Abstract
The ability to adjust one's ongoing actions in the anticipation of forthcoming task demands is considered as strong evidence for the existence of internal action representations. Studies of action selection in tool use reveal that the behaviours that we choose in the present moment differ depending on what we intend to do next. Further, they point to a specialized role for mechanisms within the human cerebellum and dominant left cerebral hemisphere in representing the likely sensory costs of intended future actions. Recently, the question of whether similar mechanisms exist in other primates has received growing, but still limited, attention. Here, we present data that bear on this issue from a species that is a natural user of tools, our nearest living relative, the chimpanzee. In experiment 1, a subset of chimpanzees showed a non-significant tendency for their grip preferences to be affected by anticipation of the demands associated with bringing a tool's baited end to their mouths. In experiment 2, chimpanzees' initial grip preferences were consistently affected by anticipation of the forthcoming movements in a task that involves using a tool to extract a food reward. The partial discrepancy between the results of these two studies is attributed to the ability to accurately represent differences between the motor costs associated with executing the two response alternatives available within each task. These findings suggest that chimpanzees are capable of accurately representing the costs of intended future actions, and using those predictions to select movements in the present even in the context of externally directed tool use.
Keywords: motor planning, action selection, chimpanzee tool use, context sensitivity, cerebral asymmetry
1. Introduction
In the late nineteenth century, Donders [1] conducted a pioneering experiment in which he contrasted the times required to execute a simple (respond to the appearance of a stimulus) versus choice (select one among two responses based on the identity of the stimulus) response. The difference in response latencies between these conditions was interpreted as reflecting the additional time required for the cognitive process of selection. One of the many things that we have learned in the intervening century is that, independent of the hand used, the human left cerebral hemisphere plays a dominant role in this fundamental process [2]. In right-handed adults (some 90% of the population), increased activity is detected in the left posterior parietal cortex (along the intraparietal sulcus, IPS), inferior frontal sulcus extending into the rostral middle frontal gyrus (rMFG) and dorsal premotor cortex (dPMC) [3]. This network of regions may form a core system for response selection.
(a). Response selection versus action selection
In the typical response-selection task, the mapping between sensory stimuli and motor responses is both fixed and explicitly known by the actor (e.g. press the left key when the light is blue, or the right key when the light is red). This differs critically from the demands that typify real-world action selection, where there are often numerous potential responses (movements) that could be used to solve the problem at hand [4]. For instance, consider the range of hand postures that might suffice to grasp a mug of coffee and bring it to one's mouth for a drink. While much remains to be learned about how this degrees-of-freedom problem is solved, it is generally accepted that action selection is informed by predictions of the motor costs that would accompany various response options. To the extent that these forecasts are accurate, they enable us to select actions that lead to successful (rewarding) solutions to the problem while minimizing costs [5].
(b). Neural substrates of response and action selection
In order to gain further insights into the role of prediction in action selection, we have used a simplified task in which participants are required to choose whether to engage an object (e.g. a handle) appearing in different orientations in an under- versus over-hand grip (e.g. a power grip). In all trials of these experiments either grip is physically possible, the question is which is preferable. We consistently find that participants prefer to grasp stimulus objects using the alternative that is perceived as least costly (or awkward) during overt execution. This is even true when they are asked to make their choices under prospective grip-selection (PGS) conditions, where movements are never actually undertaken [6]. The degree of correspondence between grip preferences in overt and PGS tasks suggests that even in the complete absence of feedback, participants are able to predict the likely motor costs of future actions with high fidelity, and select actions accordingly. Furthermore, this ability seems to be retained by many patients even during periods of acute [7] or chronic [8,9] limb disuse, or following amputation [10].
Our early functional magnetic resonance imaging work on prospective power grip selection revealed increases in the dPMC, superior parietal lobule (SPL) and along the IPS [11]. More recent findings show that prospective precision grip selection based on either hand engages the entire core network of regions implicated in response selection (left IPS, rMFG and dPMC), as introduced earlier. In addition, we find increases within a number of other brain regions including the bilateral cerebellum, SPL, pre-supplementary motor area, right dPMC, as well as in the left anterior intraparietal sulcus (aIPS) and left ventral premotor cortex (vPMC) [12]. After physical practice, these same areas come to represent PGS decisions based on the use of a formerly novel grasping tool that differs mechanically and dynamically from the natural limbs.
One possible interpretation is that these regions participate in estimating the motor costs associated with the two grip alternatives: under- or over-hand. An emerging view is that the cerebellum supports forward internal models that predict the likely sensory consequences of a motor command slightly in advance of the actual sensory feedback that accompanies movement [13,14]. These feed-forward predictions are thought to update multi-sensory estimates of the state of the body (e.g. posture of the upper limbs), represented in the parietal cortex (particularly the SPL) [13,15]. An interesting possibility is that these same predictive mechanisms might play a role in forecasting the long-range consequences of response alternatives [5,16,17]. This information could be valuable to action selection by providing a means of estimating the motor costs (energy expenditure, awkwardness) of candidate responses and their potential to achieve the desired reward state, representations that may be computed subcortically in the basal ganglia and/or brain stem [18].
(c). Cerebral asymmetry
The left cerebral asymmetry for PGS responses in vPMC and the aIPS is striking in comparison with the bilateral engagement of dPMC and the SPL. In humans, left vPMC is situated in the inferior portion of the precentral gyrus located immediately caudal to the pars opercularis (or Broca's area), which is known to be involved in a variety of language-, and a growing list of action-related functions [19,20]. In monkeys, rostral ventral premotor cortex (F5) is the putative homologue for pars opercularis [21], and is implicated in a variety of higher level motor functions including the representation of action goals [22]. Caudal ventral premotor cortex (F4), by contrast, is implicated in constructing multi-sensory representations of peripersonal space and of limb and head movements [23]. Our present understanding of vPMC functions in the human brain is limited. Though highly speculative, it is possible that with the emergence of language functions in rostral vPMC, the human left caudal vPMC has become more heavily involved in the representations of action goals. Some work demonstrating increased activity in this region during action perception appears consistent with this view [24].
There is mounting evidence for the role of the human aIPS in action representation [25,26], and more than a century of data exist implicating the left parietal and frontal cortex in manual praxis [27,28]. Asymmetrical involvement of the left aIPS in these planning tasks may be related to the fact that this region has direct anatomical connections with the vPMC [29], allowing these two areas to operate as a functional unit. Following the line of reasoning above, it may be that the left parietal asymmetry is a consequence of changes in the role of caudal vPMC precipitated by the emergence of language in rostral vPMC. This is highly speculative; however, recent findings do suggest that cerebral asymmetries in posterior parietal action representations (Brodmann area 40) are correlated with lateralization of language in Broca's area (Brodmann area 44/45) and its right hemisphere homologue [30].
(d). Context sensitivity in action selection
In speech, it is well known that articulation of a phoneme is affected by the identity of upcoming phonemes. Analogous effects of task context have been reported in a wide variety of manual behaviours including: typing, handwriting, manual aiming and prehension (see review in Johnson-Frey et al. [31]). Action-selection tasks have proven to be especially valuable in studying context effects [32], and have shed light on the properties of underlying movement representations [33]. Grip-selection tasks involving tools have proven to be particularly useful in revealing details of motor planning in human infants and adults [34]. Context effects in manual action selection have been shown to emerge during the first 2 years of life [35–38], and development can be accelerated with training [39]. While the physical properties of the effectors may contribute to some context effects [40], many of these findings are difficult to interpret without acknowledging a role for internal representations of task demands that go beyond immediately available sensory information [41,42]. This point is critical to understanding behaviours where the choice of a response can be influenced by the goal of the larger action sequence in which it is embedded. As a consequence, the responses that we choose in the present moment differ depending on how they might impact the costs of what we intend to do next.
(e). Evidence for predictive action selection in non-human primates
Whether similar predictive mechanisms exist in other species is an important and challenging question. A first step is to ask whether evidence can be found for context sensitivity in action-selection behaviours similar to those identified in humans. Several studies have tested whether, like humans, monkeys show an end-state comfort effect, i.e. whether they adapt their initial grip of an object in a way that reflects anticipation of movements required to achieve the subsequent goal of the task [43]. Across two foraging tasks, Weiss et al. [44] provided evidence for end-state comfort effects in the grip preferences of cotton-top tamarins. They later extended this finding to include lemurs, a group of primates even more distantly related to humans [45]. More recently, Nelson et al. [46] demonstrated that after a limited number of trials, most of the rhesus macaques they tested were able to develop a grip to bring a baited spoon to their mouths in an efficient manner (radial grip with the thumbside of the hand towards the bowl of the spoon) across 12 trials. An important issue is whether these behaviours reflect the learning of stimulus-response contingencies, or involve anticipation of motor costs. Evidence shows that this efficient grip was learned during the course of testing. This can be derived from the fact that, on difficult trials, the monkeys' performances improved from 28 per cent efficient grips in the first session of testing to 94 per cent in the second session. Of the six monkeys tested, three switched hands depending on the side towards which the bowl of the spoon was oriented; the remaining three used the same hand (and grip), but changed their body position relative to the spoon. No monkeys switched from an over-hand grip on the easy trials to an under-hand grip on the difficult trials. After this rapid acquisition, the monkeys maintained these efficient strategies even 1 year later when they were retested.
Given that several disparately related primate species have shown some evidence for anticipatory planning in action-selection tasks (i.e. the end-state comfort effect), it is tempting to conclude that this is a quite anciently evolved ability widely present in non-human primates, and perhaps even other mammals. While prospective planning abilities might be critical for tool-use behaviours, they would seem to have evolved in species that are not known to use tools in nature [45]. However, the existing evidence is currently limited to situations in which a simple tool is directed to the subject's mouth. In humans, anticipatory planning in the context of self-directed tool abilities emerges during the first 2 years of life [38]. However, the development of planning in externally directed tool-use tasks is more difficult and lags behind even when feedback is enhanced [37]. It is not known whether non-human primates show this more advanced form of action planning. For example, do they exhibit the end-state comfort effect when grasping a tool and directing it towards a goal located in extra-personal space?
Here, we report findings from studies (conducted between 2001 and 2004) that we designed and conducted to explore this question in our nearest living relatives, chimpanzees. Unlike lemurs, tamarins or rhesus macaques, chimpanzees both use and make tools as part of their natural ecology, and the particular individuals we tested had a long history of doing so in the laboratory in a wide variety of tasks. Like humans, chimpanzees regularly perform actions with tools that are directed towards the self (e.g. eating termites from a stick) and towards external targets (e.g. inserting a stick into a termite nest). Our strategy was to use an analogue of the procedure used by McCarty et al. [38] to study the development of the end-state comfort effect in spoon-feeding in toddlers (experiment 1), and to then use these results as a platform to study the more advanced abilities involved in using a tool to obtain a food reward in extra-personal space (experiment 2).
2. Experiment 1: grip selection in a self-directed tool-use task in chimpanzees
In our initial investigation, we sought to identify whether our chimpanzees would display an end-state comfort effect in their grip selection during a self-directed feeding task with similarities to the one used to test macaques following the study of Nelson et al. [46]. The stimulus was a horizontal tool (dowel) with either the left- or right-end baited. If action selection is influenced by the intended subsequent movement (bringing the baited end of the handle to the mouth), then we reasoned that subjects would prefer the grip that places the thumbside of the hand towards the baited end; i.e. they would prefer a radial grip. Choosing the alternative ulnar grip would be a more costly option, making it more difficult to bring the food to the mouth. Grip preferences in this test condition were compared with the control in which the choice of grip was irrelevant because both ends of the tool were baited.
(a). Method
(i). Subjects
Five adult female chimpanzees (age range = 13 years, seven months to 14 years, six months) participated in the study. The subjects were housed at the University of Louisiana and had participated in numerous dowel-use studies over a period of over 10 years [47].
(ii). Apparatus
A 30 cm long dowel (plastic pipe) was used in the experiment. In the experimental conditions, the dowel rested horizontally on two L-shaped brackets (approx. 20 cm apart and 50 cm above the floor) that were attached to the wall of the subjects' testing unit. One or both ends of the dowel could be easily covered with a highly desirable food reward (such as peanut butter or honey).
(b). Procedure
(i). Orientation and food preference
We placed 14 (unbaited) replicas of the dowel in the subjects' indoor–outdoor living environment. This allowed the chimpanzees to interact and familiarize themselves with the tool. If the apes threw the dowels out of the enclosure, then they were returned by the caretakers. We made these replicas available to the apes in their living environment every day throughout all phases of the experiment.
One week after we introduced the dowels, the primary trainer and the caretaker (hereafter referred to as the trainer) individually brought each ape into an indoor testing unit. The test unit was connected to an outdoor waiting area by a shuttle door that could be remotely opened and closed to allow the ape the opportunity to enter and exit the test unit. The apes were highly familiar with this procedure (see Povinelli [47]). The trainer inserted two dowels into the test unit and held them as the ape approached. Each dowel was baited with a different reward. The first dowel chosen to eat from was recorded. Each ape was administered 10 trials. The first reward chosen seven or more times out of 10 was considered their preferred reward. Any ape not exhibiting a preference was given both rewards randomly and equally across conditions throughout the study.
(ii). Test orientation
We conducted a series of unstructured three-trial sessions in which the dowel was placed on the bracket with food baited on both ends. The caretaker allowed each ape to enter the test unit individually, or in pairs, until such a time as they reliably took the dowel from the bracket and consumed the food. The shuttle door was opened as soon as the apes had finished eating the bait and dropped the dowel, or after 1 min from the time they entered the test unit. The apes then began testing sessions.
(iii). Testing
Testing took place in the test unit and consisted of 60 trials per ape, with a maximum of six trials per day. Before each trial began, the trainer baited a dowel on both ends and placed it horizontally on the brackets on the wall to the ape's left as he or she entered. The ape then entered the testing unit and the shuttle door was closed. After the ape grasped the dowel and consumed the reward, the trainer opened the shuttle door and allowed the ape to exit.
Testing consisted of two types of trials: (i) on test trials only, one end of the dowel was baited (on half of the test trials the right end was baited and on the other half the left end was baited) and (ii) on control trials both ends of the dowel were baited. Apes were given 40 test trials (20 baited on the right end and 20 baited on the left end) and 20 control trials. The trials were randomly administered within the following constraints: (i) the same type of trial was not administered on more than three sequential trials, (ii) the numbers of each type of trial were equal for the first and second halves of the study, and (iii) the left/right orientations of the dowels on the test trials were not the same for more than two consecutive trials.
One experimenter was present in the rear of the test unit to control the shuttle door. Every trial was recorded on video with a view that allowed excellent visibility regarding which hand the ape used to grasp the dowel, the position of the hand while reaching for the pipe and the type of grip used (figure 1).
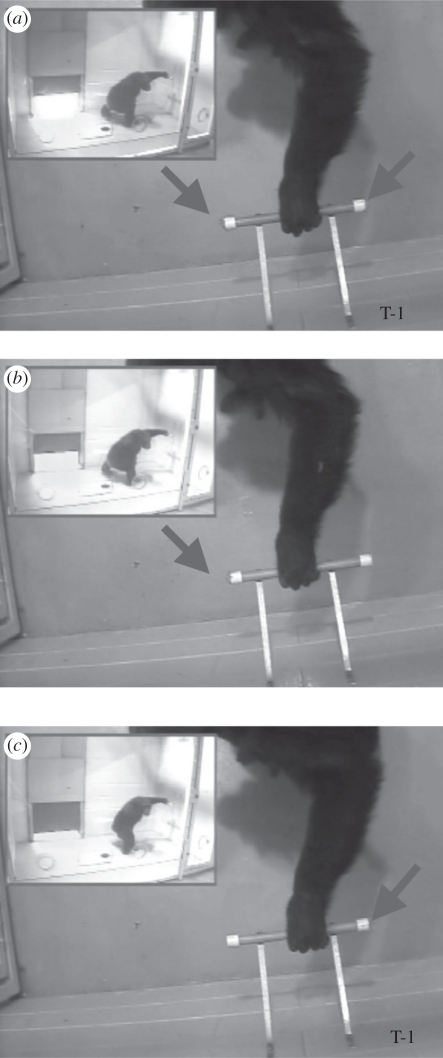
Figure 1.
The subject Brandy grasps the dowel in the three experimental conditions of experiment 1: (a) both-ends baited, (b) right-end baited, and (c) left-end baited. Note that her grip selection is the same in all three cases. Arrows indicate position of the bait (peanut butter).
(iv). Coding and inter-rater reliability
A main rater scored all trials for the orientation of the subject's thumb (up or down). This allowed for an unambiguous determination of whether their grip changed as a function of trial type (i.e. from over-hand to under-hand) as a function of how the dowel was baited (right end, left end and both ends). A secondary rater examined 25 per cent of the trials and agreed with the main rater on 96 per cent of all trials.
(c). Results and discussion
The individual apes grasped the dowel in a variety of ways (over-hand, under-hand, pincer grip, between fingers). However, each ape exhibited a striking consistency in how they did so regardless of the experimental condition. For example, figure 1a–c displays the ape Brandy grasping the dowel across the three-trial types: (i) both ends baited, (ii) right-end baited, and (iii) left-end baited. Notably, she uses the same hand and grip in each condition. Although all apes exhibited some variation, the position of their thumb upon initial grasp (towards the baited end or away from the baited end) provided a highly reliable measure of whether they switched grips as a function of what end of the dowel was baited on the test trials. Figure 2 displays the thumb position (towards or away from the baited end) for each hand (right and left). In seeming contrast to what would be expected if the apes were selecting their actions based on prediction of the motor costs that would be experienced, as a group the apes did not strongly alter their grip as a function of which end was baited. Instead, they simply grasped the dowel in a habitual fashion and inserted the baited end into their mouths. The results of the control trials confirm that the apes had a habitual grip preference: they did not depart from what they displayed in the test trials (table 1). In other words, we saw no evidence that action selection is context-sensitive.
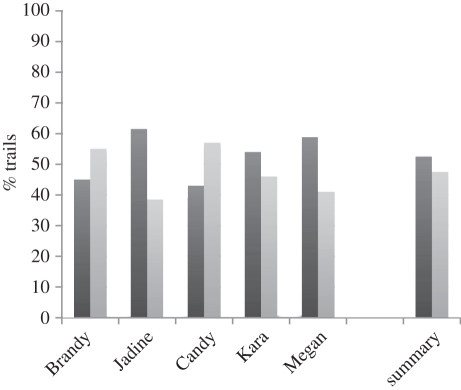
Figure 2.
Grip preferences in experiment 1. Three of the participants (Jadine, Kara and Megan) exhibit a trend towards preferring to grasp the handle with the thumb towards the baited end. This is consistent with what would be expected if the animals were taking the desired end state into consideration when selecting their grasps. These trends were not statistically reliable (see text for details; black bars, thumb towards bait; grey bars, thumb away from bait).
Table 1.
Summary of thumb placement (in per cent of trials) as a function of experimental condition.
| thumb placement | conditions |
||
|---|---|---|---|
| control | baited-left | baited-right | |
| Brandy | |||
| left | 10 | 5 | 15 |
| right | 90 | 95 | 85 |
| Jadinea | |||
| left | 75 | 95 | 78 |
| right | 25 | 5 | 22 |
| Candyb | |||
| left | 89 | 79 | 87 |
| right | 11 | 21 | 13 |
| Kara | |||
| left | 100 | 100 | 95 |
| right | 0 | 0 | 5 |
| Meganc | |||
| left | 90 | 100 | 84 |
| right | 10 | 0 | 16 |
aOne trial was inadvertently not recorded.
bAn experimental error resulted in the administration of 18 control trials and 19 baited left and 23 trials baited right test trials.
cOne trial was not codable owing to the obstruction of the stimuli by the subject's body.
Three of the five apes, however, showed a preference for the thumb towards the baited end of the dowel (radial grip), and one (Jadine) showed a fairly strong bias for the thumb towards the baited end (61.5% or 24/39 of trials; table 1). Interestingly, Jadine is the one ape that showed fairly quick improvement in a previous test, wherein the apes needed to learn to grasp a tool by a non-functional end in order to use the other functional end (see experiment 2 below; [47], experiment 13). Megan also exhibited a similar trend in favour of placing the thumb towards the baited end (59% or 23/39 of the trials; table 1). However, binomial tests (one-tailed, chance = 0.5) indicated that none of the animals (including Jadine and Megan) exhibited a statistically reliable difference between these two grip options (p < 0.09 or smaller in all cases).
These results would appear to indicate that chimpanzees were not representing forthcoming task demands and modifying their initial grip preferences accordingly. None of the individuals showed significant differences between grip preferences in the test condition and those exhibited in the control. This differs dramatically from results demonstrated by toddlers when grasping wooden spoons for self-feeding [38] and previous investigations with monkeys discussed earlier [44–47]. It is tempting to conclude that these apes lack the ability to represent the costs of forthcoming task demands and/or to adapt their responses accordingly even in a self-directed tool-use task. Indeed, in the test trials, the apes frequently wound up with the dowel in what appeared to be an awkward position. For example, an over-hand grasp (palm down) with the thumb oriented away from the baited end of the dowel left the apes in a biomechanically awkward position when bringing the tool to the mouth. However, the apes compensated for this by further rotating their wrists, and/or by tilting their heads. Although this appeared awkward to human observers, nevertheless, the apes, were adept at eating the food off the baited end of the dowel in this manner (figure 3). What we can say with confidence is that in such cases, the ape was required to exert greater motor effort in order to rotate the baited end a longer distance to reach their mouths. Nonetheless, any additional costs associated with ending the handle rotation in an awkward posture may not have been sufficient to result in anticipatory modification of the initial grip. This issue arises in grip-selection studies involving humans where participants show stronger end-state effects when the levels of accuracy for final object positioning are increased [48]. This possibility is addressed in our second experiment where the costs of choosing the incorrect initial grip would be maximal: failure to complete the trial and obtain a food reward. If we are correct, then the apes should exhibit evidence for anticipatory grip selection in this more challenging task.
Figure 3.
In experiment 1, the subject Brandy has just used an over-hand grip (palm down) with thumb pointing away from the baited end of the dowel (figure 1c). As a consequence, she must lift the dowel higher and rotate the baited end towards her mouth. This involves considerably more effort than the movement that results from the thumb towards the baited end.
3. Experiment 2: grip selection in a tool-use task directed towards an external target in chimpanzees
In this study, chimpanzees needed to use a thick dowel to dislodge and obtain a food reward. A critical feature is that the task could only be completed if the animals grasped the dowel with the thumbside of their hand towards its centre. We tested for anticipatory effects by analysing how the apes gripped the dowel when presented in a variety of different orientations.
(a). Method
(i). Subjects
The five chimpanzees from experiment 1, plus two other adult members of their group, participated in the study. At the time the study began, the apes ranged in age from 15 years, four months to 16 years, three months. The apes had participated in numerous studies similar to the one used here (i.e. using a dowel or stick to dislodge a reward from a platform; see Povinelli [47]). However, only one of these studies had systematically altered the orientation of a tool (see Povinelli [47], experiment 13). It is important to note that this previous study (and several related ones) was more cognitively demanding. It required the apes to understand that one end of the tool was functional and the other end was not, and to anticipate this in their initial grasp. In the present study, both ends of the tool were identical and equally functional.
(ii). Apparatus
The apparatus depicted in figure 4 was used. It consisted of a dowel that rested on a bracket that could be horizontally rotated, thus altering how the subject could grasp it. The goal of the task was to grasp the dowel, lift it off the bracket and poke it through a hole in the Plexiglass box to dislodge an apple (or some other round food reward). Once the ape tapped the apple with the dowel, it rolled to within their reach.
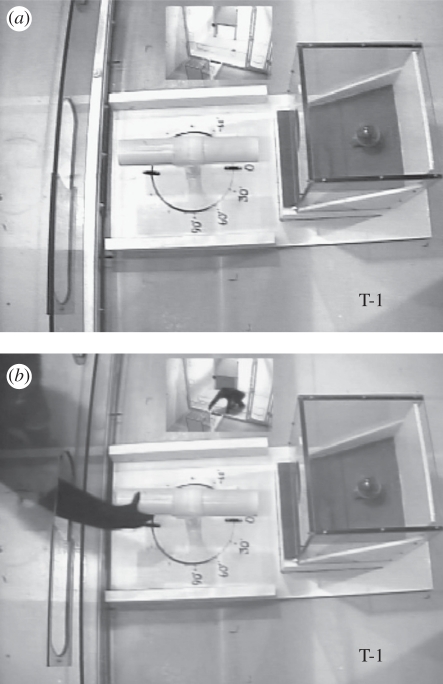
Figure 4.
Experiment 2. (a) The apparatus with the dowel in the 0° orientation. This set-up was used in the demonstration and criterion phase, as well as during the 0° trials of the testing phase. (b) An ape grasps the dowel in preparation to insert and dislodge the goal/reward.
The dowel was 46 cm long and 8 cm in diameter. In order to deter apes from gripping the centre of the dowel, a 10 cm wide Velcro strip was wrapped around the dowel's midpoint. (One ape was allowed a slightly modified dowel, owing to her aversion to the original stimulus. This dowel was 5 cm in diameter and instead of a Velcro strip, two black lines were painted on the dowel.)
(b). Procedure
(i). Familiarization with the dowel
In several sessions in the test unit, each ape was exposed to dowels of varying thicknesses, including ones with Velcro around the centre. No apparatus was present and the apes did not use the dowels to obtain food of any kind. Rather, these sessions simply helped us to identify the maximum thickness the dowel could be and still allow the apes to easily grasp it, and to ensure that the Velcro strip deterred apes from grasping the dowel in the centre.
(ii). Demonstration of the task
The trainer brought each ape into the test unit individually for a demonstration session in which the experimenter demonstrated how to use the dowel to dislodge the food reward from the apparatus. First, the apparatus (figure 4) was positioned on the trainer's side of the Lexan partition with the dowel placed on the bracket in a 0° horizontal orientation (i.e. perpendicular to the apparatus; figure 4). The apparatus was thus positioned directly in front of an opening in the Lexan partition through which the chimpanzees could reach and grasp the dowel. Next, the trainer opened the shuttle door and the ape entered the test unit. The trainer then closed the shuttle door and demonstrated the task by picking up the dowel and dislodging the food by poking it through the hole in the apparatus. The trainer then handed the food to the ape through the hole in the Lexan door. Finally, the trainer opened the shuttle door and ushered the ape out of the test unit.
(iii). Criterion
The criterion consisted of two-trial sessions that immediately followed the demonstration session. The trainer positioned the apparatus directly in front of the opening in the Lexan partition and the dowel was positioned on the bracket directly in front of the response slot (centred in front of the opening in the apparatus) on the experimenter's side of the Lexan. For all criterion trials, the dowel was oriented at 0° (figure 4). After the shuttle door was opened, the ape was given 1 min to enter the test unit and an additional maximum of 1 min to initiate the trial by touching a ready-to-respond (RTR) symbol. This resulted in the hole in the partition opening, thus allowing the ape to reach out and grasp the dowel. A complete response was defined as the ape touching the RTR symbol, grasping the dowel and inserting it through the opening in the apparatus and dislodging the food reward so that it fell down the ramp towards the ape. If the ape did not touch the RTR symbol and/or touch the dowel, then the trial was scored as no response and was immediately re-run. If the ape initiated a trial through the RTR procedure and grasped the dowel but did not complete the response, then the trial was scored as incomplete and not re-run. If any of the following conditions were met, then the response barrier was raised immediately, the ape was ushered out of the test unit, and an incomplete response was recorded: (i) the ape initially lifted the dowel off the bracket using two hands, (ii) the ape displaced the bracket from its place in the spacer board, (iii) the ape touched the apparatus with their hand or arm prior to completing the task, and/or (iv) the dowel fell out of the ape's reach before completing the task.
Any trials scored as incomplete were re-conducted at the end of all testing sessions for all apes. The participants were required to finish four complete trials across two consecutive sessions in order to move into testing.1
(iv). Testing
Testing consisted of 24 two-trial sessions per ape, for a total of 48 test trials (eight for each of six dowel orientations; see below). The configuration of the test unit and procedure remained the same as in the criterion phase except that the dowel was presented in one of the six orientations. Before each trial, the dowel was oriented at 0°, 30°, 60°, 90°, −30° or −60° (with 0° being perpendicular to the Lexan (one end pointing towards the ape and the other end pointing away), 30° being a clockwise rotation, and 90° being parallel to the Lexan partition). Each of the orientations was presented in a randomized order with the constraint that each orientation occurred once before any repeat, and all orientations occurred four times within the first and last half of the total number of trials. A complete response was defined as the ape touching the RTR symbol, grasping the dowel and contacting the dowel to the Plexiglass panel on the front of the apparatus and/or inserting the dowel through the hole in the apparatus. All trials scored as incomplete were re-run immediately. A camera was mounted directly above the bracket and dowel in order to obtain a clear view of the ape's grip.
(v). Coding and inter-rater reliability
All trials were coded for the position of the ape's thumb: either towards the centre of the dowel or away from the centre of the dowel. Critically, because of the thickness of the dowel and the biomechanical constraints of reaching through the Lexan partition and orienting the dowel into the hole in the apparatus, the apes could only dislodge the apple if they grasped the tool with the thumb pointed towards the centre. Two raters independently scored the video recordings for thumb directions and agreed on 99 per cent (332/335) of all trials (one trial was inadvertently not recorded).
(c). Results and discussion
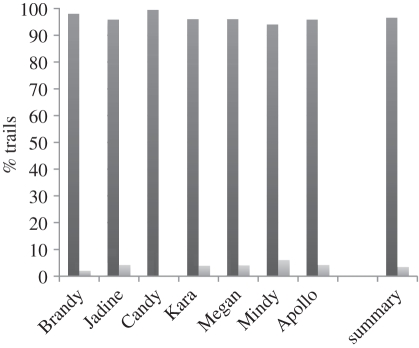
As is clear in figure 5, each of the apes displayed evidence of anticipatory grip selection in this task. All individuals exhibited a strong preference for grasping the dowel with the thumbside of their hands towards the centre of the dowel across orientations. Binomial tests (one-tailed, chance = 0.5) for each animal indicated that all of the apes exhibited a statistically reliable effect of grasping the dowel with thumb towards the centre of the dowel (p < 0.001 in all cases). These results demonstrate that as the dowel orientation was manipulated, the subjects switched either the side of the dowel they grasped (left or right) and/or their hand orientation (palm up versus down) in order to keep their thumb oriented towards the centre of the dowel. If they had not performed so, then they would have frequently grasped the dowel in a manner that made it biomechanically impossible for them to insert the dowel through the hole and dislodge the apple (without first stopping to adopt a new grip). Thus, in stark contrast to the results of experiment 1, these data provide evidence that chimpanzees are capable of anticipatory grip selection in a tool-use task. Because the apes were at ceiling levels across all dowel orientations, our results also highlight the fact that the apes did not learn to modify their grips in this manner across trials. Rather, they exhibited immediate evidence of grasping the dowel in the way that would allow for successful manipulation and dislodging of the apple.
Figure 5.
Grip preference results in experiment 2. All of the participants show some evidence of preferring to grasp the handle with the thumb towards the centre. This is consistent with what would be expected if the animals were taking the desired end state into consideration when selecting their grasps (black bars, thumb towards centre; grey bars, thumb away from centre).
As an example of the effect, consider figure 6a–d. Here, the subject Kara reaches out to grasp the right end of the dowel with her right hand (thumb pointed towards the centre of the dowel), even though the right end of the dowel is further away from her than the left end. This allows her to swing the free end of the dowel towards the hole through which the dowel needs to be inserted. If she had instead grasped the left side of the dowel with her right hand (thumb pointed away from centre), then Kara would have been unable to insert the free end into the Plexiglass box and dislodge the apple. The facts that the dowel was rotated 180° across trials in a stepped fashion, and that the apes virtually always grasped the dowel with the thumb pointing towards the centre, show that they selected the grip best suited to achieving the action goal before grasping the dowel.
Figure 6.
An illustration of the anticipatory grip-selection effect in the externally directed tool-use task: (a) the subject Kara reaches out and selects the end of the dowel that is farthest away so as to have her thumb positioned towards the centre of the dowel. (b–d) This allows the tip of the dowel to swing into a position aligned with the hole, thus allowing for easy insertion and the dislodging of the apple.
In sum, the results of experiment 2 establish that, in the context of a tool-using task, chimpanzees select from among two actions the one that will be successful. Importantly, this behaviour does not seem to have been learned over many trials. Instead, the apes' grip preferences appear to involve anticipatory planning. We hypothesize that they selected their responses through the formation of internal representations of the motor costs and probability of reward associated with the available response options. If this is correct, however, then why were their grip preferences in experiment 1, an ostensibly less difficult, self-directed task, not significantly affected by changes in the task context? The reason may be that the anticipated motor costs of obtaining the reward by placing the thumb towards or away from the baited end of the tool were simply too similar and negligible to influence grip selection. Individual apes thus chose one approach and generally stuck with it across all conditions.
4. Conclusions
Action selection is one of the fundamental problems that must be solved for behaviour to be adaptive ([49] and associated articles). There is mounting evidence that a key function of the extended motor system is generating predictions of the sensory consequences that are likely to arise from future movements, and using that information to estimate motor costs and select actions. Investigations of the grip-selection behaviours of a variety of primates suggest that these feed-forward processes are not unique to humans, and may exist in species that are not known users of tools in nature, including lemurs [45], tamarins [44] and rhesus monkeys [47]. Our findings with chimpanzees extend these previous results by demonstrating that some non-human primates are able to cope with action-selection planning even when tools are used to interact with goal objects located distally (away from the body).
Whether the neural mechanisms involved in anticipatory planning are organized similarly across primate species remains unknown. As reviewed earlier, there is mounting evidence from humans for the involvement of cerebellar, cortical and subcortical mechanisms in these predictive functions, with inferior parietal and ventral premotor cortices showing a pronounced left cerebral asymmetry. Similar to the tasks involved here, recent findings suggest that activity within these very same regions is increased when grip selection involves representing forthcoming task demands, e.g. when selecting how best to grasp a handle for the purpose of performing a subsequent rotation [50]. Whether this left-cerebral asymmetry is causally related to right-hand dominance and/or the evolution of language is unknown. However, the left-cerebral asymmetry for action planning may be coupled with right-hand dominance. Strongly left-handed individuals (who show greater incidence of atypical language organization) display increased activity in both left and right vPMC during PGS tasks involving the hands or a tool [51]. While it is true that non-human primates demonstrate hand preferences under certain circumstances [52,53], these never approach the population-level right-hand bias evident in roughly 90 per cent of humans for fine motor tasks [54,55]. The fossil and archaeological records suggest that this right-hand bias was evident very early in our lineage [56]. The emerging picture is that predictive planning may extend much further back into our primate origins, and is likely rooted in basic functions of sensorimotor control that predate handedness. The ability to predict the future based on past experiences is a core cognitive faculty of modern humans [57], and may have played a critical role in the evolution of more sophisticated forms of tool manufacture and use [58].
Acknowledgements
The research reported in this article was approved by the University of Louisiana at Lafayette Institutional Animal Care and Use Committee and was conducted in accordance with all applicable laws of the USA.
The work was funded by a James S. McDonnell Centennial Fellowship Award to D.J.P. and James S. McDonnell Foundation Collaborative activities Award to D.J.P. and S.J.F. (JSMF grant no. 21002093).
Endnote
Additional demonstration sessions were conducted for any ape that did not appear to understand the task, until it demonstrated such an understanding. These apes were given remedial training after three unsuccessful demonstrations and criterion trials (see §3c).
References
- 1.Donders F. C. 1969. On the speed of mental processes. Acta Psychol. 30, 412–431 10.1016/0001-6918(69)90065-1 (doi:10.1016/0001-6918(69)90065-1) [DOI] [PubMed] [Google Scholar]
- 2.Rushworth M. F., Nixon P. D., Wade D. T., Renowden S., Passingham R. E. 1998. The left hemisphere and the selection of learned actions. Neuropsychologia 36, 11–24 10.1016/S0028-3932(97)00101-2 (doi:10.1016/S0028-3932(97)00101-2) [DOI] [PubMed] [Google Scholar]
- 3.Schluter N. D., Krams M., Rushworth M. F., Passingham R. E. 2001. Cerebral dominance for action in the human brain: the selection of actions. Neuropsychologia 39, 105–113 10.1016/S0028-3932(00)00105-6 (doi:10.1016/S0028-3932(00)00105-6) [DOI] [PubMed] [Google Scholar]
- 4.Bernstein N. 1967. The co-ordination and regulation of movements. Oxford, UK: Pergamon Press [Google Scholar]
- 5.Shadmehr R., Krakauer J. 2008. A computational neuroanatomy for motor control. Exp. Brain Res. 185, 359–381 10.1007/s00221-008-1280-5 (doi:10.1007/s00221-008-1280-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson S. H. 2000. Thinking ahead: the case for motor imagery in prospective judgements of prehension. Cognition 74, 33–70 10.1016/S0010-0277(99)00063-3 (doi:10.1016/S0010-0277(99)00063-3) [DOI] [PubMed] [Google Scholar]
- 7.Johnson S. H. 2000. Imagining the impossible: intact motor representations in hemiplegics. Neuroreport 11, 729–732 10.1097/00001756-200003200-00015 (doi:10.1097/00001756-200003200-00015) [DOI] [PubMed] [Google Scholar]
- 8.Jenkinson P. M., Edelstyn N. M., Ellis S. J. 2009. Imagining the impossible: motor representations in anosognosia for hemiplegia. Neuropsychologia 47, 481–488 10.1016/j.neuropsychologia.2008.10.004 (doi:10.1016/j.neuropsychologia.2008.10.004) [DOI] [PubMed] [Google Scholar]
- 9.Johnson S. H., Sprehn G., Saykin A. J. 2002. Intact motor imagery in chronic upper limb hemiplegics: evidence for activity-independent action representations. J. Cogn. Neurosci. 14, 841–852 10.1162/089892902760191072 (doi:10.1162/089892902760191072) [DOI] [PubMed] [Google Scholar]
- 10.Philip B. A., Frey S. H. 2010. Intact prediction of grip selection with an amputated hand (program no. 291.13, online). San Diego, CA: Society for Neuroscience [Google Scholar]
- 11.Johnson S. H., Rotte M., Grafton S. T., Hinrichs H., Gazzaniga M. S., Heinze H. J. 2002. Selective activation of a parietofrontal circuit during implicitly imagined prehension. Neuroimage 17, 1693–1704 10.1006/nimg.2002.1265 (doi:10.1006/nimg.2002.1265) [DOI] [PubMed] [Google Scholar]
- 12.Jacobs S., Danielmeier C., Frey S. H. 2010. Human anterior intraparietal and ventral premotor cortices support representations of grasping with the hand or a novel tool. J. Cogn. Neurosci. 22, 2594–2608 10.1162/jocn.2009.21372 (doi:10.1162/jocn.2009.21372) [DOI] [PubMed] [Google Scholar]
- 13.Wolpert D. M., Ghahramani Z. 2000. Computational principles of movement neuroscience. Nat. Neurosci. 3(Suppl. 11), 1212–1217 10.1038/81497 (doi:10.1038/81497) [DOI] [PubMed] [Google Scholar]
- 14.Wolpert D. M., Miall R. C., Kawato M. 1998. Internal models in the cerebellum. Trends Cogn. Sci. 2, 338–347 10.1016/S1364-6613(98)01221-2 (doi:10.1016/S1364-6613(98)01221-2) [DOI] [PubMed] [Google Scholar]
- 15.Wolpert D. M., Goodbody S. J., Husain M. 1998. Maintaining internal representations: the role of the human superior parietal lobe. Nat Neurosci 1, 529–533 10.1038/2245 (doi:10.1038/2245) [DOI] [PubMed] [Google Scholar]
- 16.Frey S. H. 2010. Forecasting the long-range consequences of manual and tool use actions: neurophysiological, behavioral and computational considerations. In Motor control: theories, experiments and applications (eds Danion F., Latash M.), pp. 295–313 Oxford, UK: Oxford University Press [Google Scholar]
- 17.Grush R. 2004. The emulation theory of representation: motor control, imagery, and perception. Behav. Brain Sci. 27, 377–396 (discussion 396–442) [DOI] [PubMed] [Google Scholar]
- 18.Humphries M. D., Gurney K., Prescott T. J. 2007. Is there a brainstem substrate for action selection? Phil. Trans. R. Soc. B 362, 1627–1639 10.1098/rstb.2007.2057 (doi:10.1098/rstb.2007.2057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Binkofski F., Buccino G. 2004. Motor functions of the Broca's region. Brain Lang. 89, 362–369 10.1016/S0093-934X(03)00358-4 (doi:10.1016/S0093-934X(03)00358-4) [DOI] [PubMed] [Google Scholar]
- 20.Rizzolatti G., Craighero L. 2004. The mirror-neuron system. Annu. Rev. Neurosci. 27, 169–192 10.1146/annurev.neuro.27.070203.144230 (doi:10.1146/annurev.neuro.27.070203.144230) [DOI] [PubMed] [Google Scholar]
- 21.Preuss T. M., Stepniewska I., Kaas J. H. 1996. Movement representation in the dorsal and ventral premotor areas of owl monkeys: a microstimulation study. J. Comp. Neurol. 371, 649–676 (doi:10.1002/(SICI)1096-9861(19960805)371:4<649::AID-CNE12>3.0.CO;2-E) [DOI] [PubMed] [Google Scholar]
- 22.Umilta M. A., Escola L., Intskirveli I., Grammont F., Rochat M., Caruana F., Jezzini A., Gallese V., Rizzolatti G. 2008. When pliers become fingers in the monkey motor system. Proc. Natl Acad. Sci. USA 105, 2209–2213 10.1073/pnas.0705985105 (doi:10.1073/pnas.0705985105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rizzolatti G., Fogassi L., Gallese V. 2002. Motor and cognitive functions of the ventral premotor cortex. Curr. Opin. Neurobiol. 12, 149–154 10.1016/S0959-4388(02)00308-2 (doi:10.1016/S0959-4388(02)00308-2) [DOI] [PubMed] [Google Scholar]
- 24.Buccino G., Lui F., Canessa N., Patteri I., Lagravinese G., Benuzzi F., Porro C. A., Rizzolatti G. 2004. Neural circuits involved in the recognition of actions performed by nonconspecifics: an FMRI study. J. Cogn. Neurosci. 16, 114–126 10.1162/089892904322755601 (doi:10.1162/089892904322755601) [DOI] [PubMed] [Google Scholar]
- 25.Hamilton A. F., Grafton S. T. 2006. Goal representation in human anterior intraparietal sulcus. J. Neurosci. 26, 1133–1137 10.1523/JNEUROSCI.4551-05.2006 (doi:10.1523/JNEUROSCI.4551-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tunik E., Rice N. J., Hamilton A., Grafton S. T. 2007. Beyond grasping: representation of action in human anterior intraparietal sulcus. Neuroimage 36(Suppl. 2), T77–T86 10.1016/j.neuroimage.2007.03.026 (doi:10.1016/j.neuroimage.2007.03.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heilman K. M., Rothi L. J. G. 1997. Limb apraxia: a look back. In Apraxia: the neuropsychology of action (eds Rothi L. J. G., Heilman K. M.), pp. 7–18 Hove, UK: Psychology Press/Erlbaum (UK) Taylor & Francis [Google Scholar]
- 28.Leiguarda R. C., Marsden C. D. 2000. Limb apraxias: higher-order disorders of sensorimotor integration. Brain 123, 860–879 10.1093/brain/123.5.860 (doi:10.1093/brain/123.5.860) [DOI] [PubMed] [Google Scholar]
- 29.Rushworth M. F., Behrens T. E., Johansen-Berg H. 2006. Connection patterns distinguish 3 regions of human parietal cortex. Cereb. Cortex 16, 1418–1430 10.1093/cercor/bhj079 (doi:10.1093/cercor/bhj079) [DOI] [PubMed] [Google Scholar]
- 30.Kroliczak G., Piper B. J., Frey S. H. 2011. Atypical lateralization of language predicts cerebral asymmetries in parietal gesture representations. Neuropsychologia 49, 1698–1702 10.1016/j.neuropsychologia.2011.02.044 (doi:10.1016/j.neuropsychologia.2011.02.044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson-Frey S. H., McCarty M., Keen R. 2004. Reaching beyond spatial perception: effects of intended future actions on visually guided prehension. Vis. Cogn. 11, 371–399 10.1080/13506280344000329 (doi:10.1080/13506280344000329) [DOI] [Google Scholar]
- 32.Elsinger C. L., Rosenbaum D. A. 2003. End posture selection in manual positioning: evidence for feedforward modeling based on a movement choice method. Exp. Brain Res. 152, 499–509 10.1007/s00221-003-1573-7 (doi:10.1007/s00221-003-1573-7) [DOI] [PubMed] [Google Scholar]
- 33.Cohen R. G., Rosenbaum D. A. 2004. Where grasps are made reveals how grasps are planned: generation and recall of motor plans. Exp. Brain Res. 157, 486–495 10.1007/s00221-004-1862-9 (doi:10.1007/s00221-004-1862-9) [DOI] [PubMed] [Google Scholar]
- 34.Keen R. 2011. The development of problem solving in young children: a critical cognitive skill. Annu. Rev. Psychol. 62, 1–21 10.1146/annurev.psych.031809.130730 (doi:10.1146/annurev.psych.031809.130730) [DOI] [PubMed] [Google Scholar]
- 35.Chen Y. P., Keen R., Rosander K., von Hofsten C. 2010. Movement planning reflects skill level and age changes in toddlers. Child Dev. 81, 1846–1858 10.1111/j.1467-8624.2010.01514.x (doi:10.1111/j.1467-8624.2010.01514.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Claxton L. J., Keen R., McCarty M. E. 2003. Evidence of motor planning in infant reaching behavior. Psychol. Sci. 14, 354–356 10.1111/1467-9280.24421 (doi:10.1111/1467-9280.24421) [DOI] [PubMed] [Google Scholar]
- 37.Claxton L. J., McCarty M. E., Keen R. 2009. Self-directed action affects planning in tool-use tasks with toddlers. Infant Behav. Dev. 32, 230–233 10.1016/j.infbeh.2008.12.004 (doi:10.1016/j.infbeh.2008.12.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCarty M. E., Clifton R. K., Collard R. R. 1999. Problem solving in infancy: the emergence of an action plan. Dev. Psychol. 35, 1091–1101 10.1037/0012-1649.35.4.1091 (doi:10.1037/0012-1649.35.4.1091) [DOI] [PubMed] [Google Scholar]
- 39.McCarty M. E., Keen R. 2005. Facilitating problem-solving performance among 9- and 12-month-old infants. J. Cogn. Dev. 6, 209–230 10.1207/s15327647jcd0602_3 (doi:10.1207/s15327647jcd0602_3) [DOI] [Google Scholar]
- 40.Ostry D. J., Gribble P. L., Gracco V. L. 1996. Coarticulation of jaw movements in speech production: is context sensitivity in speech kinematics centrally planned? J. Neurosci. 16, 1570–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abbs J. H., Gracco V. L., Cole K. J. 1984. Control of multimovement coordination: sensorimotor mechanisms in speech motor programming. J. Mot. Behav. 16, 195–231 [DOI] [PubMed] [Google Scholar]
- 42.Arbib M. A. 1981. Perceptual structures and distributed motor control. In Handbook of neurophysiology: motor control, part 2 (ed. Brooks V. B.), pp. 1449–1480 Bethesda, MD: APA [Google Scholar]
- 43.Rosenbaum D. A., Jorgensen M. J. 1992. Planning macroscopic aspects of manual control. Hum. Mov. Sci. 11, 61–69 10.1016/0167-9457(92)90050-L (doi:10.1016/0167-9457(92)90050-L) [DOI] [Google Scholar]
- 44.Weiss D. J., Wark J. D., Rosenbaum D. A. 2007. Monkey see, monkey plan, monkey do: the end-state comfort effect in cotton-top tamarins (Saguinus oedipus). Psychol. Sci. 18, 1063–1068 10.1111/j.1467-9280.2007.02026.x (doi:10.1111/j.1467-9280.2007.02026.x) [DOI] [PubMed] [Google Scholar]
- 45.Chapman K. M., Weiss D. J., Rosenbaum D. A. 2010. Evolutionary roots of motor planning: the end-state comfort effect in lemurs. J. Comp. Psychol. 124, 229–232 10.1037/a0018025 (doi:10.1037/a0018025) [DOI] [PubMed] [Google Scholar]
- 46.Nelson E., Berthier N. E., Metevier C. M., Novak M. A. 2011. Evidence for motor planning in monkeys: rhesus macaques select efficient grips when transporting spoons. Dev. Sci. 14, 822–831 10.1111/j.1467-7687.2010.01030.x (doi:10.1111/j.1467-7687.2010.01030.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Povinelli D. J. 2000. Folk physics for apes: the chimpanzee's theory of how the world works. Oxford, UK: Oxford University Press [Google Scholar]
- 48.Short M. W., Cauraugh J. H. 1999. Precision hypothesis and the end-state comfort effect. Acta Psychol. 100, 243–252 10.1016/S0001-6918(98)00020-1 (doi:10.1016/S0001-6918(98)00020-1) [DOI] [PubMed] [Google Scholar]
- 49.Prescott T. J., Bryson J. J., Seth A. K. 2007. Introduction. Modelling natural action selection. Phil. Trans R. Soc. B 362, 1521–1529 10.1098/rstb.2007.2050 (doi:10.1098/rstb.2007.2050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marangon M., Jacobs S., Frey S. H. 2011. Evidence for context sensitivity of grasp representations in human parietal and premotor cortices. J. Neurophysiol. 105, 2536–2546 10.1152/jn.00796.2010 (doi:10.1152/jn.00796.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin K., Jacobs S., Frey S. H. 2011. Handedness-dependent and -independent cerebral asymmetries in the anterior intraparietal sulcus and ventral premotor cortex during grasp planning. Neuroimage 57, 502–512 10.1016/j.neuroimage.2011.04.036 (doi:10.1016/j.neuroimage.2011.04.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hopkins W. D., Pearson K. 2000. Chimpanzee (Pan troglodytes) handedness: variability across multiple measures of hand use. J. Comp. Psychol. 114, 126–135 10.1037/0735-7036.114.2.126 (doi:10.1037/0735-7036.114.2.126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hopkins W. D., Russell J. L. 2004. Further evidence of a right hand advantage in motor skill by chimpanzees (Pan troglodytes). Neuropsychologia 42, 990–996 10.1016/j.neuropsychologia.2003.11.017 (doi:10.1016/j.neuropsychologia.2003.11.017) [DOI] [PubMed] [Google Scholar]
- 54.Annett M. 2006. The distribution of handedness in chimpanzees: estimating right shift in Hopkins' sample. Laterality 11, 101–109 [DOI] [PubMed] [Google Scholar]
- 55.Coren S., Porac C. 1977. Fifty centuries of right-handedness: the historical record. Science 198, 631–632 10.1126/science.335510 (doi:10.1126/science.335510) [DOI] [PubMed] [Google Scholar]
- 56.Steele J., Uomini N. 2005. Humans, tools and handedness. In Stone knapping: the necessary conditions for a uniquely hominine behaviour (eds Bril B., Roux V.), pp. 217–239 Cambridge, UK: MacDonald Institute [Google Scholar]
- 57.Bar M. 2009. Predictions: a universal principle in the operation of the human brain. Introduction. Phil. Trans. R. Soc. B 364, 1181–1182 10.1098/rstb.2008.0321 (doi:10.1098/rstb.2008.0321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Faisal A., Stout D., Apel J., Bradley B. 2010. The manipulative complexity of Lower Paleolithic stone toolmaking. PLoS ONE 5, e13718. 10.1371/journal.pone.0013718 (doi:10.1371/journal.pone.0013718) [DOI] [PMC free article] [PubMed] [Google Scholar]