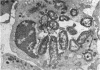Abstract
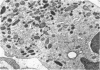
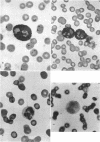
The neutrophils and monocytes of a patient with disseminated candidiasis were found to lack detectable levels of the lysosomal enzyme myeloperoxidase (MPO), although they had normal levels of other granule-associated enzymes. Leukocytes from one of the patient's sisters also lacked detectable MPO; leukocytes from his four sons contained approximately one-third of mean normal peroxidase levels. Neither the patient nor his affected relatives had experienced frequent or unusual bacterial infections.
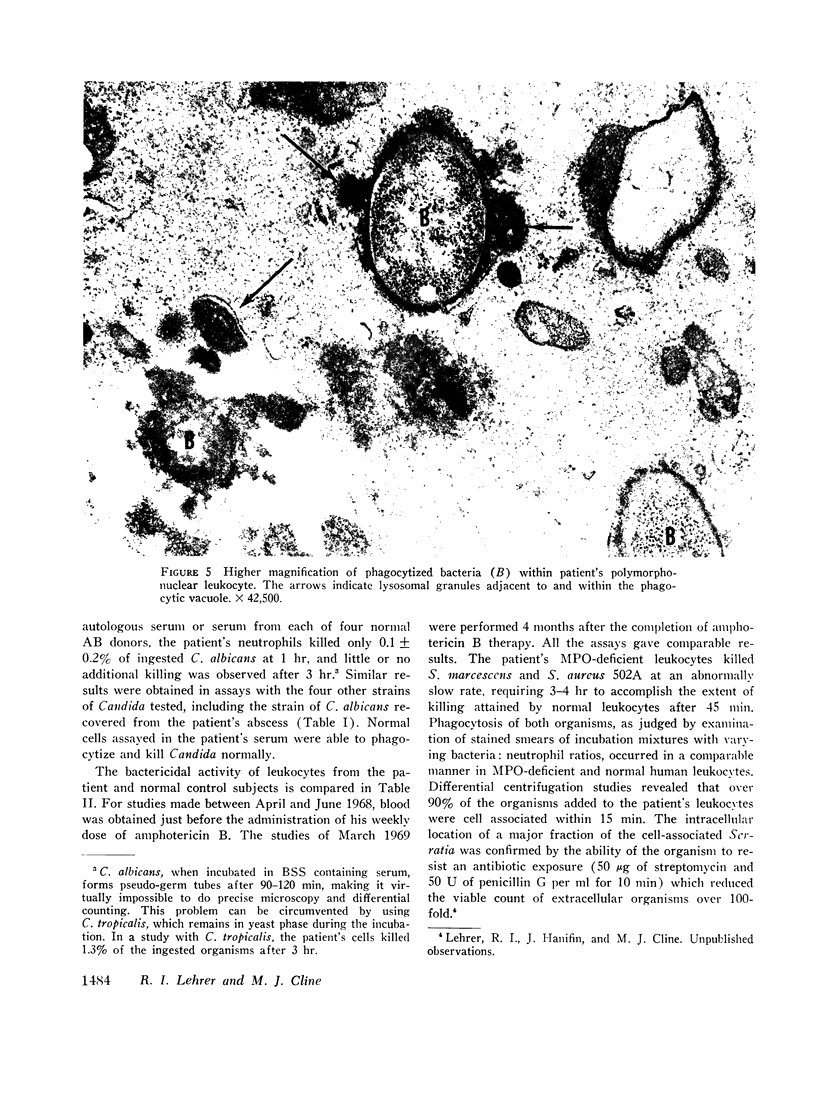
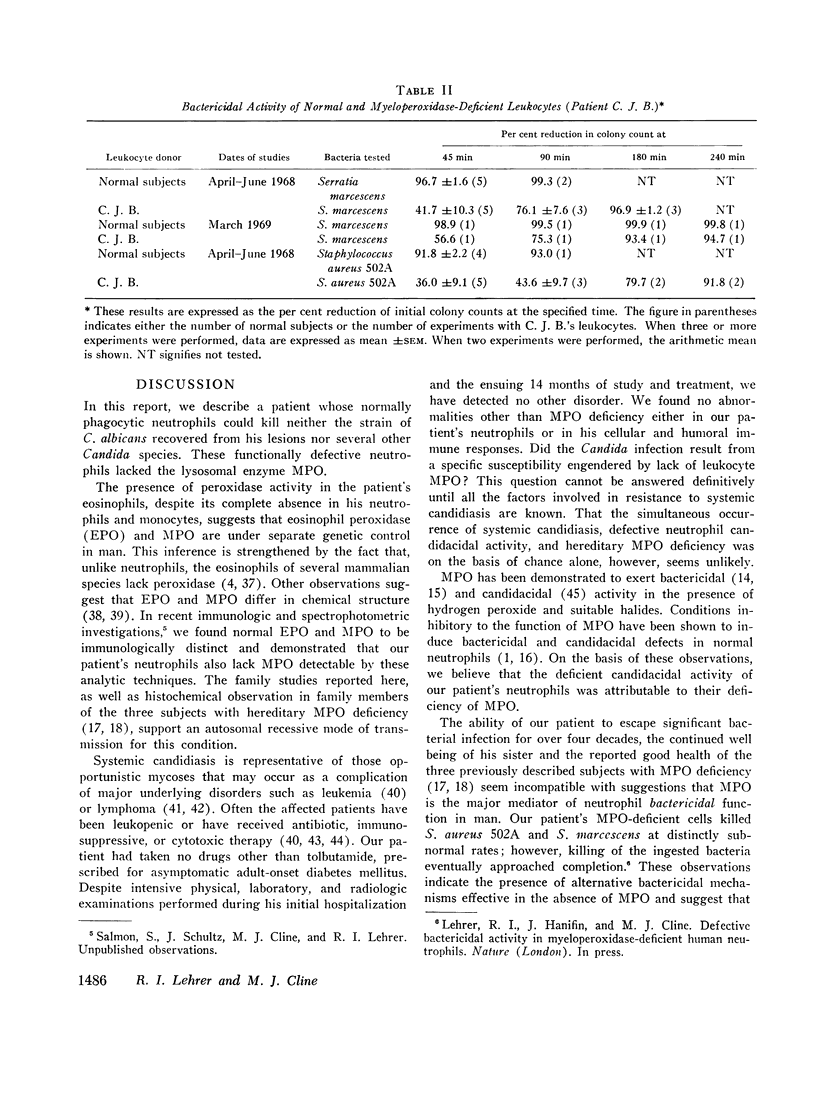
The phagocytic activity of the patient's MPO-deficient neutrophils was intact, and the cells displayed normal morphologic and metabolic responses to phagocytosis. In contrast to normal leukocytes which killed 30.5±7.3% of ingested Candida albicans in 1 hr, however, the patient's neutrophils killed virtually none. His leukocytes also failed to kill the strain of C. albicans recovered from his lesions, as well as other Candida species. These MPO-deficient neutrophils killed Serratia marcescens and Staphylococens aureus 502A at an abnormally slow rate, requiring 3-4 hr to achieve the bactericidal effect attained by normal leukocytes after 45 min. No other abnormalities in his cellular or humoral immune responses were demonstrated.
These findings suggest that hereditary MPO deficiency is transmitted as an autosomal recessive characteristic, that the homozygous state conveys enhanced susceptibility to disseminated candidiasis, and that MPO is necessary for candidacidal activity in human neutrophils. Although lending support to the suggested bactericidal role of MPO in leukocytes, the data indicate that alternative bactericidal mechanisms, effective in the absence of MPO, are functionally dominant in the human neutrophil.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AGNER K. Studies on peroxidative detoxification of purified diphtheria toxin. J Exp Med. 1950 Oct 1;92(4):337–347. doi: 10.1084/jem.92.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARCHER G. T., AIR G., JACKAS M., MORELL D. B. STUDIES ON RAT EOSINOPHIL PEROXIDASE. Biochim Biophys Acta. 1965 Apr 26;99:96–101. doi: 10.1016/s0926-6593(65)80010-8. [DOI] [PubMed] [Google Scholar]
- Arakawa T., Wada Y., Hayashi T., Kakizaki R., Chida N., Chiba R., Konno T. Uracil-uric refractory anemia with peroxidase negative neutrophils. Tohoku J Exp Med. 1965 Oct 25;87(1):52–75. doi: 10.1620/tjem.87.52. [DOI] [PubMed] [Google Scholar]
- Bodey G. P. Fungal infections complicating acute leukemia. J Chronic Dis. 1966 Jun;19(6):667–687. doi: 10.1016/0021-9681(66)90066-x. [DOI] [PubMed] [Google Scholar]
- CAULFIELD J. B. Effects of varying the vehicle for OsO4 in tissue fixation. J Biophys Biochem Cytol. 1957 Sep 25;3(5):827–830. doi: 10.1083/jcb.3.5.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLIMIE A. R., HEINRICHS W. L., FOSTER I. J. Neutrophilic alkaline phosphatase test. A review with emphasis on findings in pregnancy. Tech Bull Regist Med Technol. 1962 Jun;32:95–103. [PubMed] [Google Scholar]
- COHN Z. A., HIRSCH J. G. The isolation and properties of the specific cytoplasmic granules of rabbit polymorphonuclear leucocytes. J Exp Med. 1960 Dec 1;112:983–1004. doi: 10.1084/jem.112.6.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casazza A. R., Duvall C. P., Carbone P. P. Infection in lymphoma. Histology, treatment, and duration in relation to incidence and survival. JAMA. 1966 Aug 29;197(9):710–716. doi: 10.1001/jama.197.9.710. [DOI] [PubMed] [Google Scholar]
- EPSTEIN W. L. Induction of allergic contact dermatitis in patients with the lymphoma-leukemia complex. J Invest Dermatol. 1958 Jan;30(1):39–40. doi: 10.1038/jid.1958.8. [DOI] [PubMed] [Google Scholar]
- FALLON H. J., FREI E., 3rd, DAVIDSON J. D., TRIER J. S., BURK D. Leukocyte preparations from human blood: evaluation of their morphologic and metabolic state. J Lab Clin Med. 1962 May;59:779–791. [PubMed] [Google Scholar]
- HIRSCH J. G., STRAUSS B. STUDIES ON HEAT-LABILE OPSONIN IN RABBIT SERUM. J Immunol. 1964 Jan;92:145–154. [PubMed] [Google Scholar]
- HOWELL R. R., SEEGMILLER J. E. Uricolysis by human leukocytes. Nature. 1962 Nov 3;196:482–483. doi: 10.1038/196482a0. [DOI] [PubMed] [Google Scholar]
- HURLEY R. ACUTE DISSEMINATED (SEPTICAEMIC) MONILIASIS IN ADULTS AND CHILDREN. Postgrad Med J. 1964 Nov;40:644–653. doi: 10.1136/pgmj.40.469.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTER R. V., COLLINS H. S. The occurrence of opportunistic fungus infections in a cancer hospital. Lab Invest. 1962 Nov;11:1035–1045. [PubMed] [Google Scholar]
- Hartog M., Cline M. J., Grodsky G. M. The response of human leucocyte cultures to stimulation by tuberculin and phytohaemagglutinin measured by the uptake of radioactive thymidine. Clin Exp Immunol. 1967 Mar;2(2):217–228. [PMC free article] [PubMed] [Google Scholar]
- Higashi O., Katsuyama N., Satodate R. A case with hematological abnormality characterized by the absence of peroxidase activity in blood polymorphonuclear leukocytes. Tohoku J Exp Med. 1965 Oct 25;87(1):77–89. doi: 10.1620/tjem.87.77. [DOI] [PubMed] [Google Scholar]
- KLEBANOFF S. J. INACTIVATION OF ESTROGEN BY RAT UTERINE PREPARATIONS. Endocrinology. 1965 Feb;76:301–311. doi: 10.1210/endo-76-2-301. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967 Dec 1;126(6):1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968 Jun;95(6):2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANG E. M., UNDRITZ E., VAN OYE E. La réaction peroxydasique des éosinophiles comme moyen de taxonomie classification des mammifères. Sang. 1956;27(6):513–515. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MOORHEAD P. S., NOWELL P. C., MELLMAN W. J., BATTIPS D. M., HUNGERFORD D. A. Chromosome preparations of leukocytes cultured from human peripheral blood. Exp Cell Res. 1960 Sep;20:613–616. doi: 10.1016/0014-4827(60)90138-5. [DOI] [PubMed] [Google Scholar]
- McRipley R. J., Sbarra A. J. Role of the phagocyte in host-parasite interactions. XII. Hydrogen peroxide-myeloperoxidase bactericidal system in the phagocyte. J Bacteriol. 1967 Nov;94(5):1425–1430. doi: 10.1128/jb.94.5.1425-1430.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisler H. D., Hasenclever H. F., Levitan A. A., Henderson E. S. Serologic diagnosis of disseminated candidiasis in patients with acute leukemia. Ann Intern Med. 1969 Jan;70(1):19–30. doi: 10.7326/0003-4819-70-1-19. [DOI] [PubMed] [Google Scholar]
- REBUCK J. W., CROWLEY J. H. A method of studying leukocytic functions in vivo. Ann N Y Acad Sci. 1955 Mar 24;59(5):757–805. doi: 10.1111/j.1749-6632.1955.tb45983.x. [DOI] [PubMed] [Google Scholar]
- RECHCIGL M., Jr, EVANS W. H. ROLE OF CATALASE AND PEROXIDASE IN THE METABOLISM OF LEUCOCYTES. Nature. 1963 Sep 7;199:1001–1002. doi: 10.1038/1991001b0. [DOI] [PubMed] [Google Scholar]
- RYTOMAA T., TEIR H. Relationship between tissue eosinophils and peroxidase activity. Nature. 1961 Oct 21;192:271–272. doi: 10.1038/192271b0. [DOI] [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- SCHULTZ J., KAMINKER K. Myeloperoxidase of the leucocyte of normal human blood. I. Content and localization. Arch Biochem Biophys. 1962 Mar;96:465–467. doi: 10.1016/0003-9861(62)90321-1. [DOI] [PubMed] [Google Scholar]
- SCHULTZ J., SHMUKLER H. W. MYELOPEROXIDASE OF THE LEUCOCYTE OF NORMAL HUMAN BLOOD. II. ISOLATION, SPECTROPHOTOMETRY, AND AMINO ACID ANALYSIS. Biochemistry. 1964 Sep;3:1234–1238. doi: 10.1021/bi00897a009. [DOI] [PubMed] [Google Scholar]
- SKOOG W. A., BECK W. S. Studies on the fibrinogen, dextran and phytohemagglutinin methods of isolating leukocytes. Blood. 1956 May;11(5):436–454. [PubMed] [Google Scholar]
- Sato A. Peroxidase-negative neutrophils in Arakawahigashi's syndrome, in encephalitis Economo, further in "healthy brethren". Tohoku J Exp Med. 1965 Oct 25;87(1):94–100. doi: 10.1620/tjem.87.94. [DOI] [PubMed] [Google Scholar]
- Schultz J., Corlin R., Oddi F., Kaminker K., Jones W. Myeloperoxidase of the leucocyte of normal human blood. 3. Isolation of the peroxidase granule. Arch Biochem Biophys. 1965 Jul;111(1):73–79. doi: 10.1016/0003-9861(65)90324-3. [DOI] [PubMed] [Google Scholar]
- Undritz E. Die Alius-Grignaschi-Anomalie: Der erblich-konstitutionelle Peroxydasedefekt der Neutrophilen und Monozyten. Blut. 1966 Dec;14(3):129–136. doi: 10.1007/BF01631534. [DOI] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Cationic proteins of polymorphonuclear leukocyte lysosomes. I. Resolution of antibacterial and enzymatic activities. J Bacteriol. 1966 Feb;91(2):750–754. doi: 10.1128/jb.91.2.750-754.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]