Abstract
Multidrug resistance–associated protein 4 (MRP4, also known as Abcc4) regulates intracellular levels of cAMP and cGMP in arterial SMCs. Here, we report our studies of the role of MRP4 in the development and progression of pulmonary arterial hypertension (PAH), a severe vascular disease characterized by chronically elevated pulmonary artery pressure and accompanied by remodeling of the small pulmonary arteries as a prelude to right heart failure and premature death. MRP4 expression was increased in pulmonary arteries from patients with idiopathic PAH as well as in WT mice exposed to hypoxic conditions. Consistent with a pathogenic role for MRP4 in PAH, WT mice exposed to hypoxia for 3 weeks showed reversal of hypoxic pulmonary hypertension (PH) following oral administration of the MRP4 inhibitor MK571, and Mrp4–/– mice were protected from hypoxic PH. Inhibition of MRP4 in vitro was accompanied by increased intracellular cAMP and cGMP levels and PKA and PKG activities, implicating cyclic nucleotide-related signaling pathways in the mechanism underlying the protective effects of MRP4 inhibition. Our data suggest that MRP4 could represent a potential target for therapeutic intervention in PAH.
Introduction
Pulmonary arterial hypertension (PAH) is a severe vascular disease characterized by persistent precapillary pulmonary hypertension (PH), leading to progressive right heart failure and premature death (1, 2). PAH may be idiopathic, heritable, or associated with other diseases such as systemic sclerosis (3). The pathological mechanisms underlying this condition remain unclear. Pulmonary artery endothelial cell (PAEC) dysfunction and structural remodeling of the pulmonary vessels are early features of PAH. Pulmonary vascular remodeling includes proliferation and migration of pulmonary artery SMCs (PASMCs), leading to medial hypertrophy and increased pulmonary vascular resistance (3). Hypoxia-induced PH in mice is associated with PASMC proliferation, precapillary PH, and right heart hypertrophy (4).
Abnormalities in the homeostasis of cyclic nucleotides (namely cAMP and cGMP) are reflected in changes in vascular tone and PASMC proliferation. PASMCs from PH patients show decreased cGMP intracellular levels as well as reduced endothelial nitric oxide production, an increase in phosphodiesterase 5 expression and activity, and PASMC vasoconstriction and proliferation (5, 6). In recent years, phosphodiesterase type 5 (PDE5) inhibitors (sildenafil and tadalafil) have been approved for the treatment of PAH (7–12). The levels of cAMP have also been shown to be reduced in PASMCs from PH patients, in line with an increased expression of PDE types 1 and 3 (13, 14), also promoting PASMC proliferation. Prostacyclin analogs (epoprostenol, iloprost, and treprostinil) that increase PASMC cAMP levels have also been approved for the treatment of PAH (10, 11, 15).
In addition to hydrolysis by PDEs, cAMP and cGMP levels are also affected by a process involving active efflux out of cells. Multidrug resistance–associated protein 4 (MRP4), a member of a large family of transmembrane proteins (ATP-binding cassette transporter family class C), has been shown to function as an energy-dependent transporter for cyclic nucleotides (16, 17). MRP4 has recently been characterized as an endogenous regulator of intracellular cyclic nucleotide levels and cyclic nucleotide–mediated signaling pathways in coronary artery SMCs (18, 19).
The specific role of MRP4 in the progression of pulmonary artery vascular remodeling remains unclear. In this report, we describe the effects of MRP4 inhibition in experimental hypoxia-induced PH and also its expression in biopsy samples from PAH patients.
Results
MRP4 overexpression during PAH.
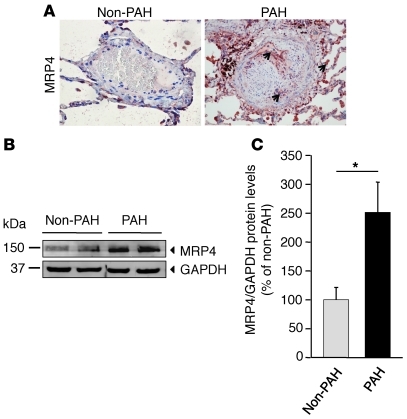
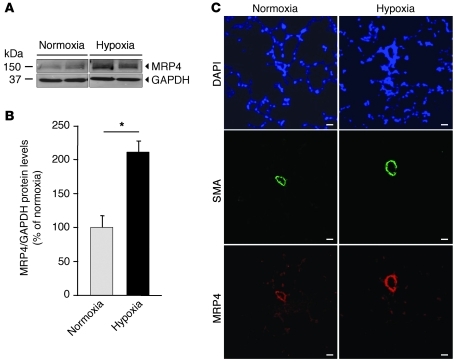
We initially compared the expression profiles of MRP4 in lung samples from normal human subjects and from patients with clinical PH. Immunohistochemistry analysis on human pulmonary arteries in sections from PAH patients revealed MRP4 expression in the media of arteries, endothelial cells, and pneumocytes (Figure 1A). In contrast, MRP4 expression was barely detectable in samples from control patients. Western blot analysis was performed on total lung extracts from PAH patients and normal subjects. In Figure 1, B and C, we show a 2.5-fold increase in MRP4 expression in the lungs of PAH individuals. Then we explored MRP4 expression profile in a model of hypoxia-induced PH in mice. We observed a 2.1-fold increase in MRP4 expression in lungs from WT mice exposed to hypoxia compared with normoxia (Figure 2, A and B). In the lung, MRP4 was constitutively expressed in pulmonary arteries (assessed by α-SMA staining), and its expression was increased in hypoxic conditions (Figure 2B).
Figure 1. MRP4 expression in control and PAH patients’ lungs.
(A) MRP4 localization assessed by immunohistochemistry in human pulmonary arteries from control and PAH patients. Arrows indicate the localization of MRP4 proteins found in endothelial cells, SMCs, and pneumocytes. (B) Western blot analysis of MRP4 in lung homogenates from patients showing controls (n = 4) and PAH (n = 4) patients. (C) Quantitative analysis of the results (*P < 0.05). Scale bar: 100 μm.
Figure 2. Increased MRP4 expression in hypoxia-induced PH in mice.
(A) Western blot analysis of MRP4 in lung homogenates from mice. Immunoblots are representative of 4 individual lungs for each group. The lanes were run on the same gel but were not contiguous. (B) Quantitative analysis of the results (*P < 0.05). (C) MRP4 and SMC actin expression assessed by immunofluorescence staining in sections of small pulmonary arteries from lungs of WT and Mrp4–/– mice in normoxia and hypoxia conditions. Scale bars: 20 μm.
Prevention of hypoxia-induced PH in MRP4-knockout mice.
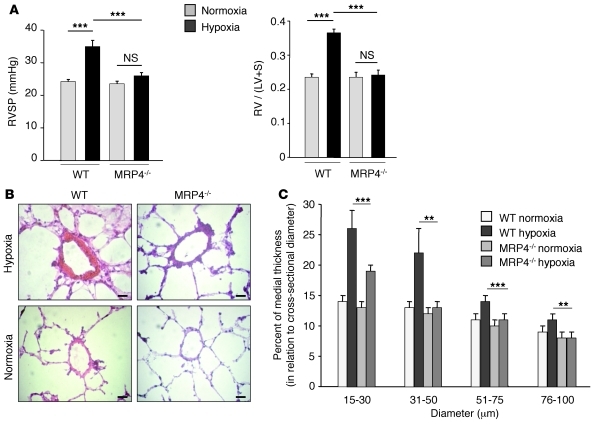
To further study the role of MRP4 on the development of hypoxia-induced PH, we exposed MRP4-knockout mice (Mrp4–/–) and WT mice to hypoxia for 4 weeks. Maintenance under normoxia was used as internal control. At baseline, as expected, MRP4 expression in the lung was completely abolished in Mrp4–/– mice (Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI45023DS1). In WT mice, hypoxia resulted in a marked increase in RV systolic pressure (RVSP) (Figure 3A), RV hypertrophy (assessed by the Fulton index) (Figure 3A), and distal pulmonary artery remodeling (Figure 3, B and C). In contrast, we were not able to detect significant changes in these parameters in Mrp4–/– mice (Figure 3, A–C). Similar results were obtained when assessing indices of vascular SMC proliferation by using Ki67 immunostaining (Supplemental Figure 2, A and B). These results demonstrate that knocking out MRP4 protects from hypoxia-induced PH development.
Figure 3. Prevention of hypoxia-induced PH in Mrp4–/– mice.
(A) RVSP (mmHg) and RV hypertrophy reflected by the RV weight over LV plus interventricular septum (S) weight ratio (RV/[LV + S] = Fulton index) in each group (n = 15, ***P < 0.001). (B) Representative H&E-stained sections of small pulmonary arteries from lungs of WT and Mrp4–/– mice in normoxia and hypoxia conditions. (C) Percentage of medial thickness of small arteries in relation to cross-sectional diameter (**P < 0.01). Scale bars: 20 μm.
Reversal of hypoxia-induced PH by MRP4 inhibitor MK571.
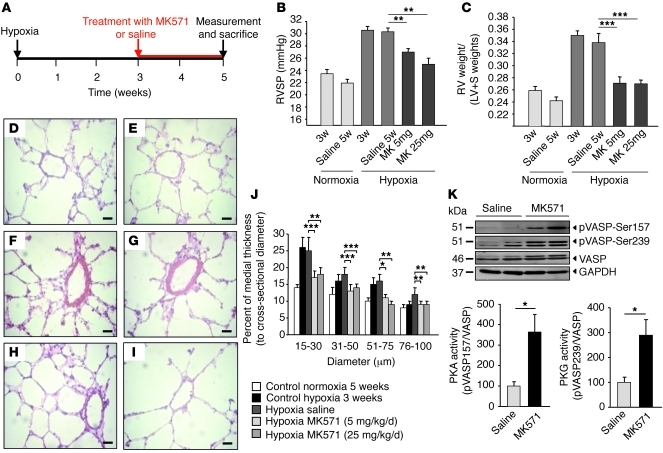
We next wished to determine whether it is possible to reverse PH induced by hypoxia in WT mice. In these experiments, we used MK571, a pharmacological inhibitor of MRP4 (20). As shown in Figure 4A, PH was induced by maintaining WT mice under hypoxia for 3 weeks. Development of PH was monitored by RVSP (Figure 4B), Fulton index (Figure 4C), and distal pulmonary artery wall thickness (Figure 4, D–J). Hypoxia-exposed WT mice were treated with either saline or MK571 (5 mg/kg/d or 25 mg/kg/d) for 2 more weeks while being maintained in hypoxic conditions. Saline-treated mice displayed all the hallmarks of PH (i.e., an increase in RVSP, Fulton index, and arterial wall thickness). However, following hypoxia, MK571-treated mice displayed lower RVSP (Figure 4B) and Fulton index (Figure 4C) and a decrease in the medial thickening of small pulmonary arteries and arterioles (Figure 4, D–J). The RV indices were similar to those observed in control animals maintained under normoxia (Figure 4C). MK571 had no effect on any physiological parameters (RVSP, mean arterial pressure), as measured in mice maintained in normoxia (data not shown). To our knowledge, this is the first demonstration in which development of hypoxia-induced PH was reversed by treatment with a compound targeting MRP4.
Figure 4. Reversal of hypoxia-induced PH after MRP4 inhibition.
(A) Schematic representation of PH-reversal experimental study design. (B) RVSP (mmHg) measured after 3 and 5 weeks (w) of normoxia and hypoxia conditions and in mice that were exposed for 5 weeks to hypoxia and treated with saline or 5 mg/kg/d or 25 mg/kg/d of MK571 (6 mice at least per group) during the last 2 weeks (***P < 0.001; **P < 0.01). (C) RV hypertrophy reflected by the Fulton index for the same groups (at least 6 mice per group). (D–I) Representative H&E-stained sections of small pulmonary arteries from the different groups: control normoxia versus hypoxia at 3 weeks (D and F), control normoxia at 5 weeks (E) versus hypoxia at 5 weeks after 2 weeks of saline (G), MK571 5 mg/kg/d (H), and 25 mg/kg/d (I) treatments. (J) Percentage of medial thickness of small arteries in relation to cross-sectional diameter. (K) Western blot analysis of total lung extracts from mice. Proteins were incubated with anti-MRP4 and anti–pVASP-Ser157 to assess PKA activity and anti–pVASP-Ser239 to assess PKG activity. Anti-VASP was used for normalization and anti-GAPDH as a loading control. Immunoblots are representative of 4 individual lungs for each groups (*P < 0.05). Scale bars: 20 μm.
To gain a better understanding of the molecular pathway(s) involved in the beneficial effect of MRP4 reversal of PH, we assessed PKA and PKG activity. Specifically, we analyzed vasodilator-stimulated phosphoprotein (VASP) phosphorylation on serine 157 and serine 239, respectively. In Figure 4K, we show increased PKA and PKG activities in lung lysates from hypertensive mice treated with MK571. Similar increases were not seen in animals receiving vehicle (saline) controls. These results suggested a possible role for PKA- and/or PKG-mediated signaling pathways in the biological effects seen following administration of an MRP4 inhibitor.
Since MK571 has also been reported to inhibit MRP1 (another member of the ATP-binding cassette transporter superfamily) (21), we measured the effect of MRP1 silencing on the cyclic nucleotide levels. MRP1 silencing in vitro by MRP1 siRNA did not increase intracellular nucleotide levels or PKA and PKG activities assessed by VASP phosphorylation (Supplemental Figure 3).
MRP4 function in PASMCs and endothelial cells.
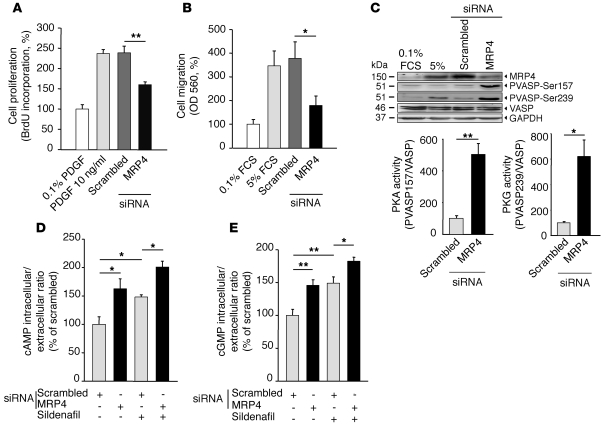
PASMC proliferation and migration are characteristics of PH (5). We next tested the biological consequences of MRP4 knockdown on proliferation and serum-induced migration on cultured human PASMCs. Compared with scrambled siRNA, MRP4 silencing led to a marked decrease in PDGF-induced proliferation (Figure 5A) and in serum-induced migration of hPASMCs (Figure 5B). Interestingly, there was no difference in apoptosis between scrambled and MRP4 siRNA–transfected cells (Supplemental Figure 4). Western blot analysis of VASP phosphorylation further revealed a significant increase in PKA and PKG activities following MRP4 silencing (Figure 5C). We then confirmed that MRP4 silencing increased intracellular/extracellular ratios of both cAMP and cGMP in hPASMCs in vitro (Figure 5, D and E). MRP4 is also a physiological transporter of endogenous substrates such as prostaglandins (22) whose accumulation may influence cyclic nucleotide homeostasis. However, inhibition of prostaglandin synthesis did not affect the increase in cAMP intracellular levels following MRP4 silencing (data not shown), thus suggesting a direct blockade of cyclic nucleotide efflux. Interestingly, we observed that changes in cyclic nucleotide levels following MRP4 silencing were further enhanced in the presence of the PDE5 inhibitor sildenafil (Figure 5, D and E). In addition, treatment with sildenafil led to an increase of MRP4 protein in sildenafil-treated WT mice (25 mg/kg/d) or in PASMCs isolated from PH patients (Supplemental Figure 5, A and B). These results further suggest that MRP4 overexpression could act in a manner to compensate for PDE5 inhibition. In contrast, PDE5 protein expression was unchanged in Mrp4–/– mice and in MRP4-silenced hPASMCs (Supplemental Figure 5, C and D). Similar results were observed for PDE3A and PDE4A isoforms (Supplemental Figure 5, E and F). Thus, MRP4 silencing inhibits both PASMC proliferation and migration, in line with the regression of pulmonary artery medial hypertrophy observed in vivo. MRP4 modifies the cyclic nucleotide homeostasis in these cells, and the combination of both PDE5 inhibitors and MRP4 inhibitors could be considered in order to provide a higher increase in cyclic nucleotide levels and further promote their antiproliferative action (23). Of note, the beneficial effect of sildenafil in PAH patients can also be partly linked to MRP4 inhibition (20).
Figure 5. Inhibition of hPASMC proliferation and migration after MRP4 silencing.
(A) Effect of MRP4 siRNA on hPASMC proliferation (assessed by BrdU incorporation) compared with scrambled siRNA. Proliferation was normalized to the value obtained in 0.1% PDGF. 4 experiments were done in triplicate (**P < 0.01). (B) Effect of MRP4 siRNA on hPASMC migration (assessed by colorimetric assay OD at 560 nm) compared with scrambled siRNA. 4 experiments were done in triplicate (*P < 0.05). (C) Western blot analysis of total lysates from hPASMC transfected with scrambled or MRP4 siRNAs for 72 hours. Proteins were incubated with anti-MRP4, anti–pVASP-Ser157 to assess PKA activity, and anti-pVASP-Ser239 to assess PKG activity. Anti-VASP was used for normalization and anti-GAPDH as a loading control. 3 experiments were done. (D and E) Intracellular/extracellular ratios of cAMP and cGMP, measured with a specific competitive enzyme immunoassay, in isolated hPASMCs transfected with scrambled or MRP4 siRNAs for 72 hours and treated or not with sildenafil (1 μM, 48 hours). 4 experiments were done in triplicate.
Because PAEC dysfunction is also characteristic of PH, we further tested the biological consequences of MRP4 knockdown in cultured human PAECs by analyzing cell migration. Compared with scrambled siRNA, MRP4 silencing resulted in a significant decrease in PDGF-induced migration (Supplemental Figure 6, A and B). This later result suggests that MRP4 silencing might also influence endothelial cell activation during PAH.
Pulmonary artery contraction and relaxation in MRP4-knockout mice.
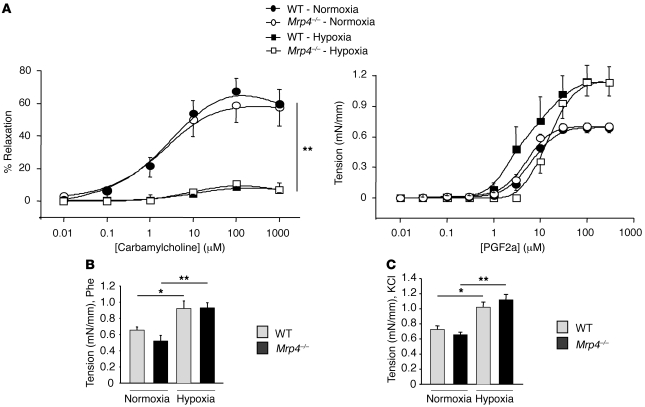
We then addressed the role of MRP4 in the relaxation and contraction of intrapulmonary arteries from WT mice and Mrp4–/– mice subjected to chronic hypoxia or maintained under normoxia. The relaxation response to carbamylcholine was not significantly different in WT and Mrp4–/– mice in normoxic or chronic hypoxic conditions (Figure 6A). However, the relaxation response to carbamylcholine was strongly reduced in mice with hypoxia-induced PH, suggesting an endothelial dysfunction, as previously described (24). The contraction to PGF2α (Figure 6A), phenylephrine (Phe) (Figure 6B), and high potassium solutions (KCl 80 mM) (Figure 6C) was similar in WT mice compared with Mrp4–/– mice. However, in WT mice as in Mrp4–/– mice, the contraction to PGF2α, Phe, and KCl (80 mM) was significantly higher in hypoxic conditions compared with normoxic conditions (Figure 6, A–C).
Figure 6. Relaxation and contraction of intrapulmonary arteries from WT and Mrp4–/– mice in normoxic or hypoxic conditions.
(A) Relaxation to carbamylcholine (0.01 to 1000 μM) in intrapulmonary arteries precontracted with either 10 μM or 30 μM of PGF2a in normoxic and chronic hypoxic mice, respectively (n = 8–13 rings, 4–5 mice and n = 10 rings, 6 mice, respectively). Relaxation is expressed as a percentage of the precontraction to PGF2a. Dose-dependent contraction to PGF2a (0.01 to 300 μM) (n = 8 rings, 3 mice in normoxia and n = 6 rings, 6 mice in chronic hypoxia) (**P < 0.001). (B) Comparison of the contraction to 10 μM phenylephrine (Phe) (n = 9–13 rings, 4–5 mice in normoxia and n = 16 rings, 6 mice in chronic hypoxia). (C) Comparison of the contraction to high potassium solutions (80 mM KCl) (n = 16 rings and 6 mice in normoxia and chronic hypoxia). (*P < 0.05).
MRP4 silencing prevents hypoxia-induced inflammatory response.
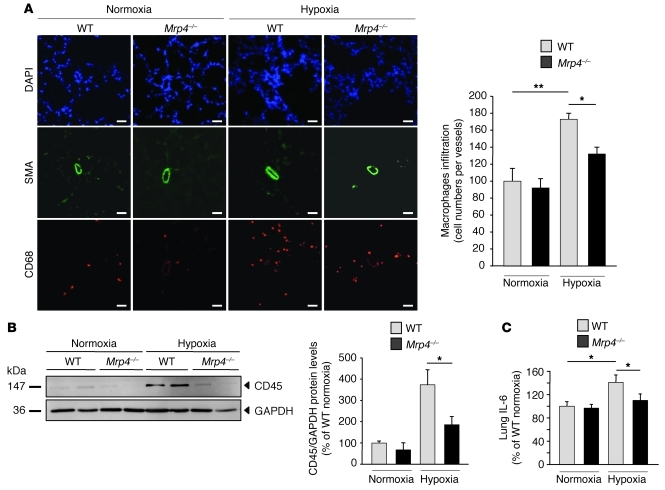
As inflammation is known to be increased in PH, we finally studied the effect of MRP4 inhibition on the inflammatory response in hypoxia-induced PH. We analyzed macrophage and lymphocyte cell recruitment in Mrp4–/– mice by measuring CD68 and CD45 expression, respectively. In both cases, we found a significant reduction in the hypoxia-induced inflammatory response in Mrp4–/– mice as compared with WT mice (Figure 7, A and B). We then measured the proinflammatory cytokine IL-6 in total lung homogenates. Under normoxic conditions, there was no difference in the IL-6 levels between WT and Mrp4–/– mice. The amount of IL-6 was, however, increased after hypoxia in WT mice but not in Mrp4–/– mice (Figure 7C). These results suggest that hypoxia-induced PH protection by MRP4 knockout is associated with a decrease in the inflammatory response.
Figure 7. Reduction of hypoxia-induced inflammatory response in Mrp4–/– mice.
(A) Lung macrophage recruitment under hypoxia conditions in WT versus Mrp4–/– mice. CD68 was used as a macrophage marker. The photographs are representative of each condition. Scale bars: 40 μm. (**P < 0.01) (B) CD45 quantification in total lung extracts from WT versus Mrp4–/– mice. Anti-GAPDH was used as a loading control, and immunoblots are representative of lungs from 4 individuals for each group. (C) Dosage of IL-6 in lung extracts determined by ELISA. The level was normalized according to the protein total quantity (*P < 0.05).
Discussion
This study identifies MRP4 as a key regulator in PH. Such evidence first came from in vivo experiments in which mice deficient for MRP4 were resistant to hypoxia-induced PH. Furthermore, in an established model of PH induced in WT mice by hypoxia, MK571, an MRP4 inhibitor, reversed PH. MRP4 deletion is particularly associated with a strong reduction in the vascular remodeling of small pulmonary arteries. We further demonstrate that MRP4 inhibition is associated with a significant reduction in PASMC proliferation and migration, a significant reduction in PAEC migration, and in turn, a significant reduction in hypoxia-induced inflammatory response. These results suggest that inhibiting MRP4 may be a useful strategy in human PAH, as MRP4 is overexpressed in human lungs from patients with PAH.
The pulmonary vascular alterations in PAH are characterized by the pathological proliferation and migration of small pulmonary artery vessel wall constituents including SMCs and endothelial cells. Several mechanisms have been proposed, such as abnormal growth responses to bone morphogenetic protein (BMP) and TGF-β (3, 25, 26), abnormal activation of RhoA (27), and hypersecretion of endothelin-1 (3), but the causal mechanism remains undetermined. Many studies have suggested a role for the dysregulation in cyclic nucleotide homeostasis observed in PASMCs. Pharmacotherapy that aims to increase intracellular levels of cyclic nucleotides has concordantly shown beneficial effects in PAH. PDE5 inhibitors and prostacyclin analogs have been approved for the treatment of PAH. Until now, the increase in cyclic nucleotide levels has been obtained either by promoting their synthesis (such as prostacyclin and nitric oxide therapies) or blocking their degradation by the use of phosphodiesterase inhibition (10). We identified a transmembrane protein called MRP4 as an endogenous regulator that has been previously shown to limit the amplitude of cyclic nucleotide signaling in vascular SMCs (19). Immunohistochemistry analysis on human pulmonary arteries in sections from PAH patients revealed MRP4 expression in the media of arteries. In contrast, MRP4 expression was barely detectable in samples from control patients. Our results suggest a preferential expression of MRP4 in proliferative SMCs and ECs in pathological conditions. In mice subjected to hypoxia, MRP4 was also upregulated. As seen for PDE1C and PDE5 that are overexpressed in PAH conditions, MRP4 could contribute to the deficiency of cyclic nucleotide intracellular levels in idiopathic PAH. The mechanism involved in the overexpression of MRP4 remains to be determined, but its overexpression in hypoxia can be explained by the presence of an HIF element in its promoter (28).
MRP4 uses the energy of ATP hydrolysis to translocate various molecules such as cyclic nucleotides across cell membranes. Here we demonstrate that MRP4 is present in PASMCs and that specific inhibition of MRP4 in PASMCs modified the intracellular content of cyclic nucleotides and resulted in an activation of the downstream pathways, namely PKA and PKG (29, 30), as shown by VASP phosphorylation. These effects were interestingly obtained without PDE inhibition. This indicates that MRP4 acts as a complementary mechanism, in addition to PDEs, to ensure intracellular cyclic nucleotide homeostasis. This was notably supported by the additional effect of concomitant PDE5 and MRP4 inhibition on the increase of cyclic nucleotide intracellular levels. These results also suggest that the combination of both PDE5 inhibitors and MRP4 inhibitors could be considered in order to provide a higher increase in cyclic nucleotide levels and further promote their antiproliferative action (23, 31–36). In addition, treatment with sildenafil led to an increase of MRP4 protein in PASMCs isolated from PH patients, further suggesting that MRP4 overexpression could act in a manner to compensate for PDE5 inhibition. In contrast, PDE5 protein expression was unchanged in Mrp4–/– mice or in MRP4-silenced hPASMCs. Similar results were observed for PDE3A and PDE4A isoforms.
We also found that MRP4 inhibition results in a notable reduction in hypoxia-induced inflammatory response. Inflammatory response is moderately increased in hypoxia-induced PH, but is markedly increased during PAH. Many studies have established the involvement of leukocytes, macrophages, and lymphocytes in complex vascular lesions of PAH (37–39). Cytokine and growth factors such as IL-6 and TGF-β1, which lead to inflammatory cell recruitment, also have an important role in the development of pulmonary vascular remodeling (40, 41). It is likely that the reduction in vascular remodeling observed after MRP4 inhibition results in a reduction in the release of proinflammatory factors from activated endothelial cells and SMCs. To our knowledge, there is no evidence that MRP4 directly controls the release of such factors or directly modulates the function of inflammatory cells. MRP4 expression has, however, been reported in blood cell progenitors (42). Whether MRP4 prevents the development of PH through a direct impact on the inflammatory process on top of its action on vascular endothelial and SMCs would deserve further investigation.
We also observed an important effect of the MRP4 inhibitor MK571 on the development of RV hypertrophy. Beyond the reduction in pulmonary pressure following MRP4 inhibition, this beneficial effect could also be linked to a more direct impact of MRP4 inhibition on the RV as previously reported with PDE5 inhibitors (43, 44). MK571 is, however, also an inhibitor of MRP1, another transporter of the ABCC family that is able to extrude leukotrienes (21), molecules acting against oxidative stress (45). However, the activation of PKA and PKG found in MK571-treated mice is unlikely to be carried by MRP1 inhibition, as MRP1 silencing in isolated PASMCs had no effect on PKA and PKG activities and on cAMP and cGMP levels. Moreover, MK571 has also been recognized as a leukotriene D4 receptor antagonist. LTD4 has been implicated in mitogen-induced proliferation of vascular SMCs (46). Inhibition of LTD4-mediated pathways could have been involved in reversal of PH in our model but is unlikely to explain the dramatic increase in PKA or PKG activities. Thus, it is more plausible that the reversal of PH in MK571-treated animals was the result of MRP4 inhibition alone. Thus, the reversal of PH in MK571-treated animals should essentially and directly be related to MRP4 inhibition.
MRP4 is also a physiological transporter of endogenous molecules such as glutathion, glucuronate, sulfate conjugates, urate, and prostaglandins (20, 22, 47–49), but none of these molecules had previously been described as modulators of PKA and PKG activity. Finally, the enhancement of PASMC apoptosis has been proposed as a potential mechanism to reverse pulmonary vascular remodeling during PH (5, 50–54). Some studies have shown the role of cyclic nucleotides in modulating apoptosis in PASMCs (6). In short-term in vitro experiments, we were, however, unable to observe an induction of apoptosis following MRP4 silencing. This could be related to a balance between the cAMP antiapoptotic effects and the cGMP proapoptotic ones (55, 56). However, the dramatic reversal of PAH observed in vivo in MK571-treated mice raises the question of whether apoptosis could be induced following long-term MRP4 inhibition.
We addressed the role of MRP4 in the relaxation and contraction of intrapulmonary arteries from WT mice and Mrp4–/– mice subjected to chronic hypoxia or maintained under normoxia. The results suggest a general hyperreactivity in intrapulmonary arteries from animals with hypoxia-induced PH in response to contractile stimuli (57). We were unable to find differences in pulmonary artery relaxation and contraction in Mrp4–/– mice even in chronic hypoxic conditions. These experiments were performed in intrapulmonary arteries with an internal diameter of 521.8 ± 8.7 μm (322.6 to 645.4 μm; n = 87), as it was not possible for technical reasons to perform such experiments in smaller vessels (15 to 50 μm in diameter). The alteration of pulmonary arterial tone and pulmonary arterial remodeling are, however, known to be higher when the vessels are smaller (58). Our histological analyses in lungs from hypoxic Mrp4–/– mice showed a major change in vascular remodeling for vessels with a lower diameter of 15 to 50 μm but not in larger vessels.
In conclusion, by acting on the possible extrusion of the cyclic nucleotides, inhibition of MRP4 not only blocks but also reverses hypoxia-induced PH in mice. In addition, targeting MRP4 can affect PH-associated proliferation and migration of vascular wall components in vitro. Collectively, we have presented both in vitro and in vivo findings strongly suggesting inhibition of MRP4 as a novel therapy for treating patients with human PAH.
Methods
Experimental procedures are described in detail in Supplemental Methods.
Reagents.
See Supplemental Methods.
Animal model of PH.
The Mrp4–/– mice were originally derived in the laboratory of John Schuetz and repeatedly backcrossed to FVB (Friend virus B-type) mice to greater than 99% FVB. Homozygous Mrp4–/– mice on an FVB background were compared with FVB WT mice kept in the same conditions. For the PH prevention protocol, 15 mice from each group (Mrp4–/– and WT, 6 weeks old) were exposed to chronic hypoxia (10% O2) in a ventilated chamber for 28 days. Animals were fed standard chow and water ad libitum. Animal studies were approved by the administrative panel on animal care at Centre de Chirurgie Expérimentale Marie Lannelongue (Le Plessis-Robinson, France). Concerning the PH reversal study, 5-week-old WT mice were maintained in hypoxia for 3 weeks, then were randomized to receive, for 2 weeks, oral vehicle or MK571 at the doses of either 5 mg/kg/d or 25 mg/kg/d. At the same time, the experiment was designed for mice in normoxia conditions. Increased hematocrit values were checked to assess the efficiency of hypoxia. For hemodynamic measurements, mice were anesthetized with an intraperitoneal injection of ketamin (100 mg/ml; MTC Pharmaceuticals) and xylazine (10 mg/kg; Bayer). A 24-gauge catheter was advanced to the RV through the right jugular vein for measurement of RV pressure with fluid-filled force transducers. RV hypertrophy was expressed as the ratio of the RV wall to LV wall plus septum weight (RV/[LV + S] – Fulton index). Experiments were performed by 2 investigators in a blinded fashion. After mice were euthanized, a thoracotomy was performed, and after exsanguination, the left lung was fixed for histology in 10% neutral buffered formalin and the right lung was snap-frozen in liquid nitrogen. H&E staining was performed on 6-μm sections according to common procedures. To assess the type of remodeling of muscular pulmonary arteries, microscopic images were analyzed using a computerized morphometric system (Leica). For each mouse, medial thickness of 50 intraracinar arteries in 4 different sections was measured and extrapolated to estimate cross-sectional diameter. Arteries were categorized according to their external diameters: category I included arteries with an external diameter between 15 and 30 μm; category II included arteries with an external diameter between 31 μm and 50 μm; category III included arteries with an external diameter between 51 μm and 75 μm; and category IV included arteries with an external diameter between 76 μm and 100 μm. Analysis was done in a blinded fashion.
Patients with idiopathic PAH and controls.
We studied lung specimens obtained at the time of lung transplantation in 8 patients with idiopathic PAH and at the time of thoracic surgery (lobectomy or pneumonectomy for localized lung cancer) in 5 control patients. This study was approved by the local ethics committee (Comité de Protection des Personnes, CPP Ile de France VII, Le Kremlin Bicêtre, France), and patients gave informed consent. See Supplemental Methods for details.
Immunofluorescence staining.
See Supplemental Methods.
Isolation and culture of PASMCs and PAECs.
Human PASMCs were isolated as previously described (59). To identify PASMCs, we examined cultured cells for expression of muscle-specific contractile and cytoskeletal proteins including α-SMA, desmin, and vinculin. Cells were cultured in SMCBM2 containing 5% FCS (FCS supplemented with 10 ng/ml epidermal growth factor, 40 ng/ml basic fibroblast growth factor, 1 mg/ml insulin) (PromoCell) and antibiotics at 37°C with 5% CO2. The cells were used between passages 3 and 6. Pulmonary artery endothelial cells were purchased from Lonza (CC-2527), cultured in EBM-2 (Lonza) containing 5% growth factors, and used between passages 2 and 3.
RNA interference and cellular experiments in PASMCs and PAECs.
Cells were transfected with siRNA (50 nM) using Lipofectamine 2000 (Invitrogen) or electroporation using Amaxa Nucleofector technology according to the manufacturer’s instructions. A colorimetric BrdU cell proliferation assay was performed according to the manufacturer’s instructions (Roche). Migration of hPASMCs was assessed using a micro Boyden Chamber QCM 24-Well Colorimetric Cell Migration Assay (Chemicon International), and apoptosis was assessed by using the ApopTag Red In Situ Apoptosis Detection Kit (Chemicon International) according to the manufacturer’s instructions. Cyclic GMP and AMP were measured by specific competitive enzyme immunoassay as recommended by the manufacturer (R&D Systems). See Supplemental Methods for details.
Quantitative real-time PCR and Western blotting.
See Supplemental Methods.
Vascular reactivity.
FVB mice were exposed to chronic hypoxia in a hypobaric chamber (380 mmHg) for 21 days. The chamber was opened three times a week for 30 minutes for animal care and cleaning. Control mice (normoxic group) were housed in room air at ambient atmospheric pressure. Mice were sacrificed using CO2 asphyxia as approved by the animal care and use local ethics committee (Comité d’éthique régional d’Aquitaine; referenced AP 21/06/2010). The left lung was rapidly removed and rinsed in Krebs solution containing 119 mM NaCl, 4.7 mM KCl, 1.17 mM MgSO4, 25 mM NaHCO3, 1.18 mM KH2PO4, 1.5 mM CaCl2, and 5. mM 5 D-glucose. Intrapulmonary arteries with an internal diameter of 515.9 ± 11.7 μm (322.6 to 638.5 μm; n = 45) for WT mice and 528 ± 12.9 μm (368.9 to 645.4 μm; n = 43) for MRP4 KO mice (intrapulmonary arteries of the first order) were then dissected free from surrounding connective tissues under binocular control. Segments of pulmonary artery (1.8–2 mm length) were mounted in a Mulvany Myograph (Multi Myograph System, model 610M; J.P. Trading), as previously described (60). Passive length-tension relationship demonstrated that the optimal resting tension corresponded to an equivalent transmural pressure of 15 and 30 mmHg in arteries from control and hypoxic mice, respectively. After 60 minutes equilibration period under resting conditions, viability of arteries was evaluated using Krebs containing 80 mM KCl. High potassium solutions were obtained by substituting an equimolar amount of KCl for NaCl from Krebs solution. Arteries developing a wall tension below 0.5 mN/mm were discarded. Concentration-response curves to PGF2α (0.01 to 300 μM) were then constructed. Relaxation was investigated by constructing concentration-response curves with carbamylcholine (as reference endothelial NO-dependent agent) (0.01 to 1000 μM) on arteries submaximally precontracted with PGF2α (10 μM and 30 μM for normoxic and chronic hypoxic conditions, respectively).
IL-6 measurements.
Levels of IL-6 were measured in total lung extracts using an ELISA kit according to the manufacturer’s instructions (R&D Systems). For each test, the quantities were normalized on the total protein concentration.
Statistics.
All quantitative data are reported as means ± SEM. Statistical analysis was performed with the Prism software package (GraphPad v3). One-way ANOVA was used to compare each parameter. Post hoc t test comparisons were performed to identify which group differences accounted for significant overall ANOVA results. P < 0.05 was considered significant. See Supplemental Methods for details.
Supplementary Material
Acknowledgments
This work was supported by grants from the Agence Nationale de la Recherche (ANR) and Fondation de France to J.S. Hulot (grant 2006005606) and by Fondation Leducq through the CAERUS network to A.-M. Lompré (research agreement 05CVD03). Y. Hara was a recipient of PhD fellowships from the Ministère de l’Enseignement Supérieur et de la Recherche (MESR), and Y. Sassi was supported by the Fondation pour la Recherche Médicale. We thank Chen Yan (University of Rochester Medical Center, Rochester, New York, USA) for providing the PDE3A antibody and Pietr Borst and Koen Van Wetering (The Netherlands Cancer Institute— Amsterdam, Netherlands) for helpful discussions and advice on the biological function of MRP4. Finally, we thank Martin Schwarz for his assistance during manuscript revision.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2011;121(7):2888–2897. doi:10.1172/JCI45023.
References
- 1.Rubin LJ. Primary pulmonary hypertension. N Engl J Med. 1997;336(2):111–117. doi: 10.1056/NEJM199701093360207. [DOI] [PubMed] [Google Scholar]
- 2.Humbert M, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122(2):156–163. doi: 10.1161/CIRCULATIONAHA.109.911818. [DOI] [PubMed] [Google Scholar]
- 3.Humbert M, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43(12 suppl S):13S–24S. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 4.Pak O, Aldashev A, Welsh D, Peacock A. The effects of hypoxia on the cells of the pulmonary vasculature. Eur Respir J. 2007;30(2):364–372. doi: 10.1183/09031936.00128706. [DOI] [PubMed] [Google Scholar]
- 5.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest. 2008;118(7):2372–2379. doi: 10.1172/JCI33452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wharton J, et al. Antiproliferative effects of phosphodiesterase type 5 inhibition in human pulmonary artery cells. Am J Respir Crit Care Med. 2005;172(1):105–113. doi: 10.1164/rccm.200411-1587OC. [DOI] [PubMed] [Google Scholar]
- 7.Zhao L, et al. Sildenafil inhibits hypoxia-induced pulmonary hypertension. Circulation. 2001;104(4):424–428. doi: 10.1161/hc2901.093117. [DOI] [PubMed] [Google Scholar]
- 8.Galie N, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005;353(20):2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 9.Galie N, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119(22):2894–2903. doi: 10.1161/CIRCULATIONAHA.108.839274. [DOI] [PubMed] [Google Scholar]
- 10.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med. 2004;351(14):1425–1436. doi: 10.1056/NEJMra040291. [DOI] [PubMed] [Google Scholar]
- 11.Galie N, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009;30(20):2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 12.Sebkhi A, Strange JW, Phillips SC, Wharton J, Wilkins MR. Phosphodiesterase type 5 as a target for the treatment of hypoxia-induced pulmonary hypertension. Circulation. 2003;107(25):3230–3235. doi: 10.1161/01.CIR.0000074226.20466.B1. [DOI] [PubMed] [Google Scholar]
- 13.Schermuly RT, et al. Phosphodiesterase 1 upregulation in pulmonary arterial hypertension: target for reverse-remodeling therapy. Circulation. 2007;115(17):2331–2339. doi: 10.1161/CIRCULATIONAHA.106.676809. [DOI] [PubMed] [Google Scholar]
- 14.Murray F, et al. Expression and activity of cAMP phosphodiesterase isoforms in pulmonary artery smooth muscle cells from patients with pulmonary hypertension: role for PDE1. Am J Physiol Lung Cell Mol Physiol. 2007;292(1):L294–L303. doi: 10.1152/ajplung.00190.2006. [DOI] [PubMed] [Google Scholar]
- 15.Hoeper MM, et al. Long-term treatment of primary pulmonary hypertension with aerosolized iloprost, a prostacyclin analogue. N Engl J Med. 2000;342(25):1866–1870. doi: 10.1056/NEJM200006223422503. [DOI] [PubMed] [Google Scholar]
- 16.Wielinga PR, van der Heijden, I, Reid G, Beijnen JH, Wijnholds J, Borst P. Characterization of the MRP4- and MRP5-mediated transport of cyclic nucleotides from intact cells. J Biol Chem. 2003;278(20):17664–17671. doi: 10.1074/jbc.M212723200. [DOI] [PubMed] [Google Scholar]
- 17.van Aubel RA, Smeets PH, Peters JG, Bindels RJ, Russel FG. The MRP4/ABCC4 gene encodes a novel apical organic anion transporter in human kidney proximal tubules: putative efflux pump for urinary cAMP and cGMP. J Am Soc Nephrol. 2002;13(3):595–603. doi: 10.1681/ASN.V133595. [DOI] [PubMed] [Google Scholar]
- 18.Sassi Y, et al. Multidrug resistance-associated protein 4 regulates cAMP-dependent signaling pathways and controls human and rat SMC proliferation. J Clin Invest. 2008;118(8):2747–2757. doi: 10.1172/JCI35067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sassi Y, Hara Y, Lompre AM, Hulot JS. Multi-drug resistance protein 4 (MRP4/ABCC4) and cyclic nucleotides signaling pathways. Cell Cycle. 2009;8(7):962–963. [PubMed] [Google Scholar]
- 20.Reid G, et al. Characterization of the transport of nucleoside analog drugs by the human multidrug resistance proteins MRP4 and MRP5. Mol Pharmacol. 2003;63(5):1094–1103. doi: 10.1124/mol.63.5.1094. [DOI] [PubMed] [Google Scholar]
- 21.Borst P, Evers R, Kool M, Wijnholds J. A family of drug transporters: the multidrug resistance-associated proteins. J Natl Cancer Inst. 2000;92(16):1295–1302. doi: 10.1093/jnci/92.16.1295. [DOI] [PubMed] [Google Scholar]
- 22.Reid G, et al. The human multidrug resistance protein MRP4 functions as a prostaglandin efflux transporter and is inhibited by nonsteroidal antiinflammatory drugs. Proc Natl Acad Sci U S A. 2003;100(16):9244–9249. doi: 10.1073/pnas.1033060100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghofrani HA, et al. Future perspectives for the treatment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54(1 suppl):S108–S117. doi: 10.1016/j.jacc.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation. 2004;109(2):159–165. doi: 10.1161/01.CIR.0000102381.57477.50. [DOI] [PubMed] [Google Scholar]
- 25.Morrell NW, et al. Altered growth responses of pulmonary artery smooth muscle cells from patients with primary pulmonary hypertension to transforming growth factor-beta(1) and bone morphogenetic proteins. Circulation. 2001;104(7):790–795. doi: 10.1161/hc3201.094152. [DOI] [PubMed] [Google Scholar]
- 26.Zhang S, et al. Bone morphogenetic proteins induce apoptosis in human pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2003;285(3):L740–L754. doi: 10.1152/ajplung.00284.2002. [DOI] [PubMed] [Google Scholar]
- 27.Loirand G, Guerin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res. 2006;98(3):322–334. doi: 10.1161/01.RES.0000201960.04223.3c. [DOI] [PubMed] [Google Scholar]
- 28.Maher JM, et al. Oxidative and electrophilic stress induces multidrug resistance-associated protein transporters via the nuclear factor-E2-related factor-2 transcriptional pathway. Hepatology. 2007;46(5):1597–1610. doi: 10.1002/hep.21831. [DOI] [PubMed] [Google Scholar]
- 29.Indolfi C, et al. Activation of cAMP-PKA signaling in vivo inhibits smooth muscle cell proliferation induced by vascular injury. Nat Med. 1997;3(7):775–779. doi: 10.1038/nm0797-775. [DOI] [PubMed] [Google Scholar]
- 30.Sinnaeve P, et al. Overexpression of a constitutively active protein kinase G mutant reduces neointima formation and in-stent restenosis. Circulation. 2002;105(24):2911–2916. doi: 10.1161/01.CIR.0000018169.59205.CA. [DOI] [PubMed] [Google Scholar]
- 31.Baliga RS, et al. Synergy between natriuretic peptides and phosphodiesterase 5 inhibitors ameliorates pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;178(8):861–869. doi: 10.1164/rccm.200801-121OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghofrani HA, Hoeper MM. [Drug combination treatment for pulmonary arterial hypertension]. Dtsch Med Wochenschr. 2006;131(49 suppl 9):S330–S333. doi: 10.1055/s-2006-957205. [DOI] [PubMed] [Google Scholar]
- 33.Ghofrani HA, et al. Nitric oxide pathway and phosphodiesterase inhibitors in pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43(12 suppl S):68S–72S. doi: 10.1016/j.jacc.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 34.Ghofrani HA, et al. Oral sildenafil as long-term adjunct therapy to inhaled iloprost in severe pulmonary arterial hypertension. J Am Coll Cardiol. 2003;42(1):158–164. doi: 10.1016/S0735-1097(03)00555-2. [DOI] [PubMed] [Google Scholar]
- 35.Simonneau G, et al. Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomized trial. Ann Intern Med. 2008;149(8):521–530. doi: 10.7326/0003-4819-149-8-200810210-00004. [DOI] [PubMed] [Google Scholar]
- 36.Zhao L, et al. Simvastatin and sildenafil combine to attenuate pulmonary hypertension. Eur Respir J. 2009;34(4):948–957. doi: 10.1183/09031936.00143508. [DOI] [PubMed] [Google Scholar]
- 37.Dorfmuller P, et al. Chemokine RANTES in severe pulmonary arterial hypertension. Am J Respir Crit Care Med. 2002;165(4):534–539. doi: 10.1164/ajrccm.165.4.2012112. [DOI] [PubMed] [Google Scholar]
- 38.Hassoun PM, et al. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54(1 suppl):S10–19. doi: 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 39.Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol. 1994;144(2):275–285. [PMC free article] [PubMed] [Google Scholar]
- 40.Steiner MK, Syrkina OL, Kolliputi N, Mark EJ, Hales CA, Waxman AB. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res. 2009;104(2):236–244. doi: 10.1161/CIRCRESAHA.108.182014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Humbert M, et al. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med. 1995;151(5):1628–1631. doi: 10.1164/ajrccm.151.5.7735624. [DOI] [PubMed] [Google Scholar]
- 42.Oevermann L, et al. Hematopoietic stem cell differentiation affects expression and function of MRP4 (ABCC4), a transport protein for signaling molecules and drugs. Int J Cancer. 2009;124(10):2303–2311. doi: 10.1002/ijc.24207. [DOI] [PubMed] [Google Scholar]
- 43.Nagendran J, et al. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation. 2007;116(3):238–248. doi: 10.1161/CIRCULATIONAHA.106.655266. [DOI] [PubMed] [Google Scholar]
- 44.Archer SL, Michelakis ED. Phosphodiesterase type 5 inhibitors for pulmonary arterial hypertension. N Engl J Med. 2009;361(19):1864–1871. doi: 10.1056/NEJMct0904473. [DOI] [PubMed] [Google Scholar]
- 45.Mueller CF, et al. Multidrug resistance protein-1 affects oxidative stress, endothelial dysfunction, and atherogenesis via leukotriene C4 export. Circulation. 2008;117(22):2912–2918. doi: 10.1161/CIRCULATIONAHA.107.747667. [DOI] [PubMed] [Google Scholar]
- 46.Panettieri RA, Tan EM, Ciocca V, Luttmann MA, Leonard TB, Hay DW. Effects of LTD4 on human airway smooth muscle cell proliferation, matrix expression, and contraction In vitro: differential sensitivity to cysteinyl leukotriene receptor antagonists. Am J Respir Cell Mol Biol. 1998;19(3):453–461. doi: 10.1165/ajrcmb.19.3.2999. [DOI] [PubMed] [Google Scholar]
- 47.Chen ZS, Lee K, Kruh GD. Transport of cyclic nucleotides and estradiol 17-beta-D-glucuronide by multidrug resistance protein 4. Resistance to 6-mercaptopurine and 6-thioguanine. J Biol Chem. 2001;276(36):33747–33754. doi: 10.1074/jbc.M104833200. [DOI] [PubMed] [Google Scholar]
- 48.Deeley RG, Westlake C, Cole SP. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev. 2006;86(3):849–899. doi: 10.1152/physrev.00035.2005. [DOI] [PubMed] [Google Scholar]
- 49.Li C, et al. Spatiotemporal coupling of cAMP transporter to CFTR chloride channel function in the gut epithelia. Cell. 2007;131(5):940–951. doi: 10.1016/j.cell.2007.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang JB, Liu YL, Sun PW, Lv XD, Bo K, Fan XM. Novel strategy for treatment of pulmonary arterial hypertension: enhancement of apoptosis. Lung. 2010;188(3):179–189. doi: 10.1007/s00408-010-9233-8. [DOI] [PubMed] [Google Scholar]
- 51.McMurtry MS, et al. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J Clin Invest. 2005;115(6):1479–1491. doi: 10.1172/JCI23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stenmark KR, Rabinovitch M. Emerging therapies for the treatment of pulmonary hypertension. Pediatr Crit Care Med. 2010;11(2 suppl):S85–S90. doi: 10.1097/PCC.0b013e3181c76db3. [DOI] [PubMed] [Google Scholar]
- 53.Schermuly RT, et al. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest. 2005;115(10):2811–2821. doi: 10.1172/JCI24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nishimura T, et al. Simvastatin rescues rats from fatal pulmonary hypertension by inducing apoptosis of neointimal smooth muscle cells. Circulation. 2003;108(13):1640–1645. doi: 10.1161/01.CIR.0000087592.47401.37. [DOI] [PubMed] [Google Scholar]
- 55.Chiche JD, et al. Adenovirus-mediated gene transfer of cGMP-dependent protein kinase increases the sensitivity of cultured vascular smooth muscle cells to the antiproliferative and pro-apoptotic effects of nitric oxide/cGMP. J Biol Chem. 1998;273(51):34263–34271. doi: 10.1074/jbc.273.51.34263. [DOI] [PubMed] [Google Scholar]
- 56.Orlov SN, et al. Activation of cAMP signaling transiently inhibits apoptosis in vascular smooth muscle cells in a site upstream of caspase-3. Cell Death Differ. 1999;6(7):661–672. doi: 10.1038/sj.cdd.4400539. [DOI] [PubMed] [Google Scholar]
- 57.Delannoy E, Courtois A, Freund-Michel V, Leblais V, Marthan R, Muller B. Hypoxia-induced hyperreactivity of pulmonary arteries: role of cyclooxygenase-2, isoprostanes, and thromboxane receptors. Cardiovasc Res. 2010;85(3):582–592. doi: 10.1093/cvr/cvp292. [DOI] [PubMed] [Google Scholar]
- 58.Rodat L, Savineau JP, Marthan R, Guibert C. Effect of chronic hypoxia on voltage-independent calcium influx activated by 5-HT in rat intrapulmonary arteries. Pflugers Arch. 2007;454(1):41–51. doi: 10.1007/s00424-006-0178-y. [DOI] [PubMed] [Google Scholar]
- 59.Eddahibi S, et al. Serotonin transporter overexpression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertension. J Clin Invest. 2001;108(8):1141–1150. doi: 10.1172/JCI12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fresquet F, et al. Role of reactive oxygen species and gp91phox in endothelial dysfunction of pulmonary arteries induced by chronic hypoxia. Br J Pharmacol. 2006;148(5):714–723. doi: 10.1038/sj.bjp.0706779. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.