Abstract
Various authors have suggested behavioural similarities between tool use in early hominins and chimpanzee nut cracking, where nut cracking might be interpreted as a precursor of more complex stone flaking. In this paper, we bring together and review two separate strands of research on chimpanzee and human tool use and cognitive abilities. Firstly, and in the greatest detail, we review our recent experimental work on behavioural organization and skill acquisition in nut-cracking and stone-knapping tasks, highlighting similarities and differences between the two tasks that may be informative for the interpretation of stone tools in the early archaeological record. Secondly, and more briefly, we outline a model of the comparative neuropsychology of primate tool use and discuss recent descriptive anatomical and statistical analyses of anthropoid primate brain evolution, focusing on cortico-cerebellar systems. By juxtaposing these two strands of research, we are able to identify unsolved problems that can usefully be addressed by future research in each of these two research areas.
Keywords: hominin, chimpanzee, Oldowan, nut cracking, experimental archaeology, cortico-cerebellar
1. Introduction
Archaeological evidence suggests that tool use has been fundamental to hominin life for at least 2.6 Myr [1,2] and probably more [3,4]. Stone knapping represents the earliest known instance of toolmaking and tool use by early hominins [5–10]. Stone tool production has therefore become diagnostic of the cognitive ability and motor skills of extinct hominins [11–14]. Following the first scientific report of chimpanzee tool use by Jane Goodall in 1964 (the use of stripped leaf stalks for termiting, sticks for ant-dipping and leaves for drinking and self-wiping [15]), numerous observations have also been made of tool use in non-human primates in the wild, as well as in controlled experimental conditions in captivity. Use of tools to crack nuts in forest-dwelling chimpanzee groups has now been widely attested (early reports included Beatty [16] in Liberia; Struhsaker & Hunkeler [17], Rahm [18], Boesch [19] in the Tai forest, Ivory Coast; and Sugiyama & Koman [20] and Sugiyama [21] in Guinea).
In contrast with the use of stone hammers to pound and crack the casings of hard food objects, stone flaking to produce cutting tools appears to be a uniquely hominin cultural trait [12]. Toth & Schick [22] suggest that modern wild chimpanzees have not acquired stone-flaking traditions because none of their feeding objects need to be accessed by cutting. In contrast, the hominins associated with Oldowan stone tools were regularly feeding on animal prey for which such cutting tools and techniques were essential [23]. The earliest direct archaeological evidence of food item processing using Oldowan stone tools is from animal bones, which show cut marks associated with stripping off edible soft tissue and also fractures associated with cracking them open to obtain edible bone marrow. Using stone tools to cut animal soft tissue is attested from marks on the surfaces of bones in the earliest archaeological record (2.5–2.6 Myr BP, associated with the extinct hominin Australopithecus garhi [24]; possibly also at 3.4 Myr BP and associated with Australopithecus afarensis [3], but see [25]).
Various authors have suggested behavioural similarities between tool use in early hominins and chimpanzee nut cracking, where nut cracking might be interpreted as a precursor of more complex stone flaking [20,26–28]. But does the production of stone cutting tools require different skills, and different levels of functional understanding, than the use of stone hammers to fracture casings of hard food objects? If so, then Oldowan stone tool production may be predictive of significant differences in the associated cognitive abilities and motor skills of modern chimpanzees and extinct hominins.
In this paper, we bring together and review two separate strands of research on chimpanzee and human tool use and cognitive abilities. Firstly, and in the greatest detail, we review our recent experimental work on behavioural organization and skill acquisition in nut-cracking and stone-knapping tasks, highlighting similarities and differences between the two tasks that may be informative for the interpretation of stone tools in the early archaeological record. Secondly, we outline a model of the comparative neuropsychology of primate tool use and review recent descriptive anatomical and statistical analyses of anthropoid primate brain evolution, focusing on the chimpanzee–human comparison. By juxtaposing these two strands of research, we are able to identify unsolved problems that can usefully be addressed by future research in each of these two research areas.
2. Functional parameters of percussive technologies
Percussion can be loosely defined as ‘a forceful, muscle-driven striking of one body against another’ [28, p. 342], but this definition does not specify the way in which force is controlled to transform an object. A tool-assisted percussive task involves delivering a blow or a series of blows with an object, typically held in the hand, in such a way that all the parameters and constraints of the task are met. This definition may be applied to activities such as hammering a nail, drumming, hitting a golf ball, cracking a nut, flaking a stone, etc. Mechanically speaking, success depends on the properties of the object being struck and on the value of the momentum of the tool (hammer, drumstick, golf club, etc.), which is defined as the product of its mass (m) and its velocity (v). For a biological system, the efficiency of a blow can be defined in terms of potential and kinetic energy. An object held in a person's hand has potential energy—energy of position—which converts to kinetic energy—the energy of motion—if the actor lets it fall to the ground. If no additional—i.e. muscular—energy is added to the system, the sum of kinetic and potential energy stays constant. For a biological system, an energy-efficient blow is one in which the minimum of muscular energy is added to the system to achieve the task goals. Indeed, for typical learned movements, the external forces and passive forces of reaction in the joints are by far the most used in movement construction. Consequently, a minimum of muscular energy is added to the system to achieve the task goal.
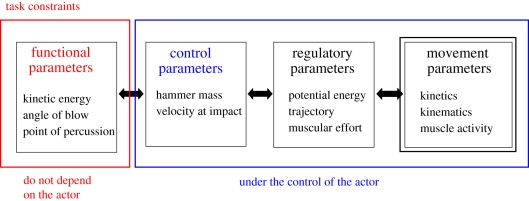
To characterize the skills needed for percussive techniques of nut cracking and stone flaking, we differentiate four layers of parameters [29] defining (respectively) the functional and deterministic task constraints, the parameters under the control of the actor performing the task and the parameters that determine effective regulation of these control parameters and movement parameters (figure 1). The functional parameters specify the topology of the task through relevant geometric and dynamical parameters, including kinetic energy, point of percussion, angle of blow and (for stone knapping) exterior platform angle. With regard to the dynamical parameter of kinetic energy (Ek = 1/2 mv2), the layer of control parameters specifies two parameters, the velocity (v) at impact and the mass of the hammer (m) that includes its substance and density, which are typically under the control of the actor. Finally, given a specific hammer, velocity can be regulated through various strategies, which depend on the actor. For example, the movement may be of large amplitude, relying on high potential energy and low muscular energy, or the opposite, with a small amplitude but with a large additional input of muscular energy. Regulatory parameters can therefore vary between actors who use alternative bodily movements to achieve the same functional output [30]. Movement parameters are those parameters that can be recorded and that allow the computation of regulatory and control parameters. These include kinematics, kinetics and muscular parameters that can be recorded through various technical means. This level of analysis will not be discussed here. For more discussion, see Biryukova & Bril [30].
Figure 1.
The three layers proposed for percussive tasks. Except for the exterior platform angle, all the parameters in some way or another have to be controlled in any percussive task. Only movement parameters are recorded and allow for computation of regulatory and control parameters (adapted from Bril et al. [16]).
(a). Nut-cracking techniques
In the case of nut cracking, the blow must be delivered in such a way that the shell cracks leaving the kernel intact. To achieve this goal, the right amount of kinetic energy must be generated and transferred to the nut in order to produce an adequate deformation of the shell so that it breaks. This depends on the hardness of the nut shell: if the kinetic energy is too high, the nut will be smashed and the kernel may be ruined, while if the kinetic energy is too low, the shell will not crack. Nut shells have evolved to be resistant to fracture, to protect the seed from predation. Consequently, their strength is largely independent of the point on the external surface receiving the percussive strike, which must therefore simply apply enough force to induce fracturing. Nut shells can be very hard indeed—macadamia nuts, for example, have an elastic modulus (a measure of the material's stiffness, or resistance to permanent deformation under compressive loading) of the order of 2–6 kN mm–2 [31], and require a force of the order of 2 kN to fracture them [32–34], while typical orally processed primate foodstuffs given in captivity have an elastic modulus in the range 0.1–350 N mm–2 [35]. The force required to fracture nuts of the species used by wild chimpanzees varies with the nut species and condition: typical forces required to fracture such nuts range from 2.8 kN for Coula edulis to 8.1 kN for Parinari excelsa, and between 9.7 and 12.5 kN for Panda oleosa ([36]; cf. [37]). Koya [38], however, found that with a hammer and anvil technique, repeated blows of much less than the force required individually to induce fracturing will still cause the palm nut shell to fail, because of the induction of micro-fractures and subsequent fatigue failure. This suggests that repetitive pounding, rather than attempting to fracture a nut by a single forceful strike, may be energetically a less costly strategy as well as one less likely to accidentally crush the kernel.
Among non-human primates, banging the food object against a hard surface is a frequently observed technique to crack open nuts. Controlled experiments as well as observations in the wild demonstrate the very fine adjustment to the constraints of the task by capuchin monkeys (e.g. [39–41]) as well as chimpanzees, to enable them to reach their goal. Wild chimpanzees (e.g. [42]) and contemporary human foragers are both reported as using a hammer and anvil technique, in which the nutshell is forcibly compressed between two hard surfaces. For energetic efficiency with the hammer and anvil technique, the blow must be elastic, the total impulse being constant before and after the blow so that, in theory, all forces are used to generate the deformation of the shell in such a way that it cracks. If the blow is a non-elastic one, a part or all of the energy will be dissipated, and it will be difficult to crack open the nut. For example, if the nut was lying on a soft surface or anvil, the energy would be absorbed by the support and the nut would not crack or would need a very high amount of energy in order to reach its breaking point. In addition, the direction of the blow must be more or less perpendicular to the surface on which the nut rests, since otherwise it would be displaced laterally (the energy being used to increase the velocity of the nut, and not to attain the goal of cracking it!). Fracturing these nuts using a stone hammer and anvil requires however only the stable placement of the nut on a sufficiently hard anvil, and delivery of a blow with a velocity vector approximately perpendicular to the plane of the anvil, of sufficient force to compress the nut and induce fracturing.
The early archaeological record also contains evidence of stone tool use in pounding or cracking open hard food objects, using techniques that are much more closely analogous to chimpanzee tool use than the production of Oldowan stone flakes. Mora & de la Torre [43] have reanalysed the stone tools from the lowest levels at Olduvai Gorge and suggested that the majority may relate to pounding hard food objects (bones, nuts), and not to producing stone flakes. Stone tool use to crack open bones would have been an essential technique in the Oldowan hominin repertoire. Pitted stones that could have been used for bone and nut cracking or for bipolar stone flaking are found in Oldowan levels at Olduvai Gorge (Tanzania) and also at Melka Kunture (Ethiopia), although the nut-cracking function is not yet directly attested for sites older than Gesher Benot Ya'aqov (Israel), where nut remains have been found in association with stones with surface pitting, and which dates to oxygen isotope stage 19 (ca 780 000 BP; [44]).
Nut cracking by a stone hammer and anvil is also found in contemporary human hunter–gatherers. The !Kung of the Kalahari desert, one of the quintessential hunter–gatherer groups of modern ethnography, process mongongo nuts for about 28 per cent of their food intake [45]. These nutshells are extremely hard and must be processed by cracking them open in this way, sometimes preceded by roasting to make the shells more brittle. Stone tools used for cracking mongongo nuts make up a disproportionately large fraction of the tools found discarded at !Kung campsites [46]. The skills involved are learned and continue to improve with experience well into adulthood. Bock [47], in a cross-sectional study conducted in 1994, experimentally measured the rate at which !Kung foragers could extract intact mongongo nut kernels from their outer shells using stone tools, and found significant effects of age (but not independently of strength) on processing rates, with efficiency in mongongo nut cracking continuing to improve through the teenage years and twenties and peaking among adults aged in the thirties and forties. Similar techniques continue to be used by subsistence and small-scale farmers: Koya & Faborode [48] found that forces of about 2 kN or higher were sufficient to induce fractures in palm nuts that are used to extract palm oil (Elaeis guineensis), and which are characteristically fractured individually by Nigerian peasant farmers who ‘break the nuts, one at a time, between two stones judging the magnitude of the applied force by experience’ [48, p. 471]. However, very often it is observed that cracking open a nut necessitates several strikes.
(b). Stone-flaking techniques
The fracture mechanics of the stone-flaking task are very different. Fine-grained stone typically has greater compressive than tensile strength (i.e. it is brittle [49]), which means that despite its hardness it can be fractured easily if force is applied in the right location and direction. The two main modes of fracture initiation to be considered here are wedge-fracturing and conchoidal fracturing [50,51].
Wedge fractures are initiated when force is applied and either detrital particles become wedged into a pre-existing flaw on the core surface, or the core surface is plastically deformed by forceful contact from a hard and sharp indenter; in both cases, the wedging causes crack initiation. This is the predominant mode of stone fracturing when the force is applied at a location far from a platform edge, or if the edge angle exceeds 90°, or if the core has many internal flaws [50, p. 688]. It is the typical fracture mode in bipolar flaking, where a pebble is placed on a hard anvil and hit with a hard hammer stone until it splits (‘the method of bipolar flaking is much like cracking a nut with a hammer’ [52, p. 131]).
Wedge fracturing corresponds to what Pelegrin [53] calls ‘split breaking’, and is the mode of flaking that seems to characterize the solutions that the captive bonobo Kanzi has developed when taught stone knapping. In split breaking, if a sufficient load is applied, the stone will break no matter how and where it is applied: essentially, all that is required is the localized application of sufficient force to the core to initiate a wedge. Consequently, the properties of the flake to be detached cannot be finely controlled; this solution is therefore fundamentally no more difficult than the solution of the nut-cracking task. When Kanzi had to face a situation where the goal was to cut through a cord to open a box containing a desirable food [54,55], he succeeded in discovering a way to produce a chip with a sharp edge. However, while Kanzi was encouraged to produce flakes with a sharp edge through direct percussion using a hard stone hammer to strike the core, he developed his own technique to get a sharp-edged piece of stone that would perfectly fit his goal by throwing the core against a hard surface. Although he was trained for quite a few years, he has never developed a technique that allows him to produce conchoidal flakes intentionally. Throwing does not seem to be a common technique to open nuts or fruits although theoretically it should have comparable functional properties to other percussive techniques, and could allow for the production of greater kinetic energy at contact (the full lever action of the whole arm could be brought to bear, with a reduced requirement for accuracy in the trajectory of the throw). Marchant & McGrew [28] relate the case of chimpanzees in the Parc National du Niokolo-Koba in Senegal throwing baobab fruits against an anvil to smash them open. However, this technique may be less likely to work on fruit and nut shells than on stone cores, because these materials differ in their elastic properties (likelihood of bouncing off a hard surface).
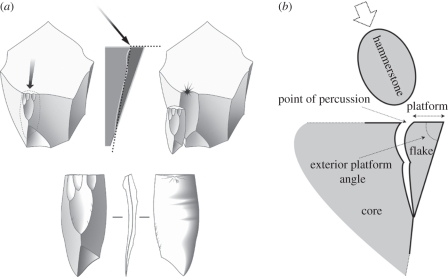
Controlled conchoidal fracturing, in contrast, requires a fuller understanding of the behaviour of the stone core under dynamic impact loading ([50]; see [56]). Hence, successful flake detachment by conchoidal fracture requires finding the appropriate point of percussion and achieving a sufficiently high degree of striking precision. Therefore, the properties of the flake to be detached can be strictly controlled by the knapper [56]. In terms of the fracture mechanics, conchoidal fracture is typically initiated with a partial Hertzian cone, caused by compression of the core at the point of impact by a hard indenter, followed by a crack propagation phase (figure 2). According to Cotterell & Kamminga [50,51], the intrinsic stiffness of the raw material means that cracks tend to propagate parallel to the plane of the external flake surface, which means that detachment is somewhat insensitive to the precise angle of contact between the platform and the percussor; when the direction and magnitude of applied force cause the crack to propagate away from that plane, the intrinsic stiffness of the material can still bring it back into that plane, producing characteristic ripples or undulations on a flake's ventral surface. In Cotterell and Kamminga's experiments, flake length (dimensionless, scaled to platform depth at the point of percussion) was found to have a greater dependence on the external platform angle and on the morphology of the dorsal surface of the developing flake (however, new experiments by Dibble & Rezek [57] suggest that the angle of the blow can also be a relevant factor). Unsuccessful flake detachment can occur when the force applied is insufficient for the given location on the core. An excess of outwardly directed force in the percussive strike may cause a hinge fracture to develop [50]. Application of insufficient force may cause the flaking energy to be consumed before the crack has propagated to the distal edge of the core, causing a step fracture; this may occur for example at locations on the core with a wide flat external surface. Hinge and step fractures are characteristic of novice stone knappers (e.g. [58]), and reflect poor understanding of the appropriate force needed to detach a flake with a feathered termination from a particular location on the core's external surface. Experienced knappers will typically choose the location much more carefully at which to apply appropriate force, and may exploit these properties of the raw material: for instance, in blade removal, a longitudinal ridge on the dorsal surface of the intended blade will be exploited because it prevents the crack spreading laterally during stiffness-controlled crack propagation [50].
Figure 2.
(a) Conchoidal fracture resulting from an angle of percussion near 40–50°, and an exterior edge angle at 70–80°. Bottom showing the characteristic feature of a flake: the swell at the point of contact or bulb of percussion is clearly visible (reproduced with permission from Pelegrin [53, p. 24]). (b) Flaking terminology.
In the case of percussive conchoidal flaking, similar to nut cracking, the blow must be an elastic blow, delivered in such a way that a flake is detached from the core responding to the mechanism of the conchoidal fracture. However, the constraints of the task, i.e. the functional parameters described above, are more numerous than in nut cracking. The shape and size of the flake depend on several parameters: the exterior platform angle, the point of percussion, the angle of the blow relative to the platform and the kinetic energy that initiates the fracture. A peculiarity of the kinetic energy necessary to produce a conchoidal fracture is the existence of a threshold value. Once a minimum effective quantity of kinetic energy is produced, an increase in this value has no impact on the flake produced, except that a value far too large may cause the flake to fragment into many pieces (reference in [29]). As such, the characteristics of the flake (its dimensions and form) depend on the convergence of multiple interrelated variables [56,57,59].
A variety of techniques for conchoidal flaking are known ethnographically, and studied experimentally (e.g. [60, p. 31]), including direct percussion with a hard or soft hammer; indirect percussion with a punch either interposed between the hard hammer and the core, or located on the opposite side of the core to the hammer (‘counter-blow’); as well as non-percussive pressure flaking techniques. In this paper, we are mainly concerned with the techniques of direct percussion with a hard hammer, which were the primary flaking techniques used in Oldowan toolmaking.
3. Mastering the functional parameters of percussive actions: experimental evidence
We now summarize a series of experiments designed to establish some of the parameters involved in skilled action in percussive tool-using tasks.
(a). Material and methods
We briefly present our methods and subject populations here; more details can be found in our papers reporting the primary results [29,32,33,56,61–66]. We designed experiments to investigate how actors of various levels of expertise develop specific behavioural traits concerning movement precision, flexibility, smoothness, regularity and optimization [67,68] (cf. figure 3). In the stone-knapping experiments in Khambhat (Gujarat, India), craftsmen of different levels of expertise have been asked to knap beads of different shapes and raw material, using an indirect percussion technique and with hammers having different properties. In the Oldowan replication study, modern experimental knappers having various amounts of practice were asked to produce conchoidal flakes of different sizes with hammers of various weights. The nut-cracking experiments with humans as well as chimpanzees were based on the same rationale, cracking nuts of different hardness with hammers of various weights. Children from 5 to 12 years of age and adults stood for actors of different levels of expertise.
Figure 3.
Illustration of the experimental setting for the different experiments. (a) Craftsman knapping a bead by means of indirect percussion by counter blow. (b) Modern experimental knapper producing a flake by direct hard-hammer percussion. (c) Chimpanzee cracking a nut (GARI, Japan Copyright © S. Hirata). (d) An 8-year-old child cracking a nut.
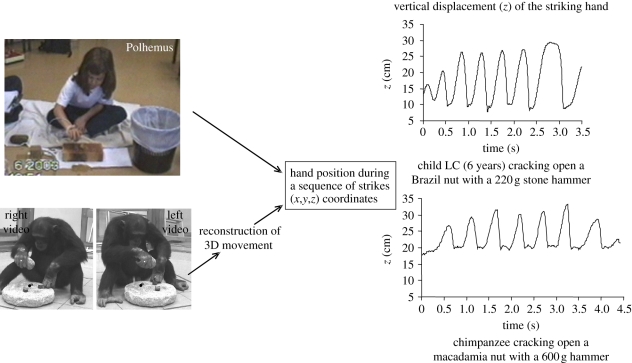
To be able to compare the movement in human stone knapping and in nut cracking by humans as well as chimpanzees, we used recording techniques that could provide data as similar as possible in all cases. Humans' knapping and nut-cracking movements were recorded with an electromagnetic recording system (either a Polhemus system or a Flock of Bird system; Ascension Technology Corporation) and an accelerometer for the Indian craftsmen. Chimpanzees' nut-cracking movements were recorded using two cameras positioned on the right and left of the animal with an angle of approximately 100°. Figure 3 shows examples of these experimental settings.
For both experiments on stone knapping with human adult subjects, sensors were attached with tape to the acromion, the exterior surface of the humerus, the posterior surface of the lower arm and to the dorsal surface of the hand following the procedure used by Biryukova et al. [69]. For children nut cracking, one sensor only was used (on the dorsal surface of the hand). As active or passive markers cannot be used for chimpanzees, we used a two-camera video-based system that allows reconstructing three-dimensional movement (figure 4). The reconstructed three-dimensional movement of the striking hand and the computation of functional parameters of the striking action necessitated frame-by-frame analysis [32,33].
Figure 4.
Recording and reconstruction of the hand movement for humans from the electromagnetic sensors, and from video cameras for the chimpanzees.
(b). Behavioural results I: control of kinetic energy in adaptation to task conditions
Kinetic energy, which is a key functional parameter to percussive tasks, involves two control parameters that are under the control of the actor: the mass of the hammer, and the velocity of the hammer at the time of contact. In the wild, chimpanzees have been observed selecting the appropriate tool, i.e. hammers and anvils of particular size, shape and materials, suggesting that they apprehend the functional properties of the nut-cracking task [20,21,42,70]. This capacity of selecting functional tools has also been shown in capuchins [40,41,71]. However, this capacity to perceive the affordances of objects as potential tools needs to be learned through experience. In an experiment where children were offered a set of 21 objects as potential tools of various degrees of functionality [61], potentially functional hammers represented 52 per cent of the objects chosen by 3 year olds, 90 per cent of the choices of 4/5 and 6/7 year olds and almost 100 per cent of choices of older children. While we have not done similar experiments exploring stone tool choice for stone knapping, experiments with Indian craftsmen also suggest that the recognition of subtle contrasts in suitability of possible hammers depends on the level of expertise [72].
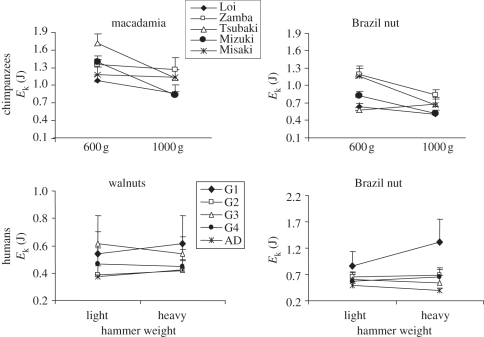
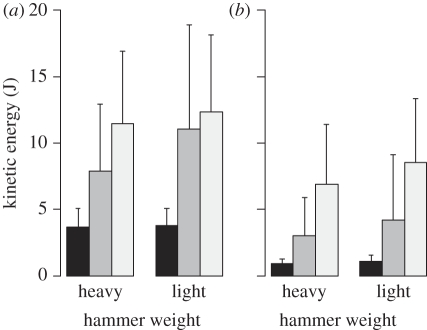
Once the hammer has been chosen, the kinetic energy depends only on the velocity at impact, which is under the control of the actor. To what degree are chimpanzees and humans able to adapt to the hammer properties to crack nuts or to knap stone? We conducted experiments to evaluate the capacity of the actor to cope with the constraints of the task by comparing the values of kinetic energy produced when the actor had to use hammers of different weights [29,32,64]. All actors (humans and chimpanzees) were able to modify the velocity they produce to the changing constraints of the task; in all cases—i.e. chimpanzees or children cracking nuts and adult humans flaking stone—the velocity was systematically greater when using a lighter hammer. However, in both tasks and in both species, a higher level of expertise was associated with a reduced velocity at impact. In the nut-cracking task, in both species, there was also evidence of an ability to adjust velocity to differences in hammer mass in order to deliver a constant level of kinetic energy at impact (figure 5; [32,64]). When looking at stone flaking, the results are quite different. While all knappers showed greater velocity when using a lighter hammer, these variations do not end up in the production of the same kinetic energy when using a light or a heavy hammer. The adaptation to the hammer weight that was observed for velocity does not transfer to kinetic energy, except for experts (figure 6; [29,56]).
Figure 5.
Kinetic energy values at the time of contact of the hammer with the nut for chimpanzees and humans when they crack nuts of two different species (different hardness) with hammers of different weights. For the chimpanzees, values are given for each chimpanzee. For humans, data are averaged for each age group, children from 5–6 years (G1) to 11–12 years (G4) and adults (AD; adapted from Bril et al. [32] and Foucart [64]).
Figure 6.
Kinetic energy at point of contact between the hammer and the core, when the task was to knap a large flake and the subjects had been previously classified as experts, intermediates and novices (adapted from Bril et al. [16]). Black grey bars, expert; light grey bars, intermediate; white bars, novice. (a) Large flake; (b) small flake.
Our results undoubtedly show that experience is a key criterion in the understanding of the constraints of the task. Experts display exactly the same kinetic energy while both intermediates and novices displayed greater kinetic energy with lighter hammers, a result that may be compared with those obtained with chimpanzees. In another set of experiments, novices produced values of kinetic energy more than three times greater compared with experts, and produced smaller flakes (figure 7). The dramatic differences in the values of the kinetic energy at impact observed between experts and novices in stone knapping contrast with the relatively small differences observed in adults and children when cracking nuts. Except for the youngest children aged 5–6 years in the more difficult situation (cracking open Brazil nuts), all the participants were able to adjust the kinetic energy delivered to the nut in different conditions of hardness of the nut and weight of the stone hammer. In the same way, in most cases chimpanzees are able to adapt to both hardness of the nut and weight of the hammer in the nut-cracking task, even though in a less fine-tuned manner.
Figure 7.
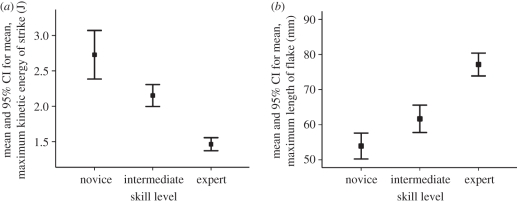
(a) Mean values for maximum kinetic energy of strike, and (b) for maximum flake length, by skill level. The task was to remove a flake (size unspecified) from a prepared single-platform core. Subjects were two experts making a total of 77 flake removals, three intermediates making a total of 131 flake removals and four novices making a total of 149 flake removals. Greater expertise is associated with less kinetic energy and larger flakes. (From F. Wenban-Smith, B. Bril, G. Dietrich, R. Rein, T. Nonaka & J. Steele 2010, unpublished data.)
We may hypothesize that the number of variables to be controlled in the case of nut cracking is much smaller than in stone knapping. In nut cracking, the amount of kinetic energy must be controlled to reach the breaking point, while stone knapping is characterized by a threshold value that must be discovered by the actor [29,56]. In nut cracking, two functional parameters only have to be controlled, kinetic energy and direction of the blow. As the nut is positioned on an anvil, the direction of the blow is approximately vertical. The exploration process necessary to find out the efficient amount of kinetic energy may be conceived as a succession of approximations that progressively converge to the right value and necessitates the control of one parameter only. The case of knapping conchoidal flakes is entirely different. The interrelationship of numerous variables to succeed in detaching the planned flake makes the task incomparably more difficult, and consequently, the exploration process of the task space will also be tremendously more complex.
(c). Behavioural results II: bimanual coordination
We have also investigated asymmetric bimanual coordination in a stone-knapping task. Previous studies have provided substantial evidence of functional asymmetries between the two hands and their underlying neural structures [73–75]. Yet, the problem of how such asymmetric bimanual activities are organized into the collective behaviour of a bimanual system still remains incompletely understood [66]. In one of the few papers addressing the issue of the coordination between the asymmetric elements, Guiard [76] proposed a ‘kinematic chain model’ to explain functional coordination in human skilled bimanual actions [75,77]. The essence of Guiard's [76,78] conceptualization is that he considers the two hands as serially assembled, instead of following a parallel assembly pattern. What is implied in serial assembly is that two different layers of activities in two hands are coupled with each other to contribute to the same output. Guiard [76] hypothesized that the outstanding manipulative efficiency of humans results not only from role differentiation between the two hands or the emergence of handedness but also from the fact that between-hand division of labour is typically functionally nested, with two hands working at two different levels of resolution in a coordinated fashion to yield a common functional outcome. In his model, one hand and/or arm performs movements that Guiard qualifies as high frequency, being more temporally and spatially precise (i.e. being faster and having a narrower target), whereas the other upper limb is low frequency, acting as a stabilizer or support, maintaining the spatial or temporal structure, and moving earlier to define the spatial reference frame. To define the group-level handedness that is specific to humans, Guiard suggested that most humans tend to learn the low-frequency role with the left hand and the high-frequency component with the right hand. Such human population-level right handedness is generally explained by reference to a left hemisphere advantage for fine temporal resolution of sensory input and motor output. Carson [79, p. 481] discusses two possible explanations for this advantage. One is that the left hemisphere may be more efficient in error correction using sensory feedback. The other is that the left hemisphere may permit more precise control of net forces and force durations (compare also [80,81]).
Non-human primates must also be observed using their hands in complex asymmetric bimanually coordinated tasks if the objective is to record hand preferences (e.g. [82]). The task that is most frequently used at present to elicit such behaviours in captive populations is the tube task [83], an extractive feeding task involving an opaque polyvinylchloride (PVC) tube containing smears of peanut butter that can be extracted if one hand holds the tube while part of the other hand is inserted into it. Hopkins et al. [83] have found a population-level right-hand preference in the tube task in three separate captive chimpanzee populations, all with large sample sizes, although the ratio of right- to left-handed individuals is lower than in humans—typically 2 : 1 in chimpanzees, as compared with 8 or 9 : 1 in humans—and, furthermore, there are very much higher frequencies of ambipreferent chimpanzees than of ambipreferent humans [84]. A similar pattern has not been reported in wild chimpanzees in nut-cracking tasks, although this may be because that task is less complex in terms of bimanual coordination.
We have not yet analysed bimanual coordination in chimpanzees in the nut-cracking task. We have however analysed bimanual coordination in human stone knapping, and this provides some insight into the correlates of expertise. In this task, the two hands have clearly differentiated functions. The hammering hand needs to be controlled in such a way as to transmit the appropriate amount of kinetic energy at impact with considerable accuracy at the point of percussion. On the other hand, the postural hand has to rotate and adjust the position of a core or rough-out to prepare for the following hammer strike, and stabilize the core or rough-out against the shock of the blow.
Professional craftsmen from two classes of workshops in Khambhat (Gujarat, India) participated in our experiment [66]. In addition to carnelian stones typically used in bead production, a new raw material—glass—was also included. Among the sub-goals that make up the task, 30 s sequences of calibration (standardization of crests to prepare for fluting) and fluting (detachment of long crests) were extracted from each trial. Dependent variables calculated for each 30 s sequence were used for the statistical analyses. The two sub-goal sequences chosen for analysis have different functional requirements. Fluting, through one strike, determines the overall shape of the product by detaching a long flake. On the other hand, calibration is more of a process of standardizing the crest to prepare for fluting. Each fluting sequence consists of small preparatory movements and several forceful strikes to detach long flakes, whereas each calibration sequence consists of a series of detachments of a number of tiny flakes.
Previous studies from Khambhat have shown that the end products produced by high-level expert craftsmen (trained with a longer apprenticeship period) had significantly greater sphericity and a smoother surface than those produced by low-level expert craftsmen (trained with a shorter apprenticeship period), and that such a group difference was amplified in the novel situation using glass rough-outs [62,63,65]. We studied asymmetric bimanual coordination of professional bead craftsmen from these two skill level groups in a naturalistic situation using recurrence methods [66]. Our key findings are that the movements of the two hands of craftsmen were controlled, reflecting the functional requirements of the task and the roles assumed by each hand, and that the skill level difference appeared in the way they were organized into a unified act. Regarding the functional specificity, among others, evidence was found in both groups that the dynamics of the displacement of the hammering hand and that of the postural hand were both relatively stable when glass was used, and that the dynamics of the displacement of the postural hand was relatively stable during fluting compared with calibration. However, only the bimanual movement coordination of highly skilled experts differentiated the functional requirements of different sub-goals. Furthermore, the dynamics of bimanual movement of high-level experts exhibited more deterministic coupling than that of low-level expert craftsmen. These results suggest that what is acquired in skilled bimanual action is adaptable and flexible nesting of differentiated functions, in which movements of two hands are modulated in such a way as to meet various functional demands of the situation.
(d). Behavioural results III: understanding of fracture mechanics
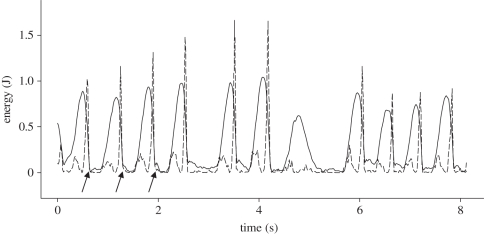
Anticipation is often considered to necessitate a high level of cognitive ability. If we define anticipatory behaviour as behaviour that prepares for the forthcoming goal, humans as well as other primates must be capable of anticipatory behaviour. The choice of a hammer adapted to the hardness of the nut prior to the actual nut-cracking activity has been observed in capuchins as well as chimpanzees [21,40,42,71]. Anticipation may be observed at the level of the striking action as well. We have been able to show how chimpanzees can modulate the kinetic energy in a sequence of strikes to crack open a macadamia nut [32]. Figure 8 shows the 11 strikes given to a nut before taking the kernel out. While the value of potential energy is constant, the kinetic energy increases up to a certain value, and remains constant for the last two hits, when probably the shell is broken; then a few low-kinetic-value strikes are given probably to take the bits of shell off. We hypothesize that this striking strategy suggests that the chimpanzee has some ‘understanding’ of the existence of a breaking point that should not be passed over.
Figure 8.
Potential (Ep, solid lines) and kinetic energy (Ek, dashed lines) during a sequence of strokes by a chimpanzee cracking a macadamia nut with a 300 g hammer. The arrows mark the time of impact of the stone hammer on the nut (adapted from Bril et al. [32]).
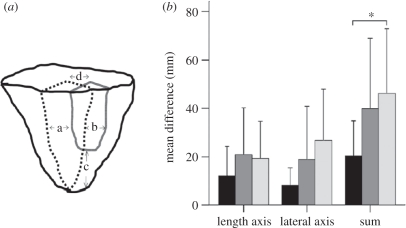
Anticipatory procedures are a great deal more complex when looking at conchoidal flaking. When planning to knap a flake with defined characteristics, a large number of interrelated features of task constraints have to be taken into account, and behaviour has to be adjusted accordingly. In a recent study, Nonaka et al. [56] have shown that only very high-level expert knappers are able to produce the flake they intended. The intentions of the knappers were analysed prior to the actual flaking, in terms of the expected shape of the detached flake and the intended percussion point. Results showed that to predict accurately the consequences of a strike entails an acute exploration of the properties of the core and of the hammer stone, to set up an interrelationship among the variables in such a way as to comply with the task constraints. In our study, only experts with approximately 20 years of part-time experience in replicating archaeological stone tools were able to predict flake dimensions that significantly correlate to the detached flakes. The fact that experts are able to accurately predict the flake to be detached suggests that under such a part-time training and practising regime, years of experience may be necessary to understand the constraints of the conchoidal fracture, and that it requires similar amounts of experience to be able to discriminate the feature of a core and the functional properties of the action that affect the morphology of the flake (figure 9).
Figure 9.
(a) The measurements made on predicted (black dotted lines) and detached flakes (grey line). a + b: difference in the lateral axis, c: difference in length, d: distance between points of percussion between predicted and detached flakes. (b) Mean differences between predicted and detached flakes for the three different expertise groups (error bars: +1s.d.). Black bars, expert; grey bars, intermediate; white bars, novice; asterisk, difference is significant at p < 0.05. Adapted from Nonaka et al. [56].
4. Brain evolution in humans and chimpanzees: issues relevant to tool use
As outlined above, we have identified similarities and contrasts in the structure and cognitive demands of two percussive tasks, nut cracking and stone flaking, and we have also discovered behavioural correlates of experience and expertise in stone-knapping tasks. These may be informative of evolutionary divergences in the behavioural capacities of the chimpanzee and human lineages. Our behavioural results reviewed above can be seen as bridging the gap between work reviewed elsewhere in this issue on monkey tool use and social learning [85], and work on human tool use and on the evolution of increasing cognitive demands in hominin Palaeolithic stone tool traditions [86,87]. Such work does not directly address African ape–human cognitive and behavioural contrasts, nor do we yet have any brain imaging observations even for a human model of the circuits activated in a nut-cracking as compared with a simple stone-flaking task (see also [88,89]).
A number of potential anatomical contrasts have been hypothesized that could explain differences in human and chimpanzee tool-making skills, but some of these have not yet been experimentally validated. Stout & Chaminade [8] have identified activation of the posterior parietal area (PP, caudal intraparietal/transverse occipital sulci) in experimental Oldowan stone flaking by modern humans. The PP is generally recognized as a site of major expansion and reorganization in humans [90,91] and may thus constitute a neural basis of what makes humans unique in tool-making skills [92]. However, because we do not have brain imaging data on areas that are activated in a nut-cracking task, we cannot be confident that the two tasks place qualitatively different demands on differing elements of cognitive systems (as opposed to quantitatively different demands on the same elements of the same cognitive systems), nor can we assume that PP contrasts found when comparing humans and monkeys will also be found when comparing humans with chimpanzees. At a more peripheral level, Walker [93] has recently proposed an explanation for the difference in strength in the human and chimpanzee limb systems (particularly the upper limb system) that may also have implications for skill in percussive task execution. He proposes that chimpanzees have fewer and larger motor units (systems of motoneurons signalling to the muscle fascicles to contract), which enables greater simultaneous force to be exerted. He suggests that, in contrast, humans have a much greater range of motor units and more small units (fewer muscle fibres per nerve), enabling us to recruit muscles for more complex but less forceful tasks. If his conjecture is correct, then chimpanzees are optimized for strength in peak loading tasks (locomotion), while humans no longer need to exert the same degree of force in their upper limbs and have therefore evolved a capacity for finer and more rapidly varying forces, but with less maximal strength. Walker's hypothesis, however, has yet to be tested.
In contrast to such work, there is also substantial evidence of continuity between humans and other primates in many fundamental aspects of brain organization relevant to skilled tool use (see also [94]). Imaging studies show that primate tool use activates a distributed network of brain areas, localized in different but interconnected anatomical structures. Brain imaging studies in humans have shown that it is important to differentiate circuits involved in conceptual semantic knowledge about tool use (often based on tasks like pantomime, visual evaluation of a tool image, hearing tool manipulation, tool naming, etc.; e.g. [95–97]), and those involved in the selection and effective use of a functional tool to solve a mechanical problem [98,99]. Thus, human tool use activates a fronto-parietal praxis network involved in hand manipulation skills, as well as regions of the cerebellum and the basal ganglia [95], while macaque tool use also activates several cortical areas (intraparietal cortex, presupplementary motor area, premotor cortex) as well as the cerebellum and the basal ganglia [100]. Human tool use also activates a network more associated with conceptual aspects of tool use involving the left inferior frontal gyrus, left posterior middle temporal gyrus and bilateral fusiform cortex ([95], cf. [101]), for which comparative analyses of tool use in monkeys are presented elsewhere in this issue (cf. [85]).
Our own recent comparative anatomical research has focused on the coupled evolution of cortical and cerebellar circuits in primates, and their implications for the evolution of motor control systems. Motor control has been described as the ‘Cinderella’ of psychology [102], but research attention is increasing thanks to a convergence of interest between psychology and neuroscience, the rise of ecological psychology and of dynamical systems approaches and an enhanced awareness of the computational complexity of skilled movement among scientists programming action planning and execution routines in robotics [102]. In parallel with this, there has been an enormous increase in scientific research on the cerebellum, a structure that contains more than half of the neurons in the entire human brain and that has traditionally been seen as mainly concerned with motor learning and movement coordination, precision and timing, but which is now also thought to be involved in higher cognitive functions (e.g. [103]).
In an extension of recent evolutionary analyses of cortico-cerebellar systems in primate brains (e.g. [104–109]), our own recent work [110] has focused on the systemic relationships of the lateral cerebellum or ‘cerebrocerebellum’. The cerebrocerebellum is crucial for execution of prehensile upper limb movements, in both feed-forward and visual feedback-guided modes of regulation of ongoing movements in action execution. It works in conjunction with primary motor cortex and parietal association cortex (via the pontine nuclei) in the organization of skilled manual actions [103,109,111]. The cerebrocerebellum is also involved in a frontal cortical circuit (primarily prefrontal) via the basal ganglia, which is involved in novel motor sequence learning (‘incremental acquisition of movements into a well-executed behaviour’ [112, p. 252]). Imaging studies suggest that these circuits are conserved: for example, recent brain imaging work with macaques also shows activation of fronto-cerebellar and fronto-parietal circuits in tasks requiring extension of tool use to novel functional demands [113].
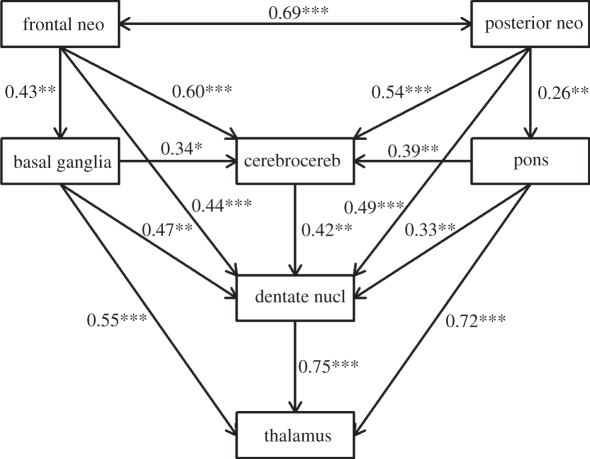
We have measured the volumes of the major cerebellar substructures in 19 living anthropoid primate species (including humans and chimpanzees), and have identified patterns of correlated and adaptive evolutionary size change in separate components of cortico-cerebellar systems within this diverse group (whose member species have not shared a common ancestor more recently than about 35 Myr BP) [110]. Our results indicate two main patterns of correlated evolution, indicative of selection acting repeatedly on integrated brain systems: one set of correlations involves the posterior neocortex, pons, cerebrocerebellum, dentate nucleus and thalamus, and the other set involves the frontal lobe, basal ganglia, cerebrocerebellum, dentate nucleus and thalamus (figure 10). We have suggested that these patterns of correlated evolution are specifically associated with selection acting to maintain the functional integrity of the two cortico-cerebellar circuits described above, and involved in the organization of skilled manual actions and in learning novel motor sequences. Our results therefore suggest that patterns of covarying size changes in neural systems involving profuse cortico-cerebellar connections are a major factor in explaining the evolution of anthropoid brain organization. We have also used phylogenetic comparative methods to reconstruct patterns of evolutionary divergence at successive phylogenetic branching events in the lineage leading to humans and chimpanzees. We infer that the Homo-Pan clade has come under strong positive selection for relative expansion of the frontal cortico-cerebellar system (with selection strongest in the human-specific branch). The marked expansion of the frontal cortico-cerebellar system in chimpanzees and humans is consistent with their increased social learning capacities, exemplified in their similar learning strategies of fine motor skills such as tool use.
Figure 10.
A summary of statistically significant bivariate correlations showing the concerted evolution of functionally inter-related structures across a sample of 19 anthropoid primate species' brains. Values indicate partial r2 values in a phylogenetically controlled comparison. The correlations are significant at probability level: *p < 0.05, **p < 0.01, ***p < 0.001. Each correlation represents the coevolutionary relation between two structures, partialling out the size of the rest of the brain (adapted from Smaers et al. [110]).
Studies of human motor-skill learning demonstrate that the transitions from an initial effortful learning phase to more established performance levels involve increased activation of the cerebrocerebellum [114–117]. Motor adaptation (dynamic adjustment to environmental changes during execution of a learned motor sequence), in particular, is dependent on the intact cortico-cerebellar system that links cerebrocerebellar areas to parietal and motor cortex [118] and is involved in both kinematic and dynamic aspects of motor control [119]. Lesion studies have provided further evidence for the role of global cerebellar deficits in profound impairments of motor adaptation (e.g. [120]). Such studies, which focus on the neuropsychology of non-declarative, incremental motor-skill learning (where ‘practice makes perfect’) and on the acquisition of capacities for motor adaptation to varying environmental constraints (analogous to the varying hammer weights that we introduced in our behavioural studies of skilled percussive action), identify roles for brain systems and components of brain systems that complement the cortical mechanisms of action understanding and action planning discussed by other contributors to this volume. The main outcome of our comparative anatomical study, as summarized in this section, is its indirect confirmation of a fundamental evolutionary continuity in the organization of such cortico-cerebellar systems in anthropoid primates (including monkeys, apes and humans). We would expect such anatomical continuities to be reflected in underlying continuities of behavioural potential for motor-skill learning, although contrasts between species in absolute brain size and (if found) in finer grained aspects of brain architecture may affect individual species' aptitudes for learning and executing complex motor sequences in particular behavioural domains.
Our behavioural studies have also identified contrasts between the chimpanzee nut-cracking and the human stone-flaking tasks in functional parameters relating to understanding of the fracture mechanics of stone cores (which are much harder to learn to predict than are the fracture properties of hard casings of nuts). As discussed above, it is worth keeping in mind that chimpanzees (or bonobos such as Kanzi) somehow fall short in being able to visualize the properties of the core so as to exploit them to produce flakes [54,55]. A functional imaging study of brain regions activated in human subjects by physical reasoning in nut-cracking versus stone-flaking tasks would elucidate this problem, but to our knowledge, no such study has yet been conducted.
5. Conclusions
We have reviewed our experimental work on the nut-cracking and stone-flaking tasks, which has been designed within a dynamical systems framework and with reference to the ecological movement in psychology [12]. In this framework, the mastering of a technical skill depends on the capacity of an organism to set up the constraints of the system according to the task demand, and to mobilize adaptively the degrees of freedom of the system. At a behavioural level, the unfolding of the action may be viewed as an emergent process, at the interface of environmental opportunities available to the organism (affordances) and the set of constraints associated with the task. Nut cracking and stone knapping differ in task conditions because conchoidal fracture of a stone core requires more precise movement control, and an asymmetrical use of both hands, characterized by the simultaneous control of at least two variables (reciprocal orientation of the core and of the trajectory of the hammer, which keeps varying during the sequences of blows). We have also shown that one of the features characterizing expert stone knappers is the ability to predict accurately the effect of a percussive strike on fracture propagation in the core, which depends on properties of the core, such as external platform angle and core surface morphology, as well as properties of the executed strike, such as kinetic energy at impact and distance of the point of impact from the platform edge.
The neuroscience of such skilled movement, and of the capacity for adaptive response to varying functional opportunities (namely the affordances of the individual core), requires further investigation (cf. [87]). In particular, it would be helpful to compare patterns of brain activation in nut cracking and stone flaking in a cohort trained in the skilful execution of both tasks. An integrated approach to the concerted evolution of systems of functionally interrelated brain structures is increasingly common in comparative neuroethological research. We expect that skilful task execution will depend on subcortical as well as cortical circuits, and we have discussed the evolutionary anatomy of the cortico-cerebellar systems as an appropriate focus for further comparative investigation.
Acknowledgements
Some of this paper was written when B.B. was a visitor at the AHRC Centre for the Evolution of Cultural Diversity, Institute of Archaeology, University College London. We thank the AHRC for funding this visit. Most of the primary research done by the co-authors and reported here was supported by the European Commission in a grant to the HANDTOMOUTH project (FP6, Contract No. 29065, NEST-2004-PATH-HUMAN).
References
- 1.Roche H. 2005. From simple flaking to shaping: stone-knapping evolution among early hominins. In Stone knapping: the necessary conditions for a uniquely hominin behavior (eds Roux V., Bril B.), pp. 35–48 Cambridge, UK: McDonald Institute for Archaeological Research [Google Scholar]
- 2.Semaw S. 2000. The world's oldest stone artefacts from Gona, Ethiopia: their implications for understanding stone technology and patterns of human evolution between 2.6–1.5 million years ago. J. Archaeol. Sci. 27, 1197–1214 10.1006/jasc.1999.0592 (doi:10.1006/jasc.1999.0592) [DOI] [Google Scholar]
- 3.McPherron S. P., Alemseged Z., Marean C. W., Wynn J. G., Reed D., Geraads D., Bobe R., Béarat H. A. 2010. Evidence for stone-tool-assisted consumption of animal tissues before 3.39 million years ago at Dikika, Ethiopia. Nature 466, 857–860 10.1038/nature09248 (doi:10.1038/nature09248) [DOI] [PubMed] [Google Scholar]
- 4.Panger M. A., Brooks A., Richmond B., Wood B. 2002. Older than the Oldowan? Rethinking the emergence of hominin tool use. Evol. Anthropol. 11, 235–245 10.1002/evan.10094 (doi:10.1002/evan.10094) [DOI] [Google Scholar]
- 5.Ambrose S. H. 2001. Paleolithic technology and human evolution. Science 291, 1748–1753 10.1126/science.1059487 (doi:10.1126/science.1059487) [DOI] [PubMed] [Google Scholar]
- 6.Roche H., Delagnes A., Grugal J.-P., Feibel C., Kibunjia M., Mourre V., Texier J. 1999. Early hominid stone tool production and technical skill 2.34 Myr ago in West Turkana, Kenya. Nature 399, 57–60 10.1038/19959 (doi:10.1038/19959) [DOI] [PubMed] [Google Scholar]
- 7.Steele J. 1999. Stone legacy of skilled hands. Nature 399, 24–25 10.1038/19869 (doi:10.1038/19869) [DOI] [PubMed] [Google Scholar]
- 8.Stout D., Chaminade T. 2007. The evolutionary neuroscience of tool making. Neuropsychologica 45, 1091–1100 10.1016/j.neuropsychologia.2006.09.014 (doi:10.1016/j.neuropsychologia.2006.09.014) [DOI] [PubMed] [Google Scholar]
- 9.Susman R. L. 1998. Hand function and tool behavior in early hominids. J. Hum. Evol. 35, 23–46 10.1006/jhev.1998.0220 (doi:10.1006/jhev.1998.0220) [DOI] [PubMed] [Google Scholar]
- 10.Wood B. 1997. The oldest whodunnit in the world. Nature 385, 292–293 10.1038/385292a0 (doi:10.1038/385292a0) [DOI] [PubMed] [Google Scholar]
- 11.Harlacker L. 2006. Knowledge and know-how in the Oldowan: an experimental approach. In Skilled production and social reproduction: aspects of traditional stone-tool technologies (eds Apel J., Knutsson K.). Uppsala, Sweden: Societas Archaeologica Upsaliensis [Google Scholar]
- 12.Roux V., Bril B. 2005. General introduction: a dynamic system framework for studying a uniquely hominin innovation. In Stone knapping: the necessary conditions for a uniquely hominin behavior (eds Roux V., Bril B.), pp. 1–18 Cambridge, UK: McDonald Institute [Google Scholar]
- 13.Stout D., Toth N., Schick K. D., Chaminade T. 2008. Neural correlates of early Stone Age toolmaking: technology, language and cognition in human evolution. Phil. Trans. R. Soc. B 363, 1939–1949 10.1098/rstb.2008.0001 (doi:10.1098/rstb.2008.0001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toth N., Schick K. D. 1993. Early stone industries and inferences regarding language and cognition. In Tools, language and cognition in human evolution (eds Gibson K. R., Ingold T.), pp. 346–362 Cambridge, UK: Cambridge University Press [Google Scholar]
- 15.Goodall J. 1964. Tool-using and aimed throwing in a community of free-living chimpanzees. Nature 201, 1264–1266 10.1038/2011264a0 (doi:10.1038/2011264a0) [DOI] [PubMed] [Google Scholar]
- 16.Beatty H. 1951. A note on the behavior of the chimpanzee. J. Mammal. 32, 118 [Google Scholar]
- 17.Struhsaker T., Hunkeler P. 1971. Evidence of tool-using by chimpanzees in the Ivory Coast. Folia Primatol. 15, 212–219 10.1159/000155380 (doi:10.1159/000155380) [DOI] [PubMed] [Google Scholar]
- 18.Rahm U. 1971. L'emploi d'outils par les chimpanzes de l'ouest de la Cote d'lvoire. Terre et Vie 25, 506–509 [Google Scholar]
- 19.Boesch C. 1978. Nouvelles observations sur les chimpanzes de la foret de Tai (Cote d'lvoire). Terre et Vie 32, 195–201 [Google Scholar]
- 20.Sugiyama Y., Koman J. 1979. Tool-using and -making behavior in wild chimpanzees at Bossou, Guinea. Primates 20, 513–524 10.1007/BF02373433 (doi:10.1007/BF02373433) [DOI] [Google Scholar]
- 21.Sugiyama Y. 1981. Observations on the population dynamics and behavior of wild chimpanzees at Bossou, Guinea, in 1979–1980. Primates 22, 435–444 10.1007/BF02381236 (doi:10.1007/BF02381236) [DOI] [Google Scholar]
- 22.Toth N., Schick K. 2009. The Oldowan: the tool making of early hominins and chimpanzees compared. Annu. Rev. Anthropol. 38, 289–305 10.1146/annurev-anthro-091908-164521 (doi:10.1146/annurev-anthro-091908-164521) [DOI] [Google Scholar]
- 23.Plummer T. 2004. Flaked stones and old bones: biological and cultural evolution at the dawn of technology. Am. J. Phys. Anthropol. 39(Suppl.), 118–164 10.1002/ajpa.20157 (doi:10.1002/ajpa.20157) [DOI] [PubMed] [Google Scholar]
- 24.de Heinzelin J., Clark J. D., White T., Hart W., Renne P., WoldeGabriel G., Beyene Y., Vrba E. 1999. Environment and behavior of 2.5-million-year-old Bouri hominids. Science 284, 625–629 10.1126/science.284.5414.625 (doi:10.1126/science.284.5414.625) [DOI] [PubMed] [Google Scholar]
- 25.Domínguez-Rodrigo M., Pickering T. R., Bunn H. T. 2010. Configurational approach to identifying the earliest hominin butchers. Proc. Natl Acad. Sci. USA 107, 20 929–20 934 10.1073/pnas.1013711107 (doi:10.1073/pnas.1013711107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boesch C., Boesch H. 1993. Diversity of tool use and tool making in wild chimpanzees. In The use of tools by human and non-human primates (eds Berthelet A., Chavaillon J.), pp. 158–168 Oxford, UK: Clarendon Press [Google Scholar]
- 27.Davidson I., McGrew W. C. 2005. Stone tools and the uniqueness of human culture. J. R. Anthropol. Inst. 11, 793–817 10.1111/j.1467-9655.2005.00262.x (doi:10.1111/j.1467-9655.2005.00262.x) [DOI] [Google Scholar]
- 28.Marchant L. F., McGrew W. C. 2005. Percussive technology: chimpanzee baobab smashing and the evolutionary modelling of hominin knapping. In Stone knapping: the necessary conditions for a uniquely hominin behavior (eds Roux V., Bril B.), pp. 341–350 Cambridge, UK: McDonald Institute for Archaeological Research [Google Scholar]
- 29.Bril B., Rein R., Nonaka T., Wenban-Smith F., Dietrich G. 2010. The role of expertise in tool use: skill differences in functional action adaptation to task constraints. J. Exp. Psychol. Hum. Percept. Perform. 36, 825–839 10.1037/a0018171 (doi:10.1037/a0018171) [DOI] [PubMed] [Google Scholar]
- 30.Biryukova E. V., Bril B. 2008. Organization of goal-directed action at a high level of motor skill: the case of stone knapping in India. Motor Control 12, 181–209 [DOI] [PubMed] [Google Scholar]
- 31.Jennings J. S., MacMillan N. H. 1986. A tough nut to crack. J. Mater. Sci. 21, 1517–1524 10.1007/BF01114704 (doi:10.1007/BF01114704) [DOI] [Google Scholar]
- 32.Bril B., Dietrich G., Foucart J., Hirata S. 2009. Tool use as a way to assess cognition: how do captive chimpanzees handle the weight of the hammer when cracking a nut? Anim. Cogn. 12, 217–235 10.1007/s10071-008-0184-x (doi:10.1007/s10071-008-0184-x) [DOI] [PubMed] [Google Scholar]
- 33.Foucart J., Bril B., Hirata S., Morimura N., Houki C., Ueno Y., Matsuzawa T. 2005. A preliminary analysis of nut-cracking movements in a captive chimpanzee: adaptation to the properties of tools and nuts. In Stone knapping: the necessary conditions for a uniquely hominin behavior (eds Roux V., Bril B.), pp. 147–158 Cambridge, UK: McDonald Institute for Archaeological Research [Google Scholar]
- 34.Schrauf C., Huber L., Visalberghi E. 2008. Do capuchin monkeys use weight to select hammer tools? Anim. Cogn. 11, 413–422 10.1007/s10071-007-0131-2 (doi:10.1007/s10071-007-0131-2) [DOI] [PubMed] [Google Scholar]
- 35.Williams S. H., Wright B. W., Troung V. D., Daubert C. R., Vinyard C. Y. 2005. Mechanical properties of foods used in experimental studies of primate masticatory function. Am. J. Primatol. 67, 329–346 10.1002/ajp.20189 (doi:10.1002/ajp.20189) [DOI] [PubMed] [Google Scholar]
- 36.Peters C. R. 1987. Nut-like oil seeds: food for monkeys, chimpanzees, humans, and probably ape-men. Am. J. Phys. Anthropol. 73, 333–363 10.1002/ajpa.1330730306 (doi:10.1002/ajpa.1330730306) [DOI] [PubMed] [Google Scholar]
- 37.Visalberghi E., Sabbatini G., Spagnoletti N., Andrade F. R. D., Ottoni E., Izar P., Fragaszy D. 2008. Physical properties of palm fruits processed with tools by wild bearded capuchins (Cebus libidinosus). Am. J. Primatol. 70, 884–891 10.1002/ajp.20578 (doi:10.1002/ajp.20578) [DOI] [PubMed] [Google Scholar]
- 38.Koya O. A. 2006. Palm nut cracking under repeated impact load. J. Appl. Sci. 11, 2471–2475 [Google Scholar]
- 39.Pouydebat E., Gorce P., Bels V., Coppens Y. 2006. Substrate optimization in nut cracking by capuchin monkeys (Cebus apella). Am. J. Primatol. 68, 1017–1024 10.1002/ajp.20291 (doi:10.1002/ajp.20291) [DOI] [PubMed] [Google Scholar]
- 40.Visalberghi E., Addessi E., Truppa V., Spagnoletti N., Ottoni E., Izar P., Fragaszy D. 2009. Selection of effective stone tools by wild bearded capuchin monkeys. Curr. Biol. 19, 213–217 10.1016/j.cub.2008.11.064 (doi:10.1016/j.cub.2008.11.064) [DOI] [PubMed] [Google Scholar]
- 41.Izawa K., Mizuno A. 1977. Palm-fruit cracking behaviour of wild black-capped capuchin (Cebus apella). Primates 18, 773–792 10.1007/BF02382930 (doi:10.1007/BF02382930) [DOI] [Google Scholar]
- 42.Sakura O., Matsuzawa T. 1991. Flexibility of wild chimpanzee nut-cracking behavior using stone hammers and anvils: an experimental analysis. Ethology 87, 237–248 10.1111/j.1439-0310.1991.tb00249.x (doi:10.1111/j.1439-0310.1991.tb00249.x) [DOI] [Google Scholar]
- 43.Mora R., de la Torre I. 2005. Percussion tools in Olduvai Beds I and II (Tanzania): implications for early human activities. J. Anthropol. Archaeol. 24, 179–192 10.1016/j.jaa.2004.12.001 (doi:10.1016/j.jaa.2004.12.001) [DOI] [Google Scholar]
- 44.Goren-Inbar N., Sharon G., Melamed Y., Kislev M. 2002. Nuts, nut cracking, and pitted stones at Gesher Benot Ya'aqov, Israel. Proc. Natl Acad. Sci. USA 99, 2455–2460 10.1073/pnas.032570499 (doi:10.1073/pnas.032570499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee R. The !Kung San: men, women, and work, in a foraging society. Cambridge,: MA: Harvard University Press; 1979. [Google Scholar]
- 46.Yellen J. E. 1977. Archaeological approaches to the present. Models for reconstructing the past. New York, NY: Academic Press [Google Scholar]
- 47.Bock J. 2005. What makes a competent adult forager? In Hunter–gatherer childhoods (eds Hewlett B., Lamb M.), pp. 109–128 New York, NY: Aldine de Gruyter [Google Scholar]
- 48.Koya O. A., Faborode M. O. 2005. Mathematical modeling of palm nut cracking based on Hertz's theory. J. Biosyst. Eng. 95, 405–412 [Google Scholar]
- 49.Luedtke B. E. 1992. An archaeologist's guide to chert and flint. Los Angeles, CA: Institute of Archaeology, University of California [Google Scholar]
- 50.Cotterell B., Kamminga J. 1987. The formation of flakes. Am. Antiquity 52, 675–708 10.2307/281378 (doi:10.2307/281378) [DOI] [Google Scholar]
- 51.Cotterell B., Kamminga J., Dickson F. D. 1985. The essential mechanics of conchoidal flaking. Int. J. Fract. 20, 205–221 10.1007/BF00125471 (doi:10.1007/BF00125471) [DOI] [Google Scholar]
- 52.Cotterell B., Kamminga J. 1990. Mechanics of pre-industrial technology. Cambridge, UK: Cambridge University Press [Google Scholar]
- 53.Pelegrin J. 2005. Remarks about archaeological techniques and methods of knapping: elements of a cognitive approach to stone knapping. In Stone knapping: the necessary conditions for a uniquely hominin behavior (eds Roux V., Bril B.), pp. 23–34 Cambridge, UK: McDonald Institute for Archaeological Research [Google Scholar]
- 54.Schick K. D., Toth N., Garufi G., Savage-Rumbaugh E. S., Rumbaugh D., Sevcik R. 1999. Continuing investigations into the stone tool-making and tool-using capabilities of a bonobo (Pan paniscus). J. Archaeol. Sci. 26, 821–832 10.1006/jasc.1998.0350 (doi:10.1006/jasc.1998.0350) [DOI] [Google Scholar]
- 55.Toth N., Schick K. D., Savage-Rumbaugh E. S., Sevcik R. A., Rumbaugh D. M. 1993. Pan the tool-maker: investigations into the stone tool-making and tool-using capabilities of a bonobo. J. Archaeol. Sci. 20, 81–91 10.1006/jasc.1993.1006 (doi:10.1006/jasc.1993.1006) [DOI] [Google Scholar]
- 56.Nonaka T., Bril B., Rein R. 2010. How do stone knappers predict and control the outcome of flaking? Implications for understanding early stone tool technology. J. Hum. Evol. 59, 155–167 10.1016/j.jhevol.2010.04.006 (doi:10.1016/j.jhevol.2010.04.006) [DOI] [PubMed] [Google Scholar]
- 57.Dibble H. L., Rezek Z. 2009. Introducing a new experimental design for controlled studies of flake formation: results for exterior platform angle, platform depth, angle of blow, velocity, and force. J. Archaeol. Sci. 36, 1945–1954 10.1016/j.jas.2009.05.004 (doi:10.1016/j.jas.2009.05.004) [DOI] [Google Scholar]
- 58.Shelley P. H. 1990. Variation in lithic assemblages: an experiment. J. Field Archaeol. 17, 187–193 [Google Scholar]
- 59.Andrefsky W., Jr 1998. Lithics: macroscopic approaches to analysis. Cambridge, UK: Cambridge University Press [Google Scholar]
- 60.Inizan M.-L., Reduron-Ballinger M., Roche H., Tixier J. 1999. Technology and terminology of knapped stone. Nanterre, Paris: Cercle de Recherches et d'Etudes Préhistoriques [Google Scholar]
- 61.Bril B., Foucart J. 2005. Enacting the perception of the affordances of potential tools. I: The case of children hammering. In Studies in perception and action VIII (eds Heft H., Marsh K. L.), pp. 3–6 Mahwah, NJ: Lawrence Erlbaum Associates, Inc [Google Scholar]
- 62.Bril B., Roux V., Dietrich G. 2005. Stone knapping: Khambhat (India), a unique opportunity? In Stone knapping: the necessary conditions for a uniquely hominin behavior (eds Roux V., Bril B.), pp. 53–71 Cambridge, UK: McDonald Institute for Archaeological Research [Google Scholar]
- 63.Bril B., Roux V., Dietrich G. 2000. Habiletés impliquées dans la taille des perles en calcédoine. Caractéristiques motrices et cognitives d'une action situeé complexe. In Cornaline de l'inde: Des Pratiques Techniques de Cambay Aux Techno-systÀmes de l'Indus (ed. Roux V.), pp. 207–332 Paris, France: Editions de la MSH [Google Scholar]
- 64.Foucart J. 2006. Etude comparee des habiletes operatoires et motrices de l'homme et du chimpanze pour une utilisation d'outils transprimatique: le cassage de noix. PhD thesis, Ecole des Hautes Etudes en Sciences Sociales, Paris, France. [Google Scholar]
- 65.Roux V., Bril B., Dietrich G. 1995. Skills and learning difficulties involved in stone knapping. World Archaeol. 27, 63–87 10.1080/00438243.1995.9980293 (doi:10.1080/00438243.1995.9980293) [DOI] [Google Scholar]
- 66.Nonaka T., Bril B. 2011. Nesting of asymmetric functions in skilled bimanual action: dynamics of hammering behavior of bead craftsmen. Hum. Mov. Sci. 81 10.1016/j.humov.2010.08.013 (doi:10.1016/j.humov.2010.08.013) [DOI] [PubMed] [Google Scholar]
- 67.Bernstein N. A. 1996. On dexterity and its development. In Dexterity and its development (eds Latash M. L., Turvey M. T.), pp. 1–235 Hillsdale, NJ: Lawrence Erlbaum Associates [Google Scholar]
- 68.Ericsson K. A., Lehmann A. C. 1996. Expert and exceptional performance: evidence of maximal adaptation to task constraints. Annu. Rev. Psychol. 47, 273–305 10.1146/annurev.psych.47.1.273 (doi:10.1146/annurev.psych.47.1.273) [DOI] [PubMed] [Google Scholar]
- 69.Biryukova E. V., Roby-Brami A., Frolov A. A., Mokhtari M. 2000. Kinematics of human arm reconstructed from spatial tracking system recording. J. Biomech. 33, 985–995 10.1016/S0021-9290(00)00040-3 (doi:10.1016/S0021-9290(00)00040-3) [DOI] [PubMed] [Google Scholar]
- 70.Kortlandt A. 1986. The use of stone tools by wild-living chimpanzees and earliest hominids. J. Hum. Evol. 15, 77–132 10.1016/S0047-2484(86)80068-9 (doi:10.1016/S0047-2484(86)80068-9) [DOI] [Google Scholar]
- 71.Visalberghi E., Fragaszy D., Ottoni E., Izar P., de Oliveira M. G., Andrade F. R. D. 2007. Characteristics of hammer stones and anvils used by wild bearded capuchin monkeys (Cebus libidinosus) to crack open palm nuts. Am. J. Phys. Anthropol. 132, 426–444 10.1002/ajpa.20546 (doi:10.1002/ajpa.20546) [DOI] [PubMed] [Google Scholar]
- 72.Bril B., Dietrich G., Byriukova L., Roby-Brami A., Roux V. 2001. Hammering, adaptation to tool properties and raw material. Poster presentation. International Workshop Knapping stone, a uniquely hominid behavior?, Abbaye des Premontrés, Pont-à-Mousson, 21–24 novembre
- 73.Kim S. G., Ashe J., Hendrich K., Ellermann J. M., Merkle H., Ugurbil K., Georgopoulos A. P. 1993. Functional magnetic resonance imaging of motor cortex: hemispheric asymmetry and handedness. Science 261, 615–617 10.1126/science.8342027 (doi:10.1126/science.8342027) [DOI] [PubMed] [Google Scholar]
- 74.McManus I. C. 1985. Handedness, language dominance and aphasia: a genetic model. Psychol. Med. 8, 1–40 [PubMed] [Google Scholar]
- 75.Sainburg R. L. 2000. Differences in control of limb dynamics during dominant and nondominant arm reaching. J. Neurophysiol. 83, 2661–2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guiard Y. 1987. Asymmetric division of labor in human skilled bimanual action: the kinematic chain as a model. J. Motor Behav. 19, 486–517 [DOI] [PubMed] [Google Scholar]
- 77.Steele J., Uomini N. 2009. Can the archaeology of manual specialization tell us anything about language evolution? A survey of the state of play. Camb. Archaeol. J. 19, 97–110 10.1017/S0959774309000067 (doi:10.1017/S0959774309000067) [DOI] [Google Scholar]
- 78.Guiard Y. 1987. Precursors to what? Theory is wanted for handedness in humans. Open peer commentary on ‘Primate handedness reconsidered’, by P. F. MacNeilage, M. G. Studdert-Kennedy & B. Lindblom. Behav. Brain Sci. 10, 276–277 10.1017/S0140525X00047816 (doi:10.1017/S0140525X00047816) [DOI] [Google Scholar]
- 79.Carson R. G. 1993. Manual asymmetries: old problems and new directions. Hum. Mov. Sci. 12, 479–506 10.1016/0167-9457(93)90001-6 (doi:10.1016/0167-9457(93)90001-6) [DOI] [Google Scholar]
- 80.Sainburg R. L., Eckhardt R. B. 2005. Optimization through lateralization: the evolution of handedness. Behav. Brain Sci. 28, 611–612 10.1017/S0140525X05440108 (doi:10.1017/S0140525X05440108) [DOI] [Google Scholar]
- 81.Wang J., Sainburg R. L. 2007. The dominant and nondominant arms are specialized for stabilizing different features of task performance. Exp. Brain Res. 178, 565–570 10.1007/s00221-007-0936-x (doi:10.1007/s00221-007-0936-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hopkins W. D., Rabinowitz D. M. 1997. Manual specialisation and tool use in captive chimpanzees (Pan troglodytes): the effect of unimanual and bimanual strategies on hand preference. Laterality 2, 267–277 10.1080/135765097397486 (doi:10.1080/135765097397486) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hopkins W. D., Fernanadez-Carriba S., Wesley M. J., Hostetter A., Pilcher D., Poss S. 2001. The use of bouts and frequencies in the evaluation of hand preferences for a coordinated bimanual task in chimpanzees (Pan troglodytes): an empirical study comparing two different indices of laterality. J. Comp. Psychol. 115, 294–299 10.1037/0735-7036.115.3.294 (doi:10.1037/0735-7036.115.3.294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hopkins W. D. 2006. Comparative and familial analysis of handedness in great apes. Psychol. Bull. 132, 538–559 10.1037/0033-2909.132.4.538 (doi:10.1037/0033-2909.132.4.538) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Macellini S., Maranesi M., Bonini L., Simone L., Rozzi S., Ferrari P. F., Fogassi L. 2012. Individual and social learning processes involved in the acquisition and generalization of tool use in macaques. Phil. Trans. R. Soc. B 367, 24–36 10.1098/rstb.2011.0125 (doi:10.1098/rstb.2011.0125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roby-Brami A., Hermsdörfer J., Roy A. C., Jacobs S. 2012. A neuropsychological perspective on the link between language and praxis in modern humans. Phil. Trans. R. Soc. B 367, 144–160 10.1098/rstb.2011.0122 (doi:10.1098/rstb.2011.0122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stout D., Chaminade T. 2012. Stone tools, language and the brain in human evolution. Phil. Trans. R. Soc. B 367, 75–87 10.1098/rstb.2011.0099 (doi:10.1098/rstb.2011.0099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Frey S. H., Povinelli D. J. 2012. Comparative investigations of manual action representations: evidence that chimpanzees represent the costs of potential future actions involving tools. Phil. Trans. R. Soc. B 367, 48–58 10.1098/rstb.2011.0189 (doi:10.1098/rstb.2011.0189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hopkins W. D., Russell J. L., Schaeffer J. A. 2012. The neural and cognitive correlates of aimed throwing in chimpanzees: a magnetic resonance image and behavioural study on a unique form of social tool use. Phil. Trans. R. Soc. B. 367, 37–47 10.1098/rstb.2011.0195 (doi:10.1098/rstb.2011.0195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Orban G. A., Van Essen D., Vanduffel W. 2004. Comparative mapping of higher visual areas in monkeys and humans. Trends Cogn. Sci. 8, 315–324 10.1016/j.tics.2004.05.009 (doi:10.1016/j.tics.2004.05.009) [DOI] [PubMed] [Google Scholar]
- 91.Zilles K. 2005. Evolution of the human brain and comparative cyto- and receptor architecture. In From monkey brain to human brain: a Fyssen Foundation symposium (eds Dehaene S., Duhamel J. R., Hauser M. D., Rizzolatti G.), pp. 41–56 Cambridge, MA: MIT Press [Google Scholar]
- 92.Orban G. A., Claeys K., Nelissen K., Smans R., Sunaert S., Todd J. T., Wardak C., Durand J. B., Vanduffel W. 2006. Mapping the parietal cortex of human and non-human primates. Neuropsychologia 44, 2647–2667 10.1016/j.neuropsychologia.2005.11.001 (doi:10.1016/j.neuropsychologia.2005.11.001) [DOI] [PubMed] [Google Scholar]
- 93.Walker A. 2009. The strength of apes and the speed of humans. Curr. Anthropol. 50, 229–234 10.1086/592023 (doi:10.1086/592023) [DOI] [PubMed] [Google Scholar]
- 94.Hopkins W. D. 2010. The comparative neuropsychology of tool use in primates with specific reference to chimpanzees and capuchin monkeys. In Primate neuroethology (eds Platt M., Ghazanfar A.), pp. 587–614 Oxford, UK: Oxford University Press [Google Scholar]
- 95.Lewis J. W. 2006. Cortical networks related to human use of tools. Neuroscience 2, 211–231 [DOI] [PubMed] [Google Scholar]
- 96.Ramayya A. G., Glasser M. F., Rilling J. K. 2010. A DTI investigation of neural substrates supporting tool use. Cereb. Cortex 20, 507–516 10.1093/cercor/bhp141 (doi:10.1093/cercor/bhp141) [DOI] [PubMed] [Google Scholar]
- 97.Johnson-Frey S. H., Newman-Norlund R., Crafton S. T. 2005. A distributed left hemisphere network active during planning of everyday tool use skills. Cereb. Cortex 15, 681–695 10.1093/cercor/bhh169 (doi:10.1093/cercor/bhh169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Goldenberg G., Hagmann S. 1998. Tool use and mechanical problem solving in apraxia. Neuropsychologia 36, 581–589 10.1016/S0028-3932(97)00165-6 (doi:10.1016/S0028-3932(97)00165-6) [DOI] [PubMed] [Google Scholar]
- 99.Goldenberg G., Spatt J. 2009. The neural basis of tool use. Brain 132, 1645–1655 10.1093/brain/awp080 (doi:10.1093/brain/awp080) [DOI] [PubMed] [Google Scholar]
- 100.Obayashi S., Suhara T., Kawabe K., Okauchi T., Maeda J., Akine Y., Onoe H., Iriki A. 2001. Functional brain mapping of monkey tool use. Neuroimage 14, 853–861 10.1006/nimg.2001.0878 (doi:10.1006/nimg.2001.0878) [DOI] [PubMed] [Google Scholar]
- 101.Johnson-Frey S. H. 2004. The neural bases of complex tool use in humans. Trends Cogn. Sci. 8, 71–78 10.1016/j.tics.2003.12.002 (doi:10.1016/j.tics.2003.12.002) [DOI] [PubMed] [Google Scholar]
- 102.Rosenbaum D. A. 2005. The Cinderella of psychology: the neglect of motor control in the science of mental life and behaviour. Am. Psychol. 60, 308–317 10.1037/0003-066X.60.4.308 (doi:10.1037/0003-066X.60.4.308) [DOI] [PubMed] [Google Scholar]
- 103.Ramnani N. 2006. The primate cortico-cerebellar system: anatomy and function. Nat. Rev. Neurosci. 7, 511–522 10.1038/nrn1953 (doi:10.1038/nrn1953) [DOI] [PubMed] [Google Scholar]
- 104.Cantalupo C., Hopkins W. D. 2010. The cerebellum and its contribution to complex tasks in higher primates: a comparative perspective. Cortex 46, 821–830 10.1016/j.cortex.2009.10.004 (doi:10.1016/j.cortex.2009.10.004) [DOI] [PubMed] [Google Scholar]
- 105.Herculano-Houzel S. 2010. Coordinated scaling of cortical and cerebellar numbers of neurons. Front. Neuroanat. 4, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.MacLeod C. E., Zilles K., Schleicher A., Rilling J. K., Gibson K. R. 2003. Expansion of the neocerebellum in Hominoidea. J. Hum. Evol. 44, 401–429 10.1016/S0047-2484(03)00028-9 (doi:10.1016/S0047-2484(03)00028-9) [DOI] [PubMed] [Google Scholar]
- 107.Rilling J. K., Insel T. R. 1998. Evolution of the primate cerebellum: differences in relative volume among monkeys, apes and humans. Brain Behav. Evol. 52, 308–314 10.1159/000006575 (doi:10.1159/000006575) [DOI] [PubMed] [Google Scholar]
- 108.Weaver A. H. 2005. Reciprocal evolution of the cerebellum and neocortex in fossil humans. Proc. Natl Acad. Sci. USA 102, 3576–3580 10.1073/pnas.0500692102 (doi:10.1073/pnas.0500692102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Whiting B. A., Barton R. A. 2003. The evolution of the cortico-cerebellar complex in primates: anatomical connections predict patterns of correlated evolution. J. Hum. Evol. 44, 3–10 10.1016/S0047-2484(02)00162-8 (doi:10.1016/S0047-2484(02)00162-8) [DOI] [PubMed] [Google Scholar]
- 110.Smaers J. B., Steele J., Zilles K. 2011. Modeling the evolution of cortico-cerebellar systems in primates. Ann. NY Acad. Sci. 1225, 176–190 10.1111/j.1749-6632.2011.06003.x (doi:10.1111/j.1749-6632.2011.06003.x) [DOI] [PubMed] [Google Scholar]
- 111.Middleton F. A., Strick P. L. 2000. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res. Rev. 31, 236–250 10.1016/S0165-0173(99)00040-5 (doi:10.1016/S0165-0173(99)00040-5) [DOI] [PubMed] [Google Scholar]
- 112.Doyon J., Penhune V., Ungerleider L. G. 2003. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia 41, 252–262 10.1016/S0028-3932(02)00158-6 (doi:10.1016/S0028-3932(02)00158-6) [DOI] [PubMed] [Google Scholar]
- 113.Obayashi S., Suhara T., Nagai Y., Maeda J., Hihara S., Iriki A. 2002. Macaque prefrontal activity associated with extensive tool use. Neuroreport 13, 2349–2354 10.1097/00001756-200212030-00036 (doi:10.1097/00001756-200212030-00036) [DOI] [PubMed] [Google Scholar]
- 114.Albus J. S. 1971. A theory of cerebellar function. Math. Biosci. 10, 25–61 10.1016/0025-5564(71)90051-4 (doi:10.1016/0025-5564(71)90051-4) [DOI] [Google Scholar]
- 115.Marr D. 1969. A theory of cerebellar cortex. J. Physiol. Lond. 202, 437–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Martin T. A., Keating J. G., Goodkin H. P., Bastian A. J., Thach W. T. 1996. Throwing while looking through prisms. 2. Specificity and storage of multiple gaze-throw calibrations. Brain 119, 1199–1211 10.1093/brain/119.4.1199 (doi:10.1093/brain/119.4.1199) [DOI] [PubMed] [Google Scholar]
- 117.Puttemans V., Wenderoth N., Swinnen S. P. 2005. Changes in brain activation during the acquisition of a multifrequency bimanual coordination task: from the cognitive stage to advanced levels of automaticity. J. Neurosci. 25, 4270–4278 10.1523/JNEUROSCI.3866-04.2005 (doi:10.1523/JNEUROSCI.3866-04.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Doyon J., Benali H. 2005. Reorganization and plasticity in the adult brain during learning of motor skills. Curr. Opin. Neurobiol. 15, 161–167 10.1016/j.conb.2005.03.004 (doi:10.1016/j.conb.2005.03.004) [DOI] [PubMed] [Google Scholar]
- 119.Diedrichsen J., Hashambhoy Y., Rane T., Shadmehr R. 2005. Neural correlates of reach errors. J. Neurosci. 25, 9919–9931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Maschke M., Gomez C. M., Ebner T. J., Konczak J. 2004. Hereditary cerebellar ataxia progressively impairs force adaptation during goal-directed arm movements. J. Neurophysiol. 91, 230–238 10.1152/jn.00557.2003 (doi:10.1152/jn.00557.2003) [DOI] [PubMed] [Google Scholar]