Abstract
A strategy for the construction of unsymmetrical cyclobutanes using C–H functionalization logic is demonstrated in the total synthesis of piperarborenine B and piperarborenine D (reported structure). These syntheses feature a new preparation of cis-cyclobutane dicarboxylates from commercially available coumalate starting materials and a divergent approach to the controlled cis or trans installation of the two distinct aryl rings found in the natural products using the first example of cyclobutane C–H arylation. The structure of piperarborenine D is reassigned to a head-to-head dimer, which was synthesized using an intramolecular [2+2] photocycloaddition strategy.
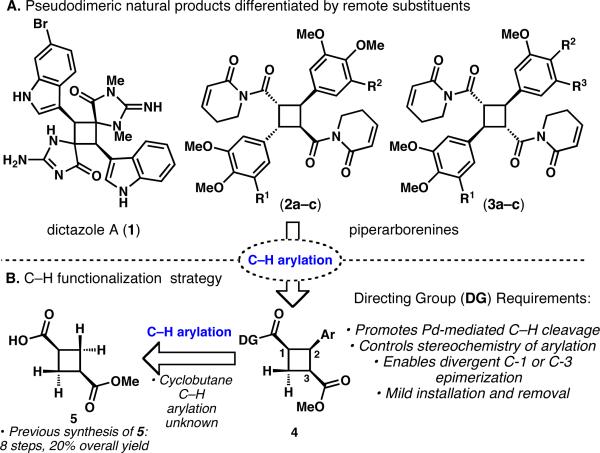
Dimeric, cyclobutane containing natural products comprise a small, yet diverse family with a variety of biological activities.1 Although no “[2+2]-ase” has been identified, it is generally presumed that such compounds originate from the direct coupling of the parent monomeric olefins in Nature. From the vantage point of synthetic strategy, cyclobutanes derived from such a heterodimerization are quite difficult to prepare in a controlled fashion. For example, dictazole A (1) and the piperarborenines (2a–2c, 3a–3c) are pseudosymmetrical cyclobutane natural products that are only differentiated by remote substituents (Figure 1A).2 While a direct [2+2] photocycloaddition is an appealing route for coupling two similar yet distinct olefins, this strategy is problematic for several reasons, including homodimerization, orientation (head-to-head versus head-to-tail) and E/Z isomerization, leading to a variety of possible structural and stereochemical outcomes.3 While crystal engineering and solid-state photochemistry techniques have provided stereodefined access to some cyclobutane classes,4 examples of direct heterodimerization of isolated olefins to form cyclobutanes are rare and are generally limited in scope.5 In this Communication, the first catalytic, transition metal-mediated C–H functionalization of cyclobutanes is reported as an alternative to [2+2] cycloaddition for the preparation of pseudosymmetrical cyclobutane structures.6,7 It is further shown for the first time that sp3 C–H arylation reactions can be conducted sequentially to access a set of stereoisomeric natural products in a practical and divergent fashion.8
Figure 1.
General C–H activation strategy for unsymmetrical cyclobutane natural products; 2a: piperarborenine A (R1,R2=H), 2b: piperarborenine B (R1=H, R2=OMe), 2c: piplartine dimer A (R1,R2=OMe), 3a: piperarborenine C (R1=OMe, R2+R3=OCH2O), 3b: piperarborenine D (R1=H, R2=R3=OMe), 3c: piperarborenine E (R1=H, R2+R3=OCH2O).
The piperarborenines (2a–2c, 3a–3c) were isolated from the stems of the creeping shrub Piper arborenscens and exhibit in vitro cytotoxicity against P-388, HT-29 and A549 cancer cell lines (IC50 < 4 μg/mL).2c This family of natural products can be classified into two distinct stereochemical classes: cis-trans-cis (2a–2c) and trans-trans-trans (3a–3c), both arising from the head-to-tail dimerization of piplartine-type monomers with varying degrees of oxidation on the aryl rings. While considering new strategies toward these cyclobutane natural products, the use of the pre-existing carboxylate functionality to guide sequential C–H arylations directly onto the cyclobutane ring was envisioned, as depicted in Figure 1. Furthermore, if these arylations could be performed sequentially, the problems of pseudosymmetry and stereochemistry would be greatly simplified. While C–H arylation reactions on cyclobutane rings have hitherto not been reported,9 the pioneering studies of Daugulis and coworkers to cross-couple sp3 C–H bonds with aryl iodides appeared to be suitable for our endeavors.10
The retrosynthesis of piperarborenine B (2b) and piperarborenine D (3b) is outlined in Figure 1B. The imide motifs found in the natural products were viewed as latent carboxylate-based directing groups for C–H functionalization, and, therefore, the dimethoxybenzene ring was replaced with a simple C–H bond. Monoarylated cyclobutane 4 was chosen as a divergent intermediate that could access both piperarborenine stereoisomers. Through selective epimerization of the C-1 or C-3 stereocenter, followed by a second C–H arylation, the synthesis of either natural product could be envisioned. The all-cis cyclobutane 4 would arise from a controlled, single C–H arylation performed on a symmetrical cyclobutane derived from acid 5. Although double arylation of this substrate could easily derail this plan, it was rationalized that the cis orientation of the ester and the directing group would hinder the rate of the second arylation due to steric and conformational effects. Furthermore, despite the seemingly simple appearance of carboxylic acid 5, the literature preparation requires a lengthy, 8-step, nonstereocontrolled sequence,11 making the first challenge a new synthesis of 1,3 substituted cyclobutane dicarboxylates.
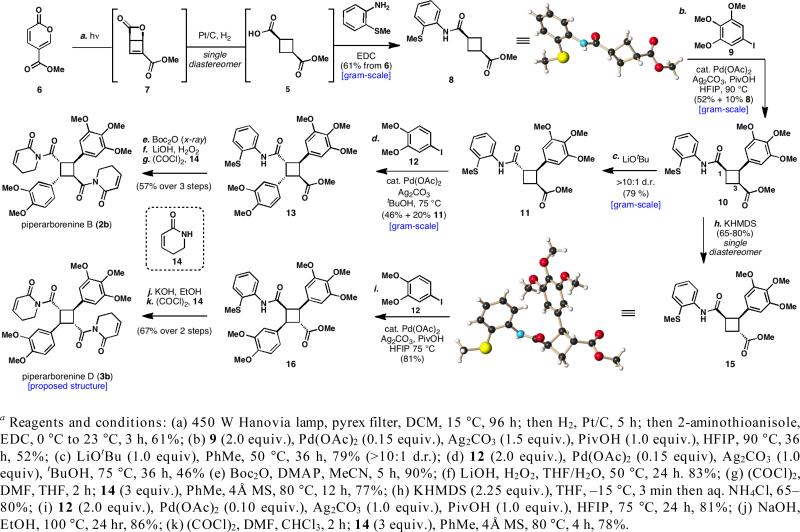
Maulide's creative work on cyclobutene synthesis12a and pioneering experiments by Corey on pyrone photochemistry12b inspired the selection of methyl coumalate (6)13 as a potential entry to acid 5. Since photochemical 4π electrocyclization to intermediate photopyrone 7 is known,12c only two reductions would be required to reach the desired product 5. After some experimentation, hydrogenation with Pt/C as a catalyst was found to reduce the photopyrone 7 to the desired carboxylic acid 5 as a single diastereomer (Scheme 1). An EDC coupling merged the directing group, 2-aminothioanisole,14 to the carboxylic acid and provided the C–H arylation substrate 8 (relative stereochemistry confirmed by X-ray crystallographic analysis). Notably, each step in this sequence was performed with DCM as the solvent, allowing all three reactions to be telescoped as a single operation in 61% isolated yield (1.0 g scale).
Scheme 1.
Total Synthesis of Piperarborenine B (2b) and Piperarborenine D (3b)a
The selective C–H cross-coupling of 8 with 3,4,5-trimethoxyiodobenzene (9) proved to be a formidable challenge. Under most conditions, the reaction was plagued with substantial decomposition, poor catalyst turnover and varying amounts of the anticipated overarylated product. After significant optimization, the addition of hexafluoroisopropanol (HFIP) and pivalic acid15 was found to be critical to the success of the reaction. This allowed the desired cyclobutane 10 to be isolated in 52% yield (1.0 g scale) with only trace amounts of overarylated byproduct formed.
With the key intermediate 10 secured, efforts were directed towards its divergent epimerization. Treatment with LiOtBu in a nonpolar solvent selectively epimerized the C-1 stereocenter to give cyclobutane 11 in 79% yield (1.6 g scale) with less than 10% of undesired epimerization products observed. Alternatively, C-3 can be selectively inverted by treatment of 10 with 2.25 equivalents of KHMDS followed by a quench of the resulting dianion with ammonium chloride. The initial amide N–H deprotonation allowed for exclusive ester enolate generation, giving 15 as a single product and diastereomer in 65–80% yield16, with its relative stereochemistry confirmed by X-ray crystallographic analysis.
With conditions secured to access either epimer, attention was then turned to the second arylation event. To this end, epimer 11 underwent a second C–H arylation reaction with 3,4-dimethoxyiodobenzene (12) in 46% yield (1.1 g scale) when t-BuOH was employed as a solvent at high concentration (1 M). Exclusive selectivity was observed for C–H insertion into the methylene C–H bond over the tertiary benzylic C–H bond.17 Curiously, HFIP and pivalic acid, critical in the first C–H arylation, resulted in inefficient cross-coupling for this substrate.
All that remained to complete the synthesis of piperarborenine B (2b) was the conversion of the ester and the directing groups into dihydropyridone rings. Using conditions developed by Evans for mild amide bond hydrolysis,18 the directing group was carbamoylated (91%, 1.8 g scale) and cleaved with lithium hydroperoxide (83%, 1.8 g scale). Fortunately, the methyl ester also hydrolyzed under these reaction conditions, and the carefully constructed stereotetrad remained intact. After conversion of both carboxylic acids to the corresponding acid chlorides and heating with dihydropyridone (14) in the presence of 4 Å molecular sieves,19 piperarborenine B (2b) was generated in 77% isolated yield (100 mg scale) and matched the reported spectral and experimental data.2b
In order to prepare the stereoisomeric piperarborenine D (3b), epimer 15 was subjected to a second round of C–H arylation with 12 to provide the desired compound 16 in 81% isolated yield. The facility with which 15 was found to undergo a second C–H arylation supports the proposed role of the methyl ester stereochemistry during the previous monoarylation reaction. Interestingly, pivalic acid and HFIP were once again pivotal for the efficiency of this cross-coupling. Refluxing 16 with sodium hydroxide in ethanol resulted in complete C-1 epimerization and full hydrolysis to the bis-carboxylic acid in 86% yield. Conversion to the bis-acid chloride and coupling with dihydropyridone (14) resulted in the proposed structure of piperarborenine D (3b) in 78% yield; however, the obtained 1H and 13C NMR spectra did not match the data given for 3 in the isolation paper.2c
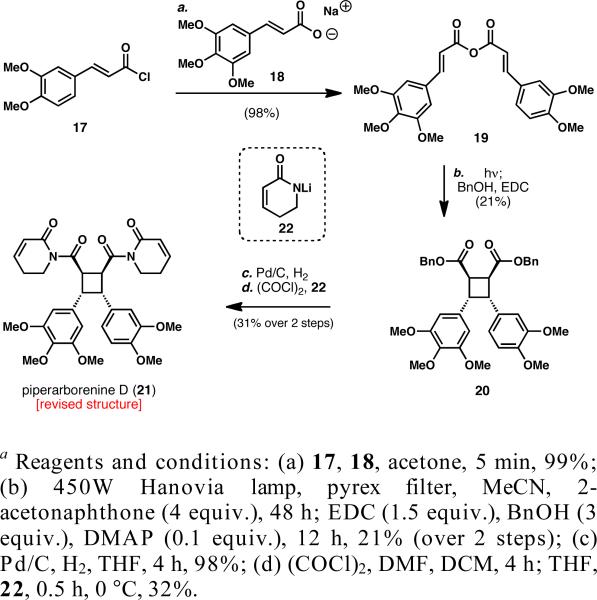
A reexamination of the original spectral data revealed a number of inconsistencies with the proposed structure, the most glaring being the excessive number of 13C NMR peaks, considering a compound that contains a σv plane of symmetry. Thus, an unsymmetrical head-to-head type dimer (21 ) that is consistent with the reported data was proposed and its preparation was pursued in order to confirm its structure. A simple, albeit unoptimized, synthesis of this heterodimeric structure was devised using an anhydride as a temporary tether for an intramolecular [2+2] photocycloaddition (Scheme 2). The mixed anhydride (19) was prepared in 98% yield by reacting 3,4-dimethoxycinnamoyl chloride (17) with sodium 3,4,5-trimethoxycinnamate (18) in acetone. While similar cinnamic anhydrides have been reported in the literature to undergo facile intramolecular [2+2] photocycloaddition,20 this particular substrate simply decomposed under most conditions tested. After a small screen of photosen-sitizers and solvents, 2’-acetonaphthone21 in acetonitrile furnished the desired photoproduct, albeit in low yield. The intermediate anhydride was immediately converted into the corresponding dibenzyl ester (20) for purification and, it was isolated in 21% yield over two steps. Hydrogenolysis of the benzyl esters and coupling of the crude diacid chloride to lithium salt 22 generated a product that was spectroscopically identical to that reported for piperarborenine D (21) in 32% yield.22
Scheme 2.
Structural Revision of Piperarborenine Da
The short routes to 2b and 3b (7 steps, 7% overall yield; 6 steps, 12% overall) demonstrate the power of C–H functionalization logic to enable a fundamentally new approach to cyclobutane natural product synthesis. Notable elements of the synthesis include: (1) a one-step, stereocontrolled preparation of 8 from methyl coumalate (6); (2) the first example of catalytic transition metal-mediated C–H activation on a cyclobutane ring; (3) the first example of sequential sp3 C–H arylation reactions performed in natural product synthesis; (4) divergent epimerization of 10 to access both proposed piperarborenine stereoisomers; (5) reassignment of the trans-trans-trans piperarborenines (3a–3c) to head-to-head cyclobutane dimers (e.g., 21).
Supplementary Material
ACKNOWLEDGMENT
Financial support for this work was provided by the NIH/NIGMS (GM-073949) and NSF (predoctoral fellowship to W.R.G.). We thank Prof. A. Rheingold and Dr. Curtis E. Moore for x-ray crystallographic measurements.
Footnotes
Supporting Information Placeholder
ASSOCIATED CONTENT
Supporting Information. Experimental procedures and characterization data for all reaction and products, including 1H NMR and 13C NMR spectra. This material is available free of charge via the Internet at http://pubs.acs.org
REFERENCES
- 1.Dembisky VM. J. Nat. Med. 2008;62:1–33. doi: 10.1007/s11418-007-0166-3. and references therein. [DOI] [PubMed] [Google Scholar]
- 2.a Dai J, Jiménez JI, Kelly M, Williams PG. J. Org. Chem. 2010;75:2399–2402. doi: 10.1021/jo902566n. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lee F-P, Chen Y-C, Chen J-J, Tsai I-L, Chen I-S. Helv. Chim. Acta. 2004;87:463–468. [Google Scholar]; c Tsai I-L, Lee F-P, Wu C-C, Duh C-Y, Ishikawa T, Chen J-J, Chen Y-C, Seki H, Chen I-S. Planta Med. 2005;71:535–542. doi: 10.1055/s-2005-864155. [DOI] [PubMed] [Google Scholar]; d Filho RB, De Souza MP, Mattos MEO. Phytochemistry. 1981;20:345–346. [Google Scholar]
- 3.Solution phase photodimerization of methyl cinnamate in the presence of BF3•Et2O produced 8 of 11 possible cycloadducts: Lewis FD, Quillen SL, Hale PD, Oxman JD. J. Am. Chem. Soc. 1988;110:1261–1267. b For the direct dimerization of piplartine to piplartine dimer A (2c) in 0.8% yield, see ref. 2d.
- 4.a Cohen MD, Schmidt GMJ. J. Chem. Soc. 2000–2013;1964 [Google Scholar]; b Ramamurthy V, Venkatesan K. Chem. Rev. 1987;87:433–481. [Google Scholar]; c Natarajan A, Ramamurthy V. In: The Chemistry of Cyclobutanes. Rappoport Z, Libeman JF, editors. Vol. 1. Chichester; Wiley: 2005. pp. 807–872. [Google Scholar]
- 5.For selected examples, see: Du J, Yoon TP. J. Am. Chem. Soc. 2009;131:14604–14605. doi: 10.1021/ja903732v.Fedorova O, Federov YV, Gulakova E, Schepel N, Alfimov M, Goli U, Saltiel J. Photochem. Photobiol. Sci. 2007;6:1097–1105. doi: 10.1039/b707093d.
- 6.For examples of stoichiometric C–H activation of cyclobutanes, see: Periana RA, Bergman RG. J. Am. Chem. Soc. 1986;108:7332–7346.Wick DD, Northcutt TO, Lachicotte RJ, Jones WD. Organometallics. 1998;17:4484–4492.Simms BL, Ibers JA. J. Organomet. Chem. 1987;330:279–289.
- 7.For selected reviews of C–H functionalization in total synthesis, see: Taber DF, Stiriba SE. Chem. Eur. J. 1998;4:990–992.Godula K, Sames D. Science. 2006;312:67–72. doi: 10.1126/science.1114731.Davies HM, Manning JR. Nature. 2008;451:417–424. doi: 10.1038/nature06485.Ishihara Y, Baran PS. Synlett. 2010;12:1733–1745.Gutekunst WR, Baran PS. Chem. Soc. Rev. 2011;40:1976–1991. doi: 10.1039/c0cs00182a.McMurray L, O'Hara F, Gaunt MJ. Chem. Soc. Rev. 2011;40:1885–1898. doi: 10.1039/c1cs15013h.Brückl T, Baxter RD, Ishihara Y, Baran PS. Acc. Chem. Res. 2011 doi: 10.1021/ar200194b. DOI: 10.1021/ar200194b. For a recent example: Wang D-H, Yu J-Q. J. Am. Chem. Soc. 2011;133:5767–5769. doi: 10.1021/ja2010225.
- 8.For examples of dimeric cyclobutane total synthesis, see: Ichikawa M, Takahashi M, Aoyagi S, Kibayashi C. J. Am. Chem. Soc. 2004;126:16553–16558. doi: 10.1021/ja0401702.Tsai AS, Bergman RG, Ellman JA. J. Am. Chem. Soc. 2008;130:6316–6317. doi: 10.1021/ja8012159.Zhang F, Jia Y. Tetrahedron. 2009;65:6840–6843.Takahashi M, Ichikawa M, Aoyagi S, Kibayashi C. Tetrahedron Lett. 2005;46:57–59.Birman VB, Jiang X-T. Org. Lett. 2004;6:2369–2371. doi: 10.1021/ol049283g.O'Malley DP, Li K, Maue M, Zografos AL, Baran PS. J. Am. Chem. Soc. 2007;129:4762–4775. doi: 10.1021/ja069035a.
- 9.A conceptually similar approach to cyclobutane function-alization using directed magnesiation has been reported, but employs harsh conditions and is limited in scope: Eaton PE, Zhang M-X, Komiya N, Yang C-G, Steele I, Gilardi R. Synlett. 2003;9:1275–1278.Zhang M-X, Eaton PE. Angew. Chem. Int. Ed. 2002;41:2169–2171.
- 10.Zaitsev VG, Shabashov D, Daugulis O. J. Am. Chem. Soc. 2005;127:13154–13155. doi: 10.1021/ja054549f.Shabashov D, Daugulis O. J. Am. Chem. Soc. 2010;132:3965–3972. doi: 10.1021/ja910900p. For other examples of intermolecular arylation of unactivated sp3 C–H bonds, see: Reddy BVS, Reddy LR, Corey EJ. Org. Lett. 2006;8:3391–3394. doi: 10.1021/ol061389j.He G, Chen G. Angew. Chem. Int. Ed. 2011;50:5192–5196. doi: 10.1002/anie.201100984.Barder TE, Walker SD, Martinelli JR, Buchwald SL. J. Am. Chem. Soc. 2005;127:4685–4696. doi: 10.1021/ja042491j.Giri R, Maugel N, Li J-J, Wang D-H, Breazzano SP, Saunders LB, Yu J-Q. J. Am. Chem. Soc. 2007;129:3510–3511. doi: 10.1021/ja0701614.Wang D-H, Wasa M, Giri R, Yu J-Q. J. Am. Chem. Soc. 2008;130:7190–7191. doi: 10.1021/ja801355s.Wasa M, Engle KM, Yu J-Q. J. Am, Chem. Soc. 2009;131:9886–9887. doi: 10.1021/ja903573p. For an application in total synthesis, see: Feng Y, Chen G. Angew. Chem. Int. Ed. 2010;49:958–961. doi: 10.1002/anie.200905134.
- 11.a Allinger NL, Tushaus NL. J. Am. Chem. Soc. 1965;30:1945–1951. [Google Scholar]; b Pecquet P, Huet F, Legraverend M, Bisagni E. Heterocycles. 1992;34:739–745. [Google Scholar]; c Bucholtz KM, Gareiss PC, Tajc SG, Miller BL. Org. Biomol. Chem. 2006;4:3973–3979. doi: 10.1039/b610727c. [DOI] [PubMed] [Google Scholar]
- 12.a Frébault F, Luparia M, Oliveira MT, Goddard R, Maulide N. Angew. Chem. Int. Ed. 2010;49:5672–5676. doi: 10.1002/anie.201000911. [DOI] [PubMed] [Google Scholar]; b Corey EJ, Streith J. J. Am. Chem. Soc. 1964;86:950–951. [Google Scholar]; c Javaheripour H, Neckers DC. J. Am. Chem. Soc. 1977;42:1844–1850. [Google Scholar]
- 13.Commercially available or prepared in two steps from malic acid ($55/kg, Sigma); Ashworth IW, Bowden MC, Dembofsky B, Levin D, Moss W, Robinson E, Szczur N, Virica J. Org. Process Res. Dev. 2003;7:74–81.
- 14.2-aminothioanisole is used instead of 8-aminoquinoline since the former does not contain a basic nitrogen and can be hydrolyzed under milder conditions (see ref. 10b).
- 15.For studies on the role of pivalic acid in C–H cleavage, see: Lafrance M, Gorelsky SI, Fagnou K. J. Am. Chem. Soc. 2007;129:14570–14571. doi: 10.1021/ja076588s.Lafrance M, Fagnou K. J. Am. Chem. Soc. 2006;128:16496–16497. doi: 10.1021/ja067144j.
- 16.The variable yield is the result of the instability of the dianion, coupled with a slow second deprotonation at low temperatures.
- 17.When more forcing conditions were employed (105 °C) , small amounts of a tentatively assigned tetraarylated byproduct could be observed by LC-MS and 1H NMR of the crude reaction mixture.
- 18.Evans DA, Carter PH, Dinsmore CJ, Barrow JC, Katz JL, Kung DW. Tetrahedron Lett. 1997;38:4535–4538. [Google Scholar]
- 19.4Å molecular sieves act as a neutral acid scavenger: Weinstock LM, Karady S, Roberts FE, Hoinowski AM, Brenner GS, Lee TBK, Lumma WC, Sletzinger M. 1975;46:3979–3982.Padwa A, Price A. J. Org. Chem. 1995;60:6258–6259.
- 20.Mascitti V, Corey EJ. Tetrahedron Lett. 2006;47:5879–5882. [Google Scholar]
- 21.Nicolaou KC, Sarlah D, Shaw DM. Angew. Chem. Int. Ed. 2007;46:4708–4711. doi: 10.1002/anie.200701552. [DOI] [PubMed] [Google Scholar]
- 22.The low yield is the result of competitive anhydride generation during formation of the bis-acid chloride.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.