Abstract
In addition to its role as an essential neurotransmitter, dopamine serves important physiologic functions in organs such as the kidney. Although the kidney synthesizes dopamine through the actions of aromatic amino acid decarboxylase (AADC) in the proximal tubule, previous studies have not discriminated between the roles of extrarenal and intrarenal dopamine in the overall regulation of renal function. To address this issue, we generated mice with selective deletion of AADC in the kidney proximal tubules (referred to herein as ptAadc–/– mice), which led to selective decreases in kidney and urinary dopamine. The ptAadc–/– mice exhibited increased expression of nephron sodium transporters, decreased natriuresis and diuresis in response to l-dihydroxyphenylalanine, and decreased medullary COX-2 expression and urinary prostaglandin E2 excretion and developed salt-sensitive hypertension. They had increased renin expression and altered renal Ang II receptor (AT) expression, with increased AT1b and decreased AT2 and Mas expression, associated with increased renal injury in response to Ang II. They also exhibited a substantially shorter life span compared with that of wild-type mice. These results demonstrate the importance of the intrarenal dopaminergic system in salt and water homeostasis and blood pressure control. Decreasing intrarenal dopamine subjects the kidney to unbuffered responses to Ang II and results in the development of hypertension and a dramatic decrease in longevity.
Introduction
Although dopamine is an essential neurotransmitter, extraneural dopamine also serves important physiologic functions. Both D1-like (D1 and D5) and D2-like (D2, D3, and D4) dopamine receptors are expressed in the mammalian kidney (1), and exogenous administration of dopamine is known to modulate solute and water transport in the mammalian kidney, mediated, at least in part, by inhibition of specific tubule transporter activity along the nephron (2, 3).
The kidney possesses an intrarenal dopaminergic system that is distinct from any neural dopaminergic input. Circulating concentrations of dopamine are normally in the picomolar range, while dopamine levels in the kidney can reach high nanomolar concentrations (4). The dopamine precursor l-dihydroxyphenylalanine (l-DOPA) is taken up by the proximal tubule via multiple amino acid transporters, including rBat, LAT2, and ASCT2 (5, 6), from the circulation or after filtration at the glomerulus and is then converted to dopamine by aromatic amino acid decarboxylase (AADC), which is also localized to the proximal tubule (7). There is evidence that intrarenal dopamine production is modulated by alterations in dietary salt intake (8, 9). Although abnormalities in dopamine production and receptor function have been associated with essential hypertension in humans and in several forms of rodent genetic hypertension (1, 10–12), previous studies have not been able to discriminate between intrarenally versus extrarenally produced dopamine in the mediation of kidney function. In the current study, we developed a mouse model with defective intrarenal dopamine production in order to test the hypothesis that the intrarenal dopaminergic system plays a significant role in regulation of renal function, blood pressure, and longevity.
Results
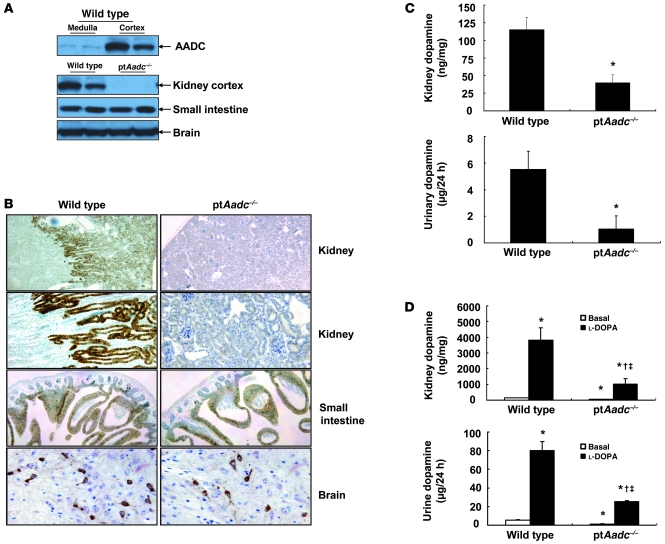
Since AADC, the enzyme responsible for intrarenal dopamine production, is localized to renal proximal tubules, we generated mice with selective proximal tubule AADC deletion (ptAadc–/–) by crossing Aadcflox7/flox7 mice with γ-GT Cre mice (ref. 13 and Supplemental Figures 1 and 2; supplemental material available online with this article; doi: 10.1172/JCI57324DS1). As expected, in wild-type mice, AADC expression was largely confined to the renal cortex and was predominantly in proximal tubules, while in ptAadc–/– mice, minimal AADC immunoreactivity was detectable in the renal cortex, although AADC expression in small intestine and in neurons was not different from that of wild-type mice (Figure 1, A and B). There were also no differences in brain and plasma dopamine levels between wild-type and ptAadc–/– mice (Supplemental Figure 3), while both kidney and urinary dopamine levels were significantly lower in ptAadc–/– mice (Figure 1C). The dopamine detected in kidneys and urine in the ptAadc–/– mice may be the result of circulating dopamine as well as residual AADC activity, since Cre-mediated approaches usually result in low residual levels of undeleted genes. Administration of the dopamine precursor, l-DOPA, increased kidney and urinary dopamine levels to a much greater extent in wild-type mice than in ptAadc–/– mice (Figure 1D).
Figure 1. AADC was selectively deleted in the renal proximal tubule in ptAadc–/– mice.
(A) AADC was primarily expressed in kidney cortex in wild-type mice. AADC expression was reduced in kidney cortex but not in small intestine or brain in ptAadc–/– mice. (B) Representative photomicrographs indicate reduced AADC expression in renal proximal tubule epithelia but not in small intestine or brain in ptAadc–/– mice. Original magnification: ×25 (first row); ×100 (second row); ×63 (third row); ×250 (fourth row). (C) Kidney dopamine levels and urinary dopamine excretion were significantly decreased in ptAadc–/– mice (*P < 0.01; n = 5 in each group). (D) Kidney dopamine levels and urinary dopamine excretion in response to l-DOPA were significantly attenuated in ptAadc–/– mice (*P < 0.01 vs. basal wild type, †P < 0.01 vs. basal ptAadc–/–, ‡P < 0.01 vs. l-DOPA wild type; n = 4 in each group).
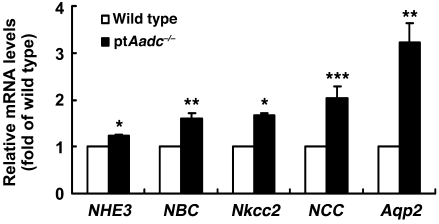
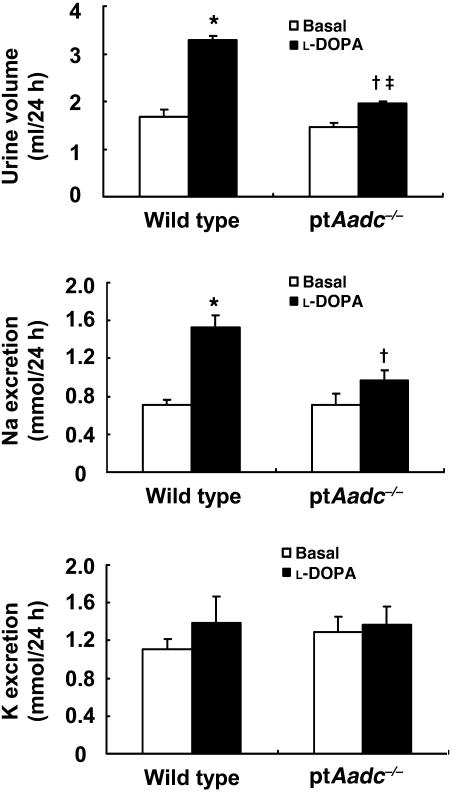
At 4 weeks of age, ptAadc–/– mice had significantly increased renal mRNA expression of transporters involved in salt reabsorption in multiple nephron segments, including proximal tubule (sodium hydrogen exchanger type 3 [NHE3], sodium-bicarbonate cotransporter [NBC]), solute carrier family 12, member 1 (Nkcc2), and solute carrier family 12, member 3 (NCC) as well as increased mRNA levels of aquaporin 2 (Aqp2), which mediates regulated water reabsorption in collecting duct (Figure 2). Although l-DOPA significantly increased natriuresis and diuresis in wild-type mice, its effects were markedly attenuated in ptAadc–/– mice (Figure 3).
Figure 2. Renal mRNA levels of sodium transporters were increased in ptAadc–/–mice.
Four-week-old wild-type and ptAadc–/– mice were studied (*P < 0.001, **P < 0.01, ***P < 0.02; n = 4 in each group).
Figure 3. l-DOPA–induced diuresis and natriuresis were attenuated in ptAadc–/– mice.
Three-month-old male mice were studied. l-DOPA induced significantly less increased urine volume and sodium excretion in ptAadc–/– mice than in wild-type mice (urine volume, *P < 0.001 vs. basal wild type, †P < 0.01 vs. basal ptAadc–/–, ‡P < 0.001 vs. l-DOPA–treated wild type) (sodium excretion, *P < 0.005 vs. basal wild type, †P < 0.05 vs. l-DOPA–treated wild type) (n = 6 in each group).
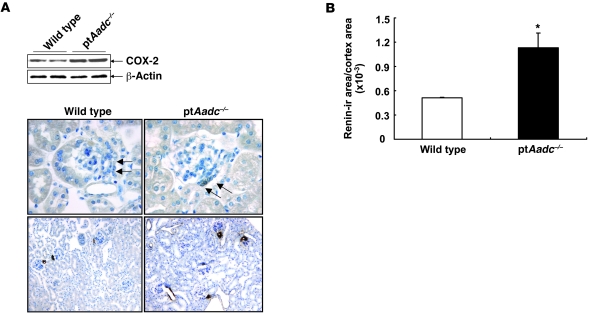
Our previous studies indicated that dopamine modulates expression of macula densa COX-2 (14). In adult wild-type mice fed normal mouse chow, macula densa immunoreactive COX-2 expression was only faintly detectable (15), while macula densa COX-2 immunoreactivity was abundant in ptAadc–/– mice (Figure 4A). Similar to COX-2 expression, renin expression in the juxtaglomerular (jg) cells increases in response to exposure to a low-salt diet and decreases in response to exposure to a high-salt diet, and macula densa COX-2 is a mediator of this regulated renin expression (16). ptAadc–/– mice had increased jg renin expression compared with that of wild-type mice (Figure 4, A and B). These studies indicate that intrarenal dopamine is an important regulator of renal renin expression.
Figure 4. Renal cortical COX-2 and renin expression increased in ptAadc–/– mice.
(A) Immunoblotting showed increased renal cortical COX-2 expression in ptAadc–/– mice. Representative photomicrographs indicated increased renal cortical COX-2 and renin expression in ptAadc–/–mice. Original magnification: ×400 (COX-2); ×100 (renin). (B) Quantitative image analysis indicated increased renal (ir) renin expression in ptAadc–/– mice (*P < 0.01; n = 4).
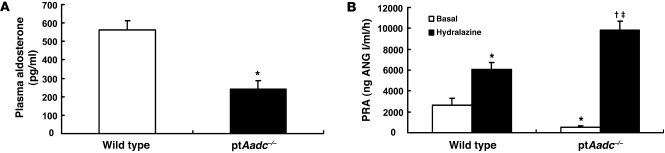
Although jg renin expression increased markedly, both plasma renin activity (PRA) and aldosterone levels were significantly decreased in ptAadc–/– mice on a normal-salt diet (Figure 5, A and B). The increased renin expression was further indicated by the response to acute administration of hydralazine to stimulate renin release. PRA increased more than 15 fold in ptAadc–/– mice, while only increasing 3 fold in wild-type mice, consistent with the increased intrarenal renin content (Figure 5B).
Figure 5. Plasma aldosterone levels and renin activity were suppressed in ptAadc–/– mice.
(A) Plasma aldosterone levels were significantly lower in ptAadc–/– than in wild-type mice (*P < 0.001; n = 6). (B) PRA was lower in untreated ptAadc–/– mice than in wild-type mice but increased more in ptAadc–/– mice than in wild-type mice after hydralazine stimulation (*P < 0.01 vs. basal wild type, †P < 0.001 vs. basal ptAadc–/–, ‡P < 0.001 vs. hydralazine stimulated wild type; n = 6 in each group).
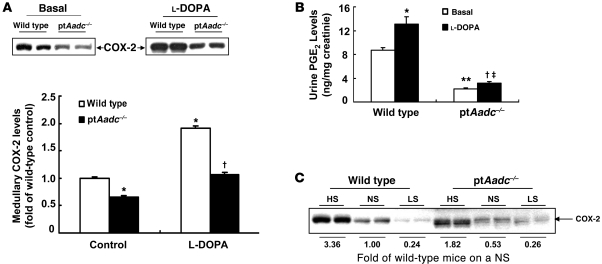
The lower PRA and plasma aldosterone levels in ptAadc–/– mice suggested relative volume expansion. In addition to localized expression in the macula densa, COX-2 is also highly expressed in mammalian kidney in the interstitial cells of the medulla, and previous studies have indicated an important role for medullary COX-2–derived prostaglandins to mediate natriuresis and diuresis (17). Basal medullary COX-2 expression was lower in ptAadc–/– mice than in wild-type mice (Figure 6A), and while l-DOPA administration markedly increased medullary COX-2 expression in wild-type mice, it produced minimal increases in ptAadc–/– mice. Similarly, baseline urinary prostaglandin E2 (PGE2) excretion was substantially higher in wild-type mice than in ptAadc–/– mice, and l-DOPA administration led to a further significant increase in wild-type mice but not in ptAadc–/– mice (Figure 6B). In wild-type mice, medullary COX-2 expression increased with a high-salt diet and decreased with a low-salt diet, but this modulation was markedly attenuated in ptAadc–/– mice (Figure 6C).
Figure 6. Renal medullary COX-2 was inhibited in ptAadc–/– mice.
(A) Renal medullary COX-2 levels were lower in ptAadc–/– mice than wild-type mice, with or without l-DOPA stimulation (*P < 0.01 vs. basal wild type, †P < 0.001 vs. l-DOPA treated wild type; n = 4 in each group). (B) Urinary PGE2 levels were significantly lower in ptAadc–/– mice than wild-type mice, with or without l-DOPA stimulation (*P < 0.05 vs. basal wild type, **P < 0.01 vs. basal wild type, †P < 0.05 vs. basal ptAadc–/–, ‡P < 0.005 vs. l-DOPA–treated wild type; n = 4 in each group). (C) High-salt diet–induced (HS-induced) renal medullary COX-2 elevations were attenuated in ptAadc–/– mice. Also shown is densitometric quantification of COX-2 immunoreactive protein in response to alterations of dietary salt intake represented as fold of expression of wild-type mice on a normal-salt diet (NS). LS, low-salt diet.
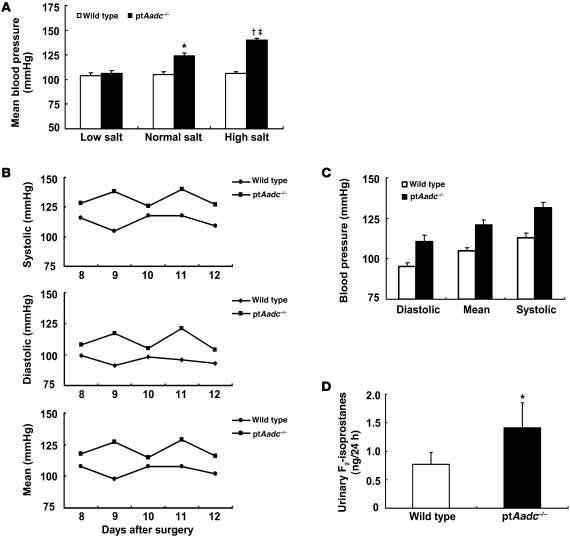
By 3 months of age, blood pressure was significantly elevated in ptAadc–/– mice compared with that in wild-type mice (mean arterial blood pressure [MAP], 124 ± 3 mmHg vs. 105± 3 mmHg, respectively; n = 6; P < 0.01) (Figure 7A). Blood pressure measurements by radiotelemetry in a subset of mice (n = 4) confirmed this elevated blood pressure (Figure 7, B and C). A high-salt diet (8% high-salt diet) for 4 weeks did not increase blood pressure in wild-type mice but led to further increases in ptAadc–/– mice (MAP, 140 ± 2 mmHg vs. 106 ± 2 mmHg, respectively; n = 6; P < 0.01). In contrast, when placed on a low-salt diet, there were no differences in blood pressure between wild-type and ptAadc–/– mice (Figure 7A). Urinary excretion of F2-isoprostane, a marker of oxidative stress (18), was significantly higher in ptAadc–/– mice in response to salt loading (1.41 ± 0.44 ng/24 hour for ptAadc–/– mice vs. 0.77 ± 0.21 ng/24 hour for wild-type mice, n = 6; P < 0.05) (Figure 7D), reflective of increased oxidative stress in high-salt diet–fed ptAadc–/– mice.
Figure 7. ptAadc–/– mice developed salt-sensitive hypertension.
(A) MBP was similar between 3-month-old ptAadc–/– mice and wild-type mice on a low-salt diet. Increasing dietary salt intake had no effect on MBP in wild-type mice but led to progressive increases in MBP in ptAadc–/– mice (*P < 0.01 vs. wild type on a normal-salt diet, †P < 0.05 vs. wild type on a high-salt diet, ‡P < 0.005 vs. ptAadc–/– on a normal-salt diet; n = 9 in each group). (B) Dynamics of systolic, diastolic, and MBP measured by radiotelemetry from 8 to 12 days after surgery (n = 4). (C) MBPs measured by radiotelemetry from 8 to 12 days after surgery. (D) Male wild-type and ptAadc–/– mice were fed an 8% high-salt diet for 4 weeks, and 24-hour urine was collected for measurement of F2-isoprostanes (*P < 0.05; n = 6).
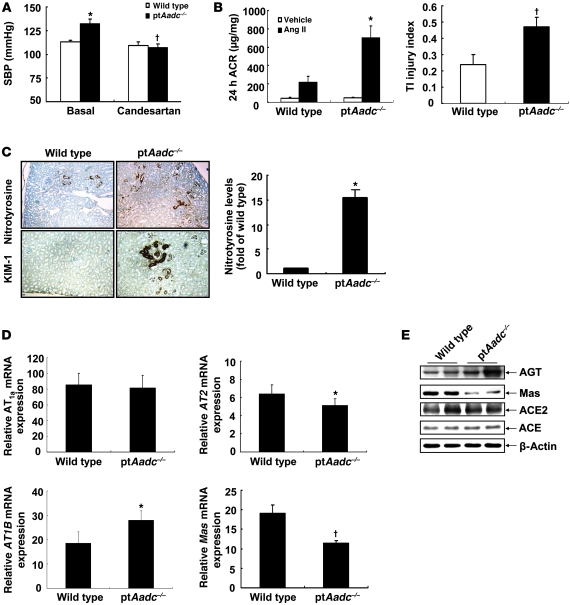
Previous studies have indicated that dopamine may modulate Ang II actions in the kidney (19, 20). Treatment of ptAadc–/– mice with the Ang II receptor 1 (AT1) blocker, candesartan, decreased blood pressure to levels seen in wild-type mice, which were unaffected by candesartan treatment (Figure 8A). Blood pressure increased more rapidly in ptAadc–/– mice during initial exposure to Ang II (0.9 mg/kg/d), but by day 5, wild-type and ptAadc–/– mice had comparable blood pressure elevations (Supplemental Figure 4). After 4 weeks of Ang II exposure, systolic blood pressure (SBP) was still comparable between groups (wild type vs. ptAadc–/– mice, 200 ± 5 mmHg vs. 205 ± 4 mmHg, respectively; n = 4), but ptAadc–/– mice had significantly more albuminuria and tubulointerstitial damage (Figure 8B). Nitrotyrosine staining, a marker of oxidative stress, and KIM-1 expression, an indicator of tubule injury, also increased (Figure 8C). In ptAadc–/– mice, there was increased renal mRNA expression of Ang II receptor, type 1b (AT1B) mRNA and decreased expression of the counterregulatory Ang II receptor, Ang II receptor, type 2 (AT2), and the Ang 1-7 receptor, Mas (Figure 8D). No comparable changes in AT1 receptor expression were seen in heart or aorta (Supplemental Figure 5). There was also increased expression of immunoreactive angiotensinogen as well as confirmation of the decreased expression of Mas protein, while there were not differences in expression of ACE or ACE2 (Figure 8E).
Figure 8. The intrarenal renin-angiotensin system was altered in ptAadc–/– mice.
(A) Antagonism of AT1 receptors with candesartan reduced SBP in ptAadc–/– mice to levels seen in wild-type mice (*P < 0.01 vs. basal wild type, †P < 0.01 vs. basal ptAadc–/–; n = 6 in each group). (B) Ang II infusion (0.9 mg/kg/d) for 4 weeks led to more significant increases in albuminuria (*P = 0.012) and tubulointerstitial (TI) injury (†P = 0.03) in ptAadc–/– mice than wild-type mice (n = 5). ACR, albumin creatinine ratio. (C) The expression of nitrotyrosine (a marker of oxidative stress) and KIM-1 (a marker of kidney injury) was much higher in Ang II–treated ptAadc–/– mice than Ang II–treated wild-type mice. Original magnification: ×63. Quantitative image analysis indicated nitrotyrosine levels were significantly higher in Ang II–treated ptAadc–/– mice than Ang II–treated wild-type mice (*P < 0.01; n = 4). (D) There were increased mRNA levels of AT1b (*P < 0.00001) but decreased mRNA levels of AT2 (*P < 0.00001) and Mas (†P < 0.0001) in ptAadc–/– mice compared with those in wild-type mice (n = 4). (E) Immunoblotting indicated increased angiotensinogen (AGT) but decreased Mas protein levels in ptAadc–/– mice.
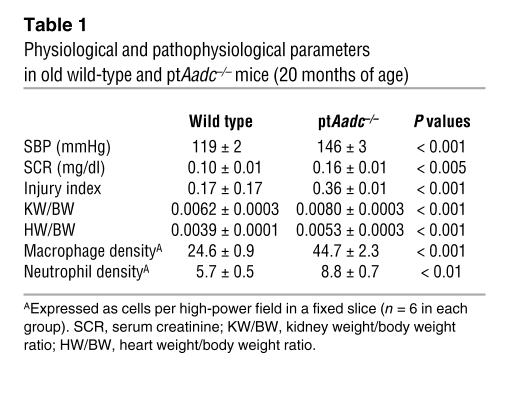
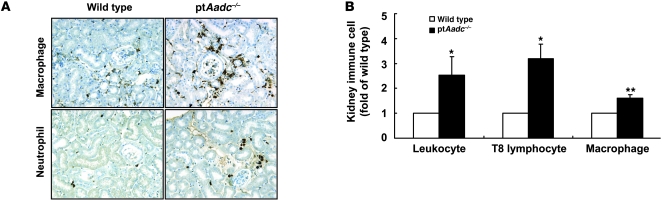
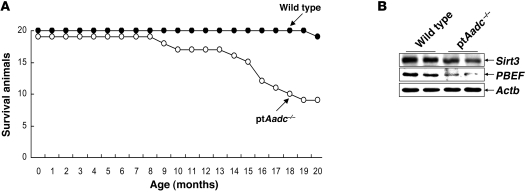
Surviving ptAadc–/– mice, sacrificed at 20 months, had increased blood pressure, increased serum creatinine, and increased renal injury index (Table 1). The kidneys of the aged ptAadc–/– mice also had increased macrophage, neutrophil, and lymphocyte infiltration (Table 1 and Figure 9). Compared with wild-type littermates, ptAadc–/– mice exhibited markedly shorter life spans. At 20 months of age, 19 out of 20 wild-type mice were still alive, while only 9 out of 19 ptAadc–/– mice had survived (Figure 10A). Expression of Sirt3 and PBEF, survival markers noted to be increased in Ang II receptor, type 1a–null mice (AT1a receptor–null mice), which have increased life spans (21), was markedly decreased in kidneys of ptAadc–/– mice (Figure 10B). These survival markers were also decreased in heart and aorta but were unchanged in liver (Supplemental Figure 6) or lung (data not shown).
Table 1 .
Physiological and pathophysiological parameters in old wild-type and ptAadc–/– mice (20 months of age)
Figure 9. Immune cell infiltration was higher in old ptAadc–/– mice than old wild-type mice.
(A) Representative photomicrographs indicated more macrophage and neutrophil infiltration in 20-month-old ptAadc–/– mice than 20-month-old wild-type mice. Original magnification: ×160. (B) FACS analysis indicated increased leukocyte, T8 lymphocyte, and macrophage infiltration in old ptAadc–/– mice than old wild-type mice (*P < 0.05, **P < 0.01; n = 3).
Figure 10. Renal dopamine deficiency was associated with shorter life spans.
(A) At 20 months of age, 10 out of 19 ptAadc–/– mice had died, while only 1 out of 20 wild-type mice had died. (B) Immunoblotting showed reduced levels of prosurvival genes Sirt3 and PBEF in ptAadc–/– mice.
Discussion
By selectively deleting proximal tubule AADC expression, we have demonstrated the importance of the intrarenal dopaminergic system in maintenance of renal function, modulation of the renin-angiotensin system, and regulation of blood pressure. Even with central and other peripheral sites of dopamine production intact, selective deletion of the kidney’s ability to generate dopamine led to profound phenotypic alterations, characterized by increased expression of salt and water transporters along the nephron, altered salt and water homeostasis, hypertension, and markedly decreased life span. A general characteristic of essential hypertension is a relative defect in renal sodium and water excretion; therefore, as demonstrated by the current studies, a dysfunctional intrarenal dopaminergic system may have profound consequences for regulation of intravascular volume and systemic blood pressure.
The current studies also demonstrate that intrarenal dopamine is a modulator of renal COX-2 expression and prostaglandin production. In previous studies, we showed that macula densa COX-2 expression decreased in response to dopamine administration (14), and in the current study, we found that macula densa COX-2 expression was increased in mice deficient in intrarenal dopamine. Furthermore, in wild-type mice, l-DOPA administration increased medullary COX-2 expression and urinary PGE2 excretion, and this effect was lost in the ptAadc–/– mice. Increases in medullary COX-2 expression in response to salt loading were also markedly blunted in ptAadc–/– mice. COX-2–derived prostaglandins are important integrators of vascular tone and salt and water homeostasis in the renal medulla, and inhibition of medullary COX-2 activity can lead to hypertension (17, 22–25). These results therefore confirm those of previous studies that suggested a potential role for dopamine to increase pronatriuretic renal medullary prostaglandin production (26, 27).
It has been suggested that dopamine and Ang II may serve counterregulatory functions in the kidney (28, 29). Our previous studies indicated that dopamine inhibits renal renin expression (15), and the current study proves that endogenously produced intrarenal dopamine modulates this expression, since mice with deficient intrarenal dopamine production had increased renal renin expression. In addition, dopamine inhibits Ang II–mediated proximal tubule reabsorption and AT1 expression (19, 30–32). In addition to vasoconstriction and stimulation of salt and water reabsorption mediated by AT1 receptors, angiotensinogen-derived peptides also mediate counterregulatory vasodilatory and natriuretic/diuretic pathways through Ang II activation of AT2 receptors and Ang 1-7 activation of Mas (33). The current study indicates that deletion of intrarenal dopamine production leads to increased angiotensinogen and AT1 expression but decreased AT2 and Mas expression. Of interest, it has been suggested that dopamine’s natriuretic effects may be mediated in part through AT2 signaling (34).
The potential importance of intrarenal dopamine to counteract renal effects of the renin-angiotensin system was further indicated by the reversal of hypertension in ptAadc–/– mice by the AT1 receptor blocker, candesartan, and by the accelerated increase in blood pressure and the greater renal damage seen in ptAadc–/– mice in response to administration of Ang II. The concentration of Ang II administered in these studies (0.9 mg/kg/d) was lower than the usual concentration used in studies designed to induce progressive renal damage (1.4 mg/kg/d) (35), because, in our preliminary studies, all ptAadc–/– mice administered this higher dose died within 4 weeks, while all wild-type mice survived, further indicating that a deficiency of intrarenal dopamine can lead to augmented responsiveness to Ang II.
A recent study by Benigni et al. found that selective deletion of AT1a increased longevity in mice (21). In contrast, the current study indicates that selective deletion of the intrarenal dopaminergic system markedly decreases life span. Although there was chronic renal injury in the aged ptAadc–/– mice, the degree of renal injury was not in itself sufficient to produce such a markedly shortened life span. It is well recognized that untreated hypertension is a risk factor for premature death, and inbred strains of mice with spontaneous hypertension have decreased life spans (36). In this regard, we hypothesize that the decreased expression of prosurvival genes in heart and vasculature is also consistent with increased cardiovascular stress in the ptAadc–/– mice.
Recent studies have demonstrated an essential role for the intrarenal renin-angiotensin system in blood pressure homeostasis and have shown the importance of activation of intrarenal AT1 receptors in development and complications of systemic hypertension (37, 38). The current study demonstrates conclusively that the intrarenal dopaminergic system plays a similarly important role in preventing the development of systemic hypertension and modulating the renal effects of the renin-angiotensin system. Thus, a dysfunctional intrarenal dopaminergic system has substantial consequences on long-term health and survival.
Methods
Generation of mice with proximal tubule-specific AADC deletion.
We used a BAC engineering strategy to produce an Aadc conditional gene targeting vector, in which 2 LoxP sites flanking exon 7 of the Aadc gene (Aadcflox7) were introduced, and a neomycin-selection cassette (Neo) flanked by 2 FRT sites was inserted into intron 7 downstream of the second LoxP site (Neoflrt). Detailed procedures and information regarding generation of Neoflrt/Aadcflox7 mice are shown in Supplemental Figure 1. The targeting construct was linearized with Sal I and electroporated into 129 ES cells. A targeting event was found in 7 out of 309 colonies resistant to G418, as demonstrated by an expected 1.4-kb product amplified by PCR. Five positive clones were confirmed by Southern blot. Two positive ES clones were used for implantation. Three male chimeras with positive targeting (with Neoflrt/Aadcflox7 allele) were obtained. These chimeras were crossed with female congenic C57BL/6 mice. Germ-line transmission of the targeted events in the F1 mice was screened by PCR and further confirmed by Southern blot, as illustrated in Supplemental Figure 2. The Neo cassette was effectively removed by crossing with FLPe mice. In our preliminary experiments, we compared AADC expression in kidney cortex and medulla in wild-type mice and Aadcflox7/flox7 mice. For immunoblotting, affinity-purified rabbit anti-AADC antibody (Chemicon, AB136) was used at 1:1,000. Expression of cortical AADC was comparable in wild-type mice and Aadcflox7/flox7 mice (data not shown). Therefore, Aadcflox7/flox7 alleles do not affect AADC expression in the kidney. Aadcflox7/flox7 mice were crossed with mice in which Cre is under the control of the γ-GT promoter to selectively delete exon 7 of the Aadc gene in the renal proximal tubule (AadcΔ7/+ mice). AadcΔ7/+ mice were intercrossed to generate AadcΔ7/Δ7 mice, in which the Aadc gene in renal proximal tubule has been deleted selectively (ptAadc–/– mice). ptAadc–/– mice were backcrossed for 10 generations onto the 129/SvJ background. Unless otherwise indicated, studies were performed both on mice on a mixed B6/129 background and the pure 129/SvJ background, and similar results were obtained; results from studies of mice on the pure 129/svj background are reported.
Animals.
All animal experiments were performed in accordance with the guidelines and with the approval of the Institutional Animal Care and Use Committee of Vanderbilt University. A subset of animals was placed on either a low-salt diet (0.02%–0.03% NaCl, ICN Biochemicals) or a high-salt diet (8% NaCl, Research Diets). The dopamine precursor l-DOPA was given in the drinking water (0.5 mg/ml), which contained 0.1% l-ascorbic acid to prevent oxidation of l-DOPA (Sigma-Aldrich) (15). To collect 24-hour urine, the animals were first acclimated individually in metabolic cages, and then 24-hour urine was collected.
For studies of chronic Ang II infusion, only wild-type and ptAadc–/– mice backcrossed to the 129/SvJ background were used. In our preliminary experiment, unilaterally nephrectomized mice were treated with Ang II (BACHEM) at a dose of 1.4 mg/kg/d through subcutaneous osmotic minipumps (model 2004, Alzet) (35). Surprisingly, all ptAadc–/– mice died within 4 weeks after initiation of Ang II infusion. Therefore, a reduced Ang II dose (0.9 mg/kg/d) was used for the reported studies. Tubule injury in response to 4 weeks of Ang II infusion was scored on H&E-stained sections (×400 magnification) and was identified by the presence of tubular dilatation, intraluminal casts, loss of brush border, and/or tubular cell swelling, blebbing, vacuolization, and detachment. All cortical fields were evaluated, and the injury score was assessed as the percentage of each field that was occupied by injured tubules and was scored as follows: 0, 0%; 1, less than 25%; 2, 25%–50%; 3, more than 50% to 75%; and 4, more than 75%. An average was then calculated for each kidney. The pathologist was blinded to the groups.
Because of the possibility that inbred lines harbor recessive mutations that might predispose to development of disorders that could negatively impact survival, the aging studies were performed in mixed B6/129 mice. It is highly unlikely that the differences seen in survival were due to variations in admixture of the 129 and B6 genomes, since the life span of both strains is similar to that of the wild-type mixed B6/129 mice.
Blood pressure measurement.
Blood pressure was measured in awake, chronically catheterized mice. Mice were anesthetized with 80 μg/g ketamine (Fort Dodge Laboratories) and 8 μg/g inactin (BYK) by i.p. administration. Mice were placed on a temperature-controlled pad. PE-10 tubing was inserted into the right carotid artery, tunneled under the skin, exteriorized, secured at the back of the neck, filled with heparinized saline, and sealed. The catheterized mice were housed individually, and blood pressure measurements were made 24 and 48 hours after surgery with a Blood Pressure Analyzer (Micro-Med) (39). Data are presented as mean blood pressure (MBP). In addition, in a subset of mice, blood pressures were monitored by radiotelemetry. Mice were anesthetized with Nembutal (50 mg/kg, i.p.). Radiotelemetric catheters (PA-C10, Data Sciences International) were inserted into the left common carotid artery with the transmitter implanted subcutaneously. Mice were housed individually. After 5 to 7 days, mice had recovered from surgery, and heart rate and blood pressure were recorded at 4-minute intervals for the duration of the study. The data from the telemetric device were collected using the Dataquest A.R.T system, version 4.0 (Data Sciences International), by way of a RPC-1 receiver placed under the mouse cage. In the subset of mice treated with either Ang II or candesartan and in the aged mice, SBP was measured with a tail-cuff microphonic manometer (40).
RNA isolation and quantitative real-time PCR.
Total RNA was isolated from kidneys using TRIzol reagents (Invitrogen) according to the manufacturer’s instructions. Quantitative PCR was performed using a TaqMan Real-Time PCR machine (7900HT, Applied Biosystems). The Master Mix and all gene probes were also purchased from Applied Biosystems. The probes used in the experiments included mouse S18 (Mm02601778); ACE1 (Mm00802048); ACE2 (Mm01159003); Mas (Mm0062713); Ang II receptors AT1a (Mm01166161), AT1b (Mm01701115), and AT2 (Mm01341373); NHE3 (Mm01352473); NBC (Mm01347935); NKCC2 (Mm00441424); NCC (Mm00490213); and AQP2 (Mm00437575).
Measurement of dopamine.
Dopamine was measured by HPLC coupled with electrochemical detection by the Neurochemistry Core Laboratory at Vanderbilt University’s Center for Molecular Neuroscience Research.
PRA.
Blood was taken from conscious mice via femoral vein in the morning, between 9:00 AM to 11:00 AM, and collected into a 75-μl hematocrit tube containing 1 μl 125 mM EDTA in its tip. The plasma was separated and frozen at –80°C until assayed. PRA was determined by radioimmunoassay (Gammacoat, DiaSorin), which measured the generation of Ang I. Plasma samples were incubated for 1 hour with excess exogenous renin substrate (plasma from rats nephrectomized 48 hours before collection) to generate Ang I. Plasma aldosterone levels were determined using RIA Kits (COAT-A-COUNT, Siemens, Siemens Medical Solutions).
Determination of urinary F2-isoprostane and PGE2.
Urinary F2-isoprostane and urinary PGE2 were measured by GC/electron capture/negative chemical ionization MS assay as previously described (18).
Flow cytometry.
After perfusion of the mice with PBS, 1 kidney was removed, minced into fragments, and digested in RPMI 1640 containing 2 mg/ml collagenase type D and 100 u/ml DNase I for 1 hour at 37°C, with intermittent agitation. Kidney fragments were passed through a 70-μm mesh (Falcon; BD Biosciences), yielding single cell suspensions. Cells were then centrifuged (800 g, 10 minutes, 8°C), resuspended in FACS buffer, kept on ice, and counted. 107 cells were incubated in 2.5 μg/ml Fc blocking solution, centrifuged again (800 g, 10 minutes, 8°C), and resuspended with FACS buffer. 106 cells were stained for 20 minutes at room temperature with antibodies, including FITC rat anti-mouse CD45 and PE/Cy7 anti-mouse F4/80 and CD8a (BD Biosciences), and then washed and resuspended in FACS buffer. After immunostaining, cells were analyzed immediately on an FACSCanto II Cytomer with DIVA software (Becton Dickinson), and off-line list mode data analysis was performed using Winlist software from Verity Software House.
Antibodies.
Affinity-purified rabbit anti-AADC antibody (AB136) was from Chemicon; rabbit anti-murine COX-2 was from Cayman Chemicals (item no. 160106); rabbit anti-renin antiserum was a gift from T. Inagami (Vanderbilt University); rat anti-mouse F4/80 (MCA497R) and Ly-6B.2 (neutrophil marker, MCA771GA) were from AbD Serotec; monoclonal anti-mouse KIM-1 (a marker of renal tubular injury, MAB1817) was from R&D Systems; rabbit anti-Mas was from Alomone (AAR-013); monoclonal anti-angiotensinogen was from ABBIOTEC (catalog no. 250551). All other antibodies were purchased from Santa Cruz Biotechnology Inc.
Immunohistochemistry and immunoblotting.
The mice were anesthetized with Nembutal (50 mg/kg, i.p.) and given heparin (1,000 units/kg, i.p.) to minimize coagulation. One kidney was removed for immunoblotting, qPCR, and flow cytometry, and the other was perfused with FPAS (3.7% formaldehyde, 10 mM sodium m-periodate, 40 mM phosphate buffer, and 1% acetic acid) through the aortic trunk. After fixation, the selected tissues were dehydrated, paraffin embedded, and immunostained as previously described (41, 42). Immunoblotting was carried out as described previously (43).
Quantitative image analysis.
Macrophage and neutrophil infiltration and nitrotyrosine immunostaining were quantified using the BIOQUANT image analysis system (R&M Biometrics) (14). Bright-field images from a Leitz Orthoplan microscope with DVC digital RGB video camera were digitized and saved as computer files. Contrast and color level adjustment (Adobe Photoshop) were performed for the entire image, i.e., no region- or object-specific editing or enhancements were performed.
Statistics.
Values are presented as mean ± SEM. ANOVA and Bonferroni t test were used for statistical analysis, and differences were considered significant when P < 0.05.
Supplementary Material
Acknowledgments
These studies were supported in part by grants from the NIH (DK62794, DK51265, DK38226, CA122620, DK61018, GM 15431, and ES 13125), by the Vanderbilt O’Brien Center (DK79341), and by funds from the Veterans Administration. We also acknowledge the helpful assistance of the Vanderbilt MMPC.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2011;121(7):2845–2854. doi:10.1172/JCI57324.
References
- 1.Felder RA, Jose PA. Mechanisms of disease: the role of GRK4 in the etiology of essential hypertension and salt sensitivity. Nat Clin Pract Nephrol. 2006;2(11):637–650. doi: 10.1038/ncpneph0301. [DOI] [PubMed] [Google Scholar]
- 2.Aperia AC. Intrarenal dopamine: a key signal in the interactive regulation of sodium metabolism. Annu Rev Physiol. 2000;62:621–647. doi: 10.1146/annurev.physiol.62.1.621. [DOI] [PubMed] [Google Scholar]
- 3.Banday AA, Lokhandwala MF. Dopamine receptors and hypertension. Curr Hypertens Rep. 2008;10(4):268–275. doi: 10.1007/s11906-008-0051-9. [DOI] [PubMed] [Google Scholar]
- 4.Zeng C, Jose PA. Dopamine receptors: important antihypertensive counterbalance against hypertensive factors. Hypertension. 2011;57(1):11–17. doi: 10.1161/HYPERTENSIONAHA.110.157727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quinones H, Collazo R, Moe OW. The dopamine precursor L-dihydroxyphenylalanine is transported by the amino acid transporters rBAT and LAT2 in renal cortex. Am J Physiol Renal Physiol. 2004;287(1):F74–F80. doi: 10.1152/ajprenal.00237.2003. [DOI] [PubMed] [Google Scholar]
- 6.Pinho MJ, Serrao MP, Soares-da-Silva P. High-salt intake and the renal expression of amino acid transporters in spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2007;292(5):F1452–F1463. doi: 10.1152/ajprenal.00465.2006. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi M, Yamaji Y, Kitajima W, Saruta T. Aromatic L-amino acid decarboxylase activity along the rat nephron. Am J Physiol. 1990;258(1 pt 2):F28–F33. doi: 10.1152/ajprenal.1990.258.1.F28. [DOI] [PubMed] [Google Scholar]
- 8.Bertorello A, Hokfelt T, Goldstein M, Aperia A. Proximal tubule Na+-K+-ATPase activity is inhibited during high-salt diet: evidence for DA-mediated effect. Am J Physiol. 1988;254(6 pt 2):F795–F801. doi: 10.1152/ajprenal.1988.254.6.F795. [DOI] [PubMed] [Google Scholar]
- 9.Baines AD. Effects of salt intake and renal denervation on catecholamine catabolism and excretion. Kidney Int. 1982;21(2):316–322. doi: 10.1038/ki.1982.24. [DOI] [PubMed] [Google Scholar]
- 10.Sidhu A, Kumar U, Uh M, Patel S. Diminished expression of renal dopamine D1A receptors in the kidney inner medulla of the spontaneously hypertensive rat. J Hypertens. 1998;16(5):601–608. doi: 10.1097/00004872-199816050-00007. [DOI] [PubMed] [Google Scholar]
- 11.Iimura O. The role of renal dopaminergic activity in the pathophysiology of essential hypertension. Jpn Heart J. 1996;37(6):815–828. doi: 10.1536/ihj.37.815. [DOI] [PubMed] [Google Scholar]
- 12.Shikuma R, et al. Dopaminergic modulation of salt sensitivity in patients with essential hypertension. Life Sci. 1986;38(10):915–921. doi: 10.1016/0024-3205(86)90259-6. [DOI] [PubMed] [Google Scholar]
- 13.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110(3):341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang MZ, Yao B, McKanna JA, Harris RC. Cross talk between the intrarenal dopaminergic and cyclooxygenase-2 systems. Am J Physiol Renal Physiol. 2005;288(4):F840–F845. doi: 10.1152/ajprenal.00240.2004. [DOI] [PubMed] [Google Scholar]
- 15.Zhang MZ, Yao B, Fang X, Wang S, Smith JP, Harris RC. Intrarenal dopaminergic system regulates renin expression. Hypertension. 2009;53(3):564–570. doi: 10.1161/HYPERTENSIONAHA.108.127035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peti-Peterdi J, Harris RC. Macula densa sensing and signaling mechanisms of renin release. J Am Soc Nephrol. 2010;21(7):1093–1096. doi: 10.1681/ASN.2009070759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi Z, et al. Opposite effects of cyclooxygenase-1 and -2 activity on the pressor response to angiotensin II. J Clin Invest. 2002;110(1):61–69. doi: 10.1172/JCI14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrow JD, Roberts LJ. The isoprostanes: unique bioactive products of lipid peroxidation. Prog Lipid Res. 1997;36(1):1–21. doi: 10.1016/S0163-7827(97)00001-5. [DOI] [PubMed] [Google Scholar]
- 19.Cheng HF, Becker BN, Harris RC. Dopamine decreases expression of type-1 angiotensin II receptors in renal proximal tubule. J Clin Invest. 1996;97(12):2745–2752. doi: 10.1172/JCI118729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H, et al. Dopamine 5 receptor mediates Ang II type 1 receptor degradation via a ubiquitin-proteasome pathway in mice and human cells. J Clin Invest. 2008;118(6):2180–2189. doi: 10.1172/JCI33637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benigni A, et al. Disruption of the Ang II type 1 receptor promotes longevity in mice. J Clin Invest. 2009;119(3):524–530. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silldorf E, Yang S, Pallone T. Prostaglandin E2 abrogates endothelin-induced vasoconstriction in renal outer medullary descending vasa recta of the rat. J Clin Invest. 1995;95(6):2734–2740. doi: 10.1172/JCI117976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cowley A, Mattson D, Lu S, Roman R. The renal medulla and hypertension. Hypertension. 1995;25(4 pt 2):663–673. doi: 10.1161/01.hyp.25.4.663. [DOI] [PubMed] [Google Scholar]
- 24.Harris RC, Breyer MD. Physiological regulation of cyclooxygenase-2 in the kidney. Am J Physiol Renal Physiol. 2001;281(1):F1–F11. doi: 10.1152/ajprenal.2001.281.1.F1. [DOI] [PubMed] [Google Scholar]
- 25.Zewde T, Mattson DL. Inhibition of cyclooxygenase-2 in the rat renal medulla leads to sodium-sensitive hypertension. Hypertension. 2004;44(4):424–428. doi: 10.1161/01.HYP.0000140924.91479.03. [DOI] [PubMed] [Google Scholar]
- 26.Huo TL, Grenader A, Blandina P, Healy DP. Prostaglandin E2 production in rat IMCD cells. II. Possible role for locally formed dopamine. Am J Physiol. 1991;261(4 pt 2):F655–F662. doi: 10.1152/ajprenal.1991.261.4.F655. [DOI] [PubMed] [Google Scholar]
- 27.Yao B, Harris RC, Zhang MZ. Intrarenal dopamine attenuates deoxycorticosterone acetate/high salt-induced blood pressure elevation in part through activation of a medullary cyclooxygenase 2 pathway. Hypertension. 2009;54(5):1077–1083. doi: 10.1161/HYPERTENSIONAHA.109.137174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen CJ, Apparsundaram S, Lokhandwala MF. Intrarenally produced angiotensin II opposes the natriuretic action of the dopamine-1 receptor agonist fenoldopam in rats. J Pharmacol Exp Ther. 1991;256(2):486–491. [PubMed] [Google Scholar]
- 29.Gesek FA, Schoolwerth AC. Hormone responses of proximal Na(+)-H+ exchanger in spontaneously hypertensive rats. Am J Physiol. 1991;261(3 pt 2):F526–F536. doi: 10.1152/ajprenal.1991.261.3.F526. [DOI] [PubMed] [Google Scholar]
- 30.Aperia A, Holtback U, Syren ML, Svensson LB, Fryckstedt J, Greengard P. Activation/deactivation of renal Na+,K(+)-ATPase: a final common pathway for regulation of natriuresis. FASEB J. 1994;8(6):436–439. doi: 10.1096/fasebj.8.6.8168694. [DOI] [PubMed] [Google Scholar]
- 31.Zeng C, et al. Activation of D3 dopamine receptor decreases angiotensin II type 1 receptor expression in rat renal proximal tubule cells. Circ Res. 2006;99(5):494–500. doi: 10.1161/01.RES.0000240500.96746.ec. [DOI] [PubMed] [Google Scholar]
- 32.Zeng C, et al. Interaction of angiotensin II type 1 and D5 dopamine receptors in renal proximal tubule cells. Hypertension. 2005;45(4):804–810. doi: 10.1161/01.HYP.0000155212.33212.99. [DOI] [PubMed] [Google Scholar]
- 33.Stegbauer J, Coffman TM. New insights into angiotensin receptor actions: from blood pressure to aging. Curr Opin Nephrol Hypertens. 2011;20(1):84–88. doi: 10.1097/MNH.0b013e3283414d40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salomone LJ, et al. Intrarenal dopamine D1-like receptor stimulation induces natriuresis via an angiotensin type-2 receptor mechanism. Hypertension. 2007;49(1):155–161. doi: 10.1161/01.HYP.0000251881.89610.ee. [DOI] [PubMed] [Google Scholar]
- 35.Lautrette A, et al. Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: a new therapeutic approach. Nat Med. 2005;11(8):867–874. doi: 10.1038/nm1275. [DOI] [PubMed] [Google Scholar]
- 36.Schlager G. Longevity in spontaneously hypertensive mice. Exp Gerontol. 1981;16(4):325–330. doi: 10.1016/0531-5565(81)90051-6. [DOI] [PubMed] [Google Scholar]
- 37.Crowley SD, et al. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest. 2005;115(4):1092–1099. doi: 10.1172/JCI200523378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crowley SD, et al. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A. 2006;103(47):17985–17990. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yao B, Harris RC, Zhang MZ. Interactions between 11beta-hydroxysteroid dehydrogenase and COX-2 in kidney. Am J Physiol Regul Integr Comp Physiol. 2005;288(6):R1767–R1773. doi: 10.1152/ajpregu.00786.2004. [DOI] [PubMed] [Google Scholar]
- 40.Cheng HF, et al. Angiotensin II attenuates renal cortical cyclooxygenase-2 expression. J Clin Invest. 1999;103(7):953–961. doi: 10.1172/JCI5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang MZ, Yao B, Cheng HF, Wang SW, Inagami T, Harris RC. Renal cortical cyclooxygenase 2 expression is differentially regulated by angiotensin II AT(1) and AT(2) receptors. Proc Natl Acad Sci U S A. 2006;103(43):16045–16050. doi: 10.1073/pnas.0602176103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang MZ, Wang JL, Cheng HF, Harris RC, McKanna JA. Cyclooxygenase-2 in rat nephron development. Am J Physiol. 1997;273(6 pt 2):F994–F1002. doi: 10.1152/ajprenal.1997.273.6.F994. [DOI] [PubMed] [Google Scholar]
- 43.Harris RC, McKanna JA, Akai Y, Jacobson HR, Dubois RN, Breyer MD. Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J Clin Invest. 1994;94(6):2504–2510. doi: 10.1172/JCI117620. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.