Abstract
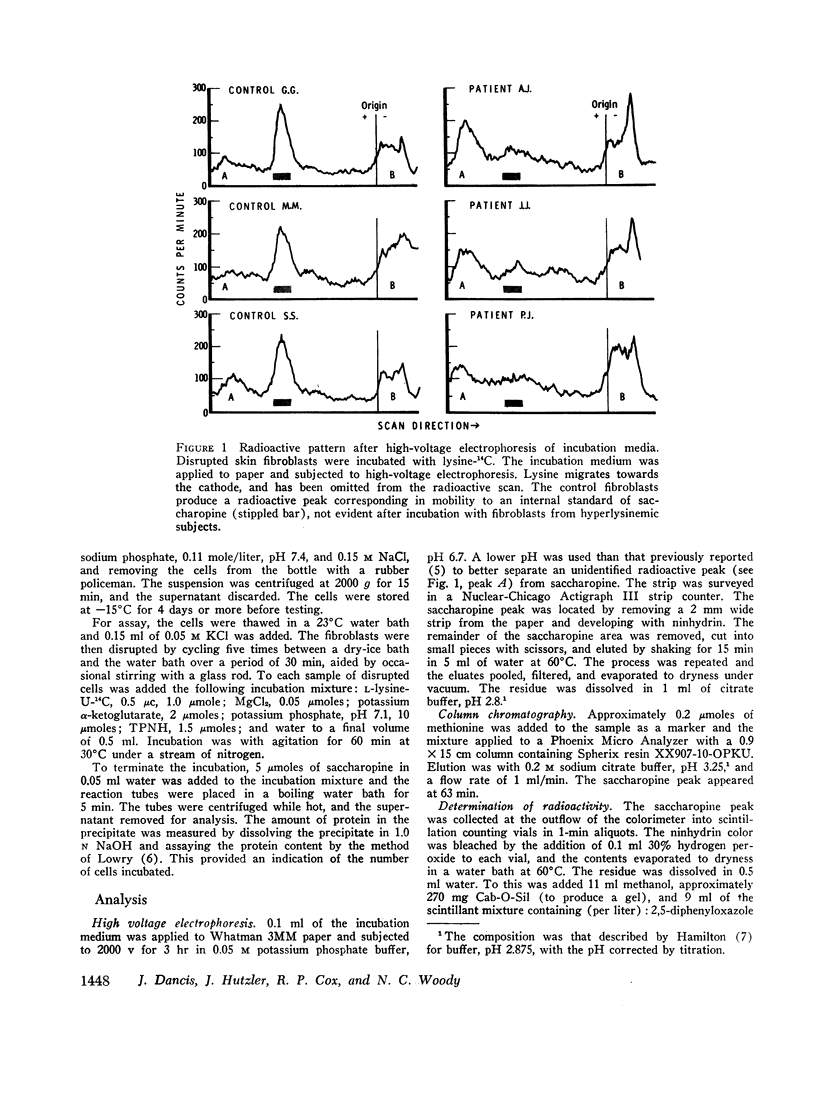
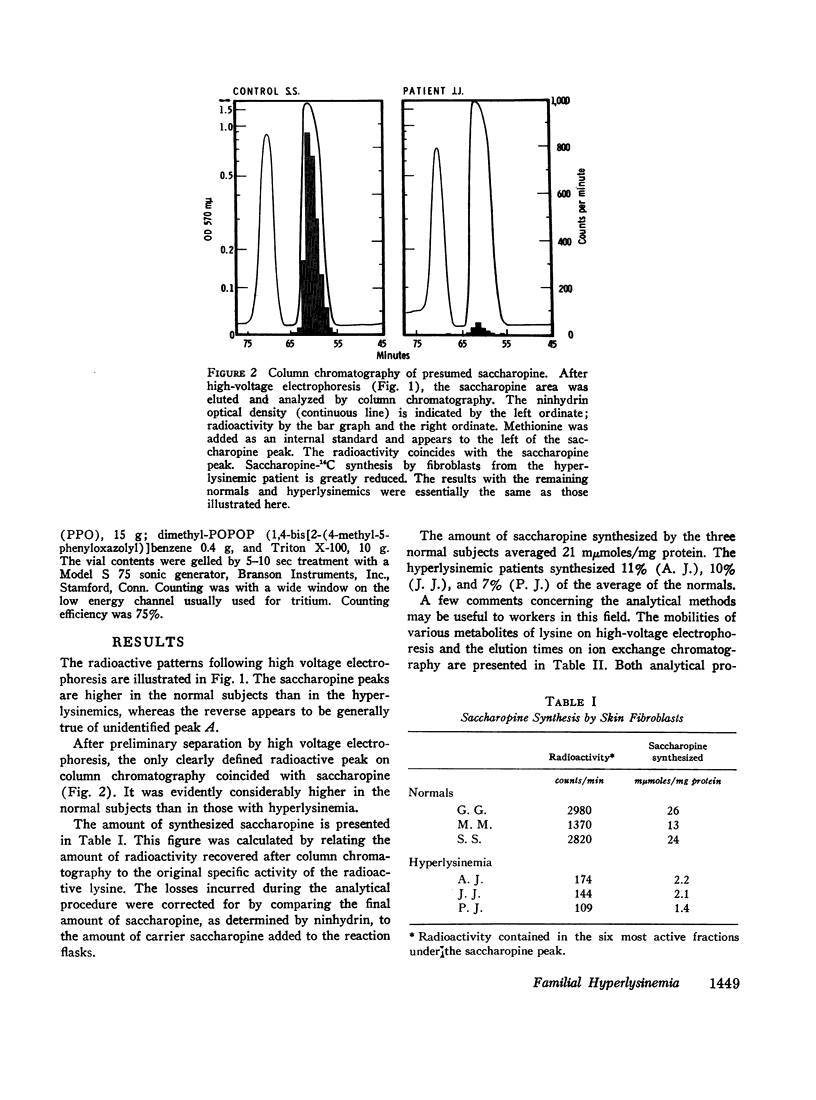
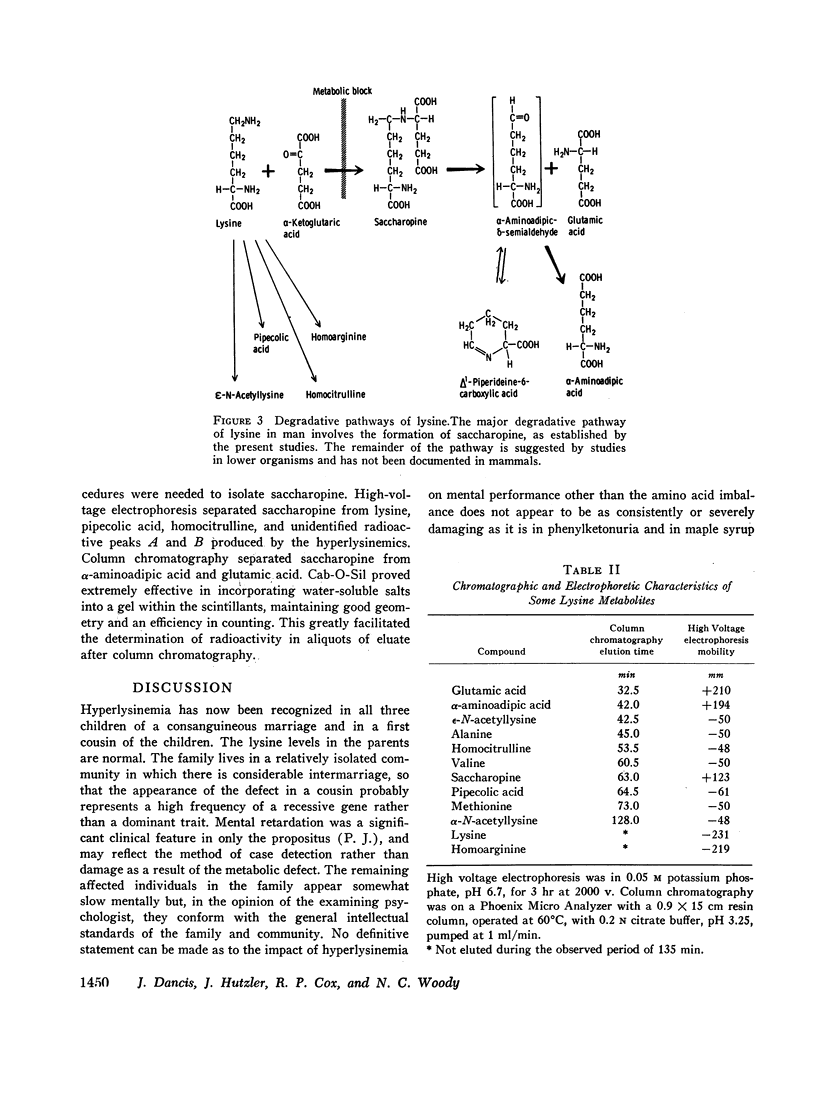
Fibroblasts grown in tissue culture from the skin of normal subjects have lysine-ketoglutarate reductase activity (lysine: α-ketoglutarate: triphosphopyridine nucleotide (TPNH) oxidoreductase (ε-N-[L-glutaryl-2]-L-lysine forming)). The activity of the enzyme is considerably reduced in the skin fibroblasts grown from three siblings with hyperlysinemia. The high concentrations of lysine in the blood of these patients, the previous demonstration in the intact subject of a reduction in the ability to degrade lysine, and the present demonstration of diminished lysine-ketoglutarate reductase activity, accurately define the metabolic defect and establish the saccharopine (ε-N-[L-glutaryl-2]-L-lysine) pathway as the major degradative pathway for lysine in the human.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong M. D., Robinow M. A case of hyperlysinemia: biochemical and clinical observations. Pediatrics. 1967 Apr;39(4):546–554. [PubMed] [Google Scholar]
- Carson N. A., Scally B. G., Neill D. W., Carré L. J. Saccharopinuria: a new inborn error of lysine metabolism. Nature. 1968 May 18;218(5142):679–679. doi: 10.1038/218679a0. [DOI] [PubMed] [Google Scholar]
- Ghadimi H., Binnington V. I., Pecora P. Hyperlysinemia associated with retardation. N Engl J Med. 1965 Sep 30;273(14):723–729. doi: 10.1056/NEJM196509302731401. [DOI] [PubMed] [Google Scholar]
- Grove J., Henderson L. M. The metabolism of D- and L-lysine in the intact rat, perfused liver and liver mitochondria. Biochim Biophys Acta. 1968 Aug 6;165(1):113–120. doi: 10.1016/0304-4165(68)90195-5. [DOI] [PubMed] [Google Scholar]
- Higashino K., Fujioka M., Aoki T., Yamamura T. Metabolism of lysine in rat liver. Biochem Biophys Res Commun. 1967 Oct 11;29(1):95–100. doi: 10.1016/0006-291x(67)90547-5. [DOI] [PubMed] [Google Scholar]
- Higashino K., Tsukada K., Lieberman I. Saccharopine, a product of lysine breakdown by mammalian liver. Biochem Biophys Res Commun. 1965 Jul 26;20(3):285–290. doi: 10.1016/0006-291x(65)90361-x. [DOI] [PubMed] [Google Scholar]
- Hutzler J., Dancis J. Conversion of lysine to saccharopine by human tissues. Biochim Biophys Acta. 1968 Apr 16;158(1):62–69. doi: 10.1016/0304-4165(68)90072-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- ROTHSTEIN M., MILLER L. L. The conversion of lysine to pipecolic acid in the rat. J Biol Chem. 1954 Dec;211(2):851–858. [PubMed] [Google Scholar]
- Saunders P. P., Broquist H. P. Saccharopine, an intermediate of the aminoadipic acid pathway of lysine biosynthesis. IV. Saccharopine dehydrogenase. J Biol Chem. 1966 Jul 25;241(14):3435–3440. [PubMed] [Google Scholar]
- WOODY N. C. HYPERLYSINEMIA. Am J Dis Child. 1964 Nov;108:543–553. doi: 10.1001/archpedi.1964.02090010545015. [DOI] [PubMed] [Google Scholar]
- Woody N. C., Hutzler J., Dancis J. Further studies of hyperlysinemia. Am J Dis Child. 1966 Dec;112(6):577–580. doi: 10.1001/archpedi.1966.02090150121014. [DOI] [PubMed] [Google Scholar]
- Woody N. C., Ong E. B. Paths of lysine degradation in patients with hyperlysinemia. Pediatrics. 1967 Dec;40(6):986–992. [PubMed] [Google Scholar]


