Abstract
The capacity of species to track changing environmental conditions is a key component of population and range changes in response to environmental change. High levels of local adaptation may constrain expansion into new locations, while the relative fitness of dispersing individuals will influence subsequent population growth. However, opportunities to explore such processes are rare, particularly at scales relevant to species-based conservation strategies. Icelandic black-tailed godwits, Limosa limosa islandica, have expanded their range throughout Iceland over the last century. We show that current male morphology varies strongly in relation to the timing of colonization across Iceland, with small males being absent from recently occupied areas. Smaller males are also proportionately more abundant on habitats and sites with higher breeding success and relative abundance of females. This population-wide spatial structuring of male morphology is most likely to result from female preferences for small males and better-quality habitats increasing both small-male fitness and the dispersal probability of larger males into poorer-quality habitats. Such eco-evolutionary feedbacks may be a key driver of rates of population growth and range expansion and contraction.
Keywords: sexual selection, habitat quality, phenotype, morphology, black-tailed godwits, shorebirds
1. Introduction
The range size and distribution of species can be greatly influenced by a wide range of ecological, behavioural and historical processes [1,2], which may play a key role in facilitating or constraining future population size and range changes in response to environmental change [3,4]. Range expansion requires dispersal of individuals to occupy new locations. However, the probability of individuals dispersing into new locations is likely to be greatly influenced by both density-dependent pressures on resources within the occupied locations and the occurrence and strength of site fidelity [5]. Many species, particularly long-lived vertebrates, have evolved very high levels of site fidelity (e.g. [6]), and recruitment to the natal area is common, although often sex-specific (e.g. [7]). Consequently, dispersal distances are typically short for the majority of individuals [8]. Numerous empirical and theoretical studies have highlighted the costs of dispersal and the concomitant likelihood of selection for site fidelity (e.g. [5,9–11]). Range expansion may therefore be most probable when there is sufficient density-dependent pressure to overcome the benefits of site fidelity, resulting in density-dependent dispersal [12].
The success of range expansion events will be influenced by the fitness of dispersing individuals and the availability of resources in the sites into which they have dispersed. Strong selection for site fidelity may result in disproportionate dispersal of less successful individuals, for whom access to increasingly limited resources within occupied areas may be most severely constrained. Alternatively, dispersing individuals may be those better adapted to conditions in newly occupied locations or habitats (e.g. [13]). For example, changes in morphology of colonist butterflies [14], bush crickets [15] and cane toads [16] during range expansion have been associated with increased dispersal ability, and trade-offs between dispersal and reproduction may have constrained the range expansion in each case. However, among mobile vertebrates, evidence for variation in dispersal probability is scarce, primarily because of a lack of long-term studies of individuals during changes in population size or range.
The Icelandic black-tailed godwit, Limosa limosa islandica, is a migratory shorebird that has undergone a sustained population increase and range expansion over the last century [17], and for which long-term tracking of marked individuals is carried out throughout the migratory range [18,19]. This provides an opportunity to compare individuals in recently colonized and traditionally occupied locations throughout the breeding range. There is substantial variation in morphology among godwits, which may influence individual dispersal probability and the fitness consequences of occupying different locations. In order to assess the potential influence of godwit morphology on the probability of dispersal during the range expansion, we first compare the morphology of individuals currently breeding in areas colonized at different times during the range expansion. Secondly, as traditionally occupied areas comprise proportionately more good-quality breeding habitat than recently colonized areas, we explore the fitness implications of dispersal to new areas by assessing the relationships between godwit morphology, breeding density, sex ratio and productivity within and between habitats of varying quality. Finally, we consider the different eco-evolutionary mechanisms that may have shaped these relationships.
2. Methods
(a). Phenotypic variation among black-tailed godwits
Measurements of bill length (exposed culmen, millimetre) and wing length (maximum chord, millimetre) were obtained for 57 female and 56 male individual godwits captured during the breeding season, on migration or on the wintering grounds [20,21], and for which breeding location was known. In addition, as bill and wing length are only weakly positively correlated (females: r = 0.089, p = 0.51, n = 57; males: r = 0.198, p = 0.14, n = 56), we calculated the ratio of bill length to wing length as an estimate of proportional wing length; lower values of this index indicate smaller individuals with relatively long wings.
(b). Characteristics of breeding areas
From 2001 to 2003, godwit breeding ecology was studied on eight marsh and five dwarf-birch bog sites in southern Iceland, the largest breeding area of Icelandic black-tailed godwits. In marshes, godwits arrive earlier in spring, breed at higher densities, have higher productivity and both adults and chicks experience higher food abundance than in dwarf-birch bogs [17,22]. Mean breeding success (the proportion of pairs on each site that fledge one or more chicks; details in [17]) was used as an index of local-scale breeding habitat quality. Breeding density (godwits km−2) was estimated from the mean of the three maximum counts during the nesting period (details in [17]).
(c). Colonization of new breeding areas: a population-scale index of habitat quality
During the 20th century, the godwit population increased rapidly and colonized lowland basins around Iceland. Extensive collation of historical and contemporary accounts of godwit distribution [22] showed a pattern of colonization of lowland basins around Iceland that has followed a buffer effect, with individuals progressively colonizing basins with lower proportions of the favoured breeding habitat [17]. Colonization rank of different basins around Iceland can thus be used as an index of large-scale breeding habitat quality, from the oldest occupied areas comprising primarily higher-quality breeding habitat to the most recently occupied areas comprising primarily poorer-quality breeding habitat [22].
(d). Sex ratio variation among breeding locations and habitats
On capture, all birds were individually colour-ringed, their plumage characteristics and biometrics were recorded for sexing, and feathers were sampled for DNA analysis (details in [21]). The vast majority (greater than 95%) of individuals were sexed using plumage characteristics and display and copulation behaviour (most birds were in pairs), and DNA analysis confirmed sexing by these techniques in all cases. These methods were used to estimate site-specific sex ratios for nine breeding sites in southern Iceland that were studied in detail (see [17] for site details). Each site was visited one to two times per week during the peak breeding season (late May–early June), and the presence of males and females within mapped territories was recorded. Sex ratio was determined as the mean ratio of females to males in visits between 15 May and 15 June (usually three visits). As the vegetation structure of the breeding sites can vary (e.g. [17,23]), it is possible that different behaviours of the sexes might produce a habitat-related detection bias. To test this, we compared the resighting probability of marked individuals of each sex (18 males and 16 females) by calculating the proportion of visits to each site on which each individual was recorded.
(e). Natal philopatry
In total, just over 500 godwit chicks have been caught and individually marked in Iceland since 1999. Feather samples allowed these chicks to be sexed by DNA analysis [21]. Subsequent sightings of some of these individuals recruiting to the breeding population allowed comparison of the average natal dispersal distances of male and female godwits. Surveys of the breeding locations of marked godwits took place opportunistically across all of Iceland from 2000 to 2008, allowing differences in the natal dispersal distances of males and female godwits to be compared.
3. Results
(a). Phenotypic variation among areas colonized at different times
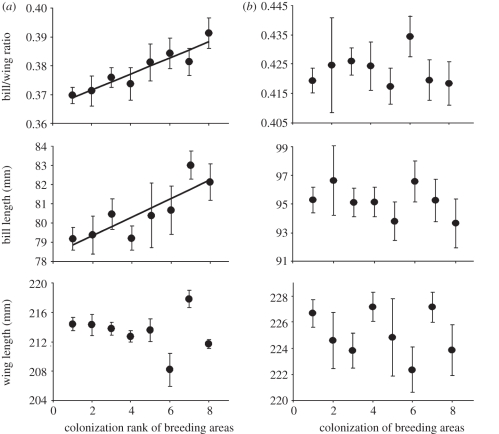
Across eight regions of Iceland ranging in date of first colonization from around 1900 to the 1990s, godwit morphology varies strongly among males, but not females (table 1 and figure 1). Male bill length and bill/wing ratio increase significantly with time since colonization (table 1), but no such relationships exist for females, which are larger than males. Among males, colonization history accounted for 88 per cent of the variation in bill/wing ratio (table 1). The variation in male morphology in relation to colonization history is primarily a consequence of small males being absent from recently occupied areas (figure 2).
Table 1.
Results of regression analyses of the relationships between (a) colonization dates of eight different regions of Iceland and (b) local-scale breeding success (percentage of pairs with fledged young per site), and phenotypic traits of male and female black-tailed godwits breeding in those areas. Significant (p < 0.05) relationships are highlighted in bold.
| r2 | p | ß | d.f. | |
|---|---|---|---|---|
| (a) pattern of breeding site colonization | ||||
| males | ||||
| wing | 0.03 | 0.662 | −0.205 | 7 |
| bill | 0.72 | 0.008 | 0.484 | 7 |
| bill/wing | 0.88 | 0.001 | 0.003 | 7 |
| females | ||||
| wing | 0.05 | 0.593 | −0.161 | 7 |
| bill | 0.18 | 0.302 | −0.184 | 7 |
| bill/wing | 0.01 | 0.868 | 0.000 | 7 |
| (b) local-scale breeding success | ||||
| males | ||||
| wing | 0.28 | 0.146 | −0.029 | 8 |
| bill | 0.64 | 0.010 | −0.042 | 8 |
| bill/wing | 0.77 | 0.002 | −14.95 | 8 |
| females | ||||
| wing | 0.14 | 0.251 | −0.026 | 10 |
| bill | 0.01 | 0.732 | −0.005 | 10 |
| bill/wing | 0.00 | 0.953 | −0.199 | 10 |
Figure 1.
Variation in morphometrics of (a) male and (b) female black-tailed godwits breeding in regions of Iceland that were colonized by godwits at different times throughout the 20th century (see table 1 for statistics). Region 1 has been occupied for the longest period and region 8 is the most recently colonized.
Figure 2.
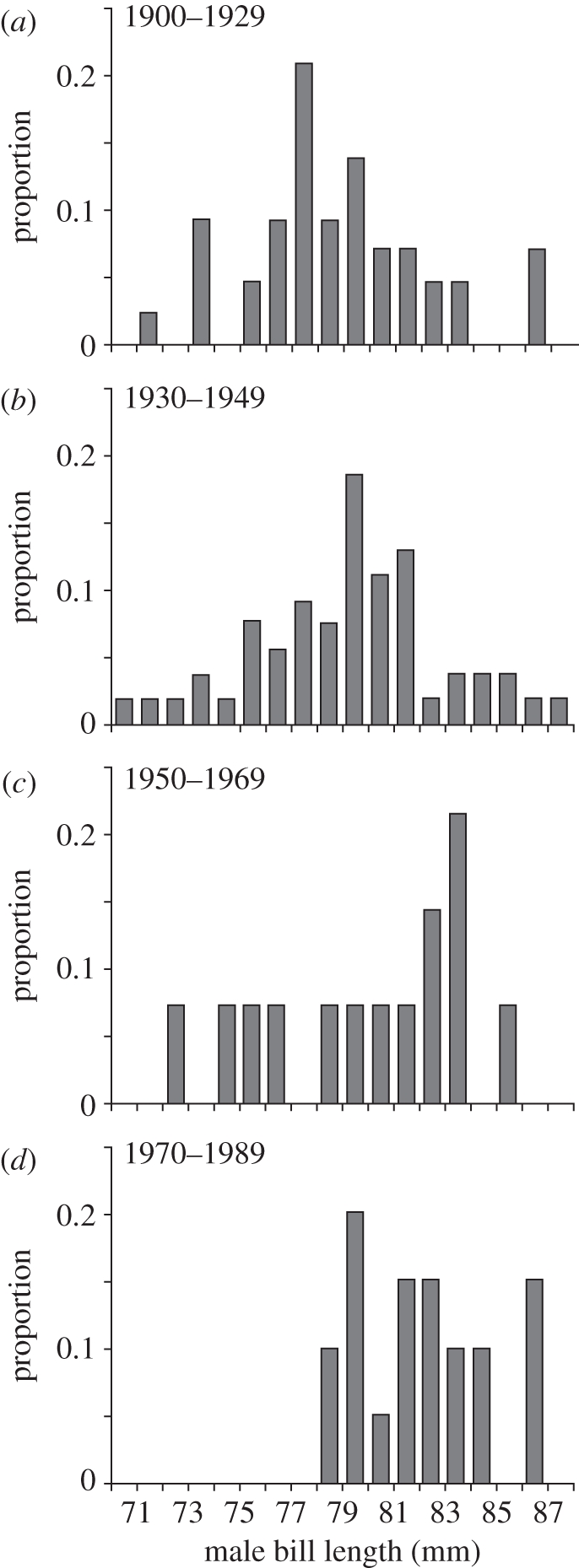
Frequency distributions of bill lengths of male black-tailed godwits breeding in regions of Iceland colonized during 1900–1929 (n = 43), 1930–1949 (n = 54), 1950–1969 (n = 14) and 1970–1989 (n = 20).
(b). Phenotypic variation in relation to breeding habitat quality
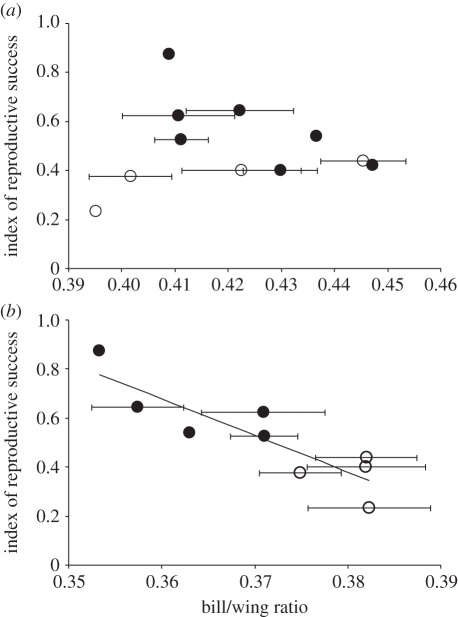
Recently occupied parts of Iceland have a greater proportionate abundance of dwarf-birch bog habitat than marshes, while traditionally occupied areas have proportionately more marsh habitat [22]. Across 13 sites in southern Iceland, breeding success (percentage of pairs fledging at least one chick) ranged from approximately 50 to 90 per cent on marsh habitats, and from approximately 20 to 40 per cent on dwarf-birch bog habitats. This variation in breeding success was unrelated to female morphology (table 1 and figure 3), but was strongly related to male morphology; males on more productive sites have significantly shorter bills and lower bill/wing ratios (table 1 and figure 3). Wings of males tended also to be shorter on more productive sites, although not significantly so, suggesting that males that are structurally smaller but with proportionately longer wings tend to occupy the more productive sites (table 1).
Figure 3.
Phenotypic variation of (a) female and (b) male black-tailed godwits in relation to average breeding success across a range of marsh (black circles) and dwarf-birch bog (white circles) sites in southern Iceland (see table 1 for statistics).
(c). Phenotypic variation in relation to sex ratios
The frequency with which individually marked godwits were observed during surveys did not differ between the two habitat types (marsh and dwarf-birch bog) nor between the sexes (G-test: G1 = 0.002, p = 0.96). These consistent detection probabilities allow sex ratios on sites to be estimated in the field as the mean ratio of males to females observed during surveys. On sites with a male-biased sex ratio, males tend to be larger and with proportionately shorter wings, whereas males on sites with a greater availability of females tend to be smaller with proportionately longer wings (figure 4). Female size and bill/wing ratio showed no relationship with sex ratio (r2 = 0.018, p = 0.69, n = 11).
Figure 4.
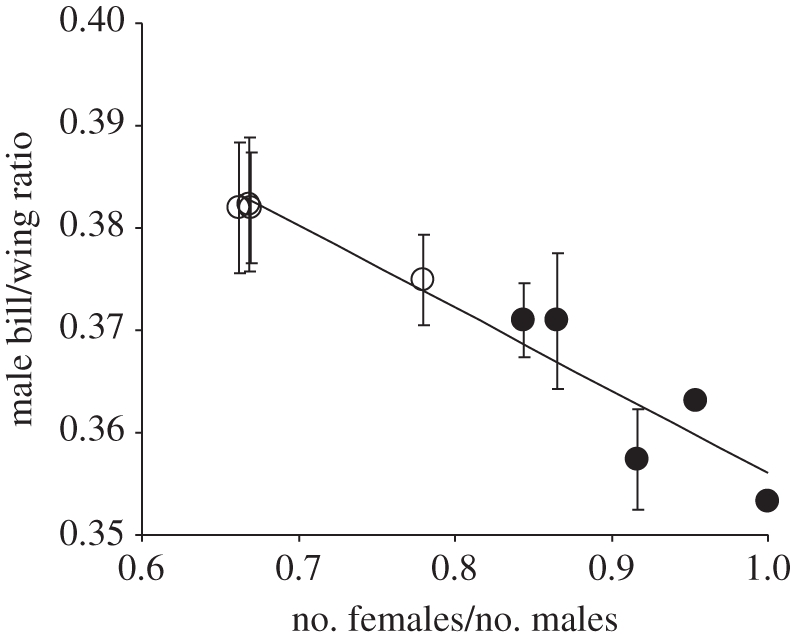
The relationship between the mean bill/wing ratio of male black-tailed godwits and the sex ratio on individual marsh (black circles) and dwarf-birch bog (white circles) sites in southern Iceland (y = −0.08x + 0.44, r2 = 0.93, p < 0.0001).
(d). Natal dispersal distances
Despite the challenge of locating recruitment sites of marked birds throughout the breeding range, and hence a small sample size, considerable differences in the natal dispersal distances of male and female godwits were apparent. Males dispersed an average of 2.3 km from their natal site (s.d. = 2.6, n = 7, range 0.5–7 km) while females dispersed an average of 48 km (s.d. = 47.8, n = 11, range 1–204 km; Mann–Whitney U-test: U = 12.0, p = 0.016).
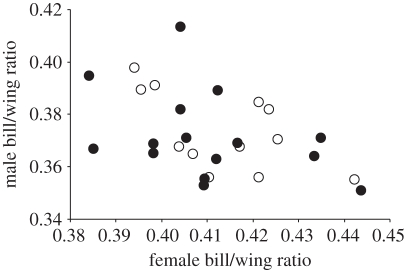
(e). Assortative pairing by phenotype
In total, morphological measurements were recorded for 27 pairs of godwits breeding at sites throughout the country and in both habitats. Overall, males with lower bill/wing ratio tended to pair with females with a larger bill/wing ratio, and the pattern was consistent across habitat types (figure 5).
Figure 5.
The association between the bill/wing ratio of paired male and female black-tailed godwits on marsh (black circles) and dwarf-birch bog (white circles) breeding habitats (r = −0.45, n = 27, p = 0.018).
4. Discussion
(a). Spatial structuring of phenotype distribution at different scales
The morphological variation exhibited by Icelandic black-tailed godwits is typical of many migratory shorebirds, and results from both sexual dimorphism and individual variation. Throughout the godwit population, the variation in male phenotype shows strong spatial structuring at a range of scales. Across the 13 intensively studied breeding sites in southern Iceland, males that occupy higher-quality breeding habitat have on average a lower bill/wing ratio than those in dwarf-birch bog habitats, the latter having lower average breeding success and relatively lower female abundance. Throughout the breeding range, smaller males are absent in the most recently colonized regions, where poor-quality dwarf-birch bog breeding habitat is more abundant. In contrast, females are larger than males and show a similar level of phenotypic variation, but no spatial structuring in relation to either local-scale breeding habitat quality or population-wide colonization patterns. As the godwits reported here are currently breeding in areas that have been colonized for differing time periods, the distribution of male phenotypes within individual areas is likely to result from phenotype-specific dispersal and recruitment. Although natal site fidelity is much stronger in males than females, the rapid range expansion means that male dispersal (probably primarily during recruitment) has clearly occurred repeatedly in recent decades. Although females also vary in body size and structure, their low level of natal philopatry would be likely to reduce any spatial structuring of morphology.
(b). Drivers of variation in the distribution of male phenotypes at local scales
Why are smaller, proportionately longer-winged males more common in sites with higher habitat quality and greater availability of females? If local adaptation of phenotypes to different habitats was producing this pattern (e.g. [13]), we might expect to see a clear step-divide in phenotype between the two habitat types, but linear trends in male size are apparent among individual study sites, across both habitat types and along a gradient of average breeding success. Although direct adaptation of male body size to habitat structure is possible, these linear trends suggest that this is unlikely to be the main driver of this pattern. Sexual selection, through competition for territories and/or mating opportunities is a more likely cause of the gradual change in male phenotype across habitats and along a gradient of local breeding success. For the Charadrii group of birds (shorebirds, seabirds and alcids), it has been hypothesized that male body size might respond more to sexual selection than female body size [24]. Among shorebirds, aerial displays by males are common [25–27] and, in one species—the dunlin Calidris alpina—smaller males have been shown to spend more time in the air and perform more costly displays [28]. In black-tailed godwits, increased competition as a consequence of the sustained increase in population size may therefore favour smaller males on higher-quality sites. The greater relative abundance of females on the higher-quality marsh breeding habitats suggests that successful competition among males for space on those habitats will also lead to increased mating opportunities. There is also some evidence for assortative mating, both within and between habitats, with smaller males tending to pair with larger females. As female size can be positively correlated with egg size and chick success in shorebirds (e.g. [29]), the fitness benefits for males pairing with larger females may skew the variance in breeding success even further in favour of small males.
(c). Large-scale patterns in male phenotype: mechanisms and implications
Throughout the godwit population, smaller males become proportionately less common as the population has colonized new, poorer-quality breeding areas (figure 1). This change has occurred within ecological time scales (e.g. within tens of generations or fewer), as this population increase has mostly taken place in the last few decades and over just a few generations [22]. A potential mechanism producing this pattern is larger males with proportionately smaller wings being less successful at attracting a mate (for example, through being less agile in display) on occupied sites, and thus more likely to disperse to new sites. As populations in newly occupied sites increase, the resulting density-dependent pressure on resources together with female preference for the smaller, more agile males may again result in higher dispersal probabilities for larger, less agile males, producing a gradient of male phenotype distributions in relation to site occupation history (figure 1). Evidence is accumulating that evolution can operate at such time scales with clear effects on population dynamics (e.g. [30,31]). Male characteristics that influence mating potential, mate choice and general access to resources, such as morphology or plumage traits, may be among those most likely to be subject to sufficient selection pressure to allow for such rapid evolutionary changes [30,32].
The Icelandic population of black-tailed godwits is experiencing strong seasonal matching of habitat quality and fitness across the migratory range, as the same individuals tend to occupy higher-quality habitats in both winter and summer, and thus experience both higher survival and greater breeding success [17]. The fitness inequality that this seasonal matching creates can reduce effective population size dramatically [17]. The skew in distribution of male body size across breeding habitats and areas of different quality may exacerbate the fitness inequality among males and thus reduce effective population size even further. These intricate interactions between ecological and evolutionary processes can thus greatly influence population level issues [33]. The range expansion of Icelandic godwits seems very likely to have been constrained by the fitness inequalities arising from female mate choice and the associated dispersal probabilities of males of differing phenotypes. Unravelling such eco-evolutionary feedbacks will be key in identifying and predicting population-level responses to environmental change.
Acknowledgements
We are indebted to the many hundreds of observers of marked birds for providing records of marked godwits, to Graham Appleton for fruitful discussions, and to Theunis Piersma and an anonymous referee for very helpful comments. W.J.S. was funded by Arcadia. The study was funded by NERC.
References
- 1.Buckley L. B., Urban M. C., Angilletta M. J., Crozier L. G., Rissler L. J., Sears M. W. 2010. Can mechanism inform species' distribution models? Ecol. Lett. 13, 1041–1054 10.1111/j.1461-0248.2010.01506.x (doi:10.1111/j.1461-0248.2010.01506.x) [DOI] [PubMed] [Google Scholar]
- 2.Guisan A., Thuiller W. 2005. Predicting species distributions: offering more than simple habitat models. Ecol. Lett. 8, 993–1009 10.1111/j.1461-0248.2005.00792.x (doi:10.1111/j.1461-0248.2005.00792.x) [DOI] [PubMed] [Google Scholar]
- 3.Atkins K. E., Travis J. M. J. 2010. Local adaptation and the evolution of species' ranges under climate change. J. Theor. Biol. 266, 449–457 10.1016/j.jtbi.2010.07.014 (doi:10.1016/j.jtbi.2010.07.014) [DOI] [PubMed] [Google Scholar]
- 4.Burton O. J., Phillips B. L., Travis J. M. J. 2010. Trade-offs and the evolution of life histories during range expansion. Ecol. Lett. 13, 1210–1220 10.1111/j.1461-0248.2010.01505.x (doi:10.1111/j.1461-0248.2010.01505.x) [DOI] [PubMed] [Google Scholar]
- 5.Bowler D. E., Benton T. G. 2005. Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biol. Rev. 80, 205–225 10.1017/S1464793104006645 (doi:10.1017/S1464793104006645) [DOI] [PubMed] [Google Scholar]
- 6.Newton I. 2008. Migration ecology of birds. London, UK: Academic Press [Google Scholar]
- 7.Clarke A. L., Sæther B. E., Røskraft E. 1997. Sex bias in avian dispersal: a reappraisal. Oikos 79, 429–438 10.2307/3546885 (doi:10.2307/3546885) [DOI] [Google Scholar]
- 8.Paradis E., Baillie S. R., Sutherland W. J., Gregory R. D. 1998. Patterns of natal and breeding dispersal in birds. J. Anim. Ecol. 67, 518–536 10.1046/j.1365-2656.1998.00215.x (doi:10.1046/j.1365-2656.1998.00215.x) [DOI] [Google Scholar]
- 9.Kokko H., Sutherland W. J. 2001. Ecological traps in changing environments. Ecological and evolutionary consequences of a behaviourally mediated Allee effect. Evol. Ecol. Res. 3, 537–551 [Google Scholar]
- 10.Peron G., Lebreton J. D., Crochet P. A. 2010. Breeding dispersal in black-headed gull: the value of familiarity in a contrasted environment. J. Anim. Ecol. 79, 317–326 10.1111/j.1365-2656.2009.01635.x (doi:10.1111/j.1365-2656.2009.01635.x) [DOI] [PubMed] [Google Scholar]
- 11.Starrfelt J., Kokko H. 2010. Parent-offspring conflict and the evolution of dispersal distance. Am. Nat. 175, 38–49 10.1086/648605 (doi:10.1086/648605) [DOI] [PubMed] [Google Scholar]
- 12.Sutherland W. J., Gill J. A., Norris K. 2002. Density dependent dispersal: concepts, evidence, mechanisms and consequences. In Dispersal (ed. Bullock J.), pp. 134–151 Oxford, UK: Blackwell Scientific Publishing [Google Scholar]
- 13.Calsbeek R., Irschick D. J. 2007. The quick and the dead: correlational selection on morphology, performance and habitat use in island lizards. Evolution 61, 2493–2503 10.1111/j.1558-5646.2007.00206.x (doi:10.1111/j.1558-5646.2007.00206.x) [DOI] [PubMed] [Google Scholar]
- 14.Hughes C. L., Hill J. K., Dytham C. 2003. Evolutionary trade-offs between reproduction and dispersal in populations at expanding range boundaries. Proc. R. Soc. Lond. B 270, S147–S150 10.1098/rsbl.2003.0049 (doi:10.1098/rsbl.2003.0049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simmons A. D., Thomas C. D. 2004. Changes in dispersal during species' range expansions. Am. Nat. 164, 378–395 10.1086/423430 (doi:10.1086/423430) [DOI] [PubMed] [Google Scholar]
- 16.Llewelyn J., Phillips B. L., Alford R. A., Schwarzkopf L., Shine R. 2010. Locomotor performance in an invasive species: cane toads from the invasion front have greater endurance, but not speed, compared to conspecifics from a long-colonised area. Oecologia 162, 343–348 10.1007/s00442-009-1471-1 (doi:10.1007/s00442-009-1471-1) [DOI] [PubMed] [Google Scholar]
- 17.Gunnarsson T. G., Gill J. A., Newton J., Potts P. M., Sutherland W. J. 2005. Seasonal matching of habitat quality and fitness in a migratory bird. Proc. R. Soc. B 272, 2319–2323 10.1098/rspb.2005.3214 (doi:10.1098/rspb.2005.3214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gill J. A., Norris K., Potts P. M., Gunnarsson T. G., Atkinson P. W., Sutherland W. J. 2001. The buffer effect and large-scale population regulation in migratory birds. Nature 412, 436–438 10.1038/35086568 (doi:10.1038/35086568) [DOI] [PubMed] [Google Scholar]
- 19.Gunnarsson T. G., Gill J. A., Atkinson P. W., Gélinaud G., Potts P. M., Croger R. E., Gudmundsson G. A., Appleton G. F., Sutherland W. J. 2006. Population-scale drivers of individual arrival times in migratory birds. J. Anim. Ecol. 75, 1119–1127 10.1111/j.1365-2656.2006.01131.x (doi:10.1111/j.1365-2656.2006.01131.x) [DOI] [PubMed] [Google Scholar]
- 20.Gunnarsson T. G., Potts P. M., Gill J. A., Croger R. E., Gelinaud G., Atkinson P. W., Gardarsson A., Sutherland W. 2005. Estimating population size in Icelandic Black-tailed Godwits Limosa limosa islandica by colour-marking. Bird Study 52, 153–158 10.1080/00063650509461385 (doi:10.1080/00063650509461385) [DOI] [Google Scholar]
- 21.Gunnarsson T. G., Gill J. A., Goodacre S., Gélinaud G., Atkinson P. W., Hewitt G., Potts P. M., Sutherland W. J. 2006. Sexing of Black-tailed godwits Limosa limosa islandica: a comparison of behavioural, molecular, biometric and field-based techniques. Bird Study 53, 193–198 10.1080/00063650609461433 (doi:10.1080/00063650609461433) [DOI] [Google Scholar]
- 22.Gunnarsson T. G., Gill J. A., Petersen A., Appleton G., Sutherland W. J. 2005. A double buffer effect in a migratory shorebird population. J. Anim. Ecol. 74, 965–971 10.1111/j.1365-2656.2005.00994.x (doi:10.1111/j.1365-2656.2005.00994.x) [DOI] [Google Scholar]
- 23.Gunnarsson T. G., Gill J. A., Appleton G. F., Gislason H., Gardarsson A., Watkinson A. W., Sutherland W. J. 2006. Large-scale habitat associations of birds in lowland Iceland: implications for conservation. Biol. Conserv. 128, 265–275 10.1016/j.biocon.2005.09.034 (doi:10.1016/j.biocon.2005.09.034) [DOI] [Google Scholar]
- 24.Lindenfors P., Szekely T., Reynolds J. D. 2003. Directional changes in sexual size dimorpishm in shorebirds, gulls and alcids. J. Evol. Biol. 16, 930–938 10.1046/j.1420-9101.2003.00595.x (doi:10.1046/j.1420-9101.2003.00595.x) [DOI] [PubMed] [Google Scholar]
- 25.Jönsson P. E., Alerstam T. 1990. The adaptive significance of parental role division and sexual size dimorphism in breeding shorebirds. Biol. J. Linn. Soc. Lond. 41, 301–314 10.1111/j.1095-8312.1990.tb00838.x (doi:10.1111/j.1095-8312.1990.tb00838.x) [DOI] [Google Scholar]
- 26.Lanctot R. B., Sandercock B. K., Kempenaers B. 2000. Do male breeding displays function to attract mates or to defend territories? The explanatory role of mate and site fidelity. Waterbirds 23, 155–164 [Google Scholar]
- 27.Miller E. H. 1983. The structure of aerial displays in three species of Calidrinae (Scolopacidae). Auk 100, 440–451 [Google Scholar]
- 28.Blomqvist D., Johansson O. C., Unger U., Larson M., Flodin L. A. 1997. Male aerial display and reversed sexual size dimorphism in the dunlin. Anim. Behav. 54, 1291–1299 10.1006/anbe.1997.0532 (doi:10.1006/anbe.1997.0532) [DOI] [PubMed] [Google Scholar]
- 29.Thompson P. S., Hale W. G. 1991. Age-related reproductive variation in the Redshank Tringa totanus. Orn. Scand. 22, 353–359 10.2307/3676508 (doi:10.2307/3676508) [DOI] [Google Scholar]
- 30.Carroll S. P., Hendry A. P., Reznick D. N., Fox C. W. 2007. Evolution on ecological time-scales. Funct. Ecol. 21, 387–393 10.1111/j.1365-2435.2007.01289.x (doi:10.1111/j.1365-2435.2007.01289.x) [DOI] [Google Scholar]
- 31.Herrel A., Huyghe K., Vanhooydonck B., Backeljau T., Breugelmans K., Grbac I., Van Damme R., Irschick D. J. 2008. Rapid large-scale evolutionary divergence in morphology and performance associated with exploitation of a different dietary resource. Proc. Natl Acad. Sci. USA 105, 4792–4795 10.1073/pnas.0711998105 (doi:10.1073/pnas.0711998105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schroeder J., Lourenço P. M., Hooijmeijer J. C. E. W., Both C., Piersma T. 2009. A possible case of contemporary selection leading to a decrease in sexual plumage dimorphism in a grassland-breeding shorebird. Behav. Ecol. 20, 797–807 10.1093/beheco/arp063 (doi:10.1093/beheco/arp063) [DOI] [Google Scholar]
- 33.Kokko H., López-Sepulcre A. 2007. The ecogenetic link between demography and evolution: can we bridge the gap between theory and data? Ecol. Lett. 10, 773–782 10.1111/j.1461-0248.2007.01086.x (doi:10.1111/j.1461-0248.2007.01086.x) [DOI] [PubMed] [Google Scholar]