Abstract
The coloration of species can have multiple functions, such as predator avoidance and sexual signalling, that directly affect fitness. As selection should favour traits that positively affect fitness, the genes underlying the trait should reach fixation, thereby preventing the evolution of polymorphisms. This is particularly true for aposematic species that rely on coloration as a warning signal to advertise their unprofitability to predators. Nonetheless, there are numerous examples of aposematic species showing remarkable colour polymorphisms. We examined whether colour polymorphism in the wood tiger moth is maintained by trade-offs between different functions of coloration. In Finland, males of this species have two distinct colour morphs: white and yellow. The efficacy of the warning signal of these morphs was tested by offering them to blue tits in the laboratory. Birds hesitated significantly longer to attack yellow than white males. In a field experiment, the survival of the yellow males was also higher than white males. However, mating experiments in the laboratory revealed that yellow males had lower mating success than white males. Our results offer an explanation for the maintenance of polymorphism via trade-off between survival selection and mating success.
Keywords: avian vision model, colour polymorphism, Parasemia plantaginis, predation, sexual selection, warning signalling
1. Introduction
Colour polymorphism provides a classic opportunity to understand the process of natural selection in maintaining biodiversity [1]. Processes involved in maintenance of colour polymorphism are of particular interest because they may be important precursors to mechanisms that cause speciation [2]. Several hypotheses have been proposed to explain the maintenance of multiple colour morphs, though none are mutually exclusive; frequency-dependent selection via predation or sexual selection being the most prominent ones [3,4].
In cryptic species, negative frequency-dependent selection often leads to polymorphism of prey [5], but selection is expected to be opposite when prey is aposematic. Aposematic prey often advertises unprofitability (i.e. spines, toxins, noxious chemicals, etc.) to predators via conspicuous coloration [6,7]. Aposematism confers protection to individuals carrying the warning signal, but the benefits are often dependent on a sufficient density of individuals displaying the signal [8–10]. Colour polymorphism is therefore not expected in aposematic organisms, like Müllerian mimics, because predator education is based on ‘strength in numbers’ of similar phenotypes [11–13] and selection is positively frequency-dependent (i.e. anti-apostatic selection) [9]. Despite the expected adaptive function of signal monomorphism in aposematic organisms, colour polymorphism has been reported in many aposematic species (e.g. [14,15]).
Conflicts among the selective pressures acting on coloration can contribute to the maintenance of colour polymorphism in aposematic species. In the strawberry poison frog (Oophaga pumilio), the aposematic coloration influences the behaviour of male conspecifics [16] and female preference [17]. Females prefer males based on familiar dorsal coloration, but female tolerance for unfamiliar colour patterns may facilitate the establishment of novel phenotypes that could be favoured further by predator bias [17]. Thus, the combined effects of sexual selection and predation may facilitate colour polymorphism [16–18].
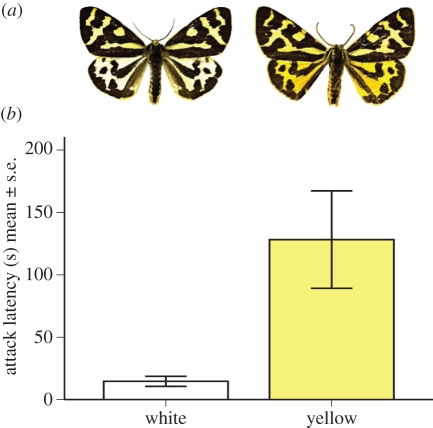
The wood tiger moth (Parasemia plantaginis) offers a particularly attractive possibility to investigate selective forces favouring colour polymorphism. Males of this species show extensive colour polymorphism both locally and on broader geographical scales. Parasemia plantaginis is widely distributed over the Northern Hemisphere and inhabits a variety of habitats [19], but rarely occurs in high densities. The genetic morphs of males have visually distinct hind wing colours ([20]; figures 1a and 2b); the most typical in Europe are yellow and white with various degrees of melanization. Parasemia plantaginis larvae and females are shown to be aposematic [21,22], and the defence chemicals (e.g. iridoid glycosides) larvae sequester are transferred to the adult females and males [20].
Figure 1.
(a) The white (left) and yellow (right) male morph of wood tiger moth. (b) Predators' hesitation (in seconds) to attack against colour morph.
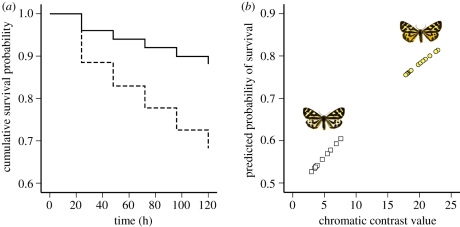
Figure 2.
(a) Survival plot of the yellow (solid line) and white (dashed line) colour morph survival in the field. The lines are the probability of surviving avian predation as a function of time (hours) based on Cox regression estimates to account for censored data during the 5 day experiment. (b) Chromatic contrast values of hind wing colour compared against different backgrounds and their relation to predicted probability of survival. Squares represent white males and circles represent yellow males.
In the first part of this study, we investigated the strength and direction of selection by predators on male coloration. We determined whether predators found male morphs aversive by offering white and yellow P. plantaginis males for blue tits under laboratory conditions. The more conspicuous morph (yellow) should have a selective advantage compared with a less conspicuous morph (white) [21,23–25] and therefore, we predicted that birds should hesitate longer and attack yellow morphs less than white morphs.
Next, we compared the survival of dead white and yellow P. plantaginis moths pinned to various different tree trunks in the field. Conspicuousness of the colour pattern depends on both receiver perception and visual background [26–28]. If an environment is heterogeneous in substrate type, the potential exists for several different colour morphs to persist, as each morph has an advantage within a given visual microhabitat [5,29]. Therefore, it is important to address the survival of different morphs in the wild where light conditions, predator community and visual environment vary. In addition, we used avian vision modelling [30] to analyse whether the conspicuousness of white and yellow morphs against different backgrounds predicted their survival. We again assumed that the more conspicuous yellow morph should be attacked less than the white morph if conspicuousness is beneficial against predators. Alternatively, the less conspicuous morph may benefit from a lower detection risk by predators (e.g. white morph on a white birch trunk). It is also possible that colour variation is neutral in terms of predation [31] and colour polymorphism can be maintained in the population.
In the second part of this study, we examined mating success and reproductive output of white and yellow males since sexual selection often plays a role in male colour polymorphism [4,32]. We hypothesized that if the conspicuous morph is favoured by increased survival against predation, then either better mating success of the less conspicuous form or signal production costs (e.g. costly pigmentation; [33,34]) could provide a plausible explanation for the observed male colour polymorphism. As mating success is shown to be dependent on an individual's condition [35–37], we forced part of the yellow and white males to excrete a defensive fluid before mating to see if males of different colour morphs are able to bear costs differently in benign and stressful conditions.
2. Material and methods
(a). Rearing of Parasemia plantaginis
Individuals for the experiments came from a laboratory stock reared under constant temperature and density. During the larval stage, food (dandelion, Taraxacum sp.) was offered ad libitum (more detailed description in Lindstedt et al. [38,39]). Adults do not feed. Males used in the assays originated from divergent selection lines for larval coloration (small and large orange patch, see Lindstedt et al. [38]. However, larval coloration does not affect male colour [20]. Both white and yellow morph are visually distinctive and easy to distinguish by the human eye (figure 1), which allowed easy categorization of individuals for the experiments. The colour classification was also confirmed by spectrophotometer measurements, which showed clear differences between the two groups based on chromatic contrast values (figure 2b).
(b). Warning signal efficacy of white and yellow morph
We conducted behavioural assays with blue tits (Parus caeruleus) in an aviary, where we tested whether predators treat yellow or white morphs as unprofitable prey by measuring the hesitation to attack and the handling time. The experiment was conducted in Konnevesi Research Station, Central Finland. Blue tits are generalist predators distributed in the same areas as P. plantaginis. Birds were captured from feeding sites between March–April and September–October 2009. A peanut-filled pre-baited trap (a box 13 × 17 × 40 cm), which could be manually operated, was used to catch the birds [21]. Captured birds (n = 36) were used only once in the experiment, ringed for the identification and immediately released back to the feeding site after the experiment.
We used a small plywood box as an experimental arena (50 × 65 × 45 cm). The temperature inside the arena was approximately 20°C. The light bulb used emitted the entire visible daylight spectrum (including UV; Litetronics, made in Germany, SPE CE 20 W, spiral-lite, 220–240 V, 50/60 Hz, 5700 K, Cape 27). Bird behaviour was observed through a small mesh-covered window, and all trials were done in a dark room to prevent disturbance to the birds. The experimental box contained a water bowl and a perch for the bird. In the opposite wall from the perch, there was a hatch from which food was offered. Between the perch and the feeding platform was a visual barrier, allowing us to determine when the bird detected the object for the first time. Prior to the experiment, the birds were familiarized with the experimental boxes for approximately 1 h, and trained to seek food from behind the visual barrier by giving them sunflower seeds. To confirm the birds' feeding motivation, they were food-deprived for 1 h before the experiment and at the beginning of the experiment a living mealworm (Tenebrio molitor) larva (weight: 0.10–0.15 mg) was offered.
In the experiment, only one living moth was offered to each bird for every trial. The moth was placed against a green background (Epiprenum pinnatum leaf), because P. plantaginis is often found resting on a green background in the wild (O.N. 2008 personal observation). In total, we used 18 white males (four from small and 14 from large orange patch size selection line of larval colour [38], and 18 yellow males (four from small and 14 from large orange patch size selection line of larval colour) of equal size (ANOVA for size: colour morph: F1,30 = 0.884, p = 0.355; line: F1,30 = 0.355, p = 0.556).
Latency to attack (i.e. hesitation) was measured in seconds from the first moment a bird detected the prey to the moment of the attack. The maximum time allowed for a bird to make an attack decision was 10 min. Handling time (in seconds) was measured from the first moment the bird attacked the prey to the time when the bird ceased handling the prey. Moth behaviour during the bird attack was scored into five categories: (i) no response (the moth was standing on the background without moving), (ii) startle (the moth flashed its forewings actively revealing its hind wings), (iii) defensive fluid (moth released an aversive defensive fluid from abdomen), (iv) escape attempt (individuals trying to fly away), and (v) feign death (when the moth relaxed its legs and remained motionless). The proportion of moth eaten was scored into four categories: 0 per cent (no observable damage), 10 per cent (head taken), 50 per cent (abdomen taken) and 100 per cent (fully eaten). The categories refer only to the proportion of the body consumed because the birds always pulled the wings off before they ate the moth (O.N. 2009 personal observation).
After the experiment, birds' hunger level was measured by offering them 12 mealworms. The weight of the mealworms consumed within 5 min was used to estimate the bird's satiation level. Birds were fed with peanuts and sunflower seeds before they were released. None of the birds were injured or died during temporary captivity.
We used analysis of variance (ANOVA) to test whether the attack latency (initial avoidance) or handling time by blue tits differed between the two male colour morphs (white and yellow). We also included larval selection line as a fixed factor into the model. Hunger level of the bird and size of the moth (pupa weight) were set as covariates in both models. We log-transformed ‘the latency of attack’ and ‘the handling time’, as neither were normally distributed to fit the assumptions of tests. The defence behaviour of the moth and proportion of the moth eaten was tested with Fisher's exact test.
(c). Predation on white and yellow morphs in the field
To study potential differences in predation on the colour morphs in natural conditions, we pinned dead adult P. plantaginis males on tree trunks to estimate avian predation pressure. Moths were kept in the freezer and thawed approximately 1 h before the experiment. The field experiment was conducted in the Åland Archipelago, southwestern Finland in summer 2008. The area is characterized by small meadows and deciduous forest patches, which are natural habitats for P. plantaginis. The avian predator community was observed once with the line transect method [40] in the early morning between 04.00 and 10.00 h, when the birds are most active. Only Passeriformes were included in the analysis, as most of the known and potential predators of P. plantaginis are within this group.
A total of seven transects were set in different open forest locations and morphs were pinned in 25 m intervals, making a total count of 40 moths (20 white : 20 yellow), and length of one transect 1000 m. Altogether 280 targets (140 white : 140 yellow) were used in the experiment. Moths were placed in visible locations enabling birds to identify them easily. Moths were always pinned on the southern side of tree trunks 1.5 m from the ground with forewings spread to 45° position. Tweezers were used to simulate this natural resting posture. Moths were pinned randomly on 13 different backgrounds, because of varying tree species composition between study sites. The majority of moths were pinned on Pinus sylvestris (36%), Betula pubescens (25%), Picea abies (13%) and Fraxinus excelsior (9%). Additional tree species comprised less than 5 per cent of study sites, respectively (see list in the electronic supplementary material, appendix table S2).
The experiment was lasted 5 days and the survival of prey specimens was checked every 24 h. A moth was determined to be attacked if we observed clear damage to the body or wings. Other cases were counted as censored values in the survival analysis to ensure that we did not inflate the predation estimate. Attacked moths were replaced with the new ones in order to keep the frequencies of available moths constant, but only the first attacks of individuals were taken into account in the analysis. We identified avian attacks by v-shaped rips and beak marks left in the body or wings [41]. We excluded missing individuals, moths which were heavily damaged by ants and beetles, covered with slime tracks (snails), and hollow exoskeletons (consumed by spiders) from the analysis [41]. However, results remained the same if we included the insect attacks and/or missing targets into the analysis.
To analyse the overall survival of white and yellow morphs in the field, we ran Cox regression analysis. We included time as the dependent variable and avian attacks as status variable, along with male colour, study site and number of predator species and their interactions as covariates. To test if vulnerability to predation differs among tree species, we constructed a separate binary logistic regression model with male colour and tree species and their interaction as a covariate. Finally, we tested whether the conspicuousness against different backgrounds predicts survival of yellow and white morphs. Chromatic contrast and achromatic contrast values of moth (see further) against the background are correlated properties, thus their effect on survival has to be analysed in two separate models. In the first model, we had survival as a binomial-dependent variable and the colour (chromatic) contrast value as a covariate. In the second model, we had survival as a binomial-dependent variable and luminosity (achromatic) contrast value as covariate. For all the results, expected beta coefficients are reported as odds ratios (OR) to describe the effect size. A value 1.00 indicates that two treatments have identical survival probabilities (i.e. event is equally likely to happen in both groups).
(d). Variation in conspicuousness and predation risk
The conspicuousness of the moths on different backgrounds was estimated with an avian vision model [42,43]. We measured the reflectance spectra of tree trunks by taking measurements from three individuals of each tree species along with the 10 measurements of both morphs. We then constructed an avian vision model to analyse contrasts between moths and backgrounds.
We used discrimination threshold modelling to predict whether the bird (blue tit) can discriminate between the colour and luminance of the white and yellow hind wing colours against several different backgrounds used in the field predation experiment. The discrimination threshold model used assumes that noise in the receptors limits discrimination ability [42,43]. The model uses information about the visual system, such as the sensitivity and relative abundance of different receptor types, and estimates of noise that arise in the photoreceptors.
We first took five measures per individual from 10 white males and 10 yellow males with an Ocean Optics (Dunedin, FL, USA) USB4000 spectrometer held at 45° to normal, with illumination by a PX-2 pulsed xenon lamp, recorded from 300 to 750 nm, expressed relative to a SpectralonTM 99 per cent white reflectance standard (Labsphere, Congleton, UK). Data were reduced to 1 nm intervals prior to analysis by selecting the first value of each nanometre. Colour of the various backgrounds (see list in the electronic supplementary material, appendix table S2) was measured similarly except with AvaSpec-2048-SPU (Avantes, USA) spectrometer with illumination by AvaLight DHS Deuterium–Halogen light source. Average spectra were taken for each stimulus type, followed by modelling of a blue tit's photon catch values for the single and double cones [44] with a standard D65 irradiance spectrum. Colour vision in birds stems from the four single cone types [45], whereas luminance discrimination apparently stems from the double cones [30]. For the colour model, we therefore used the four single cones, whereas the luminance model was based on the double cones [15]. For the discrimination model, we used a Weber fraction of 0.05 for the most abundant cone type, and the relative proportions of cone types in the blue tit retina (longwave = 1.00, mediumwave = 0.99, shortwave = 0.71 and ultraviolet sensitive = 0.37). For the results of the discrimination model, values (just noticeable differences, or ‘JNDs’) of less than 1 are indistinguishable, values between 1 and 3 are hard to distinguish unless under optimal conditions and values more than 5 are easy to tell apart under most conditions [46]. Finally, we incorporated obtained colour contrast and luminosity contrast values as covariates in the survival analysis of the field experiment data (described above).
(e). Reproductive output of white and yellow morphs
To determine whether mating success and reproductive output of males was affected by coloration, we performed a mating experiment. The experiment was conducted in a greenhouse at the University of Jyväskylä in Central Finland (62° N, 26° E) during June and July 2009. The temperature in the greenhouse varied between 20°C and 30°C during the day (approx. 20 h) and during the night (approx. 4 h) the temperature decreased to 15°C–20°C. In the northern latitudes (greater than 62° N), the flying and mating period of P. plantaginis males occurs during the midsummer when the days are very long (22 h of light). Thus, the males' colours should be visible to females, and could potentially be a target of sexual selection.
In the experiment, one virgin male was offered to a virgin female. Each ‘couple’ was placed within a separate container to control for the possibility of male–male competition. Variables measured to determine mating success included latency of copulation (the time between introduction and copulation), the duration of mating, egg production and offspring hatching success. In total, we had 42 white males (18 from small and 24 from large orange patch size selection line for larval coloration) and 43 yellow males (20 from small and 23 from large orange patch size line). Males were mated with a randomly chosen female to control any potential bias in experimenter pairing choices. Pairing was done in the evening (a mating box 10 × 13 × 12 cm) before sunset between 20.00 and 21.00. After pairing the males and females, individuals were observed continuously for 10 h from 21.00 to 07.00 (when mating naturally occurs), and their mating success (whether the male mated or not) was recorded. If males had not mated during this time they were scored as ‘unsuccessful’. After mating, males were removed from the boxes and females were allowed to lay eggs for 4 days and on the fifth day the eggs were counted. Larvae were counted on a second day after hatching to confirm that all the larvae had hatched.
In order to be able to disentangle the mating success of male morphs in benign and stressful conditions, males were divided into two treatment groups; (i) controls and (ii) manipulated, where males were forced to produce defensive fluid (e.g. [47]). The defensive fluid has a distinct odour and we have observed that males produce it when threatened and thus, it most likely has an anti-predatory function in Arctiid moths (see also [48,49]). As capital breeders, production of defence fluid must be restricted for adult P. plantaginis individuals making it costly trait to produce repetitively both in terms of resources (e.g. water) and energy (e.g. metabolic processes to synthesize and expel the fluid). In addition, releasing frequency of the defence fluid also varies among individuals under predation (table 2) and preliminary tests indicate a negative relationship between the releasing frequency and the amount of fluid exuded supporting its costliness for males (K. Suisto and C. Lindstedt, 2009 personal observation). Fluid was released from the males before the mating on the same day of the experiment. Males were forced to produce the defensive fluid only once by gently lifting the moth with tweezers. The defensive fluid produced was then drawn into a capillary tube and its volume was measured.
Table 2.
Observed behaviour and damage of moths under predation threat in the aviary.
| variable | observation | white | yellow | total | Fisher's exact |
|---|---|---|---|---|---|
| behaviour | no response | 11 | 8 | 19 | 0.505 |
| startle | 0 | 2 | 2 | 1.000 | |
| defensive fluid | 2 | 6 | 8 | 0.228 | |
| escape attempt | 2 | 1 | 3 | 1.000 | |
| feign death | 3 | 1 | 4 | 0.603 | |
| proportion eaten | 0% no damage | 3 | 6 | 9 | 0.443 |
| 10% head taken | 4 | 5 | 9 | 1.000 | |
| 50% abdomen eatena | 2 | 1 | 3 | 1.000 | |
| 100% fully eatena | 9 | 6 | 15 | 0.500 |
aNotice that wings were never eaten.
To determine whether male colour influenced mating success we used binary logistic regression. Mating success (mated or not mated) was set as the dependent variable and male colour, selection line for larval colour, defensive fluid treatment along with their possible interactions were set as covariates. We tested whether the amount of defensive fluid produced varies between morphs by setting the amount as a dependent variable, morph and selection line as fixed factors, and male weight as covariate in ANOVA. The defensive fluid volume was transformed to a logarithmic scale to fit the assumptions of ANOVA. The mating delay (in minutes) and duration of copulation (in minutes) were tested with an analysis of variance by setting the time as a dependent variable with morph, selection line for larval colour, and defensive fluid volume as fixed factors. Male weight was included as a covariate in the ANOVA. To determine if fecundity differed between the male colour morphs we also used an ANOVA. Fecundity measure (number of eggs, number of offspring) was set as a dependent variable. Colour of the male, selection line and defensive fluid treatment were set as fixed factors. Female weight was set as a covariate. Non-significant parameters were omitted from the final analyses (general protocol; p > 0.05, smallest omitted significance was p = 0.488).
3. Results
(a). Warning signal efficacy of white and yellow morph
Blue tits hesitated significantly longer to attack yellow males compared with white males (figure 1 and table 1). The average hesitation time for yellow males (mean = 128 s, s.d. = 166) was nine times longer than for white males (mean = 15 s, s.d. = 17). The handling time was not different between two morphs (table 1), even though birds' handling time for the yellow males (mean = 161 s, s.d. = 190), was approximately two times longer than for the white males (mean = 82 s, s.d. = 50). The proportion of moths eaten by blue tits did not differ between colour morphs, and both colour morphs behaved similarly when threatened by predators (table 2).
Table 1.
ANOVA-table of hesitation and handling times by blue tits.
| source of variation | d.f. | MS | F | p |
|---|---|---|---|---|
| hesitation to attack (s) | ||||
| morph | 1 | 5.347 | 9.469 | 0.005* |
| signal line | 1 | 0.438 | 0.777 | 0.386 |
| morph × signal line | 1 | 0.708 | 1.254 | 0.273 |
| male weight | 1 | 0.050 | 0.089 | 0.767 |
| satiation | 1 | 1.035 | 1.834 | 0.187 |
| error | 27 | 0.565 | ||
| handling time (s) | ||||
| morph | 1 | 0.140 | 0.792 | 0.382 |
| signal line | 1 | 0.668 | 3.791 | 0.062 |
| morph × signal line | 1 | 0.026 | 0.150 | 0.701 |
| male weight | 1 | 0.074 | 0.417 | 0.524 |
| satiation | 1 | 0.352 | 2.001 | 0.169 |
| error | 26 | 0.176 | ||
*Significant at 5%.
(b). Predation on white and yellow males in the field
Yellow males were nearly three times more likely to survive in the field compared with white males (Wald = 3.937, d.f. = 1, p = 0.047, OR = 2.727; figure 2). Total avian predation in the experiment was 33.3 per cent of all prey specimens. Of the 140 individuals of each morph, 61 white males (43.6%) and 31 yellow males (22.1%) were attacked. The survival rate of white and yellow males did not depend on the site (Wald = 0.121, d.f. = 1, p = 0.728, OR = 1.196) nor did it vary among the study sites (study site × colour morph: Wald = 0.042, d.f. = 1, p = 0.839, OR = 1.026). In addition, the amount of predator species (Wald = 0.161, d.f. = 1, p = 0.688, OR = 1.045), or their interaction with site (Wald = 0.875, d.f. = 1, p = 0.350, OR = 0.979) did not impact the survival rate of the pinned moths. When we tested whether the tree species, against which the moth was pinned, predicted survival we found that only male colour was a significant predictor (Wald = 14.153, d.f. = 1, p < 0.001, OR = 0.368). Neither the tree species (Wald = 6.534, d.f. = 12, p = 0.886) nor their interaction with male colour (Wald = 2.273, d.f. = 8, p = 0.971) predicted predation.
Both colour morphs were clearly conspicuous for avian predators against all backgrounds, though yellow males were generally more conspicuous in terms of colour (all JND values for colour contrasts varied for white males from 2.95 to 7.59 and for yellow males from 17.83 to 22.90). For luminance, JND values varied from 0.48 to 19.27 and 0.22 to 16.54 for white males and yellow males, respectively. Conspicuousness was beneficial for moths, because survival probability increased with increasing colour (chromatic) contrast against the background (Wald = 15.440, d.f. = 1, p < 0.001, OR = 1.071; figure 2). However, luminosity contrast alone did not predict predation (Wald = 0.824, d.f. = 1, p = 0.364, OR = 1.018).
(c). Reproductive output of white and yellow morphs
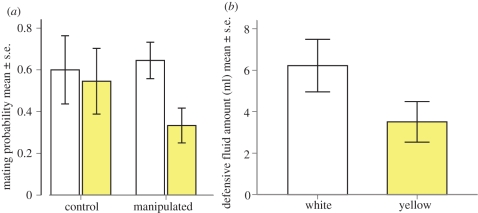
White males released more defensive fluid than yellow males (figure 3 and table 3). White males were also more than eight times more likely to mate than yellow males (Wald = 8.339, d.f. = 1, p = 0.004, OR = 8.212; figure 3). Extracting defensive fluid before the mating did not affect the mating probability (Wald = 1.490, d.f. = 1, p = 0.222, OR = 2.880), nor was there an interaction between the defensive fluid treatment and male colour (Wald = 1.177, d.f. = 1, p = 0.278, OR = 0.309). Neither the selection line (Wald = 0.097, d.f. = 1, p = 0.756, OR = 1.241) nor its interaction with the defensive fluid treatment (Wald = 0.136, d.f. = 1, p = 0.713, OR = 0.673) had an effect on mating probability. There was also no interaction between male colour and larval selection line (Wald = 3.035, d.f. = 1, p = 0.081, OR = 0.194) affecting mating probability. Male weight did not differ significantly among the treatment groups (colour morph: F1,79 = 1.887, p = 0.173; line: F1,79 = 0.032, p = 0.858; defensive fluid treatment: F1,79 = 0.008, p = 0.928), thus variation in male size does not explain the differences in mating success.
Figure 3.
(a) The mean mating probability of wood tiger moth males. On the x-axis, the ‘control’ represents individuals, which were not in the defensive fluid treatment, and ‘manipulated’, stands for the defensive fluid (i.e. droplet) treatment group. Left bars within the group (white) stands for the white males, and right bars (yellow) represent yellow males. On the y-axis is the mating probability of males, when females have no alternative choice. (b) The mean volume (mm3) of defensive fluid between two male colour morphs of wood tiger moth.
Table 3.
ANOVA-table from reproductive output experiment.
| source of variation | d.f. | MS | F | p |
|---|---|---|---|---|
| volume of defensive fluid | ||||
| morph | 1 | 6.467 | 5.291 | 0.025* |
| signal line | 1 | 0.0000424 | <0.001 | 0.995 |
| morph × signal line | 1 | 2.473 | 2.024 | 0.160 |
| male weight | 1 | 0.030 | 0.024 | 0.877 |
| error | 57 | 1.222 | ||
| mating delay (min) | ||||
| morph | 1 | 0.00000001567 | 1.783 | 0.190 |
| signal line | 1 | 0.000000579 | 0.659 | 0.422 |
| defensive fluid | 1 | 0.00000001372 | 1.561 | 0.220 |
| morph × signal line | 1 | 0.0000001519 | 0.173 | 0.680 |
| signal line × defensive fluid | 1 | 0.0000008921 | 1.015 | 0.320 |
| morph × defensive fluid | 1 | 0.0000007827 | 0.891 | 0.352 |
| error | 36 | 0.0000008789 | ||
| duration of copulation (min) | ||||
| morph | 1 | 24 296.056 | 0.930 | 0.342 |
| signal line | 1 | 8344.096 | 0.319 | 0.576 |
| defensive fluid | 1 | 188 563.120 | 7.220 | 0.011* |
| morph × signal line | 1 | 4247.928 | 0.163 | 0.689 |
| morph × defensive fluid | 1 | 70 372.151 | 2.695 | 0.110 |
| signal line × defensive fluid | 1 | 50 370.910 | 1.929 | 0.174 |
| male weight | 1 | 145 233.020 | 5.561 | 0.024* |
| signal line × defensive fluid × morph | 1 | 105 179.770 | 4.027 | 0.053 |
| error | 33 | 26 116.467 | ||
| egg number | ||||
| morph | 1 | 7.645 | 0.001 | 0.973 |
| signal line | 1 | 468.966 | 0.070 | 0.794 |
| defensive fluid | 1 | 3209.485 | 0.479 | 0.495 |
| morph × signal line | 1 | 3095.068 | 0.462 | 0.503 |
| morph × defensive fluid | 1 | 3137.048 | 0.469 | 0.500 |
| signal line × defensive fluid | 1 | 1207.520 | 0.180 | 0.675 |
| female weight | 1 | 24 581.420 | 3.672 | 0.067 |
| error | 24 | 6694.44 | ||
| offspring number | ||||
| morph | 1 | 170.262 | 0.029 | 0.866 |
| signal line | 1 | 22.905 | 0.004 | 0.951 |
| defensive fluid | 1 | 14 866.183 | 2.524 | 0.126 |
| morph × signal line | 1 | 4368.392 | 0.742 | 0.398 |
| morph × defensive fluid | 1 | 38.909 | 0.007 | 0.936 |
| signal line × defensive fluid | 1 | 5228.009 | 0.887 | 0.356 |
| female weight | 1 | 27 921.408 | 4.740 | 0.040* |
| error | 23 | 5890.859 | ||
*Significant at 5%.
The latency (i.e. mating delay) of copulation did not differ between the colour morphs (table 3). Larval coloration lines, defensive fluid treatments and their interactions did not affect mating delay (table 3). However, if we include only the males that mated, the male colour did not significantly affect the mating delay (time to start copulation). Interestingly, copulation took longer if males were forced to give defensive fluid (table 3). This could mean that the fluid may be a part of courtship signals delivered to females or it could decrease the quality of the males in other ways, prolonging the latency of copulation. Male weight also affected copulation time (table 3), which was shorter for heavier males (r = −0.330, p = 0.031).
No difference was observed in post-mating fitness differences between yellow and white males who successfully mated. We found that male colour did not affect the number of eggs or number of offspring produced (table 3). However, heavier females produced more offspring (table 3).
4. Discussion
Genetic polymorphism can be maintained if different morphs have equal mean fitness (e.g. [3,4,32]). In the present study, we demonstrate a trade-off between warning signal efficacy and mating success between yellow and white P. plantaginis males. The more conspicuous yellow males were better defended against predators, as they hesitated longer and were less likely to attack yellow males compared with white males. However, white males had better mating success in comparison with yellow males, which would lead to a higher number of egg clutches sired by white males. This trade-off can partly explain the sympatric occurrence of white and yellow male morphs.
Predators are expected to select for conspicuous [21,23,24,50,51] and convergent [11–13,52,53] warning signals in aposematic prey. In concordance, our results showed that increased colour contrast was beneficial for P. plantaginis males, with yellow males benefitting the most because of their greater conspicuousness. In addition to colour contrast, greater prey luminance contrast may increase the detection of prey, but also facilitate predator aversion [54] and memory retention of an invertebrate predator [55]. Our data showed that despite white P. plantaginis males having stronger luminosity contrast compared with the yellow males, it did not increase the survival of white males. Thus, we did not find any support for the hypothesis that the variation in predator perception and/or background coloration could maintain colour polymorphism in this system.
Selection for conspicuous morphs by predators could be relaxed if toxicity and conspicuousness are expensive to produce and maintain, but an increase in either of the components may offer equally good protection against predators [56,57]. The present study did not provide clear support for this assumption as birds were handling and consuming white and yellow males similarly. In addition, tendency to produce defensive fluid under attack did not differ among morphs, although white males excreted higher quantities of bitter smelling defensive fluid before the mating experiment. However, more detailed investigations would be needed to determine whether white and yellow males have different defence strategies before definitive conclusions can be made.
Allocation of resources to conspicuous warning coloration may have an impact on other fitness-related traits, such as reproduction [57,58]. Although we do not have any direct evidence of production or maintenance costs of male coloration, we suggest that one cost of effective warning signal expression is impaired reproductive output because the mating probability of white males was higher when compared with yellow males. Based on the current data, we cannot offer a clear reason underlying the observed female preference. Females may gain indirect benefits of mating with attractive males and having attractive sons. According to our results, male quality did not differ between colour morphs in terms of number of eggs or offspring produced per female. However, we did not measure the quality of the offspring further, thus it is possible that offspring of more attractive white males would have enhanced performance or viability. This will be a topic for future study.
In Arctiid moths, female mate choice can have more direct benefits as males leave spermatophores after mating, termed ‘nuptial gifts’, containing nutrients, water and defence chemicals [48]. Therefore, it is possible that white males could offer larger and better quality nuptial gifts. The additional nutrients may be particularly valuable for females, considering that P. plantaginis adults do not feed. Thus, one can assume that by mating with the males that can offer extra nutrients, females would gain direct benefits which could extend their individual lifespan and reproductive output. This may allow females to seek suitable egg-laying habitats longer, and thus evaluate the most suitable site for laying a clutch or to spread its clutch on several host plants (i.e. bet-hedging). Defensive chemicals transferred to females in the nuptial gifts can be used by the female for herself or for the defence of the eggs [48,59]. We did not find significant differences in the mating duration of the white and yellow males. However, larger males copulated for a shorter amount of time and individuals that were forced to produce defensive fluid mated longer, offering indirect support for the costs of producing defensive fluid. In order to test the possible differences in the spermatophore size between males of different condition and colour, further experiments are needed. In addition, tests where females are able to choose between the males are needed to see whether the results are in concordance with the current experiment when male–male competition is allowed.
Factors that contribute to the maintenance of colour polymorphisms continue to be a central focus in evolutionary research. We found that white male P. plantaginis were better at mating, but possessed a less effective warning signal. Although the mechanism behind the mate choice remains partly speculative, our results offer evidence that female mate choice along with selection by predators contributes to the maintenance of colour polymorphism in this species.
Acknowledgements
The experiment was carried out with permission from the Central Finland Regional Environmental Centre (permission no.: KSU-2007-09311/YM-23) and licence from the Experimental Animal Committee of the University of Jyväskylä (ESLH-2007-L-687/254).
We thank Martin Stevens for help with avian vision model analysis and Joanne Kitchen for checking the language. We are grateful to Hannah Rowland, the members of the Journal Club of the Department of Biological and Environmental Science in Jyväskylä and two anonymous referees for their helpful comments and suggestions. This study was financed by the Academy of Finland (Finnish Centres of Excellence in Evolutionary research), Biological Interactions graduate school and by the project 21000002561.
References
- 1.Gray S. M., McKinnon J. S. 2007. Linking color polymorphism maintenance and speciation. Trends Ecol. Evol. 22, 71–79 10.1016/j.tree.2006.10.005 (doi:10.1016/j.tree.2006.10.005) [DOI] [PubMed] [Google Scholar]
- 2.McKinnon J. S., Pierotti M. E. R. 2010. Colour polymorphism and correlated characters: genetic mechanisms and evolution. Mol. Ecol. 19, 5101–5125 10.1111/j.1365-294X.2010.04846.x (doi:10.1111/j.1365-294X.2010.04846.x) [DOI] [PubMed] [Google Scholar]
- 3.Olendorf R., Rodd F. H., Punzalan D., Houde A. E., Hurt C., Reznick D. N., Hughes K. A. 2006. Frequency-dependent survival in natural guppy populations. Nature 441, 633–636 10.1038/nature04646 (doi:10.1038/nature04646) [DOI] [PubMed] [Google Scholar]
- 4.Seehausen O., Schluter D. 2004. Male–male competition and nuptial–colour displacement as a diversifying force in Lake Victoria cichlid fishes. Proc. R. Soc. Lond. B 271, 1345–1353 10.1098/rspb.2004.2737 (doi:10.1098/rspb.2004.2737) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Endler J. A. 1988. Frequency-dependent predation, crypsis and aposematic coloration. Phil. Trans. R. Soc. Lond. B 319, 505–523 10.1098/rstb.1988.0062 (doi:10.1098/rstb.1988.0062) [DOI] [PubMed] [Google Scholar]
- 6.Poulton E. B. 1890. The colours of animals: their meaning and use. London: Kegan Paul, Trench, Trubner & Co. [Google Scholar]
- 7.Cott H. B. 1940. Adaptive coloration in animals. London: Menthuen & Co. Ltd. [Google Scholar]
- 8.Sword G. A., Simpson S. J., El Hadi O. T., Wilps H. 2000. Density-dependent aposematism in the desert locust. Proc. R. Soc. Lond. B 267, 63–68 10.1098/rspb.2000.0967 (doi:10.1098/rspb.2000.0967) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindström L., Alatalo R. V., Lyytinen A., Mappes J. 2001. Strong antiapostatic selection against novel rare aposematic prey. Proc. Natl Acad. Sci. USA 98, 9181–9184 10.1073/pnas.161071598 (doi:10.1073/pnas.161071598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Endler J., Mappes J. 2004. Predator mixes and the conspicuousness of aposematic signals. Am. Nat. 163, 532–547 10.1086/382662 (doi:10.1086/382662) [DOI] [PubMed] [Google Scholar]
- 11.Müller F. 1879. Ituna and Thyrida: a remarkable case of mimicry in butterflies. Proc. Entomol. Soc. Lond. 1879, 20–29 [Google Scholar]
- 12.Rowland H. M., Ihalainen E., Lindstrom L., Mappes J., Speed M. P. 2007. Co-mimics have a mutualistic relationship despite unequal defences. Nature 448, 64–67 10.1038/nature05899 (doi:10.1038/nature05899) [DOI] [PubMed] [Google Scholar]
- 13.Rowland H. M., Wiley E., Ruxton G. D., Mappes J., Speed M. P. 2010. When more is less: the fitness consequences of predators attacking more unpalatable prey when more are presented. Biol. Lett. 6, 732–735 10.1098/rsbl.2010.0207 (doi:10.1098/rsbl.2010.0207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brakefield P. M. 1985. Polymorphic Müllerian mimicry and interactions with thermal melanism in ladybirds and a soldier beetle: a hypothesis. Biol. J. Linnean Soc. 26, 243–267 10.1111/j.1095-8312.1985.tb01635.x (doi:10.1111/j.1095-8312.1985.tb01635.x) [DOI] [Google Scholar]
- 15.Siddiqi A., Cronin T. W., Loew E. R., Vorobyev M., Summers K. 2004. Interspecific and intraspecific views of color signals in the strawberry poison frog Dendrobates pumilio. J. Exp. Biol. 207, 2471–2485 10.1242/jeb.01047 (doi:10.1242/jeb.01047) [DOI] [PubMed] [Google Scholar]
- 16.Crothers L., Gering E., Cummings M. 2010. Aposematic signal variation predicts male–male interactions in a polymorphic poison frog. Evolution 65, 599–605 10.1111/j.1558-5646.2010.01154.x (doi:10.1111/j.1558-5646.2010.01154.x) [DOI] [PubMed] [Google Scholar]
- 17.Maan M. E., Cummings M. E. 2008. Female preferences for aposematic signal components in a polymorphic poison frog. Evolution 62, 2334–2345 10.1111/j.1558-5646.2008.00454.x (doi:10.1111/j.1558-5646.2008.00454.x) [DOI] [PubMed] [Google Scholar]
- 18.Lewis S. M., Cratsley C. K. 2008. Flash signal evolution, mate choice, and predation in fireflies. Annu. Rev. Entomol. 53, 293–321 10.1146/annurev.ento.53.103106.093346 (doi:10.1146/annurev.ento.53.103106.093346) [DOI] [PubMed] [Google Scholar]
- 19.Leraut P. Moths of Europe, volume 1: Saturnids, Lasiocampids, Hawkmoths, Tiger Moths…. Verrières-le-Buisson, France: N.A.P. Editions; 2006. (ed.) [Google Scholar]
- 20.Lindstedt C., Morehouse N., Pakkanen H., Casas J., Christides J., Kemppainen K., Lindström L., Mappes J. 2010. Characterizing the pigment composition of a variable warning signal of Parasemia plantaginis larvae. Funct. Ecol. 24, 759–766 10.1111/j.1365-2435.2010.01686.x (doi:10.1111/j.1365-2435.2010.01686.x) [DOI] [Google Scholar]
- 21.Lindstedt C., Lindström L., Mappes J. 2008. Hairiness and warning colours as components of antipredator defence: additive or interactive benefits? Anim. Behav. 75, 1703–1713 10.1016/j.anbehav.2007.10.024 (doi:10.1016/j.anbehav.2007.10.024) [DOI] [Google Scholar]
- 22.Lindstedt C., Eager H., Ihalainen E., Kahilainen A., Stevens M., Mappes J. 2011. Direction and strength of selection by predators for the color of the aposematic wood tiger moth. Behav. Ecol. 22, 580–587 10.1093/beheco/arr017 (doi:10.1093/beheco/arr017) [DOI] [Google Scholar]
- 23.Gamberale G., Tullberg B. S. 1998. Aposematism and gregariousness: the combined effect of group size and coloration on signal repellence. Proc. R. Soc. Lond. B 265, 889–894 10.1098/rspb.1998.0374 (doi:10.1098/rspb.1998.0374) [DOI] [Google Scholar]
- 24.Lindström L., Alatalo R. V., Mappes J., Riipi M., Vertainen L. 1999. Can aposematic signals evolve by gradual change? Nature 397, 249–251 10.1038/16692 (doi:10.1038/16692) [DOI] [Google Scholar]
- 25.Marples N. M., Kelly D. J., Thomas R. J. 2005. Perspective: the evolution of warning coloration is not paradoxical. Evolution 59, 933–940 [PubMed] [Google Scholar]
- 26.Endler J. A. 1980. Natural selection on color patterns in Poecilia reticulata. Evolution 34, 76–91 10.2307/2408316 (doi:10.2307/2408316) [DOI] [PubMed] [Google Scholar]
- 27.Bradbury J. W., Vehrencamp S. L. 1998. Principles of animal communication, vol. 13, 882 p Sunderland, MA: Sinauer [Google Scholar]
- 28.Chiao C., Vorobyev M., Cronin T. W., Osorio D. 2000. Spectral tuning of dichromats to natural scenes. Vision Res. 40, 3257–3271 10.1016/S0042-6989(00)00156-5 (doi:10.1016/S0042-6989(00)00156-5) [DOI] [PubMed] [Google Scholar]
- 29.Merilaita S. 2003. Visual background complexity facilitates the evolution of camouflage. Evolution 57, 1248–1254 [DOI] [PubMed] [Google Scholar]
- 30.Osorio D., Vorobyev M. 2005. Photoreceptor sectral sensitivities in terrestrial animals: adaptations for luminance and colour vision. Proc. R. Soc. B 272, 1745–1752 10.1098/rspb.2005.3156 (doi:10.1098/rspb.2005.3156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hegna R. H., Saporito R. A., Gerow K. G., Donnelly M. A. 2011. Contrasting colors of an aposematic poison frog do not affect predation. Ann. Zool. Fennici 48, 29–38 [Google Scholar]
- 32.Sinervo B., Lively C. M. 1996. The rock-paper-scissors game and the evolution of alternative male strategies. Nature 380, 240–243 10.1038/380240a0 (doi:10.1038/380240a0) [DOI] [Google Scholar]
- 33.Freitak D., Vanatoa A., Ots I., Rantala M. J. 2005. Formation of melanin-based wing patterns is influenced by condition and immune challenge in Pieris brassicae. Entomol. Exp. Appl. 116, 237–243 10.1111/j.1570-7458.2005.00330.x (doi:10.1111/j.1570-7458.2005.00330.x) [DOI] [Google Scholar]
- 34.Stoehr A. M. 2006. Costly melanin ornaments: the importance of taxon? Funct. Ecol. 20, 276–281 10.1111/j.1365-2435.2006.01090.x (doi:10.1111/j.1365-2435.2006.01090.x) [DOI] [Google Scholar]
- 35.Mappes J., Alatalo R. V., Kotiaho J., Parri S. 1996. Viability costs of condition-dependent sexual male display in a drumming wolf spider. Proc. R. Soc. Lond. B 263, 785–789 10.1098/rspb.1996.0117 (doi:10.1098/rspb.1996.0117) [DOI] [Google Scholar]
- 36.Rowe L., Houle D. 1996. The lek paradox and the capture of genetic variance by condition dependent traits. Proc. R. Soc. Lond. B 263, 1415–1421 10.1098/rspb.1996.0207 (doi:10.1098/rspb.1996.0207) [DOI] [Google Scholar]
- 37.Tomkins J. L., Radwan J., Kotiaho J. S., Tregenza T. 2004. Genic capture and resolving the lek paradox. Trends Ecol. Evol. 19, 323–328 10.1016/j.tree.2004.03.029 (doi:10.1016/j.tree.2004.03.029) [DOI] [PubMed] [Google Scholar]
- 38.Lindstedt C., Lindström L., Mappes J. 2009. Thermoregulation constrains effective warning signal expression. Evolution 63, 469–478 10.1111/j.1558-5646.2008.00561.x (doi:10.1111/j.1558-5646.2008.00561.x) [DOI] [PubMed] [Google Scholar]
- 39.Lindstedt C., Reudler Talsma J. H., Ihalainen E., Lindström L., Mappes J. 2010. Diet quality affects warning coloration indirectly: excretion costs in a generalist herbivore. Evolution 64, 68–78 10.1111/j.1558-5646.2009.00796.x (doi:10.1111/j.1558-5646.2009.00796.x) [DOI] [PubMed] [Google Scholar]
- 40.Seber G. A. F. 1973. The estimation of animal abundance. London: Charles Griffin & Co. Ltd. [Google Scholar]
- 41.Cuthill I. C., Stevens M., Sheppard J., Maddocks T., Parraga C. A., Troscianko T. S. 2005. Disruptive coloration and background pattern matching. Nature 434, 72–74 10.1038/nature03312 (doi:10.1038/nature03312) [DOI] [PubMed] [Google Scholar]
- 42.Vorobyev M., Osorio D. 1998. Receptor noise as a determinant of colour thresholds. Proc. R. Soc. Lond. B 265, 351–358 10.1098/rspb.1998.0302 (doi:10.1098/rspb.1998.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vorobyev M., Brandt R., Peitsch D., Laughlin S. B., Menzel R. 2001. Colour thresholds and receptor noise: behaviour and physiology compared. Vis. Res. 41, 639–653 10.1016/S0042-6989(00)00288-1 (doi:10.1016/S0042-6989(00)00288-1) [DOI] [PubMed] [Google Scholar]
- 44.Hart N. S., Partridge J. C., Cuthill I. C. 2000. Retinal asymmetry in birds. Curr. Biol. 10, 115–117 10.1016/S0960-9822(00)00297-9 (doi:10.1016/S0960-9822(00)00297-9) [DOI] [PubMed] [Google Scholar]
- 45.Cuthill I. C. 2006. Color perception. In Bird coloration. (eds Hill G. E., McGraw K. J.). Cambridge, MA: Harvard University Press [Google Scholar]
- 46.Stevens M. 2007. Predator perception and the interrelation between different forms of protective coloration. Proc. R. Soc. B 274, 1457–1464 10.1098/rspb.2007.0220 (doi:10.1098/rspb.2007.0220) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marples N. M., Roper T. J., Harper D. G. C. 1998. Responses of wild birds to novel prey: evidence of dietary conservatism. Oikos 83, 161–165 10.2307/3546557 (doi:10.2307/3546557) [DOI] [Google Scholar]
- 48.Weller S. J., Jacobson N. L., Conner W. E. 1999. The evolution of chemical defences and mating systems in tiger moths (Lepidoptera: Arctiidae). Biol. J. Linnean Soc. 68, 557–578 10.1111/j.1095-8312.1999.tb01188.x (doi:10.1111/j.1095-8312.1999.tb01188.x) [DOI] [Google Scholar]
- 49.Conner W. E. 2009. Tiger Moths and Woolly Bears—behaviour, ecology, and evolution of the Arctiidae, New York, Oxford University Press [Google Scholar]
- 50.Gittleman J. L., Harvey P. H. 1980. Why are distasteful prey not cryptic. Nature 286, 149–150 10.1038/286149a0 (doi:10.1038/286149a0) [DOI] [Google Scholar]
- 51.Riipi M., Alatalo R. V., Lindstrom L., Mappes J. 2001. Multiple benefits of gregariousness cover detectability costs in aposematic aggregations. Nature 413, 512–514 10.1038/35097061 (doi:10.1038/35097061) [DOI] [PubMed] [Google Scholar]
- 52.Kapan D. D. 2001. Three-butterfly system provides a field test of Müllerian mimicry. Nature 409, 338–340 10.1038/35053066 (doi:10.1038/35053066) [DOI] [PubMed] [Google Scholar]
- 53.Beatty C. D., Beirinckx K., Sherratt T. N. 2004. The evolution of Müllerian mimicry in multispecies communities. Nature 431, 63–66 10.1038/nature02818 (doi:10.1038/nature02818) [DOI] [PubMed] [Google Scholar]
- 54.Sandre S.-L., Stevens M., Mappes J. 2010. The effect of predator appetite, prey warning coloration and luminance on predator foraging decisions. Behaviour 147, 1121–1143 10.1163/000579510X507001 (doi:10.1163/000579510X507001) [DOI] [Google Scholar]
- 55.Prudic K. L., Skemp A. K., Papaj D. R. 2007. Aposematic coloration, luminance contrast, and the benefits of conspicuousness. Behav. Ecol. 18, 41–46 10.1093/beheco/arl046 (doi:10.1093/beheco/arl046) [DOI] [Google Scholar]
- 56.Darst C. R., Cummings M. E., Cannatella D. C. 2006. A mechanism for diversity in warning signals: conspicuousness versus toxicity in poison frogs. Proc. Natl Acad. Sci. USA 103, 5852–5857 10.1073/pnas.0600625103 (doi:10.1073/pnas.0600625103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Speed M. P., Ruxton G. D. 2007. How bright and how nasty: explaining diversity in warning signal strength. Evolution 61, 623–635 10.1111/j.1558-5646.2007.00054.x (doi:10.1111/j.1558-5646.2007.00054.x) [DOI] [PubMed] [Google Scholar]
- 58.Leimar O., Enquist M., Sillen-Tullberg B. 1986. Evolutionary stability of aposematic coloration and prey unprofitability: a theoretical analysis. Am. Nat. 128, 469–490 10.1086/284581 (doi:10.1086/284581) [DOI] [Google Scholar]
- 59.González A., Rossini C., Eisner M., Eisner T. 1999. Sexually transmitted chemical defense in a moth (Utetheisa ornatrix). Proc. Natl Acad. Sci. USA 96, 5570–5574 10.1073/pnas.96.10.5570 (doi:10.1073/pnas.96.10.5570) [DOI] [PMC free article] [PubMed] [Google Scholar]