Abstract
The airway is a primary portal of entry for noxious environmental stimuli that can trigger airway remodeling, which contributes significantly to airway obstruction in chronic obstructive pulmonary disease (COPD) and chronic asthma. Important pathologic components of airway remodeling include fibrosis and abnormal innate and adaptive immune responses. The positioning of fibroblasts in interstitial spaces suggests that they could participate in both fibrosis and chemokine regulation of the trafficking of immune cells such as dendritic cells, which are crucial antigen-presenting cells. However, physiological evidence for this dual role for fibroblasts is lacking. Here, in two physiologically relevant models — conditional deletion in mouse fibroblasts of the TGF-β–activating integrin αvβ8 and neutralization of αvβ8 in human COPD fibroblasts — we have elucidated a mechanism whereby lung fibroblast chemokine secretion directs dendritic cell trafficking, in a manner that is critically dependent on αvβ8-mediated activation of TGF-β by fibroblasts. Our data therefore indicate that fibroblasts have a crucial role in regulating both fibrotic and immune responses in the lung.
Introduction
Chronic obstructive pulmonary disease (COPD) and chronic asthma are major causes of morbidity and mortality worldwide and are increasing in prevalence (1–3). Airway remodeling is an essential pathophysiologic component of airway obstruction in both diseases and is thought to be caused mainly by chronic smoking or allergen exposure (1, 4). Airway remodeling is defined by the collective changes in epithelial, mesenchymal, and immune cell compartments leading to fibrotic airway wall thickening and a persistent abnormal innate and adaptive immune response, suggesting common mechanistic underpinnings (1, 4, 5).
Both innate and adaptive immune responses are implicated in the pathogenesis of COPD and asthma. Lung innate immune responses are initiated and enhanced through increased function of proinflammatory cytokines, such as IL-1β (6–13). Airway delivery of an adenoviral vector expressing IL-1β (Ad-IL-1β) and transgenic overexpression of IL-1β are associated with airway inflammation and remodeling in mice (14, 15). In addition, IL-1β is a critical mobilization signal in promoting the migration of DCs to the draining lymph nodes, where they function as APCs and initiate T cell priming (16). In COPD, this T cell priming leads to a Th1-type response characterized by production of IFN-γ and IL-17. Conversely, in asthma, a Th2-type response is primed, characterized by production of IL-4, IL-5, IL-9, and IL-13 (5, 16–20).
Specific chemokine ligands (CCL) expressed by structural cells and their receptors (CCR) expressed by DCs play a major role in DC migration (16, 21). Specifically, lung DCs migrate utilizing chemokine/receptor pairs CCL2/CCR2, CCL7/CCR2, CCL5/CCR5, and CCL20/CCR6 (22–24). CCL2 and CCL20 expression is increased in human COPD and asthma lung samples (20, 25–28). Furthermore, Ccr6–/– mice are protected from cigarette smoke–induced airway wall remodeling, and both Ccr2–/– and Ccr6–/– mice are protected from allergic pulmonary inflammation (29–31). The mechanisms guiding DC migration in the lung have not been fully elucidated, but are likely to involve the structural cells (i.e., airway epithelial cells and fibroblasts) of the compartments that DCs move through while traveling from the circulation to the airway to the mediastinal lymph node (MLN) (21). The mechanistic role played by fibroblasts in directing DC migration remains speculative (32–34).
TGF-β is a central cytokine mediating epithelial, mesenchymal, and immune cell homeostasis and has been implicated in the pathogenesis of COPD and asthma (35–39). Increased TGF-β signaling by fibroblasts has been widely implicated in airway and pulmonary fibrosis through regulation of the profibrogenic crucial components of fibrosis (15, 40–42). Aside from this role in fibrosis, TGF-β is a well-known player in immunity through the induction of immunosuppressive Tregs or the proinflammatory Th-17 cells, which secrete IL-17 (43). In experimental allergic asthma, these dualistic roles of TGF-β are further exemplified, as TGF-β signaling by airway epithelial cells can promote inflammation, while TGF-β signaling by T cells inhibits inflammation (44, 45).
TGF-β is ubiquitously expressed by all cell types in 3 isoforms in mammals, but is kept latent and bound mostly to extracellular matrix components by non-covalent association with its propeptide, the latency associated peptide (LAP) (46, 47). Thus, the activation of TGF-β is a crucial point of regulation in TGF-β function. The extracellular matrix receptor integrin αvβ8 (expressed in the lung by epithelial cells and fibroblasts) is a high-affinity receptor for the integrin-binding domain of the LAPs of TGF-β1 and -β3 and is a critical mediator of TGF-β1 and -β3 activation in vivo (47–50). Ligand-binding studies demonstrate that the LAPs of TGF-β1 and -β3 are probably the only physiologically relevant ligands for integrin αvβ8 (48). Consistent with this, mice lacking αvβ8 have a phenotype nearly identical to that of mice with a null mutation in TGF-β3 and mutation in the integrin-binding domain of TGF-β1 (50).
We have previously shown that integrin αvβ8 expression is increased in the airway fibroblasts of COPD patients, which correlates with the extent of airway wall fibrosis, and that integrin αvβ8–mediated activation of TGF-β by COPD fibroblasts mediates increased profibrogenic differentiation (40). Furthermore, we have demonstrated that airway epithelial squamous metaplasia, which is common in COPD airways, produces high levels of IL-1β, which drives airway remodeling by increasing profibrogenic differentiation of adjacent fibroblasts through increasing fibroblast expression of integrin αvβ8 and subsequent TGF-β activation (40).
Here we use in vivo mouse models that mimic airway remodeling of either COPD or asthma, coupled with conditional deletion in adult fibroblasts, to show that integrin αvβ8–mediated TGF-β activation plays a central and critical role in driving both airway fibrosis and inflammation. Furthermore, we define two αvβ8- and TGF-β–dependent chemokines secreted by lung fibroblasts, CCL2 and CCL20, and demonstrate that they are crucial mediators of DC trafficking. Finally, we extend this finding to COPD in humans by showing that human COPD lung fibroblasts have exaggerated αvβ8- and TGF-β–dependent chemokine responses to IL-1β and support increased human DC migration compared with normal lung fibroblasts.
Results
Intratracheal adenoviral IL-1β induces an airway remodeling phenotype associated with an increase in the integrin β8 subunit as well as TGF-β–responsive genes.
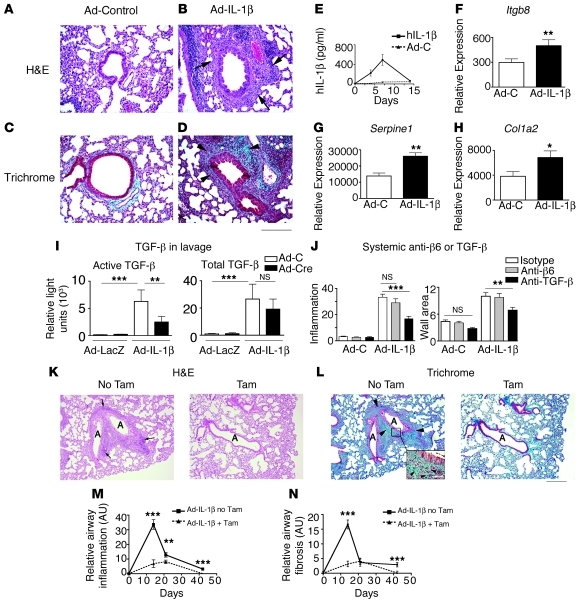
We have previously reported evidence using human COPD tissues that IL-1β increases lung fibroblast expression and function of the integrin αvβ8, the main activator of TGF-β in lung fibroblasts (40). We used intratracheal (IT) delivery of adenoviral IL-1β (Ad-IL-1β) both to establish the validity of the method as a model of airway remodeling in COPD in humans and to determine the role of the integrin β8 (Itgb8) subunit as a downstream mediator of IL-1β signaling in vivo. IT-Ad-IL-1β caused a marked increase in airway wall inflammation and fibrosis in mice after 7 days (Figure 1, A–D). This response correlated with peak expression of bioactive human IL-1β (Figure 1E) and a significant increase in mRNA for Itgb8 and the TGF-β–responsive gene plasminogen activator inhibitor (Serpine1) and collagen 1α2 (Col1a2) (Figure 1, F–H). Expression of the other major integrin subunit involved in TGF-β activation, Itgb6, was unchanged (data not shown). We were not able to assess levels of αvβ8 protein, since a suitable antibody to detect mouse αvβ8 does not currently exist (51, 52). These results demonstrate that IL-1β upregulates Itgb8 and suggest that this upregulation causes increased activation of TGF-β, which in turn induces expression of TGF-β–responsive extracellular matrix genes.
Figure 1. IT administration of adenovirus expressing human active IL-1β (IT-Ad-IL-1β) induces airway remodeling, which is dependent on αvβ8-mediated activation of TGF-β by fibroblasts.
(A–H) IT-Ad-IL-1β or Ad-control (Ad-C) was administered to adult mice to induce airway remodeling. (A–D) In Ad-IL-1β– (B) but not Ad-C–treated mice (A), 7 days after treatment, inflammation surrounds small airways (arrows, B), with increased collagen (arrowheads, D). Scale bar: 150 μm. (E) ELISA for IL-1β. (F) qPCR for Itgb8 (P = 0.01); (G) Serpine1 (P = 0.01); (H) Col1a2 (P = 0.04). (I) TGF-β bioassay of active and total TGF-β in BAL fluid from Itgb8fl/– mice (n = 6) 14 days after IT-Ad-IL-1β without or with IT-Ad-Cre. (J) Mice treated with IT-Ad-IL-1β with or without systemic administration of control antibody, anti-β6, or anti–TGF-β were evaluated at 14 days. (K–N) IT-Ad-IL-1β or Ad-C was administered to adult Itgb8fl/– mice expressing Cre-ER(T) under control of the fibroblast-specific Col1a2 promoter treated without or with tamoxifen (Tam). Tamoxifen causes specific deletion of Itgb8 in fibroblasts (Itgb8fko). (K and L) Inflammation (K, left) or fibrosis (L, left) is diminished in Itgb8fko mice (airway lumen, A). Arrows indicate inflammation in K; and arrowheads, collagen bundles, at low and high magnification (inset) in L in the presence of vehicle (K and L, left) or tamoxifen (K and L, right). Scale bar: 250 μm; 40 μm (inset). (M and N) Inflammation (M) or fibrosis (N) 7, 14, 21, or 42 days after IT-Ad-IL-1β without or with fibroblast-specific deletion of Itgb8. Shown are values minus the non–IT-Ad-IL-1β–treated controls. Raw data are shown in Supplemental Table 1. *P < 0.05, **P ≤ 0.01, ***P < 0.001.
Inactivation of Itgb8 in the airway is associated with a reduction in IL-1β–induced airway inflammation and fibrosis, which is due to decreased TGF-β activation.
We tested whether Ad-IL-1β induction of Itgb8 in the airway, in vivo, mediated increased TGF-β activation, airway inflammation, and fibrosis. We used the IT-Ad Cre deletion strategy to locally inactivate Itgb8 in postnatal Itgb8fl/– mice, since global germline inactivation of Itgb8 results in embryonic or perinatal lethality, and an anti–mouse αvβ8 neutralizing antibody does not exist. IT-Ad-IL-1β caused an approximately 50% increase in Itgb8 mRNA levels, and this increase was completely inhibited by Ad-Cre (Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI45589DS1). IT-Ad-IL-1β caused a 40-fold increase in active TGF-β levels in BAL fluid, and IT-Ad-Cre inhibited this increase by 61% (Figure 1I), while not affecting total (active plus latent) TGF-β levels (Figure 1I). The airway cell types demonstrating recombination from IT-Ad-Cre were airway epithelial cells, macrophages, and mesenchymal cells surrounding airways (Supplemental Figure 2). Coinjection of IT-Ad-Cre with Ad-IL-1β resulted in a significant decrease in airway wall inflammation at 7, 14, and 21 days, and in fibrosis at 14, 21, and 42 days after injection (Supplemental Figure 3 and Supplemental Table 1). This result was specific to conditional deletion of Itgb8, and not an artifact of Ad-Cre, since no differences in airway inflammation or fibrosis were seen in groups of wild-type mice treated with IT-Ad-IL-1β with or without Ad-Cre (Supplemental Table 1).
To test the contribution αvβ6, the other major integrin activator of TGF-β, or TGF-β itself in mediating airway inflammation and fibrosis, were treated wild-type mice with IT-Ad-IL-1β in the presence of anti-β6 or a pan–TGF-β isoform neutralizing antibody or control antibody. Treatment with anti–TGF-β markedly reduced airway inflammation and airway wall fibrosis after IT-Ad-IL-1β administration compared with control, while anti-β6 had no effect (Figure 1J and Supplemental Table 1). Taken together, these results demonstrate that TGF-β is required for the proinflammatory and profibrotic effects of IT-Ad-IL-1β and suggest that this effect is mainly due to the integrin αvβ8 and not αvβ6.
Conditional deletion of lung fibroblast αvβ8 inhibits Ad-IL-1β airway inflammation and fibrosis.
Our previous work using human COPD biospecimens led us to hypothesize that airway fibroblasts mediated the αvβ8-dependent proinflammatory and profibrotic effects of IL-1β. To determine the role of fibroblast expression of Itgb8 in airway remodeling, we used Col1a2-Cre-ER(T) mice to generate animals with tamoxifen-dependent deletion of Itgb8 in adult lung fibroblasts (Itgb8fko) (Supplemental Figure 2). Fibroblast-specific deletion inhibited IT-Ad-IL-1β–dependent airway inflammation (Figure 1, K and M) and fibrosis (Figure 1, L and N). These effects were specific to deletion of Itgb8 in fibroblasts and not due to effects of tamoxifen or Cre expression, since no effects were seen in wild-type mice (Supplemental Table 1). In addition, we used a mouse line that expresses Cre-ER(T) under control of the mouse keratin-14 promoter K14-Cre-ER(T), which drives expression in the airway basal epithelial cells, the airway epithelial cell type where ITGB8 is expressed in humans (40). Deletion of Itgb8 in airway epithelial basal cells had no significant effect on IT-Ad-IL-1β–induced airway inflammation or fibrosis (Supplemental Table 1 and ref. 53). No systemic effects of deletion of Itgb8 in adult mice were seen using Col-Cre-ER(T) and K14-Cre-ER(T) in necropsies performed at 6 months and more than 1 year, respectively. Tamoxifen-mediated deletion of Itgb8 reduced Itgb8 mRNA and TGF-β activation in cultured lung fibroblasts (Supplemental Figure 4). No differences were seen in Itgb8 levels in purified CD11c+ lung DCs (relative copy number, no tamoxifen: 6.2 ± 0.5; tamoxifen: 7.5 ± 0.4), reflecting the specificity of the Col1a2 promoter for fibroblasts. These results demonstrate that fibroblast Itgb8 expression regulates IL-1β–induced airway inflammation and fibrosis.
Conditional deletion of αvβ8 in lung fibroblasts leads to a reduction in both innate and adaptive immune responses in Ad-IL-1β–treated mice.
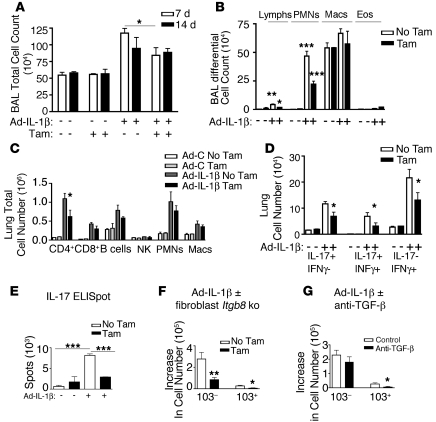
To understand the mechanistic role played by Itgb8 in regulating IL-1β–mediated airway inflammation, we next sought to determine which immune cell subsets in different anatomic lung compartments were affected by the loss of Itgb8 on fibroblasts. Examination of cells from BAL from IT-Ad-IL-1β–treated mice (7 days) revealed a significant decrease in total cell counts, and neutrophil and lymphocyte numbers from mice with conditional fibroblast deletion of Itgb8 (Itgb8fko), suggesting a role for Itgb8 in innate and adaptive immune responses (Figure 2, A and B). In whole lung digests, Ad-IL-1β induced a significant increase in numbers of CD4+ and CD8+ T cells, B cells, neutrophils, and macrophages (Figure 2C). However, with the exception of CD4+ T cells, the same increases were seen in Itgb8fko mice (Figure 2C). Specifically, IT-Ad-IL-1β induced significant increases in the numbers of several subsets of CD4+ T cells (IL-17+IFN-γ–, IL-17+IFN-γ+, IL-17–IFN-γ+ [Th1]), which were much greater than increases seen in Itgb8fko mice (Figure 2D). The decrease in IL-17–producing cells in Itgb8fko mice was reproduced by ELISpot assay (Figure 2E). Intracellular FoxP3 staining showed no differences in Treg percentages or numbers (data not shown). These results suggest a block in T cell priming in Itgb8fko mice that dampens subsequent Th1 and Th17 cell number and IL-17– and IFN-γ–dependent proinflammatory responses.
Figure 2. Fibroblast Itgb8 regulates innate and adaptive immune responses to IT-Ad-IL-1β.
(A and B) IL-β–dependent increases in lymphocyte and neutrophil numbers are inhibited in Itgb8fko mice. BAL total cell counts (A) and differential (B) 7 days after IT-Ad-IL-1β in the presence (No Tam) or absence (Tam) of Itgb8 on fibroblasts. (C) Multicolor cell surface staining for lung CD4+ and CD8+ T cells, B cells, NK cells, PMNs, and macrophages (Macs) 14 days after IT-Ad-IL-1β treatment without or with fibroblast-specific deletion of Itgb8 (n = 3). Shown are gated cell numbers. (D) Multicolor intracellular staining of CD4+ cells for IL-17 and/or IFN-γ. Three populations are identified: Th-17 cells (IL-17+IFN-γ–), IFN-γ+ Th-17 cells (IL-17+IFN-γ+), and Th1 (IL-17–IFN-γ+). Shown are cell numbers. (E) ELISpot for IL-17. Shown are number of spots/106 plated cells. (F and G) Multicolor cell surface staining for lung mDCs 14 days after IT-Ad-IL-1β treatment without or with fibroblast-specific deletion of Itgb8 (n = 6) (F) or with control IgG or systemic anti–TGF-β (n = 6) (G). Gating strategy for lung DCs is shown in Supplemental Figure 4, based on published methodology (54, 88). Shown is the increase in numbers of each subset relative to control Ad-LacZ plus corn oil or Ad-LacZ plus tamoxifen. *P < 0.05, **P < 0.01, ***P < 0.001.
Immature DCs migrate from the circulation to the airway, where they capture antigen, and then migrate to the MLN, where they prime specific T cell responses (54). Therefore, we sought to determine whether lung DCs were affected by fibroblast Itgb8 expression. Myeloid DCs (mDCs) that express CD11c represent the major airway DC subset promoting inflammation, while the plasmacytoid DCs are a minor population that is thought to attenuate inflammation (16). Two subsets of mDCs are located within the interstitium of the airway wall or within the airway epithelium, with the former expressing CD11b but not CD103 (103– DCs) and the latter not expressing CD11b but expressing CD103 (103+ DCs) (16). The 103– DC subset is the predominant subset relative to 103+ DCs (16). Both mDC subsets were increased by Ad-IL-1β and were significantly reduced in Itgb8fko mice (Figure 2F; gating shown in Supplemental Figure 5). Treatment with anti–TGF-β produced a result similar to that in Itgb8fko mice in reducing IT-Ad-IL-1β–mediated increases in numbers of 103+ DCs (Figure 2G). Very few plasmacytoid DCs were identified, and their numbers were not increased by IT-Ad-IL-1β treatment (data not shown). These results suggest that DC numbers are positively regulated by fibroblast αvβ8–mediated TGF-β activation, which leads to increased T cell–mediated immune responses.
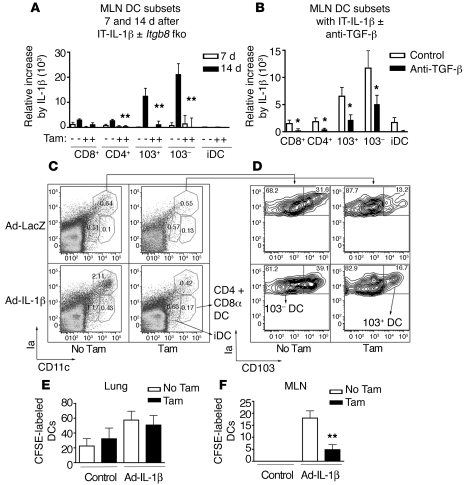
Fibroblast-specific deletion of Itgb8 alters IT-Ad-IL-1β–stimulated DC migration to the MLN.
We next determined whether DC numbers in the MLN were affected by the loss of Itgb8 on fibroblasts. The MLN contains the mDCs (103+ and 103– DCs), which originate in the airway, but also contains resident conventional DC subtypes CD8α+, CD4+, and CD8α–CD4– and inflammatory DCs. Conventional DCs can potentially acquire antigen from afferent lymphatics or from transferred antigen from itinerant mDCs from the lung, which could also lead to T cell priming (54). Therefore, we characterized the mDCs, conventional DCs, and inflammatory DCs in the MLN after IT-Ad-IL-1β treatment. In the MLN, the numbers of both 103+ and 103– DCs dramatically increased 14 days following IT-Ad-IL-1β treatment, with smaller increases in CD4+ and CD8α+ DC subsets (Figure 3A). These increases in CD4+, 103+ and 103– DCs were significantly inhibited in Itgb8fko mice (Figure 3, A–D) or pretreatment with anti–TGF-β (Figure 3B). Lung DCs are thought to undergo maturation, which facilitates migration to the MLN, a process which in other models is associated with acquisition of increased MHC-II, CCR7, and costimulatory (i.e., CD80 and CD86) molecule expression (16, 55). In the IT-Ad-IL-1β model, we could not detect significantly altered expression of CD80 or CD86 in lung mDCs (data not shown), and no changes in MHC-II expression by lung or MLN 103+ or 103– DCs were detected in Itgb8fko mice (Supplemental Figure 6). To determine whether the decreases in DC number in the lung and MLN were due to decreased DC migration to the lung or from the lung to the MLN, mice with or without fibroblast deletion of Itgb8 were injected i.v. with CFSE-labeled bone marrow–derived DCs (BMDCs), and the labeled cells were identified in the lung and MLN. There was an increase in labeled cells in both the lung and MLN after IT-Ad-IL-1β treatment, but only in the MLN was this increase significantly inhibited in Itgb8fko mice (Figure 3, E and F). Taken together, these results demonstrate that fibroblast αvβ8–mediated TGF-β activation is not required for DC maturation, but is required for DC migration to the MLN.
Figure 3. Fibroblast deletion of Itgb8 or systemic neutralization of TGF-β inhibits the IT-Ad-IL-1β–induced increase in DC subsets in the MLN due to decreased DC trafficking from the lung to the MLN.
(A) Time course of IT-Ad-IL-1β–induced increases in DCs in the MLN and dependence on Itgb8 (n = 6, each time point). Col-Cre-ER(T);Itgb8fl/– mice were treated with IT-Ad-IL-1β with or without tamoxifen and the MLN harvested 7 or 14 days later. Gating strategy and representation of CD8α+, CD4+, 103+, 103– and inflammatory DCs are identical to those in Ballesteros-Tato et al., with the exception that CD4–CD8α– DCs are not shown (54). Shown is the increase in cell number relative to the controls. (B) MLN DC subpopulations of IT-Ad-IL-1β–treated mice (14 days) without and with systemic anti–TGF-β (n = 6). Shown is the increase in cell number minus the controls. (C) MLN gating strategy (MHC-II [Ia] versus CD11c), with percentage of cells shown within each gate. (D) The percentages of gated MHC-IIhiCD11chi DCs positive or negative for 103 are shown. Indicated in C are gates used for identification of CD4+ and CD8α+ conventional DCs and inflammatory DCs (iDC). (E and F) CFSE-labeled BMDCs (2 × 106/ mouse) were injected into the lateral tail vein 12 days after IT-Ad-IL-1β or Ad-C treatment and 36 hours prior to lung (E) and MLN (F) harvest of mice without or with fibroblast deletion of Itgb8 (n = 9). Numbers of MHC-IIhiCD11c+ CFSE-labeled cells are shown. *P < 0.05, **P < 0.01.
IL-1β stimulates an Itgb8-dependent profibrogenic phenotype associated with increased CCL2 and CCL20 expression in the mouse lung.
To determine the mechanism of αvβ8-dependent DC migration to the MLN, we sought to determine αvβ8-dependent cytokine and chemokine production in vivo and in vitro. Lung expression of inflammatory mediators following IT-Ad-IL-1β treatment was assessed using real-time quantitative PCR (qPCR). IT-Ad-IL-1β significantly increased Il17a, Il17f, Ccl2, Ccl20, and Infg, and Il17a, Ccl2, Ccl20, and Infg were significantly decreased in Itgb8fko mice (Figure 4A). In addition, a cytokine antibody array was used to evaluate the changes in cytokine expression in lung homogenates from pooled groups of mice with deletion of Itgb8 from all airway cells by Ad-Cre deletion. Airway deletion of Itgb8 caused decreases in numerous lymphokines (IL-4, IL-13, IL-17, IFN-γ), proinflammatory cytokines (IL-1α, KC, TNF-α), growth factors (GM-CSF, CSF-1), and chemokines (CCL1, CCL2, CCL3, CCL5, CCL9, CCL25, CXCL5, CXCL13, and XCL1 [CCL20 was not printed on the array]) (Supplemental Figure 7). Cytokine arrays were performed in parallel from pooled supernatants of IL-1β–treated wild-type or Itgb8-deficient cultured fibroblasts (Supplemental Figure 7). CCL2 and CCL9 were both highly expressed in lung homogenates, but CCL2 was the only factor that was decreased in both by absence of Itgb8 (Supplemental Figure 7).
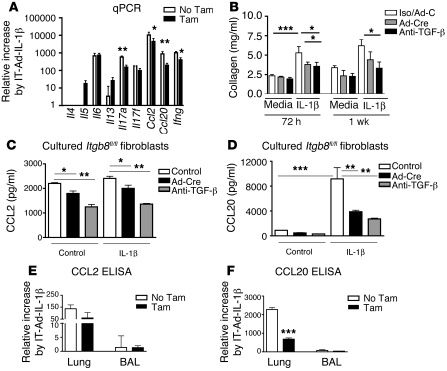
Figure 4. Cytokine profiling and collagen expression analysis reveal that IT-Ad-IL-1β induces an αvβ8-dependent profibrogenic and proinflammatory phenotype.
(A) qPCR of mouse lung homogenates 14 days after IT-Ad-IL-1β treatment without or with fibroblast deletion of Itgb8 (n = 6). Shown are copy number increases relative to control mice. (B) IL-1β treatment of mouse lung fibroblasts induces a sustained induction of collagen expression that is dependent on Itgb8 and TGF-β. Mouse lung fibroblasts were cultured from lungs of Itgb8fl/fl mice and treated with Ad-Cre or Ad-LacZ (Ad-C) (2.5 × 106 PFU) overnight prior to treatment with recombinant IL-1β (1 ng/ml) with isotype control mAb or anti–TGF-β (40 μg/ml). Cells were lysed 72 hours or 1 week later, and Sircol assay was performed to determine collagen concentration. CCL2 (C and E) and CCL20 (D and F) as measured by ELISA from Itgb8fl/fl mouse lung fibroblasts treated with Ad-Cre or anti–TGF-β (C and D) or from whole lung homogenates or BAL from Col-CreER(T);Itgb8fl/– mice 14 days after IT-Ad-IL-1β without or with tamoxifen (E and F). *P < 0.05, **P < 0.01, ***P < 0.001.
We next sought to confirm the role of fibroblasts in altered chemokine secretion. We hypothesized that a switch of fibroblasts to a profibrogenic phenotype would be associated with increased chemokine secretion. IL-1β treatment of primary cultures of mouse lung fibroblasts caused a sustained Itgb8-dependent change to a profibrogenic fibroblast phenotype, since IL-1β significantly induced collagen protein expression at 72 hours, and this increase persisted for at least 1 week and was significantly decreased by Ad-Cre–mediated deletion of Itgb8 or treatment with a pan–TGF-β neutralizing antibody (Figure 4B). CCL2 was highly expressed (8.8 ± 0.7 ng/ml/106 cells) by mouse fibroblasts, but was not significantly increased by IL-1β (9.6 ± 0.6 ng/ml/106 cells). However, CCL2 was significantly reduced by 20% following deletion of Itgb8 and 40% with treatment using anti–TGF-β (Figure 4C). In contrast, CCL20 was not as highly expressed as CCL2 by untreated fibroblasts (3.6 ± 0.7 ng/ml/106 cells), but was increased 10-fold by IL-1β (36.7 ± 11.2 ng/ml/106 cells). The majority (60%) of this IL-1β–induced expression of CCL20 was prevented by deletion of Itgb8, and 70% by treatment with anti–TGF-β (Figure 4D). ELISA of lung homogenates or BAL of IT-Ad-IL-1β–treated mice confirmed the high expression of CCL2 (458 ± 25 pg/ml lung; 5.7 ± 4.1 pg/ml BAL fluid) and CCL20 (2,398 ± 112 pg/ml lung; 82 ± 41pg/ml BAL fluid). This represented a significant IL-1β–mediated increase in CCL2 (P = 0.0031) and CCL20 (P < 0.001) in lung homogenates compared with control. The increase in CCL20 was significantly inhibited in lung homogenates of Itgb8fko mice (Figure 4F). These data demonstrate that IL-1β induces an αvβ8-mediated TGF-β–dependent profibrogenic phenotype and that this phenotype coincides with increased αvβ8-mediated TGF-β–dependent CCL20 expression in the lung.
Purified CD11c+ lung DCs showed a significant increase in Ccr7 after IT-Ad-IL-1β, and there was no significant effect on Ccr2, Ccr6, or Ccr7 expression by deletion of Itgb8 on fibroblasts (Supplemental Figure 6). These results suggest that Itgb8 expression on fibroblasts regulates DC migration through αvβ8-mediated TGF-β activation, which results in enhanced paracrine secretion of CCL20 and not through alteration of DC maturation.
Airway remodeling caused by ovalbumin challenge is also dependent on fibroblast Itgb8 expression.
Airway epithelial cells are exposed to the environment and respond to “danger” signals by engaging specific pattern recognition receptors such as NOD-like receptors or Toll-like receptors. These receptors assemble intricate signaling complexes called inflammasomes that mediate caspase-1 cleavage, which enhances the processing and secretion of bioactive IL-1β (56). Ovalbumin-challenged mice displayed significantly increased activation of caspase-1 in BAL cells (the majority of which are macrophages), which correlated with a significant increase in lung IL-1β secretion and Itgb8 mRNA (Supplemental Figure 8). These data support other reports that IL-1β is important for the ovalbumin-induced asthma phenotype (13).
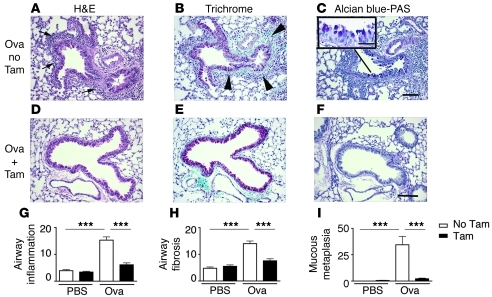
In light of these data, we tested the hypothesis that Itgb8fko mice were protected from ovalbumin-induced airway remodeling using a mouse model of chronic allergic asthma (57). Chronic ovalbumin-mediated allergic airway remodeling (inflammation, fibrosis, and mucin production) was significantly reduced by deletion of Itgb8 in fibroblasts (Figure 5, A–I), which correlated with decreased total cell count in BAL (Figure 6A) and a significant decrease in eosinophils (Figure 6B). To understand the role of Itgb8 in allergen-induced immunity, we evaluated DC numbers in ovalbumin-challenged Itgb8fko mice. Compared with controls, ovalbumin-dependent increases in DC numbers in the lung and MLN were reduced significantly in Itgb8fko mice (Figure 6, C and D). Intranasal ovalbumin conjugated to FITC was used as a tracer to measure DC antigen uptake and DC migration to the MLN. The number of labeled cells in the lung dramatically increased in ovalbumin-challenged relative to control mice, but this increase was not significantly decreased in Itgb8fko mice (Figure 6E). In contrast, in the MLN, the ovalbumin-challenged mice had an increase in labeled DCs, which was not seen in Itgb8fko mice (Figure 6F).
Figure 5. Ovalbumin-induced airway remodeling requires Itgb8 expression by fibroblasts.
Chronic ovalbumin sensitization causes airway inflammation, wall fibrosis, and increased mucin production (A–C), which is reduced by the absence of Itgb8 on fibroblasts (D–F). (A–I) Histologic (A–F) and morphometric (G–I) analysis of airway inflammation (A, D, and G); fibrosis (B, E, and H); and mucin (C, F, and I). Arrows in A indicate airway inflammation, and arrowheads in B, fibrosis. Scale bars: 150 μm; 40 μm (inset). ***P < 0.001.
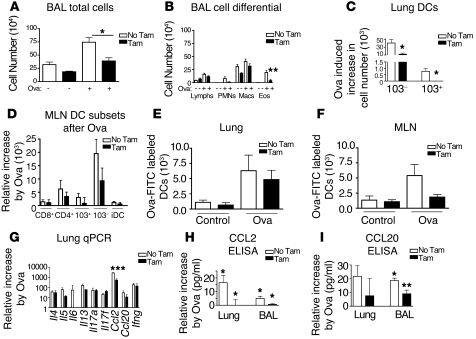
Figure 6. Ovalbumin-induced trafficking of DCs to the MLN as well as CCL2 and CCL20 expression require Itgb8 expression by fibroblasts.
(A) BAL cell counts and (B) cell differential of lymphocytes (Lymphs), PMNs, macrophages (Macs), and eosinophils (Eos) from BAL of mice after chronic ovalbumin challenge (n = 6). (C and D) Multicolor cell surface staining for lung DCs (C) or DCs from MLN (D) from mice after ovalbumin sensitization and challenge without or with fibroblast-specific deletion of Itgb8 (n = 6). Gating strategies were identical to those in Figure 2, F and G. (E and F) Ovalbumin conjugated to FITC was introduced intranasally 36 hours prior to lung (E) and MLN (F) harvest, and OVA-FITC–labeled cells were identified using gating strategies identical to those shown in Figure 3, C and D (n = 8). (G) qPCR analysis of cytokines present in lung after chronic ovalbumin challenge without or with fibroblast-specific deletion of Itgb8 (n = 6). Shown are copy number increases relative to control mice. (H and I) Ovalbumin induction of CCL2 (H) and CCL20 (I) as measured by ELISA on whole lung homogenates or BAL from Col-CreER(T);Itgb8fl/– mice after chronic ovalbumin challenge without or with tamoxifen (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001.
To determine the role of fibroblast Itgb8 in cytokine and chemokine expression in the ovalbumin model, we measured mRNAs for Il4, Il5, Il6, Il13, Il17a, Il17f, Ccl2, Ccl20, and Ifng using qPCR (n = 6) and found that they were all increased following chronic ovalbumin challenge, whereas mRNA for Ccl2 was significantly decreased by the loss of Itgb8 on fibroblasts (Figure 6G). There was a significant increase in CCL2 in the lung (P = 0.03) and BAL (P = 0.03), and CCL20 protein levels in BAL (P = 0.02) in chronic ovalbumin-challenged compared with control mice, and these increases were significantly decreased in Itgb8fko mice (Figure 6, H and I). These findings extend the data in the Ad-IL-1β model to allergen-induced inflammation and support a more general role for fibroblast αvβ8–mediated activation of TGF-β in the regulation of CCL2- and/or CCL20-dependent DC migration in airway remodeling.
IL-1β enhances αvβ8-dependent TGF-β activation, collagen expression, and proinflammatory gene expression in COPD compared with normal lung fibroblasts.
To test the relevance of our mouse model findings in humans, we extended our studies to human normal and COPD fibroblasts. Normal and COPD fibroblasts were treated with IL-1β to induce expression of ITGB8. IL-1β increased ITGB8 mRNA expression in both normal lung fibroblasts and in COPD lung fibroblasts, but the induction of ITGB8 mRNA was significantly greater in COPD fibroblasts (Figure 7A). We next determined whether the increased expression of ITGB8 by COPD relative to normal fibroblasts correlated with an induction of a profibrogenic phenotype, as measured by collagen production. Indeed, IL-1β caused a significant increase in collagen protein expression by both normal and COPD fibroblasts. This, increase was sustained, lasting at least 1 week after IL-1β treatment. One week after IL-1β treatment, collagen production was significantly increased by COPD compared with normal lung fibroblasts and was dependent on αvβ8-mediated TGF-β activation (Figure 7B). The IL-1β–mediated enhanced profibrogenic phenotype correlated with a significant increase in total TGF-β activation by COPD compared with normal fibroblasts, which was inhibited by neutralizing anti-β8 or anti–TGF-β (Figure 7C). To determine whether the profibrogenic phenotype was associated with increased cytokine and chemokine production we performed cytokine array analysis of pooled (n = 5) supernatants from IL-1β–treated normal and COPD fibroblasts. Three candidate chemokines, CCL2, CCL7, and CCL20, were highly expressed in COPD compared with normal fibroblasts, and were blocked substantially by anti-β8 (Figure 7D and Supplemental Figure 9). ELISA from supernatants of IL-1β–treated fibroblasts demonstrated an insignificant IL-1β–mediated increase in CCL2 expression by normal fibroblasts, which was not significantly enhanced in COPD compared with normal lung fibroblasts and not significantly blocked by treatment with either anti-β8 or anti–TGF-β (Figure 7E). In contrast, IL-1β markedly induced CCL20 in normal fibroblasts, and this induction was dramatically increased in COPD fibroblasts and inhibited by either anti-β8 or anti–TGF-β (Figure 7F). These data using human COPD fibroblasts corroborate findings in Ad-IL-1β–treated mice demonstrating that IL-1β induces an αvβ8-mediated TGF-β–dependent profibrogenic phenotype, which coincides with increased αvβ8-mediated TGF-β–dependent CCL20 expression.
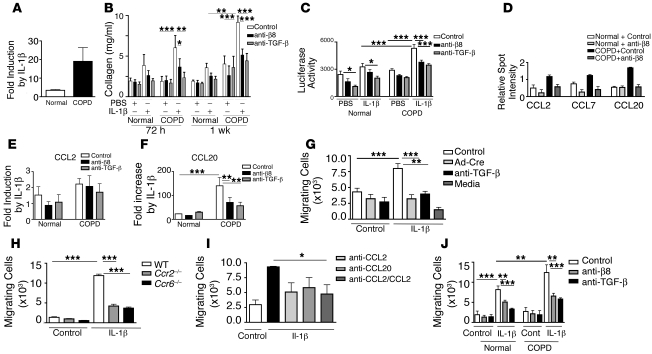
Figure 7. COPD fibroblasts have an enhanced profibrogenic phenotype and promote increased DC migration after IL-1β stimulation due to increased αvβ8-dependent TGF-β activation.
(A) ITGB8 determined by qPCR from normal and COPD fibroblasts treated with IL-1β (n = 5, P = 0.03). (B) Collagen in cell lysates from normal and COPD fibroblasts (n = 5) treated with or without IL-1β with isotype control, anti-β8, or anti–TGF-β. (C) TGF-β activation was measured by bioassay (arbitrary light units). (D) Selected human cytokine antibody array data (relative spot intensity). See Supplemental Figure 9 for complete array data. ELISA for CCL2 (E) and CCL20 (F). Results are expressed as fold increase compared with non–IL-1β–treated cells (n = 5). (G) Murine BMDC migration to fibroblast conditioned media (CM) or serum-free DMEM (Media). Mouse Itgb8fl/fl fibroblasts were treated with or without IL-1β in the presence of Ad-Cre, anti–TGF-β, or isotype control for 48 hours (n = 6). (H) Migration of wild-type, Ccr2–/–, or Ccr6–/– BMDCs (n = 3). (I) Migration of wild-type BMDCs to untreated or IL-1β–treated mouse lung fibroblast CM in the presence of anti-CCL2, anti-CCL20, or both antibodies combined (n = 3). (J) Migration of human cord blood–derived DCs to unstimulated (Control) or IL-1β–stimulated isotype control–, anti-β8–, or anti–TGF-β–treated pooled normal and COPD fibroblast CM (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001.
IL-1β enhances fibroblast-mediated αvβ8-dependent DC migration, which is exaggerated in human COPD fibroblasts.
To address the mechanism underlying altered DC trafficking mediated by lung fibroblasts, we developed an in vitro model of DC migration, which we could then extend to human studies. BMDCs demonstrated increased migration to conditioned media of Itgb8fl/fl mouse lung fibroblasts that had been treated with IL-1β. This increase was Itgb8 and TGF-β–dependent, since DC migration was significantly decreased using conditioned media from Ad-Cre– or anti–TGF-β–treated fibroblasts (Figure 7G). BMDCs from mice deficient for Ccr2 (the receptor for CCL2) or Ccr6 (the receptor for CCL20) migrated poorly to IL-1β–treated fibroblast conditioned media (Figure 7H). Neutralizing anti-CCL2, anti-CCL20, or both antibodies combined significantly inhibited IL-1β–dependent DC migration to fibroblast conditioned media (Figure 7I). Taken together, these results demonstrate that the lung fibroblast αvβ8–dependent secretion of CCL2 or CCL20 in response to IL-1β is sufficient to mediate DC migration.
To determine the relevance of fibroblast-directed DC migration in humans, we next tested the ability of human DCs to migrate to conditioned media from IL-1β–treated COPD compared with normal fibroblasts. DC cell migration to conditioned media from IL-1β–treated COPD fibroblasts was significantly greater (Figure 7J). The migration of human DCs to untreated and IL-1β–treated normal and COPD fibroblast conditioned media could be inhibited equivalently by pretreatment of fibroblasts with either anti-β8 or anti–TGF-β (Figure 7J). Taken together, these data demonstrate that IL-1β induces an αvβ8-mediated TGF-β–dependent profibrogenic phenotype in human fibroblasts, with an associated increase in CCL20 expression. Furthermore, IL-1β–induced ITGB8 expression and subsequent αvβ8-mediated TGF-β activation–dependent collagen production, chemokine secretion, and DC migration were enhanced in COPD compared with normal fibroblasts.
DC accumulation in human small airways increases by stage of COPD and correlates with integrin β8 subunit staining.
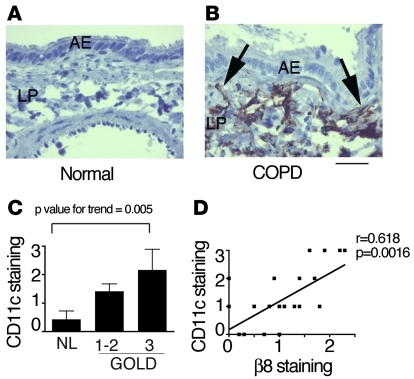
DCs were identified by morphology and CD11c immunostaining in the small airways of a cohort of COPD patients. Only rare DCs were identified within the epithelium and lamina propria of small airways from normal patients, while numerous DCs were identified in COPD small airways (Figure 8, A and B). DC immunostaining in the lamina propria was significantly enhanced with increasing severity of COPD (Figure 8C). CD11c+ DC cells were distinctive in their appearance within the lamina propria, as they possessed multiple extended cell processes emanating from the cell body. In contrast, immunostaining for integrin β8 was concentrated in spindle cells with elliptical narrow curved nuclei and a single extended cell process (40). Immunostaining for β8 and CD11c correlated significantly (Figure 8D).
Figure 8. DCs are increased in the airway wall of COPD compared with normal patients, which correlates with disease severity and β8 integrin immunostaining.
Stained sections were from a COPD cohort classified according to Global Initiative on Obstructive Lung Disease (GOLD) criteria (89), with GOLD stage 1–2 corresponding to mild to moderate and 3–4 to severe obstructive disease. Shown are sections of normal small airway wall (A) or COPD small airway wall (B) stained with ant-CD11c. DCs were identified by positive CD11c staining and the presence of long extended cellular processes (arrows in B). Airway epithelial cells (AE) and lamina propria (LP) are indicated. Scale bar: 20 μm. (C) CD11c immunostaining was graded on a 1–3 scale and compared according to disease status by GOLD stage. Normal (NL), n = 8; COPD, n = 13. ANOVA revealed a statistically significant difference (P = 0.02), and a P value for trend of 0.005. (D) CD11c immunostaining scores significantly correlated with β8 immunostaining scores (r = 0.6188, P = 0.0016).
Discussion
Our data from physiologically relevant models collectively provide functional and mechanistic in vivo evidence for a central role of the fibroblast in regulating pathologic innate and adaptive immunity. Specifically, conditional deletion of the TGF-β–activating integrin αvβ8 selectively in adult fibroblasts reduced IL-1β–induced and allergen-induced airway remodeling and secretion of CCL2 and CCL20. This cascade of events was associated with decreased DC numbers in the draining lymph node and subsequently muted adaptive immunity. We extended these studies to human disease by demonstrating that enhancement of αvβ8-mediated TGF-β activation, CCL20 expression, and DC migration is a feature of COPD fibroblasts. To further support our hypothesis that enhanced integrin β8–dependent DC trafficking in human airways may facilitate the abnormal persistent innate and adaptive immune response that characterizes human COPD, we found that increased DC accumulation in COPD airways correlates with both the stage of COPD and β8 expression.
The most likely explanation for reduction in the number of migratory DCs in the lung and lymph node that we observe with fibroblast Itgb8 deletion is altered chemokine secretion from fibroblasts. We determined that CCL2 and CCL20 are secreted at high levels by IL-1β–stimulated lung fibroblasts — 1,000 and 30 times, respectively, the levels reported to be released by cultured human airway epithelial cells stimulated with the same dose of IL-1β, on a per-cell basis, suggesting a dominant role for fibroblasts as a source of these chemokines (58, 59). Our data demonstrating αvβ8-dependent CCL2 and CCL20 expression levels in lung homogenates and BAL suggest that the respective roles for CCL2 and CCL20 may differ in different disease models, with CCL2 and CCL20 playing larger roles in allergic asthma and COPD, respectively.
Fibroblasts have been hypothesized to modulate immune responses (60), but clear in vivo demonstration of this function, and the mechanisms underlying this modulation, are lacking. Previous studies have shown that fibroblastic reticular cells, which are fibroblast-like cells of lymphoid organs, secrete chemokines and thus are thought to act as “organizers” of lymphoid tissues (61). Cytokines are released by fibroblasts in patterns that distinguish fibroblasts from different anatomic sites and disease states (33, 34, 60). These studies validate and extend recent in vitro studies suggesting that cultured skin fibroblasts stimulated with TNF/IL-1 can promote DC migration or increase the maturation of LPS-stimulated DCs by a complex feedback mechanism involving IL-1β secreted from DCs (62, 63). Our data now demonstrate that in physiologic models, fibroblast Itgb8 is required for lung DC migration. We could not detect a role for Itgb8 on fibroblasts in lung DC maturation.
The integrins αvβ6 and αvβ8 account for the majority of TGF-β activation in murine genetic models (49). However, fibroblast deletion of Itgb8 appeared to account for the majority of protection against airway remodeling in chronic asthma and Ad-IL-1β models. In addition, Ad-IL-1β did not increase the expression of Itgb6, and a neutralizing anti-αvβ6 antibody had no effect on Ad-IL-1β–mediated airway remodeling. These findings support genetic studies suggesting that Itgb6 is not an asthma or a COPD target, as mice deficient in Itgb6 develop airway hyperresponsiveness and emphysema (64, 65). The striking role for Itgb8 in promoting airway remodeling and lack of a role for Itgb6 may be due to differences in cell type distribution or differences in mechanisms of TGF-β activation. Integrin αvβ8 is expressed in the lung by epithelial cells and fibroblasts, while αvβ6 is expressed exclusively by epithelial cells (66). Integrin αvβ8 binds to latent TGF-β, and following the recruitment of a transmembrane metalloprotease, MMP14, active TGF-β is proteolytically released as a paracrine factor (48). In contrast, the integrin αvβ6 binds to latent TGF-β only on epithelial cells and alters the conformation of the LAP, and can only present active TGF-β to adjacent cells (67). Therefore, it appears that localized epithelial activation of TGF-β is insufficient, while fibroblast activation and paracrine secretion of TGF-β are required for airway remodeling.
IT-Ad-IL-1β increased active TGF-β in the BAL fluid, which was blocked by 61% with simultaneous injection of Ad-Cre. This incomplete blockade was seen despite efficient reduction of Itgb8 mRNA by Ad-Cre. Because suitable reagents are not available to assess mouse αvβ8 protein, we cannot exclude that residual αvβ8 protein remains after Ad-Cre–mediated deletion. Alternatively, other mechanisms may contribute to TGF-β activation within the alveolar lining fluid. For instance, previous literature suggests that additional integrins (i.e., αvβ3 and αvβ5) mediate activation of TGF-β in cultured fibroblasts under specific circumstances (68-72). However, αvβ3 and αvβ5 bind poorly to latent TGF-β relative to αvβ8 and αvβ6 (48, 73), and mice with deficiency of αvβ3, αvβ5, or αvβ3 and αvβ5 combined develop normally and have no evidence of TGF-β insufficiency (74). Finally, in our models, αvβ8 appears to account for the majority if not all of TGF-β effects, since Itgb8fko mice have a protective effect equal to if not a greater than a pan–TGF-β blocking antibody from IL-1β–mediated inflammation and fibrosis. Therefore, neither αvβ3, αvβ5, nor αvβ6 compensate for loss of αvβ8 in airway remodeling, in vivo.
Fibroblasts can be derived by epithelial-mesenchymal transition, from circulating fibrocytes, or from resident fibroblasts (75). We have not formally addressed the source of the fibroblasts in airway remodeling in this study. However, resident fibroblasts are likely a major source of fibroblasts in airway remodeling, because we saw no obvious epithelial or hematopoietic cell recombination in Col-Cre-ER(T) mice. Furthermore, primary cultures of lung fibroblasts, which are derived from resident fibroblasts, possess all the functional characteristics to explain the airway remodeling findings in vivo; they increase expression of αvβ8 in response to IL-1β, support αvβ8-mediated TGF-β activation, and secrete CCL2, CCL20, and collagen in an αvβ8-dependent manner.
Airway fibroblasts interact not only with immune cells, but also with adjacent airway epithelial cells to maintain homeostasis. Airway epithelial cells have recently been implicated in modulating adaptive immune responses through TLR4-dependent pathways. This recent study has shown that TLR4 expressed by airway epithelial cells is required for LPS- or allergen-induced pathology, including increases in lung DC numbers, maturation, migration to MLN, and CCL2 and CCL20 expression (76). The intriguing similarity of these findings to the findings that we present here support the hypothesis that excessive pattern recognition receptor engagement plays a significant role in airway remodeling in COPD and chronic asthma by caspase-1–mediated increased bioactive IL-1β secretion by airway epithelial cells or macrophages; this leads to the induction of αvβ8 expression and subsequent TGF-β activation by adjacent fibroblasts, which affects fibroblast chemokine secretion, DC migration, and adaptive immunity (Figure 9). However, we cannot exclude that other cytokines also influence αvβ8 expression, or that IL-1β influences αvβ8 activity (i.e., through affecting integrin activation state, or localization or activity of MMP14).
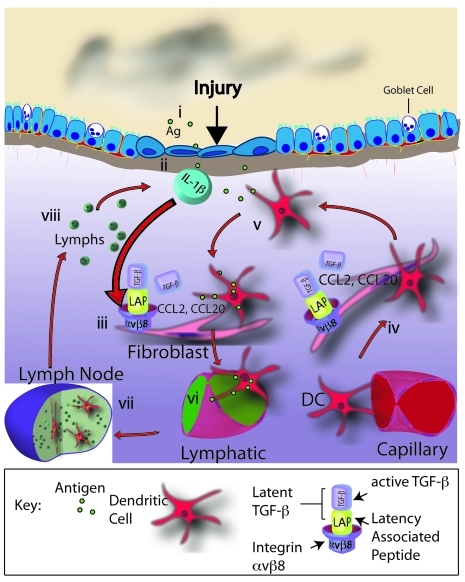
Figure 9. Model for fibroblast αvβ8–directed DC migration in airway remodeling.
(i) Injury (i.e., tobacco smoke) or antigen (Ag) leads to inflammasome activation and caspase-1–mediated cleavage and release of active IL-1β. (ii) IL-1β is released by epithelial cells or macrophages. (iii) IL-1β increases αvβ8 expression by fibroblasts, which leads to increased conversion of latent to active TGF-β. (iv) Active TGF-β binds to fibroblast TGF-β receptors, leading to increased TGF-β–dependent chemokine (CCL2, CCL20) secretion and increased recruitment of DCs from the peripheral circulation. (v) Immature DCs encounter Ag within the epithelium or lamina propria and migrate toward fibroblasts via αvβ8-dependent chemotactic gradients. (vi) Mature DCs gain access to draining lymphatics. (vii) Mature DCs enter the draining MLN, where they prime adaptive immune responses. (viii) Differentiated memory/effector lymphocytes migrate to the airway to establish pathologic inflammation.
IT delivery of active IL-1β by the adenoviral method bypasses the requirement for IL-1β activation by the inflammasome. We have exploited the Ad-IL-1β model to mechanistically dissect the pathway that is downstream of IL-1β activation and common to the pathogenic environmental stimuli that lead to airway remodeling in COPD and chronic asthma (12). The Ad-IL-1β model has many physiologically relevant features, since it recapitulates most of the morphologic and immunologic characteristics of airway remodeling in COPD in humans. Specifically, there are increases in airway wall fibrosis and airway wall inflammation; and a COPD-like innate and adaptive immune response, with increased neutrophils, mDCs, and Th17 and Th1 cells and increased production of IL-17, CCL2 and CCL20 (20).
The use of this system has provided insights into the proinflammatory role of Itgb8 and TGF-β in the lung and in COPD. TGF-β has recently been implicated as a driver of pathologic immunity through its critical role in the development of Th17 cells. This important subset of CD4+ T cells produces IL-17(A,F), a cytokine that has been implicated in the pathogenesis of asthma, COPD, psoriasis, arthritis, diabetes, multiple sclerosis, and inflammatory bowel disease (77–82). Two recent studies demonstrate that the loss of αvβ8 on DCs, which are crucial APCs, protects against experimental autoimmune encephalitis through inhibition of Th17 cell differentiation (51, 83). In the fibroblast conditional deletion model, there is no evidence of Cre-mediated deletion of Itgb8 by purified lung DCs; instead, loss of Itgb8 on fibroblasts indirectly impairs DC function through decreased trafficking to lymph nodes. Thus, Itgb8 participates in proinflammatory responses through two distinct mechanisms in different conditional deletion models, one that indirectly and one that directly influences the functional capacity of DCs to prime a pathologic immune response. Our data suggest that inhibition of integrin αvβ8–mediated TGF-β activation by lung fibroblasts represents a novel therapeutic target for airway remodeling in both COPD and asthma.
Methods
Animals.
The UCSF Committee for Animal Research reviewed and approved this study. All animals were housed in air-filtered, temperature-controlled units (20 ± 2°C) with food and water ad libitum. Male 7-week-old CD-1 mice (Charles River) were used for initial adenoviral experiments, and all other experiments were performed using C57BL/6 mice (with the few exceptions noted below) bred in the UCSF animal facility. Mice expressing a chimeric gene encoding the Cre-ER(T) fusion protein, which allows for tamoxifen-dependent recombination under the control of a fibroblast-specific regulatory sequence from the pro-alpha 2(I)collagen gene (Col-Cre-ER[T] mice, gift of Benoit de Crombrugghe, M.D. Anderson Cancer Center, Houston, Texas, USA, and Christopher Denton, Center for Rheumatology, Royal Free and University College Medical School, London, United Kingdom) were intercrossed with mice expressing one functioning Itgb8 allele with exon 4 flanked by LoxP sites (Itgb8Tm2Lfr, Itgb8fl/+) and a non-functioning Itgb8 allele with exon 4 deleted by homologous recombination (Itgb8Tm1Lfr, Itgb8–/+) (84–86). Progeny were intercrossed to create the following lines, which were used for experiments or intercrossed: Col-Cre-ER(T);Itgb8fl/–,Col-Cre-ER(T);Itgb8fl/fl, Itgb8fl/fl, and Itgb8fl/– (Itgb8–/– mice show embryonic or perinatal lethality on the C57BL/6 background) (86). Similar intercrosses were generated using keratin 14-Cre-ER(T) mice ([KRT14-creERT]20Efu/J, The Jackson Laboratory, CD-1 background), which show ligand-dependent recombination in proximal airway basal cells (53). Ccr2–/– mice were a gift of Israel Charo (UCSF); Ccr6–/– (B6.129P2-Ccr6tm1Dgen/J) mice and Rosa-LacZ reporter mice (B6.129S4-Gt[ROSA]26Sortm1Sor/J) were obtained from The Jackson Laboratory. BMDCs were cultured from adult mouse femurs, using a GM-CSF stimulation protocol, by the method of Inaba et al. (87). Detailed BMDC methodology along with cell tracing studies, intratracheal injections, tamoxifen-mediated recombination, staining for β-galactosidase, the chronic ovalbumin model organ, harvesting, BAL, airway morphometry, immunohistochemistry, genotyping, primer sequences, cell lines, reagents, flow cytometry, and migration assays are described in Supplemental Methods and Supplemental Table 2. Informed consent was obtained from tissue donors as part of an approved ongoing research protocol by the UCSF Committee on Human Research.
Statistics.
All data are reported as mean ± SEM. Comparisons between 2 different groups were determined using 2-tailed Student’s t test. One-way ANOVA was used for multiple comparisons, and the appropriate post hoc tests were used to test for statistical significance. Pearson’s correlation test was used to compare immunohistochemical staining scores. P values less than 0.05 were considered significant. All statistical analyses were performed using the software package Prism 4.0b (GraphPad Software).
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH: HL63993, ARRA HL090662, NS04415 (to S.L. Nishimura), AI068090, P30-DK26743 (to J.L. Baron), and DK06414 (to J. Publicover); a Sandler SABRE award (to S.L. Nishimura), the Burroughs Wellcome Fund and the Technical Training Foundation (to J.L. Baron), and the A.P Gianinni Foundation (to J. Publicover). We thank the Israel Charo, UCSF Liver Center and Flow Cytometry Core (Gladstone Institutes, UCSF) for Ccr2–/– mice; Dean Sheppard for helpful suggestions, discussions, and reading of the manuscript; Xiaozhu Huang for helpful suggestions with ovalbumin experiments; Charles McCulloch (Department of Epidemiology and Biostatistics, UCSF) for help with statistics; and Abul Abbas for careful reading of the manuscript.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2011;121(7):2863–2875. doi:10.1172/JCI45589.
References
- 1.Hogg JC, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 2. WHO. World health report. WHO web site. http://www.who.int/whr/2000/en/ . Accessed April 20, 2011. [Google Scholar]
- 3.Braman SS. The global burden of asthma. Chest. 2006;130(1 suppl):4S–12S. doi: 10.1378/chest.130.1_suppl.4S. [DOI] [PubMed] [Google Scholar]
- 4.Elias JA, Zhu Z, Chupp G, Homer RJ. Airway remodeling in asthma. J Clin Invest. 1999;104(8):1001–1006. doi: 10.1172/JCI8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elias J. The relationship between asthma and COPD. Lessons from transgenic mice. Chest. 2004;126(2 suppl):111S–116S. doi: 10.1378/chest.126.2_suppl_1.111S. [DOI] [PubMed] [Google Scholar]
- 6.Sabroe I, Parker LC, Dockrell DH, Davies DE, Dower SK, Whyte MK. Targeting the networks that underpin contiguous immunity in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175(4):306–311. doi: 10.1164/rccm.200606-777PP. [DOI] [PubMed] [Google Scholar]
- 7.Couillin I, et al. IL-1R1/MyD88 signaling is critical for elastase-induced lung inflammation and emphysema. J Immunol. 2009;183(12):8195–8202. doi: 10.4049/jimmunol.0803154. [DOI] [PubMed] [Google Scholar]
- 8.Mao XQ, et al. Imbalance production between interleukin-1beta (IL-1beta) and IL-1 receptor antagonist (IL-1Ra) in bronchial asthma. Biochem Biophys Res Commun. 2000;276(2):607–612. doi: 10.1006/bbrc.2000.3516. [DOI] [PubMed] [Google Scholar]
- 9.Sapey E, et al. Imbalances between interleukin-1 and tumor necrosis factor agonists and antagonists in stable COPD. J Clin Immunol. 2009;29(4):508–516. doi: 10.1007/s10875-009-9286-8. [DOI] [PubMed] [Google Scholar]
- 10.Sousa AR, Lane SJ, Nakhosteen JA, Lee TH, Poston RN. Expression of interleukin-1 beta (IL-1beta) and interleukin-1 receptor antagonist (IL-1ra) on asthmatic bronchial epithelium. Am J Respir Crit Care Med. 1996;154(4 pt 1):1061–1066. doi: 10.1164/ajrccm.154.4.8887608. [DOI] [PubMed] [Google Scholar]
- 11.Rafiq S, et al. Common genetic variation in the gene encoding interleukin-1-receptor antagonist (IL-1RA) is associated with altered circulating IL-1RA levels. Genes Immun. 2007;8(4):344–351. doi: 10.1038/sj.gene.6364393. [DOI] [PubMed] [Google Scholar]
- 12.Doz E, et al. Cigarette smoke-induced pulmonary inflammation is TLR4/MyD88 and IL-1R1/MyD88 signaling dependent. J Immunol. 2008;180(2):1169–1178. doi: 10.4049/jimmunol.180.2.1169. [DOI] [PubMed] [Google Scholar]
- 13.Wang CC, et al. Adenovirus expressing interleukin-1 receptor antagonist alleviates allergic airway inflammation in a murine model of asthma. Gene Ther. 2006;13(19):1414–1421. doi: 10.1038/sj.gt.3302798. [DOI] [PubMed] [Google Scholar]
- 14.Lappalainen U, Whitsett JA, Wert SE, Tichelaar JW, Bry K. Interleukin-1beta causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am J Respir Cell Mol Biol. 2005;32(4):311–318. doi: 10.1165/rcmb.2004-0309OC. [DOI] [PubMed] [Google Scholar]
- 15.Kolb M, Margetts PJ, Anthony DC, Pitossi F, Gauldie J. Transient expression of IL-1beta induces acute lung injury and chronic repair leading to pulmonary fibrosis. J Clin Invest. 2001;107(12):1529–1536. doi: 10.1172/JCI12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lambrecht BN, Hammad H. Biology of lung dendritic cells at the origin of asthma. Immunity. 2009;31(3):412–424. doi: 10.1016/j.immuni.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Cumberbatch M, Dearman RJ, Kimber I. Langerhans cells require signals from both tumour necrosis factor-alpha and interleukin-1 beta for migration. Immunology. 1997;92(3):388–395. doi: 10.1046/j.1365-2567.1997.00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Messmer D, et al. High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J Immunol. 2004;173(1):307–313. doi: 10.4049/jimmunol.173.1.307. [DOI] [PubMed] [Google Scholar]
- 19.Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Invest. 2008;118(11):3546–3556. doi: 10.1172/JCI36130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shan M, et al. Lung myeloid dendritic cells coordinately induce TH1 and TH17 responses in human emphysema. Sci Transl Med. 2009;1(4):4ra10. doi: 10.1126/scitranlsmed.3000154. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez D, Vollmann EH, von Andrian UH. Mechanisms and consequences of dendritic cell migration. Immunity. 2008;29(3):325–342. doi: 10.1016/j.immuni.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalo JA, et al. The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. J Exp Med. 1998;188(1):157–167. doi: 10.1084/jem.188.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schutyser E, Struyf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003;14(5):409–426. doi: 10.1016/S1359-6101(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 24.Blanco P, Palucka AK, Pascual V, Banchereau J. Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev. 2008;19(1):41–52. doi: 10.1016/j.cytogfr.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dua B, Watson RM, Gauvreau GM, O’Byrne PM. Myeloid and plasmacytoid dendritic cells in induced sputum after allergen inhalation in subjects with asthma. J Allergy Clin Immunol. 2010;126(1):133–139. doi: 10.1016/j.jaci.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Chan CK, et al. Sequential evaluation of serum monocyte chemotactic protein 1 among asymptomatic state and acute exacerbation and remission of asthma in children. J Asthma. 2009;46(3):225–228. doi: 10.1080/02770900802553805. [DOI] [PubMed] [Google Scholar]
- 27.Liu SF, Chin CH, Wang CC, Lin MC. Correlation between serum biomarkers and BODE index in patients with stable COPD. Respirology. 2009;14(7):999–1004. doi: 10.1111/j.1440-1843.2009.01608.x. [DOI] [PubMed] [Google Scholar]
- 28.Demedts IK, et al. Accumulation of dendritic cells and increased CCL20 levels in the airways of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175(10):998–1005. doi: 10.1164/rccm.200608-1113OC. [DOI] [PubMed] [Google Scholar]
- 29.Bracke KR, et al. Cigarette smoke-induced pulmonary inflammation and emphysema are attenuated in CCR6-deficient mice. J Immunol. 2006;177(7):4350–4359. doi: 10.4049/jimmunol.177.7.4350. [DOI] [PubMed] [Google Scholar]
- 30.Lukacs NW, Prosser DM, Wiekowski M, Lira SA, Cook DN. Requirement for the chemokine receptor CCR6 in allergic pulmonary inflammation. J Exp Med. 2001;194(4):551–555. doi: 10.1084/jem.194.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robays LJ, et al. Chemokine receptor CCR2 but not CCR5 or CCR6 mediates the increase in pulmonary dendritic cells during allergic airway inflammation. J Immunol. 2007;178(8):5305–5311. doi: 10.4049/jimmunol.178.8.5305. [DOI] [PubMed] [Google Scholar]
- 32.Behzad AR, McDonough JE, Seyednejad N, Hogg JC, Walker DC. The disruption of the epithelial mesenchymal trophic unit in COPD. COPD. 2009;6(6):421–431. doi: 10.3109/15412550903341471. [DOI] [PubMed] [Google Scholar]
- 33.Rollins BJ, Morrison ED, Stiles CD. Cloning and expression of JE, a gene inducible by platelet-derived growth factor and whose product has cytokine-like properties. Proc Natl Acad Sci U S A. 1988;85(11):3738–3742. doi: 10.1073/pnas.85.11.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sehgal PB, Sagar AD. Heterogeneity of poly(I) x poly(C)-induced human fibroblast interferon mRNA species. Nature. 1980;288(5786):95–97. doi: 10.1038/288095a0. [DOI] [PubMed] [Google Scholar]
- 35.Araya J, Nishimura SL. Fibrogenic reactions in lung disease. Annu Rev Pathol. 2010;5:77–98. doi: 10.1146/annurev.pathol.4.110807.092217. [DOI] [PubMed] [Google Scholar]
- 36.de Boer WI, et al. Transforming growth factor beta1 and recruitment of macrophages and mast cells in airways in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158(6):1951–1957. doi: 10.1164/ajrccm.158.6.9803053. [DOI] [PubMed] [Google Scholar]
- 37.Takizawa H, et al. Increased expression of transforming growth factor-beta1 in small airway epithelium from tobacco smokers and patients with chronic obstructive pulmonary disease (COPD). Am J Respir Crit Care Med. 2001;163(6):1476–1483. doi: 10.1164/ajrccm.163.6.9908135. [DOI] [PubMed] [Google Scholar]
- 38.Redington AE, et al. Transforming growth factor-beta 1 in asthma. Measurement in bronchoalveolar lavage fluid. Am J Respir Crit Care Med. 1997;156(2 pt 1):642–647. doi: 10.1164/ajrccm.156.2.9605065. [DOI] [PubMed] [Google Scholar]
- 39.Vignola AM, et al. Transforming growth factor-beta expression in mucosal biopsies in asthma and chronic bronchitis. Am J Respir Crit Care Med. 1997;156(2 pt 1):591–599. doi: 10.1164/ajrccm.156.2.9609066. [DOI] [PubMed] [Google Scholar]
- 40.Araya J, et al. Squamous metaplasia amplifies pathologic epithelial-mesenchymal interactions in COPD patients. J Clin Invest. 2007;117(11):3551–3562. doi: 10.1172/JCI32526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Denton CP, et al. Fibroblast-specific expression of a kinase-deficient type II transforming growth factor beta (TGFbeta) receptor leads to paradoxical activation of TGFbeta signaling pathways with fibrosis in transgenic mice. J Biol Chem. 2003;278(27):25109–25119. doi: 10.1074/jbc.M300636200. [DOI] [PubMed] [Google Scholar]
- 42.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127(3):526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 43.Li MO, Flavell RA. TGF-beta: a master of all T cell trades. Cell. 2008;134(3):392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luo X, et al. In vivo disruption of TGF-beta signaling by Smad7 in airway epithelium alleviates allergic asthma but aggravates lung carcinogenesis in mouse. PLoS One. 2010;5(4):e10149. doi: 10.1371/journal.pone.0010149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakao A, et al. Blockade of transforming growth factor beta/Smad signaling in T cells by overexpression of Smad7 enhances antigen-induced airway inflammation and airway reactivity. J Exp Med. 2000;192(2):151–158. doi: 10.1084/jem.192.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fjellbirkeland L, et al. Integrin alphavbeta8-mediated activation of transforming growth factor-beta inhibits human airway epithelial proliferation in intact bronchial tissue. Am J Pathol. 2003;163(2):533–542. doi: 10.1016/s0002-9440(10)63681-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishimura SL. Integrin-mediated transforming growth factor-beta activation, a potential therapeutic target in fibrogenic disorders. Am J Pathol. 2009;175(4):1362–1370. doi: 10.2353/ajpath.2009.090393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mu D, et al. The integrin avβ8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-β1. J Cell Biol. 2002;157(3):493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aluwihare P, et al. Mice that lack activity of alphavbeta6- and alphavbeta8-integrins reproduce the abnormalities of Tgfb1- and Tgfb3-null mice. J Cell Sci. 2009;122(pt 2):227–232. doi: 10.1242/jcs.035246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mu Z, Yang Z, Yu D, Zhao Z, Munger JS. TGFbeta1 and TGFbeta3 are partially redundant effectors in brain vascular morphogenesis. Mech Dev. 2008;125(5–6):508–516. doi: 10.1016/j.mod.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 51.Melton AC, Bailey-Bucktrout SL, Travis MA, Fife BT, Bluestone JA, Sheppard D. Expression of alphavbeta8 integrin on dendritic cells regulates Th17 cell development and experimental autoimmune encephalomyelitis in mice. J Clin Invest. 2010;120(12):4436–4444. doi: 10.1172/JCI43786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Travis MA, et al. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449(7160):361–365. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cole BB, Smith RW, Jenkins KM, Graham BB, Reynolds PR, Reynolds SD. Tracheal Basal cells: a facultative progenitor cell pool. Am J Pathol. 2010;177(1):362–376. doi: 10.2353/ajpath.2010.090870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ballesteros-Tato A, Leon B, Lund FE, Randall TD. Temporal changes in dendritic cell subsets, cross-priming and costimulation via CD70 control CD8(+) T cell responses to influenza. Nat Immunol. 2010;11(3):216–224. doi: 10.1038/ni.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hintzen G, et al. Induction of tolerance to innocuous inhaled antigen relies on a CCR7-dependent dendritic cell-mediated antigen transport to the bronchial lymph node. J Immunol. 2006;177(10):7346–7354. doi: 10.4049/jimmunol.177.10.7346. [DOI] [PubMed] [Google Scholar]
- 56.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 57.Yu M, Tsai M, Tam SY, Jones C, Zehnder J, Galli SJ. Mast cells can promote the development of multiple features of chronic asthma in mice. J Clin Invest. 2006;116(6):1633–1641. doi: 10.1172/JCI25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reibman J, Hsu Y, Chen LC, Bleck B, Gordon T. Airway epithelial cells release MIP-3alpha/CCL20 in response to cytokines and ambient particulate matter. Am J Respir Cell Mol Biol. 2003;28(6):648–654. doi: 10.1165/rcmb.2002-0095OC. [DOI] [PubMed] [Google Scholar]
- 59.van der Velden VH, et al. Interleukin-1beta and interferon-gamma differentially regulate release of monocyte chemotactic protein-1 and interleukin-8 by human bronchial epithelial cells. Eur Cytokine Netw. 1998;9(3):269–277. [PubMed] [Google Scholar]
- 60.Flavell SJ, Hou TZ, Lax S, Filer AD, Salmon M, Buckley CD. Fibroblasts as novel therapeutic targets in chronic inflammation. Br J Pharmacol. 2008;153(suppl 1):S241–S246. doi: 10.1038/sj.bjp.0707487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Link A, et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8(11):1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 62.Saalbach A, Klein C, Schirmer C, Briest W, Anderegg U, Simon JC. Dermal fibroblasts promote the migration of dendritic cells. J Invest Dermatol. 2010;130(2):444–454. doi: 10.1038/jid.2009.253. [DOI] [PubMed] [Google Scholar]
- 63.Schirmer C, Klein C, von Bergen M, Simon JC, Saalbach A. Human fibroblasts support the expansion of IL-17 producing T-cells via up-regulation of IL-23 production by dendritic cells. Blood. 2010;116(10):1715–1725. doi: 10.1182/blood-2010-01-263509. [DOI] [PubMed] [Google Scholar]
- 64.Huang XZ, et al. Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol. 1996;133(4):921–928. doi: 10.1083/jcb.133.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morris DG, et al. Loss of integrin alpha(v)beta6-mediated TGF-beta activation causes Mmp12-dependent emphysema. Nature. 2003;422(6928):169–173. doi: 10.1038/nature01413. [DOI] [PubMed] [Google Scholar]
- 66.Breuss JM, Gillett N, Lu L, Sheppard D, Pytela R. Restricted distribution of integrin beta 6 mRNA in primate epithelial tissues. J Histochem Cytochem. 1993;41(10):1521–1527. doi: 10.1177/41.10.8245410. [DOI] [PubMed] [Google Scholar]
- 67.Munger JS, et al. The integrin avβ6 binds and activates latent TGF-β1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96(3):319–328. doi: 10.1016/S0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 68.Asano Y, Ihn H, Jinnin M, Mimura Y, Tamaki K. Involvement of avβ5 integrin in the establishment of autocrine TGF-β signaling in dermal fibroblasts derived from localized scleroderma. J Invest Dermatol. 2006;126(8):1761–1769. doi: 10.1038/sj.jid.5700331. [DOI] [PubMed] [Google Scholar]
- 69.Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K. Involvement of avβ5 integrin-mediated activation of latent transforming growth factor-β1 in autocrine transforming growth factor-β signaling in systemic sclerosis fibroblasts. Arthritis Rheum. 2005;52(9):2897–2905. doi: 10.1002/art.21246. [DOI] [PubMed] [Google Scholar]
- 70.Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K. Increased expression of integrin avβ3 contributes to the establishment of autocrine TGF-β signaling in scleroderma fibroblasts. J Immunol. 2005;175(11):7708–7718. doi: 10.4049/jimmunol.175.11.7708. [DOI] [PubMed] [Google Scholar]
- 71.Wipff PJ, Hinz B. Integrins and the activation of latent transforming growth factor-β1 — an intimate relationship. Eur J Cell Biol. 2008;87(8–9):601–615. doi: 10.1016/j.ejcb.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 72.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. J Cell Biol. 2007;179(6):1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Munger JS, Harpel JG, Giancotti FG, Rifkin DB. Interactions between growth factors and integrins: latent forms of transforming growth factor-β are ligands for the integrin avβ1. Mol Biol Cell. 1998;9(9):2627–2638. doi: 10.1091/mbc.9.9.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reynolds LE, et al. Enhanced pathological angiogenesis in mice lacking β3 integrin or β3 and β5 integrins. Nat Med. 2002;8(1):27–34. doi: 10.1038/nm0102-27. [DOI] [PubMed] [Google Scholar]
- 75.Araya J, Cambier S, Morris A, Finkbeiner W, Nishimura SL. Integrin mediated TGF-β activation regulates homeostasis of the pulmonary epithelial-mesenchymal trophic unit. Am J Pathol. 2006;169(2):405–415. doi: 10.2353/ajpath.2006.060049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15(4):410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Al-Ramli W, et al. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol. 2009;123(5):1185–1187. doi: 10.1016/j.jaci.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 78.Di Stefano A, et al. T helper type 17-related cytokine expression is increased in the bronchial mucosa of stable chronic obstructive pulmonary disease patients. Clin Exp Immunol. 2009;157(2):316–324. doi: 10.1111/j.1365-2249.2009.03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Doe C, et al. Expression of the T helper 17-associated cytokines IL-17A and IL-17F in asthma and COPD. Chest. 2010;138(5):1140–1147. doi: 10.1378/chest.09-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19(3):281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 81.Wilson MS, et al. Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med. 2010;207(3):535–552. doi: 10.1084/jem.20092121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med. 2009;180(8):720–730. doi: 10.1164/rccm.200904-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Acharya M, et al. alphav Integrin expression by DCs is required for Th17 cell differentiation and development of experimental autoimmune encephalomyelitis in mice. J Clin Invest. 2010;120(12):4445–4452. doi: 10.1172/JCI43796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zheng B, Zhang Z, Black CM, de Crombrugghe B, Denton CP. Ligand-dependent genetic recombination in fibroblasts: a potentially powerful technique for investigating gene function in fibrosis. Am J Pathol. 2002;160(5):1609–1617. doi: 10.1016/S0002-9440(10)61108-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Proctor JM, Zang K, Wang D, Wang R, Reichardt LF. Vascular development of the brain requires beta8 integrin expression in the neuroepithelium. J Neurosci. 2005;25(43):9940–9948. doi: 10.1523/JNEUROSCI.3467-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu J, Motejlek K, Wang D, Zang K, Schmidt A, Reichardt LF. beta8 integrins are required for vascular morphogenesis in mouse embryos. Development. 2002;129(12):2891–2903. doi: 10.1242/dev.129.12.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Inaba K, Swiggard WJ, Steinman RM, Romani N, Schuler G, Brinster C. Isolation of dendritic cells. Curr Protoc Immunol. 2009;Chapter 3:Unit 3.7. doi: 10.1002/0471142735.im0307s86. [DOI] [PubMed] [Google Scholar]
- 88.Vermaelen K, Pauwels R. Accurate and simple discrimination of mouse pulmonary dendritic cell and macrophage populations by flow cytometry: methodology and new insights. Cytometry A. 2004;61(2):170–177. doi: 10.1002/cyto.a.20064. [DOI] [PubMed] [Google Scholar]
- 89. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of COPD. http://www.goldcopd.org . Published December 2010. Accessed May 24, 2011 . [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.