Abstract
Offspring of long-lived species should face costs of parental trade-offs that vary with overall energetic demands encountered by parents during breeding. If sex differences exist in how parents make the trade-off, sex-specific differences may exist in the contribution of each parent to those costs. Adaptations of offspring facing such costs are not well understood, but the hormone corticosterone probably plays a role. We manipulated breeding effort in Cory's shearwaters (Calonectris diomedea) to increase costs to offspring and used an integrated measure of corticosterone from chick feathers to investigate how experimental variation in parental investment influences offspring physiology. Average foraging trip duration and foraging efficiency (FE) of breeding pairs were not related to chick corticosterone, but sex biases in FE were. Adult male investment was more strongly related to chick corticosterone than was female investment. Importantly, we show for the first time suppression of adrenocortical activity in nestling Procellariiform seabirds, and explain how our results indicate an adaptive mechanism invoked by chicks facing increased costs of parental trade-offs.
Keywords: Cory's shearwater, feather corticosterone, life history, parental investment, stress physiology, trade-offs
1. Introduction
The trade-off between current reproductive effort and future survival and reproduction has been the subject of considerable research in life-history evolution [1]. Adults of long-lived species, such as many seabirds, are expected to favour their own condition over that of their young when faced with adverse circumstances during the breeding season [1–3], and offspring may therefore face costs of this parental trade-off. Although most studies on seabirds support this assertion [4–7], sex differences may exist in the extent to which males and females make the trade-off. Such differences are probably due to aspects of parental investment that differ between the sexes [8]. Male and female adult seabirds can differ in foraging strategies [9–11] ability to recover body condition [9], sensitivity to chick begging [12] and contributions to nestling diet [9,13–17]. Thus, while costs experienced by chicks are expected to vary with overall energetic demands encountered by parents, there may also be sex-specific differences in the contribution of each parent to those costs. This may be especially true when one sex is not willing or able to compensate for the other, such as during times of poor food availability when parents prioritize their own condition.
Relatively little attention has been paid to the adaptations of offspring facing costs of adverse parental decisions and how they may contribute to overall life-history strategies. One important mechanism for coping with environmental perturbations in general is activation of the vertebrate hypothalamic–pituitary–adrenal (HPA) axis in response to unpredictable noxious stimuli (i.e. ‘stressors’; [18]). The HPA axis helps vertebrates regulate energy levels through secretion of glucocorticoid (GC) hormones such as corticosterone (CORT), the primary avian GC. Nutritional challenges are known stressors characteristic of intermittent feeding of seabird chicks [19,20], and the frequency of feeding, and quality and quantity of food delivered to chicks, can influence the severity of the challenge [21,22].
Interspecific variation exists in how nestling seabirds respond with CORT to reductions in caloric intake and nutritional quality [23]. Some species increase baseline or acute stress-induced CORT secretion to promote catabolism of fat stores for increased energy availability, and to facilitate begging that encourages increased parental provisioning [19,23–25]. In doing so they risk reduced growth rate and immune response, depletion of lipid reserves, protein catabolism and impaired cognition as a result of prolonged CORT secretion [18,23,24,26–29]. In other species, nestlings respond to nutritional challenges by modulating activity of the HPA axis to suppress one or more parameters of the CORT response. This has been observed as a reduction in baseline or acute stress-induced levels [21], and also as a ‘muting’ of the response, i.e. an increase or stability in baseline with no change in stress-induced [30]. It has been proposed that this CORT suppression strategy is related to a disassociation of the nutritional state of the chick and its HPA axis [21]. Thus, although this strategy comes at a cost of a slowed growth rate, it avoids the deleterious effects of sustained elevated CORT and allows chicks to maintain protein and fat stores [21]. Why variation in CORT responses to dietary restrictions exists is not well understood [23]; however, it is apparent that CORT physiology plays a crucial role and therefore may underlie an adaptive mechanism to cope with costs of parental trade-offs [31].
A previous study of Cory's shearwaters (Calonectris diomedea) by Navarro & González-Solís [7] found that when one member of a breeding pair was experimentally handicapped via increase of flying costs (i.e. breeding effort) it decreased its parental investment and passed along the cost partly to its partner, but the cost was most strongly experienced by the offspring. Handicapped adults increased the duration and distance of foraging trips resulting in longer incubation stints for their partners and less food provisioned to chicks. In turn, chicks raised by handicapped pairs were smaller, lighter and had a lower cell-mediated immune response, and the authors suggested that poor provisioning was responsible for these effects [7]. Although foraging trip length did not differ significantly between the sexes, total mass gained while foraging was greater in males than in females [7].
Here, we suggest that nestling CORT responses to parental trade-offs can explain the effects seen in chicks from the 2007 Navarro & González-Solís paper [7], and we use an integrated measure of CORT physiology [32,33] from chick feathers collected during their experiment to explore this possibility. Feather CORT values incorporate the amplitude and duration of all CORT secretion, including response to stressors, during the period of feather growth [32,33] and thus represent a biologically relevant measure of CORT secretion [18]. We hypothesize that variation in parental investment was experienced by nestlings as variation in a nutritional stressor to which the nestling HPA axis should be sensitive. Furthermore, sex differences in how adults traded off provisioning their young in favour of their own condition should be evident in the strength of relationships between offspring CORT and each of its parents' investment.
We tested the following three predictions. First, nestling CORT should be related to variation in duration of foraging trips and foraging efficiency (FE) of parents (i.e. rate of mass gained at sea; see below) because these are measures of parental effort that vary with increasing costs to parents [7,34]. In our population, foraging costs increase with increasing trip length [7] and, at least in other populations, longer trips result in less food being delivered per day to shearwater chicks [35]. Individual differences in FE contribute to rules governing how parents allocate energy between themselves and their offspring [36] and thus influence the costs experienced by chicks. Second, nestling CORT should be differentially sensitive to male and female FE, but not foraging trip duration (TD), because the sexes differ in total mass gained at sea but not in duration of foraging trips [7]. Third, nestling CORT should be suppressed relative to controls when adult foraging costs are increased by handicapping. Suppressed HPA activity is expected to occur in nestlings of species with intermittent feeding, a prolonged nestling period to compensate for slow growth rate, and parents that are relatively insensitive to offspring demands [21,23], and Cory's shearwaters exhibit all these characteristics [7,37,38]. Our study's methodological perspective adds to a limited number of investigations into physiological adaptations of nestlings to parental reductions in food provisioning.
2. Methods
(a). Study area and field methods
For more detailed information on field methods, see [7]. Briefly, the study was conducted on Gran Canaria (15°47′18″ N; 27°50′41″ E, Canary Islands, Spain), from April to November 2004 at a breeding colony of about 150 pairs of Cory's shearwaters. Breeding pairs were randomly assigned to the control (n = 14) or experimental group (n = 28) and once the female had laid her egg, one adult from every pair (50 : 50 male : female) in the experimental group was handicapped by clipping the tips of every primary feather to increase flying costs by 5 per cent [7,39]. Thus, pairs from the experimental group included one handicapped bird and its unmanipulated partner. Additionally, during incubation, 19 control and 19 handicapped adults were each instrumented with a 10-g geolocator (GLS units, British Antarctic Survey, Cambridge, UK) to measure foraging TD and foraging locations. GLS units have a photoreceptor that measures light levels every 60 s, and they record the maximum reading within each 10-min interval with reference to an internal clock calendar. Sunset and sunrise times were estimated from thresholds in light curves; latitude was derived from day duration and longitude from the time of local midday with respect to Greenwich Mean Time and day of the year, providing two locations per day (one corresponding to midday and the other to midnight). The accuracy of the light-level geolocation is relatively low (average error approx. 186 km). However, the aim of our study was not a detailed description of the foraging trips, but a comparison of the foraging behaviour between control and handicapped birds. Any position obtained in a short period, as in the present study, is under the same accuracy error, and to avoid potential selection biases of locations we applied a homogeneous filter based solely on a velocity index (see [7] for more details). GLS units were one third the mass found to have an effect on shearwater flight performance [40], so although we cannot rule out a possible influence in our study, we believe it to be negligible and the effect balanced across treatment groups.
During incubation, we studied the changes in mass in all birds by weighing all birds every 3 days until foraging trip departure, and then again upon subsequent return. Birds were weighed between the hours of 10.00 and 12.00 using a large bag and Pesola spring balances. For those birds that we weighed 2 or 3 days before departure, we estimated the mass at departure using the last mass recorded and the proportional daily loss of mass for the appropriate sex (mean daily mass loss: males = 15.38 g d−1, females = 14.25 g d−1; calculated from incubating birds that were weighed more than once).
We sampled 28 80-day-old chicks: 10 reared by control and 18 by experimentally handicapped pairs. Chicks were ringed and weighed and their culmen, tarsus and wing were measured with digital callipers to the nearest ±0.1 mm. A single back feather was taken from each chick and stored in a paper envelope for subsequent quantification of CORT (see below). Based on exact dates of hatching, all chicks were of a comparable age when feathers were collected. All feathers were fully grown when collected, began growing when chicks were approximately 50 days old, and completed growth around 70 days of age. Aside from changes resulting from handicapping [7], adult feeding behaviour was normal throughout the feather growth period. Adults and chicks were sexed using molecular procedures [7]. Based on observations, all chicks fledged successfully and at approximately the same time.
(b). Feather CORT analysis
Feather CORT assays followed [32]. Briefly, we extracted CORT from feathers using a methanol-based technique. The length of the feather was measured, the calamus was removed and discarded, and then the sample was cut into pieces less than 5 mm2 with scissors. We then added 10 ml of methanol (HPLC grade, Fisher Scientific, Fairlawn, NJ, USA) and placed the samples in a sonicating water bath at room temperature for 30 min, followed by incubation at 50°C overnight in a shaking water bath. The methanol was then separated from feather material by vacuum filtration, using a plug of synthetic polyester fibre in the filtration funnel. The methanol extract was placed in a 50°C water bath and subsequently evaporated in a fume hood. Extract residues were reconstituted in a small volume of phosphate-buffered saline (0.05 M, pH 7.6) and frozen at −20°C until analysed by radioimmunoassay (RIA). We assessed the efficiency of the methanol extraction by including feather samples spiked with a small amount (approximately 5000 CPM) of 3H-corticosterone in the extraction. Greater than 92 per cent of the radioactivity was recoverable in the reconstituted samples. For more information about validation, see supplementary appendix S1 in [32].
Feather CORT levels were determined by RIA [41]. Measurements were performed on reconstituted methanol extracts, and samples were measured in duplicate. Samples were measured in a single assay with an intra-assay coefficient of variation of 8.7 per cent. The assay had a detectability limit (80% bound) of 14.20 pg per assay tube, but all samples were well above this value. Data values are expressed as pg CORT per mm of feather, which gives a valid estimate of CORT per unit time of feather growth [32,33] (and see [42] for validation). CORT assays were performed at the University of Saskatchewan, Canada.
(c). Variable definitions and statistical analyses
Total foraging TD and FE were defined according to [7]. TD is the total number of days between departure from the nest for foraging and subsequent return. FE is the rate of daily mass gain while foraging, calculated as total mass gained during foraging trip divided by TD. TD and FE were calculated separately for 17 males (TDmale, FEmale) and 15 females (TDfemale, FEfemale). In nine control breeding pairs we recorded both TD and FE for both partners. For these cases, we assessed the relative parental effort of breeding pairs by computing average TD and FE values for both partners (i.e. (male + female)/2; TDpair and FEpair), and assessed potential sex bias in TD and FE by computing the difference between the partners (i.e. (male − female); TDbias and FEbias).
Because Navarro & González-Solís [7] only collected feathers from a subset of chicks in their 2007 paper, we wanted to confirm that our subset of TD and FE values were not affected by a subsampling bias. We therefore used separate models with TD and FE as the response variable, adult sex and treatment as fixed factors, and included a sex × treatment interaction term. We also tested for a chick sex difference in feather CORT, as well as a possible interaction between sex and treatment, using sex and treatment as fixed factors and a sex × treatment interaction term.
To determine the influence of within-pair variation in parental investment on chick CORT, we modelled TDpair, FEpair, TDbias and FEbias individually as fixed factors in four separate models. To further confirm which sex's behaviour had the greater influence on chick CORT, we used the same pairs but modelled TDmale and TDfemale as separate terms in the same model, rather than as within-pair averages or biases, and repeated this approach for FE.
To address the relationships between parental handicapping, TD and FE, and feather CORT, we expanded our sample size by considering all cases where we had TD and FE for at least one member of a breeding pair and feather CORT data for the chick. We used CORT as the response variable in two separate models and included treatment, adult sex, behaviour (TD or FE) and a behaviour × sex interaction term as fixed factors. Non-significant interaction terms were removed from final models. All models used a normal distribution of errors and an identity link function. Data were analysed using PROC GENMOD in SAS v. 9.1 (SAS Institute, Cary, NC).
3. Results
As in the 2007 paper by Navarro & González-Solís [7], duration of adult foraging trips did not differ between the sexes for control breeding pairs (F1,16 = 2.64, p = 0.12), but we detected a non-significant trend for TDs of experimentally handicapped females to be longer than those of males (F1,13 = 4.17, p > 0.06). We acknowledge this as a potential subsampling bias because the original study did not find a difference between sexes in its larger sample of experimental adults [7], but combined the sexes for all subsequent analyses. A single CORT value was three standard deviations greater than the mean, suggesting an analytical error or an individual out of the norm for our population (e.g. an ill bird); therefore, this value was excluded from analyses. There was no significant interaction between chick sex and treatment on CORT (F1,23 = 3.13, p = 0.09), and CORT did not differ between chick sexes (F1,23 = 1.89, p = 0.18), so they were combined for subsequent analyses.
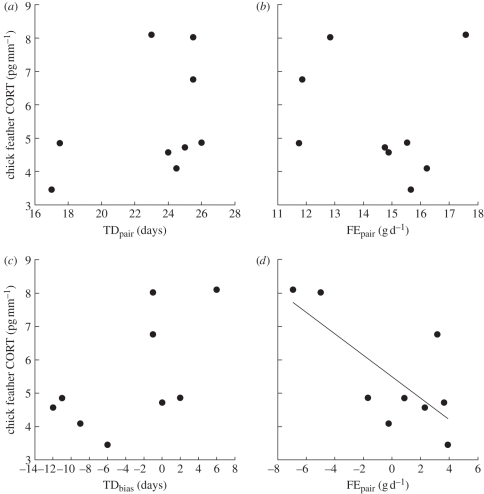
We found no significant relationship between CORT and TDpair (figure 1; F1,7 = 1.34, p = 0.28) or FEpair (F1,7 = 0.13, p = 0.73). However, when we examined the relationships between TDbias and FEbias and CORT, we found a non-significant effect of TDbias (figure 1; F1,7 = 4.64, p = 0.07) but a significant effect of FEbias (F1,7 = 8.12, p < 0.03). This implies that within control breeding pairs as TDmale increased relative to TDfemale chicks expressed relatively higher CORT levels, albeit not significantly; and as FEmale increased relative to FEfemale, chicks expressed relatively lower CORT. This sex effect was further evident in control pairs when we included FEmale and FEfemale as separate terms in the same model, because the former was significantly related to CORT (F1,6 = 10.65, p < 0.02) whereas the latter was not (F1,6 = 2.86, p = 0.14). A similar model for TD showed that neither TDmale nor TDfemale was significantly related to CORT (TDmale: F1,6 = 4.01, p = 0.09; TDfemale: F1,6 = 0.58, p = 0.48), but the trends were in the same direction as the FE models.
Figure 1.
Relationships between measures of parental investment in Cory's Shearwater breeding pairs and their nestling's feather corticosterone (CORT): (a) total duration of foraging trips (TDpair) and (b) average foraging efficiency (FEpair). The within-pair difference between males and females in (c) duration of foraging trips (TDbias) and (d) foraging efficiency (FEbias); values greater than zero indicate male bias and values less than zero indicate female bias. Data presented are for control pairs only. See text for variable definitions.
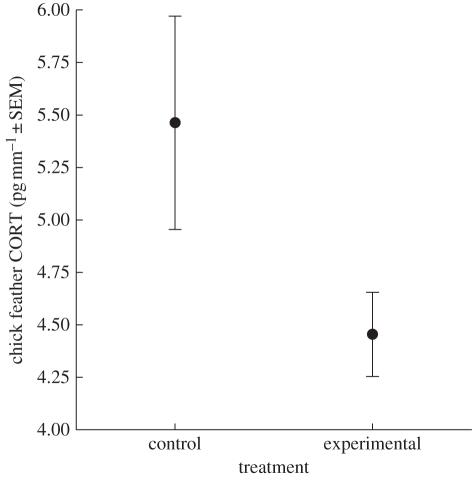
When we expanded our sample to include all cases where TD and FE were measured for at least one pair member, overall experimental chicks had significantly lower feather CORT than control chicks (figure 2; experimental = 4.45 ± 0.20 pg per mm−1, control = 5.46 ± 0.51 pg per mm−1, F1,23 = 7.08, p = 0.01). Our model of TD and chick CORT had a significant interaction between TD and adult sex (F1,27 = 5.12, p = 0.03), so we ran separate models for each sex (table 1). The final model for adult males revealed a significant positive relationship between TDmale and CORT (table 1) and experimental chicks had significantly lower CORT than controls. The final model for adult females revealed no significant relationship between TDfemale and CORT (table 1) and experimental chicks did not differ significantly from controls.
Figure 2.
Mean (± s.e.) feather corticosterone (CORT) values of Cory's shearwater chicks raised by experimentally handicapped adults (experimental; n = 17) and non-handicapped control adults (control; n = 10).
Table 1.
Summary of results from GENMOD models testing for the influence of experimental handicapping of parents, sex differences in parental foraging TD and FE, and their interaction on feather corticosterone (CORT) in Cory's shearwater chicks. Significant values are in bold.
| model term | estimate | s.e. | F-statistic (d.f.) | p-value | |
|---|---|---|---|---|---|
| males | treatment | 1.6053 | 0.6070 | 6.99 (1,14) | 0.019 |
| TD | 0.1825 | 0.0680 | 7.21 (1,14) | 0.018 | |
| TD × treatment | 0 (1,13) | 0.972 | |||
| females | treatment | 0.8274 | 0.7968 | 1.08 (1,12) | 0.320 |
| TD | −0.0958 | 0.0982 | 0.95 (1,12) | 0.349 | |
| TD × treatment | 0.02 (1,11) | 0.903 | |||
| males | treatment | 7.7083 | 2.8695 | 7.22 (1,13) | 0.018 |
| FE | −0.1020 | 0.0906 | 10.61 (1,13) | 0.006 | |
| FE × treatment | 4.91 (1,13) | 0.045 | |||
| control | −0.5393 | 0.1895 | 8.10 (1,7) | 0.025 | |
| experimental | −0.1025 | 0.0809 | 1.60 (1,6) | 0.252 | |
| females | treatment | 0.8673 | 0.7479 | 1.34 (1,12) | 0.269 |
| FE | 0.1213 | 0.0949 | 1.64 (1,12) | 0.225 | |
| FE × treatment | 0.33 (1,11) | 0.580 |
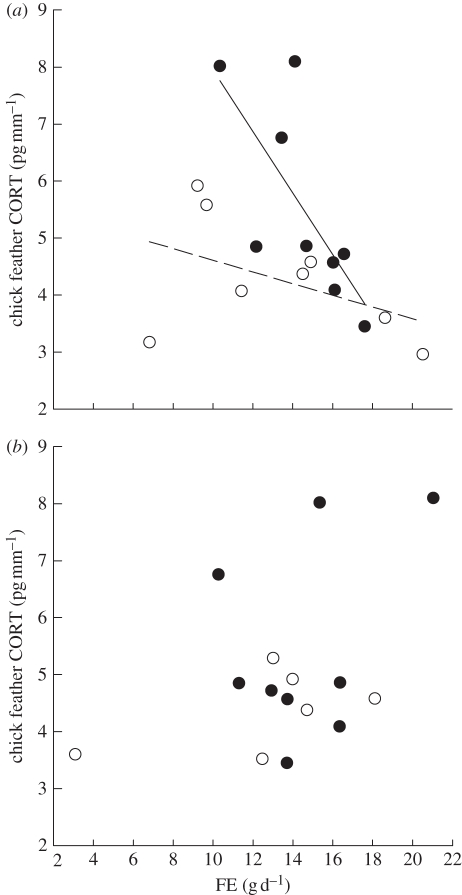
We found a significant interaction between FE and adult sex (F1,27 = 5.56, p < 0.03), so we analysed the sexes separately (table 1). The interaction between FEmale and treatment on CORT was significant, so we modelled each treatment separately for males (table 1). FEmale was negatively related to CORT in control chicks (table 1, figure 3), but was not related to CORT in experimental chicks. The interaction between FEfemale and treatment on CORT was not significant (table 1), and the final model for adult females revealed that FEfemale was not significantly related to CORT (table 1, figure 3) and did not differ between control and experimental chicks.
Figure 3.
Relationships between FE of control (filled circles and solid lines) and experimentally handicapped (open circles and dash lines) (a) male and (b) female adult Cory's shearwaters and the feather corticosterone (CORT) of their chick.
4. Discussion
Our study provides two advances in the understanding of life-history trade-offs. First, we highlight the importance of sex-biased investment to offspring physiology and show that adult male shearwaters in particular play an important role in offspring energy balance. Second, we provide experimental evidence that free-living Procellariid chicks can suppress CORT secretion as an adaptive response to cope with increased costs of parental trade-offs. This result indicates flexibility in nestling physiology during growth to better match energetic need to parental provisioning. Furthermore, we show that CORT from nestling feathers can provide information about how chicks are responding physiologically to variation in parental effort.
Costs are expected to arise in chicks when parents favour self-maintenance over provisioning their offspring, and our study suggests that sex differences in how parents resolve this trade-off differentially affects offspring CORT. In accordance with previous studies [7,19,21,24,25,30], it is probable that an overall caloric restriction was the cost of adult trade-offs to which chick CORT was responding. Responses were related to within-pair sex biases in how parents contributed to that cost. Specifically, variation in male effort was more influential than variation in female effort.
Sex differences in parental investment in our study may have been due to differences in the extent to which the sexes were willing to increase provisioning in response to chick need [43,44]. Cory's shearwaters exhibit fixed investment in reproduction and are predicted to not increase their effort as chick demands increase [7]. However, some male Procellariiform seabirds may be even less probable than females to increase effort. For example, female Manx shearwaters (Puffinus puffinus) responded to chick begging by adjusting meal size, whereas males did not [12]. Additionally, during poor food years female Wilson's storm petrels (Oceanites oceanicus) make longer foraging trips than do males, and this may be due to greater responsiveness to chick need by females than males [45]. In cases where costs of the trade-off between self-maintenance and offspring provisioning is greater in males than in females, variation in male investment could have a greater influence on chick physiology.
Importantly, we provide experimental evidence that shearwater chicks suppressed CORT secretion when faced with extended nutritional challenges. Chick CORT was most strongly related to male FE, which is a measure of parental effort that incorporates duration of foraging trips, individual quality and foraging decisions [36]. Not surprisingly, our results indicate that increased investment by control males reduced costs in their chicks. However, when we considered experimentally handicapped males, the CORT of their chicks showed no relationship with FE. This suggests that increased costs of trade-offs from handicapped males resulted in a relative insensitivity of the physiology of their chicks. Moreover, CORT was overall significantly lower in chicks raised by experimental parents compared to controls. We interpret these results as confirmation of our prediction that shearwater chicks suppress CORT secretion when adult foraging costs are experimentally increased.
Is lower CORT in experimental chicks a result of an adaptive response, or simply an expression of poor physiological functioning of birds with extended nutritional deficits? It is possible that the nutritional condition of experimental chicks was such that they were only able to mount a poor CORT response following nutritional challenges, or they were developmentally delayed and incapable of mounting a better response. However, it is unlikely that chicks expressing such compromised physiology would be able to survive to fledging without indicators of lipid or protein reserves, or muscle damage, being affected [46]. Yet, in their 2007 paper Navarro & González-Solís [7] found that levels of these biochemical parameters were similar between control and experimental chicks, and all chicks fledged at approximately the same time. Moreover, there was some overlap in the range of CORT values for each group. These data suggest that the physiology of experimental chicks was operating within normal limits. We therefore lack the evidence to support a conclusion that experimental chicks were physiologically impaired.
To the contrary, we reason that experimental chicks were within their physiological ability to handle periods of nutritional deficit. CORT suppression was therefore probably an adaptive response to cope with the increased costs of parental trade-offs. We argue that cumulative costs of parental trade-offs in experimental chicks reached a tipping point and CORT suppression allowed these birds to minimize the extent of physiological damage caused by chronically elevated CORT [18–20,23,26,29]. Experimental chicks paid for this because they were smaller, lighter and had reduced immune response [7]. Yet, these were not life-threatening energy deficits because the prolonged period of shearwater nestling growth would allow for compensatory growth [7,21,23,37,38] and survival to fledging did not differ between treatment groups [7]. CORT suppression need not entail a complete alteration of the functioning of the HPA axis, as evidence from other species indicates that even the most food-restricted individuals exhibiting CORT suppression are still able to respond to stressors [21,30].
Understanding how and why individuals manage their exposure to CORT during critical periods of post-natal development is important because CORT can affect nestling phenotype [23,47–51] and potentially fitness [52] (for reviews see [53,54]). Moreover, timing of CORT exposure during development is important [48]. In our study, handicapping of adults occurred at the onset of egg-laying and therefore increased costs were experienced by nestlings throughout their post-natal development. Whether shearwater nestlings would suppress CORT in response to less severe or shorter-term increases in costs remains to be determined. Future investigations should focus on identifying the ecological circumstances that promote a CORT suppression strategy and must consider phylogeny, mode of nestling development [55], and the type of nutritional challenge facing nestlings (i.e. feeding frequency, diet quality and/or quantity). Longitudinal studies will be essential in identifying potential long-term costs and benefits of CORT suppression.
Acknowledgements
Funding for this work was provided by the Natural Sciences and Engineering Research Council of Canada and the Stuart and Mary Houston Professorship in Ornithology (to G.R.B.), and projects REN2002-01 164 and BOS2000-0569-CO2-01 from the Spanish Government. G.D.F. was supported by a University of Saskatchewan Dean's Scholarship, a Ruby Larson Scholarship, a Malcolm A. Ramsay Memorial Award, and the Nature Saskatchewan Graduate Student Grant. Many thanks to I. Luque and V. Fachal for assistance in the laboratory. We thank S. Cabezas, G. Treen, three anonymous reviewers, and especially M. Vögeli for providing helpful suggestions on the manuscript. We gratefully acknowledge R. Knapp and E. Adkins-Regan for their efforts throughout the review process.
References
- 1.Stearns S. C. 1992. The evolution of life histories. New York, NY: Oxford University Press [Google Scholar]
- 2.Williams G. C. 1966. Natural selection, the costs of reproduction, and a refinement of Lack's principle. Am. Nat. 100, 687–690 10.1086/282461 (doi:10.1086/282461) [DOI] [Google Scholar]
- 3.Erikstad K. E., Fauchald P., Tveraa T., Steen H. 1998. On the cost of reproduction in long-lived birds: the influence of environmental variability. Ecology 79, 1781–1788 10.1890/0012-9658(1998)079[1781:OTCORI]2.0.CO;2 (doi:10.1890/0012-9658(1998)079[1781:OTCORI]2.0.CO;2) [DOI] [Google Scholar]
- 4.Mauck R. A., Grubb T. C. 1995. Petrel parents shunt all experimentally increased reproductive costs to their offspring. Anim. Behav. 49, 999–1008 10.1006/anbe.1995.0129 (doi:10.1006/anbe.1995.0129) [DOI] [Google Scholar]
- 5.Weimerskirch H., Chastel O., Ackermann L. 1995. Adjustment of parental effort to manipulated foraging ability in a pelagic seabird, the thin-billed prion Pachyptila belcheri. Behav. Ecol. Sociobiol. 36, 11–16 10.1007/BF00175723 (doi:10.1007/BF00175723) [DOI] [Google Scholar]
- 6.Weimerskirch H., Fradet G., Cherel Y. 1999. Natural and experimental changes in chick provisioning in a long-lived seabird, the Antarctic prion. J. Avian Biol. 30, 165–174 10.2307/3677126 (doi:10.2307/3677126) [DOI] [Google Scholar]
- 7.Navarro J., González-Solís J. 2007. Experimental increase of flying costs in a pelagic seabird: effects on foraging strategies, nutritional state and chick condition. Oecologia 151, 150–160 10.1007/s00442-006-0559-0 (doi:10.1007/s00442-006-0559-0) [DOI] [PubMed] [Google Scholar]
- 8.Velando A., Alonso-Alvarez C. 2003. Differential body condition regulation by males and females in response to experimental manipulations of brood size and parental effort in the blue-footed booby. J. Anim. Ecol. 72, 846–856 10.1046/j.1365-2656.2003.00756.x (doi:10.1046/j.1365-2656.2003.00756.x) [DOI] [Google Scholar]
- 9.González-Solís J., Croxall J. P., Wood A. G. 2000. Sexual dimorphism and sexual segregation in foraging strategies of northern giant petrels, Macronectes halli, during incubation. Oikos 90, 390–398 10.1034/j.1600-0706.2000.900220.x (doi:10.1034/j.1600-0706.2000.900220.x) [DOI] [Google Scholar]
- 10.González-Solís J., Croxall J. P., Wood A. G. 2000. Foraging partitioning between giant petrels Macronectes spp. and its relationship with breeding population changes at Bird Island, South Georgia. Mar. Ecol. Prog. Ser. 204, 279–288 10.3354/meps204279 (doi:10.3354/meps204279) [DOI] [Google Scholar]
- 11.Lewis S., Benvenuti S., Dall'Antonia L., Griffiths R., Money L., Sherratt T. N., Wanless S., Hamer K. C. 2002. Sex-specific foraging behaviour in a monomorphic seabird. Proc. R. Soc. B 269, 1687–1693 10.1098/rspb.2002.2083 (doi:10.1098/rspb.2002.2083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quillfeldt P., Masello J. F., Hamer K. C. 2004. Sex differences in provisioning rules and honest signalling of need in Manx shearwaters, Puffinus puffinus. Anim. Behav. 68, 613–620 10.1016/j.anbehav.2003.12.002 (doi:10.1016/j.anbehav.2003.12.002) [DOI] [Google Scholar]
- 13.Weimerskirch H., Cherel Y., CuenotChaillet F., Ridoux V. 1997. Alternative foraging strategies and resource allocation by male and female wandering albatrosses. Ecology 78, 2051–2063 10.1890/0012-9658(1997)078[2051:AFSARA]2.0.CO;2 (doi:10.1890/0012-9658(1997)078[2051:AFSARA]2.0.CO;2) [DOI] [Google Scholar]
- 14.Gray C. M., Hamer K. C. 2001. Food-provisioning behaviour of male and female Manx shearwaters, Puffinus puffinus. Anim. Behav. 62, 117–121 10.1006/anbe.2001.1717 (doi:10.1006/anbe.2001.1717) [DOI] [Google Scholar]
- 15.Hamer K. C., Quillfeldt P., Masello J. F., Fletcher K. L. 2006. Sex differences in provisioning rules: responses of Manx shearwaters to supplementary chick feeding. Behav. Ecol. 17, 132–137 10.1093/beheco/arj008 (doi:10.1093/beheco/arj008) [DOI] [Google Scholar]
- 16.Peck D. R., Congdon B. C. 2006. Sex-specific chick provisioning and diving behaviour in the wedge-tailed shearwater Puffinus pacificus. J. Avian Biol. 37, 245–251 10.1111/j.2006.0908-8857.03558.x (doi:10.1111/j.2006.0908-8857.03558.x) [DOI] [Google Scholar]
- 17.Elliott K. H., Gaston A. J., Crump D. 2010. Sex-specific behavior by a monomorphic seabird represents risk partitioning. Behav. Ecol. 21, 1024–1032 10.1093/beheco/arq076 (doi:10.1093/beheco/arq076) [DOI] [Google Scholar]
- 18.Romero L. M. 2004. Physiological stress in ecology: lessons from biomedical research. Trends Ecol. Evol. 19, 249–255 10.1016/j.tree.2004.03.008 (doi:10.1016/j.tree.2004.03.008) [DOI] [PubMed] [Google Scholar]
- 19.Kitaysky A. S., Piatt J. F., Wingfield J. C., Romano M. 1999. The adrenocortical stress-response of black-legged kittiwake chicks in relation to dietary restrictions. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 169, 303–310 10.1007/s003600050225 (doi:10.1007/s003600050225) [DOI] [Google Scholar]
- 20.Kitaysky A. S., Kitaiskaia E. V., Wingfield J. C., Piatt J. F. 2001. Dietary restriction causes chronic elevation of corticosterone and enhances stress response in red-legged kittiwake chicks. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 171, 701–709 10.1007/s003600100230 (doi:10.1007/s003600100230) [DOI] [PubMed] [Google Scholar]
- 21.Kitaysky A. S., Romano M. D., Piatt J. F., Wingfield J. C., Kikuchi M. 2005. The adrenocortical response of tufted puffin chicks to nutritional deficits. Horm. Behav. 47, 606–619 10.1016/j.yhbeh.2005.01.005 (doi:10.1016/j.yhbeh.2005.01.005) [DOI] [PubMed] [Google Scholar]
- 22.Kitaysky A. S., Kitaiskaia E. V., Piatt J. F., Wingfield J. C. 2006. A mechanistic link between chick diet and decline in seabirds? Proc. R. Soc. B 273, 445–450 10.1098/rspb.2005.3351 (doi:10.1098/rspb.2005.3351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitaysky A. S., Kitaiskaia E., Piatt J., Wingfield J. C. 2003. Benefits and costs of increased levels of corticosterone in seabird chicks. Horm. Behav. 43, 140–149 10.1016/S0018-506X(02)00030-2 (doi:10.1016/S0018-506X(02)00030-2) [DOI] [PubMed] [Google Scholar]
- 24.Kitaysky A. S., Wingfield J. C., Piatt J. F. 2001. Corticosterone facilitates begging and affects resource allocation in the black-legged kittiwake. Behav. Ecol. 12, 619–625 10.1093/beheco/12.5.619 (doi:10.1093/beheco/12.5.619) [DOI] [Google Scholar]
- 25.Harding A. M. A., Kitaysky A. S., Hall M. E., Welcker J., Karnovsky N. J., Talbot S. L., Hamer K. C., Gremillet D. 2009. Flexibility in the parental effort of an Arctic-breeding seabird. Funct. Ecol. 23, 348–358 10.1111/j.1365-2435.2008.01488.x (doi:10.1111/j.1365-2435.2008.01488.x) [DOI] [Google Scholar]
- 26.Kitaysky A. S. 1999. Metabolic and developmental responses of alcid chicks to experimental variation in food intake. Physiol. Biochem. Zool. 72, 462–473 10.1086/316684 (doi:10.1086/316684) [DOI] [PubMed] [Google Scholar]
- 27.Saino N., Suffritti C., Martinelli R., Rubolini D., Moller A. P. 2003. Immune response covaries with corticosterone plasma levels under experimentally stressful conditions in nestling barn swallows (Hirundo rustica). Behav. Ecol. 14, 318–325 10.1093/beheco/14.3.318 (doi:10.1093/beheco/14.3.318) [DOI] [Google Scholar]
- 28.Apanius V. 1998. Stress and immune defense. In Stress and behavior (eds Møller A. P., Milinski M., Slater P. J. B.), pp. 133–153 San Diego, CA: Academic Press [Google Scholar]
- 29.Sapolsky R. M., Romero L. M., Munck A. U. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 21, 55–89 10.1210/er.21.1.55 (doi:10.1210/er.21.1.55) [DOI] [PubMed] [Google Scholar]
- 30.Sears J., Hatch S. A. 2008. Rhinoceros auklet developmental responses to food limitation: an experimental study. Condor 110, 709–717 10.1525/cond.2008.8531 (doi:10.1525/cond.2008.8531) [DOI] [Google Scholar]
- 31.Ricklefs R. E., Wikelski M. 2002. The physiology/life-history nexus. Trends Ecol. Evol. 17, 462–468 10.1016/S0169-5347(02)02578-8 (doi:10.1016/S0169-5347(02)02578-8) [DOI] [Google Scholar]
- 32.Bortolotti G. R., Marchant T. A., Blas J., German T. 2008. Corticosterone in feathers is a long-term, integrated measure of avian stress physiology. Funct. Ecol. 22, 494–500 10.1111/j.1365-2435.2008.01387.x (doi:10.1111/j.1365-2435.2008.01387.x) [DOI] [Google Scholar]
- 33.Bortolotti G. R., Marchant T., Blas J., Cabezas S. 2009. Tracking stress: localisation, deposition and stability of corticosterone in feathers. J. Exp. Biol. 212, 1477–1482 10.1242/jeb.022152 (doi:10.1242/jeb.022152) [DOI] [PubMed] [Google Scholar]
- 34.Navarro J., Forero M. G., González-Solís J., Igual J. M., Becares J., Hobson K. A. 2009. Foraging segregation between two closely related shearwaters breeding in sympatry. Biol. Lett. 5, 545–548 10.1098/rsbl.2009.0150 (doi:10.1098/rsbl.2009.0150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Granadeiro J. P., Nunes M., Silva M. C., Furness R. W. 1998. Flexible foraging strategy of Cory's shearwater, Calonectris diomedea, during the chick-rearing period. Anim. Behav. 56, 1169–1176 10.1006/anbe.1998.0827 (doi:10.1006/anbe.1998.0827) [DOI] [PubMed] [Google Scholar]
- 36.Weimerskirch H., Ancel A., Caloin M., Zahariev A., Spagiari J., Kersten M., Chastel O. 2003. Foraging efficiency and adjustment of energy expenditure in a pelagic seabird provisioning its chick. J. Anim. Ecol. 72, 500–508 10.1046/j.1365-2656.2002.00720.x (doi:10.1046/j.1365-2656.2002.00720.x) [DOI] [Google Scholar]
- 37.Zino P. A., Zino F., Maul T., Biscoito J. M. 1987. The laying, incubation and fledging periods of Cory's shearwater Calonectris diomedea borealis on Selvagem Grande in 1984. Ibis 129, 393–398 10.1111/j.1474-919X.1987.tb03185.x (doi:10.1111/j.1474-919X.1987.tb03185.x) [DOI] [Google Scholar]
- 38.Warham J. 1990. The Petrels. Their ecology and breeding systems. London, UK: Academic Press [Google Scholar]
- 39.Pennycuick C. J. 1989. Variables needed for flight calculations. In Bird flight performance (ed. Pennycuick C. J.), pp. 7–17 Oxford, UK: Oxford University Press [Google Scholar]
- 40.Passos C., Navarro J., Giudici A., González-Solís J. 2010. Effects of extra mass on the pelagic behavior of a seabird. Auk 127, 100–107 10.1525/auk.2009.09036 (doi:10.1525/auk.2009.09036) [DOI] [Google Scholar]
- 41.Wayland M., Gilchrist H. G., Marchant T., Keating J., Smits J. E. 2002. Immune function, stress response, and body condition in arctic-breeding common eiders in relation to cadmium, mercury, and selenium concentrations. Environ. Res. 90, 47–60 10.1006/enrs.2002.4384 (doi:10.1006/enrs.2002.4384) [DOI] [PubMed] [Google Scholar]
- 42.Bortolotti G. R. 2010. Flaws and pitfalls in the chemical analysis of feathers: bad news–good news for avian chemoecology and toxicology. Ecol. Appl. 20, 1766–1774 10.1890/09-1473.1 (doi:10.1890/09-1473.1) [DOI] [PubMed] [Google Scholar]
- 43.Ottosson U., Backman J., Smith H. G. 1997. Begging affects parental effort in the pied flycatcher, Ficedula hypoleuca. Behav. Ecol. Sociobiol. 41, 381–384 10.1007/s002650050399 (doi:10.1007/s002650050399) [DOI] [Google Scholar]
- 44.Weimerskirch H., Mougey T., Hindermeyer X. 1997. Foraging and provisioning strategies of black-browed albatrosses in relation to the requirements of the chick: natural variation and experimental study. Behav. Ecol. 8, 635–643 10.1093/beheco/8.6.635 (doi:10.1093/beheco/8.6.635) [DOI] [Google Scholar]
- 45.Gladbach A., Braun C., Nordt A., Peter H. U., Quillfeldt P. 2009. Chick provisioning and nest attendance of male and female Wilson's storm petrels Oceanites oceanicus. Polar Biol. 32, 1315–1321 10.1007/s00300-009-0628-z (doi:10.1007/s00300-009-0628-z) [DOI] [Google Scholar]
- 46.Smith S. B., McWilliams S. R. 2009. Dietary macronutrients affect lipid metabolites and body composition of a migratory passerine, the white-throated sparrow (Zonotrichia albicollis). Physiol. Biochem. Zool. 82, 258–269 10.1086/597519 (doi:10.1086/597519) [DOI] [PubMed] [Google Scholar]
- 47.Butler M. W., Leppert L. L., Dufty A. M. 2010. Effects of small increases in corticosterone levels on morphology, immune function, and feather development. Physiol. Biochem. Zool. 83, 78–86 10.1086/648483 (doi:10.1086/648483) [DOI] [PubMed] [Google Scholar]
- 48.Dufty A. M., Clobert J., Moller A. P. 2002. Hormones, developmental plasticity and adaptation. Trends Ecol. Evol. 17, 190–196 10.1016/S0169-5347(02)02498-9 (doi:10.1016/S0169-5347(02)02498-9) [DOI] [Google Scholar]
- 49.Spencer K. A., Buchanan K. L., Goldsmith A. R., Catchpole C. K. 2003. Song as an honest signal of developmental stress in the zebra finch (Taeniopygia guttata). Horm. Behav. 44, 132–139 10.1016/S0018-506X(03)00124-7 (doi:10.1016/S0018-506X(03)00124-7) [DOI] [PubMed] [Google Scholar]
- 50.Sockman K. W., Schwabl H. 2001. Plasma corticosterone in nestling American kestrels: effects of age, handling stress, yolk androgens, and body condition. Gen. Comp. Endocrinol. 122, 205–212 10.1006/gcen.2001.7626 (doi:10.1006/gcen.2001.7626) [DOI] [PubMed] [Google Scholar]
- 51.Spencer K. A., Evans N. P., Monaghan P. 2009. Postnatal stress in birds: a novel model of glucocorticoid programming of the hypothalamic–pituitary–adrenal axis. Endocrinology 150, 1931–1934 10.1210/en.2008-1471 (doi:10.1210/en.2008-1471) [DOI] [PubMed] [Google Scholar]
- 52.Blas J., Bortolotti G. R., Tella J. L., Baos R., Marchant T. A. 2007. Stress response during development predicts fitness in a wild, long lived vertebrate. Proc. Natl Acad. Sci. USA 104, 8880–8884 10.1073/pnas.0700232104 (doi:10.1073/pnas.0700232104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Breuner C. W., Patterson S. H., Hahn T. P. 2008. In search of relationships between the acute adrenocortical response and fitness. Gen. Comp. Endocrinol. 157, 288–295 10.1016/j.ygcen.2008.05.017 (doi:10.1016/j.ygcen.2008.05.017) [DOI] [PubMed] [Google Scholar]
- 54.Bonier F., Martin P. R., Moore I. T., Wingfield J. C. 2009. Do baseline glucocorticoids predict fitness? Trends Ecol. Evol. 24, 634–642 10.1016/j.tree.2009.04.013 (doi:10.1016/j.tree.2009.04.013) [DOI] [PubMed] [Google Scholar]
- 55.Adams N. J., Cockrem J. F., Candy E. J., Taylor G. A. 2008. Non-precocial grey-faced petrel chicks (Pterodroma macroptera gouldi) show no age-related variation in corticosterone responses to capture and handling. Gen. Comp. Endocrinol. 157, 86–90 10.1016/j.ygcen.2008.02.007 (doi:10.1016/j.ygcen.2008.02.007) [DOI] [PubMed] [Google Scholar]