Abstract
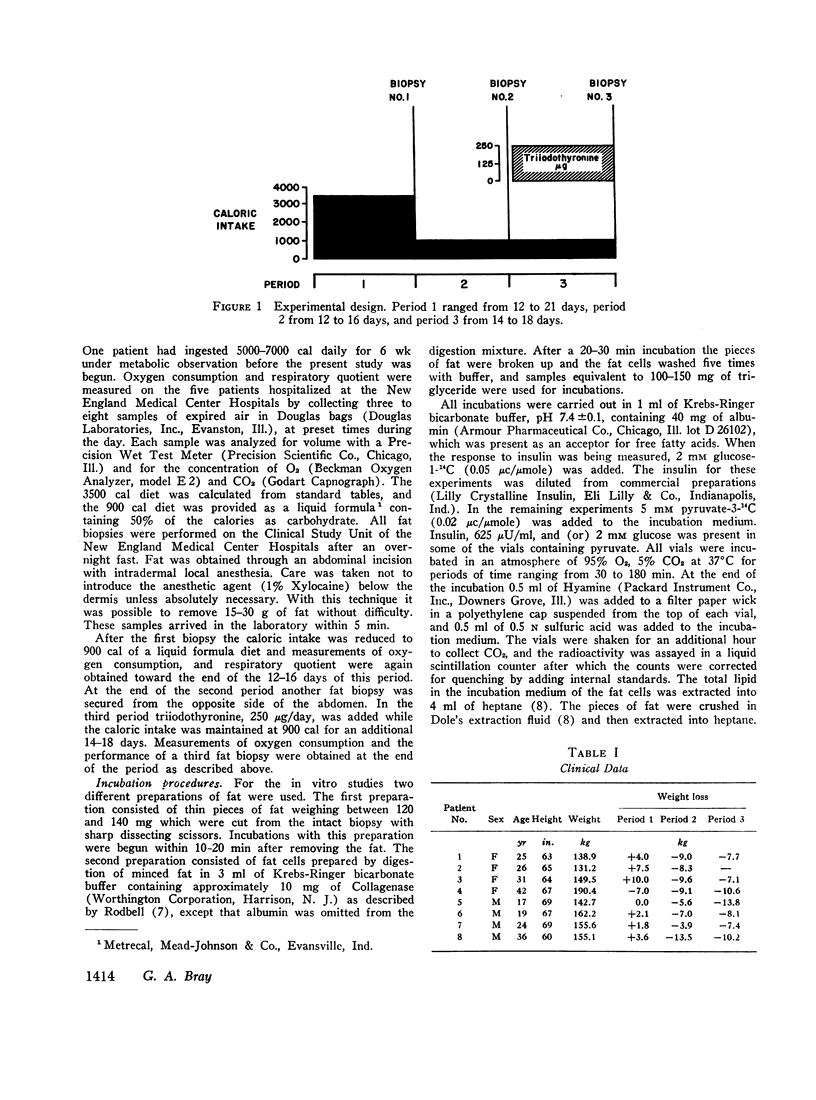
Lipogenesis and the metabolism of sn-glycerol-3-phosphate were studied in 23 fat biopsies from eight grossly obese patients. The first biopsy was obtained after a minimum of 12 days on a 3500 cal diet, the second biopsy after 2 wk on a 900 cal diet, and the third biopsy after an additional 2 wk on 900 cal supplemented with thiiodothyronine, 250 μg/day.
Oxygen consumption and respiratory quotient declined during caloric restriction. Oxygen consumption was restored to the initial level during treatment with triiodothyronine, and the respiratory quotient rose somewhat.
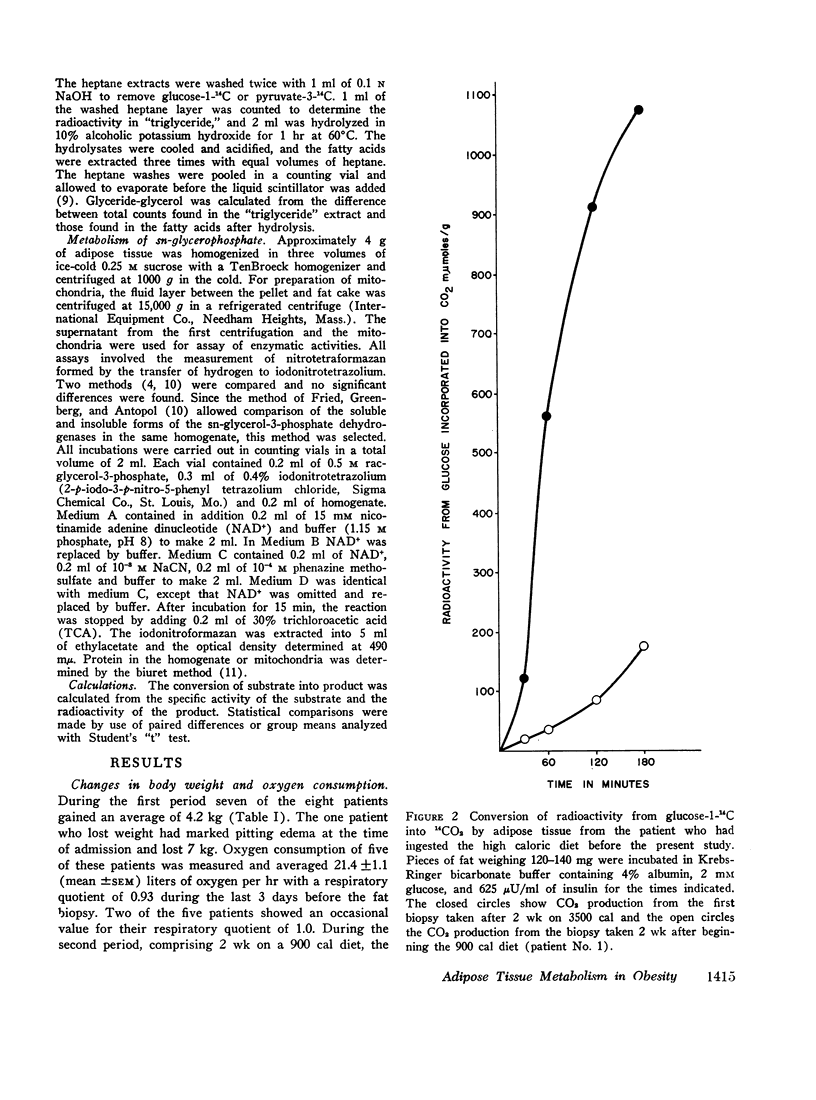
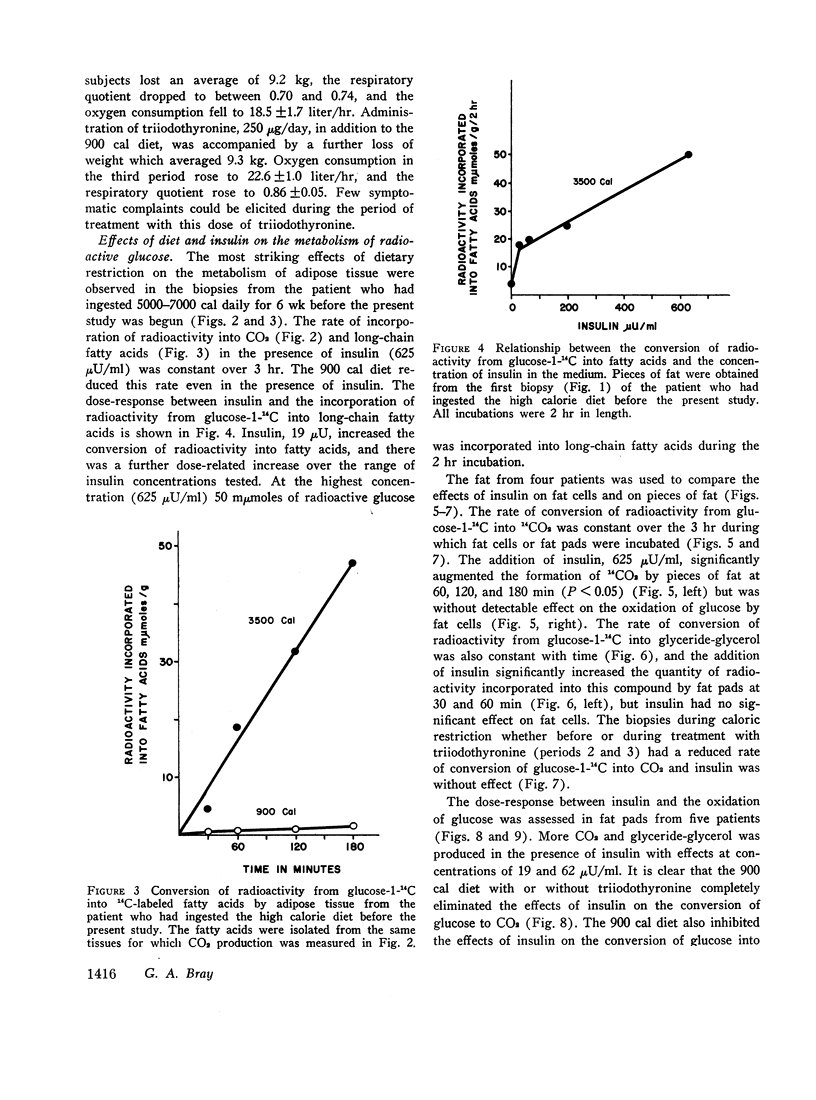
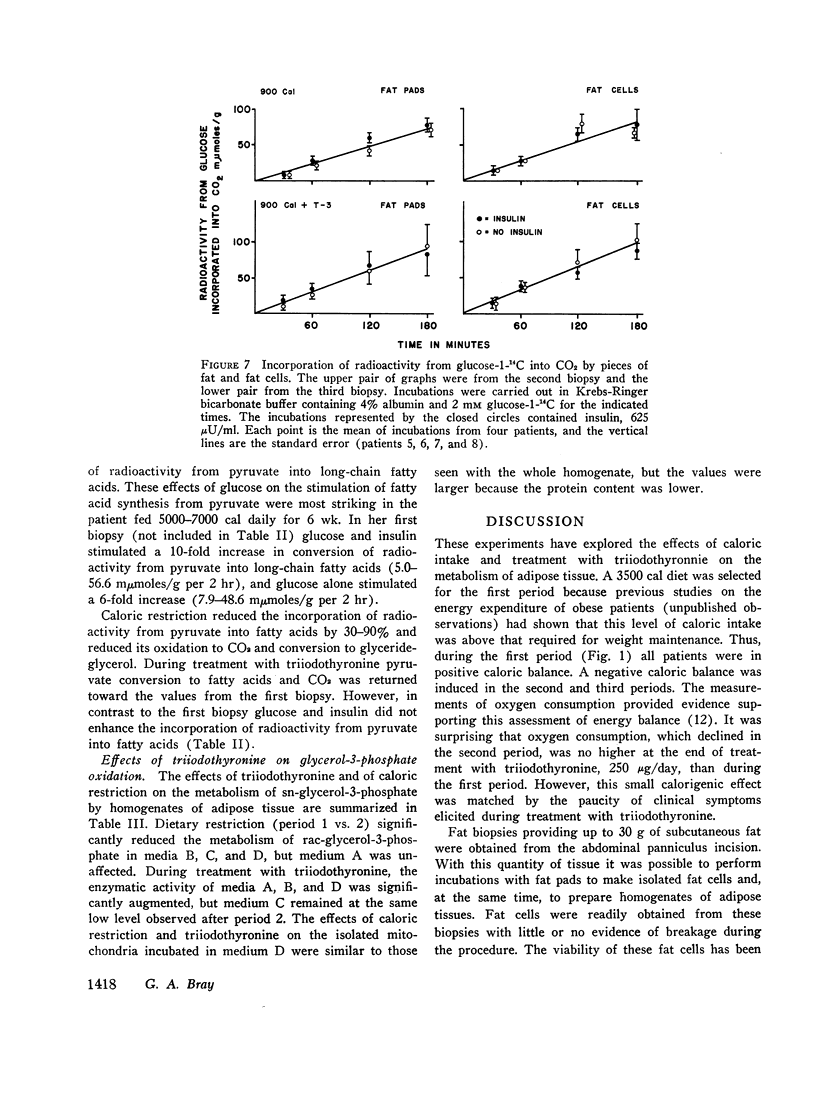
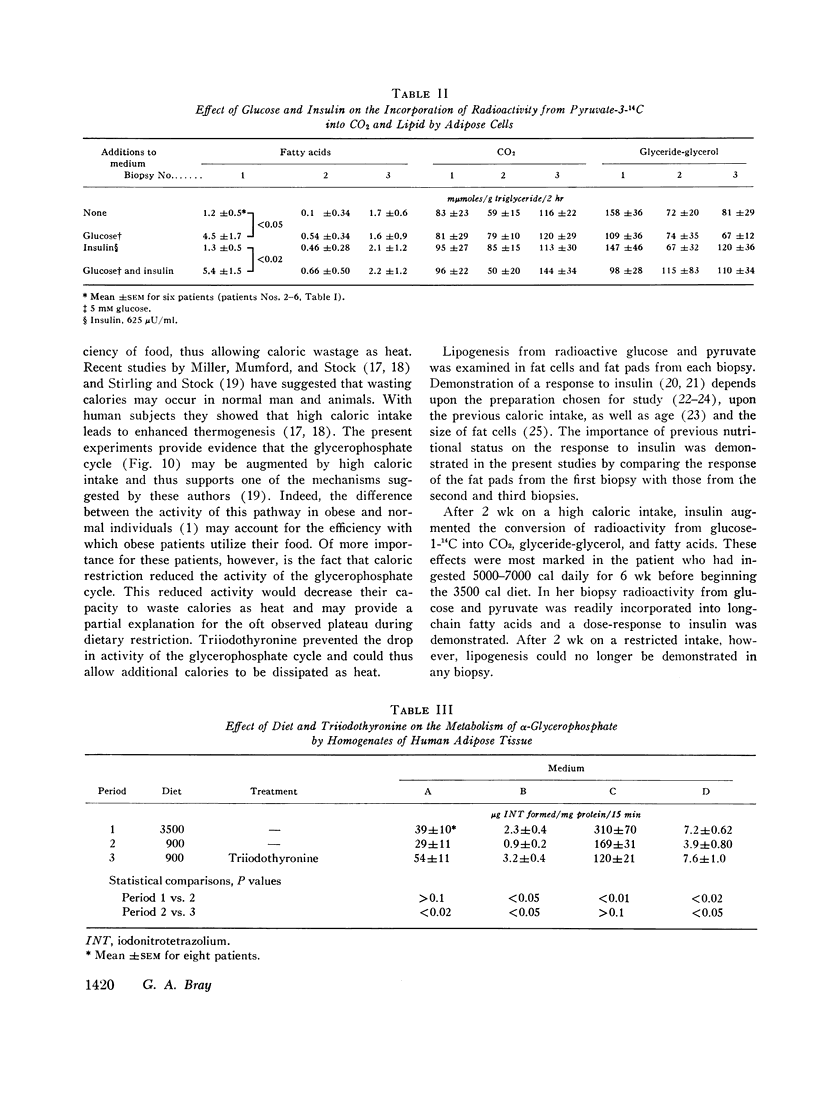
Lipogenesis from glucose and pyruvate was demonstrated in fat obtained from the first biopsy but could not be detected in the other biopsies. The incorporation of radioactivity from pyruvate into fatty acids was stimulated by the addition of glucose.
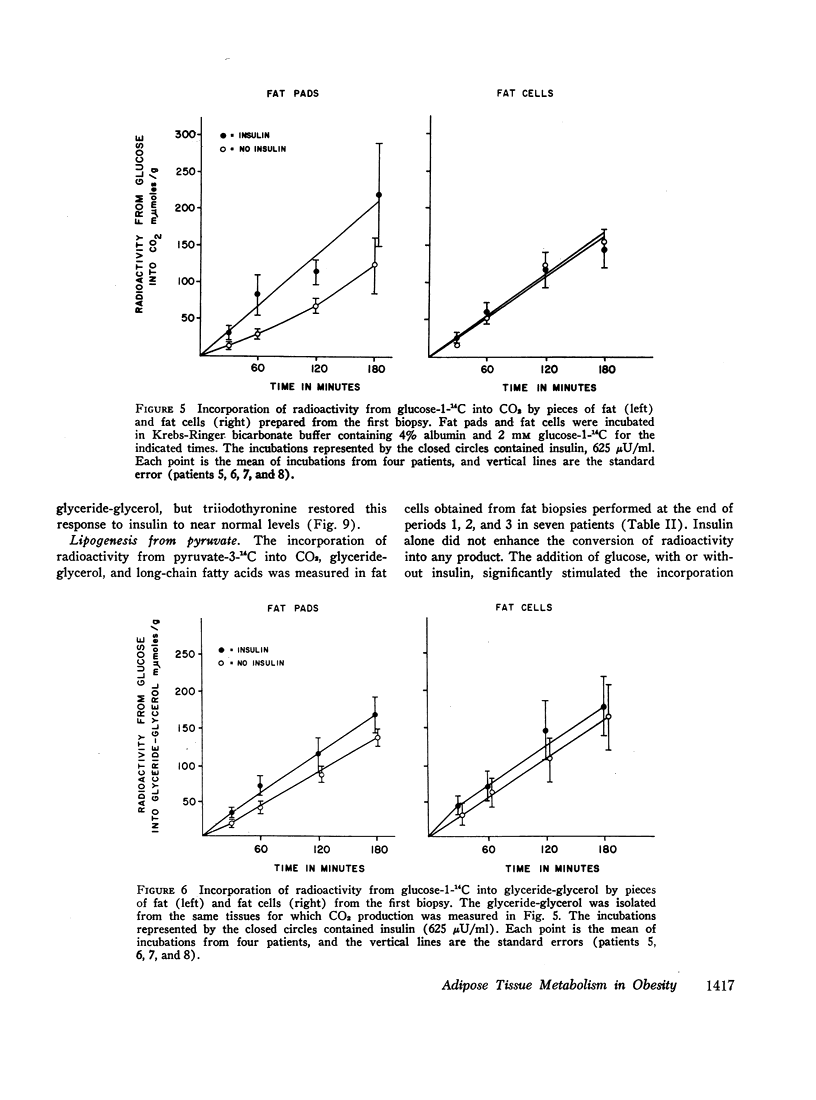
Insulin stimulated lipogenesis in pieces of fat from the first biopsy, but isolated fat cells were unaffected by insulin. After caloric restriction no effects of insulin could be detected.
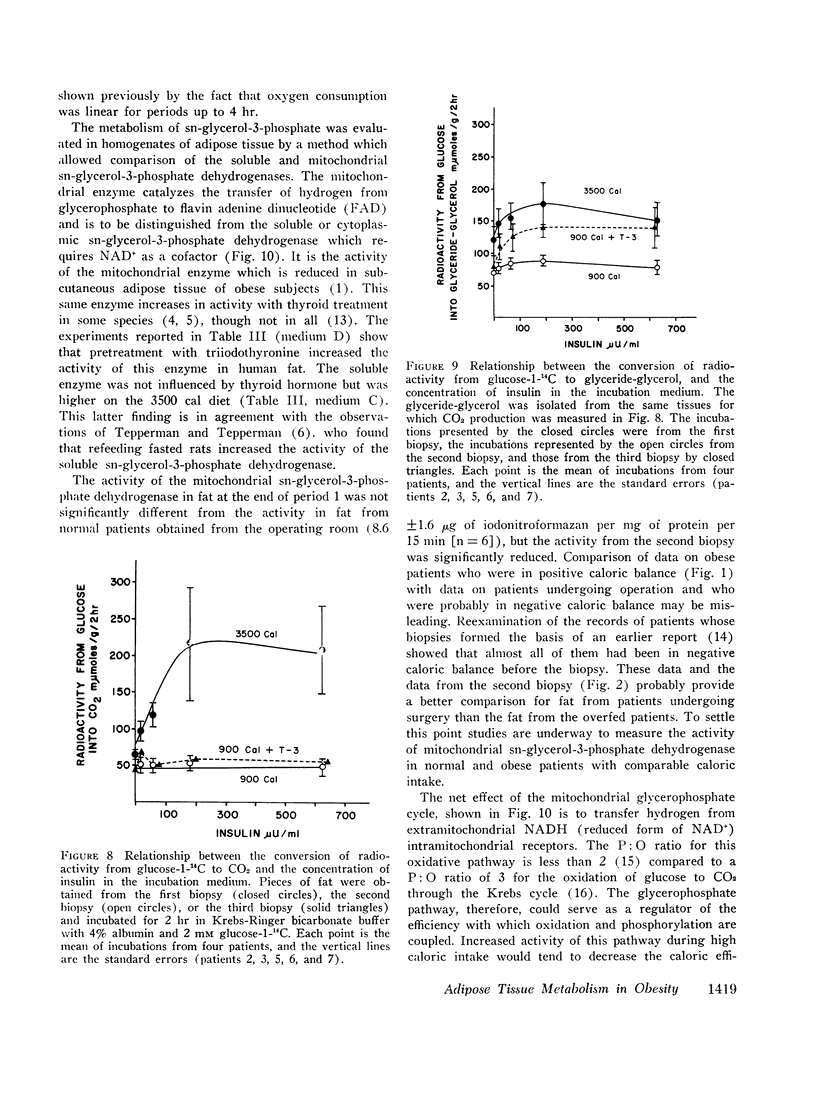
The activity of both the cytoplasmic and mitochondrial sn-glycerol-3-phosphate dehydrogenase in homogenates of adipose tissue declined with caloric restriction. Treatment with triiodothyronine enhanced the activity of the mitochondrial sn-glycerol-3-phosphate dehydrogenase but did not affect the cytoplasmic enzyme.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton D. J., Bray G. A. Metabolism of alpha-glycerol phosphate in human adipose tissue in obesity. J Clin Endocrinol Metab. 1967 Nov;27(11):1573–1580. doi: 10.1210/jcem-27-11-1573. [DOI] [PubMed] [Google Scholar]
- BRAY G. A., GOODMAN H. M. STUDIES ON THE EARLY EFFECTS OF THYROID HORMONES. Endocrinology. 1965 Feb;76:323–328. doi: 10.1210/endo-76-2-323. [DOI] [PubMed] [Google Scholar]
- Björntorp P., Martinsson A. Conversion of glucose-14C into carbon dioxide and lipids in different specimens of human subcutaneous adipose tissue. Acta Med Scand. 1967 Mar;181(3):359–366. doi: 10.1111/j.0954-6820.1967.tb15163.x. [DOI] [PubMed] [Google Scholar]
- Bray G. A., Goodman H. M. Metabolism of adipose tissue from normal and hypothyroid rats. Endocrinology. 1968 Apr;82(4):860–864. doi: 10.1210/endo-82-4-860. [DOI] [PubMed] [Google Scholar]
- Bray G. A. Lipogenesis from glucose and pyruvate in fat cells from genetically obese rats. J Lipid Res. 1968 Nov;9(6):681–686. [PubMed] [Google Scholar]
- DOLE V. P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956 Feb;35(2):150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIED G. H., GREENBERG N., ANTOPOL W. Determination of alpha-glycerophosphate oxidation with tetrazolium. Proc Soc Exp Biol Med. 1961 Jul;107:523–525. doi: 10.3181/00379727-107-26675. [DOI] [PubMed] [Google Scholar]
- Fessler A., Beck J. C. The effect of insulin on the metabolism of human adipose tissue in vitro. Biochim Biophys Acta. 1965 Jul 7;106(1):199–201. doi: 10.1016/0005-2760(65)90108-6. [DOI] [PubMed] [Google Scholar]
- Galton D. J. An enzymatic defect in a group of obese patients. Br Med J. 1966 Dec 17;2(5528):1498–1500. doi: 10.1136/bmj.2.5528.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galton D. J. Lipogenesis in human adipose tissue. J Lipid Res. 1968 Jan;9(1):19–26. [PubMed] [Google Scholar]
- Goodman H. M., Bray G. A. Role of thyroid hormones in lipolysis. Am J Physiol. 1966 May;210(5):1053–1058. doi: 10.1152/ajplegacy.1966.210.5.1053. [DOI] [PubMed] [Google Scholar]
- Gries F. A., Steinke J. Comparative effects of insulin on adipose tissue segments and isolated fat cells of rat and man. J Clin Invest. 1967 Sep;46(9):1413–1421. doi: 10.1172/JCI105633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCH J., GOLDRICK R. B. SERIAL STUDIES ON THE METABOLISM OF HUMAN ADIPOSE TISSUE. I. LIPOGENESIS AND FREE FATTY ACID UPTAKE AND RELEASE IN SMALL ASPIRATED SAMPLES OF SUBCUTANEOUS FAT. J Clin Invest. 1964 Sep;43:1776–1792. doi: 10.1172/JCI105052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard C. F., Jr, Lowenstein J. M. The effect of glycerol 3-phosphate on fatty acid synthesis. J Biol Chem. 1965 Nov;240(11):4170–4175. [PubMed] [Google Scholar]
- KAHLENBERG A., KALANT N. THE EFFECT OF INSULIN ON HUMAN ADIPOSE TISSUE. Can J Biochem. 1964 Nov;42:1623–1635. doi: 10.1139/o64-173. [DOI] [PubMed] [Google Scholar]
- Kneer P., Ball E. G. Studies on the metabolism of adipose tissue. XXI. An evaluation of the major pathways of pyruvate metabolism. J Biol Chem. 1968 Jun 10;243(11):2863–2870. [PubMed] [Google Scholar]
- LEE Y. P., LARDY H. A. INFLUENCE OF THYROID HORMONES ON L-ALPHA-GLYCEROPHOSPHATE DEHYDROGENASES AND OTHER DEHYDROGENASES IN VARIOUS ORGANS OF THE RAT. J Biol Chem. 1965 Mar;240:1427–1436. [PubMed] [Google Scholar]
- Miller D. S., Mumford P. Gluttony. 1. An experimental study of overeating low- or high-protein diets. Am J Clin Nutr. 1967 Nov;20(11):1212–1222. doi: 10.1093/ajcn/20.11.1212. [DOI] [PubMed] [Google Scholar]
- Miller D. S., Mumford P., Stock M. J. Gluttony. 2. Thermogenesis in overeating man. Am J Clin Nutr. 1967 Nov;20(11):1223–1229. doi: 10.1093/ajcn/20.11.1223. [DOI] [PubMed] [Google Scholar]
- RICH C., BIERMAN E. L., SCHWARTZ I. L. Plasma nonesterified fatty acids in hyperthyroid states. J Clin Invest. 1959 Feb;38(2):275–278. doi: 10.1172/JCI103799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Salans L. B., Knittle J. L., Hirsch J. The role of adipose cell size and adipose tissue insulin sensitivity in the carbohydrate intolerance of human obesity. J Clin Invest. 1968 Jan;47(1):153–165. doi: 10.1172/JCI105705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling J. L., Stock M. J. Metabolic origins of thermogenesis induced by diet. Nature. 1968 Nov 23;220(5169):801–802. doi: 10.1038/220801a0. [DOI] [PubMed] [Google Scholar]
- Tepperman H. M., Tepperman J. Adaptive changes in alpha-glycerophosphate-generating enzymes in rat liver. Am J Physiol. 1968 Jan;214(1):67–72. doi: 10.1152/ajplegacy.1968.214.1.67. [DOI] [PubMed] [Google Scholar]
- WINEGRAD A. I., RENOLD A. E. Studies on rat adipose tissue in vitro. I. Effects of insulin on the metabolism of glucose, pyruvate, and acetate. J Biol Chem. 1958 Aug;233(2):267–272. [PubMed] [Google Scholar]
- White L. W., Williams H. R., Landau B. R. Metabolism of pyruvate by rat adipose tissue in vitro. Arch Biochem Biophys. 1968 Aug;126(2):552–557. doi: 10.1016/0003-9861(68)90441-4. [DOI] [PubMed] [Google Scholar]



