Abstract
Sphaerodactyl geckos comprise five genera distributed across Central and South America and the Caribbean. We estimated phylogenetic relationships among sphaerodactyl genera using both separate and combined analyses of seven nuclear genes. Relationships among genera were incongruent at different loci and phylogenies were characterized by short, in some cases zero length, internal branches and poor phylogenetic support at most nodes. We recovered a polyphyletic Coleodactylus, with Coleodactylus amazonicus being deeply divergent from the remaining Coleodactylus species sampled. The C. amazonicus lineage possessed unique codon deletions in the genes PTPN12 and RBMX while the remaining Coleodactylus species had unique codon deletions in RAG1. Topology tests could not reject a monophyletic Coleodactylus, but we show that short internal branch lengths decreased the accuracy of topology tests because there were not enough data along short branches to support one phylogenetic hypothesis over another. Morphological data corroborated results of the molecular phylogeny, with Coleodactylus exhibiting substantial morphological heterogeneity. We identified a suite of unique craniofacial features that differentiate C. amazonicus not only from other Coleodactylus species, but also from all other geckos. We describe this novel sphaerodactyl lineage as a new genus, Chatogekko gen. nov. We present a detailed osteology of Chatogekko, characterizing osteological correlates of miniaturization that provide a framework for future studies in sphaerodactyl systematics and biology.
Keywords: Amazon, Chatogekko gen. nov., Coleodactylus, lizard, morphology, osteology, phylogeny, polytomy, Squamata
Introduction
Sphaerodactyl geckos (Sphaerodactylini: Sphaerodactylidae) are a species-rich group of Neotropical lizards. They comprise more than 10% of gecko species with more than 150 described species in five genera: Coleodactylus, Lepidoblepharis, Gonatodes, Pseudogonatodes, and Sphaerodactylus (Gamble, Bauer, Greenbaum & Jackman, 2008a; Kluge, 1995; Kluge, 2001; Uetz, 2010). Sphaerodactyl geckos are distributed across Central and South America and the Caribbean, including several Pacific continental and oceanic islands, e.g. Gorgona, Cocos (Harris, 1982; Harris & Kluge, 1984; Kluge, 1995; Vanzolini, 1968a). Most sphaerodactyl gecko species are active during the day and the clade is thought to be secondarily diurnal, having evolved from a nocturnal ancestor (Kluge, 1995; Röll & Henkel, 2002; Underwood, 1970; Werner, 1969). They are generally small; some Sphaerodactylus species are among the smallest known amniotes, averaging only 16 mm SVL (Hedges & Thomas, 2001; MacLean, 1985; Thomas, 1965), and the largest forms do not exceed 65 mm SVL (Rivas & Schargel, 2008).
The genus Coleodactylus is distributed in northeastern South America (Kluge, 1995) and consists of five described species: C. amazonicus (Andersson, 1918), C. brachystoma (Amaral, 1935), C. meridionalis (Boulenger, 1888), C. natalensisFreire, 1999, and C. septentrionalisVanzolini, 1980. Coleodactylus has historically been defined by the structure of the ungual sheath, the scales covering the claw, being composed of five asymmetrical scales (Kluge, 1995; Parker, 1926; Vanzolini, 1957). Coleodactylus amazonicus differs from its congeners in having an ungual sheath possessing only four asymmetrical scales; a reduction caused by the loss of the medial-most dorsal scale (Andersson, 1918; Avila-Pires, 1995; Parker, 1926; Vanzolini, 1957). Coleodactylus amazonicus also has keeled dorsal scales, while all other members of the genus have smooth scales (Avila-Pires, 1995; Vanzolini, 1957). These morphological differences cast doubt on the diagnostic reliability of the ungual sheath and other characters for the genus and/or on the allocation of C. amazonicus to Coleodactylus.
Molecular data mirror the morphological differences among Coleodactylus species. Recent molecular phylogenies recovered two deeply divergent lineages in Coleodactylus, with one clade consisting of C. amazonicus and the other made up of the remaining Coleodactylus species, the “C. meridionalis group” (Gamble, Bauer, Colli, Greenbaum, Jackman, Vitt & Simons, 2011; Geurgas & Rodrigues, 2010; Geurgas, Rodrigues & Moritz, 2008). These results were not translated into a revised taxonomy though because of poor nodal support, e.g. bootstrap values and Bayesian Posterior Probabilities, for these relationships from the molecular data. Additionally, topology tests that constrained a monophyletic Coleodactylus s.l. failed to reject the hypothesis that C. amazonicus forms a clade with the remaining Coleodactylus species (Gamble et al., 2011; Geurgas et al., 2008). Non-tree-based molecular evidence supports the distinction between C. amazonicus and the remaining Coleodactylus species. Two separate deletions of 18 and 6 bp in RAG1 occur in species of the C. meridionalis group, but not in C. amazonicus (Gamble et al., 2011; Gamble et al., 2008a; Geurgas & Rodrigues, 2010). Rare genomic events such as codon deletions and insertions (indels) are relatively homoplasy-free characters and can provide strong evidence of evolutionary history (Rokas & Holland, 2000; Simmons, Ochoterena & Carr, 2001; van Dijk, Paradis, Catzeflis & de Jong, 1999).
The sum of available data calls into question the monophyly of Coleodactylus. Coleodactylus amazonicus is morphologically distinct from the remaining Coleodactylus species. Molecular data presents a mixed picture of Coleodactylus relationships and sphaerodactyl phylogeny as a whole but, like the morphological data, casts doubt on Coleodactylus monophyly. We gathered new molecular and morphological data to address these issues. Our objectives were to: test the monophyly of Coleodactylus using a multigene molecular dataset and specifically address the failure of previous topology tests to support two distinct Coleodactylus lineages; review the morphology of C. amazonicus as a means to diagnose deeply divergent clades within Coleodactylus sensu lato; and characterize osteological correlates of miniaturization in Coleodactylus s.l.
Materials and Methods
Taxon sampling and molecular data
We assembled a nuclear gene dataset that included multiple species from each of the currently recognized genera of the New World Sphaerodactylini: Coleodactylus s.l., Gonatodes, Lepidoblepharis, Pseudogonatodes, and Sphaerodactylus. We included several Old World members of Sphaerodactylidae as outgroups, including Saurodactylus brosseti, Pristurus carteri, and two species of Teratoscincus (i.e. T. microlepis and T. przewalskii). Phylogenies were rooted with the gekkonid Hemidactylus platyurus. Locality data and GenBank accession numbers for sampled taxa are listed in the supplementary material.
We extracted genomic DNA from tissues using the DNeasy Blood and Tissue kit (Qiagen, Valencia, CA) and used PCR to amplify gene fragments of seven nuclear loci for sequencing. Six loci were protein coding regions: recombination-activating gene 1 (RAG1); recombination-activating gene 2 (RAG2); oocyte–maturation factor MOS (C-MOS); acetylcholinergic receptor M4 (ACM4 or CHRM4); phosducin (PDC); and protein tyrosine phosphatase, non-receptor type 12 (PTPN12). The seventh locus included intron 8 (in Gallus) and flanking exon regions of RNA binding motif protein, X-linked (RBMX). Primers are listed in the supplementary material. We purified PCR products using Exonuclease I and Shrimp Alkaline Phosphatase (Hanke & Wink, 1994). Big Dye sequencing was conducted at the BioMedical Genomics Center, University of Minnesota. Sequences were assembled and checked for accuracy using Sequencher 4.8 (Gene Codes, Ann Arbor, MI). We translated protein coding genes to amino acids using MacClade 4.08 (Maddison & Maddison, 1992) to confirm codon alignment and gap placement. We aligned RBMX sequences initially using T-Coffee (Notredame, Higgins & Heringa, 2000) and subsequently fine-tuned the alignment by hand.
Phylogenetic analyses
We conducted several phylogenetic analyses of the nuclear dataset. The seven loci were concatenated to conduct partitioned Maximum Likelihood (ML) analysis. We also analysed each locus separately. All ML analyses were conducted using RAxML 7.2.6 (Stamatakis, 2006). The concatenated ML analysis consisted of 19 partitions, with data partitioned by gene and by codon, except the intron RBMX, which consisted of a single partition. ML analyses of individual protein-coding loci also partitioned data by codon. All ML partitions utilized the GTR + Gamma model of sequence evolution and nodal support was estimated with 1000 bootstrap replicates (Felsenstein, 1985).
We conducted Bayesian analyses of the nuclear dataset using MrBayes 3.1.2 (Huelsenbeck & Ronquist, 2001; Ronquist & Huelsenbeck, 2003). All analyses used a neighbor-joining tree as a starting topology. Analyses of the individual genes involved two independent runs, each consisting of four parallel Markov chain Monte Carlo (MCMC) chains per run for 5 million generations and sampled every 1,000 generations. Each Bayesian analysis of the individual genes utilized a model of molecular evolution as determined by AIC in jModeltest (Posada, 2008). Analyses of the concatenated dataset partitioned data by codon with a separate partition for RBMX. Each partition utilized a model of molecular evolution as determined by AIC with model parameters estimated independently using the unlink option. The concatenated analysis involved two independent runs, each consisting of six parallel Markov chain Monte Carlo (MCMC) chains per run for 10 million generations and sampled every 1,000 generations. We assessed convergence and stationarity in all Bayesian analyses by plotting likelihood values in Tracer 1.5 (Rambaut & Drummond, 2007) as well as plotting split frequencies between independent runs using AWTY (Nylander, Wilgenbusch, Warren & Swofford, 2008).
Poor phylogenetic resolution among sphaerodactyl genera in the concatenated analyses and incongruence among individual gene trees (see results) motivated us to explore additional means of estimating phylogenetic relationships that could incorporate the sometimes diverse histories of individual genes. The probability of incomplete lineage sorting is increased when there are short internal branches (Maddison, 1997; Rosenberg & Tao, 2008), so we used two methods to estimate species trees that can accommodate individual gene genealogies. The first method, MDC (Minimized Deep Coalescence), used individual gene trees to find a species tree that minimized the number of deep coalescent events across all loci (Maddison, 1997; Maddison & Knowles, 2006). The second method, BCA (Bayesian concordance analysis), estimated the species tree possessed by the plurality of clades recovered from individual loci, the concordance tree, and also estimated the proportion of loci that shared a specific clade from the concordance tree, the concordance factor (Ane, Larget, Baum, Smith & Rokas, 2007; Baum, 2007).
We estimated the MDC tree using Mesquite 2.73 (Maddison & Maddison, 2008). This method required that “species” be identified a priori and that individuals or taxa from the analyses of separate loci be assigned to each of these “species”. Because we were interested in relationships among sphaerodactyl genera, we treated genera as “species” in the MDC analysis. We accommodated phylogenetic uncertainty associated with the reconstruction of the individual gene trees using the Augist Mesquite module (Oliver, 2008). We estimated 1,000 MDC trees with each search randomly sampling from the posterior distribution of trees from the Bayesian analyses of each of the nuclear loci. We used the subtree pruning and regrafting (SPR) heuristic search algorithm with a maximum of 100 equally parsimonious trees saved at each search. Tree weights were stored for each search in the event multiple equally parsimonious MDC trees were found. The MDC species tree was calculated as a 50% majority-rule consensus tree with bipartition frequencies providing a measure of nodal support.
We estimated the BCA tree using BUCKy 1.4.0 (Ane et al., 2007). We conducted three separate analyses, each with a different a priori discordance level among gene trees, which was controlled by the variable α (Ane et al., 2007). Setting α = 0, for example, imposes a single species tree on all of the loci, while at the other extreme setting α = ∞ forces each locus to have its own independent history. We used an interactive web-based tool (http://bigfork.botany.wisc.edu/concordance/) to calculate α values for our data. Each value for α placed a different prior on the number of possible species trees: α = 0.1 placed a high prior on 1 distinct tree; α = 1.0 placed a high prior on 2–3 species trees; and α = 10 placed a high prior on 5–6 species trees. All BUCKy analyses were run for 10,000,000 generations following a 10% burn-in.
Hypothesis testing
We tested the monophyly of Coleodactylus sensu lato using two different methods. We implemented the likelihood-based Shimodaira-Hasegawa (SH) test (Shimodaira & Hasegawa, 1999), which compared the constrained topology, a monophyletic Coleodactylus s. l., to the unconstrained maximum likelihood tree. Per-site log likelihoods were estimated in RAxML 7.2.6 (Stamatakis, 2006) and P-values calculated using CONSEL (Shimodaira & Hasegawa, 2001). We also tested alternative phylogenetic hypotheses in a Bayesian framework. We used the filter option in PAUP* 4.0b10 (Swofford, 2002) to calculate the posterior probability of a monophyletic Coleodactylus s. l. in the posterior distribution of trees from the MrBayes analyses. We tested Coleodactylus s. l. monophyly using the concatenated nuclear gene dataset and each locus separately.
Short internal branches connected the six sphaerodactyl genera in both the concatenated trees as well as individual gene trees (see results). These short internal branches not only increased the likelihood of incomplete lineage sorting, as mentioned above, but the limited number of character changes along these extremely short branches could make it difficult to adequately compare alternative hypotheses using the SH test. Some of these internal branches were so short as to have effectively zero branch length. These phenomena could explain why previous attempts to test Coleodactylus monophyly failed to adequately distinguish among competing hypotheses (Gamble et al., 2011; Geurgas et al., 2008). We examined our maximum likelihood trees for the presence of zero-length branches using a likelihood ratio test with the “describe trees” function in PAUP* 4.0b10 (Swofford, 2002). Briefly, the likelihood of the best tree was compared to the likelihood of the same tree but with a single branch collapsed to zero using the likelihood ratio test. Each of the four branches connecting the six sphaerodactyl genera were sequentially tested in this manner. A significant result meant the branch length was significantly different from zero. Significance levels were Bonferroni-corrected for the number of intergeneric branches.
Morphological data
We examined both internal and external morphological characters from specimens of several species of Sphaerodactylidae, including exemplars from each of the currently recognized sphaerodactyl genera, to assess the monophyly of Coleodactylus s.l. (see supplemental material). We also examined C. amazonicus specimens from several localities across its distributional range. We viewed osteological characters using a variation of a common clearing and double staining technique (Hanken & Wassersug, 1981). This method is especially useful for small animals in which dry skeletal preparation techniques are not suitable due to the potential risk of damage by the insects used to prepare them or to distortion caused by the drying and shrinkage of unossified portions of the skeleton. We modified the protocol in that we did not remove the integument from specimens, and used KOH only as a clearing reagent, without exposing specimens to enzymatic solutions of trypsin or pancreatin. Specimens were observed under a Leica MS6 dissecting microscope. Illustrations were traced with Adobe® Illustrator® CS3 13.0.2 directly over a series of digital photographs taken with a Nikon Coolpix 995 camera (3.1 Megapixels, 3x Optical Zoom) at different magnifications. Images were complemented with drawings made with a camera lucida.
Results
Taxon sampling and molecular data
The nuclear gene dataset consisted of 4,116 aligned base pairs from seven loci for 33 gecko taxa (Table 1). Sequence alignment was unambiguous for protein-coding regions, but several insertion/deletions (indels) were detected in five of the genes (Table 1, Fig. 1). Indels in RAG1, C-MOS and ACM4 have been commented on previously (Gamble et al., 2008a; Gamble, Simons, Colli & Vitt, 2008c; Geurgas et al., 2008). Both RBMX and PTPN12 had single codon deletions in C. amazonicus samples. The RBMX deletion occurred in the region analogous to exon 8 in chicken (Gallus).
Table 1.
Details of the seven nuclear loci used in phylogenetic analyses including: the aligned length of sequences; the number of variable sites; and the number of parsimony informative (PI) sites. The number of unique indels in protein coding regions in each locus is indicated, as is the taxonomic distribution of each indel. Some indels occurred only in a subsample of the sampled species within a genus.
| Locus | Aligned length (bp) | Variable sites | PI sites | Number of indels in coding regions |
|---|---|---|---|---|
| ACM4 | 447 | 150 | 94 | 1: within Gonatodes |
| CMOS | 384 | 157 | 97 | 2: within Gonatodes & within Coleodactylus |
| RBMX | 632 | 202 | 119 | 1: Chatogekko |
| PDC | 400 | 143 | 98 | n/a |
| PTPN12 | 1152 | 482 | 288 | 1: Chatogekko |
| RAG1 | 1095 | 533 | 344 | 4: Coleodactylus (2), within Coleodactylus, & Pristurus |
| RAG2 | 366 | 166 | 108 | n/a |
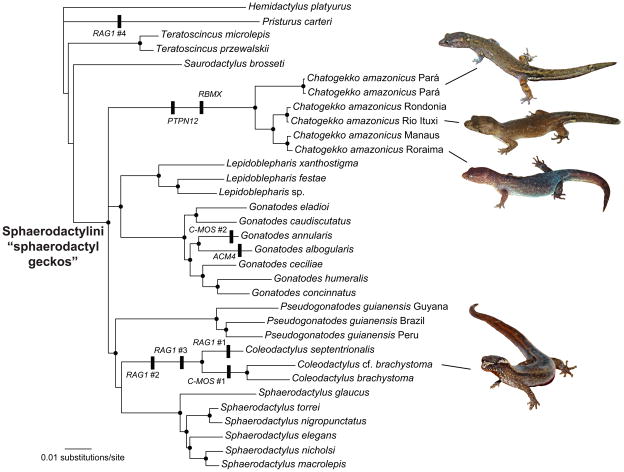
Figure 1.
Bayesian phylogeny of sphaerodactyl genera from the concatenated nuclear gene data. Nodes with black circles possess posterior probabilities > 0.95. Unique indels from protein coding regions are indicated along with the gene name. Both RAG1 and C-MOS possessed multiple unique indels and each is numbered sequentially starting with the most 5′ indel and moving in the 3′ direction. Photos by L. J. Vitt, T. Gamble, and M. Hoogmoed.
Phylogenetic analyses
Maximum likelihood and Bayesian analyses of the concatenated nuclear gene dataset were largely congruent (Fig. 1). Relationships among the sphaerodactylid outgroups were inconsistent and generally poorly supported. Several clades received high levels of support in both analyses, including: a clade consisting of Gonatodes + Lepidoblepharis; a clade consisting of Coleodactylus s.s. + Pseudogonatodes + Sphaerodactylus; and Sphaerodactylini. Generic-level sphaerodactyl clades were all well supported with the exception of Coleodactylus s.l., which was polyphyletic with regards to other sphaerodactyl genera, e.g. C. amazonicus did not form a clade with the remaining sampled Coleodactylus species. We recovered three clades within C. amazonicus. One clade consisted of individuals from eastern Amazon (Pará); the second clade consisted of individuals from southwestern Amazon (Rondônia and Rio Ituxi, Amazonas); the third clade consisted of individuals from central and northern Amazon (near Manaus, Amazonas, and Roraima). Maximum likelihood branch lengths among these C. amazonicus clades were equivalent to species level divergences within other sphaerodactyl genera and between the species Teratoscincus microlepis and T. przewalskii (Fig. 1).
Topologies among individual gene trees were largely incongruent (Fig. 2). The only well-supported nodes in all of the analyses were nodes subtending each of the sphaerodactyl genera, although, as with the concatenated analyses, Coleodactylus s.l. was polyphyletic in all loci with C. amazonicus samples forming their own clade distinct from other sampled Coleodactylus species.
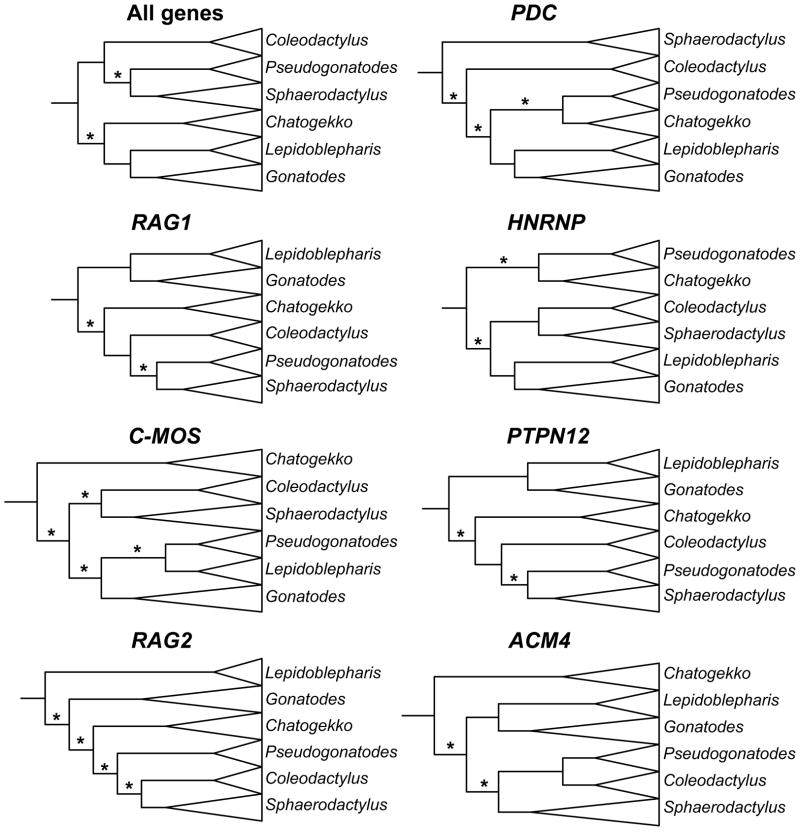
Figure 2.
Cladograms for each nuclear locus and the concatenated nuclear gene dataset illustrating relationships among sphaerodactyl genera estimated using Maximum Likelihood. Branches with lengths not significantly different than zero are indicated with an asterisk.
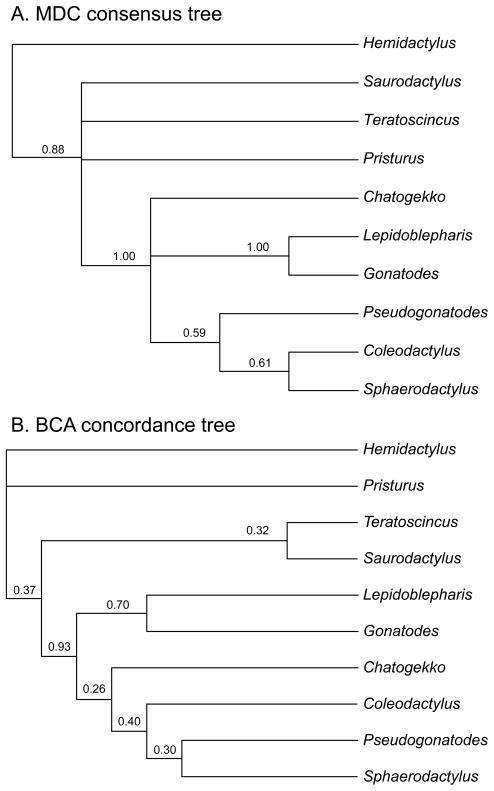
The MDC consensus tree (Fig. 3) was largely congruent with the concatenated Maximum likelihood and Bayesian trees and recovered a well-supported Sphaerodactylini consisting of three lineages: a Lepidoblepharis + Gonatodes clade; a Coleodactylus s.s. + Pseudogonatodes + Sphaerodactylus clade; and a C. amazonicus clade. BCA analyses with varying α levels produced identical concordance trees and concordance factors. The BCA tree (Fig. 3) was similar to the MDC consensus tree. While it is difficult to assess what constitutes a significant concordance factor (Baum, 2007) the Sphaerodactylini clade and Lepidoblepharis + Gonatodes clade were the only relationships that received concordance factors exceeding 0.50.
Figure 3.
Phylogenetic relationships among sphaerodactyl genera estimated using (A) MDC (minimization of deep coalescence events) and (B) BCA (Bayesian concordance analysis). Node values on the MDC tree are bipartition frequencies from 1000 replicate analyses that randomly sampled from the Bayesian posterior distributions of the individual gene trees. Node values on the BCA tree are posterior mean concordance factors.
Hypothesis testing
Results of the SH tests that constrained Coleodactylus s.l. as monophyletic were not significant (Table 2). The Bayesian posterior probability of a monophyletic Coleodactylus s.l. was zero for the concatenated data and low, but not statistically significant, for most of the individual gene analyses (Table 2).
Table 2.
Results of topological constraint tests comparing a monophyletic Coleodactylus sensu lato to the best phylogenetic estimates for seven nuclear genes analysed individually, as well as the combined analysis. Columns show the log likelihood (lnL) of the best tree, the likelihood of the tree with a monophyletic Coleodactylus s.l., the difference in likelihood values between the best tree and the constraint tree, and the P-value of the SH test. The last column shows posterior probabilities of a monophyletic Coleodactylus s.l. from the Bayesian analyses.
| Dataset | lnL of best tree | lnL of constraint tree | difference in lnL | P | Posterior Probability of alternative hypothesis |
|---|---|---|---|---|---|
| ACM4 | −2253.4118 | −2257.0046 | 3.59283 | 0.29 | 0.0566 |
| CMOS | −2034.1477 | −2034.1481 | 0.00038 | 0.15 | 0.0640 |
| RBMX | −2806.6269 | −2808.2078 | 1.58087 | 0.38 | 0.0501 |
| PDC | −2119.8431 | −2121.7696 | 1.92656 | 0.29 | 0.1280 |
| PTPN12 | −4385.4731 | −4389.2529 | 3.77983 | 0.16 | 0.0233 |
| RAG1 | −6532.1527 | −6535.0006 | 2.84790 | 0.22 | 0.0891 |
| RAG2 | −2215.4702 | −2216.7298 | 1.25966 | 0.36 | 0.0425 |
| Concatenated data | −22871.9359 | −22883.5976 | 11.66164 | 0.06 | 0.0000 |
We used the likelihood ratio test to determine whether branch lengths of any of the four branches connecting sphaerodactyl genera were significantly different from zero (Fig. 2). The concatenated data, RBMX, PTPN12, ACM4, and RAG1 had two of four internal branches with lengths not significantly different from zero. PDC had three of four branches not significantly different from zero. RAG2 and C-MOS had all four branches not significantly different from zero.
Morphological data
We recovered several morphological synapomorphies to aid in the diagnosis and description of a new genus and provide a detailed osteology to guide future research in sphaerodactyl biology and evolution. Morphological descriptions and comparisons are explained in detail below, after we address taxonomic changes.
Taxonomy
The combined morphological and molecular evidence suggested a new generic-level sphaerodactyl clade be described. We also redescribe Coleodactylus s.s. in light of our results.
Reptilia: Squamata: Sphaerodactylidae
Chatogekko Gamble, Daza, Colli, Vitt and Bauer, gen. nov
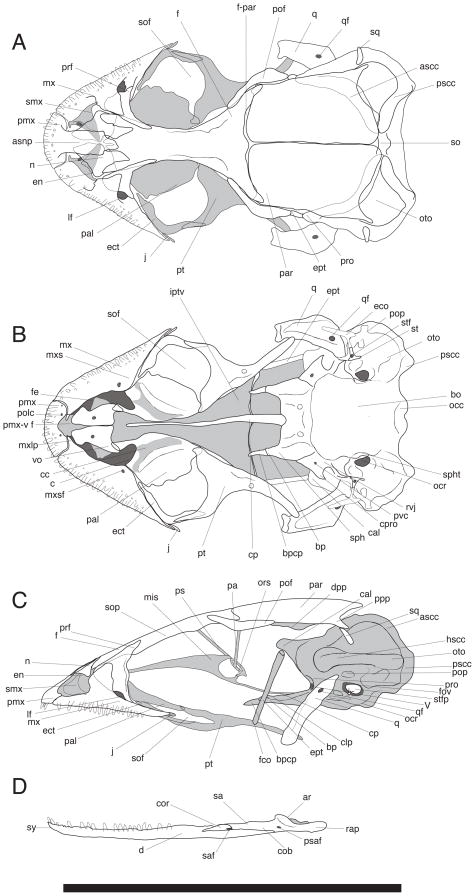
Figure 5.
Skull of Chatogekko amazonicus (USNM 290904) from Pará, Brazil. (A) dorsal, (B) ventral, (C) lateral views of the cranium. (D) labial view of the jaw. Abbreviations: ar, articular; ascc, anterior semicircular canal; asnp, ascending nasal process; bo, basioccipital; bp, basipterygoid process; bpcp, cartilaginous pad of the basipterygoid process; c, choana; cc, choanal canal; cal, crista alaris; clp, clinoid process; cob, compound bone; cor, coronoid; cp, cultriform process; cpro, crista prootica; d, dentary; dpp, decensus parietalis process; eco, extracollumella; ect, ectopterygoid; en, external nares; ept, epipterygoid; f, frontal; fco, fossa columellae; fe, fenestra exochoanalis; fov, fenestra ovalis; f-par, frontoparietal suture; hscc, horizontal semicircular canal; iptv, interpterygoid vacuity; j, jugal; lf, lacrimal foramen; mis, median interorbital septum; msy, mandibular symphysis; mx, maxilla; mxlp, maxillary lappet; mxs, maxillary shelf; mxsf, foramen of the maxillary shelf; n, nasal; occ, occipital condyle; ocr, occipital recess, ors, orbitosphenoid; oto, otooccipital; pa, pila accessoria; pal, palatine; par, parietal; pmx, premaxilla; pmx-v f, premaxillary-vomer fenestra; pof, postorbitofrontal; polc, posterior opening of the longitudinal canal; pop, paroccipital process; ppp, postparietal process; prf, prefrontal; pro, prootic; ps, planum supraseptale; psaf, posterior surangular foramen; pscc, posterior semicircular canal; pt, pterygoid; pvc, posterior opening of vidian canal; q, quadrate; qf, quadrate foramen; rap, retroarticular process; rvj, recessus vena jugularis; saf, surangular foramen; sop, subolfactory process; spht, sphenooccipital tubercle; st, stapes; stf; stapedial foramen; stfp, stapedial footplate; sa, surangular; smx, septomaxilla; so, supraoccipital; sof, suborbital fenestra; sph, sphenoid; sq, squamosal; V, incisura prootica; vo, vomer.
Figure 6.
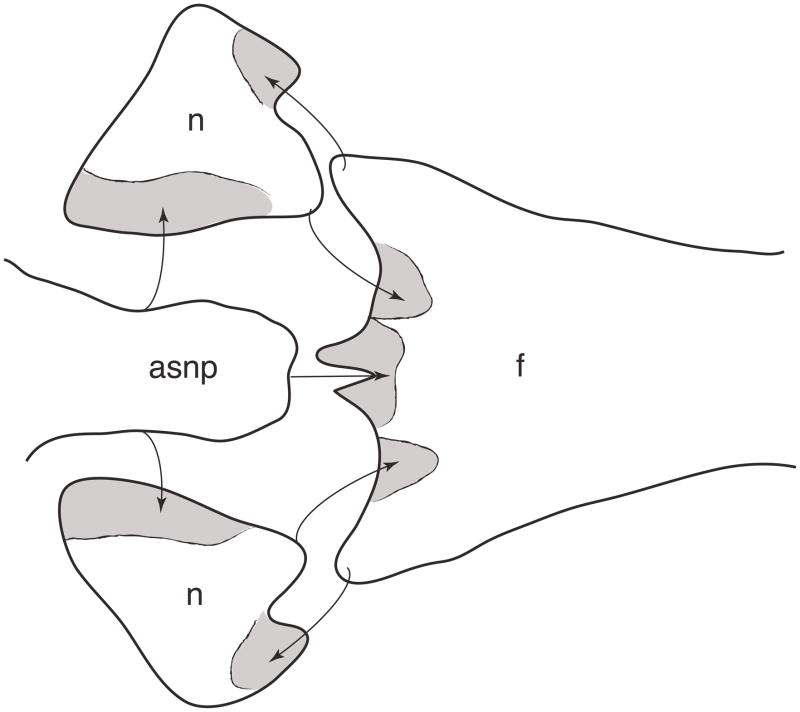
Inferred overlapping pattern among the medial bones of the snout in Chatogekko amazonicus specimen from Guyana (AMNH-R 132039). Gray areas indicate the overlap area, arrows indicate the place where each bone articulates. Abbreviations: asnp, ascending nasal process; n, nasal, f, frontal.
Type species
Sphaerodactylus amazonicus (Andersson, 1918)
Diagnosis and Description
A miniaturized species complex of diurnal sphaerodactyl geckos. Mean SVL 21mm ± 1.8, n = 41. Snout shortened. Pupil round. Body cylindrical. Dorsal scales keeled. Claws enclosed in ungual sheath consisting of 4 scales. Posterior edge of premaxilla contacts medial process of frontal bone. Posterior edge of ascending nasal process bifurcated. Palatine longer than vomer. Postparietal process of parietal in contact with supraoccipital and otooccipital, but not squamosal. Reduced paroccipital process located dorsally to fenestra ovalis.
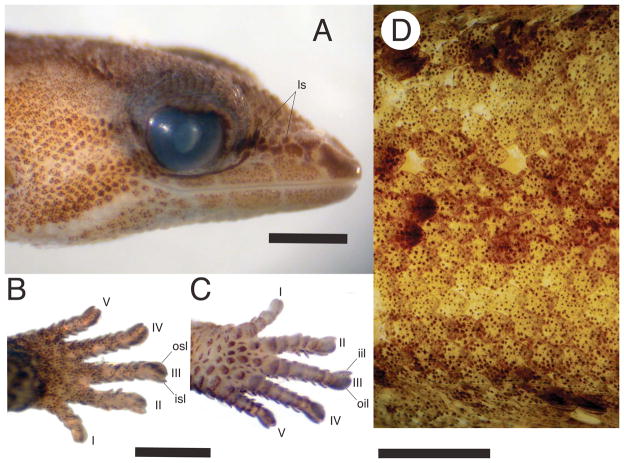
Chatogekko is distinguished from all gekkotans by the following unique combination of characters: (1) between 2 and 4 loreal scales (ls, Fig. 4A, also present in some Sphaerodactylus); (2) claws enclosed in an ungual sheath consisting of 4 scales (Avila-Pires, 1995; Parker, 1926; Vanzolini, 1957): inner supero-lateral (isl, Fig. 4B), outer supero-lateral (osl, Fig. 4B), inner infero-lateral (iil, Fig. 4C), and outer infero-lateral (oil, Fig. 4C) (ventrolaterals sensu Kluge, 1995); (3, Fig. 4D) keeled scales on dorsal body surface (Avila-Pires, 1995; Vanzolini, 1957), also present in some Sphaerodactylus; (4, Fig. 5A) external nares elongated and entering or approaching contact between prefrontal and nasals (as consequence of extensive overlapping contact of maxilla and prefrontal); (5, Fig. 5A) posterior edge of premaxilla (i.e., the ascending nasal process) contacts medial process of frontal bone (Daza, Abdala, Thomas & Bauer, 2008); (6, Fig. 5A) posterior edge of ascending nasal process bifurcated; (7, Fig. 5A) internasal contact absent; (8, Fig. 5A) jugal bone vestigial and limited to tip of maxilla; (9, Fig. 5A) postparietal process of parietal contacting supraoccipital and otooccipital, but not squamosal; (10, Fig. 5A) paroccipital process of otooccipital not visible in dorsal view; (11, Fig. 5B) paroccipital process very reduced and located dorsally to fenestra ovalis (instead of posterior as in other gekkotans) and not participating in quadrate articulation (paroccipital abutting); (12, Fig. 5B) palatine exceeds vomer substantially in length; (13) duplicipalatinate condition; (14) a three base pair deletion in coding region of exon 8 (in Gallus) of RBMX; (15) a three base pair deletion in coding region of exon 13 (in Gallus) of PTPN12.
Figure 4.
Chatogekko amazonicus specimens. A. lateral view of the head showing 2–4 loreal scales (ls), B. dorsal view of the left hand showing the inner supero-lateral and outer superolateral (isl and osl, Fig. 5B), C. ventral view of the left hand showing the inner infero-lateral and outer infero-lateral (iil and oil, Fig. 5C), and D., keeled scales along the dorsal surface of the body. A–C USNM 288775, and D MZUSP 91394.
Distribution
Central and eastern Amazonia, including the Brazilian states of Acre, Amazonas, Rondônia, Mato Grosso, Roraima, Pará, and Amapá; French Guiana; Guyana; Suriname; the Venezuelan state of Amazonas; and northern Bolivia (Avila-Pires, 1995; Gasc, 1990; Geurgas & Rodrigues, 2010; Langstroth, 2005).
Natural History
Chatogekko lives in the leaf litter in a variety of undisturbed lowland forested habitats (Vitt, Sartorius, Avila–Pires, Zani & Espósito, 2005). These geckos are active throughout the day although they do not bask (Hoogmoed, 1973). Diet is made up of small insects including springtails, mites and ticks, termites, homopterans, and larval insects (Hoogmoed, 1973; Ramos, 1981; Vitt et al., 2005). Females lay one egg per clutch and can produce several clutches during the year (Gasc, 1990; Hoogmoed, 1973). Chatogekko can be locally very abundant but appears to be negatively affected by forest fragmentation (Carvalho Jr., Lima, Magnusson & Albernaz, 2008).
Etymology
A composite word from the Spanish and Portuguese ‘Chato’, derived from the Greek ‘Platus’, meaning ‘flat’ and referring to its pug-nosed snout; and gekko from the Malay ‘gekoq’, onomatopoeic of the call of the species Gekko gecko and the common name to all limbed gekkotans. A Sri Lankan origin for the word gekko, derived from the Sinhalese word ‘gego’, is also possible (de Silva & Bauer, 2008). The name is masculine.
Species composition
Chatogekko amazonicus (Andersson, 1918). In addition, the names C. zernyi (Wettstein, 1928) and C. guimaraesi (Vanzolini, 1957) are available for populations from eastern Amazonia and southwest Amazon, respectively. See discussion for details.
ColeodactylusParker, 1926
Type species
Sphaerodactylus meridionalis (Boulenger, 1888).
Diagnosis and Description
A miniaturized species complex of diurnal sphaerodactyl geckos. SVL 20–28 mm (Avila-Pires, 1995; Vanzolini, 1968b). Snout elongate. Pupil round. Body cylindrical. Dorsal scales smooth or imbricate. Claws enclosed in an ungual sheath consisting of 5 scales.
Coleodactylus is a miniaturized species complex of diurnal sphaerodactyl geckos that can be differentiated from all other gekkotans by the following unique combination of characters: (1) claws enclosed in ungual sheath consisting of 5 scales (Avila-Pires, 1995; Parker, 1926; Vanzolini, 1957); (2) smooth or imbricate scales on dorsal body surface (Avila-Pires, 1995; Vanzolini, 1957), present in most other gekkotans; (3) ascending nasal process separates nasals approximately 1/4 their length, one of the shortest among sphaerodactyl geckos (Daza et al., 2008); (4) proximal portion of metatarsal IV not very expanded; (5) two separate deletions of 18 and six base pairs in exon 1 (in Gallus) of RAG1.
Distribution
Northern and eastern Brazil including states of Alagoas, Bahia, Ceará, Goiás, Maranhão, Mato Grosso, Mato Grosso do Sul, Pará, Paraíba, Pernambuco, Piaui, Rio Grande do Norte, Roraima, Sergipe, and Tocantins; Guyana; Suriname; the Venezuelan states of Monagas, Delta Amacuro and possibly Bolívar (Avila-Pires, 1995; Freire, 1999; Hoogmoed, 1985; Rivas & Molina R., 2001; Vanzolini, 1980).
Species composition
Coleodactylus brachystoma (Amaral, 1935); C. meridionalis (Boulenger, 1888); C. natalensisFreire, 1999; and C. septentrionalisVanzolini, 1980.
Osteology
Because most characters that differentiate Chatogekko from other sphaerodactyl geckos come from osteology, a detailed description of its skeleton would be convenient for future taxonomic differentiation of sphaerodactyl taxa. Additionally, a detailed osteology provides a baseline for future morphological research aimed at diagnosing the putative Chatogekko species. We present the osteological data in a framework that highlights the extremely small size of these lizards. It has been stated that is impossible to present a unifying model of miniaturization encompassing all lizards (Rieppel, 1984a). Even so, many anatomical similarities of the cranial structure of Chatogekko are present in other miniaturized gekkotans, especially closely related sphaerodactyl genera. Because the cranial anatomy of Sphaerodactylus roosevelti has been described in detail (Daza et al., 2008), we only highlight those structures that show differences in this new genus. We do this in the context of a descriptive approach and do not intend to imply any particular character polarity. Additionally, we review the postcranium, which has been described previously (Noble, 1921), but not in great detail.
Skull
The skull of Chatogekko is wedge shaped with a maximum width at the level of the otic capsules. It has a rounded outline in lateral view since there is a continuous curvature from the tip of the snout to the skull table. It has the shortest muzzle unit among sphaerodactylids (Fig. 5). This is especially evident in the anterorbital region, where a high degree of overlap occurs between the bones. The premaxilla has a very elongated ascending nasal process (asnp, Fig. 5A), with lateral margins that do not converge posteriorly. The last three-quarters of this process are reduced in width to a narrower projection that contacts the medial process of the frontal. In Sphaerodactylus, this process may reach the level of the frontal bone, but never contacts it directly because the nasal bones lie between them (Daza et al., 2008). The ascending nasal process is much shorter and does not reach the level of the frontal bone in Coleodactylus brachystoma. The posterior projection of the ascending nasal process varies among the specimens of Chatogekko examined and may be bifurcated or assume an almost transverse orientation.
The dorsal process of the maxilla is very narrow and exhibits an extensive overlap with the prefrontal bone. Proportionally, the external nares of Chatogekko are larger, and the prefrontal is closer to the posterior edge of this opening than in other sphaerodactyls. In Chatogekko, the approximation of the prefrontal to the external nares is mainly the result of the reduction of nasal process of the maxilla instead of being consequence of the posterior extension of the external nares, as in varanid lizards (Conrad, 2008; Conrad, Rieppel & Grande, 2008; Lee, 1997).
The orbit in Chatogekko occupies about 32% of the skull length, which is slightly more than in other sphaerodactyls (Daza et al., 2008). As in most of limbed geckos, the orbit is bounded by the postorbitofrontal, frontal, prefrontal, maxilla, and jugal (Daza & Bauer, 2010; Evans, 2008), the latter is very reduced or vestigial and contacts the tip of the posterior portion of the maxilla on the medial side. The floor of the orbit is pierced by a very large, D-shaped suborbital fenestra, which is present in all sphaerodactyls as well as the more distantly related sphaerodactylids Pristurus and Saurodactylus (Daza et al., 2008) and Euleptes (JDD, personal observation).
The rear portion of the skull is typical of miniaturized lizards (Rieppel, 1984a), which indicates how size reduction directly affects cranial structure. The basicranium is massive, being the widest part of the skull at the level of the otic capsules. The skull table is comparatively small, given that the parietals leave exposed a larger area of the basicranium. The outer margin of the basicranium (prootic, ottoccipital, and supraoccipital), as a consequence, is completely visible in dorsal view. The otooccipital area is so prominent and the horizontal semicircular canal bulges to the extent that the paroccipital process, normally seen in sphaerodactyls, is totally hidden. This paroccipital process is rudimentary and plays little or no function at all in the streptostylic quadrate articulation as in other lizards (Frazzetta, 1962; Rieppel, 1978; Versluys, 1912). The quadrate is very lightly built and articulates with the basicranium in a very anterior position, just in front of the fenestra ovalis. A quadrate foramen is present but its location is more proximal than in Sphaerodactylus. The squamosal bone is minuscule, and lost in some populations of Chatogekko. When this bone is present, it barely contacts the postparietal process of the parietal and lies against the basicranium, without contacting the quadrate or wrapping around it. Another consequence of this massive basicranium is the shape of the pterygoids, which have an almost straight medial margin (i.e., not curved or sigmoidal), and create a very wide interpterygoid vacuity posteriorly. The basipterygoid process and the cartilaginous pad that covers it are very narrow in Chatogekko.
In the palate the premaxillary-vomerine fenestra is very large and irregularly shaped, and partially invaded by the maxillary lappets. The vomer is reduced in size relative to the other palatal bones, leaving a very large fenestra exochoanalis and is partially overlapped by the septomaxilla.
Jaw
The jaw of Chatogekko is typically sphaerodactyl, very straight with an elongated dentary that extends posteriorly almost to the level of the articular surface of the craniomandibular articulation. The coronoid is low and very small, without projecting above the contour of the mandible. In lingual view, the splenial seems to be fused with the coronoid, a character that unites Pristurus with the sphaerodactyls.
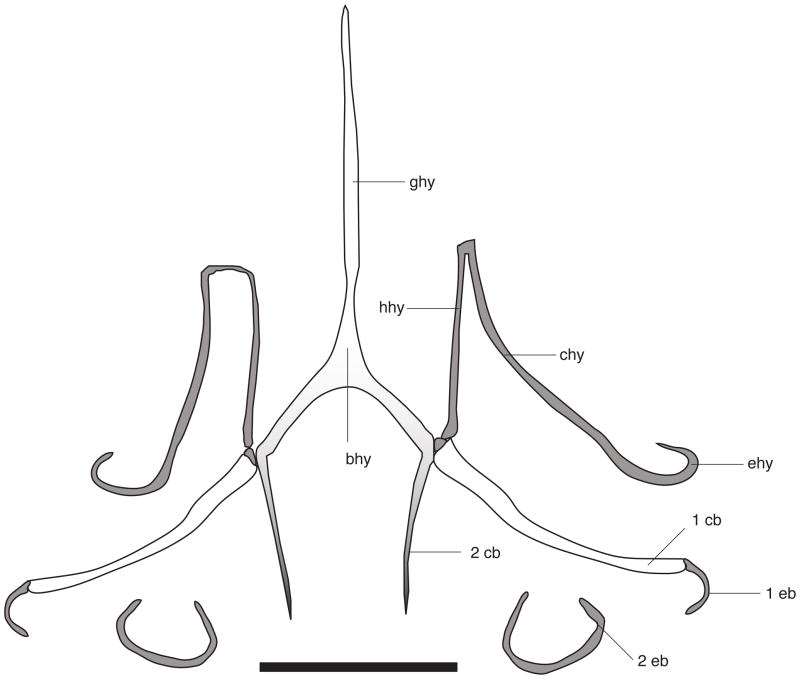
Hyoid apparatus
There are no major differences between the hyoid structure of Chatogekko (Fig. 7) and that of Sphaerodactylus macrolepis (Noble, 1921). In these two genera, medial or lateral projections of the hypohyal (hyoid cornu) do not exist. Among sphaerodactyls these are only present in Gonatodes. The second ceratobranchial (2 cb, Fig. 7) is comparatively shorter than in Sphaerodactylus and is oriented posteromedially. The second epibranchial (2 eb, Fig. 7) is not joined to the second ceratobranchial as in Sphaerodactylus.
Figure 7.
Hyoid apparatus of Chatogekko amazonicus specimen from Serra do Navio, Amapá, Brazil (AMNH R-138726). Abbreviations: 1 cb, first ceratobranchial; 1 eb, first epibranchial; 2 cb, second ceratobranchial; 2 eb, second epibranchial; bhy, basihyal; chy, ceratohyal; ehy, epihyal; ghy, glossohyal; hhy, hipohyal. Different shades of gray are meant to indicate the ossification of each element, gray = cartilaginous, white = ossified. Scale bar 1mm.
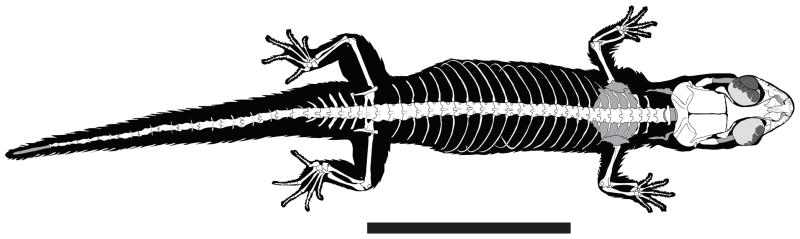
Postcranium
The postcranium of Chatogekko is 85% of the entire body length; the body and tail sections are subequal (Fig. 8). The vertebral column is composed of 47 vertebra: 26 presacral, 2 sacrals and 19 caudals. The presacral region comprises 8 cervical, 17 thoracic and 1 lumbar. In the cervical region only the atlas and the axis are ribless and the remaining six cervicals bear ribs that increase in length gradually. The atlas is fused dorsally as in all sphaerodactyls except Gonatodes, which has paired elements. The 3rd and 4th cervicals have short ribs that are widened and bifurcated distally. The ribs of the cervicals 5th– 7th are set closer to the suprascapula. The rib of the 8th cervical approaches but does not contact the sternum. The sternal ribs of the first four thoracic vertebrae are attached to the sternum directly. The 5th thoracic may be attached to the xiphisternum in specimens in which this structure is branched. The remaining thoracic vertebrae have short postxiphisternal inscriptional ribs that reduce their size gradually until becoming only a small nubbin.
Figure 8.
Articulated skeleton of Chatogekko sp. Specimen (USNM 289061) from Reserva Biologica Rio Trombetas, Pará, Brazil. Scale bar = 10 mm.
The longest rib is present on the 11th thoracic vertebra, after which ribs start to decrease in size until lost on the lumbar. A single lumbar vertebra does not differ in size from the posterior thoracic vertebrae. The two sacral vertebrae differ in structure. The first has expanded transverse processes that articulate with the pelvic girdle (illum) and posteriorly it is fused to the transverse processes of the second sacral, whereas the second has a short transverse process which are oriented anterolaterally The tail is formed by 19 caudals. The pygial vertebrae have been described as those anterior caudals devoid of fracture planes (Holder, 1960). In Chatogekko, autotomy planes are visible after the 6th caudal vertebrae, but only the first three lack hemal arches. The transverse processes are elongated and oriented posteriorly on the first five caudals; these processes gradually reduce in length distally. Beyond the 6th caudal vertebrae, centrum length increases, almost doubling the length of the presacral vertebrae.
The pectoral girdle comprises suprascapulae, scapulocoracoids, epicoracoids, clavicles, interclavicle, and sternum. The suprascapula is expanded and cartilaginous. The scapular portion of the scapulocoracoid is elongated and narrow. The scapulocoracoid fenestra is closed by a projection of the cartilaginous scapular epicoracoid bar. The anterior coracoid fenestra (i.e., anterior primary coracoid emargination) is present, but the posterior one is absent. The clavicles are expanded medially and more-or-less rotated forward. They lack the clavicular fenestra, as do Lepidoblepharis (Noble, 1921; Parker, 1926), and Gonatodes. Parker (1926) also described Coleodactylus and Pseudogonatodes with no clavicular fenestra, however, we found specimens of Coleodactylus and Pseudogonatodes with clavicular fenestrae, which indicates that this character is variable or polymorphic for these two genera; in Sphaerodactylus, the clavicle is invariably perforated (Noble, 1921) which we were able to corroborate in all species reviewed (see supplemental material). The interclavicle in Chatogekko has lateral arms, but these are very broad and almost indistinct. The sternum is shield-like and well ossified.
The pelvic girdle is formed by the fusion of the ilium, ischium, and pubis. The ischium and pubis are in close contact with their fellows, but not fused. The ischiopubic fenestra is large and compressed anteroposteriorly. In Chatogekko the ilium is constricted dorsal to the acetabulum and extends dorsally as a rod like process. The ischium is wider than the pubis, and the metischial processes are widely separated. The hypoischium is absent. The pubic symphysis is slender and capped by a small epipubic cartilage. In all sphaerodactyls, the pectineal process is large and ventrally directed. This is a very diagnostic feature, mentioned by Noble (1921) as a difference between the African ‘Gonatodes dickersoni’ (now Cnemaspis dickersoni) and the Neotropical sphaerodactyls. The rounded obturator foramen for the course of nerves lies at the boundary between the ischium and pubis. This foramen is present in all limbed gekkotans and lost in pygopodids.
The limbs are short and stout, but most typical elements of the gecko appendicular skeleton (Fabrezi, Abdala & Oliveri, 2007; Russell, 1972; Russell & Bauer, 2008) are present. One variation that occurs in sphaerodactyls is the increase in number of sesamoids on the proximal epiphyseal end of the radius with respect to other lizards. These elements have been described for a few lizards, for instance Sphaerodactylus klauberi and the xantusiid Lepidophyma gaigeae (Jerez, Mangione & Abdala, 2010). In Chatogekko and Coleodactylus there are three of these elements between the radius and the humerus (Fig. 8). This number is variable among other sphaerodactyls; for example, Pseudogonatodes and Sphaerodactylus have two, and Lepidoblepharis and Gonatodes only one. Pseudogonatodes, Coleodactylus, and Chatogekko also have sesamoids dorsal to the metacarpal-carpal and metatarsal-tarsal articulations (Fig. 5, 7). These ossifications appear sporadically in Lepidoblepharis, but not in Gonatodes.
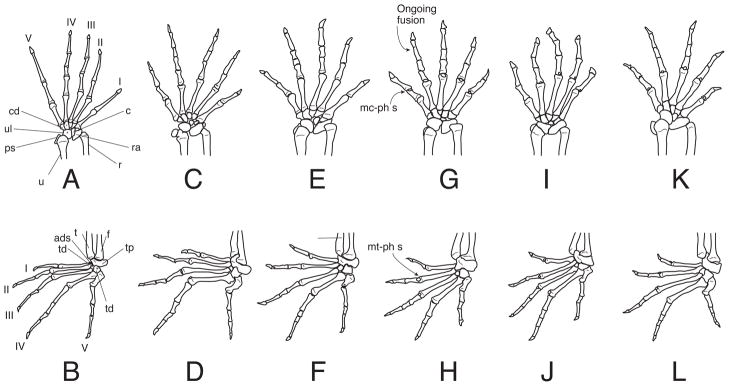
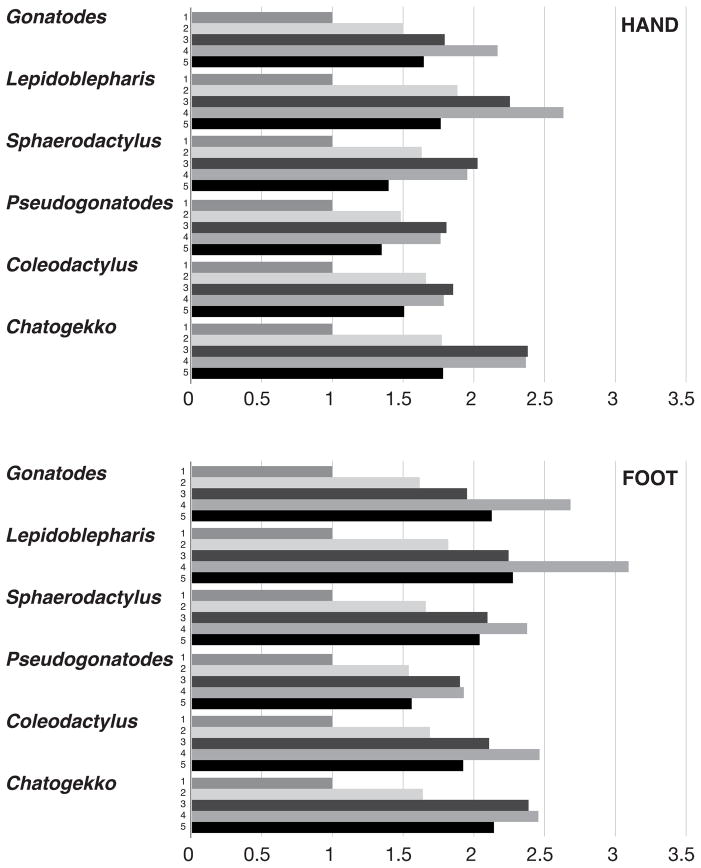
The phalangeal formulae of the manus and pes of sphaerodactyls are typically 2-3-4-5-3 and 2-3-4-5-4 (Table 3), respectively, which are primitive for squamates (Greer, 1992). One phalanx in the fourth manual digit of Pseudogonatodes, Coleodactylus, and Chatogekko and the fourth pedal digit of Pseudogonatodes are lost (Table 3, Fig. 9) The identity of the phalanx lost is hard to determine without developmental series, but it is likely that the element lost is either the ultimate or penultimate phalanx.
Table 3.
Summary of digital characteristics for each genus of sphaerodactyl gecko.
| Phalangeal formula (manus) | Phalangeal formula (pes) | Increase order of digit length (manus) | Increase order of digit length (pes) | Dorsal metacarpophalangeal sesamoids | Dorsal metatarsophalangeal sesamoids | |
|---|---|---|---|---|---|---|
| Gonatodes | 2-3-4-5-3 | 2-3-4-5-4 | 1-2-5-3-4 | 1-2-3-5-4 | no | no |
| Lepidoblepharis | 2-3-4-5-3 | 2-3-4-5-4 | 1-5-2-3-4 | 1-2-3-5-4 | no | yes |
| Sphaerodactylus | 2-3-4-5-3 | 2-3-4-5-4 | 1-5-2-4-3 | 1-2-5-3-4 | no | no |
| Pseudogonatodes | 2-3-4-4-3 | 2-3-4-4-4 | 1-5-2-4-3 | 1-2-5-3-4 | yes | yes |
| Coleodactylus | 2-3-4-4-3 | 2-3-4-5-4 | 1-5-2-4-3 | 1-2-5-3-4 | yes | yes |
| Chatogekko | 2-3-4-4-3 | 2-3-4-5-4 | 1-2-5-4-3 | 1-2-5-3-4 | yes | yes |
Figure 9.
Left manus and pes of sphaerodactyl geckos. A–B Gonatodes albogularis (UIS-R-2079); C–D Lepidoblepharis xantostigma (USNM 313791); E–F, Sphaerodactylus klauberi (UPRRP 006416); G–H Pseudogonatodes guianensis (MZUSP 94826), I–J Coleodactylus brachystoma (MZUSP uncataloged), K–L Chatogekko amazonicus (USNM 289061). Drawings not at the same scale. Abbreviations: I–V, digits; ads, anterior distal sesamoid; c, centrale; cd, distal carpal; f, fibula; mc-ph s, sesamoid dorsal to the metacarpal-phalange articulation; mt-ph s, sesamoid dorsal to the metatarsal-phalange articulation; ps, pisiform; r, radius; ra, radiale; t, tibia; td, distal tarsal; tp, proximal tarsal, u, ulna, ul, ulnare.
Discussion
Phylogeny
Phylogenetic analyses of the combined nuclear gene data, using both a concatenation approach and a gene tree approach, recovered three lineages of sphaerodactyl genera: Chatogekko; a Lepidoblepharis + Gonatodes clade; and a Pseudogonatodes + Sphaerodactylus + Coleodactylus clade. Other published molecular phylogenies have consistently recovered the Lepidoblepharis + Gonatodes clade, but have failed to recover the other clades with strong support (Gamble et al., 2011; Gamble et al., 2008a; Geurgas et al., 2008). The difficulty in recovering these clades is likely due to the short internal branches linking genera at the base of the sphaerodactyl clade. Short internal branches are a signature of rapid cladogenesis, indicating that divergences among sphaerodactyl genera occurred in a relatively short timeframe (Gamble et al., 2011; Gamble et al., 2008a). Short internal branches can also make phylogenetic reconstruction difficult (Jackman, Larson, de Queiroz & Losos, 1999; Poe & Chubb, 2004; Slowinski, 2001). Indeed, our failure to reject the hypothesis that several of those internal branches had lengths not significantly different than zero suggests hard polytomies in the molecular data (Maddison, 1989; Slowinski, 2001). One possible cause of zero-length branches is insufficient data (Poe & Chubb, 2004). This may play some role in our results as our three loci with the least amount of data: RAG2; C-MOS; and PDC, had either three or four of the four branches connecting sphaerodactyl genera with branch lengths not significantly different from zero. The remaining loci had more data, sometimes substantially so, and possessed only two of four branches with lengths not significantly different from zero. This was also the case with the concatenated data set. Close examination of which branches were statistically indistinguishable from zero shows some similarities among the loci with more data (ACM4, RAG1, PTPN12 and RBMX) and the concatenated dataset set (Fig. 2). The branch leading to the Gonatodes + Lepidoblepharis clade, for example, was always significantly different from zero, while the branch connecting Chatogekko with its sister taxon (which was not consistent and changed from tree to tree) was always not significantly different from zero. These similarities among the longer single gene datasets and their concordance with the concatenated dataset indicate we had enough data for those loci. It is likely, therefore, that two of the four branches connecting sphaerodactyl genera actually possessed zero branch lengths. These were, in the concatenated nuclear gene dataset, the branch connecting Chatogekko to its sister taxon and the branch connecting Coleodactylus to the Pseudogonatodes + Sphaerodactylus clade. Presence of a hard polytomy in the data has serious implications for our hypothesis testing. Our topology tests likely were unable to distinguish among alternative phylogenetic hypotheses because there were very little data or, in the cases of branches with zero lengths, no data supporting any one phylogenetic hypothesis over the other. This is a difficult situation for testing phylogenetic hypotheses because the lack of data means that essentially any alternative hypotheses involving these short, zero-length branches will not be rejected. The only way of evaluating alternative hypotheses when this occurs is to look to other sources of data. In our case, we had indels and morphological data providing strong evidence that Chatogekko is distinct from Coleodactylus s.s.
Polytomies in gene trees do not automatically translate to hard polytomies in the underlying species trees (Poe & Chubb, 2004; Slowinski, 2001) and the recovery of a bifurcating sphaerodactyl phylogeny is not an impossible task. We show here that rare genomic events like indels can be used to provide diagnostic characters for sphaerodactyl clades at multiple hierarchical levels. Deletions unique to Chatogekko in RBMX and PTPN12 and unique RAG1 deletions in Coleodactylus provide strong evidence that they are two separate lineages. Indels are considered relatively homoplasy-free characters and have proven useful in diagnosing numerous vertebrate clades (de Jong, van Dijk, Poux, Kappe, van Rheede & Madsen, 2003; Ericson, Johansson & Parsons, 2000; Gamble, Bauer, Greenbaum & Jackman, 2008b; Townsend, Larson, Louis & Macey, 2004; van Dijk et al., 1999). Decreasing costs for high throughput sequencing will make the identification and collection of this sort of data, e.g. indels or LINE/SINE insertions, easier and could prove useful in further untangling the phylogenetic relationships among sphaerodactyl geckos.
Taxonomy
We used molecular phylogenetic analyses to identify Chatogekko as a distinct lineage of sphaerodactyl gecko and, with a thorough examination of osteology, provided a suite of diagnostic characters for that lineage. While some of the characters used to diagnose Chatogekko have been known for a long time, e.g. Coleodactylus has smooth dorsal scales and an ungual sheath composed of five scales while Chatogekko has keeled dorsal scales and an ungual sheath composed of four scales (Vanzolini, 1957; Vanzolini, 1968a; Vanzolini, 1968b), most of our synapomorphies are new.
The discovery of generic polyphyly resulting from well-sampled phylogenetic analyses is relatively common (Amaral, Miller, Silveira, Bermingham & Wajntal, 2006; Campbell, Serb, Buhay, Roe, Minton & Lydeard, 2005; Lanyon, 1994). This problem has been particularly pervasive in geckos where digital morphology, a character suite prone to homoplasy, has played an historically important role in defining genera (Bauer, Good & Branch, 1997; Russell & Bauer, 2002). The classification of sphaerodactyl genera has been similarly dependent on digital morphology (Kluge, 1995; Vanzolini, 1957) and the historic clustering of Chatogekko with Coleodactylus s.s. was done primarily because of superficial similarities in the ungual sheath (Vanzolini, 1957). By looking beyond the digits, we were able to uncover many morphological characters unique to Chatogekko, strengthening the argument for a taxonomic change.
We recovered three deeply divergent lineages within Chatogekko. These results are consistent with Geurgas and Rodrigues (2010) and Geurgas et al. (2008), who also recovered multiple species-level lineages within C. amazonicus. The geographic distribution of the three Chatogekko lineages corresponds to three described Chatogekko species, two of which are currently synonymized with C. amazonicus. Specimens from Manaus and Roraima correspond to C. amazonicus s.s., with a type locality in the central Amazon near Manaus, Amazonas, Brazil (Andersson, 1918). Specimens from Pará likely correspond to C. zernyi, with a type locality from Taperinha, Pará, Brazil in the eastern Amazon near Santarém (Wettstein, 1928). Specimens from Rondônia and Rio Ituxi likely correspond to C. guimaraesi, with a type locality in Porto Velho, Rondônia, Brazil in the southwestern Amazon (Vanzolini, 1957). While our limited sampling is insufficient to resurrect C. zernyi and C. guimaraesi, the existence of available names for those clades makes such a decision reasonable and the eventual resurrection of these taxa seems inevitable. It should be noted that Geurgas and Rodrigues (2010) also recovered significant phylogenetic structure within Chatogekko amazonicus s.s. and C. c.f. zernyi. It is possible that splitting each species into two or more species-level lineages may be warranted although additional data would be needed to confirm this.
Coleodactylus and Chatogekko appear to be morphologically conservative and the identification of species level lineages in both genera using morphology has historically been difficult (Moretti, 2009). Our examination of Chatogekko osteology bears this out. Even though we examined specimens from three putative Chatogekko species we could not identify morphological synapomorphies for these lineages with our data. Our results mirror other morphological analyses of Chatogekko (Avila-Pires, 1995; Vanzolini, 1968b), raising the possibility that species of Chatogekko may be morphologically cryptic. A lineage-based species concept requires that species be diagnosable and genetic evidence and the molecular synapomorphies that support each of the species-level clades within Chatogekko are sufficient to satisfy the need for diagnosability (de Queiroz, 1998; de Queiroz, 2007; Sites & Marshall, 2004; Zink & McKitrick, 1995). That said, a thorough examination of morphological characters with a larger sample of specimens in light of the molecular phylogenetic hypothesis could be productive. Other means of identifying species, such as ecological niche modelling, cytogenetics, or multivariate morphometrics may also prove useful (Colli, Giugliano, Mesquita & França, 2009; Leaché, Koo, Spencer, Papenfuss, Fisher & McGuire, 2009; Oliver, Adams, Lee, Hutchinson & Doughty, 2009; Raxworthy, Ingram, Rabibisoa & Pearson, 2007).
Morphology
The skull of Chatogekko exhibits interesting modifications associated with miniaturization. The extensive overlapping pattern of the premaxilla is not typical of miniaturized gekkotans (except perhaps in the pygopodid Pletholax), although a similar pattern is found in other miniaturized lepidosaurs. The uniqueness of the Chatogekko skull compared with other small gekkotans is not surprising, given the association between morphological novelty and miniaturization in vertebrates (Hanken, 1984). The repeated evolution of this overlapping pattern in independent lineages is simply one of several possible solutions to the problems associated with extreme size reduction and highlights the novelty often found in miniaturized taxa.
Miniaturization is often associated with paedomorphosis, the retention of juvenile traits in adult organisms (Alberch, Gould, Oster & Wake, 1979; Gould, 1966; Rieppel, 1996). Gekkotans possess several paedomorphic skeletal characters such as amphicoelous vertebrae (Camp, 1923; Kluge, 1967; Werner, 1971) and paired premaxilla or parietal bones (Daza, 2008; Kluge, 1967; Kluge, 1987; Stephenson, 1960) although none of these skeletal changes are found exclusively in miniaturized forms. In fact, miniaturized species present a fused premaxilla and braincase bones more frequently than larger gekkotans (Daza, 2008). One character that might reflect paedomorphosis in Chatogekko is the slightly larger eyes proportional to the head (Daza et al., 2008), but this would have to be corroborated with a developmental series of different sized sphaerodactyl species.
Another interesting feature of the Chatogekko skull is the development of an incomplete secondary palate. A secondary palate is frequently listed as a very distinct structure in mammals, but is also present in some reptiles. A secondary palate is present in many fossil reptiles (Benton, 2005; Carroll, 1988; Romer, 1956), but among extant groups, this structure appears only in crocodilians, some turtles and some lizards (Gaffney, 1979; Greer, 1977; Iordansky, 1973; Meylan, Moody, Walker & Chapman, 2000; Presch, 1976). It has long been thought that no true secondary palate was present in lizards and the tongue was used for closing the naso-pharyngeal passages during respiration (Camp, 1923). The secondary palate in sphaerodactyls resembles that of pygopodids (Conrad, 2008) and xantusiids (Malan, 1946; Savage, 1963). In sphaerodactyls, especially in Chatogekko, the secondary palate is distinctive in that the paleochoanate condition is present, but the palatine is extremely duplicipalatinate, where this bone develops a deep choanal canal formed by the vomerine process and a ventral crest of palatine. These two structures tend to converge ventrally creating a structure that in cross section has the shape of a “C”; in this sense, the palatines roof over most of the length of the choanal tubes and the ectochoanal cartilage floors the ventral surface, and extends well posteriorly so the nasal passageway opens on the posteromedial side of the palatine (ce, Fig. 10).
Figure 10.
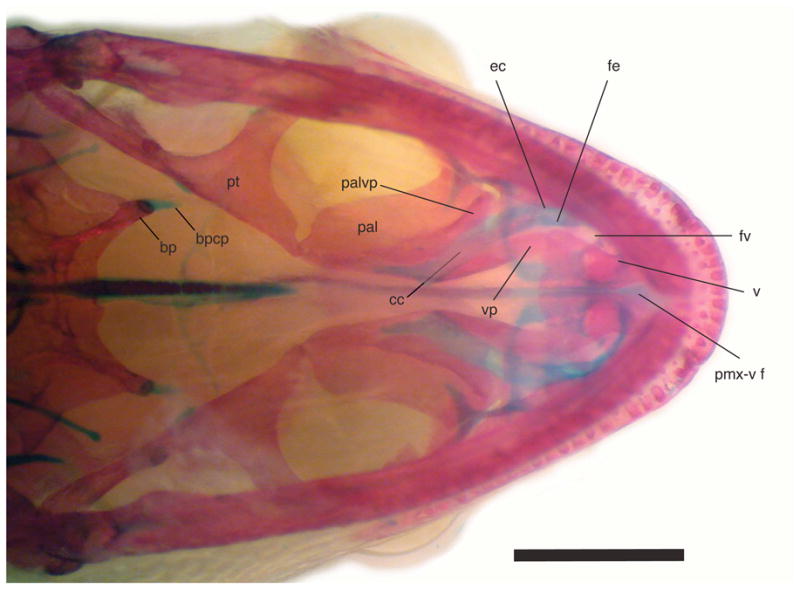
Palatal view of cleared and stained Chatogekko amazonicus specimen from Guyana (AMNH-R 132039) showing the secondary palate formed on the palatine. Abbreviations: bp, basipterygoid; bpcp, cartilaginous pad of the basipterygoid process; cc, choanal canal; ec, ectochoanal cartilage; fe, fenestra exochoanalis; pal, palatine; palvp, ventral process of the palatine; pmx-v f, premaxillary-vomer fenestra; pt, pterygoid; sof, suborbital fenestra; v, vomer; vp, vomerine process of palatine. Scale bar 1 mm.
The amount of overlap of the premaxilla with the nasal bones, and the contact of nasals have been used as phylogenetic characters (Kluge, 1976), but it has been suggested that they should be treated as independent characters because in certain forms nasal separation can be an artifact of premaxillary overlap, even if the nasals remain in contact with one another (Daza et al., 2008). This seems to be the case in all sphaerodactyls, except in Chatogekko where there is both overlap and complete separation of the nasal bones (i.e., there is no internasal contact). A similar arrangement is present the pygopodid Pletholax gracilis (Rieppel, 1984b); the chameleon Rhampholeon spectrum (Evans, 2008); the xantusiid Cricosaura typica (Savage, 1963); some miniaturized gymnophthalmids such as Bachia bicolor (Tarazona, Fabrezi & Ramirez–Pinilla, 2008), Gymnophthalmus speciosus (MacLean, 1974), Vanzosaura rubricauda (Guerra & Montero, 2009), Calyptommatus nicterus, Scriptosaura catimbau, and Nothobachia ablephara (Roscito & Rodrigues, 2011); many amphisbaenians (Montero & Gans, 2008); and to some extent in the colubrid Scaphiophis albopunctatus (Cundall & Irish, 2008). The loss of the internasal results in some substantial changes to snout configuration and to the distribution of forces; the medial laminar contact between these bones is replaced by an exclusive dorsoventral butt-lap suture with the ascending nasal process of the premaxilla. Open contact sutures are thought to work as shock absorbers or assist to allow micro-movements to dissipate forces acting between bones on the skull (Jaslow, 1989; Pritchard, Scott & Girgis, 1956), behaving in an analogous manner to the flexible material used between the slabs of concrete pavement. The loss of contact, together with the separation of nasals (dashed line in Fig. 5A), combined with the loss of a butt-lap joint with the maxilla (a suture present in other sphaerodactyls), suggest that the nasal bones will tend to be less stable and more inclined to move sideways. It has been demonstrated with three-dimensional finite element models that sutures relieve strain locally, but only at the expense of elevated strain in other regions (Moazen, Curtis, O’Higgins, Jones, Evans & Fagan, 2009). Using this reasoning, a hypothesized reduction in the medial strain on the nasals would have played an important part in the development of a posterior interlocking suture with the frontal (Fig. 6). This is purely conjectural, but is derived from the observed elaborated type of suture and comparison to a similar interlocking suture between nasals and frontal in some amphisbaenians (R. Montero, personal communication). The nasofrontal suture of Chatogekko is reciprocally overlapping; the nasal develops a narrow posterior process that overlaps the frontal bone, and the anterolateral process of the frontal overlaps the posterolateral surface of the nasal.
Characters from the postcranium were not diagnostic for Chatogekko; nonetheless it is worthwhile to comment on the occurrence of perforated clavicles among sphaerodactyl geckos. The perforation was described as variable within the gekkonid genus Cnemaspis and considered as the final stage in the thinning process of the bone, with no phylogenetic significance (Smith, 1933). This statement is not entirely true for sphaerodactyls, where similar sized species with comparable clavicles might have unperforated (e.g. Lepidoblepharis and Gonatodes) or perforated clavicles (e.g. Sphaerodactylus). In the latter, perforated clavicles are present in both small and medium sized species, indicating that this character might be diagnostic for the genus and have a phylogenetic significance at that level.
Another variable trait from the postcranium is the phalangeal formula. These characters were used in previous intergeneric cladistic analysis of sphaerodactyl geckos (Kluge, 1995). The absence of the fourth phalangeal element in the fourth finger was one of the characters that supported the sister relationship of Coleodactylus s.l. and Pseudogonatodes, likewise Pseudogonatodes was differentiated from Coleodactylus s.l. by the loss of the fourth phalangeal element in the fourth toe. A re-examination of Kluge’s (1995) data set showed that he scored the fourth phalangeal element in the fourth toe (character 12) as absent in Coleodactylus, but not in Pseudogonatodes, which is incorrect. Reanalysis of the corrected dataset does not produce any change in the topology (JDD unpublished).
We reviewed phalangeal formulae in the specimens available and encountered a problem of homology. In all sphaerodactyls, there is a minimum of four phalanges in the fourth digits of the manus and pes. Is the element lost in Coleodactylus, Pseudogonatodes, and Chatogekko the fourth (penultimate) phalanx and the remaining element the fifth (ungual)? Or is the terminal element lost and the fourth phalanx modified to develop an ungual morphology? The third phalanx of digit four seems to show a fusion of the third and fourth phalanges in the manus of Pseudogonatodes, resulting in only four phalanges in this digit. This process is symmetrical, but in the pes there is no sign of an ongoing fusion process. If a phalanx was lost, we would expect to have a shorter digit, although alternative processes such as nonossification, fusion, and reabsorption have been discussed (Shapiro, Shubin & Downs, 2007). To evaluate this, we measured the length of each digit; these measurements were converted to equivalent proportions by dividing each by the length of the shortest digit (i.e. first digit, Fig. 11). With these values, we estimated the increase order of digit length in both manus and pes (Table 3). The manus in Sphaerodactylus, Pseudogonatodes, and Coleodactylus presented an increase order of digit length of 1-5-2-4-3. In Lepidoblepharis and Gonatodes, the longest digit was the fourth and in Chatogekko the fourth digit was almost equal to the third. The situation in Lepidoblepharis is expected because this genus exhibits no reduction of any kind in the fourth digit (Fig. 9A). Sphaerodactylus exhibits similar proportions to those of Pseudogonatodes and Coleodactylus (where one phalanx is lost or fused to another) because the second phalanx of digit four is very reduced. The second digit in Gonatodes and Chatogekko is short in comparison with the other sphaerodactyls. In Chatogekko, digital proportions differ from all other sphaerodactyls, since digits two and five and digits three and four become sub-equal, but the latter are proportionally longer (Fig. 11). The pes shows a more stable pattern; in Lepidoblepharis, the order of increase of digit length is 1-2-3-5-4, while in the rest of the sphaerodactyls it is 1-2-5-3-4. The only taxon that showed element loss in the fourth finger was Pseudogonatodes, a process that is clearly demonstrated by the measurements, since the third and fourth digits become sub-equal. Developmental data would be necessary to corroborate fusion or loss of phalanges in the fourth digit of the manus in Pseudogonatodes, Coleodactylus and Chatogekko.
Figure 11.
Relative length of hand and foot digits with respect to digit number 1 in representative species from each sphaerodactyl genus. Gonatodes albogularis (UIS-R-2079), Lepidoblepharis xantostigma (USNM 313791), Sphaerodactylus klauberi (UPRRP 006416); Pseudogonatodes guianensis (MZUSP 94826), Coleodactylus brachystoma (MZUSP uncataloged), Chatogekko amazonicus (USNM 289061).
Conclusions
Small size and cryptic habits have made sphaerodactyl geckos among the most poorly studied lizard groups. Our combined use of morphological and molecular data led to the recognition and description of a new genus-level lineage of sphaerodactyl gecko, Chatogekko. Previously considered part of the genus Coleodactylus, Chatogekko possesses a unique suite of morphological and molecular characters that distinguish it from Coleodactylus s.s. Further work with additional sampling will be necessary to uncover morphological synapomorphies for three putative Chatogekko species and other potentially undescribed taxa in the genus. Our detailed osteological data will provide a framework to move forward with that research, as well as assist more generally with the systematic research of other sphaerodactyl clades. There are certainly many more sphaerodactyl species to be formally recognized and the use of multiple sources of data, including molecular data and morphology as done here, will be necessary to reveal the true diversity of this fascinating group of lizards.
Acknowledgments
We thank K. de Queiroz (USNM) for his contribution to the discussion of the secondary palate in lizards and S. Gotte (USNM) for his help with specimens and modifications to the clear and staining process; A. Herrera, P. Nunez and F. Leal for help with the photographs; D. Frost and D. Kizirian (AMNH) for loans of specimens in their care. J. Boone, R. Brumfield and the LSU Museum of Natural Science Collection of Genetic Resources, J. Campbell (UTA), M. Forstner, G. Rivas, T. Heagy, K. Krysko (FLMNH), J. McGuire (MVZ), J. Simmons (KU), J. Vindum (CAS), A. Wynn (USNM) and H. Zaher (MZUSP) kindly provided tissues from material in their care. M. Hoogmoed generously provided a photo of Chatogekko from Pará. Two anonymous reviewers provided helpful comments on the manuscript. Support for this research was provided by NSF grants DEB 0515909 and DEB 0844523 to AMB and Todd Jackman; and Dayton/Wilkie Fund, Bell Museum of Natural History to TG. TG was partially supported by T32DE007288 from the National Institute of Dental & Craniofacial Research. JDD was supported by a postdoctoral fellowship of CONICET. Fieldwork conducted by LJV and J. P. Caldwell was supported by NSF grants DEB 9200779, DEB 9505518, and DEB 0415430. Fieldwork conducted by GRC was covered by IBAMA permit 027/2003–CGFAU/LIC and supported by research fellowships from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Contributor Information
Tony Gamble, Email: gambl007@umn.edu.
Juan D Daza, Email: juand.daza@gmail.com.
Guarino R Colli, Email: grcolli@unb.br.
Laurie J Vitt, Email: vitt@ou.edu.
Aaron M Bauer, Email: aaron.bauer@villanova.edu.
Literature Cited
- Alberch P, Gould SJ, Oster GF, Wake DB. Size and shape in ontogeny and phylogeny. Paleobiology. 1979;5:296–317. [Google Scholar]
- Amaral AD. Estudos sobre lacertilios neotropicos. III. Um novo genero e duas novas especies de Geckonideos e uma nova raca de Amphisbaenideo, procedentes do Brasil Central. Memoires Instituto Butantan. 1935;9:253–257. [Google Scholar]
- Amaral FSR, Miller MJ, Silveira LF, Bermingham E, Wajntal A. Polyphyly of the hawk genera Leucopternis and Buteogallus (Aves, Accipitridae): multiple habitat shifts during the Neotropical buteonine diversification. BMC Evolutionary Biology. 2006;6:1471–2148. doi: 10.1186/1471-2148-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson LG. New lizards from South America, collected by Nils Holmgren and A. Roman. Arkiv för Zoologi. 1918;11(16):1–9. [Google Scholar]
- Ane C, Larget B, Baum DA, Smith SD, Rokas A. Bayesian estimation of concordance among gene trees. Molecular Biology and Evolution. 2007;24:412–426. doi: 10.1093/molbev/msl170. [DOI] [PubMed] [Google Scholar]
- Avila-Pires TCS. Lizards of Brazilian Amazonia (Reptilia: Squamata) Zoologische Verhandelingen. 1995;299:1–706. [Google Scholar]
- Bauer AM, Good DA, Branch WR. The taxonomy of the southern African leaf toed geckos (Squamata: Gekkonidae), with a review of Old World “Phyllodactylus” and the description of five new genera. Proceedings of the California Academy of Sciences. 1997;49:447–497. [Google Scholar]
- Baum DA. Concordance trees, concordance factors, and the exploration of reticulate genealogy. Taxon. 2007;56:417–426. [Google Scholar]
- Benton MJ. Vertebrate Palaentology. Blackwell Publishing Co; Malden, MA: 2005. [Google Scholar]
- Boulenger GA. On some reptiles and batrachians from Iguarasse, Pernambuco. Annals and Magazine of Natural History, Series 6. 1888;2:40–43. [Google Scholar]
- Camp CL. Classification of the Lizards. Bulletin of the American Museum of Natural History. 1923;48:289–481. [Google Scholar]
- Campbell DC, Serb JM, Buhay JE, Roe KJ, Minton RL, Lydeard C. Phylogeny of North American amblemines (Bivalvia, Unionoida): prodigious polyphyly proves pervasive across genera. Invertebrate Biology. 2005;124:131–164. [Google Scholar]
- Carroll RL. Vertebrate Paleontology and Evolution. W. H. Freeman and Company; New York, NY: 1988. [Google Scholar]
- Carvalho EAR, Jr, Lima AP, Magnusson WE, Albernaz ALKM. Long-term effect of forest fragmentation on the Amazonian gekkonid lizards, Coleodactylus amazonicus and Gonatodes humeralis. Austral Ecology. 2008;33:723–729. [Google Scholar]
- Colli GR, Giugliano LG, Mesquita DO, França FGR. A new species of Cnemidophorus from the Jalapão regio, in the central Brazilian cerrado. Herpetologica. 2009;65:311–327. [Google Scholar]
- Conrad JL. Phylogeny and systematics of Squamata (Reptilia) based on morphology. Bulletin of the American Museum of Natural History. 2008;310:1–182. [Google Scholar]
- Conrad JL, Rieppel O, Grande L. Re-assessment of varanid evolution based on new data from Saniwa ensidens Leidy, 1870 (Squamata, Reptilia) American Museum Novitates. 2008;(3630):1–15. [Google Scholar]
- Cundall D, Irish F. The snake skull. In: Gans C, Gaunt AS, Adler K, editors. Biology of the Reptilia, Vol. 20 Morphology H: The Skull of Lepidosauria. Ithaca, NY: Society for the Study of Amphibians and Reptiles; 2008. pp. 349–692. [Google Scholar]
- Daza JD. Unpublished PhD. University of Puerto Rico; Río Piedras: 2008. Cladistic analysis of the Gekkota (Reptilia) by means of craniological data. [Google Scholar]
- Daza JD, Abdala V, Thomas R, Bauer AM. Skull anatomy of the miniaturized gecko Sphaerodactylusroosevelti (Squamata: Gekkota) Journal of Morphology. 2008;269:1340–1364. doi: 10.1002/jmor.10664. [DOI] [PubMed] [Google Scholar]
- Daza JD, Bauer AM. The circumorbital bones of the Gekkota (Reptilia: Squamata) The Anatomical Record. 2010;293:402–413. doi: 10.1002/ar.21039. [DOI] [PubMed] [Google Scholar]
- de Jong WW, van Dijk MAM, Poux C, Kappe G, van Rheede T, Madsen O. Indels in protein–coding sequences of Euarchontoglires constrain the rooting of the eutherian tree. Molecular Phylogenetics and Evolution. 2003;28:328–340. doi: 10.1016/s1055-7903(03)00116-7. [DOI] [PubMed] [Google Scholar]
- de Queiroz K. The general lineage concept of species, species criteria, and the process of speciation: a conceptual unification and terminological recommendations. In: Howard DJ, Berlocher SH, editors. Endless Forms: Species and Speciation. Oxford, England: Oxford University Press; 1998. pp. 57–75. [Google Scholar]
- de Queiroz K. Species concepts and species delimitation. Systematic Biology. 2007;56:879–886. doi: 10.1080/10635150701701083. [DOI] [PubMed] [Google Scholar]
- de Silva A, Bauer AM. The origin of the word ‘gecko’. Bibliotheca Herpetologica. 2008;8:20–31. [Google Scholar]
- Ericson PGP, Johansson US, Parsons TJ. Major divisions in oscines revealed by insertions in the nuclear gene c-myc: A novel gene in avian phylogenetics. Auk. 2000;117:1069–1078. [Google Scholar]
- Evans SE. The skull of lizards and tuatara. In: Gans C, Gaunt AS, Adler K, editors. Biology of the Reptilia, Vol. 20 Morphology H: The Skull of Lepidosauria. Ithaca, NY: Society for the Study of Amphibians and Reptiles; 2008. pp. 1–347. [Google Scholar]
- Fabrezi M, Abdala V, Oliveri MIM. Developmental basis of limb homology in lizards. The Anatomical Record. 2007;290:900–912. doi: 10.1002/ar.20522. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Confidence-limits on phylogenies - an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Frazzetta TH. A functional consideration of cranial kinesis in lizards. Journal of Morphology. 1962;111:287–319. doi: 10.1002/jmor.1051110306. [DOI] [PubMed] [Google Scholar]
- Freire EMX. Espécie nova de Coleodactylus Parker, 1926 das Dunas de Natal, Rio Grande do Norte, Brasil, com notas sobre suas relaçoãs dicromatismo sexual no género (Squamata, Gekkonidae) Boletim do M useu Nacional Rio de Janeiro Zoologia. 1999;399:1–14. [Google Scholar]
- Gaffney ES. Comparative cranial morphology of recent and fossil turtles. Bulletin of the American Museum of Natural History. 1979;164:65–376. [Google Scholar]
- Gamble T, Bauer AM, Colli GR, Greenbaum E, Jackman TR, Vitt LJ, Simons AM. Coming to America: Multiple origins of New World geckos. Journal of Evolutionary Biology. 2011;24:231–244. doi: 10.1111/j.1420-9101.2010.02184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble T, Bauer AM, Greenbaum E, Jackman TR. Evidence for Gondwanan vicariance in an ancient clade of gecko lizards. Journal of Biogeography. 2008a;35:88–104. [Google Scholar]
- Gamble T, Bauer AM, Greenbaum E, Jackman TR. Out of the blue: a novel, trans-Atlantic clade of geckos (Gekkota, Squamata) Zoologica Scripta. 2008b;37:355–366. [Google Scholar]
- Gamble T, Simons AM, Colli GR, Vitt LJ. Tertiary climate change and the diversification of the Amazonian gecko genus Gonatodes (Sphaerodactylidae, Squamata) Molecular Phylogenetics and Evolution. 2008c;46:269–277. doi: 10.1016/j.ympev.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Gasc JP. Les Lezards de Guyane. Editions Raymond Chabaud; Paris: 1990. [Google Scholar]
- Geurgas SR, Rodrigues MT. The hidden diversity of Coleodactylus amazonicus (Sphaerodactylinae, Gekkota) revealed by molecular data. Molecular Phylogenetics and Evolution. 2010;54:583–593. doi: 10.1016/j.ympev.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Geurgas SR, Rodrigues MT, Moritz C. The genus Coleodactylus (Sphaerodactylinae, Gekkota) revisited: A molecular phylogenetic perspective. Molecular Phylogenetics and Evolution. 2008;49:92–101. doi: 10.1016/j.ympev.2008.05.043. [DOI] [PubMed] [Google Scholar]
- Gould SJ. Allometry and size in ontogeny and phylogeny. Quarterly Review of Biology. 1966;41:587–640. doi: 10.1111/j.1469-185x.1966.tb01624.x. [DOI] [PubMed] [Google Scholar]
- Greer AE. The systematics and evolutionary relationships of the scincid lizard genus Lygosoma. Journal of Natural History. 1977;11:515–540. [Google Scholar]
- Greer AE. Hyperphalangy in squamates: insight on the reacquisition of primitive character states in limb–reduced lineages. Journal of Herpetology. 1992;26:327–329. [Google Scholar]
- Guerra C, Montero R. The skull of Vanzosaura rubricauda (Squamata: Gymnophthalmidae) Acta Zoologica. 2009;90:359–371. [Google Scholar]
- Hanke M, Wink M. Direct DNA-sequencing of PCR-amplified vector inserts following enzymatic degradation of primer and dNTPs. BioTechniques. 1994;17:858–860. [PubMed] [Google Scholar]
- Hanken J. Miniaturization and its effects on cranial morphology in plethodontid salamanders, genus Thorius (Amphibia: Plethodontidae) I. Osteological variation. Biological Journal of the Linnean Society. 1984;23:55–75. [Google Scholar]
- Hanken J, Wassersug R. The visible skeleton. A new double-staintechnique reveals the native “hard” tissues. Functional Photography. 1981;16:22–26. [Google Scholar]
- Harris DM. Occasional Papers of the Museum of Zoology. 704. University of Michigan; 1982. The Sphaerodactylus (Sauria: Gekkonidae) of South America; pp. 1–31. [Google Scholar]
- Harris DM, Kluge AG. Occasional Papers of the Museum of Zoology. 706. University of Michigan; 1984. The Sphaerodactylus (Sauria: Gekkonidae) of Middle America; pp. 1–59. [Google Scholar]
- Hedges SB, Thomas R. At the lower size limit in amniote vertebrates: A new diminutive lizard from the West Indies. Caribbean Journal of Science. 2001;37:168–173. [Google Scholar]
- Holder LA. The comparative morphology of the axial skeleton in the Australian Gekkonidae. Zoological Journal of the Linnean Society. 1960;44:300–335. [Google Scholar]
- Hoogmoed MS. The Lizards and Amphisbaenians of Surinam. W. Junk; The Hague: 1973. Notes on the Herpetofauna of Surinam IV. [Google Scholar]
- Hoogmoed MS. Coleodactylus septentrionalis Vanzolini, a lizard new for the Surinamese fauna (Sauria: Gekkonidae): Notes on the herpetofauna of Suriname X. Zoologische Mededelingen. 1985;59:229–238. [Google Scholar]
- Huelsenbeck JP, Ronquist F. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Iordansky NN. The skull of the Crocodilia. In: Gans C, Parsons T, editors. Biology of the Reptilia, Vol. 4: Morphology D. London, England: Academic Press Inc; 1973. pp. 201–260. [Google Scholar]
- Jackman TR, Larson A, de Queiroz K, Losos JB. Phylogenetic relationships and tempo of early diversification in Anolis lizards. Systematic Biology. 1999;48:254–285. [Google Scholar]
- Jaslow CR. Sexual dimorphism of cranial suture complexity in wild sheep (Ovis orientalis) Zoological Journal of the Linnean Society. 1989;95:273–284. [Google Scholar]
- Jerez A, Mangione S, Abdala V. Occurrence and distribution of sesamoid bones in squamates: a comparative approach. Acta Zoologica. 2010;91:295–305. [Google Scholar]
- Kluge AG. Higher taxonomic categories of gekkonid lizards and their evolution. Bulletin of the American Museum of Natural History. 1967;135:1–60. [Google Scholar]
- Kluge AG. Phylogenetic relationships in the lizard family Pygopodidae: an evaluation of theory, methods and data. Miscellaneous Publications, Museum of Zoology, University of Michigan. 1976;152:1–72. [Google Scholar]
- Kluge AG. Cladistic relationships in the Gekkonoidea (Squamata, Sauria) Miscellaneous Publications Museum of Zoology University of Michigan. 1987;173:1–54. [Google Scholar]
- Kluge AG. Cladistic relationships of sphaerodactyl lizards. American Museum Novitates. 1995;(3139):1–23. [Google Scholar]
- Kluge AG. Gekkotan lizard taxonomy. Hamadryad. 2001;26:i–ii. 1–209. [Google Scholar]
- Langstroth RP. Adiciones probables y confirmadas para la saurofauna Boliviana. Kempffiana. 2005;1:101–128. [Google Scholar]
- Lanyon SM. Polyphyly of the blackbird genus Agelaius and the importance of assumptions of monophyly in comparative studies. Evolution. 1994;48:679–693. doi: 10.1111/j.1558-5646.1994.tb01353.x. [DOI] [PubMed] [Google Scholar]
- Leaché AD, Koo MS, Spencer CL, Papenfuss TJ, Fisher RN, McGuire JA. Quantifying ecological, morphological, and genetic variation to delimit species in the coast horned lizard species complex (Phrynosoma) Proceedings of the National Academy of Sciences of the United States of America. 2009;106:12418–12423. doi: 10.1073/pnas.0906380106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MSY. The phylogeny of varanoid lizards and the affinities of snakes. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences. 1997;352:53–91. [Google Scholar]
- MacLean WP. Feeding and locomotor mechanism of teiid lizards: Functional morphology and evolution. Papéis Avulsos de Zoologia. 1974;27:179–213. [Google Scholar]
- MacLean WP. Water-loss rates of Sphaerodactylus parthenopion (Reptilia, Gekkonidae), the smallest amniote vertebrate. Comparative Biochemistry and Physiology. A. Physiology. 1985;82:759–761. [Google Scholar]
- Maddison W. Reconstructing character evolution on polytomous cladograms. Cladistics. 1989;5:365–377. doi: 10.1111/j.1096-0031.1989.tb00569.x. [DOI] [PubMed] [Google Scholar]
- Maddison WP. Gene trees in species trees. Systematic Biology. 1997;46:523–536. [Google Scholar]
- Maddison WP, Knowles LL. Inferring phylogeny despite incomplete lineage sorting. Systematic Biology. 2006;55:21–30. doi: 10.1080/10635150500354928. [DOI] [PubMed] [Google Scholar]
- Maddison WP, Maddison DR. MacClade, analysis of phylogeny and character evolution. 3.0. Sunderland, MA: Sinauer; 1992. [DOI] [PubMed] [Google Scholar]
- Maddison WP, Maddison DR. Mesquite: a modular system for evolutionary analysis. 2.5 2008. [Google Scholar]
- Malan ME. Contributions to the comparative anatomy of the nasal capsule and the organ of Jacobson of the Lacertilia. Annals of the University of Stellenbosch. 1946;24:69–137. [Google Scholar]
- Meylan PA, Moody RTJ, Walker CA, Chapman SD. Sandownia harrisi, a highly derived trionychoid turtle (Testudines: Cryptodira) from the Early Cretaceous of the Isle of Wight, England. Journal of Vertebrate Paleontology. 2000;20:522–532. [Google Scholar]
- Moazen M, Curtis N, O’Higgins P, Jones MEH, Evans SE, Fagan MJ. Assessment of the role of sutures in a lizard skull: a computer modelling study. Proceedings of the Royal Society B-Biological Sciences. 2009;276:39–46. doi: 10.1098/rspb.2008.0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero R, Gans C. An atlas of amphisbaenian skull anatomy. In: Gans C, Gaunt AS, Adler K, editors. Biology of the Reptilia, Vol. 21, Morphology I: The Skull and Appendicular Locomotor Apparatus of Lepidosauria. Ithaca, NY: Society for the Study of Amphibians and Reptiles; 2008. pp. 621–738. [Google Scholar]
- Moretti R. Unpublished PhD dissertation. Universidade de São Paulo; 2009. Revisão taxonômica e biogeografia do gênero Coleodactylus Parker, 1926 (Squamata: Sphaerodactylidae) [Google Scholar]
- Noble GK. The bony structure and phyletic relations of Sphærodactylus and allied lacertilian genera, with the description of a new genus. American Museum Novitates. 1921;(4):1–16. [Google Scholar]
- Notredame C, Higgins DG, Heringa J. T–Coffee: A novel method for fast and accurate multiple sequence alignment. Journal of Molecular Biology. 2000;302:205–217. doi: 10.1006/jmbi.2000.4042. [DOI] [PubMed] [Google Scholar]
- Nylander JAA, Wilgenbusch JC, Warren DL, Swofford DL. AWTY (are we there yet?): a system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics. 2008;24:581–583. doi: 10.1093/bioinformatics/btm388. [DOI] [PubMed] [Google Scholar]
- Oliver JC. AUGIST: inferring species trees while accommodating gene tree uncertainty. Bioinformatics. 2008;24:2932–2933. doi: 10.1093/bioinformatics/btn556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver PM, Adams M, Lee MS, Hutchinson MN, Doughty P. Cryptic diversity in vertebrates: molecular data double estimates of species diversity in a radiation of Australian lizards (Diplodactylus, Gekkota) Proceedings of the Royal Society of London Series B-Biological Sciences. 2009;276:2001–2007. doi: 10.1098/rspb.2008.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker HW. TheNeotropical lizards of the genera Lepidoblepharis, Pseudogonatodes, Lathrogecko, and Sphaerodactylus, with the description of a new genus. Annals and Magazine of Natural History, series 9. 1926;17:291–301. [Google Scholar]
- Poe S, Chubb AL. Birds in a bush: Five genes indicate explosive evolution of avian orders. Evolution. 2004;58:404–415. [PubMed] [Google Scholar]
- Posada D. jModelTest: Phylogenetic model averaging. Molecular Biology and Evolution. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- Presch W. Secondary palate formation in microteiid lizards (Teiidae: Lacertilia) Bulletin of the Southern California Academy of Science. 1976;75:281–283. [Google Scholar]
- Pritchard JJ, Scott JH, Girgis FG. The structure and development of cranial and facial sutures. Journal of Anatomy. 1956;90:73–86. [PMC free article] [PubMed] [Google Scholar]
- Rambaut A, Drummond AJ. Tracer, version 1.5. 1.5. Distributed by authors; 2007. [Google Scholar]
- Ramos AR. Aspectos do nicho alimentar de Coleodactylus amazonicus (Sauria, Gekkonidae) Acta Amazonica. 1981;11:511–526. [Google Scholar]
- Raxworthy C, Ingram C, Rabibisoa N, Pearson R. Applications of ecological niche modeling for species delimitation: A review and empirical evaluation using day geckos (Phelsuma) from Madagascar. Systematic Biology. 2007;56:907–923. doi: 10.1080/10635150701775111. [DOI] [PubMed] [Google Scholar]
- Rieppel O. The evolution of the naso-frontal joint in snakes and its bearing on snake origins. Zeitschrift für zoologische Systematik und Evolutionsforschung. 1978;16:14–27. [Google Scholar]
- Rieppel O. Miniaturization of the lizard skull: Its function and evolutionary implications. In: Ferguson MWJ, editor. The Structure, Development and Evolution of Reptiles. London: Academic Press; 1984a. pp. 503–520. [Google Scholar]
- Rieppel O. The structure of the skull and jaw adductor musculature of the Gekkota, with comments on the phylogenetic relationships of the Xantusiidae (Reptilia: Lacertilia) Zoological Journal of the Linnean Society. 1984b;82:291–318. [Google Scholar]
- Rieppel O. Miniaturization in tetrapods: Consequences for skull morphology. In: Miller PJ, editor. Symposium of the Zoological Society of London. Oxford: Clarendon Press; 1996. pp. 47–61. [Google Scholar]
- Rivas GA, Molina RCR. Geographic distribution.Coleodactylus septentrionalis. Herpetological Review. 2001;32:275. [Google Scholar]
- Rivas GA, Schargel WE. Gecko on the rocks: an enigmatic new species of Gonatodes (Sphaerodactylidae) from Inselbergs of the Venezuelan Guayana. Zootaxa. 2008;1925:39–50. [Google Scholar]
- Rokas A, Holland PWH. Rare genomic changes as a tool for phylogenetics. Trends in Ecology & Evolution. 2000;15:454–459. doi: 10.1016/s0169-5347(00)01967-4. [DOI] [PubMed] [Google Scholar]
- Röll B, Henkel FW. Are pygopods just legless geckos? Evidence from retinal structures. Salamandra. 2002;38:73–84. [Google Scholar]
- Romer AS. Osteology of the Reptiles. University of Chicago Press; Chicago: 1956. [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Roscito JG, Rodrigues MT. Comparative cranial osteology of fossorial lizards from the tribe gymnophthalmini (Squamata, Gymnophthalmidae) Journal of Morphology. 2011;271:1352–1365. doi: 10.1002/jmor.10878. [DOI] [PubMed] [Google Scholar]
- Rosenberg NA, Tao R. Discordance of species trees with their most likely gene trees: The case of five taxa. Systematic Biology. 2008;57:131–140. doi: 10.1080/10635150801905535. [DOI] [PubMed] [Google Scholar]
- Russell AP. Unpublished PhD. University of London; 1972. The foot of gekkonid lizards: a study in comparative and functional anatomy. [Google Scholar]
- Russell AP, Bauer AM. Underwood’s classification of the geckos: a 21st century appreciation. Bulletin of The Natural History Museum (Zoology) 2002;68:113–121. [Google Scholar]
- Russell AP, Bauer AM. The appendicular locomotor apparatus of Sphenodon and normal-limbed squamates. In: Gans C, Gaunt AS, Adler K, editors. Biology of the Reptilia, Vol. 21, Morphology I: The Skull and Appendicular Locomotor Apparatus of Lepidosauria. Ithaca, NY: Society for the Study of Amphibians and Reptiles; 2008. pp. 1–465. [Google Scholar]
- Savage JM. Studies on the lizard family Xantusiidae IV. The genera. Contributions in Science, Los Angeles County Museum of Natural History. 1963;71:1–38. [Google Scholar]
- Shapiro MD, Shubin N, Downs JP. Limb diversity and digit reduction in reptilian evolution. In: Hall BK, editor. Fins into Limbs: Evolution, Development and Transformation. Chicago, IL: University of Chicago Press; 2007. pp. 225–244. [Google Scholar]
- Shimodaira H, Hasegawa M. Multiple comparisons of log-likelihoods with applications to phylogenetic inference. Molecular Biology and Evolution. 1999;16:1114–1116. [Google Scholar]
- Shimodaira H, Hasegawa M. CONSEL: for assessing the con dence of phylogenetic tree selection. Bioinformatics. 2001;17:1246–1247. doi: 10.1093/bioinformatics/17.12.1246. [DOI] [PubMed] [Google Scholar]
- Simmons MP, Ochoterena H, Carr TG. Incorporation, relative homoplasy, and effect of gap characters in sequence–based phylogenetic analyses. Systematic Biology. 2001;50:454–462. [PubMed] [Google Scholar]
- Sites JW, Marshall JC. Operational criteria for delimiting species. Annual Review of Ecology, Evolution, and Systematics. 2004;35:199–227. [Google Scholar]
- Slowinski JB. Molecular polytomies. Molecular Phylogenetics and Evolution. 2001;19:114–120. doi: 10.1006/mpev.2000.0897. [DOI] [PubMed] [Google Scholar]
- Smith MA. Remarks on some Old World geckoes. Records of the Indian Museum. 1933;35:9–19. [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: Maximum likelihood-basedphylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Stephenson NG. The comparative osteology of Australian geckos and its bearing on their morphological status. Zoological Journal of the Linnean Society. 1960;44:278–299. [Google Scholar]
- Swofford D. Phylogenetic analysis using parsimony (*and other methods) 4.0. Sunderland, MA: Sinauer; 2002. PAUP*. [Google Scholar]
- Tarazona OA, Fabrezi M, Ramirez–Pinilla MP. Cranial morphology of Bachia bicolor (Squamata: Gymnophthalmidae) and its postnatal development. Zoological Journal of the Linnean Society. 2008;152:775–792. [Google Scholar]
- Thomas R. A new gecko from the Virgin Islands. Quarterly Journal of Florida Academy of Sciences. 1965;28:117–122. [Google Scholar]
- Townsend TM, Larson A, Louis E, Macey JR. Molecular phylogenetics of Squamata: The position of snakes, amphisbaenians, and dibamids, and the root of the squamate tree. Systematic Biology. 2004;53:735–757. doi: 10.1080/10635150490522340. [DOI] [PubMed] [Google Scholar]
- Uetz P. The original descriptions of reptiles. Zootaxa. 2010;2334:59–68. doi: 10.11646/zootaxa.4375.2.5. [DOI] [PubMed] [Google Scholar]
- Underwood G. The eye. In: Gans C, editor. Biology of the Reptilia, Volume 2. Morphology B. London and New York: Academic Press; 1970. pp. 1–97. [Google Scholar]
- van Dijk MAM, Paradis E, Catzeflis F, de Jong WW. The virtues of gaps: Xenarthran (Edentate) monophyly supported by a unique deletion in alpha A–crystallin. Systematic Biology. 1999;48:94–106. doi: 10.1080/106351599260463. [DOI] [PubMed] [Google Scholar]
- Vanzolini PE. O genero Coleodactylus (Sauria, Gekkonidae) Papéis Avulsos do Departamento de Zoologia. 1957;13:1–17. [Google Scholar]
- Vanzolini PE. Geography of the South American Gekkonidae (Sauria) Arquivos de Zoologia (São Paulo) 1968a;17:85–112. [Google Scholar]
- Vanzolini PE. Lagartos Brasileiros da familia Gekkonidae (Sauria) Arquivos de Zoologia (São Paulo) 1968b;17:1–84. [Google Scholar]
- Vanzolini PE. Coleodactylus septentrionalis, sp. n., with notes on the distribution of the genus (Sauria, Gekkonidae) Papéis Avulsos de Zoologia. 1980;34:1–9. [Google Scholar]
- Versluys J. Das Streptostylie-Problem und die Bewegungen im Schädel bei Sauropsiden. Zoologische Jahrbücher, Abteilung für Anatomie und Ontogenie der Tiere. 1912;2(Suppl 15):545–716. [Google Scholar]
- Vitt LJ, Sartorius SS, Avila Pires TCS, Zani PA, Espósito MC. Small in a big world: Ecology of leaf–litter geckos in New World tropical forests. Herpetological Monographs. 2005;19:137–152. [Google Scholar]
- Werner YL. Eye size in geckos of various ecological types (Reptilia: Gekkonidae and Sphaerodactylidae) Israel Journal of Zoology. 1969;18:291–316. [Google Scholar]
- Werner YL. The ontogenetic development of the vertebrae in some gekkonid lizards. Journal of Morphology. 1971;133:41–92. doi: 10.1002/jmor.1051330104. [DOI] [PubMed] [Google Scholar]
- Wettstein O. Coleodactylus zernyi nov. spec., ein neuer Gecko aus Brasilien. Zoologischer Anzeiger. 1928;76:110–112. [Google Scholar]
- Zink RM, McKitrick MC. The debate over species concepts and its implications for ornithology. Auk. 1995;112:701–719. [Google Scholar]