Abstract
Peroxiredoxin 6 (Prdx6) is a pleiotropic oxidative stress-response protein that defends cells against reactive oxygen species (ROS)-induced damage. Curcumin, a naturally occurring agent, has diversified beneficial roles including cytoprotection. Using human lens epithelial cells (hLECs) and Prdx6-deficient cells, we show the evidence that curcumin protects cells by upregulating Prdx6 transcription via invoking specificity protein 1 (Sp1) activity against proapoptotic stimuli. Curcumin enhanced Sp1 and Prdx6 mRNA and protein expression in a concentration-dependent manner, as evidenced by western and real-time PCR analyses, and thereby negatively regulated ROS-mediated apoptosis by blunting ROS expression and lipid peroxidation. Bioinformatic analysis and DNA–protein binding assays disclosed three active Sp1 sites (−19/27, −61/69 and −82/89) in Prdx6 promoter. Co-transfection experiments with Sp1 and Prdx6 promoter–chloramphenicol acetyltransferase (CAT) constructs showed that CAT activity was dramatically increased in LECs or Sp1-deficient cells (SL2). Curcumin treatment of LECs enhanced Sp1 binding to its sites, consistent with curcumin-dependent stimulation of Prdx6 promoter with Sp1 sites and cytoprotection. Notably, disruption of Sp1 sites by point mutagenesis abolished curcumin transactivation of Prdx6. Also, curcumin failed to activate Prdx6 expression in the presence of Sp1 inhibitors, demonstrating that curcumin-mediated increased expression of Prdx6 was dependent on Sp1 activity. Collectively, the study may provide a foundation for developing transcription-based inductive therapy to reinforce endogenous antioxidant defense by using dietary supplements.
Keywords: reactive oxygen species, apoptosis, Prdx6, transcription factor, transactivation, cell survival
Many biologically relevant elements in the cellular and external environments, such as growth factors, chemicals and ultraviolet B (UVB) radiation, have been shown to initiate reactive oxygen species (ROS)-evoked deleterious signaling in cells by disruption of the antioxidant system and consequently of cellular homeostasis.1, 2, 3, 4, 5 Moreover, elevated levels of ROS have been reported in aging cells, in cells and tissues developing pathophysiology, and in tissue fluids, including the aqueous humor of cataract patients, potentially causing cell death or opacification of lens in vitro. Augmentation of the antioxidant defenses in the ocular lens has been shown to prevent or delay cataractogenesis.2, 6 Recent studies have demonstrated that a major event in the progression of age-associated disorders is the decline in expression and activity of natural antioxidant such as peroxiredoxin 6 (Prdx6).2, 4, 6, 7 However, how transcriptional machinery controls Prdx6 expression and how Prdx6 transcription could be modulated under normal physiological condition is obscure. Moreover, one of the key systems involved in maintenance of the intracellular redox state is the antioxidant defense system, which includes catalase, SODs, Gpx1, Trx and Prdxs. The Prdxs family of proteins comprises six members, Prdx1–6, each of which contains either one cysteine (1-Cys) or two (2-Cys). Redox-active Cys residues play a role in controlling intracellular ROS expression. Prdxs are important in maintaining many cellular functions, including redox control of transcription factors.6, 8 Importantly, Prdx6 (a 1-Cys Prdx), unlike classical glutathione peroxidase or other Prdxs, has the ability to reduce phospholipid hydroperoxides by such means as peroxidation of membrane phospholipids during oxidative stress, thereby controlling phospholipid turnover.2, 4, 9 Research using targeted inactivation of Prdx6 gene in under- and overexpression experiments and animal studies has shown that Prdx6 with GSH peroxidase and acidic Ca2+-independent phospholipase A2 activities is essential for cell survival.6, 7 In cells subjected to oxidative stress, Prdx6 expression is vitally important for survival.2, 3, 4
However, the potential for intracellular delivery of mature protein or DNA for therapeutic purposes has been limited owing to the impermeable nature of selective plasma membrane. Current therapies for age-related degenerative diseases have been jeopardized owing to several setbacks in DNA and/or protein delivery. Curcumin is a pharmacologically safe agent10, 11 with many activities including a powerful antioxidant function and anti-inflammatory properties.12, 13 This agent has been found to induce expression of the antioxidant enzymes in various cell types.14, 15, 16 Curcumin mediates its effects by modulating several important molecular targets, including transcription factors NF-κB, Ap1 and specificity protein 1 (Sp1).15, 17, 18, 19 Recently, curcumin has been shown to suppress NF-κB activation10, 14 and activate the transcription factor Sp1. Sp1 is a stress-inducible, antideath transcriptional factor with a wide spectrum of beneficial activity, which it exerts by binding to a GC-rich element (or GC-box) in the promoter of target genes.20 Importantly, Sp1 functionally cooperates with a large number of sequence-specific transcription factors such as NF-κB, Oct 1 and GATA-1.21 However, most studies related to identification of responsive elements in the 5′ region of human, mouse and rat Prdx6 gene promoter have described several redox-active transcription factors such as Sp1, Ap1, NRF2, NF-κB, HSF1 and LEDGF,3, 6 suggesting that Prdx6 gene is subjected to complex transcriptional regulation. These elements account for the transcriptional responses to oxidant and non-oxidant stimuli.
In this study, we demonstrate that curcumin significantly induced Sp1 mRNA and protein that physically and functionally bound to all three Sp1-responsive elements (GC-box) in 5′-proximal region of Prdx6 gene promoter in vitro and in vivo, and that curcumin enhanced Prdx6 transactivation. In addition, we showed that curcumin-mediated Sp1-enhanced activity was directly related to increased transcription of Prdx6 gene and thereby abundance of its mRNA and protein – a process that is involved in curcumin-mediated negative regulation of oxidative stress-induced death signaling in hLECs.
Results
Curcumin protected hLECs from UVB-, H2O2- or paraquat-induced cell injury
To determine an effective non-cytotoxic concentration(s), hLECs were treated with various concentrations (0–10 μM) of curcumin, and cell growth was assessed at different time points. After prolonged treatment (24, 48 or 72 h), hLECs treated with 5 μM had shown better growth, while cells treated with 10 μM showed growth inhibition (data not shown). Thus, 5 μM concentration of curcumin was chosen as the optimum effective dose and has been used throughout the study until and unless stated. In addition, to show the evidence of curcumin-mediated survival signaling, we utilized different sources to induced oxidative stress, UVB, H2O2 and paraquat.2, 22
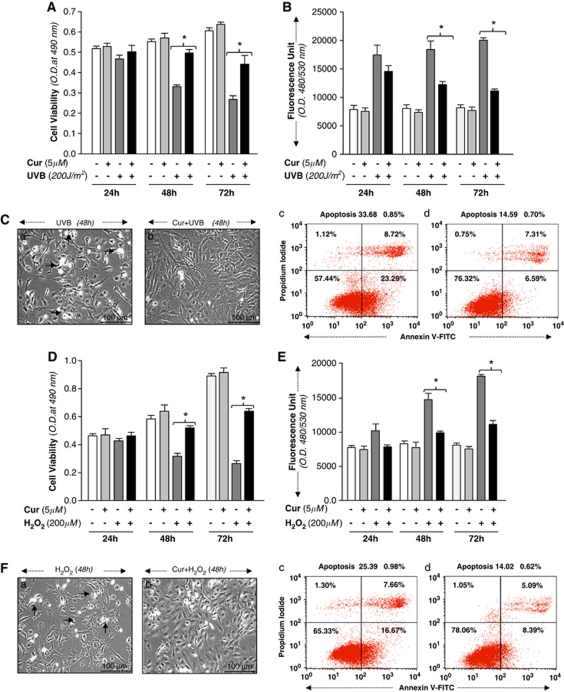
To examine whether curcumin blunted the apoptosis induced by UVB, we exposed curcumin-pretreated hLECs to variable doses of UVB radiation. Figures 1A and B illustrate time-dependent enhanced viability of hLECs (Figure 1A, dark gray bars versus black bars) and reduced expression of ROS (Figure 1B, dark gray bars versus black bars) with variable levels of UVB exposure (50, 100 or 200 J/m2) after 5 μM treatment with curcumin for 24, 48 or 72 h. Data revealed that curcumin, indeed, protected hLECs from UVB-induced stress as evidenced by photomicrographed (Figures 1Ca and b). We also conducted apoptotic cell assay. Curcumin-pretreated cells were exposed to UVB (200 J/m2), and thereafter were subjected to Annexin V-FITC binding assay, followed by FACS analysis. As shown in Figure 1C, the percentage of apoptosis increased in cells exposed to UVB (33.68±0.85%), while apoptosis was significantly reduced in the presence of curcumin (14.59±0.70). These data suggest that curcumin has the ability to attenuate UVB-induced cellular insults.
Figure 1.
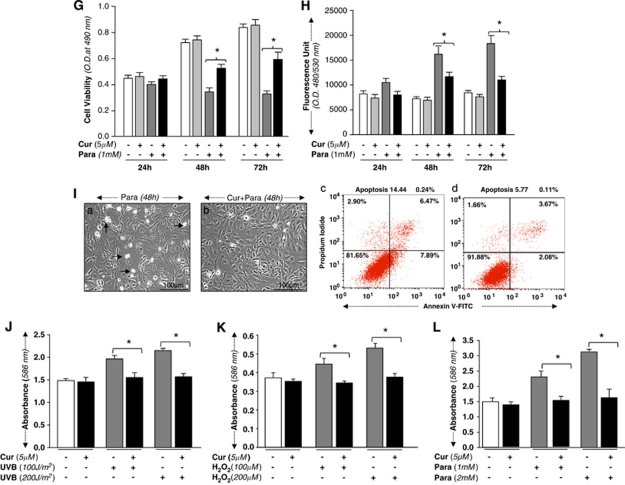
(A) Curcumin protected hLECs against UVB exposure, optimized ROS expression and reduced apoptotic cell death. Increase in survival of curcumin-treated hLECs exposed to UVB. Cells were treated with 5 μM of curcumin or dimethyl sulfoxide (DMSO) (a control vehicle). After 12 h, cells were submitted to UVB (200 J/m2), and effects on cell growth and viability were determined after 24, 48 and 72 h by MTS assay. (B) Effect of curcumin on lowering ROS expression. Cells were treated with DMSO or 5 μM of curcumin and after 12 h were exposed to UVB (200 J/m2). ROS expression was measured at 24, 48 and 72 h by replacing the medium with Hank's medium containing 10 μM H2-DCF-DA at Ex485/Em530 nm. (C, left panel) hLECs 7 × 105 were cultured and pretreated with DMSO (a) or curcumin (Cur; b) and then exposed to UVB (200 J/m2). After 48 h, cells were photomicrographed. Arrows indicate dead cells. (Right panel) Cells were trypsinized and the percentage of apoptotic cells was monitored by Annexin V-FITC staining, followed by fluorescence-activated cell sorter (FACS) analysis. A representative photomicrograph (left panel) and FACS analysis of Annexin V-FITC and PI staining were provided. Results are expressed as mean±S.D. for three replicate determinations for each treatment group, and were significant (P<0.001) compared with solvent (DMSO) control indicated by an asterisk (*). (D) Curcumin enhanced cell viability and blunted ROS expression and apoptotic cell death. hLECs 2 × 104 were cultured in 48-well plate and treated with curcumin (5 μM) or DMSO (control vehicle). Cells were subjected to H2O2 (200 μM). Cell viability was tested with MTS assay at 24, 48 and 72 h. (E) Curcumin attenuation of ROS induction by H2O2. hLECs 1 × 104 were cultured in 96-well plate, treated with curcumin (5 μM) and exposed to H2O2 (200 μM).7 ROS expression was quantified with H2-DCF-DA fluorescence dye at Ex485/Em530 nm with plate reader; results are presented as a histogram. (F) Curcumin negatively regulated the vulnerability of hLECs to H2O2-evoked apoptotic cell death. DMSO- or curcumin-treated hLECs were cultured and exposed to H2O2 (200 μM). After 48 h, cells were trypsinized and the percentage of apoptotic cells was monitored by Annexin V binding assay, followed by FACS analysis. (Left panel) Representative photomicrograph of H2O2-induced apoptosis in untreated (a) and curcumin-treated (b) cells. Arrow denotes dead cell. (Right panel) Representative FACS analysis of Annexin V-FITC and PI staining with control vehicle DMSO (c) and curcumin (d). Experiments were performed in triplicate and repeated at least three times, and the results are expressed as means±S.D. (*P<0.001). (G) Curcumin potentiated hLECs viability by blocking ROS generation and progression of apoptosis against paraquat-induced insults. Curcumin- or DMSO-treated hLECs cultured in 48-well plates containing medium were subjected to paraquat (1 mM), an oxidative stressor. Cell viability assay (MTS assay) was performed at 24, 48 and 72 h. The histogram is representative of three experiments. (H) Curcumin limited paraquat-induced ROS expression in LECs. hLECs cultured in 96-well plate were treated with DMSO (vehicle) or curcumin (5 μM), and submitted to H2-DCF-DA dye, ROS expression assay (24, 48 and 72 h) following paraquat addition as described earlier. The histogram is representative of fluorescence, which is directly proportional to intracellular ROS levels. (I) Curcumin protected hLECs from apoptotic cell death caused by paraquat-induced oxidative damage. Photomicrograph represents hLECs treated with DMSO (a) and curcumin (b) following paraquat addition. Arrow indicates white rounded dead cell (left panel). (Right panel) Representative FACS analysis of Annexin V-FITC and PI staining. hLECs were cultured and pretreated with curcumin (Cur) for 12 h and then exposed to paraquat (1 mM). After 48 h, cells were trypsinized and the percentage of apoptotic cells was monitored by Annexin V binding assay, followed by FACS analysis. Data were derived from three experiments and expressed as means±S.D. *P<0.001. (J) or (K) or (L) Curcumin delivery to cultured hLECs attenuated UVB-, H2O2- or paraquat-induced LPO process in hLECs. Cells were either treated with DMSO (a control vehicle) or curcumin (5 μM), or then exposed to stressors, UVB (J) or H2O2 (K) or paraquat (L) to generate oxidative stress. After 48 h, cells were assessed for levels of LPO using LPO assay as ascribed in commercial kit. Histogram values are mean±S.D. of three independent experiments. Asterisks indicate statistically significant difference (P<0.001 versus control). The results were derived from three experiments
To examine if curcumin would protect cells directly exposed to the most prevalent ROS, H2O2 and chemical paraquat, a producer of ROS, hLECs were treated with curcumin (5 μM). After 12 h, cells were subjected to H2O2 (200 μM) or paraquat (1 mM) for variable time intervals as shown in Figures 1D–I. Cell viability experiments showed that addition of curcumin in culture protected the cells against H2O2- and paraquat-induced death. Quantitation by staining with H2-DCF-DA dye6 established a lower prevalence of ROS in curcumin-treated cells exposed to either of the stressors (Figure 1E, dark gray bars versus black bars; Figure 1H, dark gray bars versus black bars). However, DCF fluorescence is not specific for H2O2, and other oxidants such as O2_ and NO may also oxidize H2DCF in DCF. Thus, measured fluorescence reflects overall oxidative stress in cells.23
Next, we examined and determined the type of cell death, performing Annexin V-FITC binding assay, followed by FACS analysis. Figures 1Fa and b and Figures 1Ia and b are representatives of the experiments showing photomicrographs taken after 48 h of exposure to stressors. Moreover, Annexin V-FITC and propidium iodide (PI) staining demonstrated that curcumin significantly inhibited apoptotic cell death induced by H2O2 or paraquat (Figures 1Fc versus d; Figures 1Ic versus d). In contrast, untreated cells were susceptible to identical oxidative stressors. As shown in Figures 1F and I, the percentage of apoptosis increased in cells exposed to H2O2, while the presence of curcumin significantly inhibited apoptosis (*P<0.001).
Curcumin inhibited UVB-, H2O2- or paraquat-induced lipid peroxidation and rescued hLECs
To test anti-lipid peroxidation (LPO) activity of curcumin in hLECs, cells untreated or pretreated with curcumin (5 μM) were exposed to UVB (100 or 200 J/m2) (Figure 1J), H2O2 (100 or 200 μM) (Figure 1K) or paraquat (1 or 2 mM) (Figure 1L). The cells were processed for LPO assay, which monitors levels of malondialdehyde (MDA) and 4-hydroxyalkenals in unstable lipid peroxide decomposition. As shown in earlier studies,24 LPO level was significantly retarded in treated cells compared to untreated controls (Figures 1J–L, gray bars versus black bars). Taken together, the data disclosed that curcumin has the potential to protect against LPO-induced cell injuries.
Prdx6-knockdown cells revealed that curcumin's protective efficacy was associated with Prdx6 expression
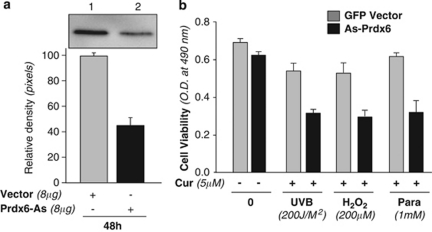
To assess if curcumin exerts its protective action, at least in part, by regulating Prdx6 expression, hLECs were transfected with Prdx6 antisense (Prdx6-As) or empty vector (Figure 2a).3, 25 After 48 h, cells were exposed to UVB or H2O2 or paraquat, and cell viability was determined by 3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2 to 4-sulfophenyl)-2H-tetrazolium salt (MTS) assay. Following normalization of transfection efficiency, data showed that the viability of even curcumin-treated cells with Prdx6-As was significantly decreased compared to that of cells transfected with vector (Figure 2, black bars versus gray bars).
Figure 2.
Reduced expression of Prdx6 affected the protective potential of curcumin against stressors. hLECs were transfected with Prdx6-As or empty vector.3, 25 After 48 h, cells of each group were pooled and harvested in 48-well plate, and subjected to stressors, followed by survival assay. A fraction of cells from each pool were used to assess the expression levels of Prdx6. (a) Western analysis of vector- (lane 1) and Prdx6-As- (lane 2) transfected cells. (b) Histogram showing the values of MTS assay of vector and Prdx6-As-transfected cells following curcumin treatment. Results are mean±S.D. of three experiments
hLECs treated with curcumin displayed enhanced expression of Prdx6 mRNA and protein
To test whether curcumin induces the expression of Prdx6 in hLECs, we investigated the effect of curcumin on the levels of Prdx6 mRNA and protein in hLECs. We treated hLECs with curcumin (2.5 or 5 μM) or vehicle containing Dulbecco's modified Eagle's medium (DMEM) medium. Real-time PCR analysis revealed the significant increase of Prdx6 mRNA in curcumin-treated cells (Figure 4b), and the maximum expression level could be detected at 5 μM curcumin concentration. Next, to determine whether Prdx6 protein was influenced by curcumin treatment, western analysis revealed increased expression of Prdx6 protein (Figure 4c, upper panel) after treatment, consistent with an increase of Prdx6 mRNA expression (Figure 4b).
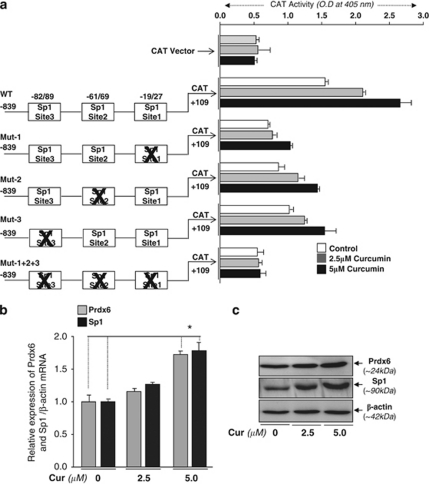
Prdx6 promoter contains three functional Sp1 sites and all sites contributes differentially in Prdx6 transcription, and responsive to curcumin treatment
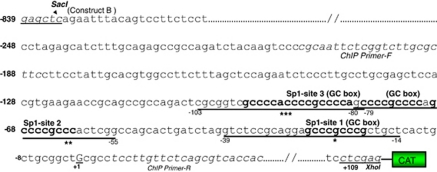
Because of our finding that curcumin induced the expression of Prdx6 mRNA, we analyzed putative transcription factor binding sites in Prdx6 promoter. In silico analysis of 5′-flanking region ranging from −839 to +109 disclosed that Prdx6 promoters contained three putative Sp1-response elements (GC-boxes) located at –19/27 (nGCCCGCCCGn), –61/69 (nCCCGCCCCGn) and –82/89 (nCCCCGCCCn) (Figures 3 and 4a). Based on the analysis of Sp1 sites, we sequentially engineered the mutant constructs mutated at each individual site, at two sites or at all three sites of wild-type chloramphenicol acetyltransferase (CAT) construct. Designated as Mut-1, Sp1-1; Mut-2, Sp1-2; Mut-3, Sp1-3 and Mut-1+2+3, these constructs were utilized for transactivation experiments in hLECs or Drosophila cell lines (SL2) (Figure 3).
Figure 3.
A construct linking the 5′-proximal regulator region of the Prdx6 promoter to CAT reporter gene. The sequence ranging from nucleotides −839 to +109 contains three putative Sp1 binding sites as predicted by MatInspector (Genomatix), a Web-based computer analysis program. The consensus sequences for the predicted Sp1 sites (G/C-boxes) are shown in bold. Asterisks denote sites of Sp1, and the sites were mutated to examine the binding affinity of each site to Sp1 and their contribution in promoter activity. Underlining is used to show the oligonucleotides employed in gel-shift and gel-shift deletion assays. Nucleotides in italics reflect primer pair used for ChIP experiments. The transcription start site is indicated by +1, and SacI and XhoI restriction sites used for marking Prdx6-CAT-constructs are shown in bold
Figure 4.
(a) Induction of Sp1-mediated transcriptional activation on the Prdx6 gene promoter by curcumin in hLECs. (Left half) Schematic representation of Sp1-site-directed mutants of Prdx6 promoter linked to CAT. (Right half) CAT activity of the mutant constructs (Mut 1 to Mut 1+2+3) and empty CAT vector in hLECs untreated (open bars) and curcumin-treated (2.5 μM, gray bars; 5 μM, black bars). Values of empty CAT vector were insignificant. All data are presented as the mean±S.D. derived from three independent experiments. Curcumin-mediated increased expression of Prdx6 mRNA and protein was Sp1 expression-dependent. hLECs were treated with 2.5 and 5 μM of curcumin or dimethyl sulfoxide (DMSO) (control vehicle). After 48 h, RNA and protein were isolated and real-time polymerase chain reaction (PCR) (b) and western analysis (c) were performed. (a) Histogram represents the data mean±S.D. obtained from three independent experiments. (c) Expression levels of Prdx6 (upper panel) and Sp1 (middle panel) following the treatment with curcumin. Lower panel shows the protein bands of β-actin. *P<0.001
To examine whether curcumin-mediated increased expression of Prdx6 transcription is Sp1-dependent, hLECs transfected with wild-type CAT or its various Sp1 site-specific mutant constructs linked to CAT or empty CAT vector (Figure 4a) were treated with curcumin at levels of 2.5 or 5 μM, and processed for CAT assay. Transfection with constructs with mutation at either site of Sp1-response elements showed inhibition of CAT activity, and that activity was Sp1-response element-dependent (Figure 4a). Importantly, the CAT activity of Prdx6 promoter with all disrupted Sp1 binding elements was significantly reduced and that was indistinguishable from CAT-vector values (Figure 4a). Furthermore, a decline in promoter activity due to disruption of one or more Sp1 sites (Mut-1, Mut-2, Mut-3 or Mut-1+2+3) suggested that all Sp1 sites were functional and contributed to controlling Prdx6 promoter activity. In parallel experiment, we examine whether curcumin alters the transcriptional activity of Prdx6 promoter. Results showed that treatment of curcumin promoted the transcriptional activity of wild-type Prdx6 promoter in LECs, but failed to promote transactivation of mutant Prdx6 promoter disrupted at Sp1 site(s) (Figure 4a, gray and black bars), suggesting that curcumin induced Prdx6 transcription via activation of Sp1.
Curcumin-mediated increased coexpression of Prdx6 and Sp1 mRNA and protein
To determine whether hLECs treated with curcumin displayed increased expression of endogenous Prdx6 mRNA and protein, hLECs were treated with curcumin at dose levels of 2.5 and 5 μM for 48 h. As expected, the expression of Sp1 (Figure 4b, black bar) and Prdx6 (Figure 4b, gray bar) was significantly increased in cells treated with curcumin. Next, we measured Sp1 and Prdx6 protein levels in curcumin-treated cells. The expression of Sp1 as well as Prdx6 was increased, and the increased expression was clearly curcumin concentration-dependent (Figure 4c, Sp1, middle panel; Prdx6, upper panel).
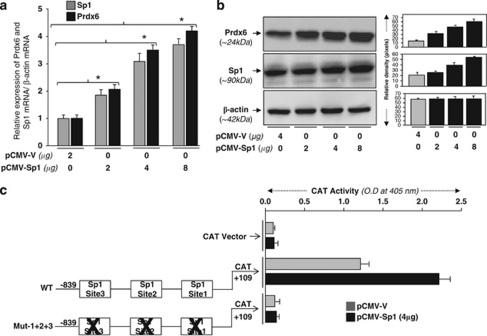
Upregulation of Prdx6 expression is Sp1 expression-dependent, and Sp1 is a regulator of Prdx6 transcription
To examine whether cells overexpressed with Sp1 displayed higher expression of endogenous Prdx6, and increasing amounts of pCMV-Sp1 co-transfection increased Prdx6 promoter activity, hLECs were overexpressed with increasing concentrations of pCMV-Sp1 (0, 2, 4 and 8 μg) or empty pCMV vector. Real-time PCR analysis showed a higher level of Prdx6 mRNA in Sp1-overexpressing cells, which was increased with increasing abundance of Sp1 (Figure 5a, gray bar and black bar; 0 versus 2 versus 4 versus 8 μg). Next, we performed western analysis using Prdx6, Sp1 and β-actin antibodies as described in ‘Materials and Methods'. Results showed Sp1-mediated upregulation of Prdx6 gene, in a dose-dependent manner. Next, we also tested the effect of Sp1 overexpression on Prdx6 promoter activity. hLECs were co-transfected with wild-type Prdx6 promoter (Figures 3 and 4a) linked to CAT or promoter constructs mutated at all Sp1 sites (Mut-1+2+3) along with pCMV-Sp1 expression construct (4 μg). Figure 5c shows that Sp1 overexpression (4 μg) significantly enhanced transcription of Prdx6 promoter (wild-type, black bar). This activation effect of Sp1 was ablated for mutant promoter (Figure 5c), demonstrating that the Sp1-responsive element in Prdx6 promoter was responsible for Sp1 activity.
Figure 5.
LECs overexpressing Sp1 displayed elevated expression of Prdx6 mRNA and protein. hLECs were transfected either with pCMV-vector (4 μg) or pCMV-Sp1 (2, 4 and 8 μg). RNA and protein were isolated at 48 h and were used to conduct real-time polymerase chain reaction (PCR) (a) and western analysis (b), respectively, using Prdx6-specific probes. (a) Histogram showing the values mean±S.D. obtained from three experiments. (b, left panel) Western analysis data showing the expression levels of Prdx6 (upper panel) in cells transfected with Sp1 plasmid at different concentrations (middle panel). (Lower panel) Membrane probed with β-actin antibody. The same membrane was probed and reprobed with antibodies following stripping and restriping to obtain relative expression of Sp1 or Prdx6 or β-actin. (Right panel) Histogram displays relative-protein band density. (c) hLECs co-transfected with Sp1 showed increased CAT activity of Prdx6 promoter. Cells were co-transfected with Prdx6-CAT or its mutant at all three Sp1 sites (4 μg) and pCMV-Sp1 (4 μg). After 72 h, CAT-ELISA was performed. (Right panel) Histogram represents the results from three independent experiments. Values of empty CAT vector were insignificant. Left panel configures the identity of wild-type and mutant constructs of Prdx6 promoter. All data are presented as the mean±S.D. from at least three independent experiments. Asterisk denotes statistically significant difference (P<0.001)
Sp1-deficient cells, SL2 and artemisinin and mithramycin A, inhibitors of Sp1, confirmed that Sp1 is a transactivator of Prdx6 promoter
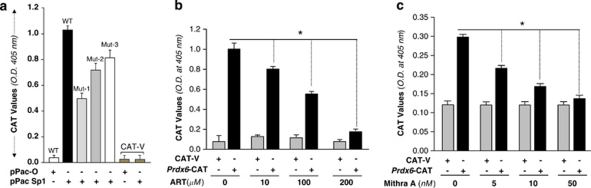
To examine and verify transcriptional activation of the Prdx6 promoter by Sp1, we performed transient co-transfection experiments in Sp1-deficient Drosophila Schneider (SL2) cells with the expression vector pPac-Sp1, encoding Sp126 (purchased from Addgene, Cambridge, MA, USA) and WT-Prdx6-CAT or Mut-1 or Mut-2 or Mut-3 (Figures 3 and 4a) reporter construct. Figure 6a shows the Sp1-dependent transcriptional activity of Prdx6 promoter in SL2 cells. Coexpression of Sp1 resulted in a ∼10-fold increase in CAT activity of WT promoter. In contrast, CAT activity of mutant promoter was reduced significantly, and each mutant promoter (disrupted Sp1 sites) displayed its contribution in transactivation of Prdx6 promoter (Figure 6a, gray bars).
Figure 6.
Activation of Prdx6-CAT promoter in Sp1-deficient Schneider cells (SL2) when expressed with Sp1 by pPac-Sp1 transfection. (a) SL2 cells were co-transfected with pPac-Sp1or pPac-0 (1 μg) along with Prdx6-CAT (2 μg) or its mutant promoter at Sp1 sites. After 48 h, CAT activities were measured. The histogram represents the results from four independent experiments. (b and c) ART and Mithra-A, inhibitors of Sp1, inhibit transcriptional activity of Prdx6 gene promoter in hLECs. Cells were transfected with Prdx6-CAT or empty CAT vector construct and treated with ART (b) or Mithra-A (c) at different concentrations. The Histogram represents the values derived from experiments. Transfection efficiencies were normalized with a plasmid secreted alkaline phosphatase basic vector. The data represent the mean±S.D. from three independent experiments. Asterisks indicate statistically significant differences (P<0.001)
To further determine whether Sp1 activates Prdx6 transcription through its responsive elements (GC-boxes), we applied the Sp1 inhibitors artemisinin (ART) and mithramycin A (Mithra-A)27, 28 and tested the effect of these agents on the CAT activity controlled by Prdx6 promoter (Figures 6b and c). hLECs were transiently transfected with WT-Prdx6-CAT promoter or empty CAT vector, and treated with increasing concentrations of ART and Mithra-A (Figures 6b and c). As shown in Figures 6b and c, ART and Mithra-A at dose levels of 200 μM and 50 nM, respectively, both showed a strong concentration-dependent inhibition of Prdx6 promoter transcription (black bars).
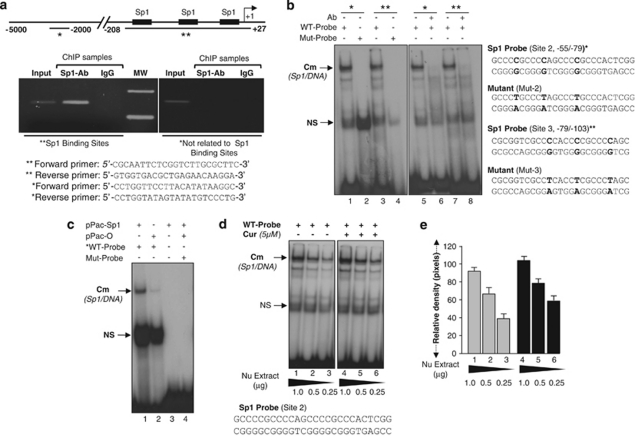
In vivo and in vitro DNA–protein binding assays disclosed that Sp1 directly and specifically bound to its response elements in the Prdx6 promoter
To study further whether Sp1 directly binds to putative Sp1-responsive site(s) (GC-box) present within specific regions of endogenous Prdx6 promoter, chromatin immunoprecipitation (ChIP) assay was performed with crosslinked nucleoproteins isolated from hLECs and antibody specific to Sp1. Transcription factor(s) contained in chromatin could be immunoprecipitated by Sp1 antibody, but not precipitated with nonspecific IgG used as control. Next, DNA isolated from immunoprecipitates was analyzed by specific primers. Figure 7a shows that, in comparison to control IgG, the Prdx6 promoter sequence was enriched and detected in the Sp1-Ab precipitates. In contrast, the Prdx6 promoter was not detected in precipitates with control IgG.
Figure 7.
(a) ChIP analysis revealed that Sp1 bound to Prdx6 promoter in vivo. ChIP assay was carried out by using ChIP-IT Express (Active Motif, Carlsbad, CA, USA). (Upper half) Schematic illustration of proximal promoter region of Prdx6-containing Sp1 binding sites. Chromatin samples prepared from LECs subjected to ChIP assay with a ChIP grade antibody against Sp1 or control IgG. The DNA fragments were used as templates and amplified by using primers designed to amplify −208 to +27 region of the Prdx6 promoter bearing Sp1 sites (**) and contiguous sequence (−2229 to −2356) to which Sp1 does not bind (*). Polymerase chain reaction (PCR) products were resolved into agarose gel and visualized with ethidium bromide staining. (Lower panel) Photograph of the amplified DNA band visualized with ethidium bromide staining. MW, molecular weight marker. (Lower half) Primers used for amplification of specific region containing Sp1 sites (**) and not related to Sp1 binding Sp1 sites (*). (b) Sp1 in the nuclear extract of hLECs bound directly to its responsive elements in the Prdx6 promoter. (Left panel) Gel-shift assay was conducted with the nuclear extracts isolated from hLECs and radiolabeled probes derived from Prdx6 promoter containing Sp1 sites. A Cm (Sp1/DNA) complex occurred (lanes 1, 3, 5 and 6) with wild-type (WT) probes; in contrast, no complex formation could be detected with mutant probe (lanes 2 and 4). (Right panel) Antibody depletion assay showing ablation of DNA and Sp1 complex (Cm). Nuclear extracts isolated from cells were incubated with antibody specific to Sp1 at 4°C. Following centrifugation, extracts were processed for gel-shift assay. No band was detected when Sp1-specific antibody was added to deplete Sp1 in nuclear extract with either of the probes with Sp1 sites (lanes 6 and 8). Bold bases denote mutated base(s). (c) Protein extracted from SL2 cells expressed with Sp1 bound to Sp1-responsive elements in Prdx6 promoter. Sp1-deficient SL2 cells were transfected with pPac-Sp1 or pPac-0 plasmids. Protein was extracted and processed with radiolabeled probes containing Sp1 sites. Reaction mix was subjected to gel-shift assay. A Cm complex was formed with extract from pPac-Sp1-transfected cells (lane 1); in contrast, no complex was evident with extracts from pPac-0-transfected cells or mutant probes (lanes 2–4). (d) Curcumin ameliorated the interaction of Sp1 with its responsive elements in the Prdx6 promoter. hLECs were cultured in the presence of dimethyl sulfoxide (DMSO) (control vehicle) or curcumin and nuclear extracts were isolated as described, and were processed for gel-shift assay using radiolabeled probes with Sp1 sites. A strong Cm (Sp1/DNA) complex occurred in cells treated with curcumin (lanes 4–6), in comparison to untreated cells (lanes 1–3). (e) Histogram represents densitometry analysis of DNA–protein complex
Sequence analyses as well as the above data demonstrated that Prdx6 promoter spanning from −208 to +27 is rich in GC and contains three GC-boxes, Sp1 binding sites. We designated these Sp1-1 (Site 1), Sp1-2 (Site 2) and Sp1-3 (Site 3) (Figure 3). Chemically synthesized oligos containing each putative GC-box element as well as their corresponding mutants (Figure 7b) were radiolabeled (32p) and were used to ascertain if Sp1 in nuclear extract directly and specifically bound to each site present in Prdx6 promoter. Gel-shift assay revealed that nuclear extracts from hLECs interacted with each probe containing Sp1 site(s) derived from Prdx6 promoter and formed shifted bands Cm with wild-type probe(s) (Figure 7b, lanes 1, 3, 5 and 7). Binding was totally eliminated when Sp1-depleted nuclear extract (preabsorbed with antibody specific to Sp1) was added to the reaction mixture (lanes 6 and 8).
Furthermore, we also performed gel-shift mobility assay using SL2 cells following pPac-Sp1 or pPac-0 (empty vector). Gel-shift experiments with protein extract isolated from Sp1-deficient SL2 cells following transfection with pPac-Sp1 or vector, pPac-0, showed that Sp1-transfected extract bound to probe derived from Prdx6 promoter-containing Sp1 site. A Cm complex occurred between WT probe and nuclear extract from Sp1 transfectants (Figure 7c, lane 1). The data revealed that Sp1 in nuclear extract directly and specifically bound to each of three sites.
Curcumin induced enhanced binding activity of Sp1 to Prdx6 promoter
To determine whether curcumin-mediated protective activity in hLECs involves an increase of DNA-binding activity of Sp1, nuclear extracts from untreated and curcumin-treated cells were examined in gel-shift mobility assay. We randomly selected an oligomer probe containing Sp1 sites (Site 2) derived from Prdx6 promoter (Figures 7b and d) and utilized it to examine DNA-binding affinity of Sp1 in nuclear extract isolated from curcumin-treated cells. Nuclear extract from curcumin-treated cells formed a stronger shifted complex designated as Cm (Figure 7d, lane 4). Titration of nuclear extracts from both treated and untreated cells and their binding to Sp1 probe further indicated that nuclear extracts from curcumin-treated cells had greater binding activity (Figure 7b, lanes 1–3 versus 4–6). A band (NS) appeared in all lanes and is nonspecific and demonstrated that not all nuclear proteins in curcumin-treated cells were altered.
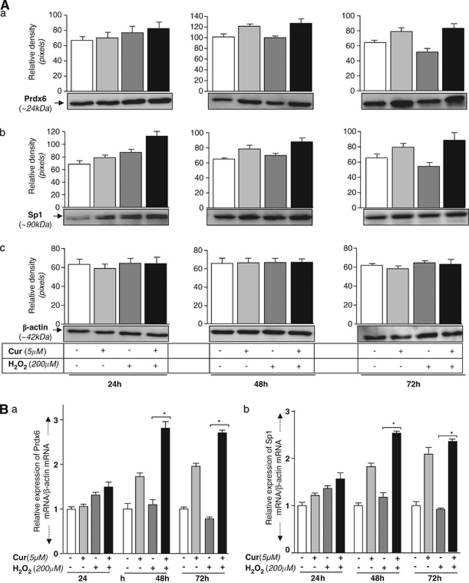
Oxidative stressors did not alter curcumin's ability to upregulate Sp1-dependent Prdx6 protein and mRNA
To investigate whether redox environment alters curcumin-induced Sp1 regulation of Prdx6 expression, we submitted untreated and curcumin-treated cells to oxidative stresses resulting from H2O2 (200 μM), paraquat (1 mM) or UVB (200 J/m2) exposure for variable time periods (24, 48 and 72 h). Western analysis of protein extracted from cells revealed that Prdx6 protein was upregulated, and the upregulation was Sp1 expression-dependent (Figures 8Aa and b, black bar). Next, we tested Prdx6 mRNA level using real-time PCR. As expected, cells treated with curcumin showed increased Prdx6 as well as Sp1 mRNA expression (Figures 8Ba and b, black bars) in a time-dependent manner. However, significantly increased Prdx6 and Sp1 mRNA were noticeable in cells co-treated with curcumin and H2O2 (48 h, black bars). We think that higher expression of Prdx6 or Sp1 may be associated with a coordinated effect of oxidative stress and curcumin, as oxidative stress may stimulate similar survival pathway(s) by cells, conferring resistance against stressors during an acute phase of stress. Similar results were obtained when cells were exposed to UVB or paraquat (data not shown).
Figure 8.
Curcumin promoted Sp1-dependent increased expression of Prdx6 protein and mRNA in hLECs facing oxidative stress. (A) Cells were exposed to 5 μM curcumin or control vehicle (dimethyl sulfoxide (DMSO)) and submitted to H2O2- (200 μM)induced oxidative stress. Cell extracts were isolated for 24, 48 and 72 h, and resolved on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by western analysis with Prdx6 (a), Sp1 (b) or β-actin (c) antibody. The histogram displays relative-protein band density. (B) Real-time polymerase chain reaction (PCR) showing expression levels of Prdx6 and Sp1 mRNA. RNA was isolated from untreated or curcumin-treated hLECs at 24, 48 or 72 h following H2O2 exposure. Real-time PCR was carried out using specific primers corresponding to Prdx6 (a) or Sp1 (b) or β-actin as internal control. Values are represented as a histogram, obtained from three independent experiments. Asterisk denotes statistically significant difference (P<0.001). Similar results were obtained from the experiments with UVB and paraquat (data not shown)
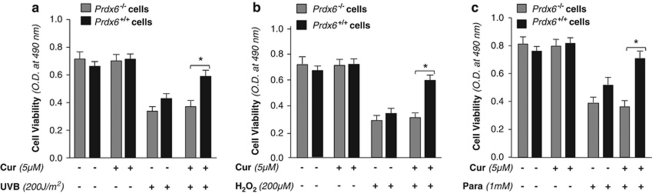
Curcumin failed to exert its protective effect against stressors in cells lacking Prdx6
To test if induction of naturally occurring Prdx6 by curcumin is responsible for curcumin-mediated protection of LECs, cells deficient in Prdx6 should be less able to resist oxidative stresses, we conducted survival assay in LECs derived from Prdx6 knockout mice (Prdx6−/−) and wild-type (Prdx6+/+) cells subjected to oxidative stressors, UVB, or H2O2 or paraquat for 48 h. As reported earlier,2, 3, 6 MTS assay revealed that Prdx6−/− cells were more susceptible to all of the stressors, and curcumin treatment did not produce protection against the oxidative stress (Figures 9a–c, gray bars). In contrast, significant curcumin-mediated cellular protection was observed in Prdx6+/+ cells (Figure 9, black bars), suggesting that Prdx6 is an essential molecule for curcumin-mediated cellular protection during stress, at least for LECs.
Figure 9.
Prdx6-deficient cells provided evidence that curcumin exerted its cytoprotective action via upregulation of Prdx6 expression in hLECs facing oxidative stresses. Wild-type (Prdx6+/+) and knockout (Prdx6−/−) LECs were cultured in 48-well plate containing DMEM medium with or without curcumin (5 μM). Cells were submitted to oxidative stressors UVB (200 J/m2) (a), or H2O2 (200 μM) (b) or paraquat (1 mM) (c). Following incubation for 48 h, viability of cells was assessed using MTS assay.2 Representative histograms displaying relative viability of untreated or curcumin-treated Prdx6−/− (gray bars) and Prdx6+/+ (black bars) LECs following oxidative stress. Asterisk denotes statistically significant difference (P<0.001)
Discussion
The abnormal processes induced by ROS-driven oxidative stress may be a major mechanism underlying abnormal physiological changes in cells that have reduced levels of cytoprotective proteins, such as Prdx6.2, 3, 6, 7, 9 Considering the well-established cytoprotective potential of Prdx6,3, 4, 6, 7, 9, 29 we studied the underlying mechanism of Prdx6 regulation and ways in which Prdx6 might be used for therapeutic intervention. Abundant evidence now exists showing that consumption of some natural compounds, including curcumin, reduces the risk of ROS-mediated disorders.14, 15, 30, 31 We found that a low concentration (5 μM) of curcumin is able to mount protection of LECs against a variety of oxidative stressors (UVB or H2O2 or paraquat), and acts by optimizing ROS levels and thereby inhibiting apoptosis (Figure 1). Our recent studies have shown that Prdx6 deficiency is a cause of initiation of ER stress, and deficient cells undergo spontaneous apoptosis. However, attenuation of apoptotic signaling in these cells as well as delaying cataractogenesis in animal model system by delivery of Prdx62, 3, 6, 7, 9, 32 clearly demonstrates the biological importance of Prdx6. Curcumin exercises its protective function by inducing the expression of antioxidant enzymes in various cell types.14, 15, 33 We found that curcumin activates Prdx6 expression in hLECs (Figures 4b and c).
The antioxidant mechanism of curcumin has been the subject of debate for more than a decade. In our investigation, curcumin-treated cells showed significant increase in Prdx6 mRNA, which was dose-dependent (Figures 4b and c). Analysis of Prdx6 gene promoter revealed the presence of three functional Sp1 sites (Figures 3 and 4a). However, Sp1-Site 1 has greater activation potential than Sites 2 and 3 (Figure 4a). This different activation potential may be explained by diversity in Sp1 core consensus elements that affects the binding affinity of Sp1 protein to each site, or, probably, recruitment of other cofactors to the sites that may cooperatively and coordinately participate in controlling Prdx6 transcription, depending on cellular requirements.34, 35 Furthermore, in vivo DNA and protein binding experiments (ChIP assay) with antibody specific to Sp1 revealed that Sp1, indeed, binds specifically to GC-rich fragments spanning from −208 to +27 nts containing GC-boxes. A gel-shift assay further showed that Sp1 binds directly to all three Sp1 response elements (Site 1: 19nGCCCGCCCGn27; Site 2: 61nCCCGCCCCGn69; and Site 3: 82nCCCCGCCCn89) present in Prdx6 promoter (Figures 3, 7b and c). However, these sequences are different from each other, and divergent from Sp1 core consensus element (nGGGGCGGGn). Curcumin has been shown to influence the activity or expression of various transcription factors such as NF-κB, Ap1, Sp1 and so forth, and thereby to modulate antioxidants expression.14, 36 Our current study clearly shows that curcumin induces the coexpression of Sp1 and Prdx6, and delivers its Prdx6 overexpression activity by enhancing Sp1 expression and thereby increasing DNA-binding and promoter activities (Figures 6 and 7d). In addition, we carried out DNA-Sp1 binding and transactivation experiments in Sp1-deficient Drosophila cells (SL2). These experiments further confirm that Sp1 functionally binds and activates transcriptional activity of Prdx6 promoter (Figures 6a and 7c). This was further supported by experiments Mithra-A and ART, known Sp1 inhibitors (Figures 6b and c). As Mithra-A directly binds to GC-rich DNA sequence, it is used to explore the sequence specificity of DNA-binding factors including Sp1.29 ART, a naturally occurring component of Artemisia annua, is able to downregulate transcription factor Sp1.27 Taken together, the data indicate that curcumin's novel mode of protective action arises from transcriptional activation of Prdx6 expression, which is in turn dependent on Sp1 expression, which is invoked by curcumin itself. Initially, Sp1 was considered a constitutive activator of housekeeping genes, but it is now known that growth factor signals influenced by curcumin or other factors may change the transcriptional activity of Sp1,37 and that several Sp1 target genes are related to cell survival and cell growth.38, 39 Curcumin has been shown to exert its biological effects through several important molecular targets, including transcription factors NF-κB, Ap1 and Sp1.15, 17, 18 Our data, however, show that Sp1 directly and specifically interacts with its site(s) (GC-boxes) in Prdx6 promoter (Figures 6 and 7a).
Curcumin has been shown to have significant antioxidant activity both in vivo and in vitro.33 Oxidative stress significantly decreases cell density in hLECs,7 UVB directly damages DNA and the process is linked to increasing ROS level.7 Among a great variety of ROS, H2O2 plays a pivotal role, because it is generated from nearly all sources of oxidative stress. Even endogenous H2O2 can enter the cells and induce cytotoxicity due to its high membrane permeability.40 Importantly, eye lenses from Prdx6-deficient mice are highly vulnerable to oxidative stress and develop cataract.6 Our current research showed that stimulation of naturally occurring Prdx6 in LECs by delivery of curcumin enhanced LECs' survival against stressors (Figures 1, 2, 8 and 9). These results highlighted the potential use of curcumin to postpone or delay the onset of cataractogenesis.
In summary, this study shows, for the first time, the novel mechanism of curcumin-mediated protection of hLECs, involving Sp1-induced upregulation of Prdx6. We found that curcumin stimulates Prdx6 gene transcription via Sp1 regulation, and that adding curcumin stimulates the Prdx6 protein and mRNA, thereby providing cytoprotection against oxidative stressors. In addition, our data reveal the presence of three functional Sp1 sites in Prdx6 promoter, each with different activation potential. These sites, we surmise, are responsible for controlling and fine tuning of Prdx6 expression in accordance with cellular requirement(s) to maintain cellular homeostasis. The results of this research should allow us to design new therapies or combinations of therapies – ‘transcription-based inductive therapies' – to attenuate deleterious signaling in situations involving physiological and environmental stress, and to take steps to prevent/delay the initiation and progression of cataractogenesis, including diseases associated with aging in general.
Materials and Methods
Cell culture
Human lens epithelial cells (hLECs) (a kind gift of Dr. Venkat N Reddy, Eye Research Institute, Oakland University, Rochester, MI, USA) were maintained in DMEM with 15% fetal bovine serum, 100 μg/ml streptomycin and 100 μg/ml penicillin in 5% CO2 environment at 37 °C as described previously. Cells were harvested and cultured in 96-, 24-, 48- or 6-well plates and 100 mm Petri dishes according to the requirement of the experiment. To examine the effect of curcumin, cells were treated with different concentrations (2.5, 5 or 10 μM in complete medium) of curcumin for variable time intervals. A stock solution of curcumin (10 mM) was prepared in DMSO and diluted in culture medium keeping the final DMSO concentration at <0.05% and same concentration of DMSO was used as vehicle. Curcumin (catalog no. C7727) and its inhibitors, ART and Mithra-A, were purchased from Sigma Aldrich (St. Louis, MO, USA).
Generation and validation of LECs isolated from lenses of Prdx6−/− and Prdx6+/+ mice
All animal experiments followed the recommendations set forth in the Statement for the Use of Animals in Ophthalmic Research by the Association for Research in Vision and Ophthalmology. Animal studies were approved by the University of Nebraska Medical Center (Omaha, NE, USA). LECs isolated from Prdx6-targeted mutants (Prdx6−/−) and wild-type (Prdx6+/+) mice were generated and maintained in DMEM with 10% fetal bovine serum, as described earlier.6 Prdx6−/− 129/Sv mice were generated at Harvard Medical School under the supervision of Dr. David R Beier. For the this study, we used Prdx6−/− mutant mice of pure 129 background, and, as controls, wild-type 129/Sv inbred mice of the same sex and age (Prdx6+/+). All animals were maintained under specific pathogen-free conditions in an animal facility. LECs were isolated from mice of identical age, and western analysis was carried out to confirm the presence of αA-crystalline, a specific marker of LECs. Cells from 3 to 5 passages were used for the experiments.
Construction of Prdx6-As
hLECs cDNA library was used to isolate Prdx6 cDNA having a full-length open reading frame. A full-length Prdx6-As construct was made by subcloning Prdx6 cDNA into a pcDNA3.1/NT-GFP-TOPO vector in reverse orientation. Plasmid was amplified following TOP 10 bacterial cells transformation as described earlier.
Cell survival assay (MTS assay)
A colorimetric MTS assay (Promega, Madison, MI, USA) was performed as described earlier.2, 6 Briefly, 2 × 104 cells were cultured in 48-well plate and pretreated with curcumin (5 μM), and after 12 h, cells were exposed to UVB (200 J/m2), H2O2 (200 μM) or paraqaut (1 mM) for 24, 48 or 72 h. This assay of cellular proliferation uses MTS (Promega). Upon being added to medium containing viable cells, MTS is reduced to a water-soluble formazan salt. The A490 nm value was measured after 4 h with an ELISA reader.
Assay for intracellular redox state
Intracellular redox state levels were measured using the fluorescent dye, H2-DCF-DA as described earlier.2, 6 Cells (1 × 104) were cultured in 96-well plates and pretreated with curcumin, and after 12 h, cells were subjected to UVB (200 J/m2), H2O2 (200 μM) or paraqaut (1 mM) for 24, 48 and 72 h. Cells were washed once with HBSS and incubated in the same buffer containing 10 μM of H2-DCF-DA. It is a nonpolar compound that is converted into a polar derivative (dichlorofluorescein) by cellular esterase following incorporation into cells. Following 30 min of incubation at room temperature, intracellular fluorescence was detected with excitation at 485 nm and emission at 530 nm (Ex485/Em530) using Spectra Max Gemini EM (Molecular Devices, Sunnyvale, CA, USA).
Apoptosis assay
Apoptosis was measured using flow cytometry to quantify the levels of oxidative stress-induced apoptotic cells. The Annexin V assay was performed using the FITC Annexin V Apoptosis Detection Kit I (BD Biosciences, San Jose, CA, USA). Briefly, hLECs (7 × 105) were seeded in 100 mm plates and treated with curcumin (5 μM), and after 12 h, cells were exposed to UVB or H2O2 or paraquat for 48 h. Cells were washed twice with ice-cold PBS (phosphate-buffered saline solution) and re-suspended in 1 × 105/100 μl of binding buffer and incubated with 5 μl of Annexin V-FITC and 5 μl of PI for 15 min at room temperature in the dark. After 15 min of incubation, 400 μl of binding buffer was added to make 500 μl final volumes and flow cytometry was performed within 1 h.
LPO assay
LPO assay was carried out according to the manufacturer's protocol (Lipid Peroxidation Microplate Assay Kit; Oxford Biomedical Research, Rochester Hills, MI, USA). The assay is based on the reaction of a chromogenic reagent, N-methyl-2-phenylindole (R1), with MDA and 4-hydroxyalkenals at 45 °C. One molecule of either MDA or 4-hydroxyalkenal reacts with 2 molecules of reagent R1 to yield a stable chromophore with maximal absorbance at 586 nm. Briefly, hLECs (7 × 105) cells were seeded in 100 mm plates and treated with curcumin, and after 12 h of treatment, cells were exposed with UVB or H2O2 or paraquat for 48 h. Cells washed twice with ice-cold PBS and total cell lysates were prepared as described.6 Equal amounts of protein were used for the assay. OD was measured at 586 nm.
Protein expression assay
Cell lysates were prepared in ice-cold radioimmunoprecipitation assay (RIPA) lysis buffer as described previously.2, 6 Equal amounts of protein samples were loaded into a 10% SDS gel, blotted onto polyvinylidene fluoride membrane (Perkin-Elmer Life Sciences, Santa Clara, CA, USA) and immunostained with primary antibodies at the appropriate dilutions: Prdx6 monoclonal antibody (Lab Frontier, Seoul, South Korea); Sp1 rabbit polyclonal and β-actin rabbit polyclonal (Abcam, Cambridge, MA, USA). Membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (1 : 1500 dilutions). Specific protein bands were visualized by incubating the membrane with luminol reagent (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and exposing to film (X-Omat; Eastman Kodak Co., Rochester, NY, USA) and recorded with a FUJIFILM-LAS-4000 luminescent image analyzer (FUJIFILM Medical Systems Inc., Stamford, CT, USA). To ascertain comparative expression and equal loading of the protein samples, the membrane stained earlier was stripped and reprobed with β-actin antibody (Abcam).
Construction of Prdx6 promoter-CAT reporter vector
The 5′-flanking region (−839 to +109 bp) (construct B) was isolated from mouse genomic DNA and sequenced. A construct of −839 bp was prepared by ligating it to basic pCAT vector (Promega) using the SacI and XhoI sites. The plasmid was amplified and used for the CAT assay. Primers used were as follows: 5′-CTTCCTCTGGAGCTCAGAATTTAC-3′ and 5′-CAGGAACTCGAGGAAGCGGAT-3′.
Site-directed mutagenesis
PCR-based site-directed mutagenesis was carried out using the QuikChange site-directed mutagenesis kit (Invitrogen, Carlsbad, CA, USA), following the company's protocol. Briefly, amino-acid exchanges Sp1 site 1 mutant (CC to GG), Sp1 site 2 mutant (C to G) and Sp1 site 3 mutant (C to G) were generated by point mutations in the Prdx6-CAT constructs. The following complementary primers were used (changed nucleotides are in boldface type and underlined):
Sp1-Mut-1for, 5′-GATCTAGGTCTCCGCAGGAGCTAGCCCGCTGCTCACTG-3′ Sp1-Mut-1rev, 5′-CAGTGAGCAGCGGGCTAGCTCCGGCGGAGACCTAGATC-3′ Sp1-Mut-2for, 5′-GCCCTGCCCTAGCCCTGCCCACTCGGCCAGCACTGATC-3′ Sp1-Mut-2rev, 5′-GATCAGTGCTGGCCGAGTGGGCAGGGCTAGGGCAGGGC-3′ andSp1-Mut-3for, 5′-GCCGCCAGACTCGCGGTCGCCTCACCTCGCCCTAGC-3′ Sp1-Mut-3rev, 5′-GCTAGGGCGAGGTGAGGCGACCGCGAGTCTGGCGGC-3′.
Epicurean Coli XL1-Blue super-competent cells (Invitrogen) were transformed with resultant plasmid, and clones were grown on Luria–Bertani/Amp Petri dishes. The plasmid was amplified, and the mutation was confirmed by sequencing.
Transfection and CAT assay
The CAT assay was performed using a CAT-ELISA kit (Roche Applied Science, Indianapolis, IN, USA). hLECs transfected/co-transfected using Superfectamine with Prdx6-CAT reporter constructs (4 μg) with or without pCMV-Sp1(4 μg) (Addgene) and pEGFP (1 μg) to SEAP vector (1 μg) and treated with different concentrations of curcumin or Sp1 inhibitors. After 72 h of incubation, cells were harvested, extracts were prepared and protein was normalized. CAT-ELISA was performed to monitor CAT activity following the manufacturer's protocol. Absorbance was measured at 405 nm using a microtiter plate ELISA reader. Transactivation activities were adjusted for transfection efficiencies using GFP/SEAP values.6
mRNA expression assay (real-time PCR)
Total RNA was isolated using the single-step guanidine thiocyanate/phenol/chloroform extraction method (Trizol reagent; Invitrogen) and converted to cDNA using Superscript II RNAase H−Reverse Transcriptase. Quantitative real-time PCR was performed with SYBR Green Master Mix (Roche Diagnostic Corporation, Indianapolis, IN, USA) in a Roche LC480 Sequence detector system (Roche Diagnostic Corporation, Indianpolis, IN, USA). PCR conditions consisted of 10-min hot start at 95 °C, followed by 45 cycles of 10 s at 95 °C, 30 s at 60 °C and 10 s at 72 °C. The primer sequences were as follows: Prdx6, 5′-GCATCCGTTTCCACGACT-3′ and 5′-TGCACACTGGGGTAAAGTCC-3′ Sp1, 5′-CCTGGATGAGGCACTTCTGT-3′ and 5′-GCCTGGGCTTCAAGGATT-3′ β-actin, 5′-CCAACCGCGAGAAGATGA-3′ and 5′-CCAGAGGCGTACAGGGATAG-3′. Expression levels of target genes were normalized to the levels of β-actin as an endogenous control in each group.
ChIP assay
ChIP was performed using the ChIP assay kit (Active Motif, Carlsbad, CA, USA) following the manufacturer's protocol. The following antibodies were used: normal mouse IgG and anti-Sp1 (Millipore, Billerica, MA, USA). Real-time PCR amplification was carried out using 4 μl of DNA sample with primers (Prdx6 promoter bearing Sp1 sites, forward primer: 5′-CGCAATTCTCGGTCTTGCGCTTC-3′ and reverse primer: 5′-GTGGTGACGCTGAGAACAAGGA-3′, positions −208/+27; and contiguous sequence to which Sp1 does not binds, forward primer: 5′-CCTGGTTCCTTACATATAAGGC-3′ and reverse primer: 5′-cctggtatagtatatgtccctg-3′, positions −2356/−2229 relative to the A in the ATG translation initiation codon) specific to the Prdx6 promoter. The amplification of soluble chromatin before immunoprecipitation was used as an input control. The program for quantification amplification was 3 min at 94 °C, 20 s at 95 °C, 30 s at 59 °C and 30 s at 72 °C for 36 cycles in 25 μl reaction volume.
DNA–protein interaction assays
Gel-shift assay was carried out using nuclear extracts2, 6, 25 isolated from hLECs to determine DNA-binding activity of Sp1 to their respective elements present in the Prdx6 promoter. Oligos consisting of putative Sp1-binding elements were commercially synthesized (Invitrogen), annealed and end-labeled with [γ-32P]ATP using T4 polynucleotide kinase (New England Biolabs, Ipswich, MA, USA). The binding reaction was performed in 20 μl of binding buffer (20 m Tris-HCl (pH 8.0), 75 m KCl, 5% (v/v) glycerol, 50 μg/ml bovine serum albumin, 0.025% (v/v) Nonidet P-40, 1 m EDTA, 5 m dithiothreitol and 1 μg of poly (dI/dC). A measure of 5 fmol of the end-labeled probe was incubated on ice for 30 min with variable concentrations of nuclear extract. The samples were then loaded on 5% polyacrylamide gel in 0.5 × TBE buffer for 2 h at 10 V/cm. The gel was dried and autoradiographed.
Statistical method
For all quantitative data collected. Statistical analysis was conducted by Student's t-test and was presented as mean±S.D. of the indicated number of experiments. A significant difference between control and treatment group was defined as P-value of <0.05 and 0.001 for three or more independent experiments.
Acknowledgments
Grant support for this study provided by the National Eye Institute, NIH (EY013394 and EY017613) (to DPS) and Research for Preventing Blindness (RPB), and also by American Health assistant Foundation (AHAF, G2009038) (NF) are gratefully acknowledged.
Glossary
- ROS
reactive oxygen species
- LECs
lens epithelial cells
- Sp1
specificity protein 1
- Prdx6
peroxiredoxin 6
- ART
artemisinin
- Mithra-A
mithramycin A
- ChIP
chromatin immunoprecipitation
- UVB
ultraviolet B
- LPO
lipid peroxidation
The authors declare no conflict of interest.
Footnotes
Edited by A Finazzi-Agró
References
- Balasubramanian D. Ultraviolet radiation and cataract. J Ocul Pharmacol Ther. 2000;16:285–297. doi: 10.1089/jop.2000.16.285. [DOI] [PubMed] [Google Scholar]
- Kubo E, Fatma N, Akagi Y, Beier DR, Singh SP, Singh DP. TAT-mediated PRDX6 protein transduction protects against eye lens epithelial cell death and delays lens opacity. Am J Physiol Cell Physiol. 2008;294:C842–C855. doi: 10.1152/ajpcell.00540.2007. [DOI] [PubMed] [Google Scholar]
- Fatma N, Singh P, Chhunchha B, Kubo E, Shinohara T, Bhargavan B, et al. Deficiency of Prdx6 in lens epithelial cells induces ER stress response-mediated impaired homeostasis and apoptosis. Am J Physiol Cell Physiol. 2011;301:C954–C967. doi: 10.1152/ajpcell.00061.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher AB. Peroxiredoxin 6: a bifunctional enzyme with glutathione peroxidase and phospholipase A activities. Antioxid Redox Signal. 2011;15:831–844. doi: 10.1089/ars.2010.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Fatma N, Kubo E, Sharma P, Beier DR, Singh DP. Impaired homeostasis and phenotypic abnormalities in Prdx6−/−mice lens epithelial cells by reactive oxygen species: increased expression and activation of TGFbeta. Cell Death Differ. 2005;12:734–750. doi: 10.1038/sj.cdd.4401597. [DOI] [PubMed] [Google Scholar]
- Kubo E, Hasanova N, Tanaka Y, Fatma N, Takamura Y, Singh DP, et al. Protein expression profiling of lens epithelial cells from Prdx6-depleted mice and their vulnerability to UV radiation exposure. Am J Physiol Cell Physiol. 2010;298:C342–C354. doi: 10.1152/ajpcell.00336.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan SA, Wang X, Wallbrandt P, Forsman-Semb K, Paigen B. Overexpression of Prdx6 reduces H2O2 but does not prevent diet-induced atherosclerosis in the aortic root. Free Radic Biol Med. 2003;35:1110–1120. doi: 10.1016/s0891-5849(03)00462-3. [DOI] [PubMed] [Google Scholar]
- Manevich Y, Fisher AB. Peroxiredoxin 6, a 1-Cys peroxiredoxin, functions in antioxidant defense and lung phospholipid metabolism. Free Radic Biol Med. 2005;38:1422–1432. doi: 10.1016/j.freeradbiomed.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Charoensuk L, Pinlaor P, Prakobwong S, Hiraku Y, Laothong U, Ruangjirachuporn W, et al. Curcumin induces a nuclear factor-erythroid 2-related factor 2-driven response against oxidative and nitrative stress after praziquantel treatment in liver fluke-infected hamsters. Int J Parasitol. 2011;41:615–626. doi: 10.1016/j.ijpara.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009;14:141–153. [PubMed] [Google Scholar]
- Griesser M, Pistis V, Suzuki T, Tejera N, Pratt DA, Schneider C. Autoxidative and cyclooxygenase-2 catalyzed transformation of the dietary chemopreventive agent curcumin. J Biol Chem. 2011;286:1114–1124. doi: 10.1074/jbc.M110.178806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci. 2009;30:85–94. doi: 10.1016/j.tips.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Mandal MN, Patlolla JM, Zheng L, Agbaga MP, Tran JT, Wicker L, et al. Curcumin protects retinal cells from light-and oxidant stress-induced cell death. Free Radic Biol Med. 2009;46:672–679. doi: 10.1016/j.freeradbiomed.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi S, Srivatava SK, Piper JT, Singhal SS, Chaubey M, Awasthi YC. Curcumin protects against 4-hydroxy-2-trans-nonenal-induced cataract formation in rat lenses. Am J Clin Nutr. 1996;64:761–766. doi: 10.1093/ajcn/64.5.761. [DOI] [PubMed] [Google Scholar]
- Tomita M, Kawakami H, Uchihara JN, Okudaira T, Masuda M, Takasu N, et al. Curcumin suppresses constitutive activation of AP-1 by downregulation of JunD protein in HTLV-1-infected T-cell lines. Leuk Res. 2006;30:313–321. doi: 10.1016/j.leukres.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Balasubramanian S, Eckert RL. Curcumin suppresses AP1 transcription factor-dependent differentiation and activates apoptosis in human epidermal keratinocytes. J Biol Chem. 2007;282:6707–6715. doi: 10.1074/jbc.M606003200. [DOI] [PubMed] [Google Scholar]
- Liu F, Pore N, Kim M, Voong KR, Dowling M, Maity A, et al. Regulation of histone deacetylase 4 expression by the SP family of transcription factors. Mol Biol Cell. 2006;17:585–597. doi: 10.1091/mbc.E05-08-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu H, Lee J, Zaman K, Kubilis J, Ferrante RJ, Ross BD, et al. Sp1 and Sp3 are oxidative stress-inducible, antideath transcription factors in cortical neurons. J Neurosci. 2003;23:3597–3606. doi: 10.1523/JNEUROSCI.23-09-03597.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merika M, Orkin SH. Functional synergy and physical interactions of the erythroid transcription factor GATA-1 with the Kruppel family proteins Sp1 and EKLF. Mol Cell Biol. 1995;15:2437–2447. doi: 10.1128/mcb.15.5.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatma N, Kubo E, Sen M, Agarwal N, Thoreson WB, Camras CB, et al. Peroxiredoxin 6 delivery attenuates TNF-alpha-and glutamate-induced retinal ganglion cell death by limiting ROS levels and maintaining Ca2+ homeostasis. Brain Res. 2008;1233:63–78. doi: 10.1016/j.brainres.2008.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Simpkins JW, Dykens JA, Cammarata PR. Oxidative damage to human lens epithelial cells in culture: estrogen protection of mitochondrial potential, ATP, and cell viability. Invest Ophthalmol Vis Sci. 2003;44:2067–2075. doi: 10.1167/iovs.02-0841. [DOI] [PubMed] [Google Scholar]
- Rukkumani R, Sri Balasubashini M, Menon VP. Protective effects of curcumin and photo-irradiated curcumin on circulatory lipids and lipid peroxidation products in alcohol and polyunsaturated fatty acid-induced toxicity. Phytother Res. 2003;17:925–929. doi: 10.1002/ptr.1254. [DOI] [PubMed] [Google Scholar]
- Fatma N, Singh DP, Shinohara T, Chylack LT., Jr Transcriptional regulation of the antioxidant protein 2 gene, a thiol-specific antioxidant, by lens epithelium-derived growth factor to protect cells from oxidative stress. J Biol Chem. 2001;276:48899–48907. doi: 10.1074/jbc.M100733200. [DOI] [PubMed] [Google Scholar]
- Courey AJ, Tjian R. Analysis of Sp1 in vivo reveals multiple transcriptional domains, including a novel glutamine-rich activation motif. Cell. 1988;55:887–898. doi: 10.1016/0092-8674(88)90144-4. [DOI] [PubMed] [Google Scholar]
- Willoughby JA, Sr, Sundar SN, Cheung M, Tin AS, Modiano J, Firestone GL. Artemisinin blocks prostate cancer growth and cell cycle progression by disrupting Sp1 interactions with the cyclin-dependent kinase-4 (CDK4) promoter and inhibiting CDK4 gene expression. J Biol Chem. 2009;284:2203–2213. doi: 10.1074/jbc.M804491200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleiman SF, Langley BC, Basso M, Berlin J, Xia L, Payappilly JB, et al. Mithramycin is a gene-selective Sp1 inhibitor that identifies a biological intersection between cancer and neurodegeneration. J Neurosci. 2011;31:6858–6870. doi: 10.1523/JNEUROSCI.0710-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Feinstein SI, Wang Y, Dodia C, Fisher D, Yu K, et al. Comparison of glutathione peroxidase 1 and peroxiredoxin 6 in protection against oxidative stress in the mouse lung. Free Radic Biol Med. 2010;49:1172–1181. doi: 10.1016/j.freeradbiomed.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese V, Bates TE, Mancuso C, Cornelius C, Ventimiglia B, Cambria MT, et al. Curcumin and the cellular stress response in free radical-related diseases. Mol Nutr Food Res. 2008;52:1062–1073. doi: 10.1002/mnfr.200700316. [DOI] [PubMed] [Google Scholar]
- Motterlini R, Foresti R, Bassi R, Green CJ. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic Biol Med. 2000;28:1303–1312. doi: 10.1016/s0891-5849(00)00294-x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Feinstein SI, Fisher AB. Peroxiredoxin 6 as an antioxidant enzyme: protection of lung alveolar epithelial type II cells from H2O2-induced oxidative stress. J Cell Biochem. 2008;104:1274–1285. doi: 10.1002/jcb.21703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryanarayana P, Krishnaswamy K, Reddy GB. Effect of curcumin on galactose-induced cataractogenesis in rats. Mol Vis. 2003;9:223–230. [PubMed] [Google Scholar]
- Naar AM, Ryu S, Tjian R. Cofactor requirements for transcriptional activation by Sp1. Cold Spring Harb Symp Quant Biol. 1998;63:189–199. doi: 10.1101/sqb.1998.63.189. [DOI] [PubMed] [Google Scholar]
- Gill G, Pascal E, Tseng ZH, Tjian R. A glutamine-rich hydrophobic patch in transcription factor Sp1 contacts the dTAFII110 component of the Drosophila TFIID complex and mediates transcriptional activation. Proc Natl Acad Sci USA. 1994;91:192–196. doi: 10.1073/pnas.91.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emili A, Greenblatt J, Ingles CJ. Species-specific interaction of the glutamine-rich activation domains of Sp1 with the TATA box-binding protein. Mol Cell Biol. 1994;14:1582–1593. doi: 10.1128/mcb.14.3.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan NY, Khachigian LM. Sp1 phosphorylation and its regulation of gene transcription. Mol Cell Biol. 2009;29:2483–2488. doi: 10.1128/MCB.01828-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierstra I. Sp1: emerging roles – beyond constitutive activation of TATA-less housekeeping genes. Biochem Biophys Res Commun. 2008;372:1–13. doi: 10.1016/j.bbrc.2008.03.074. [DOI] [PubMed] [Google Scholar]
- Kunwar A, Sandur SK, Krishna M, Priyadarsini KI. Curcumin mediates time and concentration dependent regulation of redox homeostasis leading to cytotoxicity in macrophage cells. Eur J Pharmacol. 2009;611:8–16. doi: 10.1016/j.ejphar.2009.03.060. [DOI] [PubMed] [Google Scholar]
- Mahakunakorn P, Tohda M, Murakami Y, Matsumoto K, Watanabe H, Vajaragupta O. Cytoprotective and cytotoxic effects of curcumin: dual action on H2O2-induced oxidative cell damage in NG108-15 cells. Biol Pharm Bull. 2003;26:725–728. doi: 10.1248/bpb.26.725. [DOI] [PubMed] [Google Scholar]