Abstract
Hominin evolution has involved a continuous process of addition of new kinds of cognitive capacity, including those relating to manufacture and use of tools and to the establishment of linguistic faculties. The dramatic expansion of the brain that accompanied additions of new functional areas would have supported such continuous evolution. Extended brain functions would have driven rapid and drastic changes in the hominin ecological niche, which in turn demanded further brain resources to adapt to it. In this way, humans have constructed a novel niche in each of the ecological, cognitive and neural domains, whose interactions accelerated their individual evolution through a process of triadic niche construction. Human higher cognitive activity can therefore be viewed holistically as one component in a terrestrial ecosystem. The brain's functional characteristics seem to play a key role in this triadic interaction. We advance a speculative argument about the origins of its neurobiological mechanisms, as an extension (with wider scope) of the evolutionary principles of adaptive function in the animal nervous system. The brain mechanisms that subserve tool use may bridge the gap between gesture and language—the site of such integration seems to be the parietal and extending opercular cortices.
Keywords: primates, parietal cortex, spatial integration, coordinate transformation, non-spatial cognition
1. Introduction
In the course of human evolution and human history, our ancestors have created new habitats from modified hunter–gatherer environments to agricultural landscapes with villages, and then to modern civilized technological cities. The evolution of various new cognitive capacities, including those underwriting the manufacture and use of tools and the production and comprehension of languages, has enabled these ecological transformations. Such new cognitive capacities in turn are an outcome of the dramatic expansion of the human brain and of new functional brain areas. Humans have constructed a new ‘niche’ in each of these ecological, cognitive and neural domains. ‘Niche-construction’ denotes an evolutionary process whereby the activities of organisms modify their habitat, to which in turn the organisms evolve to adapt, thus creating their own ‘ecological niche’ in the environment [1–3]. This concept will be extended in this paper to include the ‘cognitive niche’ as a newly acquired class of cognitive capacity [4], and the ‘neural niche’ as a portion of neural tissue added through expansion of the brain [5,6].
The above three classes of niche have coevolutionary interdependencies. Such interactions might have accelerated hominin evolution, which seems remarkably rapid if it was simply the product of natural selection driven by exogenous environmental change. It is possible that ecological changes to habitats have occurred not as a cause of hominin cognitive evolution, but rather as a result of it, with consequent selection pressures acting on the neural basis of behavioural adaptations to the modified environment. New brain functions would constitute the basis for further innovation in cognitive functioning and thus further modifications to the ecological niche, providing a feedback loop for ‘triadic niche constructions’. In this paper, a potential evolutionary scenario that led humans to invent successively more complex forms of tools, and eventually to acquire the language faculty, will be proposed based on this dynamic interaction. The brain's functional characteristics play a key role in the above triadic interactions. The relevant neurobiological mechanisms are explored in this paper in order that the proposed evolutionary scenario should not be seen as teleological. Finally, we will try to locate these mechanisms as an extension of the evolution of the animal nervous system in general: human higher cognitive activity can then be viewed as continuous with that of other animal species comprising the wider terrestrial ecosystem.
2. Construction of the ecological niche
(a). Hominin ecology structured through incorporating different classes of tools
Humans are peculiar, compared with non-human species, in the extent to which they try to ‘improve’ their habitual environment. To make such improvements, particularly in our modern urban environment, we make and use various kinds of tools and technologies, and often the tools themselves are incorporated into the fundamental structure of the environment to create a distinctive human ecological niche. For example, cars running on paved roads or air-conditioned skyscrapers are essentially artefacts in which to travel and to reside, yet also comprise an environment to which city inhabitants adapt both physically and perceptually. In earlier times, hominins may also have typically constructed their niche by gradually and consecutively incorporating artefacts into the habitual environment. Thus, one can ask whether the tools that comprise our environment can be classified and structured in hierarchical order and if so, how different modes of brain function subserve their use.
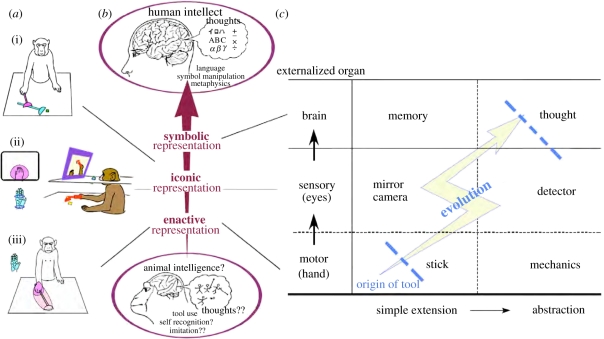
The classical definition of the tool, as used in most existing tool-use studies, is restricted to external objects held by the hand and interacting with the external environments [7]. Namely, the tools so defined and studied are the ones that extend and externalize our hand, or more generally the motor organs or effectors. Indeed, the first series of tools that early hominins are known to have used [8,9], and those used by non-human animals [7] are these ‘motor tools’ (figure 1c, bottom row). We modern humans also use tools to extend or externalize our existing sensory organs, or to support the detection of information that is outside our natural sensory range (figure 1c, middle row). The optical telescope, endoscope or stethoscope would be examples of the former and the radio telescope or Geiger counter would be examples of the latter. Non-human animals rarely use this class of ‘sensory tools’ [10]. In monkeys, our own previous studies have demonstrated that they can be trained to use a sort of endoscope only after having acquired an ability to use a motor tool (a rake) [11]—as if attaching an additional visual cue to the tip of an extended body schema that was acquired through initial training to use a rake (see §2b for details). These results suggest that the class of sensory tools comprise a higher layer, superimposed onto previously acquired motor tools as the fundamental layer. Indeed, the history of our own technology suggests that sensory tools appeared much later, after motor tools were incorporated into human cultures.
Figure 1.
Various modes of cortical body-image codings (a) and hierarchical structure of various classes of tools (c) corresponding to the putative hierarchy of internal representations (b). (a(i)) Combinatory usage of short and long rakes. (a(ii)) When monkeys used a monitor, a visual receptive field of representative intraparietal bimodal neurons was formed around the hand in the monitor, encompassing its somatosensory receptive field. (a(iii)) When monkeys used a rake to retrieve distant food, the visual receptive field encompassed the somatosensory receptive field of a representative intraparietal bimodal neuron extended along the rake. Reproduced with permission from Iriki & Sakura [6].
What then would be the tool class of the third layer? If we looked at ourselves through our own externalized eyes (the second layer of the tools), we would observe ourselves as external objects by shifting from the first-person to the third-person perspective, in other words by ‘self-objectification’ [12,13]. This leads to the perception of our own intrinsically intransitive movement as transitive, i.e. to the acquisition of a sense of the self (as the subject), and leading to the movement of ourselves or our body parts perceived as objects. We may hypothesize that once the ‘self’ has been bifurcated into a subjective self and an objective self, the mind and/or intentionality emerges as a function that bridges those fragmented ‘selves’ and reunites them; this hypothesis has been proposed in detail elsewhere [6,13]. As a result of this self-objectification and emergence of the ‘mind’, a recognition of the ‘core self’ that continues across time from the past through the present towards the future may subsequently arise. Once the future self is recognized as having a core that is identical to that of the present self, one might wish to save the present information for future use. This can be accomplished by taking notes or drawing pictures, which requires an external device for memorizing facts; thus, an ‘externalization of the brain’ is produced as the tool class of the third layer (figure 1c, top row) [6,14].
How could the three different classes of tools outlined above be incorporated into humans' habitual environments? The scenario outlined proposes that successive layers of tools (motor extensions, sensory extensions and symbolic externalized memory) can be incorporated into the environment by building on the pre-existing acquisition and incorporation of tool classes of the immediately lower level. Thus, a positive feedback would have emerged between new brain functions and resulting modifications of the habitual environment. In the course of such positive feedback processes, a brain function emerged for the mind and for future-directed ‘intentionality’, after which the feedback became guided by human intentions (‘intentional niche construction’) [6].
(b). Parietal plasticity when incorporating tools into the body schema
In using these tools, what kind of neural mechanisms and what modes of operation are employed, and what kinds of neural changes, if any, are induced upon acquisition of the ability to use tools? Our previous studies, as illustrated below, demonstrate one such example. Although Japanese macaques normally do not use tools in their natural habitat, two weeks of extensive training will enable these animals to use a hand-held rake to retrieve a distant food object located out of reach [15]. This training must imply the ability to reorganize the image of the body to one in which the rake is incorporated as an extension of the forearm. The body image is thought to form by integrating somatosensory and visual information relating to the body [16]. Thus, its modification after tool use could be physically observed as changes in the receptive field properties of the neurons that code such images [17]—when the tool was incorporated, the receptive field that codes the image of the hand was elongated to include the rake (figure 1a(iii)). This modification seemed to match the monkeys' internal states, whether or not the rake was incorporated into the image of the forearm. Here, an equivalence is established between body parts (hands) and tools, i.e. hands are extended towards tools (externalization of the innate body) or tools are assimilated into the body schema (internalization of external objects). Hence, these representations of the body image comprise an internal model of the bodily structures used to control various movements, as a concrete neural correlate of the ‘enactive representation’ [18], a class of representation that first emerges at a very early stage of postnatal development in human infants (figure 1b).
As the training proceeds further, we might postulate that the monkey's mode of representation may advance to ‘iconic (visual)’ and even close to ‘symbolic’ [18]—during human development, these appear later during childhood or after maturity. This expectation implies that motor-tool-use-trained monkeys could be further trained to use a video monitor to retrieve food that is out of their direct line of sight, and that the receptive field of the parietal neurons that code the hand and the tool incorporated into it will be activated by visual feedback when images of the hand and tool are seen on the video monitor (figure 1a(ii)) [19]. Thus, the body image is visually projected onto the distant monitor screen. In fact, we have found that monkeys that acquire the ability to use a rake and a video monitor to retrieve food objects in this way can immediately combine multiple tools purposefully to accomplish the goal [20], as if they are able to logically structure body parts using (proto-)symbolic representations (figure 1a(i)). Here, we can recognize that the hierarchical structures of motor tools/sensory tools/brain tools [18] resemble the hierarchy of representations from enactive (motor) to iconic (sensory) to symbolic (brain) structures of development, as described earlier (see figure 1a–c for these comparisons) [6]. Thus, reorganization of the modes of visuomotor integration in the parietal cortex must be crucial for the acquisition of these successively more advanced modes of representation.
3. Construction of the neural niche
(a). Brain expansion by tool-use training
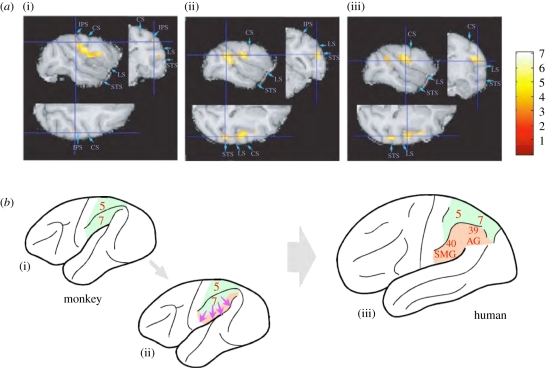
Is the neural plasticity depicted above limited within the range of the individual's learning capacity, or could it cumulatively evolve over generations? In other words, is it purely subserved by ‘cultural inheritance’, or alternatively, could it be a part of an epigenetic evolutionary mechanism in which the information embedded in the environment contributes to modification of phenotypic expression in succeeding generations? Although the latter has a flavour of Lamarckism—inheritance of acquired phenotypic traits—there may be a biological mechanism that could channel the evolution of adaptations to an environment in which cultural information is embedded. Macroscopic expansion (up to 23% of MRI grey matter signal) of cortical grey matter, including the intraparietal region, was detected in monkeys undergoing two weeks of tool-use training (figure 2a) in our recent Voxel Based Morphometry analysis (a kind of digital neuroanatomy using the magnetic resonance imaging technique) [21]. During the same period, microscopic changes (axonogenesis and synaptogenesis, as detected by tracer-injection histological examinations) [22] together with elevated expression of immediate-early genes [23] and of neurotrophic factors [24,25] were also shown to have been induced in these cortical areas. The grey matter expansion extended to include adjacent areas, such as the secondary somatosensory area (figure 2a(iii)) and the surrounding opercular cortex. Although the evidence obtained to date remains fragmentary and more detailed biological examinations are in progress, these initial findings indicate that the brain is much more adaptive than was previously believed: exposure to a novel cultural environment induces the brain to exhibit not only functional plasticity, but also extensive and persistent morphological change.
Figure 2.
Grey matter increase with improvement in rake task performance. (a) Areas where grey matter increased with increasing performance score on the rake task. Sagittal, coronal and horizontal planes with increases in grey matter, including the right intraparietal sulcus (IPS, (i)), the superior temporal sulcus (STS, (ii)) and the secondary somatosensory area (SII, (iii)), are shown. (b) Schematic illustrating how tool-use-induced expansion of the parietal cortex of monkeys (i,ii) may contribute to the establishment of a precursor for the formation of human inferior parietal areas (iii), thus creating a novel neural niche that subserves further higher cognitive functions. CS, central sulcus; LS, lateral sulcus. The colour scale indicates the t score. (a) Reproduced with permission from Quallo et al. [21].
This implies that once a novel cognitive demand, such as incorporation of motor tools into the body schema, has become embedded in the environment, modifications of brain structure would be induced automatically through the normal developmental processes in succeeding generations. The occurrence of such a plastic response during the lifespan as a result of behavioural modifications that lie within the existing adaptive capacity of individuals, and its subsequent consolidation (under selection acting on changing gene frequencies) as a default state that is stable over generations [26–28] is termed the ‘Baldwin effect’ [29,30], and comprises one potential component of the evolutionary process.
(b). Redundant and polysemic systems as pre-adaptations for a novel neural niche
What could be the biological principles that allow brain expansion as an evolutionary mechanism of the kind just outlined? Biological systems are never ultimately efficient—systems require some redundancy for stability, to avoid over-specialization that might threaten the capacity to survive new challenges. Some redundancy in brain structure would allow representational bistability, for both the originally adapted functions and functional response to the new challenge. Increased redundancy to stabilize this functional capacity for flexible adaptive responses, perhaps via rapid brain expansion, would also then allow rapid construction of new and specialized neural resources. As has been discussed earlier, monkey intraparietal neurons that normally code body image can be trained to code a tool in a way that is equivalent to that for the hand holding it [17]. Thus, these neurons are bistable or polysemous for the hand or the tool. This functional plasticity may be an inherent property at the margins of a neural coding system prepared for gradual elongation of the arm during body growth, and which can then also adapt to a ‘sudden elongation’ by using the tool. This accidentally established equivalence between body parts (hands) and tools in turn leads to additional polysemic and bistable interpretations, i.e. hands may be extended into the tool representation (externalization of the innate body) or tools may be assimilated into the body schema (internalization of external objects).
Thus, redundancy in the brain, initially adapted to stabilize this system against unexpected environmental noise (or developmental changes, following the above speculation about body growth) has occasionally allowed the system to be polysemous. This newly acquired bistable state enables the reuse of cortical systems for different functions in the future, as in the case of tool use, perhaps in combination with other parts of the brain [5,31]. This bistability, or ‘polysemy’, could enable the use of metaphors in conceptual structure—so as to comprise a novel cognitive niche, as will be described in the next section. However, how human higher cognitive functions appear to have ‘evolved’ much more quickly than might be expected from ordinary biological evolutionary processes of adaptation to changing external environmental contingencies remains an open question. Humans can induce such changes intentionally, to construct a better-fitting ecological niche [6]. The capacity of human intention, or of the human mind, to plan for the future emerged through the process described in the previous section in relation to the hierarchy of classes of tool use (motor, sensory and brain). Subsequently, the neural systems which process the information that is necessary to inhabit the tool-modified environment (the neural niche of the brain; figure 2b(ii)) could be reinforced further by extragenetic or epigenetic triggering factors embedded in such an environment.
In addition, because hominin species have attained an unusually long post-reproductive lifespan, particularly females [32], accumulation of knowledge continues over the whole lifespan of an individual, tending to peak in middle-to-old age. Thus, some extragenetic mechanisms are indispensable for inheritance of these later acquired ‘cognitive niches’ over generations to occur. Species with a short lifespan and mass reproduction adapt to environmental changes through variations in their numerous offspring, as they expect that at least a few will survive, whereas species with a long lifespan—such as primates, and most typically humans—and low birth rate do so through an individual capacity to adapt [33]. This process would be enhanced by expansion of an organ that controls adaptive behaviours, namely the brain, which are stabilized as the typical phenotype of the species through epigenetic mechanisms. The evolutionary process driving such expansion may not simply be natural selection acting on random mutations, but rather something like the Baldwin effect [29,30] as depicted earlier.
4. Construction of the cognitive niche
(a). Parietal ‘neural niches’ for processing spatial and non-spatial cognition
Debate exists on the comparative anatomy of the primate parietal cortex (figure 2b). One view claims that the inferior parietal area is evolutionarily new and uniquely expanded in humans, and that therefore the monkey posterior parietal cortex corresponds to the human superior parietal lobule. This is the position illustrated in the scheme presented in figure 2b(iii), independent of figure 2b(i) [34,35]. Evidence supporting this view would include the fact that, for example, the superior parietal lobule tends to process spatial information in a conventional way, whereas the inferior parietal lobule is often credited with non-spatial cognition. Many recent human imaging studies demonstrate that this cortical area additionally supports various forms of high-order non-spatial cognition that are not necessarily directly related to physical space itself. Indeed, a comparison of the brain areas responsible for tool-use behaviours in monkeys and humans [36] detected a patch of the parietal cortex specific to humans that might be responsible for perception and manipulation of abstract causal relationships required for human tool-use behaviours. This could be evidence of a function that was derived from a polysemic mechanism as described above, in which an additional resource of brain tissue (a neural niche) functions to enable an additional cognitive process (a cognitive niche).
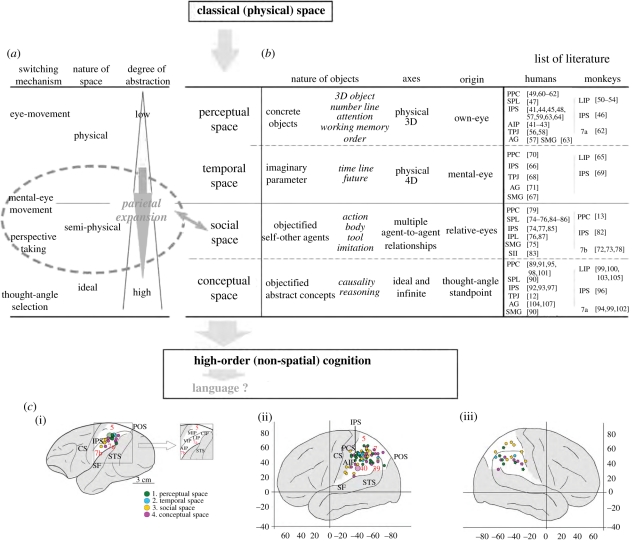
However, there is another view in which the monkey parietal cortex includes functional homologues of both regions [37–40], which allows viewing figure 2b(i–iii) as a continuum. Various kinds of non-spatial cognition can be grouped and ordered based on the levels of abstraction of the ‘objects’ and the conceptually defined spaces which are represented. The assumed coordinate systems for such ‘spaces’, with citations to research literature analysing the brain mechanisms which encode such spatial coordinates (for monkeys and humans), are summarized in figure 3 [108]. The pseudo-spatial nature of the high-order cognition supported by the posterior parietal cortex may be derived from the essential characteristics of the objects represented; alternatively, it may be derived from the nature of the pre-existing information-processing mechanisms of this area, namely as a hub for multi-sensory integration and for representing physical environmental space [109]. A meta-analysis of the references listed in figure 3 (refer to its legend for a detailed classification of various cognitive niches handled by this brain area) shows that the posterior parietal areas responsible for these novel forms of cognition are not necessarily clearly segregated, either in monkeys or in humans, and suggests a trend of gradual expansion towards the lateral sulcus as the level of abstraction increases (figure 3c) [108]. Thus, it seems that the parietal area gradually incorporated high-order cognition as it expanded during hominid evolution, while preserving its original principles of operation. This could be an example of exhibiting a novel cognitive niche by re-using the functions that have derived from a polysemic mechanism described previously. If this was the case, the gradual emergence and differentiation of functions in the transitional state might not be detected until the intensity of activation and the quantity of tissue recruited to serve this new neural niche exceeded some threshold of detection. In this sense, the above two views of parietal evolution are not necessarily mutually exclusive.
Figure 3.
(Caption opposite.)
What, then, is the explanation for this series of additions of novel cognitive niches? Mechanisms for ‘selecting’ and ‘switching’ between objects among different represented spaces (figure 3a) could be hypothesized to contribute to this process [108]. That is, initially in classical (physical) space, spatial attention towards concrete objects was typically expressed as the direction of the eye axis to represent ‘perceptual space’ ([41–64]; see legend of figure 3 for details of respective references, and also for other classes of ‘spaces’ described below). Secondarily, when such attention had to be sustained or the attending content had to be memorized, invisible ‘time’ was ‘visualized’ in the mind's eye, becoming a new virtual dimension in the existing suite of spatial-coordinate systems, namely ‘temporal space’ [65–71]. And then, once one was able to visualize an invisible virtual entity, a similar objectification process could have been extended further, enabling intentional perspective switching. Acquiring representations of ‘social space’ [13,72–87] might have accelerated this process. Via self-objectification processes [13] mentioned in the earlier section, and the development of ‘virtual eyes’ [11,12], flexible and mutually integrated representations of the bodily self, of the analogous selves of others, and of tools used as equivalents of body parts (and vice versa) may have served as a bridge between concrete physical and abstract conceptual spaces. Finally, as the posterior parietal cortex expanded in both physical volume and in range of function [6,21], a positive feedback process could have been established to achieve further human-specific forms of non-spatial conceptual cognition, or ‘conceptual space’ [12,80,81,88–107]. In this way, crucial components of human intelligence would derive their character from the precursory spatial cognition process of the parietal cortex. Language is full of spatial metaphors for abstract thoughts.
(b). Opercular cortex as a cradle for language by re-using spatial processing principles
During evolution, whenever organisms are faced with a novel and unforeseen environment, they have no other means to overcome immediate problems than to reuse any materials at hand [5,31]. Thus, cognitive capacities are extended by diverting pre-existing functions. In hominin evolution, according to the scenario outlined in the previous section, the expanded inferior parietal area and surrounding opercular region have taken on distinctive functional characteristics. Basic continuity from monkeys to humans as described above seems to be present in this general area, which includes Broca's area (anterior operculum), Wernicke's area (posterior operculum) and the middle operculum corresponding to the supramarginal and angular gyri, and which appear to be an extension of the inferior parietal lobule of the monkey brain; the continuity underwrites opercular language representations. Fundamentally, the argument from continuity implies that such representational capacity should be a simple extension of a coding system for reaching and grasping. Initially, this extension would derive from the coding of spatial integration and of a reorganized representation of space, which could be extrapolated further using a principle identical to the non-spatial higher-order coding of more abstract objects [108,110]. In particular, this extrapolation could be subserved by the ‘abstraction’ of free and unconstrained polysemous handling of the space and of the body, which would comprise a fundamental component of language representations, and perhaps also by common neural mechanisms that share a mode of information integration and processing.
Human-specific illogical cognitive biases for symmetrical inference (the tendency to incorrectly infer ‘if B then A’ from a conditional relationship ‘if A then B’) and for inference by exclusion (the tendency spontaneously to assume that an unfamiliar label goes with an unfamiliar object) involve these same brain areas [111,112]. The mind, human language and human cultural transmission, all of which contribute to the semantic inheritance of the benefits acquired during the unusually elongated human post-reproductive lifespan, are aspects of cognitive functions that have evolved recently and result from such neural niche construction. Once a fundamental syntactic SVO (subject/verb/object) structure emerges [113] and is generalized, abstraction and concept formation and their manipulation become possible and constitute a basis for further intellectual advancements, such as polysemic interpretation of phenomena (which enables metaphorical inference). Such redundancy and polysemous representation would allow equivalence and symmetric inferences and would lead to the emergence of symbols. All of these functions seem to be carried out in the expanded inferior parietal and surrounding areas in humans. Hence, the human language faculty seems to draw on these fundamental neural mechanisms, which are found in these late-myelinating brain areas, which retain a large degree of flexibility until adulthood.
5. Parietal cortex as the centre of triadic interactions
(a). A site for multiple sensory and motor integrations and coordinate transformations
The posterior parietal cortex plays a central role in multi-sensory integration and recognition of environmental space. Such integration provides a basis for the production of movements of various body parts, including eyes, hand-arm, head and whole body through transformations between different coordinate systems. The principle of neural reuse [5,31], as depicted above, seems to apply here in enabling the evolution of higher cognitive functions and thus of human cultural niches. Once these fundamentals were established in the parietal cortex, prefrontal cortex could have developed further so as to use the information for further executive functions involving working memory and syntactic operations, which are often argued to have been crucial for the evolution of human intelligence [114]. How, then, could this function have been extrapolated from the general evolution of the nervous system? In this section, we shall sketch a possible sequence of grades of gradually increasing complexity that might take us from reaching movements to tool use and language.
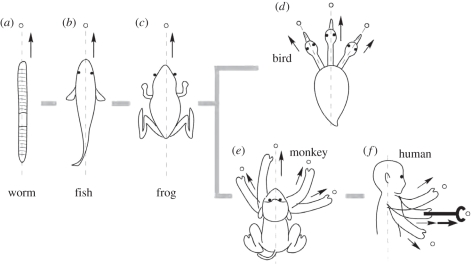
Throughout evolution from primitive protozoa to mammals, the mouth was the organ used both to grasp and to intake prey, after reaching it through locomotion along the body axis. Some animals, especially primates, finally developed the hand to reach and grasp. The target to reach (prey) is detected by sensory organs of various modalities. The nervous system links these sensory and motor apparatuses to produce appropriate actions. The site of such integration within the neuraxis expanded continuously, to finally form the parietal cortex in primates; and its further extrapolation enabled the use of tools, as depicted above. The emergence of bipedalism constituted another evolutionary path for such expansion, as it differentiated the body axis from the movement axis, thus demanding a dramatic increase in spatial information transformation. In turn, this drove the evolution of the parietal operculum in the neural niche. Figure 4 illustrates this scenario, of which fundamental behavioural and neural correlates are summarized below.
Figure 4.
Patterns of reaching-and-grasping movements in different animal species. Fundamentally, animals reach and grasp by moving towards the targets (open circles) using their whole body (a–c). Higher vertebrates developed additional organs, elongated from the trunk to reach and grasp (birds (d, neck) and primates (e, forearm)). (f) In humans, the direction of the movements became perpendicular to the axis of the body because of bipedalism, and was further extended or transformed by the use of tools. Note the differences among the different species in the relative location of the eyes (and of the vestibule) relative to the organs used to finally reach and grasp, as well as their movements relative to the trunk and to the axis of the body (dashed lines).
(i). Head reaching
Primitive animals, in which the locomotor apparatus (such as fins or limbs) has not yet evolved, ‘crawl’ with the whole body to prey (figure 4a). The ‘mouth’ is located at the front end of the body axis [115,116], where sensory organs cluster to efficiently acquire environmental information. Moving in the direction of the mouth (i.e. ‘head reaching’) is still common in extant taxa, including vertebrates. Fish swim in the water three-dimensionally along the body axis (figure 4b). Terrestrial amphibians (and essentially also reptiles and most mammalian species) were constrained to move two-dimensionally on the land surface, yet they still move in the direction of the main body axis and crawl to reach, having evolved limbs for locomotion and resistance against gravity (figure 4c). Head reaching requires information on the target from a self-centred perspective. Animals align the body axis (the direction to move) towards the object and then approach it by travelling with the whole body until arriving at the target. The neural machinery used need only be the rather stereotyped projection of the body onto the environmental space, which requires minimal resources of neural tissue, and of which even insects' tiny brains are capable [117,118].
(ii). Neck reaching
Avians further developed, from forelimbs, the wings to fly. After a flight to reach prey, two final precise reaching-and-grasping procedures emerged. Raptors grasp the prey object by a hind limb [119] and finally eat it using the mouth/beak—they use organs other than the mouth just to grasp, but not directly to eat. Species with long flexible necks, like herons or cranes, reach and grasp directly with the beak using neck movements [120,121]. Here, a discrepancy emerges between the axes of the body and the head (figure 4d). As long as the mouth and eyes remain relatively fixed, the neural processing to reach with the mouth/head remains the same. But, once the neck can move independently of the trunk, object locations need to be represented in multiple spatial-coordinate systems—not only for the original coordinates with the body/trunk at the origin, but also with the head moving relative to the body. Such transformations between coordinate systems would have required their brains to evolve further neural resources.
(iii). Arm reaching
In mammals, particularly primates, the forelimbs have evolved as apparatuses to reach and grasp, diverging from their original locomotor function. Such evolution occurred via (i) substantial elongation of the forelimbs; (ii) increased degrees of freedom of movement at the shoulder, elbow and wrist joints; and (iii) elongation of digits to grasp objects of various sizes, shapes and orientations [122–124]. These changes dramatically increased the diversity of kinds and orientations of reaching-and-grasping motions in the space around the body axis (figure 4e). However, as a trade-off, it requires complex information processing by the brain to harmonize the movement of different body parts by translating positional information between different coordinate systems—body-centred, eye–head-centred and hand-centred systems [125]. Such situations demand more neural resources and the evolution of highly developed spatial perception, resulting in the expansion of parietal cortex. This was a cradle for the further evolution of transformations and modifications between coordinate systems, even for other working spaces. These served as a preadaptation by increasing the degrees of freedom for spatial information acquisition, thereby enabling further expansion of the brain areas that are responsible for those calculations.
(iv). Rotation of moving axis
These processes could be immediately extrapolated onto further evolutionary events. One of those would be the emergence of constant bipedalism. This consists in the maintenance of an upright head-lifted posture, with locomotion perpendicular to the axis of the body trunk for the first time in evolution (figure 4f). Additional constraints emerged, i.e. visual axes became fixed to the direction of locomotion (horizontal), thus also perpendicular to the axis of the body. As a result, various axes (body, hand, head and eyes) became dissociated and the directions of locomotion and of reaching/grasping became independent, depending on the ongoing behaviour. The brain mechanisms for processing such information remain incompletely understood, and open for future investigations.
(b). Extension of axes from concrete to virtual spaces for locomotion, tool use and language
In evolution, the parietal cortex expanded initially as an adaptation to demands from the environment, perhaps for control of different movements of the various body parts, while prefrontal cortex may have expanded later to control such information coded in the parietal cortex through prefronto–parietal interactions [114]. In this way, neural mechanisms became embedded in the brain which served as pre-adaptations for further neural evolution through neural reuse, ultimately enabling the language faculties and (via prefrontal expansion) advanced modes of executive control. The emergence of bipedalism, through its associated demand on multi-sensory integration and very complex sensorimotor coordinate transformations, also pressurized expansion of the greater opercular regions of the cerebral cortex, and thereby facilitated subsequent reuse of such structures for higher cognitive functions including language.
Neural evolution in the first stage of this extension of axes put in place conditions for the emergence of tool use (derived from the usage of innate organs for a purpose not originally planned for). This polysemous pattern of organ usage, and the discovery of novel types of usage, would be key elements in inducing the re-evaluation of existing spatial structures in relation to the body axis as a second stage. The emergence of bipedalism triggered further extrapolation of such faculties and initiated the usage of the externalized body, i.e. extension of body parts into the tool representation. Such freedom from existing physical and bodily constraints in the understanding of the environmental space would allow a novel mode of spatial perception using novel tools (perspective transformation) that would be the basis for the next jump in the acquisition of abstract and transcendental thoughts—stage three. This development has served, in a final stage, as a cradle for the language faculty, principally by developing its neural basis for information processing, both for the use of polysemous and conceptual thoughts and for the articulation of oro-facial organs, to finally subserve language.
6. Conclusion
Expansion (or increase in capacity) of organs as an adaptive response to ecological pressures seems to be a general biological and evolutionary tendency to make the phenotypic system robust—the brain will not be an exception. Multi-sensory integration and coordinate transformation for the control of reaching movements in the inhabited space is an essential function of the nervous system, for which evolution finally endowed primates with a well-developed parietal cortex. The shift of body-space structure associated with the emergence of hominin bipedalism may have further pushed this trend forward to give this area, and the extended opercular cortex, further resources. Such neural enhancement (construction of the neural niche) happened to enable the processing of abstract information, detached from actual physical constraint, by applying and re-using existing principles for spatial information processing to realize novel mental functions (construction of the cognitive niche)—ultimately leading to language. Purposeful manipulation of the body image in space, required for tool use, would have accelerated interactive links between the neural and cognitive niches—tool use requires transformation of various bodily and spatial coordinates, as well as logical and sequential relations of action components.
Tools represent materialized cognitive brain functions. They have been created one after another and incorporated into hominin habitats as constituent elements (construction of the ecological niche). A human-modified environment puts pressure on succeeding generations to adapt to it, perhaps by acquiring further resources for the relevant organs. Epigenetically induced plasticity (including developmental or learning mechanisms) would participate in such processes—and this is a subject for future biological investigations. In this way, extra genomic information could be transmitted between generations via mutual interactions among ecological, neural and cognitive domains of niches, which may have contributed to hominin evolutionary processes (that is, ‘triadic niche construction’). This scenario would locate the human brain as part of an evolving holistic ecosystem.
Acknowledgements
We thank Prof. Michael Arbib and Prof. James Steele for valuable comments, and Dr Michio Tanaka and Dr Teruo Hashimoto for technical assistance. This study was supported by the Funding Program for World-leading Innovative R&D on Science and Technology.
References
- 1.Kendal J., Tehrani J. J., Odling-Smee J. 2011. Human niche construction in interdisciplinary focus. Phil. Trans. R. Soc. B 366, 785–792 10.1098/rstb.2010.0306 (doi:10.1098/rstb.2010.0306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laland K. N., Brown G. R. 2002. Sense and nonsense: evolutionary perspectives on human behaviour. Oxford, UK: Oxford University Press [Google Scholar]
- 3.Odling-Smee F. J., Laland K. N., Feldman M. W. 2003. Niche construction: the neglected process in evolution. Princeton, NJ: Princeton University Press [Google Scholar]
- 4.Pinker S. 2010. Colloquium paper: the cognitive niche: coevolution of intelligence, sociality, and language. Proc. Natl Acad. Sci. USA 107(Suppl. 2), 8993–8999 10.1073/pnas.0914630107 (doi:10.1073/pnas.0914630107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson M. L. 2010. Neural reuse: a fundamental organizational principle of the brain. Behav. Brain Sci. 33, 245–313 10.1017/S0140525X10000853 (doi:10.1017/S0140525X10000853) [DOI] [PubMed] [Google Scholar]
- 6.Iriki A., Sakura O. 2008. The neuroscience of primate intellectual evolution: natural selection and passive and intentional niche construction. Phil. Trans. R. Soc. B 363, 2229–2241 10.1098/rstb.2008.2274 (doi:10.1098/rstb.2008.2274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck B. B. 1980. Animal tool behavior. New York, NY: Garland [Google Scholar]
- 8.de la Torre I. 2011. The origins of stone tool technology in Africa: a historical perspective. Phil. Trans. R. Soc. B 366, 1028–1037 10.1098/rstb.2010.0350 (doi:10.1098/rstb.2010.0350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shennan S. 2002. Genes, memes and human history: Darwinian archeology and cultural evolution. London, UK: Thames & Hudson [Google Scholar]
- 10.Asano T. 1994. Tool using behavior and language in primates. In Behavior analysis of language and cognition (eds Hayes S. C., Hayes L. J., Sato M., Ono K.), pp. 145–148 Reno, NV: Context Press [Google Scholar]
- 11.Yamazaki Y., Namba H., Iriki A. 2009. Acquisition of an externalized eye by Japanese monkeys. Exp. Brain Res. 194, 131–142 10.1007/s00221-008-1677-1 (doi:10.1007/s00221-008-1677-1) [DOI] [PubMed] [Google Scholar]
- 12.Corradi-Dell'acqua C., Ueno K., Ogawa A., Cheng K., Rumiati R. I., Iriki A. 2008. Effects of shifting perspective of the self: an fMRI study. Neuroimage 40, 1902–1911 10.1016/j.neuroimage.2007.12.062 (doi:10.1016/j.neuroimage.2007.12.062) [DOI] [PubMed] [Google Scholar]
- 13.Iriki A. 2006. The neural origins and implications of imitation, mirror neurons and tool use. Curr. Opin. Neurobiol. 16, 660–667 10.1016/j.conb.2006.10.008 (doi:10.1016/j.conb.2006.10.008) [DOI] [PubMed] [Google Scholar]
- 14.Goldenberg G., Iriki A. 2007. From sticks to coffee-maker: mastery of tools and technology by human and non-human primates. Cortex 43, 285–288 10.1016/S0010-9452(08)70454-4 (doi:10.1016/S0010-9452(08)70454-4) [DOI] [PubMed] [Google Scholar]
- 15.Ishibashi H., Hihara S., Iriki A. 2000. Acquisition and development of monkey tool-use: behavioral and kinematic analyses. Can. J. Physiol. Pharmacol. 78, 958–966 10.1139/y00-063 (doi:10.1139/y00-063) [DOI] [PubMed] [Google Scholar]
- 16.Head H., Holmes G. 1911. Sensory disturbances from cerebral lesions. Brain 34, 102–254 10.1093/brain/34.2-3.102 (doi:10.1093/brain/34.2-3.102) [DOI] [Google Scholar]
- 17.Iriki A., Tanaka M., Iwamura Y. 1996. Attention-induced neuronal activity in the monkey somatosensory cortex revealed by pupillometrics. Neurosci. Res. (NY) 25, 173–181 [DOI] [PubMed] [Google Scholar]
- 18.Bruner J. S., Olver R. R., Greenfield P. M. 1966. Studies in cognitive growth. New York, NY: Wiley [Google Scholar]
- 19.Iriki A., Tanaka M., Obayashi S., Iwamura Y. 2001. Self-images in the video monitor coded by monkey intraparietal neurons. Neurosci. Res. (NY) 40, 163–173 [DOI] [PubMed] [Google Scholar]
- 20.Hihara S., Obayashi S., Tanaka M., Iriki A. 2003. Rapid learning of sequential tool use by macaque monkeys. Physiol. Behav. 78, 427–434 10.1016/S0031-9384(02)01006-5 (doi:10.1016/S0031-9384(02)01006-5) [DOI] [PubMed] [Google Scholar]
- 21.Quallo M. M., Price C. J., Ueno K., Asamizuya T., Cheng K., Lemon R. N., Iriki A. 2009. Gray and white matter changes associated with tool-use learning in macaque monkeys. Proc. Natl Acad. Sci. USA 106, 18 379–18 384 10.1073/pnas.0909751106 (doi:10.1073/pnas.0909751106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hihara S., Notoya T., Tanaka M., Ichinose S., Ojima H., Obayashi S., Fujii N., Iriki A. 2006. Extension of corticocortical afferents into the anterior bank of the intraparietal sulcus by tool-use training in adult monkeys. Neuropsychologia 44, 2636–2646 10.1016/j.neuropsychologia.2005.11.020 (doi:10.1016/j.neuropsychologia.2005.11.020) [DOI] [PubMed] [Google Scholar]
- 23.Iriki A. 2005. A prototype of Homo faber: a silent precursor of human intelligence in the tool-using monkey brain. In From monkey brain to human brain: a Fyssen Foundation symposium (eds Dehaene S., Duhamel J.-R., Hauser M. D., Rizzolatti G.), pp. 253–271 Cambridge, MA: MIT Press [Google Scholar]
- 24.Ishibashi H., Hihara S., Takahashi M., Heike T., Yokota T., Iriki A. 2002. Tool-use learning selectively induces expression of brain-derived neurotrophic factor, its receptor trkB, and neurotrophin 3 in the intraparietal multisensory cortex of monkeys. Brain Res. Cogn. Brain Res. 14, 3–9 10.1016/S0926-6410(02)00056-3 (doi:10.1016/S0926-6410(02)00056-3) [DOI] [PubMed] [Google Scholar]
- 25.Ishibashi H., Hihara S., Takahashi M., Heike T., Yokota T., Iriki A. 2002. Tool-use learning induces BDNF expression in a selective portion of monkey anterior parietal cortex. Brain Res. Mol. Brain Res. 102, 110–112 10.1016/S0169-328X(02)00201-2 (doi:10.1016/S0169-328X(02)00201-2) [DOI] [PubMed] [Google Scholar]
- 26.Jablonka E., Lamb M. J. 2007. Precis of evolution in four dimensions. Behav. Brain Sci. 30, 353–389 [DOI] [PubMed] [Google Scholar]
- 27.Pigliucci M. 2010. Genotype-phenotype mapping and the end of the ‘genes as blueprint’ metaphor. Phil. Trans. R. Soc. B 365, 557–566 10.1098/rstb.2009.0241 (doi:10.1098/rstb.2009.0241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tennie C., Call J., Tomasello M. 2009. Ratcheting up the ratchet: on the evolution of cumulative culture. Phil. Trans. R. Soc. B 364, 2405–2415 10.1098/rstb.2009.0052 (doi:10.1098/rstb.2009.0052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baldwin J. M. 1896. A new factor in evolution. Am. Nat. 30, 441–451 10.1086/276408 (doi:10.1086/276408) [DOI] [Google Scholar]
- 30.Bateson P. 2004. The active role of behaviour in evolution. Biol. Phil. 19, 283–298 10.1023/B:BIPH.0000024468.12161.83 (doi:10.1023/B:BIPH.0000024468.12161.83) [DOI] [Google Scholar]
- 31.Dehaene S., Cohen L. 2007. Cultural recycling of cortical maps. Neuron 56, 384–398 10.1016/j.neuron.2007.10.004 (doi:10.1016/j.neuron.2007.10.004) [DOI] [PubMed] [Google Scholar]
- 32.Harman S. M., Talbert G. B. 1985. Reproductive aging. In Handbook of the biology of aging (eds Finch C. E., Hayflick L.), pp. 457–510 New York, NY: Van Nostrand Reinhold [Google Scholar]
- 33.Reznick D., Bryant M. J., Bashey F. 2002. r- and K-selection revisited: the role of population regulation in life-history evolution. Ecology 83, 1509–1520 (doi:10.1890/0012-9658(2002)083[1509:RAKSRT]2.0.CO;2) [Google Scholar]
- 34.Brodmann K. 1909. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Leipzig, Germany: Johann Ambrosius Barth; (English translation by Laurence Garey, 2006 Brodmann's localisation in the cerebral cortex. New York, NY: Springer.) [Google Scholar]
- 35.Geschwind N. 1965. Disconnexion syndromes in animals and man. I. Brain 88, 237–294 10.1093/brain/88.2.237 (doi:10.1093/brain/88.2.237) [DOI] [PubMed] [Google Scholar]
- 36.Peeters R., Simone L., Nelissen K., Fabbri-Destro M., Vanduffel W., Rizzolatti G., Orban G. A. 2009. The representation of tool use in humans and monkeys: common and uniquely human features. J. Neurosci. 29, 11 523–11 539 10.1523/JNEUROSCI.2040-09.2009 (doi:10.1523/JNEUROSCI.2040-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arbib M. A., Bota M. 2006. Neural homologies and the grounding of neurolinguistics. In Action to language via the mirror neuron system (ed. Arbib M. A.), pp. 136–173 Cambridge, MA: Cambridge University Press [Google Scholar]
- 38.Petrides M., Pandya D. N. 2009. Distinct parietal and temporal pathways to the homologues of Broca's area in the monkey. PLoS Biol. 7, e1000170. 10.1371/journal.pbio.1000170 (doi:10.1371/journal.pbio.1000170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.von Bonin G., Bailey P. 1974. The neocortex of Macaca mulatta. Urbana, IL: University of Illinois Press [Google Scholar]
- 40.von Economo C. 1929. The cytoarchitectonics of the human cerebral cortex. London, UK: Oxford University Press [Google Scholar]
- 41.Jancke L., Kleinschmidt A., Mirzazade S., Shah N. J., Freund H. J. 2001. The role of the inferior parietal cortex in linking the tactile perception and manual construction of object shapes. Cereb. Cortex 11, 114–121 10.1093/cercor/11.2.114 (doi:10.1093/cercor/11.2.114) [DOI] [PubMed] [Google Scholar]
- 42.Durand J. B., Peeters R., Norman J. F., Todd J. T., Orban G. A. 2009. Parietal regions processing visual 3D shape extracted from disparity. Neuroimage 46, 1114–1126 10.1016/j.neuroimage.2009.03.023 (doi:10.1016/j.neuroimage.2009.03.023) [DOI] [PubMed] [Google Scholar]
- 43.Jacob S. N., Nieder A. 2009. Notation-independent representation of fractions in the human parietal cortex. J. Neurosci. 29, 4652–4657 10.1523/JNEUROSCI.0651-09.2009 (doi:10.1523/JNEUROSCI.0651-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cappelletti M., Lee H. L., Freeman E. D., Price C. J. 2010. The role of right and left parietal lobes in the conceptual processing of numbers. J. Cogn. Neurosci. 22, 331–346 10.1162/jocn.2009.21246 (doi:10.1162/jocn.2009.21246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castelli F., Glaser D. E., Butterworth B. 2006. Discrete and analogue quantity processing in the parietal lobe: a functional MRI study. Proc. Natl Acad. Sci. USA 103, 4693–4698 10.1073/pnas.0600444103 (doi:10.1073/pnas.0600444103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tudusciuc O., Nieder A. 2009. Contributions of primate prefrontal and posterior parietal cortices to length and numerosity representation. J. Neurophysiol. 101, 2984–2994 10.1152/jn.90713.2008 (doi:10.1152/jn.90713.2008) [DOI] [PubMed] [Google Scholar]
- 47.Knops A., Thirion B., Hubbard E. M., Michel V., Dehaene S. 2009. Recruitment of an area involved in eye movements during mental arithmetic. Science 324, 1583–1585 10.1126/science.1171599 (doi:10.1126/science.1171599) [DOI] [PubMed] [Google Scholar]
- 48.Franklin M. S., Jonides J. 2009. Order and magnitude share a common representation in parietal cortex. J. Cogn. Neurosci. 21, 2114–2120 10.1162/jocn.2008.21181 (doi:10.1162/jocn.2008.21181) [DOI] [PubMed] [Google Scholar]
- 49.Malhotra P., Coulthard E. J., Husain M. 2009. Role of right posterior parietal cortex in maintaining attention to spatial locations over time. Brain 132, 645–660 10.1093/brain/awn350 (doi:10.1093/brain/awn350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Balan P. F., Gottlieb J. 2009. Functional significance of nonspatial information in monkey lateral intraparietal area. J. Neurosci. 29, 8166–8176 10.1523/JNEUROSCI.0243-09.2009 (doi:10.1523/JNEUROSCI.0243-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oristaglio J., Schneider D. M., Balan P. F., Gottlieb J. 2006. Integration of visuospatial and effector information during symbolically cued limb movements in monkey lateral intraparietal area. J. Neurosci. 26, 8310–8319 10.1523/JNEUROSCI.1779-06.2006 (doi:10.1523/JNEUROSCI.1779-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sereno A. B., Amador S. C. 2006. Attention and memory-related responses of neurons in the lateral intraparietal area during spatial and shape-delayed match-to-sample tasks. J. Neurophysiol. 95, 1078–1098 10.1152/jn.00431.2005 (doi:10.1152/jn.00431.2005) [DOI] [PubMed] [Google Scholar]
- 53.Sereno A. B., Maunsell J. H. 1998. Shape selectivity in primate lateral intraparietal cortex. Nature 395, 500–503 10.1038/26752 (doi:10.1038/26752) [DOI] [PubMed] [Google Scholar]
- 54.Gifford G. W., Cohen Y. E. 2005. Spatial and non-spatial auditory processing in the lateral intraparietal area. Exp. Brain Res. 162, 509–512 10.1007/s00221-005-2220-2 (doi:10.1007/s00221-005-2220-2) [DOI] [PubMed] [Google Scholar]
- 55.Medendorp W. P., Goltz H. C., Vilis T. 2005. Remapping the remembered target location for anti-saccades in human posterior parietal cortex. J. Neurophysiol. 94, 734–740 10.1152/jn.01331.2004 (doi:10.1152/jn.01331.2004) [DOI] [PubMed] [Google Scholar]
- 56.Hu S., Bu Y., Song Y., Zhen Z., Liu J. 2009. Dissociation of attention and intention in human posterior parietal cortex: an fMRI study. Eur. J. Neurosci. 29, 2083–2091 10.1111/j.1460-9568.2009.06757.x (doi:10.1111/j.1460-9568.2009.06757.x) [DOI] [PubMed] [Google Scholar]
- 57.Zenon A., Filali N., Duhamel J. R., Olivier E. 2010. Salience representation in the parietal and frontal cortex. J. Cogn. Neurosci. 22, 918–930 10.1162/jocn.2009.21233 (doi:10.1162/jocn.2009.21233) [DOI] [PubMed] [Google Scholar]
- 58.Schendan H. E., Amick M. M., Cronin-Golomb A. 2009. Role of a lateralized parietal-basal ganglia circuit in hierarchical pattern perception: evidence from Parkinson's disease. Behav. Neurosci. 123, 125–136 10.1037/a0013734 (doi:10.1037/a0013734) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Phillips J. S., Velanova K., Wolk D. A., Wheeler M. E. 2009. Left posterior parietal cortex participates in both task preparation and episodic retrieval. Neuroimage 46, 1209–1221 10.1016/j.neuroimage.2009.02.044 (doi:10.1016/j.neuroimage.2009.02.044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McNab F., Varrone A., Farde L., Jucaite A., Bystritsky P., Forssberg H., Klingberg T. 2009. Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science 323, 800–802 10.1126/science.1166102 (doi:10.1126/science.1166102) [DOI] [PubMed] [Google Scholar]
- 61.Kawasaki M., Watanabe M., Okuda J., Sakagami M., Aihara K. 2008. Human posterior parietal cortex maintains color, shape and motion in visual short-term memory. Brain Res. 1213, 91–97 10.1016/j.brainres.2008.03.037 (doi:10.1016/j.brainres.2008.03.037) [DOI] [PubMed] [Google Scholar]
- 62.Rawley J. B., Constantinidis C. 2009. Neural correlates of learning and working memory in the primate posterior parietal cortex. Neurobiol. Learn Mem. 91, 129–138 10.1016/j.nlm.2008.12.006 (doi:10.1016/j.nlm.2008.12.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oztekin I., McElree B., Staresina B. P., Davachi L. 2009. Working memory retrieval: contributions of the left prefrontal cortex, the left posterior parietal cortex, and the hippocampus. J. Cogn. Neurosci. 21, 581–593 10.1162/jocn.2008.21016 (doi:10.1162/jocn.2008.21016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mitchell D. J., Cusack R. 2008. Flexible, capacity-limited activity of posterior parietal cortex in perceptual as well as visual short-term memory tasks. Cereb. Cortex 18, 1788–1798 10.1093/cercor/bhm205 (doi:10.1093/cercor/bhm205) [DOI] [PubMed] [Google Scholar]
- 65.Leon M. I., Shadlen M. N. 2003. Representation of time by neurons in the posterior parietal cortex of the macaque. Neuron 38, 317–327 10.1016/S0896-6273(03)00185-5 (doi:10.1016/S0896-6273(03)00185-5) [DOI] [PubMed] [Google Scholar]
- 66.Bueti D., Walsh V., Frith C., Rees G. 2008. Different brain circuits underlie motor and perceptual representations of temporal intervals. J. Cogn. Neurosci. 20, 204–214 10.1162/jocn.2008.20017 (doi:10.1162/jocn.2008.20017) [DOI] [PubMed] [Google Scholar]
- 67.Coull J. T., Vidal F., Nazarian B., Macar F. 2004. Functional anatomy of the attentional modulation of time estimation. Science 303, 1506–1508 10.1126/science.1091573 (doi:10.1126/science.1091573) [DOI] [PubMed] [Google Scholar]
- 68.Davis B., Christie J., Rorden C. 2009. Temporal order judgments activate temporal parietal junction. J. Neurosci. 29, 3182–3188 10.1523/JNEUROSCI.5793-08.2009 (doi:10.1523/JNEUROSCI.5793-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Calton J. L., Dickinson A. R., Snyder L. H. 2002. Non-spatial, motor-specific activation in posterior parietal cortex. Nat. Neurosci. 5, 580–588 10.1038/nn0602-862 (doi:10.1038/nn0602-862) [DOI] [PubMed] [Google Scholar]
- 70.Beudel M., Renken R., Leenders K. L., de Jong B. M. 2009. Cerebral representations of space and time. Neuroimage 44, 1032–1040 10.1016/j.neuroimage.2008.09.028 (doi:10.1016/j.neuroimage.2008.09.028) [DOI] [PubMed] [Google Scholar]
- 71.Oliveri M., Koch G., Salerno S., Torriero S., Lo Gerfo E., Caltagirone C. 2009. Representation of time intervals in the right posterior parietal cortex: implications for a mental time line. Neuroimage 46, 1173–1179 10.1016/j.neuroimage.2009.03.042 (doi:10.1016/j.neuroimage.2009.03.042) [DOI] [PubMed] [Google Scholar]
- 72.Fogassi L., Ferrari P. F., Gesierich B., Rozzi S., Chersi F., Rizzolatti G. 2005. Parietal lobe: from action organization to intention understanding. Science 308, 662–667 10.1126/science.1106138 (doi:10.1126/science.1106138) [DOI] [PubMed] [Google Scholar]
- 73.Ishida H., Nakajima K., Inase M., Murata A. 2010. Shared mapping of own and others' bodies in visuotactile bimodal area of monkey parietal cortex. J. Cogn. Neurosci. 22, 83–96 10.1162/jocn.2009.21185 (doi:10.1162/jocn.2009.21185) [DOI] [PubMed] [Google Scholar]
- 74.Pelphrey K. A., Morris J. P., McCarthy G. 2004. Grasping the intentions of others: the perceived intentionality of an action influences activity in the superior temporal sulcus during social perception. J. Cogn. Neurosci. 16, 1706–1716 10.1162/0898929042947900 (doi:10.1162/0898929042947900) [DOI] [PubMed] [Google Scholar]
- 75.Muhlau M., et al. 2005. Left inferior parietal dominance in gesture imitation: an fMRI study. Neuropsychologia 43, 1086–1098 10.1016/j.neuropsychologia.2004.10.004 (doi:10.1016/j.neuropsychologia.2004.10.004) [DOI] [PubMed] [Google Scholar]
- 76.Jackson P. L., Meltzoff A. N., Decety J. 2006. Neural circuits involved in imitation and perspective-taking. Neuroimage 31, 429–439 10.1016/j.neuroimage.2005.11.026 (doi:10.1016/j.neuroimage.2005.11.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dinstein I., Gardner J. L., Jazayeri M., Heeger D. J. 2008. Executed and observed movements have different distributed representations in human aIPS. J. Neurosci. 28, 11 231–11 239 10.1523/JNEUROSCI.3585-08.2008 (doi:10.1523/JNEUROSCI.3585-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamazaki Y., Yokochi H., Tanaka M., Okanoya K., Iriki A. 2010. Potential role of monkey inferior parietal neurons coding action semantic equivalences as precursors of parts of speech. Soc. Neurosci. 5, 105–117 10.1080/17470910802625306 (doi:10.1080/17470910802625306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Culham J. C., Valyear K. F. 2006. Human parietal cortex in action. Curr. Opin. Neurobiol. 16, 205–212 10.1016/j.conb.2006.03.005 (doi:10.1016/j.conb.2006.03.005) [DOI] [PubMed] [Google Scholar]
- 80.Johnson-Frey S. H., Newman-Norlund R., Grafton S. T. 2005. A distributed left hemisphere network active during planning of everyday tool use skills. Cereb. Cortex 15, 681–695 10.1093/cercor/bhh169 (doi:10.1093/cercor/bhh169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lewis J. W. 2006. Cortical networks related to human use of tools. Neuroscientist 12, 211–231 10.1177/1073858406288327 (doi:10.1177/1073858406288327) [DOI] [PubMed] [Google Scholar]
- 82.Obayashi S., Suhara T., Kawabe K., Okauchi T., Maeda J., Akine Y., Onoe H., Iriki A. 2001. Functional brain mapping of monkey tool use. Neuroimage 14, 853–861 10.1006/nimg.2001.0878 (doi:10.1006/nimg.2001.0878) [DOI] [PubMed] [Google Scholar]
- 83.Agnew Z., Wise R. J. 2008. Separate areas for mirror responses and agency within the parietal operculum. J. Neurosci. 28, 12 268–12 273 10.1523/JNEUROSCI.2836-08.2008 (doi:10.1523/JNEUROSCI.2836-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shmuelof L., Zohary E. 2008. Mirror-image representation of action in the anterior parietal cortex. Nat. Neurosci. 11, 1267–1269 10.1038/nn.2196 (doi:10.1038/nn.2196) [DOI] [PubMed] [Google Scholar]
- 85.Yamakawa Y., Kanai R., Matsumura M., Naito E. 2009. Social distance evaluation in human parietal cortex. PLoS ONE 4, e4360. 10.1371/journal.pone.0004360 (doi:10.1371/journal.pone.0004360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Neggers S. F., Van der Lubbe R. H., Ramsey N. F., Postma A. 2006. Interactions between ego- and allocentric neuronal representations of space. Neuroimage 31, 320–331 10.1016/j.neuroimage.2005.12.028 (doi:10.1016/j.neuroimage.2005.12.028) [DOI] [PubMed] [Google Scholar]
- 87.Decety J., Jackson P. L., Sommerville J. A., Chaminade T., Meltzoff A. N. 2004. The neural bases of cooperation and competition: an fMRI investigation. Neuroimage 23, 744–751 10.1016/j.neuroimage.2004.05.025 (doi:10.1016/j.neuroimage.2004.05.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goldenberg G., Spatt J. 2009. The neural basis of tool use. Brain 132, 1645–1655 10.1093/brain/awp080 (doi:10.1093/brain/awp080) [DOI] [PubMed] [Google Scholar]
- 89.Lewis J. W., Brefczynski J. A., Phinney R. E., Janik J. J., DeYoe E. A. 2005. Distinct cortical pathways for processing tool versus animal sounds. J. Neurosci. 25, 5148–5158 10.1523/JNEUROSCI.0419-05.2005 (doi:10.1523/JNEUROSCI.0419-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pelgrims B., Andres M., Olivier E. 2009. Double dissociation between motor and visual imagery in the posterior parietal cortex. Cereb. Cortex 19, 2298–2307 10.1093/cercor/bhn248 (doi:10.1093/cercor/bhn248) [DOI] [PubMed] [Google Scholar]
- 91.Schicke T., Muckli L., Beer A. L., Wibral M., Singer W., Goebel R., Rösler F., Röder B. 2006. Tight covariation of BOLD signal changes and slow ERPs in the parietal cortex in a parametric spatial imagery task with haptic acquisition. Eur. J. Neurosci. 23, 1910–1918 10.1111/j.1460-9568.2006.04720.x (doi:10.1111/j.1460-9568.2006.04720.x) [DOI] [PubMed] [Google Scholar]
- 92.Zatorre R. J., Halpern A. R., Bouffard M. 2010. Mental reversal of imagined melodies: a role for the posterior parietal cortex. J. Cogn. Neurosci. 22, 775–789 10.1162/jocn.2009.21239 (doi:10.1162/jocn.2009.21239) [DOI] [PubMed] [Google Scholar]
- 93.Champod A. S., Petrides M. 2007. Dissociable roles of the posterior parietal and the prefrontal cortex in manipulation and monitoring processes. Proc. Natl Acad. Sci. USA 104, 14 837–14 842 10.1073/pnas.0607101104 (doi:10.1073/pnas.0607101104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kamigaki T., Fukushima T., Miyashita Y. 2009. Cognitive set reconfiguration signaled by macaque posterior parietal neurons. Neuron 61, 941–951 10.1016/j.neuron.2009.01.028 (doi:10.1016/j.neuron.2009.01.028) [DOI] [PubMed] [Google Scholar]
- 95.Liston C., Matalon S., Hare T. A., Davidson M. C., Casey B. J. 2006. Anterior cingulate and posterior parietal cortices are sensitive to dissociable forms of conflict in a task-switching paradigm. Neuron 50, 643–653 10.1016/j.neuron.2006.04.015 (doi:10.1016/j.neuron.2006.04.015) [DOI] [PubMed] [Google Scholar]
- 96.Nakahara K., Hayashi T., Konishi S., Miyashita Y. 2002. Functional MRI of macaque monkeys performing a cognitive set-shifting task. Science 295, 1532–1536 10.1126/science.1067653 (doi:10.1126/science.1067653) [DOI] [PubMed] [Google Scholar]
- 97.Pessoa L., Rossi A., Japee S., Desimone R., Ungerleider L. G. 2009. Attentional control during the transient updating of cue information. Brain Res. 1247, 149–158 10.1016/j.brainres.2008.10.010 (doi:10.1016/j.brainres.2008.10.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shomstein S., Yantis S. 2006. Parietal cortex mediates voluntary control of spatial and nonspatial auditory attention. J. Neurosci. 26, 435–439 10.1523/JNEUROSCI.4408-05.2006 (doi:10.1523/JNEUROSCI.4408-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stoet G., Snyder L. H. 2004. Single neurons in posterior parietal cortex of monkeys encode cognitive set. Neuron 42, 1003–1012 10.1016/j.neuron.2004.06.003 (doi:10.1016/j.neuron.2004.06.003) [DOI] [PubMed] [Google Scholar]
- 100.Ogawa T., Komatsu H. 2009. Condition-dependent and condition-independent target selection in the macaque posterior parietal cortex. J. Neurophysiol. 101, 721–736 10.1152/jn.90817.2008 (doi:10.1152/jn.90817.2008) [DOI] [PubMed] [Google Scholar]
- 101.Hampshire A., Gruszka A., Fallon S. J., Owen A. M. 2008. Inefficiency in self-organized attentional switching in the normal aging population is associated with decreased activity in the ventrolateral prefrontal cortex. J. Cogn. Neurosci. 20, 1670–1686 10.1162/jocn.2008.20115 (doi:10.1162/jocn.2008.20115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Crowe D. A., Chafee M. V., Averbeck B. B., Georgopoulos A. P. 2004. Participation of primary motor cortical neurons in a distributed network during maze solution: representation of spatial parameters and time-course comparison with parietal area 7a. Exp. Brain Res. 158, 28–34 10.1007/s00221-004-1876-3 (doi:10.1007/s00221-004-1876-3) [DOI] [PubMed] [Google Scholar]
- 103.Freedman D. J., Assad J. A. 2009. Distinct encoding of spatial and nonspatial visual information in parietal cortex. J. Neurosci. 29, 5671–5680 10.1523/JNEUROSCI.2878-08.2009 (doi:10.1523/JNEUROSCI.2878-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hoenig K., Scheef L. 2009. Neural correlates of semantic ambiguity processing during context verification. Neuroimage 45, 1009–1019 10.1016/j.neuroimage.2008.12.044 (doi:10.1016/j.neuroimage.2008.12.044) [DOI] [PubMed] [Google Scholar]
- 105.Yang T., Shadlen M. N. 2007. Probabilistic reasoning by neurons. Nature 447, 1075–1080 10.1038/nature05852 (doi:10.1038/nature05852) [DOI] [PubMed] [Google Scholar]
- 106.Tosoni A., Galati G., Romani G. L., Corbetta M. 2008. Sensory-motor mechanisms in human parietal cortex underlie arbitrary visual decisions. Nat. Neurosci. 11, 1446–1453 10.1038/nn.2221 (doi:10.1038/nn.2221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Turriziani P., Oliveri M., Bonni S., Koch G., Smirni D., Cipolotti L. 2009. Exploring the relationship between semantics and space. PLoS ONE 4, e5319. 10.1371/journal.pone.0005319 (doi:10.1371/journal.pone.0005319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yamazaki Y., Hashimoto T., Iriki A. 2009. The posterior parietal cortex and non-spatial cognition. F1000 Biol. Rep. 1, 74. 10.3410/B1-74 (doi:10.3410/B1-74) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Arbib M. A., Lee J. 2008. Describing visual scenes: towards a neurolinguistics based on construction grammar. Brain Res. 1225, 146–162 10.1016/j.brainres.2008.04.075 (doi:10.1016/j.brainres.2008.04.075) [DOI] [PubMed] [Google Scholar]
- 110.Almor A., Smith D. V., Bonilha L., Fridriksson J., Rorden C. 2007. What is in a name? Spatial brain circuits are used to track discourse references. Neuroreport 18, 1215–1219 10.1097/WNR.0b013e32810f2e11 (doi:10.1097/WNR.0b013e32810f2e11) [DOI] [PubMed] [Google Scholar]
- 111.Ogawa A., Yamazaki Y., Ueno K., Cheng K., Iriki A. 2010. Neural correlates of species-typical illogical cognitive bias in human inference. J. Cogn. Neurosci. 22, 2120–2130 10.1162/jocn.2009.21330 (doi:10.1162/jocn.2009.21330) [DOI] [PubMed] [Google Scholar]
- 112.Ogawa A., Yamazaki Y., Ueno K., Cheng K., Iriki A. 2010. Inferential reasoning by exclusion recruits parietal and prefrontal cortices. Neuroimage 52, 1603–1610 10.1016/j.neuroimage.2010.05.040 (doi:10.1016/j.neuroimage.2010.05.040) [DOI] [PubMed] [Google Scholar]
- 113.Hihara S., Yamada H., Iriki A., Okanoya K. 2003. Spontaneous vocal differentiation of coo-calls for tools and food in Japanese monkeys. Neurosci. Res. (NY) 45, 383–389 [DOI] [PubMed] [Google Scholar]
- 114.Deacon T. 1997. The symbolic species. London, UK: Penguin Press [Google Scholar]
- 115.Carroll S. B., Grenier J. K., Weatherbee S. D. 2005. From DNA to diversity: molecular genetics and the evolution of animal design, 2nd edn Malden, MA: Blackwell Publishing [Google Scholar]
- 116.De Robertis E. M., Sasai Y. 1996. A common plan for dorsoventral patterning in Bilateria. Nature 380, 37–40 10.1038/380037a0 (doi:10.1038/380037a0) [DOI] [PubMed] [Google Scholar]
- 117.Mizunami M., Yokohari F., Takahata M. 1999. Exploration into the adaptive design of the arthropod ‘microbrain’. Zool. Sci. 16, 703–709 10.2108/zsj.16.703 (doi:10.2108/zsj.16.703) [DOI] [PubMed] [Google Scholar]
- 118.Mizunami M., Yokohari F., Takahata M. 2004. Further exploration into the adaptive design of the arthropod ‘microbrain’: I. Sensory and memory-processing systems. Zool. Sci. 21, 1141–1151 10.2108/zsj.21.1141 (doi:10.2108/zsj.21.1141) [DOI] [PubMed] [Google Scholar]
- 119.Ward A. B., Weigl P. D., Conroy R. M. 2002. Functional morphology of raptor hindlimbs: implications for resource partitioning. Auk 119, 1052–1063 10.1642/0004-8038(2002)119[1052:FMORHI]2.0.CO;2 (doi:10.1642/0004-8038(2002)119[1052:FMORHI]2.0.CO;2) [DOI] [Google Scholar]
- 120.Martin G. R., Katzir G. 1994. Visual fields and eye movements in herons (Ardeidae). Brain Behav. Evol. 44, 74–85 10.1159/000113571 (doi:10.1159/000113571) [DOI] [PubMed] [Google Scholar]
- 121.White C. R., Day N., Butler P. J., Martin G. R. 2007. Vision and foraging in cormorants: more like herons than hawks? PLoS ONE 2, e639. 10.1371/journal.pone.0000639 (doi:10.1371/journal.pone.0000639) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ankel-Simons F. 2007. Primate anatomy: an introduction, 3rd edn Burlington, VT: Academic Press [Google Scholar]
- 123.Larson S. G., Stern J. T., Jr 2006. Maintenance of above-branch balance during primate arboreal quadrupedalism: coordinated use of forearm rotators and tail motion. Am. J. Phys. Anthropol. 129, 71–81 10.1002/ajpa.20236 (doi:10.1002/ajpa.20236) [DOI] [PubMed] [Google Scholar]
- 124.Marzke M. W. 1996. Evolution of the hand and the bipedality. In Handbook of human symbolic evolution (eds Lock A., Peters C. R.), pp. 126–154 Oxford, UK: Oxford University Press [Google Scholar]
- 125.Buneo C. A., Andersen R. A. 2006. The posterior parietal cortex: sensorimotor interface for the planning and online control of visually guided movements. Neuropsychologia 44, 2594–2606 10.1016/j.neuropsychologia.2005.10.011 (doi:10.1016/j.neuropsychologia.2005.10.011) [DOI] [PubMed] [Google Scholar]