Abstract
The species richness of flower-visiting insects has declined in past decades, raising concerns that the ecosystem service they provide by pollinating crops and wild plants is threatened. The relative commonness of different species with shared ecological traits can play a pervasive role in determining ecosystem functioning, but information on changes in abundances of pollinators over time is lacking. We gathered data on relative abundances of bumble-bee species in Swedish red clover fields during three periods in the last 70 years (1940s, 1960s and present), and on clover seed yields since 1921. We found drastic decreases in bumble-bee community evenness, with potential consequences for level and stability of red clover seed yield. The relative abundances of two short-tongued bumble-bees have increased from 40 per cent in the 1940s to entirely dominate present communities with 89 per cent. Average seed yield declined in recent years and variation in yield doubled, suggesting that the current dependence on few species for pollination has been especially detrimental to stability in seed yield. Our results suggest a need to develop management schemes that promote not only species-rich but also more evenly composed communities of service-providing organisms.
Keywords: Bombus spp., ecosystem service, pollination, Trifolium pratense, red clover
1. Introduction
Observations of declines in species richness of pollinating insects [1] have raised serious concerns, resulting in international agreements to safeguard pollinator diversity [2]. Particular attention has been given to the ubiquitous and well-documented disappearance of bumble-bee species (Hymenoptera, Apidae, Bombus spp.) in the last half-century [3–5]. Because a majority of plant species depend on pollination by insects [6,7], these declines in pollinator species richness and distributional range have spurred an animated debate on whether the ecosystem service that wild bees provide in the form of crop pollination is also threatened [1,8].
The controversy partly springs from differences in appreciation of whether a diverse wild pollinator community benefits crop pollination. The idea has been put forward that biodiversity is closely linked to ecosystem functioning [9]. This has mainly been interpreted such that a large part of the functioning of an ecosystem can be explained by differences between species in the community, and much effort has been invested in experimentally separating effects for ecosystem functioning of species richness per se. This pioneering work has demonstrated that decreased diversity tends to reduce ecosystem process rates and stability [10,11], but it has also highlighted our limited ability to predict biodiversity and ecosystem functioning relationships in nature from small-scale experiments with relatively species-poor communities, where the relative abundance distribution in the community is not considered.
It is increasingly appreciated that not only the number of species but also the relative commonness of different species, or groups of species with shared ecological traits, can play a pervasive role in determining the ecosystem functioning provided by a community [12]. Human-induced environmental changes are also thought to lead to rapid shifts in community composition that precede extinctions [13]. To better understand how global change, via its effects on species communities, may have affected ecosystem functioning and the delivery of ecosystem services, it would therefore be useful to have historic information not only on changes in species numbers (e.g. [1]), but also on how the relative abundances and dominance patterns in service-providing species have changed in the last century. Changes in community evenness, however, remain poorly documented. Available information is rarely sampled from comparable environments, making it hard to separate large-scale community changes from shifts resulting from local habitat changes.
Fortunately, crop pollination, which for many crops is provided by naturally occurring communities [7], has been a recurring focus of agronomic research. Notable efforts were made from the 1940s to the 1960s to explore pollination and seed production of red clover (Trifolium pratense L.), which is an important forage crop. Seed set in red clover is entirely dependent on pollination by insects, mainly bumble-bees (Bombus spp.). Remarkably detailed records of the bumble-bee community composition can be found in the annals of national agronomic research. In the years 1942–1946, Bertil Schwan [14] initiated investigations with intense sampling of bumble-bee abundances in 10 geographically separated red clover fields grown for seed across Sweden. During 1959–1961, similar detailed inventories were done in 21 red clover fields in south and central Sweden (table 1). By again measuring bumble-bee community composition in 44 red clover seed fields during 2008, 2009 and 2010, we were able to examine possible shifts in relative (but not absolute) abundance of bumble-bee species groups in a comparable environment.
Table 1.
Summary of sampling schemes and sources for available bumble-bee studies in red clover seed fields 1942–2010.
| period | years | number of fields visiteda | sampled area per visit (m2) | number of visits to each field in a season | average number of bumble-bees per year and fieldb | source |
|---|---|---|---|---|---|---|
| 1940s | 1942 | 7 | 25 | 10–20 daily | 1140 | [14] |
| 1943–1946 | 10 | 100–400 | 2–3 daily | 2439 | [14] | |
| 1949 | 1 | — | — | 1440 | [15] | |
| 1960s | 1959–1960 | 11 | — | — | — | [16]c |
| 1961 | 16 | — | — | — | [17]c | |
| 1962 | 1 | — | — | 7142 | [18] | |
| 1962 | 1 | 100–200 | 1 | — | [19]c | |
| present | 2008 | 14 | 200 | 2 | 234 | |
| 2009 | 41 | 100 | 3–5 | 118 | ||
| 2010 | 28 | 100 | 3–5 | 58 |
aSome fields were revisited in several years.
bAverage total number of bumble-bees recorded in a field over a season.
cOnly proportional species composition (not abundances) are reported.
The decline of key generalist pollinators such as bumble-bees has raised concerns for the integrity of pollination networks and the delivery of their services [20,21]. A lower number of service-providing species is predicted to reduce both the level and stability of the service, as the presence of several species that differ in their response to a changing environment can buffer against variation in ecosystem functioning in fluctuating environments [22]. This effect is expected to be exacerbated in uneven communities [12]. To understand whether trends and variability in red clover harvests might have significantly changed over the period for which we examined bumble-bee communities, we collected and analysed yearly average seed yields per hectare from national statistics collected from 1921 to 2009.
2. Methods
(a). Study sites
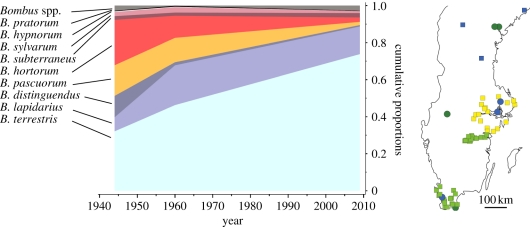
Data on bumble-bee abundances were collected from the literature and from sampling in arable fields cultivated for red clover seed in 2008, 2009 and 2010 (table 1). Clover was previously cultivated in the far north of Sweden. Today the northern-most cultivation for clover seed can be found north of Umeå city at 63.8° latitude. Only sites situated south of the latitude of 64° are therefore considered in the current study (figure 1).
Figure 1.
Map of visited sites and detected proportional shifts in bumble-bee community composition in red clover seed fields in the last 70 years. Blue circles, all three periods; green circles, 1940s and present; blue squares, 1940s; yellow squares, 1960s; green squares, present. Proportion of bumble-bee abundance for the different species is presented as cumulative proportions for the communities averaged among sites and years within each period. Averages and standard error variation for the data on which the figure is based are presented in electronic supplementary material, table S1.
Since the exact locations of historic fields were not available, we matched fields based on the name of the farm or village mentioned in the historic records, and we inventoried current red clover seed fields situated at a maximum distance of 2 km from that site. In presently urbanized areas where clover cultivations occurred historically, we chose the closest available clover field. This happened only for two sites, in the region of Uppland between the cities of Uppsala and Stockholm, where the distances between historic and present sites were 17 and 39 km, respectively.
Some sites were visited during several years in a period. In the 1940s, 10 unique sites were visited, 21 sites were visited in the 1960s and 44 sites were surveyed in the present day. Of these, three were visited in all three periods, and a further four were visited in the 1940s and again in the present day, giving a total of 65 unique sites (figure 1 and table 1).
(b). Bumble-bees
In all time periods, bumble-bee abundances were recorded in arable fields of flowering red clover (Trifolium pratense L.) intended for seed production. Sampling intensity differed or was not reported among studies and between periods. Detailed descriptions of sampling schemes were lacking for studies in the 1940s. Several of the studies in the 1960s only report proportional bumble-bee species composition, and no abundances or methods descriptions are reported. This makes any comparisons of abundances between and within these periods spurious. Sampling was, however, intense enough to obtain reliable estimates of proportional abundances within any period (table 1).
In 2008 (14 sites), 2009 (41 sites) and 2010 (28 sites), bumble-bees were collected along 1 m wide and 50 m long transects in the red clover seed fields; there were four transects located 4 and 12 m from the field edge in 2008, and two transects located 8 and 100 m from the field edge (or for smaller fields in the field centre) in 2009 and 2010 (table 1). In 2008, each site was visited twice, and in 2009 and 2010 three to five times (mean 3.6 and 4.0 visits per site, respectively), to cover the main flowering period of the red clover fields. Sampling was done between 25 June and 29 July 2008, 26 June and 20 August 2009, and 5 July and 10 August 2010, on days with warm, sunny and calm weather. The collected bumble-bees were put in individual tubes filled with 70 per cent ethanol, and brought to the laboratory for species determination. Bumble-bees were determined to species following Løken [23], Prŷs-Jones & Corbet [24] and Edwards & Jenner [25].
Only non-parasitic bumble-bees are considered in the analysis, as parasitic bumble-bees are rarely found in red clover fields. Some bumble-bee species are hard to distinguish and were often clumped into groups in the historic records. Hence, the resolution of species determinations varied between periods and was greatest in the most recent data. Prior to analysis we therefore summed observed species into common denominator species groups across periods, as follows. The Bombus terrestris group comprised the summed abundance in each field of B. terrestris (L.), Bombus lucorum (L.), and more rarely Bombus soroeensis (Fabricius), Bombus cryptarum (Fabricius), Bombus magnus (Vogt) and Bombus sporadicus (Nylander). The Bombus lapidarius group consisted of B. lapidarius (L.) and a low proportion of Bombus ruderarius (Müller). The Bombus pascuorum group consisted of B. pascuorum (Scopoli) and a low proportion of Bombus muscorum (L.). The following species were separated in the datasets: Bombus distinguendus (Morawitz), Bombus hortorum (L.), Bombus subterraneus (L.), Bombus sylvarum (L.), Bombus hypnorum (L.) and Bombus pratorum (L.). Other observed individuals noted as Bombus spp. in the historic records were counted as one additional species group in the analysis. A few individuals of Bombus jonellus (Kirby) were found in 2009 and these were included in the Bombus spp. group as B. jonellus was not identified in any previous period.
(c). Red clover yields
In Sweden, red clover seed yields per hectare harvested by farmers have been collected or published by the Swedish association for seed-growing farmers (Frö- och Oljeväxtodlarna). We searched their periodical Svensk Frötidning and their extension journal Meddelande från Sveriges Fröodlareförbund, published since 1932, and on their Web page (http://www.svenskraps.se/medlem), for data on national mean seed yields per hectare. We found that mean yield per hectare was sometimes reported as a single value, but more commonly split into two or three classes of early, late and sometimes also mid-late red clover varieties. In the latter cases, the sample sizes of each group, which are needed to calculate means across variety classes, were not fully provided. To maximize data coverage, we therefore used average yearly yield for late varieties when two classes were used, and mid-late varieties when three classes were used. We made this choice because available information on sample sizes indicated that these classes were consistently most common. Moreover, early varieties disappeared from 1990 and onwards in the records.
We found published national mean yield data from 1921, 1923–1935, 1937–1942, 1944 and 1955, which had been collected at seed exhibitions. In 10 years, the number of fields included in the estimation of the mean yield per hectare was reported with a mean of 126 (maximum = 212, minimum = 83, s.e. = 13) fields per year. From the period 1971–1994, we found mean yield data that had been collected by national authority in connection with a subsidy scheme. From this period, the area of red clover seed fields on which the mean was based was reported in all but 1 year, with an average of 1334 hectares per year (maximum = 2465, minimum = 182, s.e. = 128). Mean yield data from 1997 to 2009 have been collected by the Swedish association for seed-growing farmers and can be found at the association Web page, based on a mean of 78 fields per year (maximum = 82, minimum = 74, s.e. = 1.5). In addition to these three main sources of yield statistics, we found additional national mean yield values from 1950, 1963, 1969 and 1996 without descriptions of the collection methodology.
(d). Statistical analysis
We tested for differences among the three time periods in average observed species-richness per field, community evenness (measured with the Simpson's evenness index) and proportional abundance of individual species. All proportions were arcsine and square root transformed, and species richness was log10-transformed prior to analyses. All statistical analyses were performed with Proc Mixed in SAS v. 9.2 for Linux (SAS, Cary, NC, USA). We tested for changes in bumble-bee community composition in a mixed model with site and site within period as random factors. We employed the contain method to calculate the degrees of freedom in all analyses [26].
The mean number of bumble-bees collected per field was typically an order of magnitude higher in the 1940s and 1960s compared with the present data (table 1), possibly indicating that the sampling effort within each site was considerably higher in past periods. An alternative explanation is that abundances were higher in the past. Higher sampling effort might, in any case, increase observed measures of species richness from earlier periods [27]. However, given that the community is quite species-poor, it is possible that more sampling in previous periods only has a minor influence on species richness, and this can be tested with rarefaction. Because we lack information on sampling effort across periods, we used individual-based rarefaction to obtain a standardized measure of species richness [28]. Information on the number of individuals sampled from each field and year was only available from the 1940s and present. For these two periods, mean species richness was calculated from 47 bumble-bees re-sampled with 1000 iterations in EcoSim v. 7.71 [29]. The choice of a minimum of 47 collected individuals in a field allowed us to resample a fair number of individuals and still only exclude nine out of 121 observations in which a lower total number of individuals had been sampled. We used the same mixed model set-up as described above to test for differences between the 1940s and present periods in rarefied species richness, which was log10-transformed prior to analysis. Yield over years was analysed with linear and quadratic regression analysis.
3. Results
(a). Bumble-bees
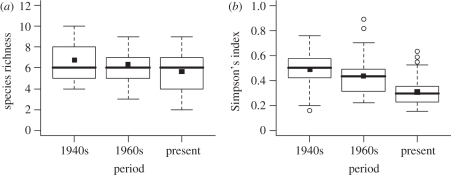
The average proportion of B. terrestris observed in a clover field in a year has increased from 32 per cent in the 1940s to 46 per cent in the 1960s, and then to 74 per cent in the present (figure 1 and table 1). Bombus lapidarius is another bumble-bee species that has increased, from 8 to 15 per cent in the last 70 years. Species that were previously common, such as B. hortorum, B. pascuorum, B. distinguendus and B. sylvarum, are presently rarely found in clover fields. Only the less common B. subterraneus and B. pratorum remain constant across periods. These radical shifts in community composition in the past 70 years are also reflected in a significant decrease in average evenness in the bumble community (figure 2 and table 2), and are paralleled by a decrease in average species richness per red clover field per year (figure 2 and table 2). We found that rarefied species richness per field per year followed the same trend and was somewhat higher in the 1940s (mean = 5.08, s.d. = 0.22, n = 38) compared with present day (mean = 4.36, s.d. = 0.14, n = 74; F1,6 = 5.4, p = 0.058). We found three species of parasitic bumble-bee (Bombus campestris (Panzer), Bombus rupestris (Fabricius) and Bombus vestalis (Geoffroy)), making up 0.13 per cent of all observed bumble-bees during 2008–2010.
Figure 2.
Changes between periods in bumble-bee (a) observed species richness and (b) community evenness measured with the Simpson's index. The box-plots show average (point) and median (horizontal line in the box) value per clover field per year in three time periods in the last 70 years. The bottom and top of the box are the first and third quartiles. The whiskers show either the maximum value, or 1.5 times the difference between the first and third quartiles, corresponding roughly to two standard deviations. Observations outside the range of the whiskers are plotted individually.
Table 2.
Results from statistical analysis of differences between time periods in arcsine transformed proportional abundances, evenness and log10-transformed species richness of bumble-bees in clover seed fields. Community evenness was measured with the Simpson's index. Results are presented from a mixed model, with site and site within period as random factors.
| species | mixed model results |
||
|---|---|---|---|
| d.f. | F | p | |
| B. terrestris | 2,8 | 48.9 | <0.0001 |
| B. lapidarius | 2,8 | 3.9 | 0.07 |
| B. pascuorum | 2,8 | 30.4 | 0.0002 |
| B. distinguendus | 2,8 | 29.1 | 0.0002 |
| B. hortorum | 2,8 | 51.3 | <0.0001 |
| B. subterraneus | 2,8 | 0.54 | 0.60 |
| B. sylvarum | 2,8 | 4.8 | 0.04 |
| B. hypnorum | 2,8 | 8.2 | 0.01 |
| B. pratorum | 2,8 | 2.6 | 0.14 |
| Bombus spp. | 2,8 | 19.5 | 0.0008 |
| evenness | 2,8 | 12.9 | 0.003 |
| species richness | 2,8 | 9.6 | 0.008 |
(b). Red clover yields
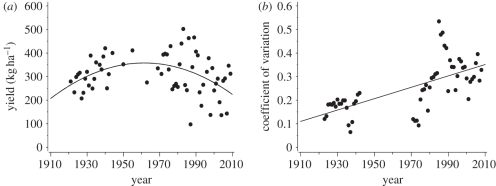
The best fit for trends in average seed yield was obtained with a quadratic regression where yields first increased from 1921 and onward, and then showed a decline in average seed yield in recent years (quadratic regression: first-order term F1,60 = 10.6, pslope = 0.002; second-order term F1,60 = 10.7, pslope = 0.002; figure 3). We found a marked increase in the between-year variability of yield levels, with a doubled coefficient of variation in the last 20 years calculated from 5 year moving average with minimum four values (linear regression: F1,56 = 42.6, pslope < 0.0001; figure 3).
Figure 3.
Trends in red clover seed yields in the last 90 years. (a) Yearly statistic of yield per hectare. (b) Variability in yield measured presented as the coefficient of variation calculated from 5 year moving average (with minimum four values).
4. Discussion
We found that the community of bumble-bees that visit flowering red clover fields for seed production has radically changed in relative species abundance in the last 70 years (figure 1). In particular, B. terrestris and B. lapidarius are now the consistently dominant species in clover field bumble-bee communities in Sweden. In contrast, species that were previously common, such as B. hortorum, B. pascuorum, B. distinguendus and B. sylvarum, are presently only rarely found. Especially conspicuous is the negative trend for B. distinguendus: in the 1940s this species constituted on average 12 per cent of the populations, but it is now entirely absent in the south of Sweden, and categorized as near threatened on the national red list for threatened species in Sweden and critically endangered in nearby Denmark.
Certainly, in the last 70 years, several different large-scale environmental changes have taken place that could explain these pervasive shifts in bumble-bee species richness and community composition. The primary causes for the decline of bumble-bees are probably land-use conversion and intensification linked to changed agricultural practices, resulting in loss of nest and flower resources for bumble-bees [3,30]. In Sweden, large areas of key bumble-bee habitat, such as hay meadows and semi-natural pastures, have been converted into arable land or forest. In combination with concurrent removal of other semi-natural landscape elements, such as ditches and field margins and the diverse flora they probably once had, this is probably the most important cause for the observed decline of several bumble-bee species [31,32]. Remaining nest and flower resources have become spatially disassociated in the modern landscape, so that only highly mobile super-generalists such as B. terrestris and B. lapidarius are able to exploit and even benefit from ephemeral and spatially separated resources such as mass-flowering crops [33]. In addition, the introduction of pesticides to agriculture in the last 70 years may have added pressure on bumble-bees and other pollinating insects [3].
The above explanations would, however, suggest that current complex landscapes, with low pesticide input, and where ample nest and flower resources are available within range, will harbour an intact bumble-bee fauna. Although such landscapes clearly are beneficial for bumble-bees [34], the results presented here indicate that the historic shifts in bumble-bee community composition are substantially larger than the differences between present-day communities. The results thus clarify that the relative declines or increases of certain species have taken place at a national scale in Sweden. One possible (but not much explored) explanation for this is that declining species are outcompeted by superior competitor species, such as B. terrestris and B. lapidarius [35].
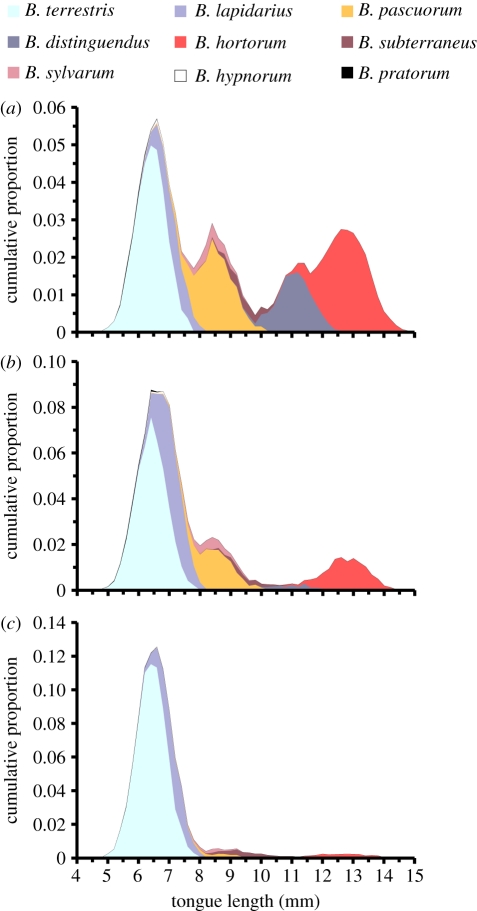
Fabaceae plants in general and red clover in particular have been identified as key pollen and nectar sources for bumble-bees [36]. Red clover was previously commonly cultivated across Sweden. A first national survey in 1939 measured 19 993 ha of red clover seed crop [37]. During the period of 2006–2010, the average corresponding area was 2061 ha (i.e. 90 per cent lower; http://www.svenskraps.se/vallfro/areal_vallfro_medel.asp). Intensively farmed agricultural plains have gradually become dominated by crop production without legumes in the rotation [30]. In areas where livestock and milk production are now concentrated, the forage is produced in fertilized, grass-dominated meadows, where early and repeated harvesting for silage disrupts the late flowering of red clover. A suggested reason for why certain bumble-bee species have been more sensitive to these changes than others is that they differ in proboscis (tongue) length and diet breadth: short-tongued species are generalists who are better adapted to exploit open flowers that have probably become prevalent in current agricultural landscapes, whereas long-tongued species are more specialized to flowers with deep corollas, such as red clover [36]. This fits well with our results, where the short-tongued B. terrestris and B. lapidarius are increasingly dominant at the expense of B. hortorum, B. pascuorum, B. distinguendus and B. sylvarum, which all have longer tongues. These changes in relative species abundance have also led to clear shifts in tongue length distribution within the communities, as seen in figure 4.
Figure 4.
Relative frequencies of bumble-bee tongue lengths in red clover fields across Sweden in the (a) 1940s, (b) 1960s and (c) present. Ten thousand individuals were distributed among species according to national average relative abundance in each time period. Tongue lengths were then drawn from a distribution based on available mean and standard deviation in tongue length for each species, such that cumulative proportion in the community within each 0.20 mm tongue length interval was obtained. Tongue length estimates were obtained from Goulson et al. [36] for B. subterraneus and from Goulson et al. [38] for all other species.
Bumble-bees are important ecosystem service providers as they pollinate crops and wild flowers. A lower number of service-providing species is predicted to reduce both the level and stability of the service, and this effect is expected to be especially evident in uneven communities, particularly if the dominant species happen to be poor service providers [12]. The efficiency of the now-ubiquitous short-tongued B. terrestris and B. lapidarius as pollinators of flowers with long corollas has also been questioned, as they frequently rob nectar without pollinating the flower [35,39]. In practice, these are today the only two wild pollinator species significantly contributing to red clover pollination. Our results do show a decline in average seed yield in recent years, and this might be a result of a decline in pollination of the crop. However, this interpretation is complicated by the introduction of tetraploid varieties in recent years. Tetraploid varieties were grown on 49 per cent of the fields during 2008–2010. Compared with traditional diploid varieties, tetraploids have larger biomass that is negatively correlated with seed yield, and this could explain a decrease in seed yield. Tetraploid varieties also have longer flower tubes that can increase nectar-robbing by short-tongued bumble-bees such as B. terrestris and B. lapidarius [40]. There is a great general need to further explore the responses and dependencies of insect pollination for different crop varieties [7].
Given the drastic and large-scale shifts of the bumble-bee community composition, we also expect that between-year variability of clover seed yield will have increased, as yields are expected to closely follow the between-season fluctuations in abundance of the dominant bumble-bee species. We also note a marked increase in the between-year variability of yield levels, with a doubled coefficient of variation in the last 20 years. This observation is in accordance with the theoretical prediction that yield levels of a crop that is heavily dependent on a supporting ecosystem service will vary more when the service is delivered by fewer species [22]. Comparable information on bumble-bee abundances between years is, however, entirely lacking, and the time series within periods are too short to obtain reliable estimates of parallel changes in the stability of bumble-bee abundance. We can therefore not confirm a causal link between variation in bumble-bee abundances and harvest. In addition, we would need information on pest attacks on the flowers by seed-eating weevils and the biological control of these by parasitoids, as these organisms also play an important role in determination of final seed set in red clover [41].
The substantial increase in harvest variability for a crop that is heavily dependent on two ecosystem services (pollination and biological control) is worrying and deserves further attention. Our results add to a picture of substantially increased variability in pollinator-dependent crop yields globally [42]. More consistent monitoring and documenting of occurrence and abundance of service-providing species such as bumble-bees are necessary to assess whether service-providing organisms that decline in species numbers are also going through the process of drastically changed dominance patterns. This information is necessary for understanding the consequences for long-term and large-scale stability of ecosystem services delivered by communities. Meanwhile, recovery of historic records of abundances and comparison of these with current records, together with experimental studies identifying mechanisms determining the population dynamics and regulation of service-providing organisms, emerge as research priorities. From a policy perspective, the results of the current report suggest an urgent need to develop management schemes that promote not only more species-rich but also more evenly composed communities of service-providing organisms.
Acknowledgements
We thank Lorenzo Marini and David W. Inouye for useful comments. Linda Öhlund at Svalöf Weibull Seeds AB, Camilla Winqvist, field assistants and seed-growing farmers are thanked for the sampling of bumble-bees and access to clover fields. Funding was provided to R.B. and H.G.S. by the Swedish research council for environment, agricultural sciences and spatial planning (FORMAS), and by the EU in the FP7 project ‘STEP—Status and Trends of European Pollinators’ (244090).
References
- 1.Biesmeijer J. C., et al. 2006. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313, 351–354 10.1126/science.1127863 (doi:10.1126/science.1127863) [DOI] [PubMed] [Google Scholar]
- 2.Potts S. G., Biesmeijer J. C., Kremen C., Neumann P., Schweiger O., Kunin W. E. 2010. Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25, 345–354 10.1016/j.tree.2010.01.007 (doi:10.1016/j.tree.2010.01.007) [DOI] [PubMed] [Google Scholar]
- 3.Goulson D., Lye G. C., Darvill B. 2008. Decline and conservation of bumble bees. Annu. Rev. Entomol. 53, 191–208 10.1146/annurev.ento.53.103106.093454 (doi:10.1146/annurev.ento.53.103106.093454) [DOI] [PubMed] [Google Scholar]
- 4.Williams P. H., Osborne J. L. 2009. Bumblebee vulnerability and conservation world-wide. Apidologie 40, 367–387 10.1051/apido/2009025 (doi:10.1051/apido/2009025) [DOI] [Google Scholar]
- 5.Cameron S. A., Lozier J. D., Strange J. P., Koch J. B., Cordes N., Solter L. F., Griswold T. L. 2011. Patterns of widespread decline in North American bumble bees. Proc. Natl Acad. Sci. USA 108, 662–667 10.1073/pnas.1014743108 (doi:10.1073/pnas.1014743108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kearns C. A., Inouye D. W., Waser N. M. 1998. Endangered mutualisms: the conservation of plant–pollinator interactions. Annu. Rev. Ecol. Syst. 29, 83–112 10.1146/annurev.ecolsys.29.1.83 (doi:10.1146/annurev.ecolsys.29.1.83) [DOI] [Google Scholar]
- 7.Klein A.-M., Vaissière B. E., Cane J. H., Steffan-Dewenter I., Cunningham S. A., Kremen C., Tscharntke T. 2007. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B 274, 303–313 10.1098/rspb.2006.3721 (doi:10.1098/rspb.2006.3721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghazoul J. 2005. Buzziness as usual? Questioning the global pollination crisis. Trends Ecol. Evol. 20, 367–373 10.1016/j.tree.2005.04.026 (doi:10.1016/j.tree.2005.04.026) [DOI] [PubMed] [Google Scholar]
- 9.Schulze E. D., Mooney H. A. 1994. Biodiversity and ecosystem function. Berlin, Germany: Springer [Google Scholar]
- 10.Balvanera P., Pfisterer A. B., Buchmann N., He J.-S., Nakashizuka T., Raffaelli D., Schmid B. 2006. Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol. Lett. 9, 1146–1156 10.1111/j.1461-0248.2006.00963.x (doi:10.1111/j.1461-0248.2006.00963.x) [DOI] [PubMed] [Google Scholar]
- 11.Cardinale B. J., Srivastava D. S., Duffy J. E., Wright J. P., Downing A. L., Sankaran M., Jouseau C. 2006. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 443, 989–992 10.1038/nature05202 (doi:10.1038/nature05202) [DOI] [PubMed] [Google Scholar]
- 12.Hillebrand H., Bennett D. M., Cadotte M. W. 2008. Consequences of dominance: a review of evenness effects on local and regional ecosystem processes. Ecology 89, 1510–1520 10.1890/07-1053.1 (doi:10.1890/07-1053.1) [DOI] [PubMed] [Google Scholar]
- 13.Chapin F. S., et al. 2000. Consequences of changing biodiversity. Nature 405, 234–242 10.1038/35012241 (doi:10.1038/35012241) [DOI] [PubMed] [Google Scholar]
- 14.Schwan B. 1953. Iakttagelser rörande rödklöverpollinerande insekter åren 1942–1946. Meddelande från Sveriges Fröodlareförbund 2, 34–61 [Google Scholar]
- 15.Julén G. 1953. Erfarenheter från fröodlingsförsök med rödklöverstammar i Götaland. Meddelande från Sveriges Fröodlareförbund 2, 83–101 [Google Scholar]
- 16.Fridén F. 1960. Humlestudier inom Mälar-Hjälmarområdet år 1960. Svensk Frötidning 29, 144–150 [Google Scholar]
- 17.Åkerberg E. 1961. Humlestudier inom Mälar-Hjälmarområdet år 1961. Svensk Frötidning 30, 151–158 [Google Scholar]
- 18.Fridén F. 1967. Jämförande undersökningar av insektspopulationer i frövallar. Meddelande från Sveriges Fröodlareförbund 8, 83–95 [Google Scholar]
- 19.Umaerus M., Åkerberg E. 1967. Undersökningar åren 1960–62 över förekomst av pollinerande insekter inom Svalöf-området. Meddelande från Sveriges Fröodlareförbund 8, 7–26 [Google Scholar]
- 20.Memmott J., Waser N. M., Price M. V. 2004. Tolerance of pollination networks to species extinctions. Proc. R. Soc. Lond. B 271, 2605–2611 10.1098/rspb.2004.2909 (doi:10.1098/rspb.2004.2909) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fortuna M. A., Bascompte J. 2006. Habitat loss and the structure of plant–animal mutualistic networks. Ecol. Lett. 9, 278–283 10.1111/j.1461-0248.2005.00868.x (doi:10.1111/j.1461-0248.2005.00868.x) [DOI] [PubMed] [Google Scholar]
- 22.Yachi S., Loreau M. 1999. Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proc. Natl Acad. Sci. USA 96, 1463–1468 10.1073/pnas.96.4.1463 (doi:10.1073/pnas.96.4.1463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Løken A. 1973. Studies on Scandinavian bumble bees (Hymenoptera, Apidae). Norw. J. Entomol. 20, 1–218 [Google Scholar]
- 24.Prŷs-Jones O. E., Corbet S. A. 1987. Bumblebees. Cambridge, UK: Cambridge University Press [Google Scholar]
- 25.Edwards E., Jenner M. Field guide to the bumblebees of Great Britain & Ireland. Eastbourne, UK: Ocelli; 2005. [Google Scholar]
- 26.Littell R. C., Milliken G. A., Stroup W. W., Wolfinger R. D., Schabenberger O. 2006. SAS for mixed models, 2 ed. Cary, NC: SAS Institute Inc [Google Scholar]
- 27.Magurran A. E. 2004. Measuring biological diversity. Oxford, UK: Blackwell Publishing [Google Scholar]
- 28.Gotelli N. J., Colwell R. K. 2001. Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 4, 379–391 10.1046/j.1461-0248.2001.00230.x (doi:10.1046/j.1461-0248.2001.00230.x) [DOI] [Google Scholar]
- 29.Gotelli N. J., Entsminger G. L. 2010. EcoSim: Null models software for ecology. Version 7. Jericho, VT: Acquired Intelligence Inc. & Kesey-Bear; See http://garyentsminger.com/ecosim/index.htm [Google Scholar]
- 30.Carvell C., Roy D. B., Smart S. M., Pywell R. F., Preston C. D., Goulson D. 2006. Declines in forage availability for bumblebees at a national scale. Biol. Conserv. 132, 481–488 10.1016/j.biocon.2006.05.008 (doi:10.1016/j.biocon.2006.05.008) [DOI] [Google Scholar]
- 31.Bommarco R., Biesmeijer J. C., Meyer B., Potts S. G., Pöyry J., Roberts S. P. M., Steffan-Dewenter I., Öckinger E. 2010. Dispersal capacity and diet breadth modify the response of wild bees to habitat loss. Proc. R. Soc. B 277, 2075–2082 10.1098/rspb.2009.2221 (doi:10.1098/rspb.2009.2221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams N. M., Crone E. E., Roulston T. H., Minckley R. L., Packer L., Potts S. G. 2010. Ecological and life-history traits predict bee species responses to environmental disturbances. Biol. Conserv. 143, 2280–2291 10.1016/j.biocon.2010.03.024 (doi:10.1016/j.biocon.2010.03.024) [DOI] [Google Scholar]
- 33.Westphal C., Steffan-Dewenter I., Tscharntke T. 2003. Mass flowering crops enhance pollinator densities at a landscape scale. Ecol. Lett. 6, 961–965 10.1046/j.1461-0248.2003.00523.x (doi:10.1046/j.1461-0248.2003.00523.x) [DOI] [Google Scholar]
- 34.Rundlöf M., Nilsson H., Smith H. G. 2008. Interacting effects of farming practice and landscape context on bumblebees. Biol. Conserv. 141, 417–426 10.1016/j.biocon.2007.10.011 (doi:10.1016/j.biocon.2007.10.011) [DOI] [Google Scholar]
- 35.Diekötter T., Kadoya T., Peter F., Wolters V., Jauker F. 2010. Oilseed rape crops distort plant–pollinator interactions. J. Appl. Ecol. 47, 209–214 10.1111/j.1365-2664.2009.01759.x (doi:10.1111/j.1365-2664.2009.01759.x) [DOI] [Google Scholar]
- 36.Goulson D., Hanley M. E., Darvill B., Ellis J. S., Knight M. E. 2005. Causes of rarity in bumblebees. Biol. Conserv. 122, 1–8 10.1016/j.biocon.2004.06.017 (doi:10.1016/j.biocon.2004.06.017) [DOI] [Google Scholar]
- 37.Witte H. 1940. 1939 års inventering av till frötäkt avsedda arealer utav rödklöver, alsikeklöver och timotej. Svensk frötidning 9, 65–67 [Google Scholar]
- 38.Goulson D., Lye G. C., Darvill B. 2008. Diet breadth, coexistence and rarity in bumblebees. Biodivers. Conserv. 17, 3269–3288 10.1007/s10531-008-9428-y (doi:10.1007/s10531-008-9428-y) [DOI] [Google Scholar]
- 39.McGregor S. E. 1976. Insect pollination of cultivated crop plants. Washington, DC: USDA Agriculture Handbook [Google Scholar]
- 40.Nørgaard Holm S. 1966. The utilization and management of bumble bees for red clover and alfalfa seed production. Annu. Rev. Entomol. 11, 155–182 10.1146/annurev.en.11.010166.001103 (doi:10.1146/annurev.en.11.010166.001103) [DOI] [Google Scholar]
- 41.Kreuss A., Tscharntke T. 1994. Habitat fragmentation, species loss, and biological control. Science 264, 1581–1584 10.1126/science.264.5165.1581 (doi:10.1126/science.264.5165.1581) [DOI] [PubMed] [Google Scholar]
- 42.Garibaldi L. A., Aizen M. A., Klein A. M., Cunningham S. A., Harder L. 2011. Global growth and stability of agricultural yield decrease with pollinator dependence. Proc. Natl Acad. Sci. USA 108, 5909–5914 10.1073/pnas.1012431108 (doi:10.1073/pnas.1012431108) [DOI] [PMC free article] [PubMed] [Google Scholar]