Abstract
Macaques can efficiently use several tools, but their capacity to discriminate the relevant physical features of a tool and the social factors contributing to their acquisition are still poorly explored. In a series of studies, we investigated macaques' ability to generalize the use of a stick as a tool to new objects having different physical features (study 1), or to new contexts, requiring them to adapt the previously learned motor strategy (study 2). We then assessed whether the observation of a skilled model might facilitate tool-use learning by naive observer monkeys (study 3). Results of study 1 and study 2 showed that monkeys trained to use a tool generalize this ability to tools of different shape and length, and learn to adapt their motor strategy to a new task. Study 3 demonstrated that observing a skilled model increases the observers' manipulations of a stick, thus facilitating the individual discovery of the relevant properties of this object as a tool. These findings support the view that in macaques, the motor system can be modified through tool use and that it has a limited capacity to adjust the learnt motor skills to a new context. Social factors, although important to facilitate the interaction with tools, are not crucial for tool-use learning.
Keywords: body schema, tool selection, sensorimotor experience, action perception, mirror neurons
1. Introduction
It is well acknowledged that several primate species are capable of selecting and using tools. In chimpanzees and capuchin monkeys, the flexible and the extensive use of tools have been widely documented both in free-living [1–3] and captive populations [4,5]. More scattered and limited are reports on tool use in free-ranging populations of macaques. Most of these studies documented the use of tools only in a limited number of individuals and often the reports are anecdotal [6]. More recently, it has been reported that long-tailed macaques of a wild population in Thailand regularly use stones as tools to crack shelled seafood [7]. Despite this example, there is still a general agreement in the scientific community that macaques are not skilled tool users. However, this picture becomes more complex, if one considers studies on macaques under more controlled experimental conditions or in captivity, where long-lasting observations are feasible.
In fact, more detailed descriptions are available on captive and free-ranging provisioned groups of macaques, in which prolonged observations allowed researchers to understand which factors facilitate the acquisition of tool use or prevent individuals from acquiring new behaviours [8–13].
A series of laboratory experiments demonstrated that macaques are capable of learning the use of tools for retrieving food out of reach [14,15]. Under certain circumstances, the process of tool-use learning may require a relatively short time of training through instrumental conditioning procedures. Other laboratory studies demonstrated that macaques can learn to use even more complex tools, such as pliers, requiring a higher level of coordination and handedness [16]. Together, these studies indicate that macaques are capable of refining their motor representations and have the cognitive potential to include the tools within their expanded motor repertoire. This is also supported by neurophysiological studies indicating that, after tool-use learning, motor representations and body schema change in the parietal and premotor cortices [16,17].
An important issue that has been very scarcely investigated is how can monkeys discriminate the appropriate tool for a given task. Recent work in capuchin monkeys showed that they can select the most adequate tool for extracting food protected in a nut shell based on functional features such as weight and shape [2]. Few studies have explored in detail how non-human animals represent tools and, in particular, whether they distinguish between functional and non-functional objects based on their physical features [14,15,18]. In a series of experiments on object knowledge, Hauser [19] assessed in cotton-top tamarins the capacity of understanding which properties of a tool are relevant to its functioning. Once monkeys learned to use canes to retrieve food out of reach, they were presented with a variety of new tools with different colours, shapes, textures and sizes. The results showed that, on average, monkeys chose the functional tool. This has been interpreted as a demonstration that they use a strategy based on an understanding of the means-end relationship [19]. However, it cannot be excluded that trial-and-error processes, based on the sensorimotor experience with the tool, could have intervened. This latter interpretation would also be consistent with the conclusions reached by Visalberghi & Limongelli [20], based on their studies on capuchin monkeys.
Considering the reports so far reviewed about tool use in macaques in the wild, captivity and in more controlled experimental conditions [6,8–15], the issue of tool selection and of the underlying cognitive processes remains still poorly investigated in this taxon.
The process of tool-use acquisition can also be facilitated by social factors. Several studies showed in different species of macaques the possibility to acquire new behaviours through social-based learning. This social transmission of tool use can account for traditions and cultures so well developed and documented in primates [21].
One of the possibilities for a naive subject to learn a new behaviour is to observe an expert individual performing the action. The acquisition of the new behaviour will be probably linked also to the frequency with which the subject observes the demonstrator performing that action [22]. Other factors that increase the likelihood of social learning new behaviours are the attention that the subject pays towards the observed behaviour and its proximity to the demonstrator [22–30]. Thus, the acquisition of a new behaviour in naive subjects should be faster in those individuals with greater opportunity to observe and learn from expert models [22,26,27]. This observer–demonstrator paradigm has been typically used in captivity and in laboratory settings [21].
The series of experiments we present here had two main objectives and were organized into two main parts.
The first part was focused on the issue of individual learning processes. In particular, it was aimed at investigating whether, and to what extent, monkeys previously trained to use a tool for retrieving food could generalize their capacity across different tools with novel features and different contexts requiring the adjustment of the learned motor strategy.
In the second part, the monkeys that were employed for individual learning experiments during the first part, served as demonstrators of tool use for completely naive macaque monkeys. In this observer–demonstrator paradigm, we explored the possible presence of social learning processes in the observing individuals.
2. Study 1: selection of tools based on their physical properties
Capuchin monkeys and chimpanzees are capable of selecting tools with different features in relation to their behavioural purposes [2,5]. However, this ability is still largely unexplored in macaques. The main aims of this study were twofold: (i) to assess how the learned capacity to use a tool in a specific task can be generalized to other types of tools having the same length, and (ii) to verify whether monkeys can select, among tools of different shapes, one of appropriate length to enable food retrieval.
Monkeys were first trained to use a stick in order to retrieve a creamy food (yogurt) out of arm's reach. Then, they were presented with two novel elongated tools of different shape, in addition to the stick. In one condition, all tools were functional for retrieving food, in another condition only one was functional, the other two being too short for this purpose.
We analysed the animals' choice in both conditions.
(a). Material and methods
(i). Subjects
The experimental subjects were two male pigtailed macaque monkeys (Macaca nemestrina), here identified as M1 and M2. Both monkeys were captive born, mother-reared until they were 2–3 years old and then individually housed at the Primate Section of the Department of Neuroscience, University of Parma. At the time of testing, they were both 5 years old. Both monkeys were singly housed in cages (175 × 100 × 100 cm) allowing them visual and auditory contact with other monkeys (Macaca nemestrina and Macaca mulatta) housed in the same room.
To maintain a high motivation to the task, during experiments, subjects were mildly food-deprived, receiving their daily food only at the end of each testing session. Food consisted of fresh fruits, vegetables, bread, seeds and monkey chow. Water was always available.
(ii). Apparatus and training procedures
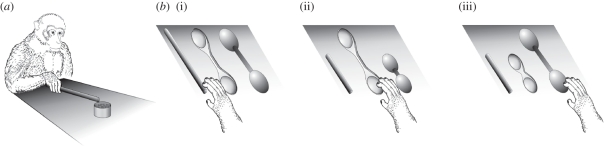
The basic set-up employed in this study is shown in figure 1a.
Figure 1.
(a) Basic experimental set-up employed in study 1. (b) Experimental conditions and features of the tools used for testing tool selection during study 1: b(i) all three tools functional (3F); b(ii) functional spoon (FS) and non-functional stick and egg-shaped tools; b(iii) functional egg-shaped tool (FE) and non-functional stick and spoon.
During the first phase, monkeys sat in their home cages. They were allowed to retrieve the food (yogurt) from a transparent Plexiglas container (inner diameter 6.5 cm, height 5.5 cm) by means of a wooden stick (diameter 1 cm, length 22 cm). The container was located in front of the monkey cage, screwed on a plywood table (length 70 cm, width 75 cm, height 32 cm from the floor of the cage) outside the monkey reaching space (44.5 cm from the cage bars). In this phase, none of the monkeys spontaneously succeeded or attempted to use the tool for retrieving the food.
In the second phase, we tried to facilitate the monkeys to individually learn tool use. The experimenter inserted the stick into the container filled with yogurt, and then monkeys were allowed to retrieve the tool and lick the food from it (10 sessions lasting 10 min, 10 trials per session). The intent underlying this procedure was that of prompting part of the motor sequences that the monkeys had to perform to accomplish the task (e.g. grasping the tool already inserted into the glass, bringing it to the mouth and eating the yogurt). Even though monkeys easily succeeded in retrieving the tool from the container and eating the yogurt in all trials, subsequently they did not show any attempt at spontaneous tool use. This rendered it necessary to introduce a shaping procedure. In the first part of this procedure, monkeys were first reinforced with food whenever they touched and grasped the tool. Then, they received food whenever they extended the arm while holding the tool and, finally, whenever they touched the container with it. This training procedure was employed twice a day for four consecutive weeks, until the monkeys successfully performed the correct action sequence with at least 90 per cent correct trials per session, for at least three consecutive sessions.
The training was recorded with a digital camcorder CANON MVX250i, and the video clips were subsequently analysed to evaluate the rate of success in the task.
(iii). Experimental task
Monkeys were tested in their home cage. In each session (5 min long), three wooden tools differing in shape (spoon, egg-shaped and stick; figure 1b), but not in texture and colour, were used. Each tool could be presented in one of two versions, either ‘functional’ or ‘non- functional’. The functional tools were 22 cm long and enabled the monkey to reach for the food, while the non-functional ones were only 11 cm long, thus not long enough to reach it. The three tools were simultaneously presented to the monkey on the same plywood table previously used during the training phase, in the following combinations:
— all three functional (3F);
— functional spoon (FS), non-functional stick and egg-shaped tool; and
— functional egg-shaped tool (FE), non-functional stick and spoon.
Each combination was presented three times, resulting in a total number of nine sessions for each animal. In each session, monkeys were free to interact with any of the available tools and try to use each of them for reaching the food. The order of presentation was as follows: 3F, FS, FE, FS, FE, 3F, FE, 3F, FS. There was no session in which the only functional tool was the stick, because this was the most familiar to the animal and could have biased its choice.
(iv). Behavioural analysis
All sessions were video recorded with a digital camcorder CANON MVX250i and the tapes independently analysed by two experimenters familiar with the experimental procedure. The frequency of interactions of the monkey with each of the available tools in each session was assessed. An interaction was defined as the grasping of a tool followed by the attempt to insert it into the container, regardless of the outcome of the attempt (the rate of success was always above 90%). For each session, we also scored which tool was the first contacted by the monkey, in order to verify whether its choice was based on an evaluation of the suitability of the physical features of the tool in relation to task requirements, or on a mere trial-and-error learning process.
(v). Statistical analysis
χ2-tests were applied to assess whether there was a preference for a specific type of tool during the 3F sessions. The same test was then employed in the sessions in which only one tool was functional (i.e. FS and FE) to assess whether the general choice frequency for the functional tool was higher than that expected based on chance. Furthermore, in these sessions, we also tested whether the frequency of choice of the functional tool as first was higher than chance.
(b). Results
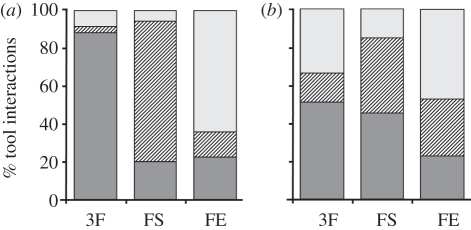
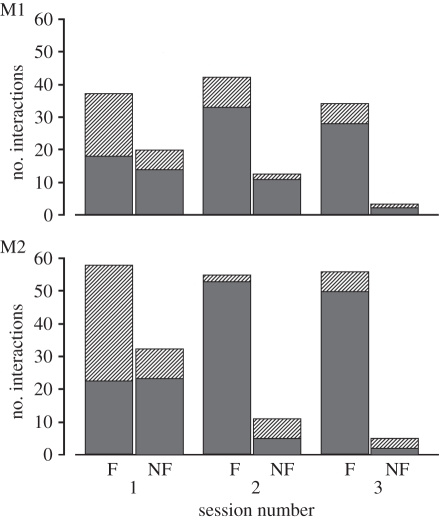
The results of study 1 are summarized in figure 2. In the sessions in which all tools were functional (i.e. 3F), both monkeys preferred the stick to retrieve food significantly above chance level (M1 χ2 = 218.49, p < 0.001 and M2 χ2 = 16.95, p < 0.001). During the sessions in which only one tool was functional (FS and FE), both monkeys more frequently used the appropriate tool (M1 χ2 = 115.996, p < 0.001 and M2 χ2 = 8.00, p < 0.005). More specifically, both M1 and M2 used the egg-shaped tool in the FE sessions (χ2 = 43.29, p < 0.001 and χ2 = 8.40, p < 0.005, respectively) more frequently than expected by chance. As far as the spoon is concerned, both monkeys chose it more frequently during FS sessions, although M1 did it at a higher frequency with respect to chance level (χ2 = 74.67, p < 0.001), while M2 did not reach a statistically significant level (χ2 = 0.76, n.s.).
Figure 2.
Percentage of tool interactions with each of the available tools. Each histogram represents the interactions during the sessions with all tool types: functional (3F), functional spoon (FS) and functional egg-shaped tool (FE) for (a) M1 and (b) M2. Light grey bars, egg-shaped; striped bars, spoon; dark grey bars, stick.
In the sessions in which only one tool was functional, the first-grasped tool was randomly chosen by both monkeys, contradicting the hypothesis that the functional tool was mostly preferred as a ‘first choice’ (M1 χ2 = 0.75, n.s. and M2 χ2 = 0.83, n.s.).
It is worth noting that, in all conditions, during the first interaction with an unfamiliar tool, the two monkeys never brought it to the mouth, but they used it to retrieve the food by applying the same motor patterns employed with the familiar tool. In only a few occasions, we did record exploratory behaviours consisting of tool manipulation without using it.
3. Study 2: generalization of tool-use strategy to different environmental contexts
If tool-use skill acquired in a certain situation is based on a causal understanding of the physical properties of the object used as a tool, this capacity should be easily transferred to contexts different from that in which learning occurred. This transfer, here referred to as generalization, may be expressed through newly adapted behavioural strategies with various levels of complexity, depending on the contextual demands. The aim of this study was to assess whether monkeys capable of using a stick to extract food from a container (study 1) could generalize the learned skills to a new contextual setting.
More specifically, a transparent cylindrical container was located inside the monkeys' home cage, firmly fixed to the cage bars. Monkeys could directly interact with the container, but the small aperture on the top of it and its elongated shape allowed them to retrieve the food only by inserting the stick into the container (figure 3). Therefore, when compared with study 1, monkeys could adopt a wider range of motor strategies to retrieve the food, but it was designed so that the previously learned one was not effective in this context. Furthermore, we verified whether the presence/absence of food inside the container, which represents the monkey's behavioural goal, could affect its attempts to reach for the food.
Figure 3.
Schematic set-up and apparatus employed in study 2.
(a). Material and methods
(i). Subjects
The experimental subjects were the same as in study 1.
(ii). Apparatus and procedures
The food (yogurt) was put into a cylindrical Plexiglas container (internal diameter 3 cm, height 18 cm) positioned inside the cage and firmly attached to the bars. At the beginning of each trial, the tool, a stick identical to that used during study 1, was placed on the floor of the cage. The monkey had to grasp the stick and to insert it completely inside the container to obtain the food.
The study was divided into four phases: Baseline, Familiarization/Facilitation, Practice and Food/No food test. During Baseline (six sessions, each 5 min long), we evaluated the frequency of interactions between the monkey's hand and the tool. Since none of the monkeys succeeded in getting food by using the tool, we introduced the Familiarization/Facilitation phase. This phase consisted of a single experimental session, lasting 40 min, during which the experimenter inserted the stick twice into the container, so that the monkey could simply retrieve the stick and lick the yogurt. We assessed the monkeys' behaviour scoring both their failed attempts and autonomously performed trials of successful tool use during the whole session. The Practice phase consisted of three sessions, each 10 min long, in which the monkey was given the tool to accomplish autonomously the same task. The fourth phase, Food/No food test, consisted of six sessions. In three of them, the container was filled with yogurt (Food sessions, F), while in the other three it was empty (No food sessions, NF). Before using it for NF sessions, the container was thoroughly washed to remove residual food or smells. Sessions F and NF were alternated and lasted 15 min each: during the first 5 min of each session, the monkeys were given the possibility to explore the container, filled or empty, allowing them to familiarize with the container in the absence of the tool. Subsequently, the experimenter introduced the tool in the cage and the monkey was allowed to use it for the next 10 min of the session.
(iii). Behavioural analysis
All the experimental phases were recorded with a digital camcorder CANON MVX250i and the videos were off-line independently analysed by two coders familiar with the experimental phases. The frequency of interactions of the monkeys with the tool was scored. Since it was difficult to systematically describe and categorize the numerous patterns of behaviour each monkey displayed with the tool, we limited our analysis to a few items relative to those events in which the monkey grasped the tool and directed it towards the aperture on top of the container, trying to insert it although unsuccessfully (‘attempt’). When an interaction ended with the tool completely inserted into the container in F sessions, enabling food retrieval, it was considered as ‘insertion’.
(iv). Statistical analysis
χ2-tests were used to compare the number of interactions with the tool and insertions during F and NF phases, against those expected by chance.
(b). Results
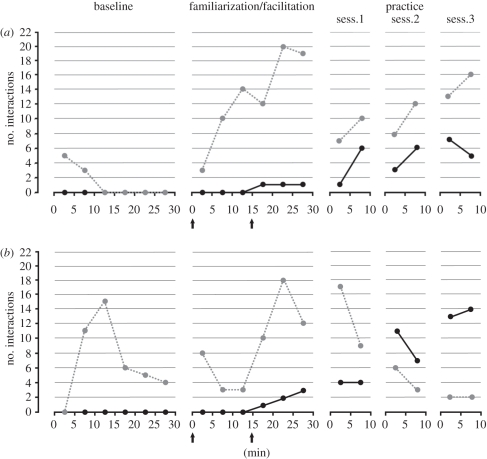
Figure 4 shows the time course of the frequency of interactions with the tool (in terms of insertion and attempt, separately) of both monkeys across subsequent sessions and phases. During the Baseline phase, none of the subjects succeeded in retrieving the food with the tool. From the beginning of this phase, both monkeys tried to directly access the food by biting the container or probing their fingers inside it. After a few minutes in which they failed to reach the food with this strategy, both of them grasped the stick and brought it in proximity to the container. More specifically, M2 made several attempts to insert the tool into the container, but always without success. The tool was sometimes handled by the monkey with its arms outside of the cage bars and lifted above the container, attempting to insert it. In other cases, the monkey manipulated the tool very vigorously with both hands in proximity to the container opening. Such episodes of tool manipulation were usually highly variable, jerky and poorly coordinated, so that in several cases, the tool fell on the floor. All of these behaviours decreased over time. M1 also approached the container with the stick and manipulated the tool in its proximity. However, when compared with M2, it performed only a few clear attempts of insertion, and its manipulations of the tool rapidly decreased over time.
Figure 4.
Number of tool insertions and insertion attempts performed by (a) M1 and (b) M2 during the Baseline, Familiarization/Facilitation and Practice phases. The arrows indicate the time points when the experimenter inserted the stick inside the container, providing the monkey with a cue about part of the motor sequence to be done. Grey circles with dashed line, attempt; black circles with continuous line, insertion.
During the Familiarization/Facilitation phase, following the first tool insertion by the experimenter, both monkeys rapidly increased their rate of attempts to use the tool for retrieving food (M1 χ2 = 49.44, p < 0.001; M2 χ2 = 6.00, p < 0.05). In the first minutes after stick extraction, both monkeys licked the tool and tried to lick the spilled drops of yogurt near the opening of the container or to probe with the fingers into the container to extract the left-over drops. These activities lasted a few minutes before monkeys started to use the tool again.
Interestingly, among other attempts, M1 tried on one occasion to replicate the exact motor pattern that was effective in study 1, namely, sitting in a cage sector far from the container and then extending the arm holding the stick, pointing with it and attending to the container. Furthermore, both monkeys started to show some successful insertions of the tool into the container. In particular, M1 succeeded in retrieving food autonomously for the first time after 30 insertion attempts, 20 min after the beginning of the Familiarization/Facilitation phase, while M2 succeeded for the first time after 15 insertion attempts, 20 min after the beginning of the same phase.
During the Practice phase, the monkeys' rate of success tended to increase across sessions, but the difference between the first and the last Practice session was significant only in M2 (M1 χ2 = 1.80, n.s.; M2 χ2 = 9.52, p < 0.01).
In the Food/No food phase (figure 5), the number of interactions with the tool, calculated by pooling insertions and attempts frequencies, was significantly higher in the F than in NF condition (M1 χ2 = 39.02, p < 0.001; M2 χ2 = 65.06, p < 0.001). Nevertheless, both monkeys also tried in a consistent number of cases to insert the stick into the container in the NF condition, succeeding on several occasions in reaching the bottom of the container with the tip of the stick. Interestingly, after tool insertion in the empty container, both monkeys often extracted the stick and brought it to the mouth, smelling and licking it, thus behaving similarly to in the F condition. However, the frequency of interactions with the tool (taking together insertions and attempts frequencies) remained similar across sessions in the F condition (M1 χ2 = 0.84, n.s.; M2 χ2 = 0.14, n.s.), while it decreased significantly across sessions in the NF condition (M1 χ2 = 12.17, p < 0.005; M2 χ2 = 25.12, p < 0.001).
Figure 5.
Number of tool insertions and attempts performed by M1 and M2 during the sessions in which the container was alternatively filled with yogurt (Food sessions, F) or empty (No Food sessions, NF). Striped bars, attempts; grey bars, insertions.
4. Study 3: effect of social facilitation on tool use
Previous studies have shown that macaques can sometimes benefit from observing the behaviour of skilled individuals for learning novel actions [6,31]. However, it is still unclear whether the observation of a skilled conspecific can facilitate tool-use learning in an observer monkey, and what information macaques can extract by observing other individuals using a tool [21].
The aim of the present study was to assess the behavioural responses of naive macaque monkeys after exposure to a trained conspecific using a rake for retrieving a piece of food out of arm's reach. We scored the observers' behaviour both after they were exposed to the performing model and during the model's performance.
For this study, we used a rake as tool because it requires minimal handling abilities and effort, and it has to be moved mainly in a two-dimensional space in order to achieve the goal (i.e. retrieving the food placed on the table in front of the monkey).
(a). Material and methods
(i). Subjects
The subjects were nine male Macaca mulatta, aged from 5 to 7 years. They were all naive to the use of tools. The two Macaca nemestrina employed in study 1 and study 2 acted as models. The procedures to train the model monkeys to use the rake were similar to those described by Iriki and co-workers [32,33].
The rearing and housing conditions of the animals were the same as described in study 1 and study 2. The use of animals of the same genus (Macaca), but of different species (M. mulatta) with respect to the model (M. nemestrina) as observers should not represent theoretical problems, given the similarity in the general body morphology as well as in the patterns of communicative and non-communicative behaviours shared by the two species [34]. Finally, our monkeys were very familiar with each other and the nature of the task did not require any species-specific behaviour.
(ii). Apparatus
The handle of the rake was a wooden stick of 1 cm diameter and 35 cm length, while the head was a wooden splint (12.5 × 4.5 cm), fixed to the handle in its centre.
The model and the observer were in different cages, one facing the other and separated by plywood working tables on which the tool and the food were placed. We used the same type of working table described in the first study. The experimenter placed small pieces of food (a piece of apple) on the table top by introducing them from the bottom of the table, unseen by the monkeys, through holes (diameter 1.5 cm each). This procedure was employed in order to prevent the monkeys from being distracted by the experimenter's action and to reduce interference with their behaviour. One hole was made on the side of the table where the demonstrator was located, 57 cm outside its cage, so that it could be reached only by using the rake. A second hole was made on the same side, but at 34 cm from the cage, so that the monkey could retrieve the food from it by hand. A third hole was made on the side of the table where the observer was located, 57 cm outside its cage.
(iii). Tasks and procedure
The experimental setting is shown in figure 6. Each trial started with the experimenter placing a piece of food on the table, on the side of the model monkey, through the hole located at 57 cm from the model's cage, so that the food was completely out of reach. Thus, the model monkey had to use the rake to reach the food and drag it along the table until it was graspable with the hand. In each phase, the model received a piece of apple every 15 s.
Figure 6.
Set-up and apparatus employed in study 3. The picture shows the model retrieving a small piece of food using the rake while the observer is looking at the action.
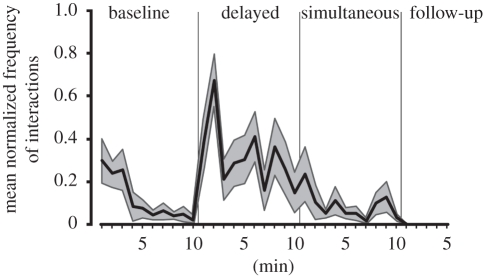
The experiment was divided into four phases: Baseline, Observation-Delayed tool interaction, Observation-Simultaneous tool interaction and Follow-up.
During Baseline (10 sessions, 10 min each), the model was given pieces of food by the experimenter through the closest hole present on its side of the table. While the model was engaged in grasping with the hand and eating the food, the observers were allowed to interact with the tool in order to retrieve a piece of food placed by the experimenter on the observer's side of the table, out of arm's reach.
The Observation-Delayed tool interaction phase consisted of 10 experimental sessions (25 min each). First, the model performed the correct food retrieving behaviour with the rake 40 times within a 10 min period (one trial every 15 s). Then, after a 5 min break, the observer was given a tool identical to that of the model for the following 10 min. The observer thus had the possibility to easily reach and grasp the rake in order to retrieve a piece of food placed on the table out of arm's reach. During this 10 min period, the model was given pieces of food by the experimenter through the hole closest to its cage, so that it could reach for and grasp it with the hand.
During Observation-Simultaneous tool interaction (10 sessions, 10 min each), the model and the observer were simultaneously provided with a rake and they could use it to reach a piece of food introduced by the experimenter every minute through the two farthest holes, on each side of the table.
During the Follow-up phase (five sessions, 10 min each), the model was not present and the observer monkeys alone could interact with their own rake for the duration of the whole experimental session. This phase was aimed at assessing whether the observation of a conspecific using a tool in the previous phases affected the number of tool interactions by the observers in the absence of any model.
(iv). Behavioural analysis
All sessions were video recorded with a digital camcorder CANON MVX250i and the tapes analysed independently by two experimenters not blind to the experimental phases. A third scorer blind to the experimental conditions analysed 20 per cent of the sessions, showing a high concordance with the scores attributed by non-blind experimenters (Kendall τ = 0.89, p < 0.001). The number of interactions of the monkeys' hands with the tool (touching or grasping) and the monkeys' attempts to use the tool for retrieving food were scored.
(v). Statistical analysis
The frequency of hand–tool interaction for each subject in each session has been normalized by dividing each value by the higher value recorded for that subject among the sessions of each specific phase. By this procedure, we obtained values ranging from 0 to 1, which allowed us to pool data of all tested subjects. A one-way repeated measure ANOVA, eventually followed by Newman–Keuls post hoc tests, was used in order to compare the number of hand–tool interactions of the observing monkeys among subsequent experimental phases.
(b). Results
None of the observing subjects tried to use the tool to retrieve food. However, the comparison of the number of hand–tool interactions revealed a significant difference among conditions (F = 15.109, p < 0.001). The post hoc test showed that only the interactions performed during the Observation-Delayed tool interaction were significantly more frequent with respect to those in all other experimental phases (p < 0.01; figure 7).
Figure 7.
Mean normalized frequency of hand–tool interactions of all the observing subjects during the Baseline, Observation-Delayed tool interaction, Observation-Simultaneous tool interaction and Follow-up phases. Grey shaded area represents 1 s.e.
5. Discussion
(a). Individual learning and understanding of the physical properties of a tool
The results of the first study demonstrate that monkeys in the current experimental setting were not capable of using a tool for retrieving food. Nevertheless, they could easily do so after a shaping procedure. Subsequently, they also showed the capacity to generalize the previously learned motor pattern to the use of unfamiliar tools. Moreover, the monkeys demonstrated they were capable of selecting a functional tool based on its length, despite its shape not being familiar to them. However, this capacity does not appear to be based on an understanding of the functional properties of the tool, but had to be achieved through a fast trial-and-error learning process.
Previous captive studies on macaques have reported that the spontaneous use of tools sometimes occurs [9,10], but the relatively natural setting of these experiments renders it difficult to identify which type of learning process leads to the result. Other studies showed that, under more controlled experimental conditions, macaques can learn to use tools without much practice or sensorimotor experience [14]. Surprisingly, some of the monkeys in this latter study succeeded in using the tool after only a few minutes of interaction with it, promoting the authors to interpret this finding as a sign of insightful behaviour. However, the macaques were partially restrained in a primate chair, so that the number of relevant stimuli with which they could interact was limited, thus increasing the possibility of interacting with the tool (a rake) that was presented on a table in front of them. It is, therefore, possible that the monkey succeeded not because of ‘insight’, but because this situation highly facilitated trial-and-error learning. Our data indicate that in the absence of any physical restraint and with a relatively easy sensorimotor task, the monkeys did not learn the use of the tool and could not accidentally obtain the food, a key factor in producing associative learning through operant conditioning.
After the shaping procedure, the monkeys became capable of using the stick. However, when presented with tools of different shapes (irrelevant feature for task accomplishment) and different length (relevant feature), both monkeys did not select the tool of adequate length for retrieving food in the very first trials. Instead, they first randomly chose a tool, grasped it and tried to use it for retrieving food. If the first attempt succeeded, then they continued to use that tool more frequently, otherwise they selected by chance another one. Despite the random choice observed in the first trial, overall the monkeys demonstrated a more frequent use of the functional tool. These results might, therefore, reflect a very rapid trial-and-error learning process.
In the condition in which all three tools were functional, monkeys (especially M1) tended to choose more frequently the stick which they had previously learned to use. It is possible that, in this condition, the familiarity with the stick based on previous sensorimotor experience facilitated its use, in line with previous data [14]. This issue could have been solved by presenting the monkey with three completely new tools, in order to verify its capacity for actively selecting the functional one regardless of its similarity to previously employed tools. Some observations made before the formal experiment on M1, after it learned to use the stick, showed that when the monkey was presented with only one unfamiliar tool, it demonstrated the capacity to generalize the use of the tool to the new objects despite their different and novel shapes. This supports the idea that monkeys possess a certain capacity to select objects as tools based on their general physical properties and regardless of their familiarity. Future experimental studies should investigate specifically the effect of familiarity on the capacity of tool selection.
From the motor point of view, the fact that the monkeys applied the same pattern of arm extension and tool insertion into the container when using the unfamiliar tools suggests that, despite the fact that they were not capable of identifying the correct physical properties which differentiate functional from non-functional tools, they could generalize the tool-use strategy previously learnt with one object to new objects, differing in shape, size and weight. When facing novel situations, they seem capable of rapidly learning these new associations.
Which factors might contribute to such a generalization process? Study 2 was aimed at investigating this issue in more depth.
(b). Are generalization capacities of tool use based on a comprehension of the means-ends relationship?
The two macaque monkeys employed in study 1 were presented with a new contextual situation, requiring them to use the stick with a completely new motor strategy.
At the beginning of the Baseline phase, both monkeys approached the container and attempted to extract the food by inserting their fingers or by biting it. Subsequently, they also made several attempts to retrieve food with the tool. Although M1 made fewer attempts than M2, it still used the stick and interacted with it in proximity to the container. Furthermore, both monkeys persisted in performing these manipulative behaviours for a long time. These observations induce us to interpret the monkeys manipulative behaviours as rudimentary attempts to use the tool, thus suggesting that they did know what to do, but not how to do it. Together, these findings also indicate that both monkeys ‘conceived’ the tool as a means to achieve the goal and that, once they had retrieved their neural motor representation for tool use, they tried to adjust it to a new context.
The difficulty observed in accomplishing the task with new motor strategies could be due to the previous sensorimotor practice and prolonged training which they experienced during study 1. This long-lasting training, in which the same tool-use behaviour was repeated in several sessions, could have resulted in reinforcing the link between the behavioural patterns used by the monkey and the type of target stimulus, thus favouring motor stereotypy. In support of this view, the results of study 2 showed that one of the two monkeys (M1), in the very first stages of the experiment, attempted to insert the tool into the container by applying the same motor patterns (arm extension and wrist rotation/flexion) learnt during study 1.
During the Familiarization/Facilitation phase, after the tool was inserted by the experimenter into the container, both monkeys persistently attempted to insert the tool into the container in spite of their repeated failures. These repeated attempts could be based on a primitive and associative form of representation of means-ends relationship. Thus, after having extracted the tool with the food at the beginning of this phase, the monkeys could have transferred to the new contextual situation the link between the tool and the consequence of its use in the presence of a container with food. The final posture of the forelimb before the stick extraction movement could have facilitated the creation of a new motor pattern linking the forelimb sensorimotor representation occurring during the attempt, with the posture taken during grasping of the inserted tool. Moreover, the fact that this posture and tool extraction was followed by a reward, very likely increased the number of attempts and the probability to interact successfully with the container. In agreement with our interpretation, a study on free-ranging Japanese macaques showed that some individuals started to insert a stick into a pipe in order to retrieve the food trapped inside it after they were trained to pull the tool that was previously inserted by the experimenter [6]. Although, in that study, there was no description of the attempts and of the motor patterns used by the monkeys, factors similar to those described in the present study might have played a role.
The number of successful insertions rapidly increased within three sessions following the Familiarization/Facilitation phase. Although the monkeys had a limited time of exposure to the task, it is likely that the high success rate they obtained in the following practice phase depended, at least in part, on the time spent in manipulating the tool in proximity of the container in this phase.
Once the monkeys had learned how to perform the task, we directly assessed whether their behaviour was actually guided by a representation of the behavioural goal. In the Food/No Food test, we verified that monkeys rigidly applied the newly acquired motor strategy of tool use regardless of whether the food was present or not in the container. Probably, a further trial-and-error learning process enabled them to extinguish tool-use behaviour when the action did not lead to the reward. This lack of behavioural flexibility, as also evidenced in the first part of study 2, could be due to the high frequency of repetition of the previously learned behaviour, and can also be well framed within a neurophysiological perspective.
It has been demonstrated that neurons in areas of the monkey parietal and premotor cortices undergo changes as a consequence of tool use [16,32,33]. In one study, in which monkeys were trained to use different tools to grasp food morsels, it has been found that motor neurons of the ventral premotor cortex, normally active during hand grasping, also fired when the monkeys grasped the food with the tools, regardless of the exact movement sequence required for the purpose [16]. This finding clearly exemplifies that hand movements and associated goals are tightly linked in a unitary representation at the single neuron level, that is referred to as a ‘motor act’ [35]. As a consequence, the monkeys in our study very likely have motor representations in which goals and means are tightly linked to each other, and therefore cannot be processed independently. This could justify why the apparent monkeys' knowledge of the motor goal cannot be flexibly used to adapt their motor pattern to a novel context and to take into account the absence/presence of reward. In another study [32], authors investigated the properties of bimodal (somatosensory and visual) neurons of the parietal cortex in monkeys trained to use a rake to retrieve food out of reach. The results showed that the use of tools modified the size of the peripersonal receptive fields of the studied bimodal neurons and their modifications depended on whether the monkey actively used the tool. Altogether, these and other findings [17] suggest that the use of tools modifies the body schema and generates new motor representations, as if the tool becomes part of the body. Although the creation of new motor representations is indicative of brain plasticity, these representations tend to be rigidly used in strict relation to the tool-use behaviour in which they have been created.
(c). Social learning processes of tool use: from behavioural data to possible neurophysiological mechanisms
There is no clear consensus in the literature about the social learning abilities of macaques. While some studies showed that after a relatively long exposure to a demonstrator using a tool other individuals rarely acquire the new behaviour [8,10,11,36], several other studies have reported the social transmission and maintenance of novel behaviours in macaques [7,37], a phenomenon that is considered very important in creating new cultural achievements [38,39], especially where behaviours relative to food habits are concerned [40].
In the present study, we explored this issue in adult rhesus macaques with the aim of clarifying the social factors and cognitive processes possibly underlying the learning of tool use in this species. The main evidence provided by our study contrasts with the idea that macaques can learn tool use through a rapid observational learning process. Although during the Observation-Delayed tool interaction phase observers increased the frequency of tool manipulation, they never attempted to use the tool.
Beyond the possible cognitive limitations affecting the monkeys' capability to learn by observation from conspecifics, other factors may further contribute in explaining such failure. Among these factors, social tolerance is crucial since it allows individuals to observe others in close proximity and to directly participate in their activities. Rhesus macaques, instead, are known to be socially intolerant, especially during food processing [41]. Although they gather together while foraging, they do not tolerate the close proximity of other individuals, members of the same troop. Our data seem to support an impact of social intolerance on observational learning. In fact, when observers could use the tool simultaneously with the model, they sometimes seemed to be inhibited by the model. This behavioural inhibition could reflect either a species-specific trait or the lack of social relations between the model and observer. We also cannot exclude the occurrence of behavioural extinction since, in the last few sessions of the Observation-Delayed tool interaction period, observers decreased their hand interactions with the tool.
Finally, another factor that could have contributed to the poor social learning abilities showed by the monkeys in the present study is the perspective from which they viewed the demonstrator using the tool, that is, a third-person perspective. To observe an action in a third-person rather than a first-person perspective implies additional cognitive operations such as mental rotation and transformation of the perceptual appearance of the observed action into a correspondent motor plan. Interestingly, it has been recently demonstrated that observing an action from first- or third-person perspective can activate different subpopulations of mirror neurons in the ventral premotor cortex [42]. Furthermore, other neurophysiological studies showed that when two monkeys sitting near each other are allowed to interact in a competitive situation, parietal neurons present complex combinatorial responses to ‘of self’ and ‘other's’ motion [43]. It is, therefore, possible that these neural mechanisms enable the monkeys to exploit the sight of other's action from several perspectives in order to better organize an appropriate response.
The behavioural and cognitive processes responsible for social learning in macaques have for long been at the centre of debate. Complex phenomena such as some form of imitation have been shown in macaques and other monkeys [44,45], and some authors argued that similar mechanisms may underlie tool-use learning in capuchin monkeys and chimpanzees [46]. Conversely, others have proposed that mechanisms different from imitation, such as stimulus or social enhancement, play a major role in promoting the individual discovery of how to use a tool [21,23].
In the current study, although monkeys did not learn by observation the use of the tool, they were clearly facilitated in interacting with it by observing the model's action. This effect is quite robust, but in the absence of any apparent reward deriving from the manipulative activity, the interaction with the tool decreases after a few sessions. Despite the social enhancement of manipulative behaviours with the tool during the Observation-Delayed tool interaction, naive observers never attempted to use it. From the observer's perspective, the model's activities with the tool might have enhanced the salience of the object, thus affecting its visual attention and interest towards it. However, considering the time lag between the observation and the execution phase, this explanation seems unlikely. Despite not being mutually exclusive with the stimulus-enhancement-hypothesis, it is also possible that the observation of grasping actions per se exerts facilitating effects on the observers' behaviour. For example, previous studies have demonstrated that in macaques and capuchin monkeys, the observation of and the listening to eating actions facilitate the performance of the same actions [47,48].
The presence of mirror neurons in the monkey motor cortex has prompted the idea that monkeys, as well as humans, understand the goal of an observed action by mapping its visual description onto the corresponding cortical motor representation [49]. According to this view, motor representation in the observer's brain enables him/her to directly understand the behavioural goal of the acting agent.
In the present experiment, it is very likely that observer monkeys could understand the goal of the model's action when it grasped the tool and when it licked it to eat the yogurt. All these actions were familiar to the observers and were part of their motor repertoire. Do they also have an understanding of the action made with the tool? Clearly, when monkeys are exposed to the observation of an action which they master because of a prolonged sensorimotor training, mirror neurons in their premotor cortex fire during observation of actions performed with the tool [50]. More interestingly, after prolonged visual exposure to an action performed by an experimenter using a tool, some premotor mirror neurons have been shown to respond specifically to the observation of tool actions [47]. In this latter study, however, when tested in their home cage, monkeys were not capable of using the same tool to which they were previously visually exposed. This is in line with the present findings showing an increased interaction of the observed monkey with the tool in the Observation-Delayed tool interaction phase. However, the lack of evidence of tool use indicates that monkeys do not have the ability to translate the visual description of the observed unfamiliar action into the motor programmes necessary for copying its behavioural goal.
6. Conclusions and evolutionary remarks
The literature on tool use shows that there is a discontinuity among different primate species, such as apes, capuchin monkeys and macaques. Macaques appear not to be proficient tool users, as testified by the very few reports on this topic in the wild [7]. Nevertheless, there is consistent evidence that, in this genus, the plasticity of the motor system is such to include tools as part of its ‘vocabulary’ of motor representations. In fact, not only can macaques be trained to use a variety of tools, but their use can be partially generalized to other objects and contexts. Interestingly, Iriki and co-workers [33,51] showed direct evidences of tool use-induced anatomical modifications in the temporal and parietal cortices, and the development of new cortico-cortical connections. Furthermore, this plasticity process appears to involve regions that are crucial for hand grasping [52]. In an evolutionary perspective, it is possible that cortical areas more susceptible to modifications as a result of tool use became more specialized for this function and separated from those just involved in sensorimotor transformation for hand grasping [53,54], supporting the idea that the use of tools required brain changes that determined the appearance of a new network.
An important capacity underlying tool use is that of combining single motor acts in action sequences. The construction of complex action sequences implies neural structures capable of dealing with and integrating spatial and temporal features. For example, several studies reported the use of hammers and anvils in order to crack nuts in capuchin monkeys [55,56] and chimpanzees [57,58]. These activities may require complex behavioural patterns, such as the selection of an efficient hammer, its transportation to the location of the anvil, and the choice of the most appropriate motor pattern (trajectory, force, etc.). Thus, the brain of these primates is involved in several cognitive operations: individuation of a final goal, planning of the whole motor sequence necessary to reach this goal, a mental representation of the goal even in the actual absence of the sensory elements that drive the final part of the action sequence. This mental representation involves the capacity to travel in both space and time. Neurophysiological investigations have demonstrated the presence of circuits involved in motor planning and organization of sequential actions [59–61].
The issue of sequential organization of behaviour extends beyond the use of tools and embraces several other domains and, among them, speech. In fact, in speech, sequential organization is very important both in phonological articulation and for building a syntactic structure. Although the neural mechanisms underlying these processes can be the subject of investigation only in humans, many anatomical and functional data suggest that the neural substrates of sequential organization in non-human primates have provided the raw material for extending the properties of the cortical motor system to the domain of articulatory speech [62–65].
From the anatomical point of view, the rostral part of the macaque ventral premotor cortex has been considered, on the basis of anatomical location and cytoarchitectonic properties, as homologous to part of the human Broca's area. Functionally, neuroimaging studies in humans demonstrated that this latter area activates not only during speech production, but also during execution and observation of mouth and hand motor acts [63]. The same motor areas (ventral premotor cortex and posterior part of inferior frontal gyrus) involved in speech production and hand/mouth action organization seem to play an important role in tool use [66–68]. This brain regional overlap suggests that a basic organization of the motor system for hand and mouth actions has been exploited for the emergence of new functions that nonetheless rely, at least in part, on the same mechanisms.
Acknowledgements
All experimental protocols complied with the European law on the humane care and use of laboratory animals and were approved by the Veterinarian Animal Care and Use Committee of the University of Parma, as well as by the Italian Ministry of Health.
We thank Valentina Sclafani for helping us in data analysis. This work has been supported by 029065 FP6 European grant Hand to Mouth.
References
- 1.Ottoni E. B., Izar P. 2008. Capuchin monkey tool use: overview and implications. Evol. Anthropol. 17, 171–178 10.1002/evan.20185 (doi:10.1002/evan.20185) [DOI] [Google Scholar]
- 2.Visalberghi E., Addessi E., Truppa V., Spagnoletti N., Ottoni E., Izar P., Fragaszy D. 2009. Selection of effective stone tools by wild bearded capuchin monkeys. Curr. Biol. 19, 213–217 10.1016/j.cub.2008.11.064 (doi:10.1016/j.cub.2008.11.064) [DOI] [PubMed] [Google Scholar]
- 3.McGrew W. C. 1992. Chimpanzee material culture: implications for human evolution. Cambridge, UK: Cambridge University Press [Google Scholar]
- 4.Fragaszy D., Izar P., Visalberghi E., Ottoni E. B., De Oliveira M. G. 2004. Wild capuchin monkeys (Cebus libidinosus) use anvils and stone pounding tools. Am. J. Primatol. 64, 359–366 10.1002/ajp.20085 (doi:10.1002/ajp.20085) [DOI] [PubMed] [Google Scholar]
- 5.Boesch C., Head J., Robbins M. 2009. Complex tool sets for honey extraction among chimpanzees in Loango National Park, Gabon. J. Hum. Evol. 56, 560–569 10.1016/j.jhevol.2009.04.001 (doi:10.1016/j.jhevol.2009.04.001) [DOI] [PubMed] [Google Scholar]
- 6.Tokida E., Tanaka I., Takefushi H., Hagiwara T. 1994. Tool-use in Japanese macaques: use of stones to obtain fruit from a pipe. Anim. Behav. 47, 1023–1030 10.1006/anbe.1994.1140 (doi:10.1006/anbe.1994.1140) [DOI] [Google Scholar]
- 7.Gumert M. D., Kluck M., Malaivijitnond S. 2009. The physical characteristics and usage patterns of stone axe and pounding hammers used by long-tailed macaques in the Andaman Sea region of Thailand. Am. J. Primatol. 71, 594–608 10.1002/ajp.20694 (doi:10.1002/ajp.20694) [DOI] [PubMed] [Google Scholar]
- 8.Beck B. B. 1976. Tool use in captive pigtailed macaques. Primates 17, 301–310 10.1007/BF02382787 (doi:10.1007/BF02382787) [DOI] [Google Scholar]
- 9.Westergaard G. C. 1988. Lion-tailed macaques (Macaca silenus) manufacture and use tools. J. Comp. Psychol. 102, 152–159 10.1037/0735-7036.102.2.152 (doi:10.1037/0735-7036.102.2.152) [DOI] [PubMed] [Google Scholar]
- 10.Zuberbühler K., Gygax L., Harley N., Kummer H. 1996. Stimulus enhancement and spread of spontaneous tool use in a colony of long-tailed macaques. Primates 37, 1–12 10.1007/BF02382915 (doi:10.1007/BF02382915) [DOI] [Google Scholar]
- 11.Ducoing A. M., Thierry B. 2005. Tool use in Tonkeana macaques (Macaca tonkeana). Anim. Cogn. 8, 103–113 10.1007/s10071-004-0240-0 (doi:10.1007/s10071-004-0240-0) [DOI] [PubMed] [Google Scholar]
- 12.Leca J. B., Gunst N. A., Watanabe K., Huffman M. A. 2007. A new case of fish-eating in Japanese macaques: implications for social constraints on the diffusion of feeding innovation. Am. J. Primatol. 69, 821–828 10.1002/ajp.20401 (doi:10.1002/ajp.20401) [DOI] [PubMed] [Google Scholar]
- 13.Leca J. B., Gunst N. A., Huffman M. A. 2010. The first case of dental flossing by a Japanese macaque (Macaca fuscata): implications for the determinants of behavioral innovation and the constraints on social transmission. Primates 51, 13–22 10.1007/s10329-009-0159-9 (doi:10.1007/s10329-009-0159-9) [DOI] [PubMed] [Google Scholar]
- 14.Ishibashi H., Hihara S., Iriki A. 2000. Acquisition and development of monkey tool-use: behavioral and kinematic analyses. Can. J. Physiol. Pharmacol. 78, 958–966 10.1139/y00-063 (doi:10.1139/y00-063) [DOI] [PubMed] [Google Scholar]
- 15.Hihara S., Obajashi S., Tanaka M., Iriki A. 2003. Rapid learning of sequential tool use by macaque monkey. Physiol. Behav. 78, 427–434 10.1016/S0031-9384(02)01006-5 (doi:10.1016/S0031-9384(02)01006-5) [DOI] [PubMed] [Google Scholar]
- 16.Umiltà M. A., Escola L., Intskirveli I., Grammont F., Rochat M., Caruana F., Jezzini A., Gallese V., Rizzolatti G. 2008. When pliers become fingers in the monkey motor system. Proc. Natl Acad. Sci. USA 105, 2209–2213 10.1073/pnas.0705985105 (doi:10.1073/pnas.0705985105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maravita A., Iriki A. 2004. Tools for the body (schema). Trends Cogn. Sci. 8, 79–86 10.1016/j.tics.2003.12.008 (doi:10.1016/j.tics.2003.12.008) [DOI] [PubMed] [Google Scholar]
- 18.Santos L. R., Miller C., Hauser M. D. 2003. Representing tools: how two non human primate species distinguish between the functionally relevant and irrelevant features of a tool. Anim. Cogn. 6, 269–281 10.1007/s10071-003-0171-1 (doi:10.1007/s10071-003-0171-1) [DOI] [PubMed] [Google Scholar]
- 19.Hauser M. D. 1997. Artifactual kinds and functional design features: what a primate understands without language. Cognition 64, 285–308 10.1016/S0010-0277(97)00028-0 (doi:10.1016/S0010-0277(97)00028-0) [DOI] [PubMed] [Google Scholar]
- 20.Visalberghi E., Limongelli L. 1994. Lack of comprehension of cause-effect relations in tool-using capuchin monkeys (Cebus apella). J. Comp. Psychol. 108, 15–22 10.1037/0735-7036.108.1.15 (doi:10.1037/0735-7036.108.1.15) [DOI] [PubMed] [Google Scholar]
- 21.Fragaszy D., Visalberghi E. 2004. Socially-biased learning in monkeys. Anim. Learn. Behav. 32, 24–35 10.3758/BF03196004 (doi:10.3758/BF03196004) [DOI] [PubMed] [Google Scholar]
- 22.Nahallage C. A. D., Huffman M. A. 2007. Acquisition and development of stone handling behavior in infant Japanese macaques. Behaviour 144, 1193–1215 10.1163/156853907781890959 (doi:10.1163/156853907781890959) [DOI] [Google Scholar]
- 23.Coussi-Korbel S., Fragaszy D. M. 1995. On the relationship between social dynamics and social learning. Anim. Behav. 50, 1441–1453 10.1016/0003-3472(95)80001-8 (doi:10.1016/0003-3472(95)80001-8) [DOI] [Google Scholar]
- 24.Whiten A. 2000. Primate culture and social learning. Cogn. Sci. 24, 477–508 10.1207/s15516709cog2403_6 (doi:10.1207/s15516709cog2403_6) [DOI] [Google Scholar]
- 25.van Schaik C. P. 2003. Local traditions in orangutans and chimpanzees: social learning and social tolerance. In The biology of traditions: models and evidence (eds Fragaszy D., Perry S.), pp. 159–186 Cambridge, UK: Cambridge University Press [Google Scholar]
- 26.van Schaik C. P., Fox E. A., Fechtman L. 2003. Individual variation in the rate of use of tree-hole tools among wild orang-utans: implications for hominin evolution. J. Hum. Evol. 44, 11–23 10.1016/S0047-2484(02)00164-1 (doi:10.1016/S0047-2484(02)00164-1) [DOI] [PubMed] [Google Scholar]
- 27.Perry S., Manson J. H. 2003. Traditions in monkeys. Evol. Anthropol. 12, 71–81 10.1002/evan.10105 (doi:10.1002/evan.10105) [DOI] [Google Scholar]
- 28.Lonsdorf E. 2006. What is the role of mother in the acquisition of termite-fishing behaviours in the wild chimpanzees (Pan troglodytes schweinfurthii)? Anim. Cogn. 9, 36–46 10.1007/s10071-005-0002-7 (doi:10.1007/s10071-005-0002-7) [DOI] [PubMed] [Google Scholar]
- 29.Moscovice L. R., Snowdon C. T. 2006. The role of social context and individual experience in novel task acquisition in cotton-top tamarins (Saguinus oedipus). Anim. Behav. 7, 933–943 10.1016/j.anbehav.2005.09.007 (doi:10.1016/j.anbehav.2005.09.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Range F., Huber L. 2007. Attention of common marmosets: implications for social learning experiments. Anim. Behav. 73, 1033–1041 10.1016/j.anbehav.2006.07.015 (doi:10.1016/j.anbehav.2006.07.015) [DOI] [Google Scholar]
- 31.Hirata S., Watanabe K., Kawai M. 2001. Sweet potato washing revisited. In Primate origins of human cognition and behavior (ed. Matsuzawa T.), pp. 487–508 Tokyo, Japan: Springer [Google Scholar]
- 32.Iriki A., Tanaka M., Iwamura Y. 1996. Coding of modified body schema during tool use by macaque postcentral neurones. Neuroreport 14, 2325–2330 [DOI] [PubMed] [Google Scholar]
- 33.Hihara S., Notoya T., Tanaka M., Ichinose S., Ojima H., Obayashi S., Fujii N., Iriki A. 2006. Extension of corticocortical afferents into the anterior bank of the intraparietal sulcus by tool-use training in adult monkeys. Neuropsychologia 44, 2636–2646 10.1016/j.neuropsychologia.2005.11.020 (doi:10.1016/j.neuropsychologia.2005.11.020) [DOI] [PubMed] [Google Scholar]
- 34.Van Hooff J. A. R. A. M. 1967. The facial displays of Catarrhine monkeys and apes. In Primate Ethology (ed. Morris D.), pp. 7–68 London, UK: Weidenfeld & Nicholson [Google Scholar]
- 35.Rizzolatti G., Camarda R., Fogassi L., Gentilucci M., Luppino G., Matelli M. 1988. Functional organization of inferior area 6 in the macaque monkey. Exp. Brain Res. 71, 491–507 10.1007/BF00248742 (doi:10.1007/BF00248742) [DOI] [PubMed] [Google Scholar]
- 36.Chauvin C., Berman C. M. 2004. Intergenerational transmission of behavior. In Macaque societies: a model for the study of social organization. (eds Thierry B., Singh M., Kaumanns W.), pp. 209–234 Cambridge, UK: Cambridge University Press [Google Scholar]
- 37.Leca J., Gunst N., Huffman M. 2010. Indirect social influence in the maintenance of the stone-handling tradition in Japanese macaques, Macaca fuscata. Anim. Behav. 79, 117–126 10.1016/j.anbehav.2009.09.035 (doi:10.1016/j.anbehav.2009.09.035) [DOI] [Google Scholar]
- 38.Itani J., Nishimura A. 1973. The study of intrahuman culture in Japan. A review. In Precultural primate behavior (ed. Menzel E. V.), pp. 26–55 Basel, Switzerland: Karger [Google Scholar]
- 39.Huffman M., Nahallage C. A. D., Leca J. 2008. Cultured monkeys. Social learning cast in stones. Curr. Dir. Psychol. Sci. 17, 410–414 10.1111/j.1467-8721.2008.00616.x (doi:10.1111/j.1467-8721.2008.00616.x) [DOI] [Google Scholar]
- 40.Huffman M. A. 1996. Acquisition of innovative cultural behaviours in nonhuman primates: a case study of SH, a socially transmitted behavior in Japanese macaques. In Social learning in animals: roots of culture (eds Galef B. G., Jr, Heyes C.), pp. 267–289 San Diego, CA: Academic Press [Google Scholar]
- 41.Thierry B., Iwaniuk A. N., Pellis S. M. 2000. The influence of phylogeny on the social behaviour of macaques (Primates: Cercopithecidae, genus Macaca). Ethology 106, 713–728 10.1046/j.1439-0310.2000.00583.x (doi:10.1046/j.1439-0310.2000.00583.x) [DOI] [Google Scholar]
- 42.Caggiano V., Fogassi L., Rizzolatti G., Pomper J. K., Thier P., Giese M. A., Casile A. 2011. View-based encoding of actions in mirror neurons of area f5 in macaque premotor cortex. Curr. Biol. 21, 144–148 10.1016/j.cub.2010.12.022 (doi:10.1016/j.cub.2010.12.022) [DOI] [PubMed] [Google Scholar]
- 43.Fujii N., Hihara S., Iriki A. 2007. Dynamic social adaptation of motion-related neurons in primate parietal cortex. PLoS ONE 2, 1–8 10.1371/journal.pone.0000397 (doi:10.1371/journal.pone.0000397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferrari P. F., Visalberghi E., Paukner A., Fogassi L., Ruggero A., Suomi S. J. 2006. Neonatal imitation in rhesus macaques. PLoS Biol. 4, 1501–1508 10.1371/journal.pbio.0040302 (doi:10.1371/journal.pbio.0040302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bugnyar T., Huber L. 2000. True imitation in marmosets. Anim. Behav. 60, 195–202 10.1006/anbe.2000.1457 (doi:10.1006/anbe.2000.1457) [DOI] [PubMed] [Google Scholar]
- 46.Whiten A., McGuigan N., Marshall-Pescini S., Hopper L. M. 2009. Emulation, imitation over-imitation, the scope of culture for child and chimpanzee. Phil. Trans. R. Soc. B 364, 2417–2428 10.1098/rstb.2009.0069 (doi:10.1098/rstb.2009.0069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferrari P. F., Maiolini C., Addessi E., Fogassi L., Visalberghi E. 2005. The observation and hearing of eating actions activates motor programs related to eating in macaque monkeys. Behav. Brain Res. 161, 95–101 10.1016/j.bbr.2005.01.009 (doi:10.1016/j.bbr.2005.01.009) [DOI] [PubMed] [Google Scholar]
- 48.Galloway A. T., Addessi E., Fragaszy D. M., Visalberghi E. 2005. Social facilitation of eating familiar food in tufted capuchins (Cebus apella): does it involve behavioral coordination? Int. J. Primatol. 26, 181–189 10.1007/s10764-005-0729-7 (doi:10.1007/s10764-005-0729-7) [DOI] [Google Scholar]
- 49.Gallese V., Fadiga L., Fogassi L., Rizzolatti G. 1996. Action recognition in the premotor cortex. Brain 119, 593–609 10.1093/brain/119.2.593 (doi:10.1093/brain/119.2.593) [DOI] [PubMed] [Google Scholar]
- 50.Rochat M., Caruana F., Jezzini A., Escola L., Intskirveli I., Grammont F., Gallese V., Rizzolatti G., Umiltà M. A. 2010. Responses of mirror neurons in area F5 to hand and tool grasping observation. Exp. Brain Res. 204, 605–616 10.1007/s00221-010-2329-9 (doi:10.1007/s00221-010-2329-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quallo M. M., Price C. J., Ueno K., Asamizuya T., Cheng K., Lemon R. N., Iriki A. 2009. Gray and white matter changes associated with tool-use learning in macaque monkeys. Proc. Natl Acad. Sci. USA 106, 18 379–18 384 10.1073/pnas.0909751106 (doi:10.1073/pnas.0909751106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Obayashi S., Suhara T., Kawabe K., Okauchi T., Maeda J., Akine Y., Onoe H., Iriki A. 2001. Functional brain mapping of monkey tool use. Neuroimage 14, 853–861 10.1006/nimg.2001.0878 (doi:10.1006/nimg.2001.0878) [DOI] [PubMed] [Google Scholar]
- 53.Peeters R., Simone L., Nelissen K., Fabbri-Destro M., Vanduffel W., Rizzolatti G., Orban G. A. 2009. The representation of tool use in humans and monkeys: common and uniquely human features. J. Neurosci. 29, 11 523–11 539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson-Frey S. H. 2004. The neural bases of complex tool use in humans. Trends Cogn. Sci. 8, 71–78 10.1016/j.tics.2003.12.002 (doi:10.1016/j.tics.2003.12.002) [DOI] [PubMed] [Google Scholar]
- 55.Visalberghi E., Spagnoletti N., Ramos da Silva E. D., Andrade F. R., Ottoni E., Izar P., Fragaszy D. 2009. Distribution of potential suitable hammers and transport of hammer tools and nuts by wild capuchin monkeys. Primates 50, 95–104 10.1007/s10329-008-0127-9 (doi:10.1007/s10329-008-0127-9) [DOI] [PubMed] [Google Scholar]
- 56.de Resende B. D., Ottoni E. B., Fragaszy D. M. 2008. Ontogeny of manipulative behavior and nut-cracking in young tufted capuchin monkeys (Cebus apella): a perception-action perspective. Dev. Sci. 11, 82–400 10.1111/j.1467-7687.2008.00731.x (doi:10.1111/j.1467-7687.2008.00731.x) [DOI] [PubMed] [Google Scholar]
- 57.Inoue-Nakamura N., Matsuzawa T. 1997. Development of stone tool use by wild chimpanzees (Pan troglodytes). J. Comp. Psychol. 111, 159–173 10.1037/0735-7036.111.2.159 (doi:10.1037/0735-7036.111.2.159) [DOI] [PubMed] [Google Scholar]
- 58.McGrew W. C. 2010. In search of the last common ancestor: new findings on wild chimpanzees. Phil. Trans. R. Soc. B 27, 3267–3276 10.1098/rstb.2010.0067 (doi:10.1098/rstb.2010.0067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tanji J., Hoshi E. 2008. Role of the lateral prefrontal cortex in executive behavioral control. Physiol. Rev. 88, 37–57 10.1152/physrev.00014.2007 (doi:10.1152/physrev.00014.2007) [DOI] [PubMed] [Google Scholar]
- 60.Rozzi S., Ferrari P. F., Bonini L., Rizzolatti G., Fogassi L. 2008. Functional organization of inferior parietal lobule convexity in the macaque monkey: electrophysiological characterization of motor, sensory and mirror responses and their correlation with cytoarchitectony areas. Eur. J. Neurosci. 28, 1569–1588 10.1111/j.1460-9568.2008.06395.x (doi:10.1111/j.1460-9568.2008.06395.x) [DOI] [PubMed] [Google Scholar]
- 61.Fogassi L., Ferrrai P. F., Gesierich B., Rozzi S., Chersi F., Rizzolatti G. 2005. Parietal lobe: from action organization to intention understanding. Science 308, 662–667 10.1126/science.1106138 (doi:10.1126/science.1106138) [DOI] [PubMed] [Google Scholar]
- 62.Rizzolatti G., Arbib M. G. 1998. Language within our grasp. Trends Neurosci. 21, 188–194 10.1016/S0166-2236(98)01260-0 (doi:10.1016/S0166-2236(98)01260-0) [DOI] [PubMed] [Google Scholar]
- 63.Fogassi L., Ferrari P. F. 2007. Mirror neurons and the evolution of embodied language. Curr. Dir. Psychol. Sci. 16, 136–141 10.1111/j.1467-8721.2007.00491.x (doi:10.1111/j.1467-8721.2007.00491.x) [DOI] [Google Scholar]
- 64.Gentilucci M., Corballis M. C. 2006. From manual gesture to speech: a gradual transition. Biobehav. Rev. 30, 949–960 10.1016/j.neubiorev.2006.02.004 (doi:10.1016/j.neubiorev.2006.02.004) [DOI] [PubMed] [Google Scholar]
- 65.Pulvermüller F., Fadiga L. 2010. Active perception: sensorimotor circuits as a cortical basis for language. Nat. Rev. Neurosci. 11, 351–360 10.1038/nrn2811 (doi:10.1038/nrn2811) [DOI] [PubMed] [Google Scholar]
- 66.Goldenberg G., Hermsdörfer J., Glindermann R., Rorden C., Karnath H. O. 2007. Pantomime of tool use depends on integrity of left inferior frontal cortex. Cereb. Cortex 7, 2769–2776 10.1093/cercor/bhm004 (doi:10.1093/cercor/bhm004) [DOI] [PubMed] [Google Scholar]
- 67.Kroliczak G., Frey S. H. 2009. A common network in the left cerebral hemisphere represents planning of tool use pantomimes and familiar intransitive gestures at the hand-independent level. Cereb. Cortex 19, 2396–2410 10.1093/cercor/bhn261 (doi:10.1093/cercor/bhn261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jacobs S., Danielmeier C., Frey S. H. 2010. Human anterior intraparietal and ventral premotor cortices support representations of grasping with the hand or a novel tool. J. Cogn. Neurosci. 22, 2594–2608 10.1162/jocn.2009.21372 (doi:10.1162/jocn.2009.21372) [DOI] [PubMed] [Google Scholar]