Abstract
Winter chilling is of central importance in the phenology of temperate annual and perennial plants. Chilling accelerates flowering through the process of vernalization and breaks both bud and seed dormancy, permitting the onset of growth in the spring. The quantitative effects of chilling in floral promotion in winter annual Arabidopsis accessions are well-documented, but very little is known about the basic physiology underlying summer annual responses to winter chilling, which acts on seeds within the soil seed bank. Here, we analyse the response of wild accessions to extended chilling in seeds, and explore the interaction between seed-maturation temperature and chilling responses. We show that two weeks of chilling induces secondary dormancy, and that this time period is not dependent on seed-maturation temperature. In addition, we found that seeds for most accessions set under simulated summer conditions in the laboratory are unable to overwinter in the soil seed bank, as they germinate without light during extended chilling treatments. This shows that these seeds are committed to re-establishment in the same growing season. Understanding how winter chilling affects the timing of Arabidopsis phenology will enable us to explore the genetics behind adaptation to changing climates, and inform rational approaches to breeding crops with improved performance under new climate scenarios and develop a systems ecology of Arabidopsis.
Keywords: life history, chilling, Arabidopsis, seed dormancy
1. Introduction
Arabidopsis thaliana is an excellent model system for understanding adaptation and evolution [1]. Two decades of genetic research have revealed much detail of the molecular functions of plants that underlie their behaviour in field and laboratory conditions. Gene networks controlling Arabidopsis phenology, including flowering, germination and growth control are now well understood, as is the genetic basis of interactions with other organisms, including pathogen resistance, responses to herbivory and adaptations to competition with nearby plants. Importantly, these genetic pathways have been found to be widely conserved throughout both flowering and non-flowering plants, showing that techniques applied to predict Arabidopsis behaviour will be broadly applicable across all plants in an ecosystem.
Chilling, defined as the experience of non-freezing low temperatures, is an important signal for plant life histories. Chilling is well-known to induce pathways producing cold acclimation, which lead to the acquisition of freezing tolerance [2]. During winter, accumulation of a memory of prolonged chilling is important for the acceleration of flowering by vernalization, and for controlling the loss of bud dormancy, bud burst and germination in the spring [3]. It is well-documented that climate change is advancing these events in spring [4]. In addition, chilling can be important in autumn phenology, affecting the timing of leaf fall and in some horticultural species, notably apple, the onset of bud dormancy [5,6]. Of particular importance in the UK is the fact that many of our fruit crops are sensitive to freezing injury if frosts coincide with spring flowering. Thus, there is concern that climate change may cause early flowering, but that the continuing stochastic chances of frosts will increase the chances of losing crop yield to late cool episodes. Conversely, lack of winter chilling may result in a failure to break dormancy that can result in lower yields of fruit crops. Evidence for this has recently been observed among vegetation on the Tibetan plateau [7]. Understanding the genetic basis of these traits is key to breeding new varieties that will outperform current favourites under new climate regimes.
Very little is known of how chilling responses are controlled in seeds, but much is generally known about how plants can measure extended periods of cold through the study of vernalization. In vernalization, the function of the central regulator, FLOWERING LOCUS C (FLC), is to provide an integrated memory of recent temperatures and thus allow the plant to detect the passing of winter through vernalization [8]. FLC encodes a floral repressor whose expression is gradually reduced as chilling time increases, and thus provides a memory of winter. Because of their high FLC expression in the absence of vernalization, winter annuals flower only in the second calendar year of growth. In summer annuals, which do not require vernalization for flowering and therefore flower in the same calendar year as germination, a genetic change has taken place that results in plants with constitutively low FLC activity, even in the absence of vernalization [9]. The summer annual habit has evolved independently several times, and most of these occurrences have been tracked to changes in the FRIGIDA (FRI) locus, a key activator of FLC expression [9,10]. In contrast, very few accessions have been identified in which FLC itself is modified, which has led to the hypothesis that FLC may have a pleiotropic function not related to flowering control, for which purpose selection for functional alleles is maintained. At least two candidates for this function have been suggested: firstly, quantitative trait loci (QTLs) for FLC have a strong effect on the plant's ability to survive drought [11], whereas FLC has also been shown to influence seed [12]. Importantly, a latitudinal cline in the magnitude of the vernalization response has been discovered, with northern accessions from Scandinavia requiring more chilling units (CUs) to repress FLC than more southerly ecotypes. Interestingly, however, neither the haplotype at the FRI locus nor the expression level of FLC is subject to the same cline, suggesting that other genetic factors are important [10,13]. One important finding is that the maintenance of the epigenetic silencing of FLC in the warm requires a longer period of cold in the Scandinavian accessions, suggesting that variation in epigenetic processes contributes to this cline [13]. Evidence suggests that selection for non-functional FRI haplotypes has been recent, and that this has lead to an increase in rapid cycling summer annuals among wild Arabidopsis accessions [14]. One possibility is that these rapid cycling ecotypes evolved in response to changing local conditions associated with the increase in farming after the last ice age. Such studies show that understanding the genetic control of important life-history traits can lead to insights into adaptation and evolution, and that pleiotropy is an important phenomenon as the same genetic pathways can control multiple life-history traits. Pleiotropy is an obvious consequence of the fact that measurement of either daily or annual timing is a key driver of fitness for biological organisms, and that plants have one mechanism to measure time. For instance, circadian oscillators present in all eukaryotes (and some prokaryotes) are important for measuring timing, and their perturbation has costs for individual traits, including growth rate and fecundity [15]. In this context, it is clearly important that multiple loci have roles in both germination cueing and reproductive timing, including genes required for gibberellin signalling, light responsiveness, circadian oscillations and temperature responses.
2. Towards an integrated genetic understanding of plant phenology
In order to understand the range of evolutionary trajectories available to plants, we can consider the roles of important signalling pathways in turn: how they affect each developmental transition. A second consideration is the relative importance of each pathway, given by the dynamic range of phenotypic outputs caused by the potential loss of the pathway, or the contrasting saturating response obtained by increasing the sensitivity of each pathway to maximum. Integrating these with environmental data would show how each theoretical variant would perform in any set of seasonal environments. An example here is a study [16], whereby for different sowing times the contribution of different molecular pathways toward the control of time to flowering is predicted by a simple thermal time model.
In Arabidopsis, temperature during seed maturation is a key determinant of dormancy, with photoperiod less important [17,18]. Some mutants with abnormal flowering time also have abnormal dormancy [12,19], so time to flowering affects dormancy in two distinct manners: (i) the direct impact of the genetic consequences of altered flowering time gene levels on seed dormancy and (ii) the indirect effect of the time of year in which seed maturation occurs, and the prevailing temperature during this time. Germination in turn also affects time to flowering, so understanding the interactions between the environment, genetics and the expression of life-history traits is a non-obvious problem, the solution of which will help predict responses to changing climates, and help develop rational approaches to breeding crops with new properties. Thus, understanding control of dormancy is only possible in the context of whole plant life history. Yet, the physiology and genetics of seed responses to chilling are only poorly defined, so determining what parameters need to be measured and how these are influenced by genes and the environment is an important first step in simulating seed behaviour over temperate winters. Integrating a genetic understanding of seed responses to chilling with our current understanding of the genetics of cold responsiveness at later stages in the life cycle will enable us to understand for the first time how genetic changes at single loci will affect whole plant life history.
3. Understand the role of chilling in arabidopsis summer annual phenology
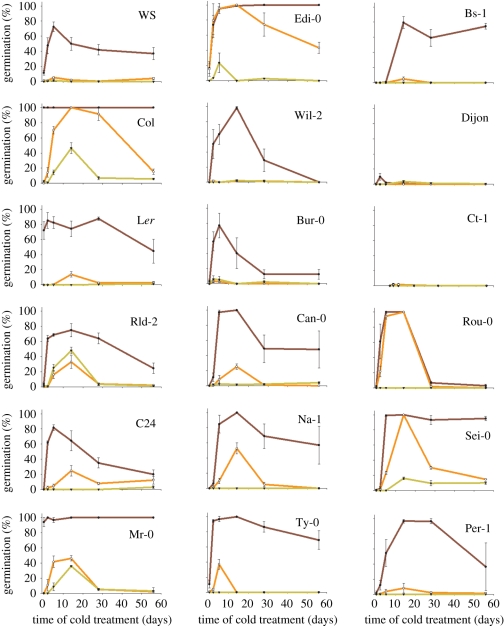
Dormancy in any seed is best identified by failure of germination given ideal conditions for germination to occur [20]. Loss of dormancy in mature Arabidopsis seeds depends on three key variables: environmental temperature (especially chilling), after-ripening in the low hydrated state and soil nitrate levels. Although brief chilling spells break dormancy, extended chilling times have also been shown to be capable of inducing a secondary dormant state in the strongly dormant ecotype Cape Verde Islands (CVI) [21], but it is not clear whether this behaviour is universal across accessions. Hence, there is a complex relationship between the accumulation of CUs and seed behaviour. In order to understand the importance of seed chilling in Arabidopsis life history, it is important to determine when chilling is perceived, and how much is necessary to break dormancy or to induce secondary dormancy. A second important determination is whether seeds in the seed bank have enough dormancy, or light requirement for germination, to accumulate enough CUs to enter secondary dormancy without germinating in the process. To investigate these traits as a function of seed-maturation temperature, we subjected wild Arabidopsis accessions, including laboratory strains, to extended periods of chilling, and recorded germination frequency after subsequent incubation in warm light conditions to examine the post-chilling dormancy state (figure 1). As observed previously [18], lowering the seed-maturation temperature generally resulted in an increase in primary dormancy at harvest, with the exception of Catania-1 (Ct-1) and Dijon, which showed strong dormancy at harvest irrespective of seed-maturation conditions. This is likely owing to a strong genetic component of their dormancy: Dijon, for instance, has been shown to have very high transcript levels of the dormancy-inducing delay of germination 1 (DOG1) gene [22]. The common laboratory strains Columbia (Col) and Landsberg erecta (Ler) showed low dormancy when matured at 20°C, such that no chilling was required for germination, but induced high levels of dormancy at lower maturation temperatures. In general, chilling up to 14 days steadily increased germination frequency, but further increasing the duration of chilling resulted in the onset of secondary dormancy. This trait was shared by most strains that showed chilling-responsive germination, and although lower seed-maturation temperature increased overall dormancy levels, it did not affect the timing of induction of secondary dormancy in most cases (figure 1): a higher resolution time series would confirm this. Thus, the ability for chilling to induce secondary dormancy observed in CVI [21] is conserved across most, if not all Arabidopsis accessions studied, and can be viewed as a function of the seed-maturation temperature. In many cases, secondary dormancy could not be induced if seed was matured at 20°C, including Basel-1 (Bs-1) and Seis am Schlern (Sei-0), an accession from high altitude in central Europe, as well as Col and Ler. This experiment suggests that Arabidopsis seeds overwintering in the seed bank will enter a secondary dormant state upon chilling which is likely maintained until broken by an as yet unknown stimulus in spring. This is an important observation, as it suggests that attempts to uncover the signal that breaks seed dormancy in the spring and permits germination will not be successful unless the appropriate secondary dormant state is studied.
Figure 1.
Analysis of the response of 18 wild accessions of Arabidopsis thaliana (L. Heynh) to extended simulated winter chill, by incubation in the dark at 4°C. The effect of chilling on dormancy was assessed by testing germination frequency when seeds are placed under favourable conditions at 22°C for 7 days. Germination is shown as mean and standard error of five seed batches per genotype per treatment. Brown lines indicate seed matured at 20°C, orange lines 15°C and yellow lines 10°C.
This experiment revealed that maintenance of the non-germinating state during dark chilling is important for seasonal behaviour, because seed that germinates during the accumulation of CUs cannot enter secondary dormancy and overwinter in the seed bank. Thus, we directly scored whether seeds germinated during the chilling treatment itself, without any light (table 1). Strikingly, seeds from all accessions where dormancy could be broken by chilling also germinated in the dark at 4°C if the seed was matured at 20°C. Exceptions again were the highly dormant Ct-1 and Dijon strains, where little germination was recorded under any conditions. However, across all accessions, seed matured at either 15 or 10°C was completely resistant to germination at 4°C in the dark. Thus, seed matured under cooler conditions can complete the transition from primary to secondary dormancy in the dark, and this likely pre-adapts them to overwintering in the soil seed bank. Arabidopsis is traditionally thought of as a species with a light requirement for germination. However, under laboratory conditions, chilling can often substitute the requirement for light and promote germination [23].
Table 1.
Germination of seeds matured at the indicated temperatures after 28 days at 4°C in the dark. Germination was scored as nearly all seeds germinated (triple plus, ‘+++’), some un-germinated seeds (double plus, ‘++’), sporadic germination only (plus, ‘+’) or no germination at all (0).
| ecotype | 20°C | 15°C | 10°C | ecotype | 20°C | 15°C | 10°C |
|---|---|---|---|---|---|---|---|
| Bs-1 | +++ | + | 0 | Mr-0 | +++ | 0 | 0 |
| Bur-0 | + | 0 | 0 | Na-1 | +++ | 0 | 0 |
| C24 | ++ | 0 | 0 | Per-1 | ++ | 0 | 0 |
| Can-0 | ++ | 0 | 0 | Rld-2 | ++ | 0 | 0 |
| Col-0 | +++ | + | 0 | Rou-0 | + | 0 | 0 |
| Ct-1 | + | 0 | 0 | Sei-0 | +++ | 0 | 0 |
| Dijon | 0 | 0 | 0 | Ty-0 | +++ | 0 | 0 |
| Edi-0 | +++ | + | 0 | Wil-2 | + | 0 | 0 |
| Ler | +++ | 0 | 0 | WS | ++ | 0 | 0 |
In order to understand how daily chilling varied throughout the year, and at what times of the year seeds would be expected to receive enough daily CUs to enter secondary dormancy, we analysed the annual variation in chilling at York, UK, using historic weather data. Using this approach, we can map our standard laboratory chilling conditions onto a standard autumn and ask at what time of year wild species would be expected to accumulate the same number of CUs as in our standard laboratory experiments. We used a standard thermal time definition of CUs as degree hours below a maximum threshold temperature above which no chilling is registered. Thus, the number of CUs accumulated during any 1 h period was defined as:
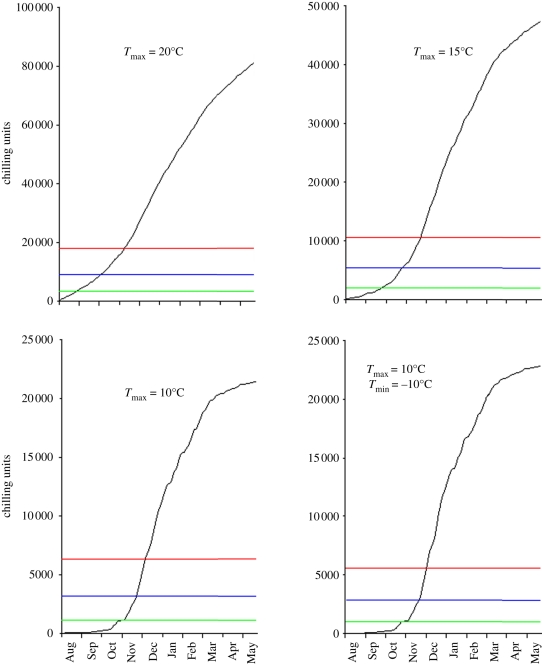
| 3.1 |
where Tmax indicates the maximum temperature at which temperature is registered as chilling by Arabidopsis seeds, T is the temperature in degree Celsius and the minimum CUs experienced in any hour is zero. We restricted the initial minimum value of T to 0°C, so that subzero temperatures have no further chilling effect in line with previous works [24,25]. Such simple threshold models describing the effect of chilling on dormancy release have been successful, but require determination of the upper ceiling for a chilling response [26]. Tmax is known to vary genetically between species, and has not been estimated for our system. Therefore, we simulated the accumulation of chilling hours over a winter cycle in York using a range of ceiling temperatures from 8 to 20°C (figure 2). These simulations then serve as useful guides to understanding the resolution required in determining the upper chilling ceiling experimentally, and the magnitude of the consequences in predictability when using an unrepresentative value. Overlaying a typical annual accumulation of CUs with those levels used in laboratory experiments shows that seeds with weak dormancy (normally overcome with around 3–5 days of chilling at 4°C) would have their dormancy broken before the end of August in York, with a high ceiling threshold, whereas reduction of the threshold to 10°C or lower delayed this until mid-October. Therefore, the ceiling below which temperatures induce a chilling response in dormancy levels is a key parameter in determining the time of year of germination. The maximum dormancy-breaking effect of chilling, occurring after 14 days at 4°C in the laboratory, would occur in mid-November using a 10°C ceiling, whereas secondary dormancy would be expected to be established shortly afterwards, by the end of November. So, as the winter enters December, our data predict that most Arabidopsis in the seed bank would most likely be in secondary dormant phase. Using the upper values of either 15 or 20°C significantly advanced the timing of maximum dormancy-breaking chill into the beginning of September, showing that error in the use of an incorrect ceiling temperature could result in discrepancies of over one month between observed and expected germination time in autumn. Interestingly, the time between the point of maximum dormancy breakage, and the onset of full secondary dormancy is predicted to be longer the higher the upper ceiling of chilling perception. This is because the rate of accumulation of CUs early in the autumn is lower than the rate in late-November. Thus, a non-obvious consequence of an increase in the upper ceiling of chilling perception is the extension of the period during which chilling-responsive seeds remain in the seed bank with a low dormancy level and therefore a high probability of germination. To test whether lowering the minimum threshold for chilling altered the rate of accumulation of CUs, we lowered the level to −10°C. This made very little difference to the rate of accumulation of CUs in our standard York autumn. These simulations show that while determining the upper ceiling for chilling is essential, there is no need to experimentally determine the lower limit.
Figure 2.
Testing the rigour in which chilling units (CUs) need to be defined to develop a thermal time model of Arabidopsis seed dormancy and germination responses to chilling. The accumulation of CUs, expressed as estimated degree hours (see §5) during a typical autumn in York. The effect of varying the upper threshold below which the temperature is defined as chilling between 8 and 20°C on the rate and timing of CU accumulation is presented. The number of CUs shown in figure 1 to break the dormancy of low dormancy accessions set at 20°C (5 days at 4°C; green line), for maximum dormancy loss (14 days at 4°C; blue line) and re-introduction of secondary dormancy (28 days at 4°C; red line) are shown, in order to estimate the date these may occur in a wild setting.
4. Discussion
Dormancy in seeds is determined by the interaction between genetics and the environment during seed maturation. The seed-maturation environment is in turn determined by seed dormancy and germination, because the time of year of germination determines the timing of flowering and seed set. In this paper, we have argued that understanding the genetics and physiology of seed dormancy and other life-history traits will allow the reconstruction of the control of Arabidopsis life-history traits at the genetic level. This is essential because understanding the behaviour of extant wild accessions gives limited insight into the role that environmental selection for novel variants may play in plant responses to climate change. Because all possible genetic variants can be synthesized in the laboratory, this will provide important information on the range of life histories available for selection, and their phenology in synthetic novel climate regimes can be predicted. A good example of this approach is described by Kover et al. [27] where variation provided by 19 parent ecotypes is condensed into a relatively small population of recombinant inbred lines, which can then be screened for traits of interest.
Our data show that extended chilling of multiple Arabidopsis accessions leads to secondary dormancy, as has been described previously for CVI ecotype [21]. Interestingly, the duration of chilling that leads to secondary dormancy varies little between ecotypes, or in response to seed-maturation temperature (although the actual germination frequency, indicative of the dormancy depth, does vary). This is true even for common laboratory strains if seed is set at low maturation temperatures. Importantly, we show that maturation at warmer temperatures leads to an inability to enter secondary dormant states, as extended chilling during dark imbibition leads to germination even in the absence of light (table 1). This observation demonstrates that in the presence of sufficient soil moisture, these seeds are committed to germination in the same growing season as seed set. Whether these plants complete another life cycle prior to winter, or function as winter annuals overwintering in the vegetative state is yet to be determined. The increase in germination and then decrease as chilling times are extended has strong parallels with vernalization, where shorter two-week chilling spells often increase flowering time a little, whereas longer vernalization periods cause a marked decrease [28] suggesting a similar mechanism may be at work.
The definition of a CU may have a genetic basis, and our analysis shows that the definition has an effect on the predicted timing of germination of chilling-responsive seeds in the autumn, as well as a smaller effect on the timing of the onset of secondary dormancy. If chilling is detected at temperatures below 20°C, then germination of chilling-responsive low dormant seeds is advanced into August. If threshold temperature is set at 10°C, then this is delayed into September or even further into mid-October, using local temperature series measured at the University of York, UK. Thus, defining this threshold is a key step in understanding chilling responses in natural populations. During vernalization, temperatures below 10°C are accepted to result in a long-term reduction in FLC transcript levels. Temperatures in the range of 10–16°C actually cause an increase in FLC expression, compared with base temperatures of 20–22°C [29]. This type of threshold may have a genetic basis, and therefore may vary between strains or species. Our analysis predicts that whatever threshold for the ceiling of chilling is taken, seeds capable of entering secondary dormancy will have done to by the end of November under conditions found in York, UK. We also found that there is no theoretical need to determine the lower limit for chilling, as this is unlikely to affect our simulations of Arabidopsis phenology (figure 2). Empirical data will be required to establish the validity of the thermal time model for Arabidopsis chilling, or whether moving to a ‘chilling portion’ dynamic model will be necessary, as is used in predicting dormancy in perennial fruit crops [30]. These predictions will have wider utility in understanding Arabidopsis summer annual phenology, and because genes governing phenology are widely conserved among angiosperms, this approach could have utility in understanding pleiotropic consequences during plant breeding. In addition, it will be possible to establish tipping points in gene networks where the control of phenology might break down without selection of novel combinations of genes, that are not obvious from simple observational studies.
5. Material and methods
(a). Plant material and germination assays
Seeds for all lines were obtained from the Nottingham Arabidopsis Stock Centre (www.nasc.ac.uk). Plants for seed production were grown until flowering in standard long days (16 L : 8 D cycles) at a light level of 100 µmol m−2 s−1 at 20°C until flowering. During seed production, plants were either maintained at 20°C or transferred to either 15°C or 10°C, using Sanyo MLR350 growth chambers. Seed was harvested from the mother plant when approximately 50 per cent of the siliques had dehisced. Seeds were then sown directly on 0.9 per cent water agar plates and cold-treated at 4°C in the dark using a Sanyo MIR154 incubator. Germination was scored at the end of the chilling period where indicated. To test whether chilling had affected dormancy, after chilling, plates were removed to a Sanyo MLR350 growth chamber running a 12 L : 12 D cycle at 22°C for 7 days and germination frequencies scored. In all cases, five seed batches from five distinct mother plants were used as biological replicates.
(b). Chilling unit accumulation model
Historic temperature data for York were measured by the Department of Electronics, University of York, and raw data for maximum, minimum and mean daily temperatures are available online (http://weather.elec.york.ac.uk/archive.html). The daily temperature distribution was assumed to be 6 h of each day close to the minimum, 6 h close to maximum and 12 h close to the daily average. Accumulation of CUs was estimated for temperatures between a maximum threshold (Tmax) above which no chilling is perceived, and a minimum threshold (Tmin) where chilling is maximized and further decreasing temperature had no additional effect, in line with previous approaches [25]. For temperatures (T) between these thresholds, hourly CUs were calculated using the following formula: CUh − 1 = Tmax − T with CU set to a minimum value of 0 [25]. This model accurately predicts Arabidopsis responses to chilling during floral promotion [16] but further work is ongoing to test the validity of this model for the simulation of Arabidopsis seed germination in response to chilling.
References
- 1.Metcalf C. J., Mitchell-Olds T. 2009. Life history in a model system: opening the black box with Arabidopsis thaliana. Ecol. Lett. 12, 593–600 10.1111/j.1461-0248.2009.01320.x (doi:10.1111/j.1461-0248.2009.01320.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomashow M. F. 1999. Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 571–599 10.1146/annurev.arplant.50.1.571 (doi:10.1146/annurev.arplant.50.1.571) [DOI] [PubMed] [Google Scholar]
- 3.Penfield S. 2008. Temperature perception and signal transduction in plants. New Phytol. 179, 615–628 10.1111/j.1469-8137.2008.02478.x (doi:10.1111/j.1469-8137.2008.02478.x) [DOI] [PubMed] [Google Scholar]
- 4.Fitter A. H., Fitter R. S. 2002. Rapid changes in flowering time in British plants. Science 296, 1689–1691 10.1126/science.1071617 (doi:10.1126/science.1071617) [DOI] [PubMed] [Google Scholar]
- 5.Greer D. H., Wünsche J. N., Norling C. L., Wiggins H. N. 2006. Root-zone temperatures affect phenology of bud break, flower cluster development, shoot extension growth and gas exchange of ‘Braeburn’ (Malus domestica) apple trees. Tree Physiol. 26, 105–111 10.1093/treephys/26.1.105 (doi:10.1093/treephys/26.1.105) [DOI] [PubMed] [Google Scholar]
- 6.Piao S. L. 2007. Net carbon dioxide losses of northern ecosystems in response to autumn warming. Nature 451, 49–52 10.1038/nature06444 (doi:10.1038/nature06444) [DOI] [PubMed] [Google Scholar]
- 7.Yu H., Luedeling E., Xu J. 2010. Winter and spring warming result in delayed spring phenology on the Tibetan Plateau. Proc. Natl Acad. Sci. USA 107, 22 151–22 156 10.1073/pnas.1012490107 (doi:10.1073/pnas.1012490107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheldon C. C., Rouse D. T., Finnegan E. J., Peacock W. J., Dennis E. S. 2000. The molecular basis of vernalization: the central role of FLOWERING LOCUS C (FLC). Proc. Natl Acad. Sci. USA 97, 3753–3758 10.1073/pnas.060023597 (doi:10.1073/pnas.060023597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gazzani S., Gendall A. R., Lister C., Dean C. 2003. Analysis of the molecular basis of flowering time variation in Arabidopsis accessions. Plant Physiol. 132, 1107–1114 10.1104/pp.103.021212 (doi:10.1104/pp.103.021212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johanson U., West J., Lister C., Michaels S., Amasino R., Dean C. 2000. Molecular analysis of FRIGIDA, a major determinant of natural variation in Arabidopsis flowering time. Science 290, 344–347 10.1126/science.290.5490.344 (doi:10.1126/science.290.5490.344) [DOI] [PubMed] [Google Scholar]
- 11.McKay J. K., Richards J. H., Mitchell-Olds T. 2003. Genetics of drought adaptation in Arabidopsis thaliana. I. Pleiotropy contributes to genetic correlations among ecological traits. Mol. Ecol. 12, 1137–1151 10.1046/j.1365-294X.2003.01833.x (doi:10.1046/j.1365-294X.2003.01833.x) [DOI] [PubMed] [Google Scholar]
- 12.Chiang G. C., Barua D., Kramer E. M., Amasino R. M., Donohue K. 2009. Major flowering time gene, FLOWERING LOCUS C, regulates seed germination in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 106, 11 661–11 666 10.1073/pnas.0901367106 (doi:10.1073/pnas.0901367106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shindo C., Lister C., Crevillen P., Nordborg M., Dean C. 2006. Variation in the epigenetic silencing of FLC contributes to natural variation in Arabidopsis vernalization response. Genes Dev. 20, 3079–3083 10.1101/gad.405306 (doi:10.1101/gad.405306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toomajian C., et al. 2006. A nonparametric test reveals selection for rapid flowering in the Arabidopsis genome. PLoS Biol. 4, e137. 10.1371/journal.pbio.0040137 (doi:10.1371/journal.pbio.0040137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dodd A. N., Salathia N., Hall A., Kévei E., Tóth R., Nagy F., Hibberd J. M., Millar A. J., Webb A. A. 2005. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 309, 630–633 10.1126/science.1115581 (doi:10.1126/science.1115581) [DOI] [PubMed] [Google Scholar]
- 16.Wilczek A. M., et al. 2009. Effects of genetic perturbation on seasonal life history plasticity. Science 323, 930–934 10.1126/science.1165826 (doi:10.1126/science.1165826) [DOI] [PubMed] [Google Scholar]
- 17.Munir J., Dorn L. A., Donohue K., Schmitt J. 2001. The effect of maternal photoperiod on seasonal dormancy in Arabidopsis thaliana (Brassicaceae). Am. J. Bot. 88, 1240–1249 10.2307/3558335 (doi:10.2307/3558335) [DOI] [PubMed] [Google Scholar]
- 18.Schmuths H., Bachmann K., Weber W. E., Horres R., Hoffmann M. H. 2006. Effects of preconditioning and temperature during germination of 73 natural accessions of Arabidopsis thaliana. Ann. Bot. 97, 623–634 10.1093/aob/mcl012 (doi:10.1093/aob/mcl012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Penfield S., Hall A. 2009. A role for multiple circadian clock genes in the response to signals that break seed dormancy in Arabidopsis. Plant Cell 21, 1722–1732 10.1105/tpc.108.064022 (doi:10.1105/tpc.108.064022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bewley J. D. 1997. Seed germination and dormancy. Plant Cell 9, 1055–1066 10.1105/tpc.9.7.1055 (doi:10.1105/tpc.9.7.1055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finch-Savage W. E., Cadman C. S., Toorop P. E., Lynn J. R., Hilhorst H. W. 2007. Seed dormancy release in Arabidopsis Cvi by dry after-ripening, low temperature, nitrate and light shows common quantitative patterns of gene expression directed by environmentally specific sensing. Plant J. 51, 60–78 10.1111/j.1365-313X.2007.03118.x (doi:10.1111/j.1365-313X.2007.03118.x) [DOI] [PubMed] [Google Scholar]
- 22.Bentsink L., Jowett J., Hanhart C. J., Koornneef M. 2006. Cloning of DOG1, a quantitative trait locus controlling seed dormancy in Arabidopsis. Proc. Natl Acad. Sci. USA 103, 17 042–17 047 10.1073/pnas.0607877103 (doi:10.1073/pnas.0607877103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Penfield S., Josse E. M., Kannangara R., Gilday A. D., Halliday K. J., Graham I. A. 2005. Cold and light control seed germination through the bHLH transcription factor SPATULA. Curr. Biol. 15, 1998–2006 10.1016/j.cub.2005.11.010 (doi:10.1016/j.cub.2005.11.010) [DOI] [PubMed] [Google Scholar]
- 24.Batlla D., Benech-Arnold R. L. 2009. Predicting changes in dormancy level in natural seed soil banks Plant. Mol. Biol. 73, 3–13 [DOI] [PubMed] [Google Scholar]
- 25.Luedeling E., Zhang M., Girvetz E. H. 2009. Climatic changes lead to declining winter chill for fruit and nut trees in California during 1950–2099. PLoS ONE 4, e6166. 10.1371/journal.pone.0006166 (doi:10.1371/journal.pone.0006166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Batlla D., Verges V., Benech-Arnold R. L. 2003. A quantitative analysis of seed responses to cycle-doses of fluctuating temperatures in relation to dormancy level. Development of a thermal-time model for Polygonum aviculare L. seeds. Seed Sci. Res. 13, 197–207 10.1079/SSR2003137 (doi:10.1079/SSR2003137) [DOI] [Google Scholar]
- 27.Kover P. X., Valdar W., Trakalo J., Scarcelli N., Ehrenreich I. M., Purugganan M. D., Durrant C., Mott R. 2009. A multiparent advanced generation inter-cross to fine-map quantitative traits in Arabidopsis thaliana. PLoS Genet. 5, e1000551. 10.1371/journal.pgen.1000551 (doi:10.1371/journal.pgen.1000551) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chandler J., Martinez-Zapater J. M., Dean C. 2000. Mutations causing defects in the biosynthesis and response to gibberellins, abscisic acid and phytochrome B do not inhibit vernalization in Arabidopsis fca-1. Planta 210, 677–682 10.1007/s004250050059 (doi:10.1007/s004250 050059) [DOI] [PubMed] [Google Scholar]
- 29.Blázquez M. A., Ahn J. H., Weigel D. 2003. A thermosensory pathway controlling flowering time in Arabidopsis thaliana. Nat. Genet. 33, 168–171 10.1038/ng1085 (doi:10.1038/ng1085) [DOI] [PubMed] [Google Scholar]
- 30.Erez A., Fishman S. 1998. The dynamic model for chilling evaluation in peach buds. Acta Hortic. 465, 507–510 [Google Scholar]