping the total parasite dose constant at 100 cercariae per fish. The proportions were 100:0, 90:10, 75:25, 50:50, 25:75, 10:90 and 0:100, from snail A and B in a pair, respectively. Ten rainbow trout were exposed individually to parasites from each combination of the six snail pairs and the seven genotype proportions, totalling 420 fish (data for single genotype exposures (100:0 and 0:100) came from experiment 1). Both experiments were conducted at the same time, and the exposures took place in containers with 0.5 l of water (17°C) and lasted for 30 min. After the exposure, fish were placed in 35 × 35 × 35 cm mesh cages, one group of 10 fish in each. The cages were placed randomly in five holding tanks (1500 l, continuous water flow, 17°C) so that the treatments from the first (single versus double infection) and the second experiment (genotype proportions and snail pair) were assigned to multiple tanks. This minimized any possible tank effect. Fish were maintained in these conditions for 48 h to allow parasite establishment after which they were euthanized with an overdose of MS-222 anaesthetic and dissected for the number of parasites in the eye lenses.
(c). Statistical analysis
In the first experiment, parasite infection success in natural single- and double-genotype exposures was analysed using a nested ANOVA with the number of genotypes as a fixed factor and snail as a random factor nested within the number of genotypes. In the second experiment, analysis on artificial genotype mixtures was carried out using an ANOVA with genotype proportion as a fixed factor and snail pair as a random factor. A polynomial contrast (quadratic prediction) was used to test if more even mixtures of genotypes resulted in higher infection success. All data fulfilled the assumptions of equal variance and normal distribution. Fish length was not different between the groups exposed to one or two parasite genotypes (nested ANOVA: F1,197 = 0.460, p = 0.498 (number of genotypes); F20,197 = 1.294, p = 0.186 (snail nested within the number of genotypes)) in the first experiment, or to different genotype proportions in artificial mixtures (nested ANOVA: F6,377 = 0.549, p = 0.771 (genotype proportion); F35,377 = 1.127, p = 0.290 (snail pair nested within the genotype proportion)) in the second experiment, and was therefore not included in the ANOVA models. Two fish died during the experiments and were excluded from data leaving a total of 219 and 419 fish for the first and second analysis, respectively. All analyses were conducted using SPSS v. 16.0.
3. Results
There was no difference in the mean cercarial output from the snails infected with one (mean ± s.e. = 17 366 ± 2106 cercariae 3 h−1) or two (18 287 ± 2207 cercariae 3 h−1) parasite genotypes (t-test: t20 = 0.301, p = 0.767). Moreover, genotype proportions had no effect on the cercarial production of the double-infected snails (Pearson correlation: r = −0.213, n = 10, p = 0.556).
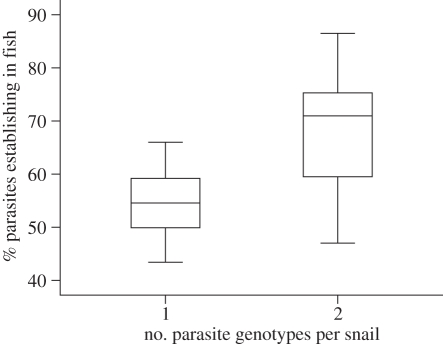
In both experiments, all fish became infected. In the first experiment, cercariae originating from double-genotype infected snails had a significantly higher infection success (25.5% difference) on fish than cercariae from single-genotype infected snails (mean ± s.e. = 68.6 ± 3.7 versus 54.7 ± 2.0 parasites per fish, respectively) (nested ANOVA: F1,197 = 12.167, p = 0.002; figure 1). We also found a significant variation in infection success of cercariae originating from different snail individuals (F20 ,197 = 2.559, p = 0.0005). However, the infection success was not affected by the actual proportions of the two parasite genotypes in double-infected snails (6.7–46.7% of the emerging cercariae were produced by the less common genotype, but this did not correlate with the infection success (Pearson correlation: r = −0.117, n = 99, p = 0.247)).
Figure 1.
Box plot showing the infection success of Diplostomum pseudospathaceum cercariae on naive rainbow trout. Parasites originated from either single- (left, n = 12) or double-genotype infected (right, n = 10) Lymnaea stagnalis snails. Infection success is defined as the proportion of parasites establishing in the eye lenses of the fish.
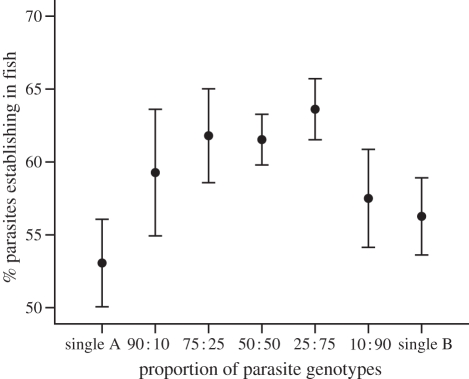
In the second experiment, artificial genotype mixtures had a higher infection success than single genotypes. The largest difference (16.0%) was observed when genotypes were mixed in equal proportions (polynomial contrast: F6,377 = 2.798, p = 0.011; quadratic prediction provided the best fit (p < 0.001) and captured the shape of the relationship; figure 2). There was also a significant difference among the snail pairs (ANOVA: F5,377 = 4.172, p = 0.005 (snail pair); F6,377 = 2.146, p = 0.077 (genotype proportion); F30,377 = 1.304, p = 0.135 (interaction)). Relatedness among the co-infecting parasite genotypes was low and did not have any effect on parasite infection success either in the first or in the second experiment (see appendix B in the electronic supplementary material).
Figure 2.
Mean infection success (±s.e.) of Diplostomum pseudospathaceum cercariae in single-genotype infections and in artificial mixtures of two genotypes in different proportions. Mixtures were produced by randomly assigning the 12 single-infected snails (figure 1) to six pairs (snails denoted by A and B in each pair).
4. Discussion
The dominating paradigm in host–parasite research is that interactions among parasite genotypes are antagonistic. Alternative hypotheses predicting that parasite genotypes could facilitate each other's success have received very little attention. Most of the examples showing facilitative interactions among parasite genotypes discuss cooperation in related pathogen strains and are motivated by kin selection [15–17]. In this paper, we examined the costs of competition for production of transmission stages, and the benefits of co-exposures for the establishment of the transmission stages, in consecutive hosts of a trematode parasite. We postulated that the advantage of co-infection in the establishment of transmission stages could be mediated by parasite diversity, which compromises host immune defences and leads to higher average parasite infection success. In our snail-fish-trematode system, the parasites first multiply in the snail, relying heavily on host resources (high host exploitation rate), and are then transmitted to fishes, where they are less dependent on host resources and use the fishes mainly as a vehicle to reach the definitive host (low host exploitation rate). Therefore, the competition is probably stronger in the snail host and the opportunity for facilitation is higher during transmission/establishment to/in the fish host. The optimal strategy for the parasite would then be to avoid co-infection and competition in the snail, and promote simultaneous attack with another genotype during transmission and establishment in the fishes. Our results are in accordance with this prediction.
We found that double-genotype infections produced fewer cercariae per parasite genotype, as predicted by the competition hypothesis. The most likely explanation for this is the reduced host resources available for each co-infecting genotype in a competitive situation. The idea of resource limitation is supported by the fact that the daily production of cercariae from the snails decreases with the age of infection [18], although this has not been investigated in relation to the number of parasite genotypes within a snail. Moreover, we observed that the relative proportions of the two parasite genotypes in double-infected snails had no effect on cercarial production. This is surprising as it could be expected that more equal proportions of the genotypes would intensify competition and host exploitation rate, resulting in higher production of transmission stages. The lack of such a pattern, however, suggests that genotypes do not actively increase their exploitation rate while sharing a host, which again supports the overall conclusion that the magnitude of cercarial production is mainly driven by the available host resources for each genotype.
The results from the first experiment indicated that cercariae from these natural double-genotype infections had a higher infection success in fishes when compared with single-genotype infections, supporting the facilitation hypothesis. To our knowledge, these data are the first to show higher infection success of natural mixed-genotype infections in a macroparasite. For example, Davies et al. [5] studied the effect of double-genotype infections in Biomphalaria glabrata snails on the cercarial infection success of Schistosoma mansoni in mice and found no difference when compared with cercariae originating from single-genotype infections in the snails. To our knowledge, the only study where such effects have previously been reported dealt with transmission success in mixed-strain infections of the rodent malaria Plasmodium chabaudi in mice showing higher transmission to mosquitoes when compared with single-strain infections [19]. Our results suggest that although the average infection success of genotypes from double-infected snails was higher, the per genotype production of transmission stages was lower than in single-infected snails. More specifically, while cercariae from double-infected snails had, on average, 25.5 per cent higher infection success when compared with single-genotype infections, each parasite genotype in double-infected snails produced, on average, 50 per cent less cercariae. This suggests that in cases where the proportions of the genotypes in the snail are close to 50 : 50, the benefit from higher infection success is eroded and both genotypes actually suffer from co-infection in terms of reduced production of infective stages. The overall transmission success could decrease further if double infection decreased snail survival, thus shortening the period of cercarial production. We do not know if this is the case in our system, but it has been described in other snail–trematode interactions [5].
In the second experiment, we determined whether artificial mixtures of the parasite genotypes originating from single-infected snails also lead to higher infection success in fishes when compared with a single-genotype attack, and if the success increased when genotypes were mixed in more equal proportions. It is important to note that this set-up excluded the possible confounding effect of competition in the snail host and thus the differences in the infection success should come from the responses of the fishes. We found that artificially mixed genotypes were more successful than single genotypes, and that the difference peaked when genotypes were mixed in equal proportions (figure 2). This suggests that the increase in infection success in double infections, as already found in the first experiment, primarily originates from the heterogeneity of exposure and not from specific characteristics of cercariae produced under conditions of within-snail competition. Earlier studies on parasites with complex life cycles have reported increased [20], decreased [21] or unaltered [22] infection success in artificial mixtures of parasite genotypes, suggesting species-specific variation in these interactions. In other systems, infection success was also shown to depend on relatedness of the co-infecting parasite genotypes [23–25], but this was not the case in this system. The low level of relatedness among the parasite genotypes is not surprising given the lack of genetic structure among the parasite populations even over a large geographical scale [12]. In our experiment, the infection success on fishes was highest when genotypes were mixed in equal proportions, which supports the hypothesis that higher infection success was owing to a decrease in the effectiveness of the host defence to cope with simultaneous attack by two genotypes. This effect, however, was absent in naturally double-infected snails in the first experiment (see above) despite the considerable variation in genotype proportions, which suggests that interactions exist between the within-snail competition and the fish's ability to respond. The exact mechanism underlying these different results, however, is unknown. Taken together, our results suggest that parasites suffer from co-existence in the snail, but infection of individual genotypes on fishes is facilitated by the diversity of exposure.
We point out that we did not determine the relative proportions of the co-infecting genotypes after establishment in fishes. It is possible, or even expected, that the interaction between individual genotypes and the fish host involves complex processes where one genotype may benefit disproportionally when compared with the other. In extreme cases, one genotype may also suffer from co-infection. As the parasite life cycle includes a sexual stage and co-infections occur between random genotypes, natural selection should operate on the average infection success of genotypes exhibiting particular traits. This is what our result showed. The average success of a parasite genotype co-infecting the fish with another genotype was higher than the average success of a single genotype. Also, average cercarial production rate per genotype in the snail host was lower if a genotype shared the snail with another genotype. We assume that the key difference between the snail and the fish host is that opportunity for competition between the parasite genotypes in fishes is low because metacercariae are unlikely to consume fish resources to a significant extent. An eye lens of an individual fish can harbour hundreds of metacercariae [26–28], suggesting that competition for space within the lens is also likely to be negligible in infection intensities observed in wild fishes and used in this experiment.
Higher success of parasite genotypes that attack simultaneously with others may have significant evolutionary consequences for parasite transmission strategies. In this system, there are several potential mechanisms for how co-exposure could emerge. First, snail habitats are restricted to shallow littoral zones of water bodies concentrating high numbers of hosts in a relatively small area and creating infection ‘hotspots’ [29]. Second, the release of cercariae from snails takes place synchronously within a few weeks during summer [9] and mainly during the day [18]. Given that natural fish hosts of these parasites inhabit the same areas as the snails, and actively move within and between the infection ‘hotspots’, a simultaneous co-exposure to multiple parasite genotypes is likely to be the norm rather than an exception. Infection of fishes by multiple genotypes may be advantageous also later in the life cycle as it decreases the probability of inbreeding when parasites become transmitted to an avian definitive host and reproduce sexually [30]. Overall, our results suggest that facilitative parasite genotype interactions could be much more common than previously acknowledged, while this does not exclude the possibility for competition in hosts where exploitation rates are high. Earlier, such facilitation has mainly been considered in the context of kin selection of related pathogen strains where the term ‘cooperation’ is typically used [15–17], but studies considering advantages for transmission to the next host are rare [31]. However, further empirical and theoretical studies on cost–benefit ratios associated with single versus multiple genotype interactions are needed in different systems to address the generality of these findings.
Acknowledgements
All experiments were carried out with permission from Finnish Regional State Administrative Agency (license no. ESLH-2008-05938/Ym-23) and they complied with the animal care legislation of Finland.
We thank S. Viinikainen for help in the laboratory, O. Seppälä for discussions, and Kayla King for reading and commenting on the manuscript. The study was supported by grants from the Academy of Finland (A.K., project number 121993), Finnish Academy Centre of Excellence of Evolutionary Research (A.K., C.R., K.-R.L. and J.J.), Swiss National Science Foundation (J.J., project number 31003A-113666), and ETH-CCES projects BioChange and GeDiHap (J.J.).
References
- 1.Read A. F., Taylor L. H. 2001. The ecology of genetically diverse infections. Science 292, 1099–1102 10.1126/science.1059410 (doi:10.1126/science.1059410) [DOI] [PubMed] [Google Scholar]
- 2.Woolhouse M. E. J., Webster J. P., Domingo E., Charlesworth B., Levin B. R. 2002. Biological and biomedical implications of the co-evolution of pathogens and their hosts. Nat. Genet. 32, 569–577 10.1038/ng1202-569 (doi:10.1038/ng1202-569) [DOI] [PubMed] [Google Scholar]
- 3.Kuris A. M., Lafferty K. D. 1994. Community structure: larval trematodes in snail hosts. Ann. Rev. Ecol. Syst. 25, 189–217 10.1146/annurev.es.25.110194.001201 (doi:10.1146/annurev.es.25.110194.001201) [DOI] [Google Scholar]
- 4.de Roode J. C., et al. 2005. Virulence and competitive ability in genetically diverse malaria infections. Proc. Natl Acad. Sci. USA 102, 7624–7628 10.1073/pnas.0500078102 (doi:10.1073/pnas.0500078102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies C. M., Fairbrother E., Webster J. P. 2002. Mixed strain schistosome infections and the evolution of parasite virulence. Parasitology 124, 31–38 10.1017/S0031182001008873 (doi:10.1017/S0031182001008873) [DOI] [PubMed] [Google Scholar]
- 6.Jokela J., Schmid-Hempel P., Rigby M. C. 2000. Dr. Pangloss restrained by the Red Queen: steps towards a unified defence theory. Oikos 89, 267–274 10.1034/j.1600-0706.2000.890207.x (doi:10.1034/j.1600-0706.2000.890207.x) [DOI] [Google Scholar]
- 7.Chappell L. H., Hardie L. J., Secombes C. J. 1994. Diplostomiasis: the disease and host-parasite interactions. In Parasitic diseases of fish (eds Pike A. W., Lewis J. W.), pp. 59–86 Dyfed, UK: Samara Publishing Limited [Google Scholar]
- 8.Waadu G. D. B., Chappell L. H. 1991. Effect of water temperature on the ability of Diplostomum spathaceum miracidia to establish in lymnaeid snails. J. Helminthol. 65, 179–185 10.1017/S0022149X00010671 (doi:10.1017/S0022149X00010671) [DOI] [PubMed] [Google Scholar]
- 9.Karvonen A., Seppälä O., Valtonen E. T. 2004. Parasite resistance and avoidance behaviour in preventing eye fluke infections in fish. Parasitology 129, 159–164 10.1017/S0031182004005505 (doi:10.1017/S0031182004005505) [DOI] [PubMed] [Google Scholar]
- 10.Criscione C. D., Blouin M. S. 2004. Life cycles shape parasite evolution: comparative population genetics of salmon trematodes. Evolution 58, 198–202 10.1111/j.0014-3820.2004.tb01587.x (doi:10.1111/j.0014-3820.2004.tb01587.x) [DOI] [PubMed] [Google Scholar]
- 11.Reusch T. B. H., Rauch G., Kalbe M. 2004. Polymorphic microsatellite loci for the trematode Diplostomum pseudospathaceum. Mol. Ecol. Notes 4, 577–579 10.1111/j.1471-8286.200400740.x (doi:10.1111/j.1471-8286.200400740.x) [DOI] [Google Scholar]
- 12.Louhi K.-R., Karvonen A., Rellstab C., Jokela J. 2010. Is the population genetic structure of complex life cycle parasites determined by the geographic range of the most motile host? Infect. Genet. Evol. 10, 1271–1277 10.1016/j.meegid.201008.013 (doi:10.1016/j.meegid.201008.013) [DOI] [PubMed] [Google Scholar]
- 13.Queller D. C., Goodnight K. F. 1989. Estimating relatedness using genetic markers. Evolution 43, 258–275 10.2307/2409206 (doi:10.2307/2409206) [DOI] [PubMed] [Google Scholar]
- 14.Hardy O. J., Vekemans X. 2002. SPAGeDi: a versatile computer program to analyse spatial genetic structure at the individual or population levels. Mol. Ecol. Notes 2, 618–620 10.1046/j.1471-8286.2002.00305.x (doi:10.1046/j.1471-8286.2002.00305.x) [DOI] [Google Scholar]
- 15.Griffin A. S., West S. A., Buckling A. 2004. Cooperation and competition in pathogenic bacteria. Nature 430, 1024–1027 10.1038/nature02744 (doi:10.1038/nature02744) [DOI] [PubMed] [Google Scholar]
- 16.Buckling A., Brockhurst M. A. 2008. Kin selection and the evolution of virulence. Heredity 100, 484–488 10.1038/sj.hdy.6801093 (doi:10.1038/sj.hdy.6801093) [DOI] [PubMed] [Google Scholar]
- 17.Nogueira T., Rankin D. J., Touchon M., Taddei F., Brown S. P. 2009. Horizontal gene transfer of the secretome drives the evolution of bacterial cooperation and virulence. Curr. Biol. 19, 1683–1691 10.1016/j.cub.200908.056 (doi:10.1016/j.cub.200908.056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karvonen A., Kirsi S., Hudson P. J., Valtonen E. T. 2004. Patterns of cercarial production from Diplostomum spathaceum: terminal investment or bet-hedging? Parasitology 129, 87–92 10.1017/S0031182004005281 (doi:10.1017/S0031182004005281) [DOI] [PubMed] [Google Scholar]
- 19.Taylor L. H., Walliker D., Read A. F. 1997. Mixed-genotype infections of the rodent malaria Plasmodium chabaudi are more infectious to mosquitoes than single-genotype infections. Parasitology 115, 121–132 10.1017/S0031182097001145 (doi:10.1017/S0031182097001145) [DOI] [PubMed] [Google Scholar]
- 20.Wedekind C. 1997. The infectivity, growth, and virulence of the cestode Schistocephalus solidus, in its first intermediate host, the copepod Macrocyclops albidus. Parasitology 115, 317–324 10.1017/S0031182097001406 (doi:10.1017/S0031182097001406) [DOI] [PubMed] [Google Scholar]
- 21.Rauch G., Kalbe M., Reusch T. B. H. 2008. Partitioning average competition and extreme-genotype effects in genetically diverse infections. Oikos 117, 399–405 10.1111/j.20070030-1299.16301.x (doi:10.1111/j.20070030-1299.16301.x) [DOI] [Google Scholar]
- 22.Keeney D. B., Bryan-Walker K., Khan N., King T. M., Poulin R. 2009. The influence of clonal diversity and intensity-dependence of trematode infections in an amphipod. Parasitology 136, 339–348 10.1017/S0031182008005416 (doi:10.1017/S0031182008005416) [DOI] [PubMed] [Google Scholar]
- 23.Jäger I., Schjørring S. 2006. Multiple infections: relatedness and time between infections affect the establishment and growth of the cestode Schistocephalus solidus in its stickleback host. Evolution 60, 616–622 10.1111/j.0014-3820.2006.tb01141.x (doi:10.1111/j.0014-3820.2006.tb01141.x) [DOI] [PubMed] [Google Scholar]
- 24.Koskella B., Giraud T., Hood M. E. 2006. Pathogen relatedness affects the prevalence of within-host competition. Am. Nat. 168, 121–126 10.1086/505770 (doi:10.1086/505770) [DOI] [PubMed] [Google Scholar]
- 25.López-Villavicencio M., Courjol F., Gibson A. K., Hood M. E., Jonot O., Shykoff J. A., Giraud T. 2011. Competition, cooperation among kin, and virulence in multiple infections. Evolution 65, 1357–1366 10.1111/j.1558-5646.2010.01207.x (doi:10.1111/j.1558-5646.2010.01207.x) [DOI] [PubMed] [Google Scholar]
- 26.Chappell L. H. 1969. The parasites of the three-spined stickleback Gasterosteus aculeatus L. from a Yorkshire pond. II. Variation of the parasite fauna with sex and size of fish. J. Fish Biol. 1, 339–347 10.1111/j.1095-8649.1969.tb03881.x (doi:10.1111/j.1095-8649.1969.tb03881.x) [DOI] [Google Scholar]
- 27.Wootten R. 1974. Observations on strigeid metacercariae in the eyes of fish from Hanningfield Reservoir, Essex, England. J. Helminthol. 48, 73–83 10.1017/S0022149X00022628 (doi:10.1017/S0022149X00022628) [DOI] [PubMed] [Google Scholar]
- 28.Karvonen A., Paukku S., Seppälä O., Valtonen E. T. 2005. Resistance against eye flukes: naïve versus previously infected fish. Parasitol. Res. 95, 55–59 10.1007/s00436-004-1246-x (doi:10.1007/s00436-004-1246-x) [DOI] [PubMed] [Google Scholar]
- 29.Jokela J., Lively C. M. 1995. Spatial variation in infection by digenetic trematodes in a population of fresh-water snails (Potamopyrgus antipodarum). Oecologia 103, 509–517 10.1007/BF00328690 (doi:10.1007/BF00328690) [DOI] [PubMed] [Google Scholar]
- 30.Rauch G., Kalbe M., Reusch T. B. H. 2005. How a complex life cycle can improve a parasite's sex life? J. Evol. Biol. 18, 1069–1075 10.1111/j.1420-9101.200500895.x (doi:10.1111/j.1420-9101.200500895.x) [DOI] [PubMed] [Google Scholar]
- 31.Clavijo G., Williams T., Muñoz D., Caballero B., Lόpez-Ferber M. 2010. Mixed genotype transmission bodies and virions contribute to the maintenance of diversity in an insect virus. Proc. R. Soc. B 277, 943–951 10.1098/rspb.20091838 (doi:10.1098/rspb.20091838) [DOI] [PMC free article] [PubMed] [Google Scholar]