Abstract
OBJECTIVES
To examine whether use of opioids or benzodiazepines is associated with increased risk of community-acquired pneumonia in older adults.
DESIGN
Population-based case-control study.
SETTING
An integrated healthcare delivery system.
PARTICIPANTS
Community-dwelling, immunocompetent adults aged 65–94 from 2000–2003. Presumptive pneumonia cases were identified from health plan automated data and validated through medical record review. Two matched controls were selected for each case with pneumonia, matched on age, sex and calendar year.
MEASUREMENTS
Information about opioid and benzodiazepine use came from computerized pharmacy data. Information on covariates including comorbid illnesses and functional and cognitive status came from medical record review and electronic health data.
RESULTS
1039 validated cases of pneumonia and 2022 matched controls were identified. 13.9% (144/1039) of cases and 8.0% (161/2022) of controls used prescription opioids (adjusted OR 1.38, 95% CI 1.08 to 1.76, vs. nonuse). Risk was highest for opioids categorized as immunosuppressive based on immunologic studies (OR 1.88 [95% CI, 1.26 to 1.79] vs. nonuse), while for non-immunosuppressive opioids the OR was 1.23 (95% CI, 0.89 to 1.69). Risk was highest in the first 14 days of use (OR 3.24 [95% CI, 1.64 to 6.39] vs. nonuse). For long-acting opioids, the OR was 3.43 [95% CI, 1.44 to 8.21] vs. nonuse, while for short-acting opioids it was 1.27 [95% CI, 0.98 to 1.64]. No increased risk was seen for current benzodiazepine use (OR 1.08, 95% CI 0.80 to 1.47, compared to nonuse).
CONCLUSION
Use of opioids but not benzodiazepines was associated with increased pneumonia risk. The differences in risk seen for different opioid regimens warrant further study.
Keywords: pneumonia, epidemiology, opioids, adverse drug effects, benzodiazepines
INTRODUCTION
Prescription opioids and benzodiazepines are widely used, opioids for acute or chronic pain and benzodiazepines for conditions including insomnia and anxiety. In 2002, 18% of U.S. adults received at least one opioid prescription, and the prevalence of use increased with age.1 An estimated 2 million U.S. adults over age 65 are using opioids long-term for non-cancer pain.2 These numbers may rise due to recommendations from the American Geriatrics Society promoting opioids over nonsteroidal anti-inflammatory drugs for older adults with moderate to severe persistent pain.3 There are few recent data for benzodiazepine use, but a 1998 study found that one in 10 people over 65 in the U.S. was using a benzodiazepine.4
Both opioids and benzodiazepines plausibly could increase risk of pneumonia, a common infection with serious consequences in older adults. Both medication classes cause sedation, which may increase the risk of aspiration,5 and both can cause respiratory depression. Moreover, in human and animal studies, some opioids suppress the immune system. They inhibit macrophages and natural killer cells,6–11 alter cytokine production,9, 11, 12 and impair migration of macrophages and neutrophils.12–14 In a mouse model of pneumonia due to Streptococcus pneumoniae, mice pre-treated with morphine had increased pulmonary inflammation, bacterial dissemination, and mortality compared to mice pre-treated with placebo.12, 14 These effects appeared to be mediated by a decrease in chemokines including TNF-α, IL-1, IL-6, and MIP-2 in bronchoalveolar lavage fluid and lung tissue.12, 14 In vitro, alveolar macrophages treated with morphine released less MIP-2, a chemoattractant for neutrophils.14 Morphine inhibited NF-kB-dependent gene transcription in these cells, suggesting a mechanism for these effects.14 Thus, immunologic studies have demonstrated plausible biologic mechanisms by which opioids may increase infection risk, including risk of pneumonia.
Particularly noteworthy are immunologic studies showing that immune effects may differ across opioid medications. Among commonly used opioids, several suppress the immune system (e.g. morphine, codeine and fentanyl), while others appear not to (e.g. hydrocodone and oxycodone).11, 15–17 This has important implications for clinical practice, because in many cases, physicians could readily select a non-immunosuppressive opioid in place of one that is believed to be immunosuppressive. Despite an extensive immunologic literature, the association between opioid use and infection has scarcely been examined in human clinical or epidemiologic studies. The only two studies that have been conducted18, 19 provide limited insight because they examined specialized populations and included few patients with infections. Neither study attempted to classify opioids according to their immunosuppressive effects. Three studies have examined the association of pneumonia with benzodiazepine use, with conflicting results.20–22
We examined the association between use of opioids or benzodiazepines and pneumonia risk in a population-based case-control study in which all pneumonia cases were validated and information on potential confounders came from detailed medical record review.23 We hypothesized that pneumonia risk would be elevated among people using benzodiazepines and opioids compared to nonusers, and that risk would be highest for long-acting opioids and opioids deemed to be immunosuppressive in prior immunologic studies.
METHODS
Overview and Setting
We analyzed existing data from a nested case-control study of community-acquired pneumonia (CAP) among older adults within Group Health (GH), an integrated healthcare delivery system. This study was approved by the GH Human Subjects Review Committee with a waiver of consent.
Source population
The study population included GH members’ ages 65–94 years with at least 2 years of continuous enrollment who were community-dwelling and not immunosuppressed. Eligibility was based on computerized pharmacy, laboratory, and utilization data and confirmed by medical record review. Immunosuppression was defined as having serious cancer, recent cancer treatment, or chronic kidney disease, or receiving certain immunosuppressive medications or medications for human immunodeficiency virus (details in Appendix Table 1, online). Because the original study23 examined pneumonia risk in relation to influenza vaccination, eligibility was initially determined as of September 1 in each study year (2000, 2001 and 2002). Because we wished to examine opioid and benzodiazepine use shortly before pneumonia onset, we also excluded people who based on computerized diagnosis and pharmacy data developed the above exclusionary conditions during follow-up in each study year. These people were considered eligible up until the date they developed an exclusionary condition but were excluded from the study population thereafter.
Selection of cases and controls
The methods used to identify and validate pneumonia cases have been described previously.24 We identified presumptive cases using International Classification of Diseases, version 9 codes (480 to 487.0 or 507.0) and validated them through review of chest radiograph reports and hospitalization records. Medical record abstractors reviewed the electronic text of chest radiograph reports for descriptions of qualifying findings such as an infiltrate or consolidation. We did not re-interpret the radiographs themselves. Many GH patients who require hospitalization receive this care outside of GH. For these patients, we did not have access to radiograph reports but rather to hospital admission, discharge, and consultation notes. Cases were judged to have pneumonia if a chest radiograph report described a parenchymal infiltrate not known to be chronic or, for hospitalized cases, if the final physician assessment was that pneumonia caused the illness present at admission. Cases of hospital-acquired pneumonia or massive aspiration were excluded. Massive aspiration was identified through medical record review and was considered to be present if a person with no prior evidence of acute respiratory illness developed pneumonia after a sudden, massive insult (such as near-drowning, loss of consciousness due to a stroke or seizure, or choking on food).
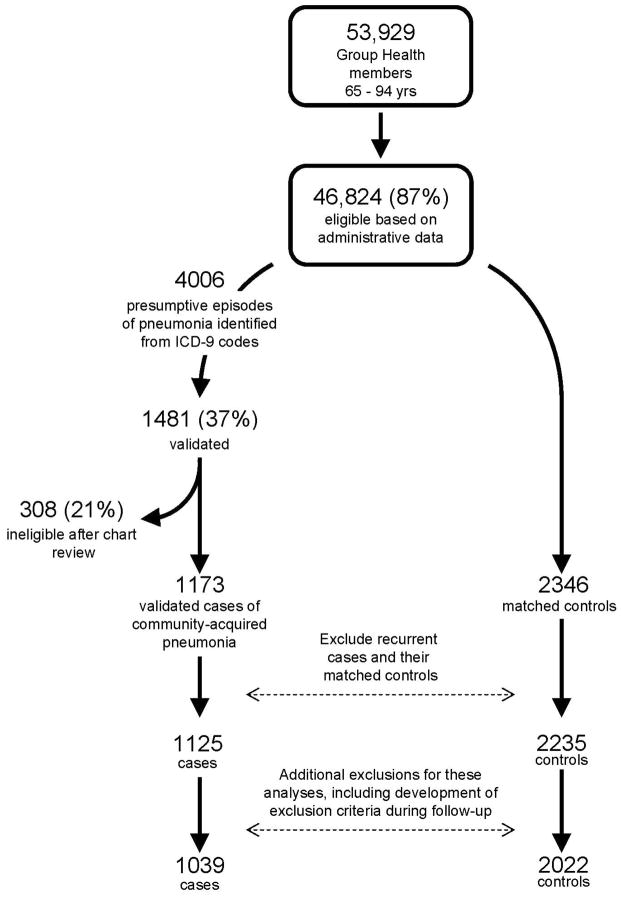
Because of the aims of the original study, cases were identified from September 1 until the end of influenza season in each study year (2000–1, 2001–2, and 2002–3).23 Cases were assigned an index date representing the date of pneumonia diagnosis, defined as the date of the first positive chest radiograph or, for hospitalized cases without a positive chest radiograph at GH, the date of hospital admission. For people with multiple episodes of pneumonia, we included only the first episode. Up to 2 controls were selected for each case matched on age, sex, and calendar year. Controls were required to have survived and remained enrolled in GH through their matched case’s diagnosis date (incidence density sampling.) Controls were assigned the same index date as their matched case. Figure 1 shows the flow of potential subjects through the study.
Figure 1.
Selection of cases and controls for inclusion in the study. For this study, we extended the time period during which eligibility was assessed by obtaining additional administrative and pharmacy data to identify exclusion criterion after September 1 of each study year. This process led to the exclusion of an additional 86 cases and 213 controls from the original study. Many of these (61 cases and 17 controls) were people who developed an exclusion criterion during follow-up (after September 1 of their study year). Some additional people were excluded because they were found to have used an immunosuppressive medication during baseline (23 cases, 29 controls) or because the case was discovered to have been hospital-acquired (2 cases and their 4 matched controls). The other 163 controls were dropped because their matched cases had been excluded due to one of the above criteria.
Measures of drug exposure
GH’s computerized pharmacy database provided information on prescription fills including medication name, strength, route of administration, date dispensed, quantity dispensed, and days supplied. In prior studies, 96% of GH members in this age group reported filling all or almost all of their prescriptions at GH pharmacies.25 Oral, transdermal, and sublingual medications were included. Opioids used in cough suppressants were excluded because they might have been prescribed for pneumonia symptoms. We defined “current use” as having filled 1 or more prescriptions between 5 and 60 days prior to index date. Fills in the 4 days prior to index were excluded because these may have been prescribed for symptoms of pneumonia. Thus, for example, a person who filled an opioid prescription 3 days prior to index date but had no other fills in the prior 12 months would have been characterized as a nonuser. We chose a relatively liberal definition of “current use” to allow for sporadic or inconsistent use. “Past use” was defined as filling at least one prescription between 61 and 365 days prior to index date but none from 5–60 days before index date, while “nonuse” was defined as filling no prescriptions from 5–365 days before index.
For current users, we defined initiation of use as follows. Each prescription fill was assigned a run-out date based on the date of the fill and the days supplied. Use was considered continuous if the next prescription was filled no later than 90 days after the previous fill’s run-out date. We chose 90 days to allow for intermittent or sporadic use, because based on the immunologic literature we believed it was important to identify “new initiators” accurately, including only people who truly were initiating these medications after a period of non-use. We defined the date of initiation as the date of the first fill in the most recent episode of continuous use.
We classified opioid medications as long-acting or short-acting and, based on prior immunologic studies, as immunosuppressive or non-immunosuppressive (Table 1).11, 15, 16, 26–28. For current opioid users who initiated use more than 90 days prior to index date (chronic long-term users), we estimated their daily dose (EDD) in morphine equivalents as follows. We identified prescription fills which overlapped the 90-day window prior to index date. For each fill, we multiplied the number of pills by the strength and by a conversion factor to calculate morphine equivalent doses (MEDs).28 We then summed the MEDs across prescriptions (accounting for the fact that some prescriptions overlapped this time period only partially) and divided by 90 days (the length of the time window) to get each current user’s EDD. We did not calculate an EDD for people who initiated use less than 90 days prior to index date because this would require strong assumptions about intensity of use. We categorized the EDD as 1 to 19 mg, 20 to 49 mg, or ≥ 50 mg daily in morphine equivalents.
Table 1.
Opioid Classification Scheme and Use of Individual Medications by Study Subjects
| Medication | Number of prescriptions dispensed* | Half-life | Immunosuppressive† | Morphine equivalent dose‡ | |
|---|---|---|---|---|---|
| n | % | ||||
| Hydrocodone | 1,117 | 34.2% | Short | No | 1.0 |
| Codeine | 805 | 24.6% | Short | Yes | 0.15 |
| Oxycodone (short acting) | 670 | 20.5% | Short | No | 1.5 |
| Propoxyphene | 304 | 9.3% | Short | Unknown | 0.23 |
| Morphine (sustained release) | 138 | 4.2% | Long | Yes | 1.0 |
| Oxycodone (controlled release) | 65 | 2.0% | Long | No | 1.5 |
| Morphine (immediate release) | 45 | 1.4% | Short | Yes | 1.0 |
| Meperidine | 44 | 1.3% | Short | Unknown | 0.1 |
| Hydromorphone | 27 | 0.8% | Short | No | 4.0 |
| Fentanyl (transdermal) | 23 | 0.7% | Long | Yes | 2.4 |
| Tincture of opium | 13 | 0.4% | Unknown | Unknown | 10.0 |
| Tramadol | 11 | 0.3% | Short | No | 0.10 |
| Methadone | 3 | 0.1% | Long | Yes | 3.0 |
| Levorphanol | 1 | 0.0% | Long | Unknown | 11.0 |
|
| |||||
| Total | 3,266 | 100% | |||
Number of prescriptions dispensed between 5–365 days prior to index date for cases and controls.
Conversion factors are based on information from multiple sources and were arrived at by consensus of several investigators in a prior study of opioid medication use.28 For tincture of opium, the conversion factor is based on information from Thomson Micromedex.27 To use conversion factors, multiple the amount of opioid by the conversion factor to get the amount of morphine. E.g. 200 mg of codeine is equivalent to 200 * 0.15 = 30 mg of morphine, and 20 mg of oxycodone is equivalent to 20 * 1.5 = 30 mg of morphine.
Benzodiazepines were classified according to half-life (Appendix Table 2, online). To measure intensity of use, we converted benzodiazepine prescriptions to total standardized doses (TSD) based on the minimum effective daily dose reported in standard references29 and summed TSD over the 12 months prior to index date. Among current benzodiazepine users, “heavy use” was defined as receiving at least 150 TSD in the prior 365 days.
Measurement of other covariates
Information about other characteristics came from electronic databases and detailed medical record review. The medical record review focused on the 2 year period prior to September 1 of each year (defined hereafter as the baseline period) and was conducted in the same manner for cases and controls. It assessed demographic characteristics, height and weight, smoking status, and the presence and severity of health conditions hypothesized to be associated with pneumonia risk. We assessed the severity of comorbid illnesses using measures such as long-term steroid use for lung disease (receiving oral steroids to be taken at least 3 times per week for at least 3 months), home oxygen use, and whether ejection fraction (EF) was measured. Functional status measures were defined based on documentation in the chart and included need for assistance with ambulation or bathing, use of home health services, and whether a subject was ever described as frail by medical personnel. Cognitive status was defined based on the presence of a physician diagnosis of dementia in the chart. We obtained information from computerized pharmacy data about medications expected to be associated with pneumonia risk, including medications for chronic lung or heart disease. Electronic data sources provided information about the number of outpatient visits in the 1 year prior to September 1 of each year.
Analysis
We first examined characteristics of pneumonia cases and controls, calculating proportions for categorical variables and medians and interquartile ranges for continuous variables. Similar analyses explored characteristics associated with current use of opioids or benzodiazepines among control subjects. We used multivariable conditional logistic regression to estimate adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for the risk of CAP, comparing current use of each medication class to nonuse. Opioid and benzodiazepine use were examined in the same model, so risk estimates for each medication class are adjusted for the other. We selected the following potential confounders for our final model a priori, based on review of the literature and clinical plausibility: asthma, chronic obstructive pulmonary disease (COPD), COPD hospitalization, use of home oxygen, long-term steroid use for lung disease, whether forced expiratory volume in one second (FEV1) was measured, congestive heart failure (CHF), CHF hospitalization, whether EF was measured, stroke, number of outpatient visits in 1-year baseline period (a continuous variable), need for assistance with ambulation or bathing, dementia, and use of inhaled bronchodilators, inhaled corticosteroids, oral corticosteroids, or furosemide. To test whether the association between opioid or benzodiazepine use and pneumonia was modified by use of the other drug class, we included interaction terms in the model and used an omnibus Wald test to assess statistical significance.
Additional analyses examined the risk of pneumonia according to medication half-life, recency of initiation, and intensity of use. For opioids, we also examined risk for groups categorized by their immunosuppressive effects based on the published immunologic literature.
We carried out sensitivity analyses to assess the robustness of our results. First, we applied a stricter definition of “current use,” in which current use was defined as having filled an opioid prescription which would have been expected to last beyond 7 days prior to index date. The goal was to increase the likelihood that “current users” were using opioids on or shortly before the index date. Second, to disentangle the potential effects of opioid half-life vs. immunosuppression, we repeated the analysis of immunosuppressive versus non-immunosuppressive opioids stratified by opioid half-life. Third, we performed sensitivity analyses excluding people with any use of oral corticosteroids during baseline or follow-up. We did this to remove any bias which could arise if patients with an inflammatory condition experienced a disease flare and were prescribed an opioid and an oral corticosteroid at the same time, resulting in steroid-induced immunosuppression. Fourth, to address the bias which could arise if opioids or benzodiazepines were prescribed to treat pneumonia symptoms, we repeated our primary analysis excluding prescriptions filled within 10 days prior to index date. Fifth, we carried out analyses focused on the three most commonly used opioids: codeine, hydrocodone, and oxycodone. We grouped hydrocodone and oxycodone together because according to the immunologic literature, neither is immunosuppressive. Finally, as a post-hoc analysis, we repeated the analysis of chronic long-term opioid use applying a more stringent definition, in which use was considered continuous only if there was no more than a 30 day gap between the expected end date of one prescription and the start date of the next. The intention was to identify a group of chronic long-term users who were filling prescriptions on a regular basis, because we hypothesized that if any increased risk were associated with chronic use, it would be most likely to be seen for this group.
Analyses were carried out using SAS version 9.1 (SAS Institute, Incorporated, Cary, North Carolina, United States) and Stata version 9.2 (StataCorp LP, College Station, Texas, United States).
RESULTS
1039 cases of CAP and 2022 controls were included in these analyses. Their median age was 77, and about half were male (Table 2). Among people with CAP, 352 (33.9%) were hospitalized, and 48 (4.6%) died within 30 days of their index date. A greater proportion of cases than controls had comorbid illnesses, particularly chronic lung or heart disease, as well as more severe disease and functional impairment (Table 2).
Table 2.
Characteristics of Pneumonia Cases and Controls
| Characteristic* | Pneumonia cases (N=1,039) n (%)† | Controls (N=2,022) n (%)† |
|---|---|---|
| Median age (IQR), years‡ | 77 (71 – 82) | 77 (71 – 82) |
| Male‡ | 525 (50.5) | 1030 (50.9) |
| Median BMI (IQR), kg/m2† | 26.5 (23.4 – 30.6) | 27.3 (24.5 – 30.6) |
| (N=736) | (N=1341) | |
| Current smoker | 94/1027 (9.2) | 101/1972 (5.1) |
| Asthma | 185 (17.8) | 158 (7.8) |
| Chronic obstructive pulmonary disease (COPD) | 324 (31.2) | 227 (10.4) |
| Hospitalized for COPD | 50 (4.8) | 14 (0.7) |
| Home oxygen use | 83 (8.0) | 18 (0.9) |
| Long term steroid use for lung disease | 28 (2.7) | 6 (0.3) |
| FEV1 measured | 166 (16.0) | 78 (3.9) |
| Congestive heart failure (CHF) | 192 (18.5) | 141 (7.0) |
| Hospitalized for CHF | 33 (3.2) | 16 (0.8) |
| Ejection fraction measured | 72 (6.9) | 35 (1.7) |
| Myocardial infarction | 126 (12.1) | 199 (9.8) |
| Coronary revascularization | 145 (14.0) | 224 (11.1) |
| Stroke | 87 (8.4) | 140 (6.9) |
| Swallowing disorder leading to aspiration | 13 (1.3) | 9 (0.4) |
| Alcoholism | 16 (1.5) | 22 (1.1) |
| Diabetes mellitus | 172 (16.6) | 300 (14.8) |
| Dementia | 48 (4.6) | 68 (3.4) |
| At least one functional impairment | 191 (18.4) | 232 (11.5) |
| Requires assistance bathing | 22 (2.1) | 15 (0.7) |
| Requires assistance walking | 184 (17.7) | 221 (10.9) |
| Any use of home health services | 150 (14.4) | 125 (6.2) |
| Frail§ | 84 (8.1) | 58 (2.9) |
| Bronchodilator use|| | 138 (13.3) | 51 (2.5) |
| Furosemide use|| | 231 (22.2) | 206 (10.2) |
| Inhaled corticosteroid use|| | 244 (23.5) | 159 (7.9) |
| Insulin or oral hypoglycemic use|| | 114 (11.0) | 188 (9.3) |
| Oral corticosteroid use|| | 262 (25.2) | 191 (9.4) |
| Received pneumococcal vaccine¶ | 951 (91.5) | 1846 (91.3) |
| Received influenza vaccine** | 630 (60.6) | 1245 (61.6) |
| Number of outpatient visits, median (IQR)†† | 11 (6 – 17) | 8 (5 – 14) |
Abbreviations: BMI, body mass index; IQR, interquartile range; FEV1, forced expiratory volume in 1 second.
All characteristics are defined as of September 1, and the time period of interest is the 2 year baseline period unless otherwise stated.
Results are provided as n (%) unless otherwise stated. Less than 5% of people had missing values for all characteristics except for BMI, which was missing for 29% (303/1039) of cases and 34% (681/2022) of controls.
Matching variable used in selection of controls.
Ever described as frail by medical personnel (from chart review).
Defined from computerized pharmacy data as filling at least one prescription for a medication in this class during the 2 year baseline period.
Any history of receiving pneumococcal vaccine.
Receipt of the current year’s influenza vaccine prior to index date.
Number of visits in 1-year baseline.
Hydrocodone was the most commonly dispensed opioid, followed by codeine and oxycodone (Table 1). About 7% of fills were for long-acting opioids. Compared to people using only short-acting agents, people using long-acting opioids had longer duration of use (median 747 versus 91 days) and higher estimated daily doses (median 51.3 versus 10.0 mg in morphine equivalents). Among benzodiazepines, lorazepam was most commonly used, followed by temazepam and diazepam (Appendix Table 2, online). About one-fourth of benzodiazepine dispensings were for long-half-life medications. Among controls, people currently using opioids or benzodiazepines were more likely than nonusers to be female, to smoke, and to have chronic lung disease and functional impairment (Appendix Tables 3 and 4, online).
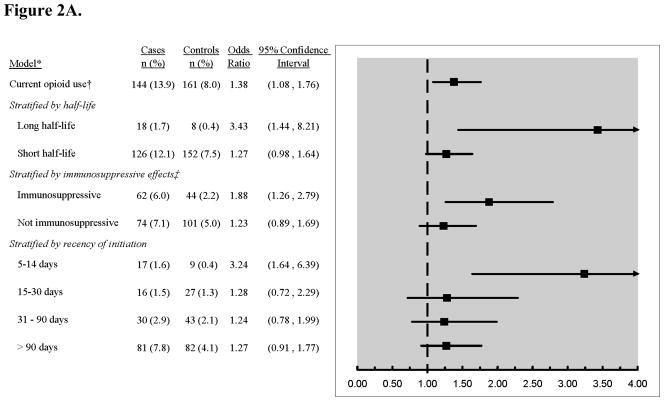
Among people with pneumonia, 13.9% were current opioid users, compared to 8.0% of controls (adjusted OR 1.38, 95% CI 1.08 to 1.76, compared to nonuse; Table 3). No association was seen for past use. The association was stronger for long-acting than short-acting opioids and for opioids predicted to be immunosuppressive compared to those predicted not to be immunosuppressive (Figure 2A). Pneumonia risk was elevated for current use that began from 5 to 14 days prior to index date (OR 3.24, 95% CI 1.64 to 6.39) and was closer to the null for use begun more than 90 days prior to index date (OR 1.27, 95% CI 0.91 to 1.77). Among chronic users, no pattern was seen for pneumonia risk in relation to estimated daily dose. Compared to nonuse, the adjusted OR for CAP for an EDD of 1 to 19 mg morphine equivalents was 1.05 (95% CI 0.71 to 1.56); for 20 to 49 mg, 2.30 (95% CI 1.10 to 4.83); and for ≥ 50 mg, 1.37 (95% CI 0.64 to 2.92).
Table 3.
Risk of Community-Acquired Pneumonia in Relation to Use of Opioids and Benzodiazepines
| Cases (N=1,039) n (%) |
Controls (N=2,022) n (%) |
Odds ratio (95% CI) | ||
|---|---|---|---|---|
| Adjusted for matching variables† | Fully adjusted‡ | |||
| Opioid use* | ||||
| No use | 695 (66.9) | 1527 (75.5) | 1.00 (Ref.) | 1.00 (Ref.) |
| Current use | 144 (13.9) | 161 (8.0) | 1.91 (1.55, 2.36) | 1.38 (1.08, 1.76) |
| Past use | 200 (19.2) | 334 (16.5) | 1.31 (1.12, 1.54) | 1.06 (0.88, 1.27) |
| Benzodiazepine use* | ||||
| No use | 892 (85.9) | 1796 (88.8) | 1.00 (Ref.) | 1.00 (Ref.) |
| Current use | 87 (8.4) | 94 (4.6) | 1.63 (1.26, 2.11) | 1.08 (0.80, 1.47) |
| Past use | 60 (5.8) | 132 (6.5) | 0.82 (0.63, 1.07) | 0.62 (0.46, 0.85) |
Abbreviations: CAP, community-acquired pneumonia; CHF, congestive heart failure; CI, confidence interval; FEV1, forced expiratory volume in 1 second.
Current use is defined as having ≥1 fill for a medication in the specified class within 5–60 days prior to the case’s pneumonia diagnosis date. Non-users had no fills from 5–365 days prior to index date. Past users had ≥ 1 fill from 61–365 days prior to index date but none in the 5–60 days prior to index date.
Adjusted only for age, sex, and index date.
Adjusted for matching variables above and for asthma, chronic obstructive pulmonary disease (COPD) and history of COPD hospitalization, use of home oxygen, long-term steroid use for lung disease, FEV1 measured, congestive heart failure (CHF) and history of CHF hospitalization, ejection fraction measured, stroke, dementia, need for assistance with ambulation or bathing, number of outpatient visits, and use of the following medications: inhaled bronchodilators, inhaled corticosteroids, oral corticosteroids, or furosemide
Figure 2.
Figure 2A: Risk estimates for the association between current opioid use and risk of pneumonia, stratified by characteristics of exposure. *Adjusted for matching variables (age, sex, index date), comorbidities, and functional and cognitive status measures. Referent group is non-users. †Current use was defined as receiving ≥ 1 opioid prescription in the 5–60 days prior to index date. ‡According to human and animal studies.7–9
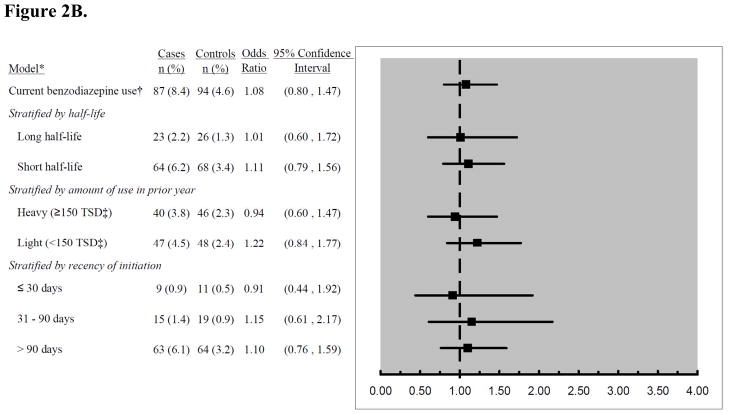
Figure 2B: Risk estimates for the association between current benzodiazepine use and risk of pneumonia, stratified by characteristics of exposure. *Adjusted for matching variables (age, sex, index date), comorbidities, and functional and cognitive status measures. Referent group is non-users. †Current use was defined as receiving ≥ 1 benzodiazepine prescription in the 5–60 days prior to index date. ‡Total standardized doses.
Current benzodiazepine use was present in 8.4% of cases and 4.6% of controls, but this difference disappeared after other variables were taken into account (adjusted OR 1.08, 95% CI 0.80 to 1.47, compared to nonuse). There was no interaction between current opioid and benzodiazepine use (p=0.69). No increased risk was seen for use of long-half life benzodiazepines or heavy use (Figure 2B). For use initiated within the past 30 days, the adjusted OR was 0.91 (95% CI 0.44 to 1.92) compared to nonuse.
Similar results were obtained in sensitivity analyses using a stricter definition for “current use”. In these analyses, 7.4% of cases and 3.5% of controls were current opioid users (adjusted OR 1.43, 95% CI 1.00–2.05). A second sensitivity analysis examined whether the elevated risk seen with use of immunosuppressive opioids was due primarily to use of long-half-life medications. The risk of pneumonia in people using short-acting, immunosuppressive opioids (primarily codeine) was elevated compared to nonusers (OR 1.69, 95% CI 1.10 to 2.61), while for short-acting opioids classified as non-immunosuppressive it was 1.17 (95% CI 0.85 to 1.63). The odds ratio for pneumonia associated with current opioid use in people not receiving oral corticosteroids was 1.29 (95% CI 0.98 to 1.71, vs. nonuse) and in analyses excluding prescriptions filled within 10 days prior to the index date it was 1.24 (0.97 to 1.60). In analyses focusing on the three most commonly used opioids, only current use of codeine was associated with higher pneumonia risk compared to nonuse (OR 1.93; 95% CI, 1.22 to 3.06). No increased risk was seen for current use of oxycodone and/or hydrocodone (OR 1.10, 95% CI 0.78 to 1.55, compared to nonuse). Using a stricter definition of chronic long-term opioid use did not alter results substantially; with the stricter definition, the OR for current opioid users who had initiated use at least 90 days prior to index date was 1.41 (95% CI 0.95 to 2.08) compared to nonusers.
DISCUSSION
We observed higher pneumonia risk with current use of prescription opioids in this study of community-dwelling older adults. Risk was highest with recent initiation of use (within 14 days) and with use of long-acting opioids or those classified as immunosuppressive. No association was seen for current benzodiazepine use.
This is the first large epidemiologic study in a general population to examine opioid use and risk of infection. Strengths of the study include that it is population-based, strengthening generalizability, and that pneumonia outcomes were validated through medical record review. We made extensive efforts to account for potential confounders such as comorbid illnesses and functional and cognitive status, which were ascertained from medical records. Information about opioid and benzodiazepine use came from computerized pharmacy data, eliminating recall bias. Our hypotheses about different pneumonia risk with immunosuppressive vs. nonimmunosuppressive opioids have a clear physiologic and mechanistic basis and were developed a priori based on published immunologic studies.11, 12, 14–16
Several limitations of this study should be considered in interpreting these results. First, residual confounding is always a possibility in observational studies. We had limited measures of functional and cognitive impairment, which could have led to bias. Second, we lacked information about the indication for opioid use. Some patients may have been prescribed opioids for a prodromal illness or for early symptoms of pneumonia. We did not have data about the timing of pneumonia onset, so we addressed this problem in our primary analyses by excluding opioid fills that occurred between 1 and 4 days prior to the case’s diagnosis date. In sensitivity analyses, we excluded fills occurring between 1 and 10 days prior to this date, and the adjusted OR decreased from 1.38 to 1.24. It is difficult to know which of these risk estimates is more valid. If opioids have acute immunosuppressive effects, as suggested by studies in animals,11, 12, 14–16 and if these effects wane with continuing exposure,8 then the 10-day sensitivity analysis may have excluded the very time period in which an elevated risk would be expected to be present. If so, it may underestimate the association between opioid use and pneumonia. The best solution would be to collect more precise data about the onset of infection in future studies. Third, this study included few opioid users, leading to low power for some comparisons. We cannot provide a definite answer about whether there is a modest but clinically important increase in risk with use of short-acting opioids or with chronic long-term use. Fourth, there could be misclassification of exposure if people took opioids sporadically or took medications not prescribed to them. We did not have information about illicit opioid use. Finally, we studied patients within a single health care system, which could limit generalizability.
Despite these limitations, our findings are important because millions of older adults are exposed to opioids,1, 2 and there is a plausible biologic mechanism for increased risk of infection in opioid users. Until now, this question has received little study. One study of nursing home residents with chronic pain examined outcomes of chronic opioid use and did not observe an association with pneumonia,19 but the number of pneumonia cases was very small. In addition, the study’s inclusion criteria may have led to bias, because patients who discontinued opioids were excluded altogether. This group may disproportionately include patients who experienced adverse events. Another study examined infectious complications in patients hospitalized for severe burns.18 People who developed an infection had received opioids at higher dosages and for longer duration than controls. To our knowledge, no clinical or epidemiologic study has examined infection risk related to opioid use in a general population.
One noteworthy finding in our study is a substantially higher pneumonia risk soon after initiation of opioids (OR 3.24) that was not seen with long-term use (OR 1.27 for ≥ 90 days of use). This pattern is consistent with some prior immunologic studies which suggest that opioid-induced immunosuppression wanes with continuing exposure to opioids. For instance, in one study, natural killer cell activity was suppressed after 4 but not 14 days of exposure to morphine.8 The time frame over which opioids’ immunosuppressive effects might wane is not clear, and immunologic studies have yielded conflicting results.8, 30–32
Regarding benzodiazepine use, two population-based case-control studies reported conflicting findings: one observed decreased CAP risk with benzodiazepine use (adjusted OR 0.46, 95 % CI 0.23 to 0.94, compared to nonuse),20 while the second found no association.21 Reasons for the discrepancy are unclear because the two studies used very similar methods. Both studies assessed medication use from self-report, which may lead to bias, and both defined use as any use in the prior year, which may be less relevant than use shortly before pneumonia onset. Taken together with prior findings, our results suggest that benzodiazepine use is not associated with increased risk of pneumonia.
Further research about prescription opioids and infection risk is urgently needed, particularly because our findings could have major implications for clinical practice. A randomized trial would address concerns about confounding but likely would not be feasible for logistical and ethical reasons.33 The relatively low incidence of pneumonia (about 3 per 1000 per year in this age group34) would necessitate a large sample size and lengthy follow-up. Observational studies have potential to advance knowledge in this area but will need to address specific methodologic challenges. Future studies should collect detailed information about the timing of pneumonia onset (to address potential reverse causation) and about potential confounding factors including comorbid illnesses. Our prior work suggests that such studies will need to go beyond administrative data to measure comorbidity, because such data may not be adequate to account for confounding in studies of medications and pneumonia risk.23, 35, 36
In conclusion, because millions of older adults are currently exposed to opioids,1, 2 and against a backdrop of immunologic studies suggesting that opioids impair immune function, our findings demonstrate the urgent need for additional investigation of the association between opioid use and infection.
Supplementary Material
Acknowledgments
Funding sources and related paper presentations:
Dr. Dublin was funded by a Paul Beeson Career Development Award from the National Institute on Aging, grant K23AG028954, by the Branta Foundation, and by Group Health Research Institute internal funds. The Beeson award is also supported by the Hartford and Starr Foundations and Atlantic Philanthropies. Mr. Walker receives support from the Branta Foundation. Dr. Von Korff was funded by a grant from the National Institute on Drug Abuse (grant R01DA022557). Group Health Research Institute internal funds covered the data collection and analysis. This work was presented as on oral presentation at the International Conference on Pharmacoepidemiology and Therapeutic Risk Management on August 20, 2010.
Sponsors’ Role: The sponsors played no role in study design; the collection, analysis or interpretation of data; the writing of the report; nor in the decision to submit the manuscript for publication.
Footnotes
Authors’ Contributions: Study conception and design: SD, MLJ, JCN, NSW, LAJ; acquisition of data: SD, MLJ, JCN, NSW, LAJ; data analysis: SD, RLW; interpretation of results: all authors; drafting or revision of the manuscript: all authors.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, the National Institute on Drug Abuse, or the National Institutes of Health.
Conflict of Interest
SD received a Merck/American Geriatrics Society New Investigator Award and holds a grant from the Branta Foundation. RW is funded in part by Dr. Dublin’s grant from the Branta Foundation. JCN has done statistical consulting for Glaxo Smith Kline. MVK has grants from Johnson & Johnson (grant for public domain research on predicting back pain outcomes) and the National Institute on Drug Abuse. LAJ has grants from Novartis, Sanofi Pasteur, Glaxo Smith Kline, and Pfizer.
References
- 1.Williams RE, Sampson TJ, Kalilani L, et al. Epidemiology of opioid pharmacy claims in the United States. J Opioid Manag. 2008;4:145–152. doi: 10.5055/jom.2008.0019. [DOI] [PubMed] [Google Scholar]
- 2.Boudreau D, Von Korff M, Rutter CM, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf. 2009;18:1166–1175. doi: 10.1002/pds.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Geriatrics Society Panel on the Pharmacological Management of Persistent Pain in Older Persons. Pharmacological Management of Persistent Pain in Older Persons. Pain Med. 2009;10:1062–1083. doi: 10.1111/j.1526-4637.2009.00699.x. [DOI] [PubMed] [Google Scholar]
- 4.Gleason PP, Schulz R, Smith NL, et al. Correlates and prevalence of benzodiazepine use in community-dwelling elderly. J Gen Intern Med. 1998;13:243–250. doi: 10.1046/j.1525-1497.1998.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huxley EJ, Viroslav J, Gray WR, et al. Pharyngeal aspiration in normal adults and patients with depressed consciousness. Am J Med. 1978;64:564–568. doi: 10.1016/0002-9343(78)90574-0. [DOI] [PubMed] [Google Scholar]
- 6.Shavit Y, Ben-Eliyahu S, Zeidel A, et al. Effects of fentanyl on natural killer cell activity and on resistance to tumor metastasis in rats. Dose and timing study. Neuroimmunomodulation. 2004;11:255–260. doi: 10.1159/000078444. [DOI] [PubMed] [Google Scholar]
- 7.Beilin B, Martin FC, Shavit Y, et al. Suppression of natural killer cell activity by high-dose narcotic anesthesia in rats. Brain Behav Immun. 1989;3:129–137. doi: 10.1016/0889-1591(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 8.Shavit Y, Terman GW, Lewis JW, et al. Effects of footshock stress and morphine on natural killer lymphocytes in rats: Studies of tolerance and cross-tolerance. Brain Res. 1986;372:382–385. doi: 10.1016/0006-8993(86)91149-2. [DOI] [PubMed] [Google Scholar]
- 9.House RV, Thomas PT, Bhargava HN. In vitro evaluation of fentanyl and meperidine for immunomodulatory activity. Immunol Lett. 1995;46:117–124. doi: 10.1016/0165-2478(95)00035-4. [DOI] [PubMed] [Google Scholar]
- 10.Beilin B, Shavit Y, Hart J, et al. Effects of anesthesia based on large versus small doses of fentanyl on natural killer cell cytotoxicity in the perioperative period. Anesth Analg. 1996;82:492–497. doi: 10.1097/00000539-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Sacerdote P, Manfredi B, Mantegazza P, et al. Antinociceptive and immunosuppressive effects of opiate drugs: A structure-related activity study. Br J Pharmacol. 1997;121:834–840. doi: 10.1038/sj.bjp.0701138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Barke RA, Charboneau R, et al. Morphine impairs host innate immune response and increases susceptibility to Streptococcus pneumoniae lung infection. J Immunol. 2005;174:426–434. doi: 10.4049/jimmunol.174.1.426. [DOI] [PubMed] [Google Scholar]
- 13.Malik AA, Radhakrishnan N, Reddy K, et al. Morphine-induced macrophage apoptosis modulates migration of macrophages: Use of in vitro model of urinary tract infection. J Endourol. 2002;16:605–610. doi: 10.1089/089277902320913314. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Barke RA, Charboneau R, et al. Morphine induces defects in early response of alveolar macrophages to Streptococcus pneumoniae by modulating TLR9-NF-kappa β signaling. J Immunol. 2008;180:3594–3600. doi: 10.4049/jimmunol.180.5.3594. [DOI] [PubMed] [Google Scholar]
- 15.Budd K. Pain management: is opioid immunosuppression a clinical problem? Biomed Pharmacother. 2006;60:310–317. doi: 10.1016/j.biopha.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Sacerdote P. Opioids and the immune system. Palliat Med. 2006;20 (Suppl 1):s9–15. [PubMed] [Google Scholar]
- 17.Sacerdote P, Bianchi M, Gaspani L, et al. The effects of tramadol and morphine on immune responses and pain after surgery in cancer patients. Anesth Analg. 2000;90:1411–1414. doi: 10.1097/00000539-200006000-00028. [DOI] [PubMed] [Google Scholar]
- 18.Schwacha MG, McGwin G, Jr, Hutchinson CB, et al. The contribution of opiate analgesics to the development of infectious complications in burn patients. Am J Surg. 2006;192:82–86. doi: 10.1016/j.amjsurg.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Won A, Lapane KL, Vallow S, et al. Long-term effects of analgesics in a population of elderly nursing home residents with persistent nonmalignant pain. J Gerontol A Biol Sci Med Sci. 2006;61:165–169. doi: 10.1093/gerona/61.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Almirall J, Bolibar I, Balanzo X, et al. Risk factors for community-acquired pneumonia in adults: A population-based case-control study. Eur Respir J. 1999;13:349–355. doi: 10.1183/09031936.99.13234999. [DOI] [PubMed] [Google Scholar]
- 21.Almirall J, Bolibar I, Serra-Prat M, et al. New evidence of risk factors for community-acquired pneumonia: A population-based study. Eur Respir J. 2008;31:1274–1284. doi: 10.1183/09031936.00095807. [DOI] [PubMed] [Google Scholar]
- 22.Vergis EN, Brennen C, Wagener M, et al. Pneumonia in Long-term Care: A Prospective Case-Control Study of Risk Factors and Impact on Survival. Arch Intern Med. 2001;161:2378–2381. doi: 10.1001/archinte.161.19.2378. [DOI] [PubMed] [Google Scholar]
- 23.Jackson ML, Nelson JC, Weiss NS, et al. Influenza vaccination and risk of community-acquired pneumonia in immunocompetent elderly people: A population-based, nested case-control study. Lancet. 2008;372:398–405. doi: 10.1016/S0140-6736(08)61160-5. [DOI] [PubMed] [Google Scholar]
- 24.Nelson JC, Jackson M, Yu O, et al. Impact of the introduction of pneumococcal conjugate vaccine on rates of community acquired pneumonia in children and adults. Vaccine. 2008;26:4947–4954. doi: 10.1016/j.vaccine.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Saunders KW, Davis RL, Stergachis A. Group Health Cooperative. In: Strom BL, editor. Pharmacoepidemiology. 4. Chichester: John Wiley & Sons; 2005. p. 234. [Google Scholar]
- 26.Sacerdote P, Bianchi M, Manfredi B, et al. Effects of tramadol on immune responses and nociceptive thresholds in mice. Pain. 1997;72:325–330. doi: 10.1016/s0304-3959(97)00055-9. [DOI] [PubMed] [Google Scholar]
- 27.Opium. Micromedex 10 Updated periodically. Greenwood Village, Colo: Thomson Reuters (Healthcare) Inc; 2010. [Google Scholar]
- 28.Von Korff M, Saunders K, Thomas Ray G, et al. De facto long-term opioid therapy for noncancer pain. Clin J Pain. 2008;24:521–527. doi: 10.1097/AJP.0b013e318169d03b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gray SL, Eggen AE, Blough D, et al. Benzodiazepine use in older adults enrolled in a health maintenance organization. J Am Geriatr Soc. 2003;11:568–576. [PubMed] [Google Scholar]
- 30.De Waal EJ, Van Der Laan JW, Van Loveren H. Effects of prolonged exposure to morphine and methadone on in vivo parameters of immune function in rats. Toxicology. 1998;129:201–210. doi: 10.1016/s0300-483x(98)00077-8. [DOI] [PubMed] [Google Scholar]
- 31.Singhal PC, Sharma P, Kapasi AA, et al. Morphine enhances macrophage apoptosis. J Immunol. 1998;160:1886–1893. [PubMed] [Google Scholar]
- 32.Freier DO, Fuchs BA. A mechanism of action for morphine-induced immunosuppression: corticosterone mediates morphine-induced suppression of natural killer cell activity. J Pharmacol Exp Ther. 1994;270:1127–1133. [PubMed] [Google Scholar]
- 33.Chapman CR, Lipschitz DL, Angst MS, et al. Opioid pharmacotherapy for chronic non-cancer pain in the United States: A research guideline for developing an evidence-base. J Pain. 2010;11:807–829. doi: 10.1016/j.jpain.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 34.Jackson ML, Neuzil KM, Thompson WW, et al. The burden of community-acquired pneumonia in seniors: Results of a population-based study. Clin Infect Dis. 2004;39:1642–1650. doi: 10.1086/425615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dublin S, Walker RL, Jackson ML, et al. Use of proton pump inhibitors and H2 blockers and risk of pneumonia in older adults: A population-based case-control study. Pharmacoepidemiol Drug Saf. 2010;19:792–802. doi: 10.1002/pds.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dublin S, Jackson ML, Nelson JC, et al. Statin use and risk of community acquired pneumonia in older people: Population based case-control study. BMJ. 2009;338:b2137. doi: 10.1136/bmj.b2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.