Abstract
Natural odors, generally composed of many monomolecular components, are analyzed by peripheral receptors into component features and translated into spatiotemporal patterns of neural activity in the olfactory bulb. Here we will discuss the role of the olfactory cortex in the recognition, separation and completion of those odor-evoked patterns, and how these processes contribute to odor perception. Recent findings regarding the neural architecture, physiology and plasticity of the olfactory cortex, principally the piriform cortex, will be described in the context of how this paleocortical structure creates odor objects.
1. Introduction
For most organisms, chemical cues in the environment (odorants) guide behaviors critical for survival, including reproduction, mother-infant interactions, finding food and avoiding predators. The basic components of olfactory systems which transduce odorants into odor percepts have remained remarkably consistent over millions of years of evolution and across varied ecological niches. At the periphery is a diverse array of sensory receptors tuned either to specific molecules (Jones et al., 2007; Suh et al., 2004), or much more commonly to submolecular features (Araneda et al., 2000). Sensory neurons expressing the same odorant receptor converge onto glomeruli in the olfactory bulb (vertebrates) or antennal lobe (invertebrates), producing a unique, odorant-specific spatial pattern of activity in second order neurons (Johnson and Leon, 2007; Lin et al., 2006). The odor-evoked spatiotemporal pattern of second order neuron activity is then projected to the olfactory cortical areas (vertebrates, especially mammals) or mushroom bodies (invertebrates), where odor quality appears to be encoded in a sparse and distributed manner in striking contrast to the spatial patterns in the olfactory bulb (Perez-Orive et al., 2002; Rennaker et al., 2007; Stettler and Axel, 2009). Several excellent reviews of olfaction, covering topics from the periphery to perception have been recently published (e.g., (Davis, 2011; Gottfried, 2010; Mori and Sakano, 2011; Su et al., 2009)).
Here, we focus on the mammalian olfactory cortex. The olfactory cortex serves as point of anatomical convergence for olfactory bulb output neurons, mitral/tufted cells, conveying information about distinct odorant features extracted in the periphery. This convergence is an important early step in the ultimate formation of perceptual odor objects, such as the aroma coffee or rose. Odor object formation, however also requires an experience-dependent process, largely mediated by plasticity of intrinsic intracortical association fibers that helps bind the activity of ensembles of distributed, co-active cortical neurons responding to particular olfactory bulb output patterns. In addition to the converging olfactory bulb projection onto individual cortical neurons, this projection is also divergent, producing distributed parallel processing streams to different subregions of the olfactory cortex. Based on the anatomy of these divergent projection patterns and the anatomy and physiology of the diverse olfactory cortical target structures, odorant information can be transformed in a variety of ways to ultimately enrich odor perception and motivate odor-guided behavior. Thus, the olfactory cortex appears to play a crucial role in the translation of inhaled molecular features into rich, emotion and memory tinged perceptions called odors.
2. Basic anatomy of the olfactory cortex
The olfactory cortex is defined as those forebrain areas receiving direct olfactory bulb (mitral/tufted cell) input. In rodents this includes the majority of the ventrolateral brain, ventral to the rhinal fissure including the anterior olfactory nucleus, tenia tecta, olfactory tubercle, cortical nuclei of the amygdala, anterior and posterior piriform cortex and lateral entorhinal cortex (Cleland and Linster, 2003). For the most part, these same regions can be identified in the human brain as well, though they lie along the ventromedial edge of the temporal lobe, at the base of the olfactory peduncle. All regions of the olfactory cortex send projections back to the olfactory bulb. There are also strong commissural projections between the bilateral olfactory cortical subregions via the anterior commissure. Thus, while the olfactory sensory neurons project exclusively to the ipsilateral olfactory bulb, cortical neurons have access to bilateral input (Kikuta et al., 2008; Wilson, 1997).
With the exception of the lateral entorhinal cortex, the olfactory cortex is paleocortical, primarily consisting of three layers (Fig. 1). Layer I is a plexiform layer which includes pyramidal cell apical dendrites and the mitral/tufted cell axons as they leave the lateral olfactory tract, as well as association fibers. Layer II is a cell body layer, largely consisting of pyramidal cell bodies. Layer III includes cell bodies of deeper pyramidal cells, pyramidal cell basal dendrites and a variety of interneurons. This same general pattern holds true throughout the different subregions of the olfactory cortex, though with important regional differences in cell classes and local connectivity (e.g., (Brunjes et al., 2005; Wesson and Wilson, 2011)).
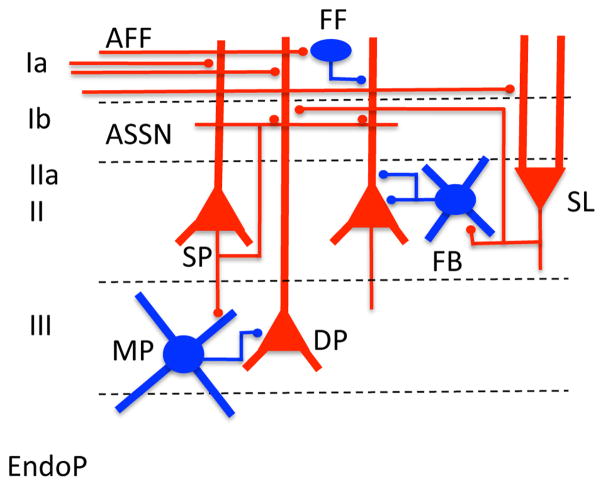
Figure 1.
Major local circuit components of the piriform cortex. See text for circuit description. Abbreviations: FF = feedforward inhibition mediated by interneurons in Layer I; FB = feedback inhibition mediated by interneurons in Layers II and III; MP = multipolar interneuron; SL = semilunar pyramidal cell; SP = superficial pyramidal cell; DP = deep pyramidal cell; ASSN = association fibers; AFF = afferent fibers; EndoP = endopiriform nucleus. Excitatory neurons depicted in red, inhibitory in blue.
Piriform cortex is the largest subregion of olfactory cortex. For a detailed anatomical review see (Neville and Haberly, 2004). Mitral/tufted cell axons are localized to the most superficial Layer Ia. Layer Ib contains intrinsic intracortical association fiber axons as well as commissural fibers. Layer I also contains somata of a small populations of inhibitory interneurons. Layer II contains the somata of superficial pyramidal cells, with apical dendrites extending into Layer I and basal dendrites into Layer III. Layer IIa, a thin, superficial component of Layer II contains the somata of the pyramidal cell-like semilunar cells. These cells lack basal dendrites and appear to preferentially receive input from mitral/tufted cells, with relatively less input from association fibers (Suzuki and Bekkers, 2011). Unlike other pyramidal cells, they do not project back to the olfactory bulb. Layer III contains the somata of deep pyramidal cells, as well as a variety of interneurons. At least five classes of piriform cortical GABAergic interneurons have been identified (Suzuki and Bekkers, 2010a; Young and Sun, 2009). Deep to layer III lies the endopiriform nucleus. Whether the endopiriform nucleus should be considered piriform cortical layer IV is unclear, though the two structures are highly interconnected. The endopiriform nucleus contains dense local and extended excitatory interconnections with relatively low levels of GABAergic interneurons (Behan and Haberly, 1999; Ekstrand et al., 2001). This combination of autoexcitation and low inhibition makes the endopiriform highly susceptible to seizure development (Behan and Haberly, 1999). It sends strong, dispersed output throughout the piriform cortex and other perirhinal structures. These characteristics have led to the hypothesis (Behan and Haberly, 1999) that the endopiriform nucleus may be involved in generating sharp-waves in olfactory cortex similar to those described in the hippocampal formation (Buzsaki, 1986), and that these sharp-waves may contribute to plasticity and odor memory. In fact as described below, sharp-waves have recently been described in piriform cortex (Manabe et al., 2011).
Understanding the role of olfactory cortex in odor perception has been the focus of a variety of theoretical and computational models (Ambros-Ingerson et al., 1990; Granger and Lynch, 1991; Haberly, 1985; Haberly, 2001; Haberly and Bower, 1989; Hasselmo et al., 1990; Linster et al., 2009). An underlying theme of many of these is olfactory cortex as auto-associative combinatorial array, capable of content addressable memory. Here, we use this model as an organizing framework to describe recent advances in understand olfactory cortical structure and function.
3. Olfactory cortex as an auto-associative combinatorial array
The basic model describes the olfactory cortex in terms of a combinatorial, auto-associative array capable of content addressable memory (Haberly, 2001). Put simply, the model proposes that unique combinations of odorant features, encoded in the spatio-temporal pattern of olfactory bub glomerular output, can be synthesized, stored and recalled in the activity of distributed ensembles of olfactory cortical pyramidal cells. The olfactory cortex thus serves as a pattern recognition device to deal with the complex, dynamic combinatorial patterns of olfactory bulb output. The activity of individual cortical pyramidal cells reflects not only the unique combination of ongoing odorant feature input from mitral/tufted cells, but also the past history of synaptic input to that cell from its co-active partners within the distributed pyramidal cell ensemble (auto-association). This historical/memorial component of the pattern recognition process supports synthetic processing of odor mixtures through the experience-dependent formation of odor objects, and further promotes pattern completion in the face of degraded inputs. Thus, a familiar odor (i.e., combination of odorant features and the corresponding spatiotemporal pattern of glomerular activation) induces activity in a distributed, non-topographic ensemble of cortical neurons (content-addressable memory) in part due to direct, convergent afferent input, and in part due to association fiber inputs between co-active cells that have been strengthened during past experience with that odor. These combined processes promote both odor discrimination and perceptual stability (Fig. 3).
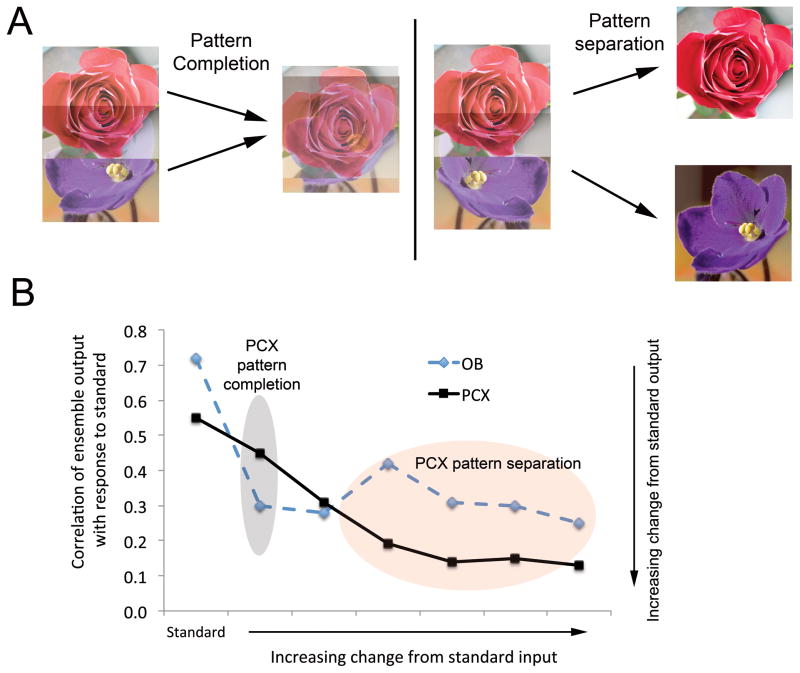
Figure 3.
(A) Pattern separation allows a decorrelation between two scents with highly overlapping component features into two distinct odor objects. Pattern completion can reduce the distinction between two overlapping scents by recapitulating one familiar odor object from the partial input driven by the other. (B) Piriform cortical neural ensembles perform pattern separation and completion relative to olfactory bulb input – a function common to auto-associative arrays and similar to processes in the hippocampal system (adapted from Barnes et al., 2008). See text for description.
In more detail, the model posits several basic circuit components. Although each of these components has had some experimental support in the past (see (Haberly, 2001; Neville and Haberly, 2004; Wilson et al., 2004), recent work with new techniques has solidified this foundation, as well as added important new details. The model includes the following network features: 1) distributed, overlapping input from olfactory bulb output neurons to a large population of pyramidal cells spread non-topographically across the piriform cortex. This distributed input would maximize opportunities for convergence of input from afferent fibers conveying information from different, spatially dispersed glomeruli. 2) Distributed, sparse, auto-associative intra-cortical connections, wherein individual pyramidal cells not only receive input from the olfactory bulb but also from other olfactory cortical pyramidal cells. This auto-associative connectivity is sparse with individual cell-cell connections relatively weak, but further expands the opportunity for convergence of input regarding different odorant features. 3) Together, the afferent and intrinsic synaptic inputs result in sparse, spatially distributed pyramidal cell odor-evoked activity, in contrast to the odor-specific spatial activity patterns observed in olfactory bulb. 4) The intra-cortical association fibers are capable of activity-dependent associative plasticity, which helps link ensembles of co-active cells. Thus, ensembles of cells that were co-active during prior odor stimulation become more strongly bound through enhancement of association fiber synaptic strength. This leads to a more reliable ensemble response to familiar odors, enhancing discriminability of the familiar pattern from other similar patterns. It can also lead to activation of the entire ensemble even if the input pattern is degraded; a process known as pattern completion which can promote perceptual stability. 5) Inhibitory interneurons create temporal patterning of pyramidal cell activity resulting in odor-evoked cortical oscillations, which can enhance synchrony of afferent and intrinsic synaptic activity onto individual neurons as well as synchrony of co-active neurons. 6) Synaptic plasticity is regulated by neuromodulatory inputs from the basal forebrain and brainstem. 7) Due to differences in local circuitry and top-down inputs, different subregions of the olfactory cortex may play different roles in odor coding, with more rostral regions dedicated to synthetic processing of odor object quality and increasingly complex associations (odor categories, learned hedonics, context, etc) mediated by more caudal regions.
Below, we summarize new data within the context of this model. While experimental data supporting some aspects of the model have existed (Haberly, 2001), this review will emphasize exciting recent findings that both provide new detail and further clarify our view of this important region.
Feature 1: Distributed afferent input
Previous data using small injections of horseradish peroxidase or similar strategies have supported the view of broad, non-topographic distribution of olfactory bulb input to the piriform cortex (Buonviso et al., 1991; Ojima et al., 1984). More recent work has explored this question in greater detail. Electroporation of tetramethylrhodamine (TMR)-dextran into identified glomeruli (Sosulski et al., 2011) or viral labeling of mitral/tufted cells from specific glomeruli (Ghosh et al., 2011) allowed tracing output projections to the cortex from individual glomeruli. Mitral and tufted cells from specific glomeruli projected throughout olfactory cortex, with no identifiable spatial pattern in the piriform cortex. Output from different glomeruli showed similar diffuse projections, providing ample opportunity for convergence of input from different glomeruli onto individual target neurons. The broad anatomical distribution of fibers projecting from individual glomeruli to the piriform cortex is associated with a broad distribution pre-synaptic mitral/tufted cell activity following stimulation of individual glomeruli. Using transgenic mice expressing synaptopHluorin in mitral/tufted cells and either electrically stimulating individual glomeruli or delivering odor pulses revealed broad, overlapping patterns of pre-synaptic afferent activity in piriform cortex (Mitsui et al., 2011). This technique is particularly useful for such mapping because, as discussed elsewhere, spatial patterns of odor-evoked post-synaptic cortical activity will reflect both afferent and intrinsic fiber driven responses, and thus are not a good indicator of purely afferent input patterns.
While the output of individual glomeruli is distributed across the piriform cortex, individual cortical neurons receive input from broadly distributed glomeruli, in a classic divergent-convergent pattern. Thus, using rabies virus-dependent retrograde labeling with mono-trans-synaptic control, infection of small numbers of piriform cortical neurons resulted in labeling of mitral cells from broadly scattered glomeruli (Miyamichi et al., 2011). This provides an anatomical substrate for synthesis of co-occurring odorant features. In fact, piriform cortical neurons may require co-activation of multiple glomeruli to drive spiking activity. Photo-uncaging of glutamate with precise spatial patterns of photo-stimulation in the olfactory bulb glomerular layer with intracellular recording of piriform cortex pyramidal cells in vivo showed that individual cells were responsive to specific spatial patterns of glomerular activation (Davison and Ehlers, 2011). Single glomerular activation was ineffective at driving cortical neurons. Similar results were reported in an in vitro olfactory bulb-piriform cortex slice (Apicella et al., 2010). Of course cortical association fiber activity contributes to this pyramidal cell activity, but the results strongly suggest convergence of multiple glomerular input onto individual pyramidal cells. Interestingly, similar convergence of odor feature information onto individual neurons appears to occur in the zebrafish dorsal pallium, the homolog of mammalian olfactory cortex (Yaksi et al., 2009).
The efficacy of individual afferent fibers in driving cortical pyramidal cells is also consistent with a convergence requirement. Although afferent fiber glutamatergic synapses onto piriform cortical pyramidal cells are relatively strong, layer II pyramidal cells require co-activation of multiple afferent fibers to reach spike threshold (Franks and Isaacson, 2006; Suzuki and Bekkers, 2006, 2011). However, subclasses of pyramidal cells show differential sensitivity to afferent input. Semi-lunar cells, which have apical dendrites with large spines located selectively within Layer Ia and thus anatomically appear highly sensitive to afferent input, are in fact more strongly depolarized by afferent input than superficial pyramidal cells in Layer II (Suzuki and Bekkers, 2011). In addition, semi-lunar cells have no basal dendrites (Neville and Haberly, 2004), and thus appear to be primarily tuned to afferent input with only minimal responses to association fiber input (Suzuki and Bekkers, 2011). Thus, these cells may have unique contributions to the intra-cortical association fiber system described below. For example, semilunar cells form a major component of the association fiber input to superficial pyramidal cells, forming in essence a second layer of processing in piriform cortex (Suzuki and Bekkers, 2011). Interestingly, semi-lunar cells are also profoundly affected by loss of afferent input, showing rapid apoptosis following either olfactory bulbectomy (Capurso et al., 1997; Heimer and Kalil, 1978) or naris occlusion (Leung and Wilson, 2003).
Finally, afferent input synapses also demonstrate strong paired-pulse facilitation (Neville and Haberly, 2004; Suzuki and Bekkers, 2006), which could help translate the complex temporal patterns of olfactory bulb output (Spors et al., 2006; Wachowiak and Cohen, 2001) in to unique patterns of activity in cortical target neurons. The temporal structure of both glomerular activation and mitral/tufted cell odor-evoked spike trains appears to convey important information about odor quality (Friedrich, 2006; Friedrich and Laurent, 2001; Shusterman et al., 2011), intensity (Meredith, 1986) and perhaps associative meaning (Doucette et al., 2011).
Together these new data satisfy the requirement of a distributed, overlapping pattern of afferent input from olfactory bulb glomeruli to the piriform cortex as required by the model.
Feature 2: Auto-associative intrinsic connections
An auto-associative circuit requires a robust intrinsic excitatory network connecting elements within the circuit. This intrinsic network helps bind distributed co-active neurons into an ensemble unique to a given input. Recent use of both axonal tracing and electrophysiological techniques have added to past data (e.g., (Haberly, 2001)) describing this association fiber network. For example, reconstruction of axons from individual pyramidal neurons has demonstrated far reaching axonal projections extending for millimeters throughout the piriform cortex and into other olfactory cortical regions (Johnson et al., 2000). The axons shown no patchiness in terminal fields and appear to make a small number of synapses onto a large number of other cortical neurons (Johnson et al., 2000). More recently, optogenetic techniques have further demonstrated that these intrinsic connections can reinforce or suppress the effectiveness of afferent input, depending on the relative timing between the two pathways (Franks et al., 2011). Association fibers strongly drive inhibitory interneurons in addition to providing direct excitatory input to pyramidal cells, thus temporal patterning of activity plays a role in effectiveness of association fiber action. As noted above, the relative strength of association fiber input varies with cell type..
These association fiber connections are an important component in driving odor-evoked activity. In some cases, pyramidal cells that do not respond directly to stimulation of individual glomeruli, do respond when specific combinations of glomeruli are activated, suggesting a role for intrinsic excitatory connections in driving this activity (Davison and Ehlers, 2011). More direct evidence comes from the fact that selective blockade of association fibers robustly reduces pyramidal cell odor response and narrows receptive field width (range of effective odor stimuli) (Poo and Isaacson, 2011).
Feature 3: Sparse, distributed non-topographic odor-evoked ensemble activity
Given the anatomy of the afferent and intrinsic excitatory circuitry, the model predicts that odor-evoked activity will be spatially distributed across the piriform cortex, with no topographic relationship to the beautiful spatial patterns of olfactory bulb glomerular layer activity. This sparse, ensemble encoding allows extremely large numbers of patterns (odor objects) to be stored in content addressable memory. This has now been confirmed with a variety of techniques, including 2-deoxyglucose (Cattarelli et al., 1988), single-unit electrode arrays (Rennaker et al., 2007), voltage-dependent dye imaging (Litaudon et al., 1997), immediate early gene mapping (Illig and Haberly, 2003), and optical imaging (Mitsui et al., 2011; Stettler and Axel, 2009). Neighboring neurons are as likely to respond to different odors as they are to respond to the same odors (Rennaker et al., 2007; Stettler and Axel, 2009) and there appears to be no spatial patterning at any scale (Stettler and Axel, 2009). As noted above, these spatially distributed patterns of activation reflect both afferent input termination patterns and association fiber activity (Poo and Isaacson, 2011).
In general, piriform cortical neurons show very low spontaneous activity rates (Poo and Isaacson, 2009), particularly compared to mitral/tufted cells (Wilson, 1998a). Odor evoked excitatory responses are also less robust than mitral/tufted cell responses, though odor-evoked instantaneous firing frequencies recorded intracellularly can exceed 200Hz (Wilson, 1998a). Afferent input from a single glomerulus to a pyramidal cell evokes only a weak excitation, with activation of multiple glomeruli required to reach threshold (Davison and Ehlers, 2011). Excitatory responses in individual pyramidal cells are narrowly tuned (Poo and Isaacson, 2009), with tuning (breadth of odor responsiveness) even more narrow in more posterior regions of the piriform (Litaudon et al., 2003), at least in anesthetized rodents. Together, these features define sparse odor coding in piriform cortex.
It has previously been demonstrated that rodents can detect, discriminate and learn about different spatial patterns of olfactory bulb activation (Mouly et al., 2001; Roman et al., 1987). Recent work using optogenetic stimulation techniques has demonstrated similar behavioral outcome with activation of distributed piriform cortical pyramidal cells (Choi et al., 2011). Associating activation of the distributed pyramidal cells with aversive or appetitive rewards can conditioned learned approach or avoidance behaviors, similar to natural odor stimulation. Activation of around 500 cells was sufficient to mediate this behavior (Choi et al., 2011). The fact that such a small ensemble of neurons (0.5% of the piriform cortical population) can drive behavior is consistent with Marr’s model of archicortex and allows for high capacity storage of many odor objects (Marr, 1971).
Feature 4: Associative synaptic plasticity
Another critical component of the model, as well as more general models of content addressable memory (Rolls and Treves, 1998), is synaptic plasticity of the intracortical association fiber system. This plasticity serves as the heart of the content addressable memory functioning in piriform cortex.
Association fiber synapses exhibit robust NMDA-dependent long-term potentiation (Kanter and Haberly, 1990; Poo and Isaacson, 2007). Associative learning with odors can increase synaptic currents evoked by association fiber stimulation (Saar et al., 2002), as well as dendritic spine density in regions of the apical dendritic where association fibers terminate (Knafo et al., 2001). Furthermore, this learning induced synaptic potentiation interferes with in vitro induction of long-term potentiation and enhances pre-disposition toward long-term depression induction, suggesting a common mechanism with NMDA dependent long-term potentiation (Lebel et al., 2001).
In addition to the intrinsic association fibers, in some circumstances afferent synapses can also express long-term potentiation (Patil et al., 1998; Poo and Isaacson, 2007; Roman et al., 1993; Sevelinges et al., 2004). Synaptic plasticity at this synapse appears to be most robust in very young animals (Best and Wilson, 2003; Poo and Isaacson, 2007) or in situations which elevate acetylcholine (Patil et al., 1998), though the magnitude of this plasticity still does not reach that expressed by association fiber synapses (see Development below).
However, while afferent synapses show reduced long-term potentiation, they do show robust and behaviorally important short-term depression (Best and Wilson, 2004). The piriform cortex displays rapid adaptation to stable odor input (Wilson, 1998a), and this cortical adaptation to odor is associated with afferent synaptic depression recorded intracellularly, in vivo (Wilson, 1998b). The recovery of odor responses occurs within about 2 min, as does the synaptic depression (Best and Wilson, 2004). This cortical adaptation is mediated by pre-synaptic metabotropic receptors (group III) which reduce glutamate release from mitral/tufted cell axons during repetitive stimulation (Best and Wilson, 2004). Pharmacological blockade of mGluRIII receptors within the piriform cortex prevents afferent synaptic depression, cortical odor adaptation, and short-term behavioral habituation (Bell et al., 2008; Best et al., 2005; Yadon and Wilson, 2005). Noradrenergic inputs to piriform cortex can also reduce synaptic depression (Best and Wilson, 2004), potentially via pre-synaptic beta receptors on mitral cell axons. Activation of noradrenergic beta receptors can inhibit mGluRIII receptor function via a protein kinase A dependent phosphorylation (Cai et al., 2001). Loud sounds which elevate norepinephrine within the piriform cortex (Smith et al., 2009) can induce dishabituation of odor-evoked behavioral responses (Smith et al., 2009). The behavioral dishabituation is blocked by intra-cortical infusion of the noradrenergic beta receptor antagonist propranolol (Smith et al., 2009).
The synaptic depression is homo-synaptic, leaving afferent inputs conveying information from other non-active mitral/tufted cells (and glomeruli) intact (Best and Wilson, 2004). Thus, homosynaptic depression may contribute to the fact that short-term cortical adaptation (Wilson, 2000) and short-term behavioral habituation are highly odor specific (Fletcher and Wilson, 2002; McNamara et al., 2008). However, homosynaptic depression is not sufficient to account for habituation specificity between highly overlapping input patterns (Linster et al., 2009). Potentiation of association fiber synapses also plays a major role in this odor-specificity. In a computational model of the olfactory system which incudes olfactory sensory neurons, olfactory bulb neurons and piriform cortex (Linster et al., 2007), cortical odor adaptation was induced if afferent homosynaptic depression was included in the model. However, this cortical adaptation was only minimally odor specific. In contrast, if long-term potentiation was included in association fiber synapses, and odor exposure was sufficiently long to induce familiarization, then cortical adaptation was highly odor specific (Linster et al., 2009). The same constraints hold true in vivo. The specificity of cortical odor adaptation and of behavioral odor habituation is dependent on how familiar the odors are (e.g., duration of exposure (Fletcher and Wilson, 2002; Wilson, 2003), and this specificity can be disrupted by pharmacological disruption of normal synaptic plasticity in association fiber synapses, for example with modulation of piriform cortical acetylcholine muscarinic receptors (Fletcher and Wilson, 2002; Wilson, 2001).
These results support the prediction that potentiation of association fiber synapses helps bind members of a co-active ensemble response to a given odor object, and that with this binding of spatially distributed neurons, discrimination and odor acuity improve. A second hypothesized consequence of this network effect is pattern completion. Computational models of piriform cortex have demonstrated that optimal associative plasticity in association fiber synapses helps store a template of familiar odor patterns which allow “filling-in” features of degraded inputs and full response to an odor object (Barkai et al., 1994; Hasselmo et al., 1992). Either too much or too little plasticity can result in excessive or impaired pattern completion and thus, impaired recognition and discrimination (Hasselmo and McGaughy, 2004). Recent work has directly tested the pattern completion ability of piriform cortical circuits (Barnes et al., 2008; Wilson, 2009). Complex mixtures of monomolecular odorants were “morphed” by either removing individual components (10 component mix, 10 component mix with 1 missing, 10 component mix with 2 missing, etc.) or by replacing individual components with a novel contaminant. Ensembles of mitral/tufted cells decorrelated (responded significantly differently between) all the various mixture morphs and the standard 10 component mixture. This is consistent with a pattern separation role for the olfactory bulb, similar to that of the hippocampal dentate gyrus (Sahay et al., 2011). In contrast, piriform cortical single-unit ensembles failed to decorrelate the 10 component mixture from that missing a single component, despite the fact that sufficient information was available in the olfactory bulb. This suggests that the piriform cortical ensembles completed the slightly degraded input, and responded as if the entire odor object was present. As more components were removed, or novel contaminants added, piriform cortical ensembles decorrelated the mixtures even more strongly than the olfactory bulb (Barnes et al., 2008). Behavioral discrimination performance in a two-alternative choice task mirrored the cortical ensemble decorrelation – mixtures not de-correlated by the cortex were difficult for the animals to discriminate (Barnes et al., 2008). These results suggest that, as originally hypothesized (Haberly, 2001), the piriform cortex can perform pattern completion which contributes to perceptual stability. Interestingly, new data suggest that the boundary between cortical pattern completion and separation is experience dependent. Cortical pattern completion can be enhanced in tasks requiring odor generalization and pattern separation can be enhanced in tasks requiring fine odor acuity (Chapuis and Wilson, 2010). These changes in olfactory cortical processing lead to changes in perceptual acuity in both rodents (Chapuis and Wilson, 2010; Chen et al., 2011; Fletcher and Wilson, 2002) and humans (Li et al., 2008; Li et al., 2006)
Feature 5: Inhibition
An auto-excitatory cortical network such as the piriform cortex is susceptible to run-away excitation and seizure activity. Thus, synaptic inhibition plays an important role in maintaining circuit function within manageable extremes. However, inhibition can also play important roles in shaping receptive fields of individual neurons, and imposing temporal structure in pyramidal spike trains and circuit oscillations. These factors have been assumed in past cortical models but were never fully developed (Haberly, 2001). We now know that there are a large variety of inhibitory interneurons in piriform cortex falling into perhaps five different classes based on morphology, location and physiological properties (Suzuki and Bekkers, 2010a, b; Young and Sun, 2009; Zhang et al., 2006), and these new data suggest unique roles for different cell classes. Interneurons have somata in all three layers of the piriform, and connections that can either remain within the same layer or spread to other layers. In addition to inter-laminar connections, the connectivity and function of inhibitory interneurons may also vary over the anterior-posterior extent of the piriform cortex. For example, pyramidal cells in anterior piriform cortex appear to be under stronger inhibitory control from interneurons more caudal to them than interneurons more rostral (Luna and Pettit, 2010). Rostral-caudal gradients may be particularly important in the piriform due to the fact that afferent input travels as a rostral-caudal wave over the cortex, rather than as a more synchronous input from thalamic inputs to large spatial extents of sensory neocortex.
Layer I GABAergic inhibitory interneurons, which are believed to mediate feedforward inhibition by receiving direct mitral/tufted cell input (Stokes and Isaacson, 2010) are more broadly tuned to odors than pyramidal cells (Miyamichi et al., 2011; Poo and Isaacson, 2009). These interneurons are hypothesized to have either a lower threshold or receive greater convergence of mitral/tufted cell inputs than pyramidal cells (Poo and Isaacson, 2009). Thus, while pyramidal cells express excitatory responses to relatively few odors in a test stimulus set, the same cells show broadly tuned inhibitory responses. Thus, as in other systems, inhibition can play an important role in shaping stimulus receptive fields.
Interneurons in Layers II and III are more typically targets of intracortical association fiber inputs or input from non-piriform sources. These GABAergic interneurons tend to terminate on pyramidal cell proximal dendrites, soma or axon initial segments and can be highly effective at blocking pyramidal cell output either via shunting inhibition or action potential blockade (Luna and Schoppa, 2008).
GABAergic interneurons in each layer also show a dichotomy in their response to excitatory synaptic input. A subset of interneurons in each layer show strong initial response to excitatory input evoking spiking output, while another subset show weaker initial responses but facilitation over repeated stimulation (Suzuki and Bekkers, 2010a). Suzuki and Bekkers suggest these differences in synaptic physiology could allow a temporal segregation of activity, with different interneurons producing output at different phases of the respiratory cycle.
The respiratory cycle is a strong source of oscillations throughout the olfactory pathway, however, several other spontaneous and induced oscillations are also prominent. For example, beta (15–35 Hz) and gamma (35–90 Hz) frequency oscillations can be robustly evoked in the piriform cortex, generally in phase with the 2–4 Hz respiratory cycle. Current source density analyses suggest that these higher frequencies oscillations derive from the cyclical afferent-association fiber activity loop, shaped by synaptic inhibition (Ketchum and Haberly, 1993). More recently, in vivo whole cell recordings from piriform cortex pyramidal cells supported this by showing that pyramidal cell spiking was phase locked to beta frequency oscillations and that this phase locking was partially governed by synaptic inhibition (Poo and Isaacson, 2009). As mentioned above, precise timing of pyramidal cell activity can reinforce temporal convergence of afferent synaptic excitation driven by the current odor input with association fiber synaptic excitation which reflects both ongoing sensory input and previous experience (due to experience-dependent synaptic potentiation during past odor stimuli). This temporal convergence may promote associative synaptic plasticity to help store a template of the now familiar odor pattern. It should be noted that inhibitory synapses themselves may express experience-dependent plasticity (Brosh and Barkai, 2009), further helping shape coding of familiar odors.
In summary, as in other systems, piriform cortical synaptic inhibition serves to shape receptive fields, sensory evoked responses and the temporal structure of cell output. Inhibitory neurons are not only targeted by afferent and intracortical excitatory inputs, but are also the targets of inputs from other regions (Luna, 2011; Mouly and Di Scala, 2006) and neuromodulators (Neville and Haberly, 2004). Thus, this suggests a role for inhibition in modulating cortical processing in a state- or experience-dependent manner.
Feature 6: Neuromodulation
The olfactory cortex is the target of neuromodulatory input from the noradrenergic nucleus locus coeruleus, the cholinergic nucleus of the horizontal limb of the diagonal band and the serotonergic raphe nucleus (Shipley and Ennis, 1996). These modulatory inputs shape cortical processing and circuit plasticity. For example, activation of the locus coeruleus enhances odor evoked responses and respiratory entrainment of piriform cortical neurons to the respiratory cycle (Bouret and Sara, 2002). This enhancement may have multiple contributing mechanisms, including NE effects in the olfactory bulb (Jiang et al., 1996), modulation of cortical association fiber synaptic efficacy (Hasselmo et al., 1997), and the cortical dishabituation mechanism described above (Smith et al., 2009). It nonetheless demonstrates the importance of behavioral state and noradrenergic tone on piriform cortical activity.
ACh similarly modulates activity and plasticity in the piriform cortex. ACh to the olfactory bulb and olfactory cortex derives from the horizontal limb of the diagonal band, as opposed to the medial septum which is the primary cholinergic input to the entorhinal cortex and hippocampal formation. Cholinergic muscarinic receptor activation selectively suppresses intrinsic association fiber synapses, with minimal effect on afferent fiber synapses (Hasselmo and Bower, 1992). ACh also modulates pyramidal cell excitability (Barkai and Hasselmo, 1994) and association fiber synaptic plasticity (Hasselmo and Barkai, 1995; Patil et al., 1998). Disruption of normal cholinergic activity within the piriform cortex impairs odor memory and discrimination of similar odors (De Rosa and Hasselmo, 2000; Fletcher and Wilson, 2002; Linster et al., 2001; Ravel et al., 1992; Saar et al., 2001; Wilson, 2001).
Feature 7: Sub-regional functional specialization
The piriform cortex encompasses the largest area within the olfactory cortex, and thus has received the most experimental attention. However, based on differences in anatomy between the piriform cortex and other olfactory cortical areas, it has been proposed that the olfactory cortex serves a parallel processing function, with different subregions supporting different functions due to different afferent inputs and different local circuits (Haberly, 2001). Again, recent evidence has supported that view.
Olfactory bulb output to the olfactory cortex varies by subregion. For example, while output from an individual glomerulus projects widely throughout anterior and posterior piriform cortex, projections to the cortical nuclei of the amygdala (COA) are more patchy, with different glomeruli projecting to different locations (Sosulski et al., 2011). Furthermore, all regions of the olfactory bulb project to the piriform cortex, while the COA is more strongly targeted by the dorsal olfactory bulb (Miyamichi et al., 2011). The loss of odor specific spatial patterns of input in the piriform cortex, and their at least partial maintenance in the COA may suggest a more labeled line mechanism of processing in the COA as opposed to the distributed, content addressable process in the piriform cortex. This more direct, odor-specific processing in COA may contribute to apparent innate hedonic responses to some odors (Khan et al., 2007; Kobayakawa et al., 2007).
The anterior olfactory nucleus (AON) can be divided into several subregions, and has a three layered structure roughly similar to that of the piriform cortex (Brunjes et al., 2005). The principal cell type is the pyramidal cell, and membrane and synaptic properties of pyramidal cells within the anterior olfactory nucleus are similar to those within the piriform cortex (McGinley and Westbrook, 2011). The majority of AON receives distributed olfactory bulb input, though the AON pars externa is more topographically organized relative to the bulb (Brunjes et al., 2005; Miyamichi et al., 2011). Individual neurons in AON respond to diverse odorants and odorant mixtures that activate spatially disparate olfactory bulb glomeruli (Lei et al., 2006) suggesting convergence of odorant feature input onto individual AON neurons. There appears to be no odor-specific spatial patterning of activity (Kay et al., 2011), similar to that seen in piriform cortex. In fact, Haberly has hypothesized that much of the initial odorant feature convergence involved in the early stages of building odor objects may occur in the AON (Haberly, 2001), allowing piriform cortex to perform more higher order associations between the odor objects and hedonics, context and other odors (see below).
The olfactory tubercle receives olfactory input dominated by tufted cells from the ventral olfactory bulb (Scott et al., 1980; Wesson and Wilson, 2011). This input may also show a patchy distribution like the COA, though this has not been quantified (Sosulski et al., 2011). Despite the direct olfactory bulb input, the olfactory tubercle has been primarily studied as a region involved in reward and addiction given its developmental and anatomical association with the ventral striatum (Heimer, 2003; Ikemoto, 2007). Thus, until recently relatively little has been known about its sensory physiology or role in olfaction (for a full review see: (Wesson and Wilson, 2011)). The major cell types show a diversity of physiological properties ranging from regular spiking to bursting that covary with cell morphology (Chiang and Strowbridge, 2007). Interestingly one class of bursting cells shows a strong initial burst to depolarization followed by an extended refractory period, suggesting it may play a specialized role in signal detection and stimulus onset. Olfactory tubercle neurons respond to odor (Murakami et al., 2005; Wesson and Wilson, 2010), and single-units respond differentially to different odors (Kikuta et al., 2008; Wesson and Wilson, 2010). Interestingly, tubercle single-units also show multi-sensory responses, with single-unit capable of responding to both odor and sound (Wesson and Wilson, 2010). The behavioral significance of this convergence is not known, but the data further emphasize that olfactory cortex, as is increasingly apparent in many sensory systems (Lakatos et al., 2007), is not a simple, uni-sensory cortex.
Thus, based on the anatomy and limited known sensory physiology, information leaving the olfactory bulb targets distinctly different olfactory cortical subregions, each of which transform that information in distinct ways and presumably with distinct impact on odor guided behavior. This regional specialization extends to the piriform cortex itself, which can be divided into at least two distinct sub-areas. The anterior and posterior piriform cortices have been demonstrated to process odors in distinct ways in both humans (Gottfried et al., 2006; Kirkwood et al., 1995) and rodents (Kadohisa and Wilson, 2006; Litaudon et al., 2003; Moriceau and Sullivan, 2004). It has been suggested that more caudal regions of the olfactory cortex are anatomically and functionally more similar to higher order association cortex than primary sensory cortex. In rodents, the division between anterior and posterior piriform cortex occurs as the lateral olfactory tract axons ends and Layer Ia reduces substantially in thickness. These more caudal regions receive input directly from mitral cells, but their relative contribution to pyramidal cell input diminishes in favor of association fiber input. Thus, while activity in anterior regions is strongly influenced by mitral cell afferent input, activity in more posterior regions becomes dominated by intracortical fiber input the olfactory cortex and other neighboring regions. This shift is even apparent in local field potential recordings which suggest a strong coherence between the anterior piriform cortex and olfactory bulb, while the posterior piriform cortex is more strongly coherent with the entorhinal cortex than with the olfactory bulb (Chabaud et al., 1999). Similarly, single-units in posterior piriform show less robust odor responses and are less in phase with respiration than anterior piriform neurons (Litaudon et al., 2003). Importantly, the nature of information encoded by these two regions also differs. In both humans (Gottfried et al., 2002; Howard et al., 2009) and rodents (Kadohisa and Wilson, 2006), anterior piriform cortex appears to encode information related to structural or perceptual identity of the odor, i.e., “banana”. More posterior regions, perhaps in accord with the dominance of association fiber input, appear to encode the perceptual category of odor, i.e., “fruity”.
The posterior piriform may also be involved in building search templates prior to odor sampling that assist in odor identification (Kirkwood et al., 1995). Using fMRI, Zelano et al., demonstrated that expectation of the arrival of a specific odor target creates target-specific patterns of activity in both the anterior and posterior piriform. At the arrival of the odor, anterior piriform activity appeared to continue reflecting the expected odor, while posterior piriform activity rapidly shifted to the actual, perceived odor. Further analyses, perhaps using higher temporal resolution techniques are warranted. Nonetheless, these results further emphasize the region-specific distributed processing of odor information across the olfactory cortex.
Finally, the most caudal region of the olfactory cortex is the lateral entorhinal cortex (LEC). Neurons in Layer II of the LEC receive input from the olfactory bulb and piriform cortex and their axons form the lateral perforant path into the hippocampal formation (Agster and Burwell, 2009; Haberly and Price, 1978; Kerr et al., 2007). Surprisingly little is known about the olfactory sensory physiology of the LEC. In awake rats, about a third of LEC single-units sampled (45/128 units) responded to odors (Young et al., 1997). It is important to note, as described below that the LEC not only receives input from the olfactory system but is also sends a strong feedback to both the olfactory bulb and piriform cortex (Ferry et al., 2006; Mouly and Di Scala, 2006). Work ongoing in our lab is currently further exploring LEC sensory physiology and top-down control of piriform cortex odor coding (Wilson, 2011).
4. Piriform cortex as a component of a larger network
As is true with any brain region, the piriform cortex functions within a larger context of forebrain activity. Direct, reciprocal connections have been demonstrated between all or parts of the olfactory cortex and the orbitofrontal cortex (Illig, 2005), amygdala (Majak et al., 2004) and perirhinal areas such as the entorhinal cortex (Haberly and Price, 1978; Kerr et al., 2007). These diverse connections add substantially to the richness of information available to the olfactory cortex, in terms of context, hedonic valence, reward and expectation. In fact, single-units in piriform cortex of rats performing an odor discrimination task show changes in activity relative to several components of the task in addition to odor sampling, including approach to the odor port prior to odor onset and entry to the water reward port (Schoenbaum and Eichenbaum, 1995; Zinyuk et al., 2001).
Associative odor learning modifies piriform cortical odor-evoked activity as assessed with single-unit recording (Calu et al., 2007; Roesch et al., 2007; Zinyuk et al., 2001), ensemble recording (Chapuis and Wilson, 2010; Kadohisa and Wilson, 2006), local field potential recording (Chapuis et al., 2009; Martin et al., 2006), 2-deoxyglucose uptake (Moriceau and Sullivan, 2004), and c-fos immune-reactivity (Datiche et al., 2001). These evoked response changes may reflect synaptic or neural plasticity within the olfactory cortex itself (Brosh and Barkai, 2004; Saar and Barkai, 2003), or reflect changes in functional connectivity within the larger network of which the olfactory cortex is a part (Martin et al., 2007; Martin et al., 2004). For example, odor learning modifies the synaptic strength of both olfactory bulb and orbitofrontal cortex projections to the piriform cortex (Cohen et al., 2008). As described above, this rich experience-dependent plasticity may be involved not only in associating odors with context or outcome, but also in helping modify sensory acuity for the familiar or learned odor (Chapuis and Wilson, 2010; Chen et al., 2011; Kadohisa and Wilson, 2006).
In addition to experience-dependent changes in functional connectivity of the olfactory cortex, connectivity is also influenced by behavioral state. Single-unit and local field potential responses to odor in the anterior piriform cortex are greatly reduced during slow-wave sleep (Barnes et al., 2011; Murakami et al., 2005; Wilson, 2010) and certain stages of anesthesia (Fontanini and Bower, 2005). Although there is a circadian rhythm in olfactory sensitivity in rodents (Granados-Fuentes et al., 2011), the sleep related cortical hyposensitivity is rapid, is selective to slow-wave sleep and not REM, and does not appear in the olfactory bulb (Barnes et al., 2011; Murakami et al., 2005; Wilson, 2010). Piriform cortical activity during slow-wave sleep is dominated by sharp-waves (Manabe et al., 2011), similar to those observed in hippocampus (Buzsaki, 1986), and single-unit activity during these sharp-waves is shaped by recent odor experience (Wilson, 2010). This latter observation may suggest an opportunity for odor “replay” during slow-wave sleep while the cortex is otherwise hypo-responsive to afferent input. Such replay could help consolidate intracortical association fiber plasticity underlying memory of new odor objects (Wilson, 2010), as well as send a strong excitatory feedback to olfactory bulb that could be critical for survival of odor-specific populations of newborn granule cells (Manabe et al., 2011).
While the piriform cortex is in this hypo-responsive state, it becomes more strongly coherent with other regions such as the dorsal hippocampus and basolateral amygdala compared to waking or fast-wave activity, as assessed with local field potential recordings and fMRI (Wilson et al., 2011; Wilson and Yan, 2010). This change in functional connectivity toward more central circuits during a time of reduced sensitivity to afferent input, may be important for consolidation of odor memory, perhaps allowing association of information about odor quality with context and emotion. In fact, the time spent in slow-wave sleep is enhanced following odor learning (Eschenko et al., 2008; Magloire and Cattarelli, 2009). Following odor fear conditioning, the magnitude of this increase as recorded in the piriform cortex is significantly correlated the intensity of the odor-evoked fear the following day (Barnes et al., 2011).
From these specific examples, it is clear that the olfactory cortex does not function in isolation, but rather is modulated by top-down influences and the strength of those influences can be modified by past experience and current state. Furthermore, the olfactory cortex provides a strong feedback to its primary afferent, the olfactory bulb – a feedback which again can be modified by experience (Gao and Strowbridge, 2009).
5. Piriform cortex across the lifespan
As a cortical structure with non-topographic inputs, relatively little is known about the ontogeny of the olfactory cortex. Afferent- and odor-evoked piriform cortical activity emerge relatively early in the postnatal rat (Illig, 2007; Schwob et al., 1984). In fact, the neonatal piriform cortex and its input, the olfactory bulb, are required for survival dependent behaviors in the infant rat, including orienting to the mother and nipple attachment (Greer et al., 1982; Hofer et al., 1976; Moriceau and Sullivan, 2004; Raineki et al., 2010; Roth and Sullivan, 2005; Singh and Tobach, 1975; Sullivan et al., 1990). Indeed, it was pups’ dependence on maternal odor for survival that led to the old notion that maternal odor was a pheromone (Leon et al., 1977). However, extensive research has demonstrated that the maternal odor is associatively learned perinatally, and a novel odor paired with maternal care or sensory stimuli mimicking maternal care (i.e. tactile stimulation or milk), takes on the characteristics of maternal odor to enable pups to contact the mother and nipple attach (Hofer et al., 1976; Pedersen et al., 1982; Raineki et al., 2010; Roth and Sullivan, 2005; Sullivan et al., 1990). This artificial maternal odor appears to produce olfactory bulb and piriform cortex responses similar to the natural maternal odor (Raineki et al., 2010; Roth and Sullivan, 2005; Sullivan et al., 1990).
The rules applying to neocortical development, with thalamic afferents invading the cortical plate from below, and the subsequent emergence of multiple layers and topographically organized cortical columns, are not appropriate for the paleocortex (Sarma et al., 2010; Schwob and Price, 1984). Nonetheless, several similarities with neocortical (and hippocampal) development do apply. For example, during early development afferent inputs to the piriform cortex show robust plasticity in response to manipulations of sensory input (Best and Wilson, 2003), or as expressed by NMDA-dependent synaptic potentiation (Poo and Isaacson, 2007). Within a few weeks, however, this plasticity subsides, suggesting a sensitive period for afferent plasticity. In the case of NMDA-dependent long-term potentiation, the critical period termination coincides with a down-regulation of NMDA receptor mediated currents (Franks and Isaacson, 2005). This NMDA receptor down-regulation can be delayed by sensory deprivation, suggesting an activity dependent role in shaping afferent synapses during early development (Franks and Isaacson, 2005). While afferent synapses show an early sensitive period for plasticity, association fiber synapses do not (Best and Wilson, 2003; Poo and Isaacson, 2007). Plasticity in association fiber synapses is maintained throughout life and, as described above remain critical for odor learning and perception. These developmental characteristics of afferent and association fiber plasticity match those reported in the thalamocortical visual system (Crair and Malenka, 1995; Kirkwood et al., 1995).
Finally, while age and dementia related changes in olfactory perception are well documented (Albers et al., 2006; Murphy, 1999), relatively little is known about normal aging in the olfactory cortex. However, recent studies have suggested a possible role for the piriform cortex in dementia related olfactory perceptual losses. In both humans with Alzheimer’s disease (Li et al., 2010a; Wang et al., 2010) and mice over-expressing human amyloid precursor protein (Wesson et al., 2011; Wesson et al., 2010), piriform cortical dysfunction correlated strongly with odor perceptual or memory impairments. While amyloid beta burden can induce pathology throughout the olfactory system from the olfactory sensory neurons (Talamo et al., 1989) to the entorhinal cortex (Braak and Braak, 1992), the piriform cortex appears to be a major contributor to the overall sensory decline.
6. Summary and open questions
The olfactory cortex is divided into several subregions based on local anatomy and patterns of afferent input producing a parallel, distributed processing of olfactory bulb odor-evoked spatiotemporal activity patterns. The piriform cortex functions as a pattern recognition device capable of content addressable memory which allows storage of familiar input patterns across ensembles of distributed neurons through plasticity of intracortical association fiber synapses binding these dispersed neurons. This form of synthetic pattern recognition allows formation of odor objects from complex odorant features. Odor object processing allows for pattern completion in the face of degraded inputs which facilitates perceptual stability. As input patterns further diverge from familiar, stored templates, cortical pattern separation comes to dominate which promotes perceptual discrimination. The plasticity within the olfactory cortex and between the cortex and its monosynaptic partners allows experience-dependent change in odor coding and perceptual acuity (perceptual learning). Modulatory inputs and state-dependent changes in functional connectivity further allow adjustments in odor coding and association of odor quality with context and hedonics. Together, these processes place neural plasticity and memory at the heart of odor perception (Stevenson and Wilson, 2007; Wilson and Stevenson, 2003), similar to object perception in other sensory systems and spatial memory in the hippocampal formation.
A window is opening to allow a view of what the olfactory cortex contributes to odor perception and how, but a myriad of questions remain. While some are beginning to be addressed, much work lies ahead. A small sample of these questions include, where does conscious perception of odors occur within the brain (Li et al., 2010b)? How does attention to odor influence processing and perception (Plailly et al., 2008)? What are the effects of top-down influences on olfactory cortical sensory physiology and perception (Martin et al., 2007; Mouly and Di Scala, 2006)? Is the reduced ability to perceptually analyze odorant mixtures into their components (Laing and Francis, 1989) due to the lack of a spatial cortical code? How is odor intensity encoded in the olfactory cortex (Anderson et al., 2003)? Why does the olfactory system seem so sensitive to neurodegenerative disease (Li et al., 2010a; Wesson et al., 2010)? How do the rules for ontogeny of a non-topographic cortex differ from those involved in topographic neocortex (Sarma et al., 2010; Schwob and Price, 1984)? Pursuing these questions will not only further our understanding of olfaction, but also about how very simple circuits produce such profound outcomes as the scent of a rose.
Figure 2.
Piriform cortical ensemble processing of odorants. (A) Odorant features, encoded as spatiotemporal patterns in olfactory bulb glomeruli and their outputs, converge in the piriform cortex through broadly dispersed, non-topographic projections. Information is also distributed through broad intracortical association fibers projections. Neurons activated by a given odor (black cells) are broadly distributed. Spiking in individual neurons can driven directly by afferent input (cell a), directly by association fiber input (cell b), or the combination (cell c). (B) Given the distributed afferent and association fiber projections, different odorants (X and Y) can activate widely distributed, overlapping ensembles of neurons. As described in the text, plasticity of association fiber synapses allows for pattern completion in the event of degraded familiar afferent inputs.
Acknowledgments
Funding : Work described here has been funded by DC03906, DC008982 and AG037693 to DAW and MH091451 and DC009910 to RMS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agster KL, Burwell RD. Cortical efferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. Hippocampus. 2009;19:1159–1186. doi: 10.1002/hipo.20578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers MW, Tabert MH, Devanand DP. Olfactory dysfunction as a predictor of neurodegenerative disease. Curr Neurol Neurosci Rep. 2006;6:379–386. doi: 10.1007/s11910-996-0018-7. [DOI] [PubMed] [Google Scholar]
- Ambros-Ingerson J, Granger R, Lynch G. Simulation of paleocortex performs hierarchical clustering. Science. 1990;247:1344–1348. doi: 10.1126/science.2315702. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, Gabrieli JD, Sobel N. Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci. 2003;6:196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Apicella A, Yuan Q, Scanziani M, Isaacson JS. Pyramidal cells in piriform cortex receive convergent input from distinct olfactory bulb glomeruli. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:14255–14260. doi: 10.1523/JNEUROSCI.2747-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araneda RC, Kini AD, Firestein S. The molecular receptive range of an odorant receptor. Nat Neurosci. 2000;3:1248–1255. doi: 10.1038/81774. [DOI] [PubMed] [Google Scholar]
- Barkai E, Bergman RE, Horwitz G, Hasselmo ME. Modulation of associative memory function in a biophysical simulation of rat piriform cortex. J Neurophysiol. 1994;72:659–677. doi: 10.1152/jn.1994.72.2.659. [DOI] [PubMed] [Google Scholar]
- Barkai E, Hasselmo ME. Modulation of the input/output function of rat piriform cortex pyramidal cells. J Neurophysiol. 1994;72:644–658. doi: 10.1152/jn.1994.72.2.644. [DOI] [PubMed] [Google Scholar]
- Barnes DC, Chapuis J, Chaudhury D, Wilson DA. Odor fear conditioning modifies piriform cortex local field potentials both during conditioning and during post-conditioning sleep. PLoS ONE. 2011;6:e18130. doi: 10.1371/journal.pone.0018130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes DC, Hofacer RD, Zaman AR, Rennaker RL, Wilson DA. Olfactory perceptual stability and discrimination. Nat Neurosci. 2008;11:1378–1380. doi: 10.1038/nn.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan M, Haberly LB. Intrinsic and efferent connections of the endopiriform nucleus in rat. J Comp Neurol. 1999;408:532–548. [PubMed] [Google Scholar]
- Bell H, Chenoweth B, Wilson DA. Neurobehavioral consequences of cortical adaptation disruption during ontogeny. Neurosci Lett. 2008;445:47–52. doi: 10.1016/j.neulet.2008.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best AR, Thompson JV, Fletcher ML, Wilson DA. Cortical metabotropic glutamate receptors contribute to habituation of a simple odor-evoked behavior. J Neurosci. 2005;25:2513–2517. doi: 10.1523/JNEUROSCI.5298-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best AR, Wilson DA. A postnatal sensitive period for plasticity of cortical afferents but not cortical association fibers in rat piriform cortex. Brain Res. 2003;961:81–87. doi: 10.1016/s0006-8993(02)03847-7. [DOI] [PubMed] [Google Scholar]
- Best AR, Wilson DA. Coordinate synaptic mechanisms contributing to olfactory cortical adaptation. J Neurosci. 2004;24:652–660. doi: 10.1523/JNEUROSCI.4220-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret S, Sara SJ. Locus coeruleus activation modulates firing rate and temporal organization of odour-induced single-cell responses in rat piriform cortex. Eur J Neurosci. 2002;16:2371–2382. doi: 10.1046/j.1460-9568.2002.02413.x. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. The human entorhinal cortex: normal morphology and lamina-specific pathology in various diseases. Neuroscience research. 1992;15:6–31. doi: 10.1016/0168-0102(92)90014-4. [DOI] [PubMed] [Google Scholar]
- Brosh I, Barkai E. Learning-induced long-term synaptic modifications in the olfactory cortex. Curr Neurovasc Res. 2004;1:389–395. doi: 10.2174/1567202043362090. [DOI] [PubMed] [Google Scholar]
- Brosh I, Barkai E. Learning-induced enhancement of feedback inhibitory synaptic transmission. Learn Mem. 2009;16:413–416. doi: 10.1101/lm.1430809. [DOI] [PubMed] [Google Scholar]
- Brunjes PC, Illig KR, Meyer EA. A field guide to the anterior olfactory nucleus (cortex) Brain Res Brain Res Rev. 2005;50:305–335. doi: 10.1016/j.brainresrev.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Buonviso N, Revial MF, Jourdan F. The Projections of Mitral Cells from Small Local Regions of the Olfactory Bulb: An Anterograde Tracing Study Using PHA-L (Phaseolus vulgaris Leucoagglutinin) Eur J Neurosci. 1991;3:493–500. doi: 10.1111/j.1460-9568.1991.tb00836.x. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Hippocampal sharp waves: their origin and significance. Brain Res. 1986;398:242–252. doi: 10.1016/0006-8993(86)91483-6. [DOI] [PubMed] [Google Scholar]
- Cai Z, Saugstad JA, Sorensen SD, Ciombor KJ, Zhang C, Schaffhauser H, Hubalek F, Pohl J, Duvoisin RM, Conn PJ. Cyclic AMP-dependent protein kinase phosphorylates group III metabotropic glutamate receptors and inhibits their function as presynaptic receptors. J Neurochem. 2001;78:756–766. doi: 10.1046/j.1471-4159.2001.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calu DJ, Roesch MR, Stalnaker TA, Schoenbaum G. Associative Encoding in Posterior Piriform Cortex during Odor Discrimination and Reversal Learning. Cereb Cortex. 2007;17:1342–1349. doi: 10.1093/cercor/bhl045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capurso SA, Calhoun ME, Sukhov RR, Mouton PR, Price DL, Koliatsos VE. Deafferentation causes apoptosis in cortical sensory neurons in the adult rat. J Neurosci. 1997;17:7372–7384. doi: 10.1523/JNEUROSCI.17-19-07372.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattarelli M, Astic L, Kauer JS. Metabolic mapping of 2-deoxyglucose uptake in the rat piriform cortex using computerized image processing. Brain Res. 1988;442:180–184. doi: 10.1016/0006-8993(88)91449-7. [DOI] [PubMed] [Google Scholar]
- Chabaud P, Ravel N, Wilson DA, Gervais R. Functional coupling in rat central olfactory pathways: a coherence analysis. Neurosci Lett. 1999;276:17–20. doi: 10.1016/s0304-3940(99)00773-9. [DOI] [PubMed] [Google Scholar]
- Chapuis J, Garcia S, Messaoudi B, Thevenet M, Ferreira G, Gervais R, Ravel N. The way an odor is experienced during aversive conditioning determines the extent of the network recruited during retrieval: a multisite electrophysiological study in rats. J Neurosci. 2009;29:10287–10298. doi: 10.1523/JNEUROSCI.0505-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapuis J, Wilson DA. Society for Neuroscience (San Diego, CA) 2010. Effect of olfactory learning on pattern separation and completion processes in the piriform cortex. [Google Scholar]
- Chen CF, Barnes DC, Wilson DA. Generalized versus stimulus-specific learned fear differentially modifies stimulus encoding in primary sensory cortex of awake rats. Journal of Neurophysiology. 2011 doi: 10.1152/jn.00721.2011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang E, Strowbridge BW. Diversity of neural signals mediated by multiple, burst-firing mechanisms in rat olfactory tubercle neurons. Journal of Neurophysiology. 2007;98:2716–2728. doi: 10.1152/jn.00807.2007. [DOI] [PubMed] [Google Scholar]
- Choi GB, Stettler DD, Kallman BR, Bhaskar ST, Fleischmann A, Axel R. Driving opposing behaviors with ensembles of piriform neurons. Cell. 2011;146:1004–1015. doi: 10.1016/j.cell.2011.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland TA, Linster C. Central olfactory structures. Handbook of olfaction and gustation. 2003:165–180. doi: 10.1016/B978-0-444-63855-7.00006-X. [DOI] [PubMed] [Google Scholar]
- Cohen Y, Reuveni I, Barkai E, Maroun M. Olfactory learning-induced long-lasting enhancement of descending and ascending synaptic transmission to the piriform cortex. J Neurosci. 2008;28:6664–6669. doi: 10.1523/JNEUROSCI.0178-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair MC, Malenka RC. A critical period for long-term potentiation at thalamocortical synapses. Nature. 1995;375:325–328. doi: 10.1038/375325a0. [DOI] [PubMed] [Google Scholar]
- Datiche F, Roullet F, Cattarelli M. Expression of Fos in the piriform cortex after acquisition of olfactory learning: an immunohistochemical study in the rat. Brain Res Bull. 2001;55:95–99. doi: 10.1016/s0361-9230(01)00499-3. [DOI] [PubMed] [Google Scholar]
- Davis RL. Traces of Drosophila memory. Neuron. 2011;70:8–19. doi: 10.1016/j.neuron.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison IG, Ehlers MD. Neural circuit mechanisms for pattern detection and feature combination in olfactory cortex. Neuron. 2011;70:82–94. doi: 10.1016/j.neuron.2011.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa E, Hasselmo ME. Muscarinic cholinergic neuromodulation reduces proactive interference between stored odor memories during associative learning in rats. Behav Neurosci. 2000;114:32–41. [PubMed] [Google Scholar]
- Doucette W, Gire DH, Whitesell J, Carmean V, Lucero MT, Restrepo D. Associative cortex features in the first olfactory brain relay station. Neuron. 2011;69:1176–1187. doi: 10.1016/j.neuron.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrand JJ, Domroese ME, Johnson DM, Feig SL, Knodel SM, Behan M, Haberly LB. A new subdivision of anterior piriform cortex and associated deep nucleus with novel features of interest for olfaction and epilepsy. J Comp Neurol. 2001;434:289–307. doi: 10.1002/cne.1178. [DOI] [PubMed] [Google Scholar]
- Eschenko O, Ramadan W, Molle M, Born J, Sara SJ. Sustained increase in hippocampal sharp-wave ripple activity during slow-wave sleep after learning. Learn Mem. 2008;15:222–228. doi: 10.1101/lm.726008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry B, Ferreira G, Traissard N, Majchrzak M. Selective involvement of the lateral entorhinal cortex in the control of the olfactory memory trace during conditioned odor aversion in the rat. Behavioral Neuroscience. 2006;120:1180–1186. doi: 10.1037/0735-7044.120.5.1180. [DOI] [PubMed] [Google Scholar]
- Fletcher ML, Wilson DA. Experience modifies olfactory acuity: acetylcholine-dependent learning decreases behavioral generalization between similar odorants. J Neurosci. 2002;22:RC201. doi: 10.1523/JNEUROSCI.22-02-j0005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanini A, Bower JM. Variable coupling between olfactory system activity and respiration in ketamine/xylazine anesthetized rats. J Neurophysiol. 2005;93:3573–3581. doi: 10.1152/jn.01320.2004. [DOI] [PubMed] [Google Scholar]
- Franks KM, Isaacson JS. Synapse-specific downregulation of NMDA receptors by early experience: a critical period for plasticity of sensory input to olfactory cortex. Neuron. 2005;47:101–114. doi: 10.1016/j.neuron.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Franks KM, Isaacson JS. Strong single-fiber sensory inputs to olfactory cortex: implications for olfactory coding. Neuron. 2006;49:357–363. doi: 10.1016/j.neuron.2005.12.026. [DOI] [PubMed] [Google Scholar]
- Franks KM, Russo MJ, Sosulski DL, Mulligan AA, Siegelbaum SA, Axel R. Recurrent circuitry dynamically shapes the activation of piriform cortex. Neuron. 2011;72:49–56. doi: 10.1016/j.neuron.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich RW. Mechanisms of odor discrimination: neurophysiological and behavioral approaches. Trends Neurosci. 2006;29:40–47. doi: 10.1016/j.tins.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Friedrich RW, Laurent G. Dynamic optimization of odor representations by slow temporal patterning of mitral cell activity. Science. 2001;291:889–894. doi: 10.1126/science.291.5505.889. [DOI] [PubMed] [Google Scholar]
- Gao Y, Strowbridge BW. Long-term plasticity of excitatory inputs to granule cells in the rat olfactory bulb. Nature Neuroscience. 2009;12:731–733. doi: 10.1038/nn.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Larson SD, Hefzi H, Marnoy Z, Cutforth T, Dokka K, Baldwin KK. Sensory maps in the olfactory cortex defined by long-range viral tracing of single neurons. Nature. 2011;472:217–220. doi: 10.1038/nature09945. [DOI] [PubMed] [Google Scholar]
- Gottfried JA. Central mechanisms of odour object perception. Nat Rev Neurosci. 2010;11:628–641. doi: 10.1038/nrn2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, Deichmann R, Winston JS, Dolan RJ. Functional heterogeneity in human olfactory cortex: an event-related functional magnetic resonance imaging study. J Neurosci. 2002;22:10819–10828. doi: 10.1523/JNEUROSCI.22-24-10819.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, Winston JS, Dolan RJ. Dissociable codes of odor quality and odorant structure in human piriform cortex. Neuron. 2006;49:467–479. doi: 10.1016/j.neuron.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Granados-Fuentes D, Ben-Josef G, Perry G, Wilson DA, Sullivan-Wilson A, Herzog ED. Daily rhythms in olfactory discrimination depend on clock genes, but not the suprachiasmatic nucleus. Journal of Biological Rhythms. 2011 doi: 10.1177/0748730411420247. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger R, Lynch G. Higher olfactory processes: perceptual learning and memory. Curr Opin Neurobiol. 1991;1:209–214. doi: 10.1016/0959-4388(91)90080-q. [DOI] [PubMed] [Google Scholar]
- Greer CA, Stewart WB, Teicher MH, Shepherd GM. Functional development of the olfactory bulb and a unique glomerular complex in the neonatal rat. The Journal of Neuroscience. 1982;2:1744–1759. doi: 10.1523/JNEUROSCI.02-12-01744.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberly LB. Neuronal circuitry in olfactory cortex: anatomy and functional implications. Chemical Senses. 1985;10:219–238. [Google Scholar]
- Haberly LB. Parallel-distributed processing in olfactory cortex: new insights from morphological and physiological analysis of neuronal circuitry. Chem Senses. 2001;26:551–576. doi: 10.1093/chemse/26.5.551. [DOI] [PubMed] [Google Scholar]
- Haberly LB, Bower JM. Olfactory cortex: model circuit for study of associative memory? Trends Neurosci. 1989;12:258–264. doi: 10.1016/0166-2236(89)90025-8. [DOI] [PubMed] [Google Scholar]
- Haberly LB, Price JL. Association and commissural fiber systems of the olfactory cortex of the rat. I. systems originating in the piriform cortex and adjacent areas. J Comp Neurol. 1978;178:711–740. doi: 10.1002/cne.901780408. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Anderson BP, Bower JM. Cholinergic modulation of cortical associative memory function. J Neurophysiol. 1992;67:1230–1246. doi: 10.1152/jn.1992.67.5.1230. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Barkai E. Cholinergic modulation of activity-dependent synaptic plasticity in the piriform cortex and associative memory function in a network biophysical simulation. J Neurosci. 1995;15:6592–6604. doi: 10.1523/JNEUROSCI.15-10-06592.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Bower JM. Cholinergic suppression specific to intrinsic not afferent fiber synapses in rat piriform (olfactory) cortex. J Neurophysiol. 1992;67:1222–1229. doi: 10.1152/jn.1992.67.5.1222. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Linster C, Patil M, Ma D, Cekic M. Noradrenergic suppression of synaptic transmission may influence cortical signal-to-noise ratio. J Neurophysiol. 1997;77:3326–3339. doi: 10.1152/jn.1997.77.6.3326. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, McGaughy J. High acetylcholine levels set circuit dynamics for attention and encoding and low acetylcholine levels set dynamics for consolidation. Prog Brain Res. 2004;145:207–231. doi: 10.1016/S0079-6123(03)45015-2. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Wilson MA, Anderson BP, Bower JM. Associative memory function in piriform (olfactory) cortex: computational modeling and neuropharmacology. Cold Spring Harb Symp Quant Biol. 1990;55:599–610. doi: 10.1101/sqb.1990.055.01.057. [DOI] [PubMed] [Google Scholar]
- Heimer L. A new anatomical framework for neuropsychiatric disorders and drug abuse. The American journal of psychiatry. 2003;160:1726–1739. doi: 10.1176/appi.ajp.160.10.1726. [DOI] [PubMed] [Google Scholar]
- Heimer L, Kalil R. Rapid transneuronal degeneration and death of cortical neurons following removal of the olfactory bulb in adult rats. The Journal of Comparative Neurology. 1978;178:559–609. doi: 10.1002/cne.901780310. [DOI] [PubMed] [Google Scholar]
- Hofer MA, Shair H, Singh P. Evidence that maternal ventral skin substances promote suckling in infant rats. Physiology & Behavior. 1976;17:131–136. doi: 10.1016/0031-9384(76)90279-1. [DOI] [PubMed] [Google Scholar]
- Howard JD, Plailly J, Grueschow M, Haynes JD, Gottfried JA. Odor quality coding and categorization in human posterior piriform cortex. Nat Neurosci. 2009;12:932–938. doi: 10.1038/nn.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain research reviews. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illig KR. Projections from orbitofrontal cortex to anterior piriform cortex in the rat suggest a role in olfactory information processing. J Comp Neurol. 2005;488:224–231. doi: 10.1002/cne.20595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illig KR. Developmental changes in odor-evoked activity in rat piriform cortex. Neuroscience. 2007;145:370–376. doi: 10.1016/j.neuroscience.2006.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illig KR, Haberly LB. Odor-evoked activity is spatially distributed in piriform cortex. J Comp Neurol. 2003;457:361–373. doi: 10.1002/cne.10557. [DOI] [PubMed] [Google Scholar]
- Jiang M, Griff ER, Ennis M, Zimmer LA, Shipley MT. Activation of locus coeruleus enhances the responses of olfactory bulb mitral cells to weak olfactory nerve input. J Neurosci. 1996;16:6319–6329. doi: 10.1523/JNEUROSCI.16-19-06319.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Leon M. Chemotopic odorant coding in a mammalian olfactory system. J Comp Neurol. 2007;503:1–34. doi: 10.1002/cne.21396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DM, Illig KR, Behan M, Haberly LB. New features of connectivity in piriform cortex visualized by intracellular injection of pyramidal cells suggest that “primary” olfactory cortex functions like “association” cortex in other sensory systems. J Neurosci. 2000;20:6974–6982. doi: 10.1523/JNEUROSCI.20-18-06974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones WD, Cayirlioglu P, Kadow IG, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445:86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- Kadohisa M, Wilson DA. Separate encoding of identity and similarity of complex familiar odors in piriform cortex. Proc Natl Acad Sci U S A. 2006;103:15206–15211. doi: 10.1073/pnas.0604313103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanter ED, Haberly LB. NMDA-dependent induction of long-term potentiation in afferent and association fiber systems of piriform cortex in vitro. Brain Res. 1990;525:175–179. doi: 10.1016/0006-8993(90)91337-g. [DOI] [PubMed] [Google Scholar]
- Kay RB, Meyer EA, Illig KR, Brunjes PC. Spatial distribution of neural activity in the anterior olfactory nucleus evoked by odor and electrical stimulation. The Journal of comparative neurology. 2011;519:277–289. doi: 10.1002/cne.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr KM, Agster KL, Furtak SC, Burwell RD. Functional neuroanatomy of the parahippocampal region: the lateral and medial entorhinal areas. Hippocampus. 2007;17:697–708. doi: 10.1002/hipo.20315. [DOI] [PubMed] [Google Scholar]
- Ketchum KL, Haberly LB. Synaptic events that generate fast oscillations in piriform cortex. J Neurosci. 1993;13:3980–3985. doi: 10.1523/JNEUROSCI.13-09-03980.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan RM, Luk CH, Flinker A, Aggarwal A, Lapid H, Haddad R, Sobel N. Predicting odor pleasantness from odorant structure: pleasantness as a reflection of the physical world. J Neurosci. 2007;27:10015–10023. doi: 10.1523/JNEUROSCI.1158-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuta S, Kashiwadani H, Mori K. Compensatory rapid switching of binasal inputs in the olfactory cortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:11989–11997. doi: 10.1523/JNEUROSCI.3106-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Lee HK, Bear MF. Co-regulation of long-term potentiation and experience-dependent synaptic plasticity in visual cortex by age and experience. Nature. 1995;375:328–331. doi: 10.1038/375328a0. [DOI] [PubMed] [Google Scholar]
- Knafo S, Grossman Y, Barkai E, Benshalom G. Olfactory learning is associated with increased spine density along apical dendrites of pyramidal neurons in the rat piriform cortex. Eur J Neurosci. 2001;13:633–638. doi: 10.1046/j.1460-9568.2001.01422.x. [DOI] [PubMed] [Google Scholar]
- Kobayakawa K, Kobayakawa R, Matsumoto H, Oka Y, Imai T, Ikawa M, Okabe M, Ikeda T, Itohara S, Kikusui T, et al. Innate versus learned odour processing in the mouse olfactory bulb. Nature. 2007;450:503–508. doi: 10.1038/nature06281. [DOI] [PubMed] [Google Scholar]
- Laing DG, Francis GW. The capacity of humans to identify odors in mixtures. Physiol Behav. 1989;46:809–814. doi: 10.1016/0031-9384(89)90041-3. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Chen CM, O’Connell MN, Mills A, Schroeder CE. Neuronal oscillations and multisensory interaction in primary auditory cortex. Neuron. 2007;53:279–292. doi: 10.1016/j.neuron.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel D, Grossman Y, Barkai E. Olfactory learning modifies predisposition for long-term potentiation and long-term depression induction in the rat piriform (olfactory) cortex. Cerebral Cortex. 2001;11:485–489. doi: 10.1093/cercor/11.6.485. [DOI] [PubMed] [Google Scholar]
- Lei H, Mooney R, Katz LC. Synaptic integration of olfactory information in mouse anterior olfactory nucleus. J Neurosci. 2006;26:12023–12032. doi: 10.1523/JNEUROSCI.2598-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon M, Galef BG, Jr, Behse JH. Establishment of pheromonal bonds and diet choice in young rats by odor pre-exposure. Physiol Behav. 1977;18:387–391. [Google Scholar]
- Leung CH, Wilson DA. Trans-neuronal regulation of cortical apoptosis in the adult rat olfactory system. Brain Res. 2003;984:182–188. doi: 10.1016/s0006-8993(03)03129-9. [DOI] [PubMed] [Google Scholar]
- Li W, Howard JD, Gottfried JA. Disruption of odour quality coding in piriform cortex mediates olfactory deficits in Alzheimer’s disease. Brain. 2010a;133:2714–2726. doi: 10.1093/brain/awq209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Howard JD, Parrish TB, Gottfried JA. Aversive learning enhances perceptual and cortical discrimination of indiscriminable odor cues. Science. 2008;319:1842–1845. doi: 10.1126/science.1152837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Lopez L, Osher J, Howard JD, Parrish TB, Gottfried JA. Right orbitofrontal cortex mediates conscious olfactory perception. Psychological Science. 2010b;21:1454–1463. doi: 10.1177/0956797610382121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Luxenberg E, Parrish T, Gottfried JA. Learning to smell the roses: experience-dependent neural plasticity in human piriform and orbitofrontal cortices. Neuron. 2006;52:1097–1108. doi: 10.1016/j.neuron.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DY, Shea SD, Katz LC. Representation of natural stimuli in the rodent main olfactory bulb. Neuron. 2006;50:937–949. doi: 10.1016/j.neuron.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Linster C, Garcia PA, Hasselmo ME, Baxter MG. Selective loss of cholinergic neurons projecting to the olfactory system increases perceptual generalization between similar, but not dissimilar, odorants. Behav Neurosci. 2001;115:826–833. doi: 10.1037//0735-7044.115.4.826. [DOI] [PubMed] [Google Scholar]
- Linster C, Henry L, Kadohisa M, Wilson DA. Synaptic adaptation and odor-background segmentation. Neurobiol Learn Mem. 2007;87:352–360. doi: 10.1016/j.nlm.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Linster C, Menon AV, Singh CY, Wilson DA. Odor-specific habituation arises from interaction of afferent synaptic adaptation and intrinsic synaptic potentiation in olfactory cortex. Learn Mem. 2009;16:452–459. doi: 10.1101/lm.1403509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litaudon P, Amat C, Bertrand B, Vigouroux M, Buonviso N. Piriform cortex functional heterogeneity revealed by cellular responses to odours. Eur J Neurosci. 2003;17:2457–2461. doi: 10.1046/j.1460-9568.2003.02654.x. [DOI] [PubMed] [Google Scholar]
- Litaudon P, Mouly AM, Sullivan R, Gervais R, Cattarelli M. Learning-induced changes in rat piriform cortex activity mapped using multisite recording with voltage sensitive dye. Eur J Neurosci. 1997;9:1593–1602. doi: 10.1111/j.1460-9568.1997.tb01517.x. [DOI] [PubMed] [Google Scholar]
- Luna VM. The Piriform Cortex Utilizes Different Microcircuits to Process Cortical and Amygdaloid Synaptic Inputs. Association for Chemoreception Sciences Annual meeting; St. Pete’s Beach, FL. 2011. [Google Scholar]
- Luna VM, Pettit DL. Asymmetric rostro-caudal inhibition in the primary olfactory cortex. Nat Neurosci. 2010;13:533–535. doi: 10.1038/nn.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna VM, Schoppa NE. GABAergic circuits control input-spike coupling in the piriform cortex. J Neurosci. 2008;28:8851–8859. doi: 10.1523/JNEUROSCI.2385-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magloire V, Cattarelli M. Delayed changes of sleep duration after rewarded olfactory discrimination learning in the rat. Behav Brain Res. 2009;205:568–571. doi: 10.1016/j.bbr.2009.08.039. [DOI] [PubMed] [Google Scholar]
- Majak K, Ronkko S, Kemppainen S, Pitkanen A. Projections from the amygdaloid complex to the piriform cortex: A PHA-L study in the rat. J Comp Neurol. 2004;476:414–428. doi: 10.1002/cne.20233. [DOI] [PubMed] [Google Scholar]
- Manabe H, Kusumoto-Yoshida I, Ota M, Mori K. Olfactory cortex generates synchronized top-down inputs to the olfactory bulb during slow-wave sleep. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:8123–8133. doi: 10.1523/JNEUROSCI.6578-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D. Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- Martin C, Beshel J, Kay LM. An olfacto-hippocampal network is dynamically involved in odor-discrimination learning. J Neurophysiol. 2007;98:2196–2205. doi: 10.1152/jn.00524.2007. [DOI] [PubMed] [Google Scholar]
- Martin C, Gervais R, Chabaud P, Messaoudi B, Ravel N. Learning-induced modulation of oscillatory activities in the mammalian olfactory system: the role of the centrifugal fibres. J Physiol Paris. 2004;98:467–478. doi: 10.1016/j.jphysparis.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Martin C, Gervais R, Messaoudi B, Ravel N. Learning-induced oscillatory activities correlated to odour recognition: a network activity. Eur J Neurosci. 2006;23:1801–1810. doi: 10.1111/j.1460-9568.2006.04711.x. [DOI] [PubMed] [Google Scholar]
- McGinley MJ, Westbrook GL. Membrane and synaptic properties of pyramidal neurons in the anterior olfactory nucleus. Journal of neurophysiology. 2011;105:1444–1453. doi: 10.1152/jn.00715.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara AM, Magidson PD, Linster C, Wilson DA, Cleland TA. Distinct neural mechanisms mediate olfactory memory formation at different timescales. Learn Mem. 2008;15:117–125. doi: 10.1101/lm.785608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith M. Patterned response to odor in mammalian olfactory bulb: the influence of intensity. J Neurophysiol. 1986;56:572–597. doi: 10.1152/jn.1986.56.3.572. [DOI] [PubMed] [Google Scholar]
- Mitsui S, Igarashi KM, Mori K, Yoshihara Y. Genetic visualization of the secondary olfactory pathway in Tbx21 transgenic mice. Neural Systems & Circuits. 2011;1:5. doi: 10.1186/2042-1001-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamichi K, Amat F, Moussavi F, Wang C, Wickersham I, Wall NR, Taniguchi H, Tasic B, Huang ZJ, He Z, et al. Cortical representations of olfactory input by trans-synaptic tracing. Nature. 2011;472:191–196. doi: 10.1038/nature09714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Sakano H. How is the olfactory map formed and interpreted in the mammalian brain? Annual Review of Neuroscience. 2011;34:467–499. doi: 10.1146/annurev-neuro-112210-112917. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Unique neural circuitry for neonatal olfactory learning. J Neurosci. 2004;24:1182–1189. doi: 10.1523/JNEUROSCI.4578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouly AM, Di Scala G. Entorhinal cortex stimulation modulates amygdala and piriform cortex responses to olfactory bulb inputs in the rat. Neuroscience. 2006;137:1131–1141. doi: 10.1016/j.neuroscience.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Mouly AM, Fort A, Ben-Boutayab N, Gervais R. Olfactory learning induces differential long-lasting changes in rat central olfactory pathways. Neuroscience. 2001;102:11–21. doi: 10.1016/s0306-4522(00)00476-0. [DOI] [PubMed] [Google Scholar]
- Murakami M, Kashiwadani H, Kirino Y, Mori K. State-dependent sensory gating in olfactory cortex. Neuron. 2005;46:285–296. doi: 10.1016/j.neuron.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Murphy C. Loss of olfactory function in dementing disease. Physiol Behav. 1999;66:177–182. doi: 10.1016/s0031-9384(98)00262-5. [DOI] [PubMed] [Google Scholar]
- Neville KR, Haberly L. Olfactory cortex. The synaptic organization of the brain. 2004:415–454. [Google Scholar]
- Ojima H, Mori K, Kishi K. The trajectory of mitral cell axons in the rabbit olfactory cortex revealed by intracellular HRP injection. J Comp Neurol. 1984;230:77–87. doi: 10.1002/cne.902300107. [DOI] [PubMed] [Google Scholar]
- Patil MM, Linster C, Lubenov E, Hasselmo ME. Cholinergic agonist carbachol enables associative long-term potentiation in piriform cortex slices. J Neurophysiol. 1998;80:2467–2474. doi: 10.1152/jn.1998.80.5.2467. [DOI] [PubMed] [Google Scholar]
- Pedersen PE, Williams CL, Blass EM. Activation and odor conditioning of suckling behavior in 3-day-old albino rats. J Exp Psychol Anim Behav Process. 1982;8:329–341. [PubMed] [Google Scholar]
- Perez-Orive J, Mazor O, Turner GC, Cassenaer S, Wilson RI, Laurent G. Oscillations and sparsening of odor representations in the mushroom body. Science. 2002;297:359–365. doi: 10.1126/science.1070502. [DOI] [PubMed] [Google Scholar]
- Plailly J, Howard JD, Gitelman DR, Gottfried JA. Attention to odor modulates thalamocortical connectivity in the human brain. J Neurosci. 2008;28:5257–5267. doi: 10.1523/JNEUROSCI.5607-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo C, Isaacson JS. An early critical period for long-term plasticity and structural modification of sensory synapses in olfactory cortex. J Neurosci. 2007;27:7553–7558. doi: 10.1523/JNEUROSCI.1786-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo C, Isaacson JS. Odor representations in olfactory cortex: “sparse” coding, global inhibition, and oscillations. Neuron. 2009;62:850–861. doi: 10.1016/j.neuron.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo C, Isaacson JS. A major role for intracortical circuits in the strength and tuning of odor-evoked excitation in olfactory cortex. Neuron. 2011;72:41–48. doi: 10.1016/j.neuron.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Moriceau S, Sullivan RM. Developing a neurobehavioral animal model of infant attachment to an abusive caregiver. Biological Psychiatry. 2010;67:1137–1145. doi: 10.1016/j.biopsych.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravel N, Vigouroux M, Elaagouby A, Gervais R. Scopolamine impairs delayed matching in an olfactory task in rats. Psychopharmacology (Berl) 1992;109:439–443. doi: 10.1007/BF02247720. [DOI] [PubMed] [Google Scholar]
- Rennaker RL, Chen CF, Ruyle AM, Sloan AM, Wilson DA. Spatial and temporal distribution of odorant-evoked activity in the piriform cortex. J Neurosci. 2007;27:1534–1542. doi: 10.1523/JNEUROSCI.4072-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch MR, Stalnaker TA, Schoenbaum G. Associative encoding in anterior piriform cortex versus orbitofrontal cortex during odor discrimination and reversal learning. Cereb Cortex. 2007;17:643–652. doi: 10.1093/cercor/bhk009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Treves A. Neural Networks and Brain Function. OxFord: Oxford University Press; 1998. [Google Scholar]
- Roman F, Staubli U, Lynch G. Evidence for synaptic potentiation in a cortical network during learning. Brain Res. 1987;418:221–226. doi: 10.1016/0006-8993(87)90089-8. [DOI] [PubMed] [Google Scholar]
- Roman FS, Chaillan FA, Soumireu-Mourat B. Long-term potentiation in rat piriform cortex following discrimination learning. Brain Res. 1993;601:265–272. doi: 10.1016/0006-8993(93)91719-9. [DOI] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Memory of early maltreatment: neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol Psychiatry. 2005;57:823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Saar D, Barkai E. Long-term modifications in intrinsic neuronal properties and rule learning in rats. Eur J Neurosci. 2003;17:2727–2734. doi: 10.1046/j.1460-9568.2003.02699.x. [DOI] [PubMed] [Google Scholar]
- Saar D, Grossman Y, Barkai E. Long-lasting cholinergic modulation underlies rule learning in rats. J Neurosci. 2001;21:1385–1392. doi: 10.1523/JNEUROSCI.21-04-01385.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saar D, Grossman Y, Barkai E. Learning-induced enhancement of postsynaptic potentials in pyramidal neurons. J Neurophysiol. 2002;87:2358–2363. doi: 10.1152/jn.2002.87.5.2358. [DOI] [PubMed] [Google Scholar]
- Sahay A, Wilson DA, Hen R. Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron. 2011;70:582–588. doi: 10.1016/j.neuron.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma AA, Richard MB, Greer CA. Developmental Dynamics of Piriform Cortex. Cerebral cortex. 2010 doi: 10.1093/cercor/bhq199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Eichenbaum H. Information coding in the rodent prefrontal cortex. I. Single-neuron activity in orbitofrontal cortex compared with that in pyriform cortex. J Neurophysiol. 1995;74:733–750. doi: 10.1152/jn.1995.74.2.733. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Haberly LB, Price JL. The development of physiological responses of the piriform cortex in rats to stimulation of the lateral olfactory tract. J Comp Neurol. 1984;223:223–237. doi: 10.1002/cne.902230206. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Price JL. The development of axonal connections in the central olfactory system of rats. J Comp Neurol. 1984;223:177–202. doi: 10.1002/cne.902230204. [DOI] [PubMed] [Google Scholar]
- Scott JW, McBride RL, Schneider SP. The organization of projections from the olfactory bulb to the piriform cortex and olfactory tubercle in the rat. J Comp Neurol. 1980;194:519–534. doi: 10.1002/cne.901940304. [DOI] [PubMed] [Google Scholar]
- Sevelinges Y, Gervais R, Messaoudi B, Granjon L, Mouly AM. Olfactory fear conditioning induces field potential potentiation in rat olfactory cortex and amygdala. Learn Mem. 2004;11:761–769. doi: 10.1101/lm.83604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley MT, Ennis M. Functional organization of olfactory system. J Neurobiol. 1996;30:123–176. doi: 10.1002/(SICI)1097-4695(199605)30:1<123::AID-NEU11>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Shusterman R, Smear MC, Koulakov AA, Rinberg D. Precise olfactory responses tile the sniff cycle. Nature Neuroscience. 2011;14:1039–1044. doi: 10.1038/nn.2877. [DOI] [PubMed] [Google Scholar]
- Singh PJ, Tobach E. Olfactory bulbectomy and nursing behavior in rat pups (Wistar DAB) Developmental Psychobiology. 1975;8:151–164. doi: 10.1002/dev.420080207. [DOI] [PubMed] [Google Scholar]
- Smith JJ, Shionoya K, Sullivan RM, Wilson DA. Auditory stimulation dishabituates olfactory responses via noradrenergic cortical modulation. Neural Plast. 2009;2009:754014. doi: 10.1155/2009/754014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosulski DL, Lissitsyna Bloom M, Cutforth T, Axel R, Datta SR. Distinct representations of olfactory information in different cortical centres. Nature. 2011;472:213–216. doi: 10.1038/nature09868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spors H, Wachowiak M, Cohen LB, Friedrich RW. Temporal dynamics and latency patterns of receptor neuron input to the olfactory bulb. J Neurosci. 2006;26:1247–1259. doi: 10.1523/JNEUROSCI.3100-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettler DD, Axel R. Representations of odor in the piriform cortex. Neuron. 2009;63:854–864. doi: 10.1016/j.neuron.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Stevenson RJ, Wilson DA. Odour perception: an object-recognition approach. Perception. 2007;36:1821–1833. doi: 10.1068/p5563. [DOI] [PubMed] [Google Scholar]
- Stokes CC, Isaacson JS. From dendrite to soma: dynamic routing of inhibition by complementary interneuron microcircuits in olfactory cortex. Neuron. 2010;67:452–465. doi: 10.1016/j.neuron.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su CY, Menuz K, Carlson JR. Olfactory perception: receptors, cells, and circuits. Cell. 2009;139:45–59. doi: 10.1016/j.cell.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh GS, Wong AM, Hergarden AC, Wang JW, Simon AF, Benzer S, Axel R, Anderson DJ. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA, Wong R, Correa A, Leon M. Modified behavioral and olfactory bulb responses to maternal odors in preweanling rats. Brain Res Dev Brain Res. 1990;53:243–247. doi: 10.1016/0165-3806(90)90013-o. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Bekkers JM. Neural coding by two classes of principal cells in the mouse piriform cortex. J Neurosci. 2006;26:11938–11947. doi: 10.1523/JNEUROSCI.3473-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Bekkers JM. Distinctive Classes of GABAergic Interneurons Provide Layer-Specific Phasic Inhibition in the Anterior Piriform Cortex. Cereb Cortex. 2010a doi: 10.1093/cercor/bhq046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Bekkers JM. Inhibitory neurons in the anterior piriform cortex of the mouse: classification using molecular markers. The Journal of comparative neurology. 2010b;518:1670–1687. doi: 10.1002/cne.22295. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Bekkers JM. Two layers of synaptic processing by principal neurons in piriform cortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:2156–2166. doi: 10.1523/JNEUROSCI.5430-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talamo BR, Rudel R, Kosik KS, Lee VM, Neff S, Adelman L, Kauer JS. Pathological changes in olfactory neurons in patients with Alzheimer’s disease. Nature. 1989;337:736–739. doi: 10.1038/337736a0. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, Cohen LB. Representation of odorants by receptor neuron input to the mouse olfactory bulb. Neuron. 2001;32:723–735. doi: 10.1016/s0896-6273(01)00506-2. [DOI] [PubMed] [Google Scholar]
- Wang J, Eslinger PJ, Doty RL, Zimmerman EK, Grunfeld R, Sun X, Meadowcroft MD, Connor JR, Price JL, Smith MB, Yang QX. Olfactory deficit detected by fMRI in early Alzheimer’s disease. Brain Research. 2010;1357:184–194. doi: 10.1016/j.brainres.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DW, Borkowski AH, Landreth GE, Nixon RA, Levy E, Wilson DA. Sensory network dysfunction, behavioral impairments, and their reversibility in an Alzheimer’s β-amyloidosis mouse model. J Neurosci. 2011 doi: 10.1523/JNEUROSCI.2085-11.2011. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DW, Levy E, Nixon RA, Wilson DA. Olfactory dysfunction correlates with amyloid-beta burden in an Alzheimer’s disease mouse model. J Neurosci. 2010;30:505–514. doi: 10.1523/JNEUROSCI.4622-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DW, Wilson DA. Smelling sounds: olfactory-auditory sensory convergence in the olfactory tubercle. J Neurosci. 2010;30:3013–3021. doi: 10.1523/JNEUROSCI.6003-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DW, Wilson DA. Sniffing out the contributions of the olfactory tubercle to the sense of smell: hedonics, sensory integration, and more? Neuroscience and Biobehavioral Reviews. 2011;35:655–668. doi: 10.1016/j.neubiorev.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA. Binaral interactions in the rat piriform cortex. J Neurophysiol. 1997;78:160–169. doi: 10.1152/jn.1997.78.1.160. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Habituation of odor responses in the rat anterior piriform cortex. J Neurophysiol. 1998a;79:1425–1440. doi: 10.1152/jn.1998.79.3.1425. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Synaptic correlates of odor habituation in the rat anterior piriform cortex. J Neurophysiol. 1998b;80:998–1001. doi: 10.1152/jn.1998.80.2.998. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Odor specificity of habituation in the rat anterior piriform cortex. J Neurophysiol. 2000;83:139–145. doi: 10.1152/jn.2000.83.1.139. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Scopolamine enhances generalization between odor representations in rat olfactory cortex. Learn Mem. 2001;8:279–285. doi: 10.1101/lm.42601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA. Rapid, experience-induced enhancement in odorant discrimination by anterior piriform cortex neurons. J Neurophysiol. 2003;90:65–72. doi: 10.1152/jn.00133.2003. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Pattern separation and completion in olfaction. Ann N Y Acad Sci. 2009;1170:306–312. doi: 10.1111/j.1749-6632.2009.04017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA. Single-unit activity in piriform cortex during slow-wave state is shaped by recent odor experience. J Neurosci. 2010;30:1760–1765. doi: 10.1523/JNEUROSCI.5636-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA. Lateral entorhinal cortex top-down modulation of odor coding in the piriform cortex. Society for Neuroscience Annual Meeting Abstracts; Washington, DC. 2011. [Google Scholar]
- Wilson DA, Best AR, Sullivan RM. Plasticity in the olfactory system: lessons for the neurobiology of memory. Neuroscientist. 2004;10:513–524. doi: 10.1177/1073858404267048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA, Hoptman MJ, Gerum SV, Guilfoyle DN. State-dependent functional connectivity of rat olfactory system assessed by fMRI. Neuroscience letters. 2011 doi: 10.1016/j.neulet.2011.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DA, Stevenson RJ. The fundamental role of memory in olfactory perception. Trends Neurosci. 2003;26:243–247. doi: 10.1016/S0166-2236(03)00076-6. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Yan X. Sleep-like states modulate functional connectivity in the rat olfactory system. Journal of neurophysiology. 2010;104:3231–3239. doi: 10.1152/jn.00711.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadon CA, Wilson DA. The role of metabotropic glutamate receptors and cortical adaptation in habituation of odor-guided behavior. Learn Mem. 2005;12:601–605. doi: 10.1101/lm.41405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksi E, von Saint Paul F, Niessing J, Bundschuh ST, Friedrich RW. Transformation of odor representations in target areas of the olfactory bulb. Nature Neuroscience. 2009;12:474–482. doi: 10.1038/nn.2288. [DOI] [PubMed] [Google Scholar]
- Young A, Sun QQ. GABAergic inhibitory interneurons in the posterior piriform cortex of the GAD67-GFP mouse. Cereb Cortex. 2009;19:3011–3029. doi: 10.1093/cercor/bhp072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young BJ, Otto T, Fox GD, Eichenbaum H. Memory representation within the parahippocampal region. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1997;17:5183–5195. doi: 10.1523/JNEUROSCI.17-13-05183.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Szabo G, Erdelyi F, Rose JD, Sun QQ. Novel interneuronal network in the mouse posterior piriform cortex. J Comp Neurol. 2006;499:1000–1015. doi: 10.1002/cne.21166. [DOI] [PubMed] [Google Scholar]
- Zinyuk LE, Datiche F, Cattarelli M. Cell activity in the anterior piriform cortex during an olfactory learning in the rat. Behav Brain Res. 2001;124:29–32. doi: 10.1016/s0166-4328(01)00212-1. [DOI] [PubMed] [Google Scholar]