Abstract
HDL cholesterol activates endothelial cell production of the atheroprotective signaling molecule NO, and it promotes endothelial repair. In this issue of the JCI, Besler et al. provide new data indicating that HDL from stable coronary artery disease (CAD) or acute coronary syndrome patients inhibits rather than stimulates endothelial NO synthesis and endothelial repair. This may be related to decreased HDL-associated paraoxonase 1 (PON1) activity. These observations support the concept that the cardiovascular impact of HDL is not simply related to its abundance, and the translation of the present findings to prospective studies of CAD risk and to evaluations of HDL-targeted therapeutics is a logical future goal.
HDL and cardiovascular disease
Numerous epidemiological studies indicate that the risk of cardiovascular disease is inversely related to HDL cholesterol levels (1). In addition, mice with elevated LDL due to the expression of a human apolipoprotein B transgene have more severe atherosclerosis if they also have low HDL due to deficiency of the major apolipoprotein of HDL, apoA-I (2). Furthermore, providing apoA-I or HDL improves vascular disease in hypercholesterolemic animal models (3, 4), and studies in humans have suggested that the infusion of apoA-I mimetics or reconstituted HDL particles has the potential to reverse atherosclerosis (5). However, genetically based variations in HDL levels in humans do not correspond directly with relative incidence or severity of cardiovascular disease (6), and therapies that effectively cause increases in HDL levels have not yielded clear-cut decreases in cardiovascular disease (7, 8). This was particularly the case in a trial of torcetrapib, an inhibitor of cholesteryl ester transfer protein, which yielded a 72% increase in HDL levels but an actual increase in cardiovascular events (9). Thus, the cardiovascular impact of HDL is not simply related to the abundance of the lipoprotein, and there is a great need for increased understanding of the biological actions of HDL and the development of assays to assess HDL function in humans. In the current issue of the JCI, Besler and colleagues provide important new insights into the atheroprotective actions of HDL in a comparison of the endothelial functions of HDL obtained from healthy individuals and HDL from patients with stable coronary artery disease (CAD) or acute coronary syndrome (10), designated as HDLHealthy and HDLCAD, respectively, throughout this commentary.
Biological actions of HDL
The classical function of HDL is to mediate cholesterol efflux from peripheral tissues and cells such as macrophages and transport it back to the liver in a process known as reverse cholesterol transport (RCT). RCT likely plays an important role in the cardiovascular protective capacity of HDL (11). A measure of the “cholesterol efflux capacity” of HDL was recently reported to be inversely associated with atherosclerosis in humans even after adjusting for HDL cholesterol levels (12).
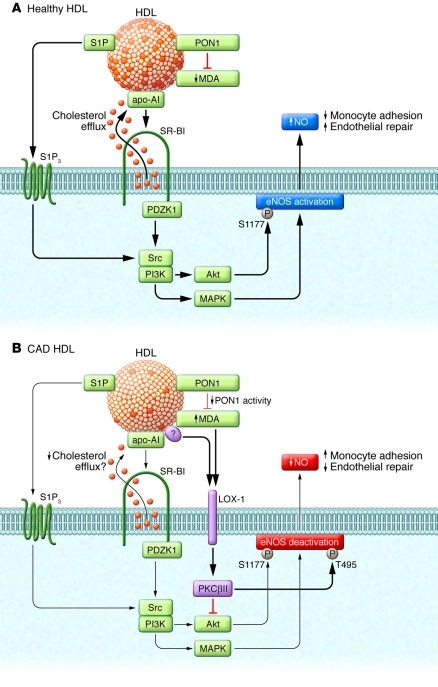
In addition to promoting RCT, it is now well appreciated that HDL has direct actions on the endothelium that may also contribute to the cardiovascular protective characteristics of the lipoprotein (13). In endothelial cells, HDL from healthy individuals activates the production of the antiatherosclerotic and antithrombotic signaling molecule NO by eNOS (14), it blunts adhesion molecule expression (13), attenuates tissue factor and E-selectin expression (13), and promotes endothelial cell migration, thereby optimizing endothelial repair and the integrity of the intimal layer (15). eNOS activation by HDL entails apoA-I–dependent binding of the lipoprotein to scavenger receptor class B, type I (SR-BI) in endothelial cells; this causes cholesterol efflux that is sensed by SR-BI and initiates a signaling cascade involving the activation of Src family kinase(s), PI3K, and Akt, which phosphorylates eNOS at Ser1177 to increase enzyme activity (Figure 1A). These processes are dependent on the adaptor protein PDZK1, which binds to the extreme C-terminus of SR-BI. SR-BI–, Src-, and PI3K-dependent Erk MAPK activation is also required for eNOS activation by HDL. In addition, HDL-associated sphingosine-1-phosphate (S1P) and related molecules may activate the lysophospholipid receptor S1P3 to stimulate eNOS (16).
Figure 1. Changes in endothelial HDL action in coronary artery disease.
(A) HDLHealthy stimulates eNOS through multiple mechanisms. HDL binding to SR-BI via apoA-I causes cholesterol efflux (orange circles) that is sensed by SR-BI, leading to PDZK1-dependent activation of Src family kinase(s), PI3K, and Akt, which phosphorylates eNOS Ser1177 and thereby increases enzyme activity. SR-BI–, Src-, and PI3K-dependent Erk MAPK activation is also required for eNOS activation by HDL. HDL-associated S1P and related molecules may activate the lysophospholipid receptor S1P3 to stimulate eNOS. HDLHealthy contains active PON1, which suppresses the formation of oxidized lipids and lipoproteins such as MDA. The net effect of eNOS activation by HDLHealthy is to blunt endothelial cell–monocyte adhesion and promote endothelial repair. (B) HDLCAD activates inhibitory signaling that suppresses eNOS activation. HDLCAD has reduced PON1 activity that potentially leads to greater formation of MDA, which activates LOX-1 and thereby stimulates PKCβ. PKCβ inhibits stimulatory Akt and eNOS phosphorylation events, and it enhances the inhibitory phosphorylation of eNOS at Thr495. As a result, HDLCAD has an impaired capacity to favorably influence endothelial cell–monocyte adhesion or endothelial repair. It is unknown whether the loss in PON1 activity leads to alterations in other HDL constituents besides MDA that activate LOX-1. Whether HDLCAD has decreased capacity to evoke cholesterol efflux from endothelial cells — possibly via MDA-related modification of apoA-I, resulting in attenuated SR-BI–dependent signaling — is also unknown. In addition, it is unknown whether endothelial cell signaling activated by cargo molecules such as S1P is altered in HDLCAD.
Adverse endothelial effects of HDL from CAD patients
Besler and colleagues now report that whereas HDL from healthy individuals (HDLHealthy) causes an increase in bioavailable endothelium-derived NO, HDL from patients with stable CAD or acute coronary syndrome (HDLCAD) causes no increase or an actual decrease in NO (10). This is related to decreased activating eNOS Ser1177 phosphorylation and increased inactivating eNOS Thr495 phosphorylation by HDLCAD (Figure 1B). They also show that in an eNOS-dependent manner, HDLHealthy promotes endothelial repair and blunts NF-κB activation and VCAM-1 expression, thereby preventing endothelial cell–monocyte adhesion, whereas HDLCAD lacks these properties. They interrogated the basis for the adverse effects of HDLCAD on endothelial function, finding that total binding of HDLCAD to endothelium is decreased, but relative SR-BI–dependent binding is not altered. However, the researchers did not use endothelial cells to evaluate the effect of HDLCAD on cholesterol efflux, which is critically involved in endothelial HDL SR-BI–mediated signaling to eNOS. When the efflux capacity of HDL is specifically altered, the relative activation of eNOS changes in parallel (17). If HDLCAD has a blunted capacity to promote cholesterol efflux from endothelial cells, this might help to explain the observed impairment in NO generation. This could be experimentally tested by the ex vivo addition of phosphatidylcholine (18) to enhance the endothelial cell cholesterol efflux capacity of HDLCAD and determination of whether doing so restores the ability to generate NO.
Besler and colleagues additionally demonstrated that through a process involving the endothelial multiligand receptor known as lectin-type oxidized LDL receptor 1 (LOX-1), HDLCAD activates endothelial PKCβII, which in turn inhibits Akt-activating phosphorylation (Akt-Ser473) and eNOS-activating phosphorylation (eNOS-Ser1177) events and NO production (Figure 1B). Recognizing that endothelial LOX-1 is activated by oxidized lipids, they then evaluated the potential role of malondialdehyde (MDA) and found that MDA content is increased in HDLCAD compared with HDLHealthy. They also show that the addition of MDA to HDLHealthy blunts endothelial NO production and activates endothelial PKCβII in a LOX-1–dependent fashion. Since HDL-associated paraoxonase 1 (PON1) diminishes MDA formation, they then evaluated PON1 and found that although its abundance is nearly doubled in HDLCAD versus HDLHealthy, its enzyme activity is markedly decreased in HDLCAD. The researchers further found that PON1 inactivation in HDLHealthy leads to greater PKCβII activation, decreased activating eNOS-Ser1177 phosphorylation and increased inactivating eNOS-Thr495 phosphorylation, blunted NO production, increased monocyte–endothelial cell adhesion, and impaired endothelial repair. These findings suggest a potential mechanistic link between decreased PON1 activity in HDLCAD and exaggerated PKCβII activation and impaired eNOS and endothelial function. Furthermore, they showed that HDL from Pon1–/– mice fails to stimulate NO production or to antagonize endothelial inflammatory activation, and that supplementation of Pon1–/– HDL with purified PON1 restores these functions (10). Since cholesterol-free lipoprotein particles consisting solely of apoA-I and phosphatidylcholine are sufficient to cause eNOS activation (17), similar to HDLCAD, the Pon1–/– HDL must contain component(s) that blunt eNOS activation such as MDA, which decreases efflux capacity via the modification of apoA-I (19). Although the available evidence implicates MDA, the modified component of HDLCAD and Pon1–/– HDL that is directly responsible for LOX-1– and PKCβII-mediated eNOS inactivation requires further clarification. It is also unclear what causes the downregulation of PON1 activity in HDLCAD, even though its abundance is increased. In any case, the findings by Besler et al. importantly indicate that HDL-associated PON1 has a major impact on endothelial function, which is consistent with the reported inverse relationship between PON1 activity and cardiovascular disease development (20).
Remaining questions
The work by Besler and colleagues has provided valuable evidence that HDL from CAD patients differs from HDL from healthy individuals in its capacity to invoke signaling in endothelial cells that induces eNOS activation and subsequent antiatherogenic and antiinflammatory processes. It also supports the concept that the cardiovascular impact of HDL is not simply related to its abundance. Furthermore, the findings suggest that assays of HDL action on endothelium may increase our ability to assign cardiovascular disease risk, and they may enhance our understanding of the outcomes of future trials testing HDL-targeted therapies. However, there are a number of remaining questions. Do the differences in endothelial intracellular signaling observed in cell culture in response to HDLHealthy versus HDLCAD reflect disparities in HDL-induced signaling in vivo? Are there differences in other critical endothelial cell phenotypes in vivo besides repair, such as leukocyte adhesion? The authors also appropriately point out that it remains unknown whether their findings represent a cause or a consequence of CAD. In this regard, the homogeneity of the functional defect in HDLCAD observed in this study is surprising, and additional cross-sectional studies with a wider spectrum of cardiovascular disease incidence and severity as well as prospective studies are now warranted. Combined with the recent study of atherosclerotic vascular disease and HDL macrophage cholesterol efflux (12), the work by Besler et al. indicates that we no longer need to ponder, but instead can conclude that measures of HDL function are clinically relevant. If we are earnest in our desire to harness the cardiovascular protective potential of HDL, we must go well beyond the quantification and even successful modification of HDL abundance, and reliably quantify and ultimately take therapeutic advantage of the bases for differences in HDL function.
Acknowledgments
This work was supported by NIH grant HL58888 (to P.W. Shaul). Additional support was provided by the Crystal Charity Ball Center for Pediatric Critical Care Research and the Lowe Foundation (to P.W. Shaul).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2011;121(7):2545–2548. doi:10.1172/JCI57671.
See the related article beginning on page 2693.
References
- 1.Gordon DJ, Rifkind BM. High-density lipoprotein — the clinical implications of recent studies. N Engl J Med. 1989;321(19):1311–1316. doi: 10.1056/NEJM198911093211907. [DOI] [PubMed] [Google Scholar]
- 2.Hughes SD, Verstuyft J, Rubin EM. HDL deficiency in genetically engineered mice requires elevated LDL to accelerate atherogenesis. Arterioscler Thromb Vasc Biol. 1997;17(9):1725–1729. doi: 10.1161/01.atv.17.9.1725. [DOI] [PubMed] [Google Scholar]
- 3.Forrester JS, Shah PK. Emerging strategies for increasing high-density lipoprotein. Am J Cardiol. 2006;98(11):1542–1549. doi: 10.1016/j.amjcard.2006.06.059. [DOI] [PubMed] [Google Scholar]
- 4.Williams KJ, Feig JE, Fisher EA. Rapid regression of atherosclerosis: insights from the clinical and experimental literature. Nat Clin Pract Cardiovasc Med. 2008;5(2):91–102. doi: 10.1038/ncpcardio1086. [DOI] [PubMed] [Google Scholar]
- 5.Mendez AJ. The promise of apolipoprotein A-I mimetics. Curr Opin Endocrinol Diabetes Obes. 2010;17(2):171–176. doi: 10.1097/MED.0b013e3283373cb5. [DOI] [PubMed] [Google Scholar]
- 6.Khera AV, Rader DJ. Discovery and validation of new molecular targets in treating dyslipidemia: the role of human genetics. Trends Cardiovasc Med. 2009;19(6):195–201. doi: 10.1016/j.tcm.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briel M, et al. Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: systematic review and meta-regression analysis. BMJ. 2009;338:b92. doi: 10.1136/bmj.b92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh IM, Shishehbor MH, Ansell BJ. High-density lipoprotein as a therapeutic target: a systematic review. JAMA. 2007;298(7):786–798. doi: 10.1001/jama.298.7.786. [DOI] [PubMed] [Google Scholar]
- 9.Barter PJ, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357(21):2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 10.Besler C, et al. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J Clin Invest. 2011;121(7):2693–2708. doi: 10.1172/JCI42946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duffy D, Rader DJ. Update on strategies to increase HDL quantity and function. Nat Rev Cardiol. 2009;6(7):455–463. doi: 10.1038/nrcardio.2009.94. [DOI] [PubMed] [Google Scholar]
- 12.Khera AV, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364(2):127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mineo C, Deguchi H, Griffin JH, Shaul PW. Endothelial and antithrombotic actions of HDL. Circ Res. 2006;98(11):1352–1364. doi: 10.1161/01.RES.0000225982.01988.93. [DOI] [PubMed] [Google Scholar]
- 14.Yuhanna IS, et al. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat Med. 2001;7(7):853–857. doi: 10.1038/89986. [DOI] [PubMed] [Google Scholar]
- 15.Seetharam D, et al. High-density lipoprotein promotes endothelial cell migration and reendothelialization via scavenger receptor-B type I. Circ Res. 2006;98(1):63–72. doi: 10.1161/01.RES.0000199272.59432.5b. [DOI] [PubMed] [Google Scholar]
- 16.Saddar S, Mineo C, Shaul PW. Signaling by the high-affinity HDL receptor scavenger receptor B type I. Arterioscler Thromb Vasc Biol. 2010;30(2):144–150. doi: 10.1161/ATVBAHA.109.196170. [DOI] [PubMed] [Google Scholar]
- 17.Assanasen C, et al. Cholesterol binding, efflux, and a PDZ-interacting domain of scavenger receptor-BI mediate HDL-initiated signaling. J Clin Invest. 2005;115(4):969–977. doi: 10.1172/JCI200523858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yancey PG, et al. High density lipoprotein phospholipid composition is a major determinant of the bi-directional flux and net movement of cellular free cholesterol mediated by scavenger receptor BI. J Biol Chem. 2000;275(47):36596–36604. doi: 10.1074/jbc.M006924200. [DOI] [PubMed] [Google Scholar]
- 19.Shao B, et al. Modifying apolipoprotein A-I by malondialdehyde, but not by an array of other reactive carbonyls, blocks cholesterol efflux by the ABCA1 pathway. J Biol Chem. 2010;285(24):18473–18484. doi: 10.1074/jbc.M110.118182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Regieli JJ, et al. Paraoxonase variants relate to 10-year risk in coronary artery disease: impact of a high-density lipoprotein-bound antioxidant in secondary prevention. J Am Coll Cardiol. 2009;54(14):1238–1245. doi: 10.1016/j.jacc.2009.05.061. [DOI] [PubMed] [Google Scholar]