Abstract
The world is changing at an unprecedented rate. In such a situation, we need to understand the nature of the change and to make predictions about the way in which it might affect systems of interest; often we may also wish to understand what might be done to mitigate the predicted effects. In ecology, we usually make such predictions (or forecasts) by making use of mathematical models that describe the system and projecting them into the future, under changed conditions. Approaches emphasizing the desirability of simple models with analytical tractability and those that use assumed causal relationships derived statistically from data currently dominate ecological modelling. Although such models are excellent at describing the way in which a system has behaved, they are poor at predicting its future state, especially in novel conditions. In order to address questions about the impact of environmental change, and to understand what, if any, action might be taken to ameliorate it, ecologists need to develop the ability to project models into novel, future conditions. This will require the development of models based on understanding the processes that result in a system behaving the way it does, rather than relying on a description of the system, as a whole, remaining valid indefinitely.
Keywords: ecological forecasts, environmental change, systems biology, global climate models (GCMs), philosophy of modelling, phenomenological models
1. Introduction
The statement that the world's environment is facing a period of unprecedented change has become a truism. The ‘anthropogenic cocktail’ of climate change, habitat loss and degradation are combining to put considerable pressure on the world's ecosystems [1]. We know that the natural world is already being affected because we can observe effects consistent with climate change that range from the loss of animal and plant species [2], to shifts in distributional ranges [3], and, in particular, to changes in phenology [4–6]. At present, our understanding of the vulnerability of ecological systems, and the services provided by them, to impacts arising from environmental change is relatively weak [7]. In addition, although it is clear that the ecology of the Earth is essential for human health and well-being and that the services provided by the world's ecosystems are important both economically and culturally, it is equally clear that these services are poorly understood [8,9]. Society needs answers to questions about the likely future state of the natural world in order to inform decisions about appropriate land-use policies and strategies, and to allow assessment of the risks posed by climate change to different ecological systems and to different environments [10–13].
In the first half of the twentieth century, the majority view of climatologists seems to have been that the climate was a self-regulating system, such that in understanding future climates ‘we can safely accept the past performance as an adequate guide for the future’ [14]. However, the discovery that the concentration of carbon dioxide in the atmosphere was increasing at a rate similar to that which would be predicted from the combustion of fossil fuels with no feedback processes removing it from the atmosphere [15] was incompatible with the view that the climate system would equilibrate. In the decades since, the science of climatology has changed significantly, no longer viewing the climate as a self-regulating system, and now uses some of the largest computer models in the world to make forecasts of future climates. Such forecasts include the modified climates that are predicted to occur in the future owing to anthropogenic activity resulting in the release of greenhouse gases such as carbon dioxide into the atmosphere. As a result of this modelling effort, which has been synthesized in the series of reports (to date four) from the Intergovernmental Panel on Climate Change (IPCC), we have an increasingly detailed understanding of the likely impact of changing concentrations of greenhouse gases on climate [16]. The physical science of climate change currently suggests that over the next century there will be 1.8–4.0°C (depending on emissions scenario) increase in mean global surface temperature, with associated changes in the amount and distribution of precipitation and rises in sea level [16]. In addition to providing this synthesis, the IPCC has paved the way for the translation of climate science into policy [17].
The scale of the likely changes in the physical environment (e.g. temperature change of 1.8–4°C) is worthy of consideration because few people would be able to detect directly a change in mean temperature of this magnitude against the background of normal, highly variable weather. Despite the fact that these changes will not be easy to discern directly, the consensus view is that climate change is a serious problem. The perceived seriousness of the problem arises not from the temperature change itself but from its likely impact on non-physical elements of the world. The indirect effects of the changes in the physical environment—changes in the landscape, the demise of charismatic species, drought-induced crop failures and by changes in the services received from ecosystems—will affect most (if not all) people. In other words, it is the impact of the physical changes on the biological world, and not the scale of the physical changes themselves, that give the issue of climate change its seriousness. We therefore have the paradox that our state of knowledge about the aspect of the problem that will affect people the greatest amount is simultaneously the aspect about which we know least.
At one level, there is a childishly easy answer to the question ‘what is the impact of climate change on the biological world?’ Given that, for obvious physical reasons, temperatures decrease with both altitude and latitude, most people would expect that as climate change proceeds, species ranges should move polewards and upwards. A general answer, such as this, provides some information but is insufficiently specific to provide anything other than a likely, and very general, direction of travel. A more robust answer, which gives specific information about systems of interest, will be required. The critical step that needs to be taken to tackle the problem of understanding the ecological impact of environmental change is the development of realistic models of ecological systems, which can be projected into future changed conditions to make accurate predictions about the state of the system in those conditions. In accordance with this, prediction is going to have to be an important function of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services [18]. Therefore, there is an urgent need to develop the ability to produce ecological forecasts, so as to be able to predict the future state of ecosystems and parts of ecosystems [19]. This will be challenging, as ecological systems are both complex and noisy at all organizational levels.
2. Modelling philosophy
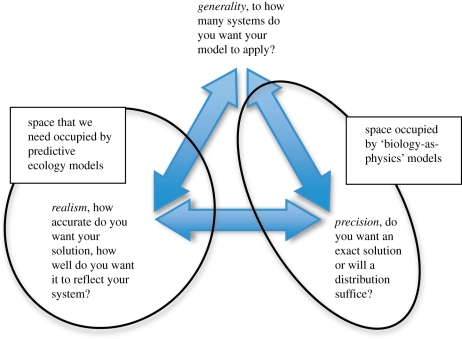
Models represent real world phenomena in simplified forms in order to generate understanding of those phenomena [20,21]; in ecology, models are typically mathematical objects [22]. A modeller attempting to represent even a simple ecological system has to cope with its inherent complexity. As it is impossible to capture every possible element of a system in a model, modellers need to take decisions about which of its features to include and which to disregard. These decisions are, or at least should be, guided by the aims that the modeller is attempting to achieve by creating a model. Three common aspirations for models are that they be general (the model's conclusions can be applied to a wide range of real-life systems—in other words, it captures some basic essence that applies to many possible systems), realistic (achieved when a model's conclusions have a close match to a real-life system—in other words, it accurately captures the way in which a particular system functions) and precise (the model's predictions for a specific set of circumstances have little or no uncertainty; figure 1). In 1966, Levins suggested that while it would be desirable to ‘work with manageable models that maximized generality, realism and precision’, simultaneous maximization of these three different desiderata could not, in practice, be achieved [23]. He described various modelling strategies that had been adopted to cope with the inability to simultaneously maximize desiderata, all of which involved trading-off one desideratum in order to achieve greater performance in the other two (e.g. sacrificing realism to achieve generality and precision). While these are all reasonable strategies for simplifying a complex phenomenon into a tractable form [24], adopting any of them (or indeed any other idealization strategy) will have consequences that will result in the resulting model being more or less well suited for particular types of problem.
Figure 1.
Schematic of modelling trade-offs. A modeller has to decide what characteristics their model will emphasize. Philosophical considerations suggest that they cannot have a model that maxmizes all these desirable characteristics.
Although, some philosophers have cast doubt on the existence of Levins' claimed trade-offs [20,25,26], the consensus in the philosophical literature is that in model building at least some of these trade-offs exist [27–29], and recent work has claimed to have formalized proofs for the existence of trade-offs between desiderata (e.g. generality and precision) as posited by Levins ([28] and R. De Langhe, unpublished data; http://www.ugent.academia.edu/RogierDeLanghe/Papers/132229/A-general-argument-for-tradeoffs-in-model-building). In the particular case of ecology, it seems inevitable that there should be a trade-off between realism and precision, as any realistic model will contain stochastic effects, and stochasticity will inevitably reduce precision. Similarly, there must be a trade-off between realism and generality. A realistic model will explicitly consider biological characteristics specific to the system, which will inevitably reduce the generality of the predictions. For an ecologist attempting to model a complex ecological system, the important consequence of the existence of these trade-offs is that if one wishes to maximize one of these desiderata, then one has to sacrifice at least one of the other two (figure 1). In other words, if these trade-offs exist, then the modeller will have to decide which of these characteristics they would like the model to possess and allow this decision to inform model development.
Given the demand from society for answers to questions about the ecological impact of environmental change, it would seem reasonable to give some thought to what modelling strategies might be most appropriate in order to provide the forecasts that will be necessary to answer such questions and what characteristics they would need to have to make them useful. It does not seem unreasonable to suggest that:
— forecasts will need to be realistic, probably reflecting specific systems and allow judgements to be made about potential action that may be taken; and
— forecasts will be needed about the state of systems of interest in novel environmental conditions, as almost by definition these are what is created by the environmental changes with which we are concerned.
In §3, I will ask whether the prevalent modelling approaches used in ecology meet these pre-requisites.
3. Current approaches to ecological modelling
(a). ‘Biology-as-physics’
In 1966, Levins identified his second set of modelling idealization strategies as those that sacrificed realism to generality and precision. He suggested that most physicists who entered biology worked in this manner, setting up simple equations from which they could obtain precise answers [23]. This tradition of modelling has a long history [30] and can be traced back to the work in the early part of the twentieth century of the mathematical physicist Volterra, who used simple differential equations to examine trends in prey and predatory fish populations in the Adriatic [31]. Anderson & May [32,33] used essentially the same equations over 50 years later in their highly influential papers on the population biology of diseases. Such models have the advantage of mathematical tractability; they can be solved analytically to give precise, point answers and can be easily interrogated to determine the sensitivity of the model to its parameters. There is a tendency to regard simple models, such as these, as being general by virtue of their simplicity. While it seems intuitively obvious that simple models will never accurately reflect any particular system (and so they lack realism), it does not seem obvious that a simple model should necessarily have generality. It might be more true to claim that, at their best, simple models provide general, rather than specific, insight. For example, the conclusion that the rate of change in the size of a population of organisms infected with a disease will be influenced by the birth rate, the disease-independent mortality rate and the mortality rate of infected individuals (eqn 4, [32]) is generally useful. However, if this is to be applied more specifically, then new terms need to be added that increasingly make the results less general (e.g. compare eqn 4, [32] representing the general case, with eqn 14 [33] that presents a highly simplified model for human populations affected by malaria). Even when this is achieved, the output of such models need further modification; otherwise they suggest, for example, that every outbreak of a disease will be identical [33].
As they emphasize analytical precision and aim for generality, biology-as-physics models lose realism and are usually difficult to apply to specific systems [34]. An approach with this emphasis is unlikely to meet the first pre-requisite for forecasting the ecological impact of environmental changes—as it will be difficult to make realistic predictions for specific systems that will inform us about the nature of change predicted in that system and what action might be taken to ameliorate it.
(b). Phenomenological
If we are concerned about the impact of environmental change on ecological systems then, almost by definition, the future into which we would like to project our predictions will be novel. It is well appreciated that models based on phenomenological descriptions of data (such as simple statistical associations) should not be used to predict beyond the realm of existing data [35–37]. Phenomenological models are excellent at describing what has happened and have been a powerful tool in the analysis of datasets, including those concerned with environmental change but should not be projected beyond the regions within which data were collected [21]. If one does so, then there is a high possibility of errors occurring when the association between two variables (e.g. gender and rate of change in sprint performance [35], climatic constraints and species ranges [10], financial incentives and the induction of conservation favourable behaviours [38]) that hold for the regions of parameter space in which data were collected break down in other hitherto unobserved regions. Twain [39] provided an excellent illustration of the problems of extrapolating outside the range of observed data:
In the space of one hundred and seventy-six years the Lower Mississippi has shortened itself two hundred and forty-two miles. That is an average of a trifle over one mile and a third per year. Therefore, any calm person, who is not blind or idiotic, can see that in the Old Oolitic Silurian Period, just a million years ago next November, the Lower Mississippi River was upwards of one million three hundred thousand miles long, and stuck out over the Gulf of Mexico like a fishing-rod. And by the same token any person can see that seven hundred and forty-two years from now the Lower Mississippi will be only a mile and three-quarters long.
Twain [39]
Phenomenological models, based on assumed causal relationships derived statistically from data, fail to fulfil the second pre-requisite for forecasting the ecological impact of environmental change—that of projection into novel conditions. To predict a system's behaviour in novel conditions requires models that capture the important underlying mechanisms that drive the behaviour of the system [40–42]. This distinction is observed in climatological research in which the description of historical datasets (such as carbon dioxide time series [15] or temperature time series [43]) is conducted with, sometimes complex, statistical analyses. However, forecasting the effects of future greenhouse gas concentrations on temperature and other aspects of the world's climate is done using process-based systems models [44]. These models rely on an understanding of the underlying mechanisms that interact to influence the climate system. It is because of this that they can predict the effect of previously unknown levels of greenhouse gases with climate change emerging as a property of the system under these novel conditions [44,45].
4. An alternative approach
If a system is defined as a set of interacting or interdependent entities forming an integrated whole, then ecology is clearly concerned with systems. It is a truism that all populations, communities and ecosystems are composed of interacting individual organisms. Despite this, ecologists typically treat populations, communities and ecosystems as entities that can be studied in isolation and usually define themselves as having interests addressing a single level of organization. Population dynamics or community structure are usually seen as properties of the population or community. However, any population or ecosystem attribute arises from individuals and the way they live, interact and die. Demography, growth rates, extinction rates, community structure and epidemiology are all emergent properties of individual-based systems [46,47]. If we could understand these individual decisions and model them in an appropriate manner, then, as has been suggested, we could treat population or community dynamics as emergent properties of the system [40,42,48].
Each individual is itself an emergent property of the morphological, physiological, cellular, metabolic, genetic systems below; and contributes to the hierarchical levels ‘above’ by interacting with other individuals and contributing to population and community dynamics. However, the individual is also clearly the fundamental level of biological function; it is at this level that interactions with the environment occur and at which selection operates (through the differential survival and/or reproduction of individuals and through them their genes). The challenge for a systems approach will be to decide what processes at lower hierarchical levels are necessary to predict the emergent properties of higher hierarchical levels. For example, does predicting the population dynamics of a specific system require information on how genes and environment shape individual phenotypes and with dynamics arising as the sum of individuals' life histories across the population? Understanding ecosystem dynamics will probably not require the inclusion of every individual within the community, but may require some of the spatio-temporal heterogeneity in between-species interactions that arises from individual-level heterogeneities. The requirement to consider a priori which mechanisms at lower organizational levels may drive the system at the level of interest (for example, population dynamics emerges from individuals and their behaviour, which in turn depends on genes and environment; community dynamics depends on interactions between species that will have means and variances that depend on individuality) is one benefit of conceptualizing ecological systems as systems.
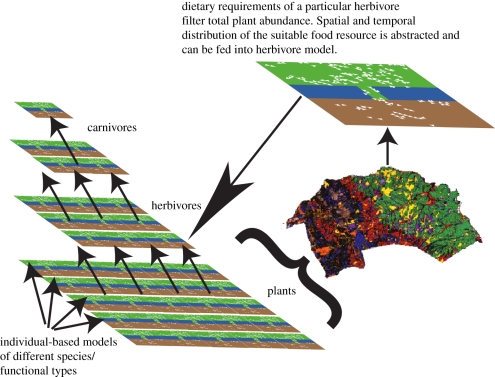
A systems approach to ecology would imply that processes operating within a system were modelled such that changes in the system (e.g. population dynamics, community structure, ecosystem services) emerged as properties of the system. A robust understanding of these processes allows the prediction of the future state of the system in a novel environment and so allows better prediction of the impacts of environmental change on the biological world. This is something with which non-systems approaches struggle [10,11,49–53], because they require us to assume that a description of the system will remain valid indefinitely. There are many methods (e.g. [54,55]) that can be used to cross levels of organization and therefore abstract the necessary and sufficient information from the levels below [23]. A ‘necessary and sufficient’ abstraction makes intuitive sense—a predator may not care which individual it consumes, while for an individual prey the decision matters greatly. Therefore, at one trophic level, which individual dies is a matter of concern; while at higher level it is not (figure 2). This general issue is similar to the problem faced in developing a digital cell or organism, in which it will be neither necessary nor desirable to replicate every metabolic pathway in every cell in the organism [57,58]. Simplifying between-individual variation by grouping individuals into trait-groups (such as size groups, or functional groups) is a commonly used way of reducing complexity and attempting to carry sufficient information between levels [40–42].
Figure 2.
Schematic of coupled species models, interacting to produce an ecosystem model. The basic structure is a layered series of dynamic models: the vegetation model feeds location-specific parameters into herbivore models to give, for example, food supply; the herbivore layer then feeds into the carnivore layer in a comparable fashion. This is shown for a single herbivore but could in theory be conducted separately for all herbivores, and similar abstractions could be conducted for models in other layers. The three-dimensional vegetation map was produced by the Environmental Change Network, and represents the Moor House NNR in the Pennines (UK) [56].
Considering a system as a system, and modelling it as such, is a common approach in science. For example, climate change predictions come from very complex models [45] that use well-understood, deterministic but nonlinear, physical relationships (e.g. the Navier–Stokes equation) within the atmosphere and oceans. Global climate models (GCMs) can predict the effect that previously unknown amounts of greenhouse gases entering the atmosphere would have on climate because they are process-based [44], and the phenomenon of climate change emerges from the model under these novel conditions. The use of such models has played a large part in convincing the public and policy-makers that climate change is occurring and that its cause is anthropogenic [16]. Similarly, systems biology has emerged as a response to the molecular revolution in the past twenty years [59]. The stimulus of new, large datasets produced by high-throughput technologies, coupled with development in computational power led to the emergence of systems biology as a coherent discipline at the end of the twentieth century [59,60]. This is combined with the recognition that the behaviour of a complex system (like a metabolic network) cannot be understood by reducing the system to a sample of simple chemical reactions. There are many definitions of systems biology, but they typically emphasize that examining the interactions between its component parts is the best way of understanding the behaviour of a system. Patterns emerge at one level of a hierarchy because of the processes internal to the system rather than properties of the system. Most definitions stress that a systems approach is typified by iterated cycles of data collection, analysis, computational modelling and prediction, which is usually followed by further cycles in which the model's predictions are tested and the model iteratively refined. Thus, explicitly, systems approaches combine data plus mathematical analysis with a computational approach to generate predictions. The approach used in systems biology is therefore very different from that which we conventionally see in ecology.
System-specific models, by definition, could not be applied beyond their specific system; yet we would wish to be able to produce general conclusions. Empiricists are familiar with the problem of achieving generality of conclusions and how this can be achieved through replication at the appropriate scale. The results of any experiment or set of observations can be generalized to the sampled population. Similarly, if multiple, alternative models of a particular phenomenon converged on similar solutions then generality would be achieved. If models of multiple systems show the same processes to be important then we begin to achieve a generality of understanding about fundamental ecological mechanisms. This approach to generality is taken by the climatological community: although the various competing GCMs give predictions that vary substantially in their forecast for the global mean annual temperature for the same scenario, they agree on the trajectory. These differences are not seen as a significant obstacle to progress and it has been possible to draw robust conclusions, by which policy decisions have increasingly been informed, about the changes in mean global temperatures, sea level and precipitation by combining the outputs of sets of models [16]. The instances where alternative models give divergent outputs are often used to inform model development, or to suggest further research [61].
It is increasingly clear that it is not appropriate to treat the process of evolutionary change as being separate from the ecological context in which it occurs [62]. At the very least, the timescales are comparable; for example, ecological succession in a forest community is likely to take place on longer timescales than the evolution of insect species within that forest. The distinction between the ‘evolutionary play’ and the ‘ecological theatre’ [63] is not appropriate if we want to understand biological diversity and function [62,64,65]. Thus, applied ecological problems (such as predicting species' ranges, responses to environmental change, harvesting, designing nature reserves), even if only predicting a few years or decades into the future, should consider the potential for model parameters to be variables that evolve [66], at least for species with generation times substantially less than the model run time. Ecosystem dynamics ultimately emerge from the actions of, and interactions between, individuals, and considerable research effort has been invested in gaining insight into general rules governing behaviour [67,68]. Both behavioural ecology and life-history theory have been successful in providing explanations for why individual organisms do what they do, rather than simply describing what they do. The fact that we know organisms will act as if to maximize fitness gives us a conceptual basis for modelling the decision-making behaviour of organisms and a way of inferring the likely behaviour of organisms for which there is limited information [69,70]. It might also allow us to account for the fact that ecological and evolutionary change are intertwined—population dynamics are the product of the realized life histories (a product of selection) of individuals within the population, while the strength of selection is modified by properties of the population (e.g. density) [65]. Further recognizing this organizing principle in ecology would reveal the relationship between biological processes at the individual-level and the population, community or ecosystem results of these processes.
Any predictions that emerge from ecological forecasting models will have substantial uncertainty. This uncertainty will originate from three main sources:
— some will be the result of imprecision in the estimation of biological parameters used in the model; this source of uncertainty may suggest fruitful areas of future research that would enable reduction in the output error of the model;
— some will be the result of the inherent stochasticities of any ecological system; the multiplication of stochastic effects may mean that confidence intervals on predictions inevitably will be large; and
— another inevitable source of uncertainty will be due to variance in the physical predictions of the environment into which biological predictions are being made; any downstream models will inherit this variance.
The multiplication of stochastic effects may mean that confidence intervals on predictions are likely to be large but they would be realistic measures of our uncertainty, which will truly reflect our ability to predict outcomes in the real world [71]. These models would allow us to see effects that, at present, are obscured by a fear of tackling the complexity inherent in understanding ecology as the product of its component parts, rather than as a system simplified to the point of tractability [72].
Systems ecology was a term prevalent in the 1960s and was used to describe the modelling approach taken by the International Biological Program (IBP, for a description of which see [73,74]). Levins described this approach as sacrificing generality for precision and realism, and he was equally critical of both this and biology-as-physics, giving preference to his own methodology that he characterized as maximizing realism and generality at the expense of precision [23]. In 1968, Levins turned his attention fully to the methods used by the IBP (which he labelled FORTRAN ecology) and suggested that it trimmed ‘the scope of theory to its narrowest role of programming curve fitting’ [75]. This attack probably contributed to the end of the IBP and to this approach to the analysis of ecological systems; it also allowed theoretical population biologists to ignore Levins' equally robust criticisms of the biology-as-physics approach [76], which—since the 1970s—has dominated modelling in ecology, especially population ecology [30]. In some ways, the alternative approach described here is not dissimilar to the systems ecology of the 1960s, in that it advocates a more holistic systems approach. There are, however, some important differences: the use of functional types potentially provides a method to abstract features of a system and allows patterns to be discerned while not requiring us to include everything in a model; the substantial advances in evolutionary biology since the 1960s mean we have the ability to understand decision-making in organisms, thus improving the theoretical underpinning of our models; we have vastly more computing power at our disposal than was available in the 1960s, which makes approaches to ecological modelling that could not have been conceivably used in the 1960s feasible now [77]. The systems ecology of the 1960s can be seen as a response to a perceived ecological crisis around the utilization, management and preservation of natural resources [74,75]; similarly the concern in society today about the ecological impact of anthropogenic environmental change might provide the impetus for ecology in the twenty-first century to adapt its approach to address these issues.
5. Existing applications of systems approaches in ecological modelling
There is an established literature examining community succession and ecosystem service provision in forests that has made substantial use of models similar to those advocated here [40–42,78,79]. The population dynamics of forest trees have been modelled by tracking the fate of every individual tree in the Great Mountain Forest (CT, USA) and predictions made for the state of this secondary regrowth forest over 1000 years [79]. A related study started with detailed data on individual trees in one location over a 12-year period and was able to model 100 years of forest dynamics in the Great Lakes region of the USA [41]. A similar model describing the growth, reproduction and mortality dynamics of trees, but which does not model the fate of every tree individually, has been used to make predictions about ecosystem productivity, biomass and carbon storage for forest communities in tropical South America [78], while a model related to this was used to make similar predictions for the forests of northeastern USA and Quebec [80]. A comparable model has been developed, apparently independently, in Europe, which has been used to simulate the effects of climate change on community structure and productivity in Austrian forests [81–83], while another process-based model has been used to model changes in ranges of individual tree species in North America [84,85]. It is also worth noting that these forest models are based on the growth characteristics, physiology and behaviour of trees; they do not require knowledge of, for example, the mycorrhizal community or the microbial community involved in decay. These data would be interesting, and their incorporation could be potentially valuable, but a lack of knowledge about this aspect of forest ecology did not impede generation of an understanding of the forest community, which perhaps runs counter to an ecologist's prejudice that the exclusion of an important ecological process would invalidate the conclusions of the model.
The forest models described earlier are systems approaches to the problem of modelling forest communities. They are based on characteristics of individual trees, and forest-level features are emergent properties of the models. They were not designed either to examine the ecological impact of environmental change or to act as models of the ecosystem as a whole. However, such a model could be used as a basis for describing the forest habitat of a herbivore of interest (figure 2). An ecosystem model that coupled vegetation (two types of trees as well as food crops), herbivore (humans) and hydrology models was sufficiently accurate to produce a reasonable description of the current state of a Himalayan valley from historical starting points and could generate predictions about its potential future state [86]. While the forest models contain a single trophic layer [79] and the model of human activity in a wooded valley contains two [86], Caron-Lormier et al. [87] present a process-based model of a highly simplified arable ecosystem with four trophic layers, using functional types of various invertebrates rather than species. These examples demonstrate that the production of coupled ecosystem models capturing key elements in a food chain is achievable.
The call to move beyond descriptive models to developing process-based predictive models is not new [34,48,62,64,65,88–90] but has so far gone largely unheeded. Ecology is highly quantitative, but has remained constrained by an approach that assumes that only simple models can be useful. The inaccuracy of ecological models and their lack of utility for applied questions has been criticized previously, e.g. [34,91]. Simplified models of ecological systems are, and have been, extremely valuable and can provide insight into ecological phenomena [92]. We do, however, have to recognize the strengths and weaknesses of different approaches and simple models will not be helpful when accuracy is required and/or when answers are needed for specific systems. If a conservation manager needs to make decisions about strategies for halting the decline of a particular species, or information is needed for a fishery about harvesting rates or the impact of climate change on agricultural pollinators needs to be understood, then a simple, general analytical model is unlikely to be helpful. Modelling approaches should be adapted to their purpose. A systems approach is likely to offer advantages over other approaches when we are concerned about the impact of environmental change or when we are interested in specific, accurate answers to problems.
Acknowledgements
Thanks to Tim Benton and Ken Norris for the conversations, thoughts and discussions that led up to this piece of work. Thanks also to Robert Muetzelfeldt, whose comments have significantly improved this manuscript.
References
- 1.Travis J. M. J. 2003. Climate change and habitat destruction: a deadly anthropgenic cocktail. Proc. R. Soc. Lond. B 270, 467–473 10.1098/rspb.2002.2246 (doi:10.1098/rspb.2002.2246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnosky A. D., et al. 2011. Has the Earth's sixth mass extinction already arrived? Nature 471, 51–57 10.1038/nature09678 (doi:10.1038/nature09678) [DOI] [PubMed] [Google Scholar]
- 3.Wilson R. J., Gutiérrez D., Gutiérrez J., Matrtínez D., Agudo R., Monserrat V. J. 2005. Changes to the elevational limits and extent of species ranges associated with climate change. Ecol. Lett. 8, 1138–1146 10.1111/j.1461-0248.2005.00824.x (doi:10.1111/j.1461-0248.2005.00824.x) [DOI] [PubMed] [Google Scholar]
- 4.Crick H. Q. P., Dudley C., Glue D. E., Thomson D. L. 1997. UK birds are laying eggs earlier. Nature 388, 526. 10.1038/41453 (doi:10.1038/41453) [DOI] [Google Scholar]
- 5.Visser M. E., et al. 2003. Variable responses to large-scale climate change in European Parus populations. Proc. R. Soc. Lond. B 270, 367–372 10.1098/rspb.2002.2244 (doi:10.1098/rspb.2002.2244) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Visser M. E., Hollemann L. J. M. 2001. Warmer springs disrupt the synchrony of oak and winter moth phenology. Proc. R. Soc. Lond. B 268, 289–294 10.1098/rspb.2000.1363 (doi:10.1098/rspb.2000.1363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischlin A., et al. 2007. Ecosystems, their properties, goods, and services. In Climate change 2007: impacts, adaptation and vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (eds Parry M. L., Canziani O. F., Palutikof J. P., van der Linden P. J., Hansen C. E.), pp. 211–272 Cambridge, UK: Cambridge University Press [Google Scholar]
- 8.Hindmarch C., Harris J., Morris J. 2006. Growth and sustainability: integrating ecosystem services into economics. Biologist 53, 135–142 [Google Scholar]
- 9.Millennium Ecosystem Assessment 2005. Ecosystems and human well-being: synthesis. Washington, DC: Island Press [Google Scholar]
- 10.Huntley B., Green R. E., Collingham Y. C., Willis S. G. 2008. A climatic atlas of European breeding birds. Barcelona, Spain: Lynx Editions; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Settele J., et al. 2008. Climatic risk atlas of European butterflies. Sofia, Bulgaria: Pensoft [Google Scholar]
- 12.Walmsley C. A., Smithers R. J., Berry P. M., Harley M., Stevenson M. J., Catchpole R. 2007. MONARCH—Modelling Natural Resource Responses to Climate Change: a synthesis for biodiversity conservation. Oxford, UK: UKCIP [Google Scholar]
- 13.Commission of the European Communities 2009. Adapting to climate change: towards a European framework for action. See http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=COM:2009:0147:FIN:EN:PDF [Google Scholar]
- 14.Landsberg H. E. 1946. Climate as a natural resource. Sci. Mon. 63, 293–298 [PubMed] [Google Scholar]
- 15.Keeling C. D. 1960. The concentration and isotopic abundances of carbon dioxide in the atmosphere. Tellus 12, 200–203 10.1111/j.2153-3490.1960.tb01300.x (doi:10.1111/j.2153-3490.1960.tb01300.x) [DOI] [Google Scholar]
- 16.IPCC 2007. Climate change 2007: synthesis report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Geneva, Switzerland: IPCC [Google Scholar]
- 17.Stern N. 2007. The economics of climate change: the Stern review. Cambridge, UK: Cambridge University Press [Google Scholar]
- 18.Marris E. 2010. New UN science body to monitor biosphere. Nature 465, 859. 10.1038/465859a (doi:10.1038/465859a) [DOI] [PubMed] [Google Scholar]
- 19.Clark J. S., et al. 2001. Ecological forecasts: an emerging imperative. Science 293, 657–660 10.1126/science.293.5530.657 (doi:10.1126/science.293.5530.657) [DOI] [PubMed] [Google Scholar]
- 20.Weisberg M. 2006. Forty years of ‘the strategy’: Levins on model building and idealization. Biol. Phil. 21, 623–645 10.1007/s10539-006-9051-9 (doi:10.1007/s10539-006-9051-9) [DOI] [Google Scholar]
- 21.Sutherland W. J. 2006. Predicting the ecological consequences of environmental change: a review of the methods. J. Appl. Ecol. 43, 599–616 10.1111/j.1365-2664.2006.01182.x (doi:10.1111/j.1365-2664.2006.01182.x) [DOI] [Google Scholar]
- 22.Weisberg M. 2007. Who is a modeler? Brit. J. Phil. Sci. 58, 207–233 10.1093/bjps/axm011 (doi:10.1093/bjps/axm011) [DOI] [Google Scholar]
- 23.Levins R. 1966. The strategy of model building in population ecology. Am. Sci. 54, 421–431 [Google Scholar]
- 24.Weisberg M. 2007. Three kinds of idealization. J. Phil. 104, 639–659 [Google Scholar]
- 25.Orzack S. H., Sober E. 1993. A critical assessment of Levins's the strategy of model building in population biology (1966). Q. Rev. Biol. 68, 533–546 10.1086/418301 (doi:10.1086/418301) [DOI] [Google Scholar]
- 26.Orzack S. H. 2005. Discussion: what, if anything, is ‘the strategy of model building in population biology?’ A comment on Levins (1966) and Odenbaugh (2003). Phil. Sci. 72, 479–485 10.1086/498475 (doi:10.1086/498475) [DOI] [Google Scholar]
- 27.Odenbaugh J. 2003. Complex systems, trade-offs and mathematical modeling: Richard Levins' ‘strategy of model building in population biology’ revisited. Phil. Sci. 70, 1496–1507 10.1086/377425 (doi:10.1086/377425) [DOI] [Google Scholar]
- 28.Matthewson J., Weisberg M. 2008. The structure of tradeoffs in model building. Synthese 170, 169–190 10.1007/s11229-008-9366-y (doi:10.1007/s11229-008-9366-y) [DOI] [Google Scholar]
- 29.Weisberg M. 2004. Qualitative theory and chemical explanation. Phil. Sci. 71, 1071–1081 10.1086/428011 (doi:10.1086/428011) [DOI] [Google Scholar]
- 30.Kingsland S. E. 1985. Modeling nature: episodes in the history of population ecology. Chicago, IL: Chicago University Press [Google Scholar]
- 31.Volterra V. 1926. Fluctuations in the abundance of a species considered mathematically. Nature 118, 558–560 10.1038/118558a0 (doi:10.1038/118558a0) [DOI] [Google Scholar]
- 32.Anderson R. M., May R. M. 1979. Population biology of infectious diseases. I. Nature 280, 361–367 10.1038/280361a0 (doi:10.1038/280361a0) [DOI] [PubMed] [Google Scholar]
- 33.May R. M., Anderson R. M. 1979. Population biology of infectious diseases. II. Nature 280, 455–461 10.1038/280455a0 (doi:10.1038/280455a0) [DOI] [PubMed] [Google Scholar]
- 34.Simberloff D. 1981. The sick science of ecology. Eidema 1, 49–54 [Google Scholar]
- 35.Rice K. 2004. Sprint research runs into a credibility gap. Nature 432, 147. 10.1038/432147b (doi:10.1038/432147b) [DOI] [PubMed] [Google Scholar]
- 36.Reichmann W. J. 1964. Use and abuse of statistics. Harmondsworth, UK: Pelican [Google Scholar]
- 37.Grafen A., Hails R. S. 2002. Modern statistics for the life sciences. Oxford, UK: Oxford University Press [Google Scholar]
- 38.Milner-Gulland E. J. 2012. Interactions between human behaviour and ecological systems. Phil. Trans. R. Soc. B 367, 270–278 10.1098/rstb.2011.0175 (doi:10.1098/rstb.2011.0175) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Twain M. 1883. In Life on the Mississippi, ch. 17, p. 79 London, UK: Chatto and Windus [Google Scholar]
- 40.Purves D., Pacala S. 2008. Predictive models of forest dynamics. Science 320, 1452–1453 10.1126/science.1155359 (doi:10.1126/science.1155359) [DOI] [PubMed] [Google Scholar]
- 41.Purves D. W., Lichstein J. W., Strigul N., Pacala S. W. 2008. Predicting and understanding forest dynamics using a simple tractable model. Proc. Natl Acad. Sci. USA 105, 17 018–17 022 10.1073/pnas.0807754105 (doi:10.1073/pnas.0807754105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strigul N., Pristinski D., Purves D., Dushoff J., Pacala S. 2008. Scaling from trees to forests: tractable macroscopic equations for forest dynamics. Ecol. Monogr. 78, 523–545 10.1890/08-0082.1 (doi:10.1890/08-0082.1) [DOI] [Google Scholar]
- 43.Mann M. E., Bradley R. S., Hughes M. K. 1998. Global-scale temperature patterns and climate forcing over the past six centuries. Nature 392, 779–787 10.1038/33859 (doi:10.1038/33859) [DOI] [Google Scholar]
- 44.Schmidt G. A. 2007. The physics of climate modeling. Phys. Today 60, 72–73 10.1063/1.2709569 (doi:10.1063/1.2709569) [DOI] [Google Scholar]
- 45.Moss R. H., et al. 2010. The next generation of scenarios for climate change research and assessment. Nature 463, 747–756 10.1038/nature08823 (doi:10.1038/nature08823) [DOI] [PubMed] [Google Scholar]
- 46.Benton T. G., Plaistow S. J., Coulson T. N. 2006. Complex population dynamics and complex causation: devils, details and demography. Proc. R. Soc. B 273, 1173–1181 10.1098/rspb.2006.3495 (doi:10.1098/rspb.2006.3495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uchmánski J., Kowalczyk K., Ogrodowczyk P. 2008. Evolution of theoretical ecology in last decades: why did individual-based modelling emerge. Ecol. Questions 10, 13–18 10.2478/v10090-009-0002-3 (doi:10.2478/v10090-009-0002-3) [DOI] [Google Scholar]
- 48.Levin S. A. (ed.) 2005. Individual-based modelling and ecology. Princeton, NJ: Princeton University Press [Google Scholar]
- 49.Hampe A. 2004. Bioclimate envelope models: what they detect and what they hide. Glob. Ecol. Biogeogr. 13, 469–471 10.1111/j.1466-822X.2004.00090.x (doi:10.1111/j.1466-822X.2004.00090.x) [DOI] [Google Scholar]
- 50.Pearson R. G., Dawson T. P. 2003. Predicting the impacts of climate change on the distribution of species: are bioclimatic envelope models useful? Glob. Ecol. Biogeogr. 12, 361–371 10.1046/j.1466-822X.2003.00042.x (doi:10.1046/j.1466-822X.2003.00042.x) [DOI] [Google Scholar]
- 51.Beale C. M., Lennon J. J., Gimona A. 2008. Opening the climate envelope reveals no macroscale associations with climate in European birds. Proc. Natl Acad. Sci. USA 105, 14 908–14 912 10.1073/pnas.0803506105 (doi:10.1073/pnas.0803506105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beale C. M., Lennon J. J., Gimona A. 2009. European bird distributions still show few climate associations. Proc. Natl Acad. Sci. USA 106, E41–E43 10.1073/pnas.0902229106 (doi:10.1073/pnas.0902229106) [DOI] [Google Scholar]
- 53.Huntley B., Collingham Y. C., Willis S. G., Green R. E. 2008. Potential impacts of climatic change on European breeding birds. PLoS ONE 3, e1439. 10.1371/journal.pone.0001439 (doi:10.1371/journal.pone.0001439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Needham C. J., Bradford J. R., Bulpitt A. J., Westhead D. R. 2007. A primer on learning in Bayesian networks for computational biology. PLoS Comput. Biol. 3, e129. 10.1371/journal.pcbi.0030129 (doi:10.1371/journal.pcbi.0030129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Renken H., Mumby P. J. 2009. Modelling the dynamics of coral reef macroalgae using a Bayesian belief network approach. Ecol. Model. 220, 1305–1314 10.1016/j.ecolmodel.2009.02.022 (doi:10.1016/j.ecolmodel.2009.02.022) [DOI] [Google Scholar]
- 56.Eddy A., Welch D., Rawes M. 1969. The vegetation of the Moor House National Nature Reserve in the northern Pennines, England. Vegetatio 16, 239–284 [Google Scholar]
- 57.de Jong H. 2002. Modeling and simulation of genetic regulatory systems: a literature review. J. Comp. Biol. 9, 67–103 10.1089/10665270252833208 (doi:10.1089/10665270252833208) [DOI] [PubMed] [Google Scholar]
- 58.Tomita M. 2001. Whole-cell simulation: a grand challenge of the 21st century. Trends Biotech. 19, 205–210 10.1016/S0167-7799(01)01636-5 (doi:10.1016/S0167-7799(01)01636-5) [DOI] [PubMed] [Google Scholar]
- 59.Westerhoff H. V., Palsson B. O. 2004. The evolution of molecular biology into systems biology. Nat. Biotechnol. 22, 1249–1252 10.1038/nbt1020 (doi:10.1038/nbt1020) [DOI] [PubMed] [Google Scholar]
- 60.Kitano H. 2002. Systems biology: a brief overview. Science 295, 1662–1664 10.1126/science.1069492 (doi:10.1126/science.1069492) [DOI] [PubMed] [Google Scholar]
- 61.Sitch S., et al. 2008. Evaluation of the terrestrial carbon cycle, future plant geography and climate-carbon cycle feedbacks using five dynamic global vegetation models (DGVMs). Glob. Change Biol. 14, 1–25 10.1111/j.1365-2486.2008.01626.x (doi:10.1111/j.1365-2486.2008.01626.x) [DOI] [Google Scholar]
- 62.Coulson T., Benton T. G., Lundberg P., Dall S. R. X., Kendall B. E. 2006. Putting evolutionary biology back in the ecological theatre: a demographic framework mapping genes to communities. Evol. Ecol. Res. 8, 1155–1171 [Google Scholar]
- 63.Hutchinson G. E. 1965. The ecological theater and the evolutionary play. New Haven, CT: Yale University Press [Google Scholar]
- 64.Coulson T., Benton T. G., Lundberg P., Dall S. R. X., Kendall B. E., Gaillard J. M. 2006. Estimating individual contributions to population growth: evolutionary fitness in ecological time. Proc. R. Soc. B 273, 547–555 10.1098/rspb.2005.3357 (doi:10.1098/rspb.2005.3357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kokko H., Lopez-Sepulcre A. 2007. The ecogenetic link between demography and evolution: can we bridge the gap between theory and data? Ecol. Lett. 10, 773–782 10.1111/j.1461-0248.2007.01086.x (doi:10.1111/j.1461-0248.2007.01086.x) [DOI] [PubMed] [Google Scholar]
- 66.McInerny G. J., Turner J. R. G., Wong H. Y., Travis J. M. J., Benton T. G. 2009. How range shifts induced by climate change affect neutral evolution. Proc. R. Soc. B 276, 1527–1534 10.1098/rspb.2008.1567 (doi:10.1098/rspb.2008.1567) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Krebs J. R., Davies N. B. 1993. An introduction to behavioral ecology, 3rd edn. Oxford, UK: Blackwell Scientific [Google Scholar]
- 68.Stearns S. C. 1992. The evolution of life histories. Oxford, UK: Oxford University Press [Google Scholar]
- 69.Grafen A. 2007. The formal Darwinism project: a mid-term report. J. Evol. Biol. 20, 1243–1254 10.1111/j.1420-9101.2007.01321.x (doi:10.1111/j.1420-9101.2007.01321.x) [DOI] [PubMed] [Google Scholar]
- 70.Grafen A. 1999. Formal Darwinism, the individual-as-maximizing-agent analogy, and bet-hedging. Proc. R. Soc. Lond. B 266, 799–803 10.1098/rspb.1999.0708 (doi:10.1098/rspb.1999.0708) [DOI] [Google Scholar]
- 71.Forster M., Sober E. 1994. How to tell when simpler, more unified, or less ad hoc theories will provide more accurate predictions. Brit. J. Phil. Sci. 45, 1–35 10.1093/bjps/45.1.1 (doi:10.1093/bjps/45.1.1) [DOI] [Google Scholar]
- 72.Wimsatt W. C. 2006. Reductionism and its heuristics: making methodological reductionism honest. Synthese 151, 445–475 10.1007/s11229-006-9017-0 (doi:10.1007/s11229-006-9017-0) [DOI] [Google Scholar]
- 73.Watt K. E. F. 1966. Systems analysis in ecology. New York, NY: Academic Press [Google Scholar]
- 74.Watt K. E. F. 1968. Ecology and resource management: a quantitative approach. New York, NY: McGraw-Hill [Google Scholar]
- 75.Levins R. 1968. Review: ecological engineering: theory and technology. Q. Rev. Biol. 43, 301–305 10.1086/405813 (doi:10.1086/405813) [DOI] [Google Scholar]
- 76.Palladino P. 1991. Defining ecology: ecological theories, mathematical models, and applied biology in the 1960s and 1970s. J. Hist. Biol. 24, 223–243 10.1007/BF00209430 (doi:10.1007/BF00209430) [DOI] [Google Scholar]
- 77.Odenbaugh J. 2006. The strategy of ‘the strategy of model building in population biology’. Biol. Phil. 21, 607–621 10.1007/s10539-006-9049-3 (doi:10.1007/s10539-006-9049-3) [DOI] [Google Scholar]
- 78.Moorcroft P. R., Hurtt G. C., Pacala S. W. 2001. A method for scaling vegetation dynamics: the ecosystem demography model (ED). Ecol. Monogr. 71, 557–586 10.1890/0012-9615(2001)071[0557:AMFSVD]2.0.CO;2 (doi:10.1890/0012-9615(2001)071[0557:AMFSVD]2.0.CO;2) [DOI] [Google Scholar]
- 79.Pacala S. W., Canham C. D., Saponara J., Silander J. A., Kobe R. K., Ribbens E. 1996. Forest models defined by field measurements: estimation, error analysis and dynamics. Ecol. Monogr. 66, 1–43 10.2307/2963479 (doi:10.2307/2963479) [DOI] [Google Scholar]
- 80.Medvigy D., Wofsy S. C., Munger J. W., Hollinger D. Y., Moorcroft P. R. 2009. Mechanistic scaling of ecosystem function and dynamics in space and time: ecosystem demography model version 2. J. Geophys. Res. 114, G01002. 10.1029/2008JG000812 (doi:10.1029/2008JG000812) [DOI] [Google Scholar]
- 81.Seidl R., Lexer M. J., Jäger D., Hönninger K. 2005. Evaluating the accuracy and generality of a hybrid patch model. Tree Physiol. 25, 939–951 10.1093/treephys/25.7.939 (doi:10.1093/treephys/25.7.939) [DOI] [PubMed] [Google Scholar]
- 82.Lexer M. J., Hönninger K. 2001. A modified 3D-patch model for spatially explicit simulation of vegetation composition in heterogeneous landscapes. Forest Ecol. Manag. 144, 43–65 10.1016/S0378-1127(00)00386-8 (doi:10.1016/S0378-1127(00)00386-8) [DOI] [Google Scholar]
- 83.Lexer M. J., Hönninger K., Scheifinger H., Matulla C., Groll N., Kromp-Kolb H., Schadauer K., Starlinger F., Englisch M. 2002. The sensitivity of Autrian forests to scenarios of climatic change: a large-scale risk assessment based on a modified gap model and forest inventory data. Forest Ecol. Manag. 162, 53–72 10.1016/S0378-1127(02)00050-6 (doi:10.1016/S0378-1127(02)00050-6) [DOI] [Google Scholar]
- 84.Morin X., Thuiller W. 2009. Comparing niche- and process-based models to reduce prediction uncertainty in species range shifts under climate change. Ecology 90, 1301–1313 10.1890/08-0134.1 (doi:10.1890/08-0134.1) [DOI] [PubMed] [Google Scholar]
- 85.Morin X., Viner D., Chuine I. 2008. Tree species range shifts at a continental scale: new predictive insights from a process-based model. J. Ecol. 96, 784–794 10.1111/j.1365-2745.2008.01369.x (doi:10.1111/j.1365-2745.2008.01369.x) [DOI] [Google Scholar]
- 86.Bithell M., Brasington J. 2009. Coupling agent-based models of subsistence farming with individual-based forest models and dynamic models of water distribution. Environ. Model Softw. 24, 173–190 10.1016/j.envsoft.2008.06.016 (doi:10.1016/j.envsoft.2008.06.016) [DOI] [Google Scholar]
- 87.Caron-Lormier G., Bohan D. A., Hawes C., Raybould A., Haughton A. J., Humphry R. W. 2009. How might we model an ecosystem? Ecol. Model. 220, 1935–1949 10.1016/j.ecolmodel.2009.04.021 (doi:10.1016/j.ecolmodel.2009.04.021) [DOI] [Google Scholar]
- 88.Grimm V. 1999. Ten years of individual-based modelling in ecology: what have we learned and what could we learn in the future? Ecol. Model. 115, 129–148 10.1016/S0304-3800(98)00188-4 (doi:10.1016/S0304-3800(98)00188-4) [DOI] [Google Scholar]
- 89.Judson O. P. 1994. The rise of the individual-based model in ecology. Trends Ecol. Evol. 9, 9–14 10.1016/0169-5347(94)90225-9 (doi:10.1016/0169-5347(94)90225-9) [DOI] [PubMed] [Google Scholar]
- 90.Sutherland W. J., Norris K. 2002. Behavioural models of population growth rates: implications for conservation and prediction. Phil. Trans. R. Soc. B 357, 1273–1284 10.1098/rstb.2002.1127 (doi:10.1098/rstb.2002.1127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peters R. H. 1991. A critique for ecology. Cambridge, UK: Cambridge University Press [Google Scholar]
- 92.Odenbaugh J. 2005. Idealized, inaccurate but successful: a pragmatic approach to evaluating models in theoretical ecology. Biol. Phil. 20, 231–255 10.1007/s10539-004-0478-6 (doi:10.1007/s10539-004-0478-6) [DOI] [Google Scholar]