Abstract
A ketogenic diet (KD) is a high-fat, low-carbohydrate metabolic regimen; its effectiveness in the treatment of refractory epilepsy suggests that the mechanisms underlying its anticonvulsive effects differ from those targeted by conventional antiepileptic drugs. Recently, KD and analogous metabolic strategies have shown therapeutic promise in other neurologic disorders, such as reducing brain injury, pain, and inflammation. Here, we have shown that KD can reduce seizures in mice by increasing activation of adenosine A1 receptors (A1Rs). When transgenic mice with spontaneous seizures caused by deficiency in adenosine metabolism or signaling were fed KD, seizures were nearly abolished if mice had intact A1Rs, were reduced if mice expressed reduced A1Rs, and were unaltered if mice lacked A1Rs. Seizures were restored by injecting either glucose (metabolic reversal) or an A1R antagonist (pharmacologic reversal). Western blot analysis demonstrated that the KD reduced adenosine kinase, the major adenosine-metabolizing enzyme. Importantly, hippocampal tissue resected from patients with medically intractable epilepsy demonstrated increased adenosine kinase. We therefore conclude that adenosine deficiency may be relevant to human epilepsy and that KD can reduce seizures by increasing A1R-mediated inhibition.
Introduction
A ketogenic diet (KD) is an alternative metabolic treatment for epilepsy, and multiple retrospective and prospective studies confirm its clinical benefits (1, 2). The high-fat, low-carbohydrate composition of a KD forces ketone- rather than glucose-based metabolism, but it is not known how this shift leads to anticonvulsant consequences. Primary applications of KD therapy include pediatric and medically refractory epilepsy; its use is increasing globally, and clinical benefits are similar across cultures and age groups (1, 2). Despite its success, side effects and requisite strict compliance have limited widespread use of KD, and a diet-based approach is often considered as a last resort. Better understanding of the mechanisms involved in the anticonvulsant actions of a KD could lead to development of pharmacological strategies that take advantage of beneficial aspects and limit problems associated with diet therapy.
Adenosine acting at adenosine A1 receptors (A1Rs) is a logical candidate for the effects of KD therapy (3). Adenosine is well-established as a powerful anticonvulsant (4), and endogenous adenosine acting at A1Rs is an important seizure-control mechanism (5). Moreover, deletion of A1Rs and increased adenosine clearance by elevated adenosine kinase (ADK) both cause spontaneous intrahippocampal electrographic seizures (6) and increase the brain’s susceptibility to injury (7). Conversely, therapeutic adenosine augmentation is highly effective in controlling seizures (8). Adenosine is the core of ATP, a key molecule in basic biochemistry and a ligand at its own family of G protein–coupled cell surface receptors. Thus, adenosine is a homeostatic bioenergetic network regulator involved in metabolism and ongoing neuronal activity and is well-positioned to translate metabolic changes into altered brain activity. Using 3 lines of transgenic mice that all exhibit electrographic seizures as a result of deficient adenosine signaling (6), we present here what we believe to be the first direct evidence that adenosine acting at A1Rs contributes to the therapeutic effects of KDs.
Results and Discussion
A1R activation suppresses seizures.
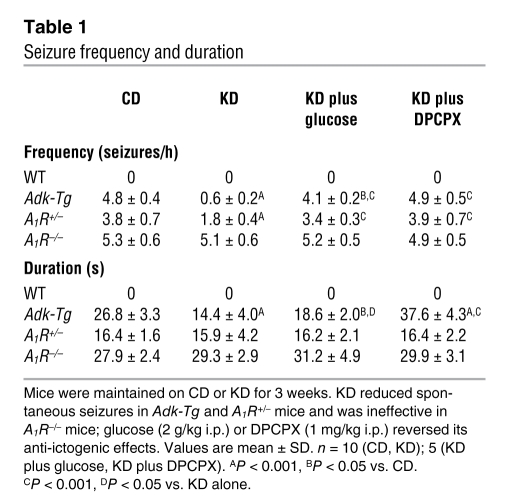
In line with our previous demonstration (6), A1R+/– mice, A1R–/– mice, and Adk-Tg mice (based on expression of a ubiquitously expressed Adk transgene on an Adk-null background) maintained on a control diet (CD) experienced regular electrographic hippocampal seizures consistent with deficient adenosine/A1R signaling (Figure 1). Seizure frequency and duration were similar between Adk-Tg and A1R–/– mice and significantly lower in A1R+/– mice (but still elevated above WT; Table 1). Behavioral (clinical) seizures were never observed in the mutants, and electrographic seizures were never observed in the WT group.
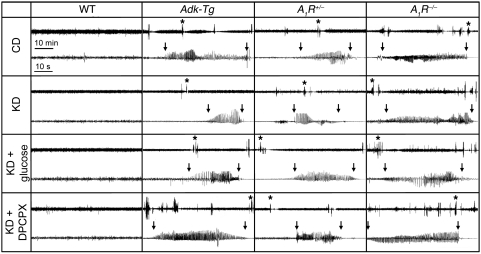
Figure 1. Seizure suppression by KD depends on A1R activation.
Representative EEG recordings from the CA3 of WT and transgenic mice reflect seizure distribution over a 1-hour time span (top traces) and individual seizures at higher resolution (1 minute; bottom traces). Asterisks in top traces denote the individual seizures chosen. Beginning and end of seizures are marked by vertical arrows. Traces from CD-fed animals showed baseline seizure activity in all mutants and lack of seizures in WT. KD almost completely abolished seizures in Adk-Tg mice; rare seizures were of reduced duration, as shown. KD reduced seizure activity in A1R+/– mice, had no effect in A1R–/– mice. Treatment with glucose or DPCPX reversed KD effects. See Table 1 for quantitation.
Table 1 .
Seizure frequency and duration
To quantify the effects of KD, WT and transgenic mice were fed CD or KD. At 3 weeks, KD nearly abolished seizures in Adk-Tg mice and significantly reduced the duration of remaining seizures (Table 1 and Figure 1). If KD suppresses seizures in Adk-Tg mice by elevating adenosine in brain, we predicted that its seizure-suppressing potential would be reduced in A1R+/– mice, which have only 50% of this receptor and lowered sensitivity to adenosine (9). As expected, KD-fed A1R+/– mice experienced a significant, but lesser (approximately 50%), reduction in seizure frequency, although seizure duration was not changed compared with CD-fed A1R+/– mice (Table 1). We further predicted that spontaneous seizures in A1R–/– mice would be resistant to the beneficial effects of KD if its protective mechanism involves activation of A1Rs. Indeed, KD was completely ineffective in affecting seizures in A1R–/– mice. These findings demonstrate that KD suppresses seizures caused by adenosine deficiency (Adk-Tg) or reduced adenosine signaling (A1R+/–), but has no effect in the absence of A1Rs (A1R–/–).
Seizures are restored with glucose or A1R blocker treatment.
To determine the extent to which the reduced frequency of spontaneous seizures in Adk-Tg and A1R+/– mice was specifically the result of the low-carbohydrate nature of KD, we injected glucose into KD-fed mice of each genotype. In Adk-Tg and A1R+/– mice, which displayed reduced seizures after KD, glucose injection increased seizure frequency significantly within 30–90 minutes; in Adk-Tg mice, the increase did not quite reestablish the baseline CD-fed seizure phenotype (Table 1 and Figure 1).
The loss of KD-induced seizure suppression in A1R–/– mice, combined with the reduced efficacy of KD in A1R+/– compared with Adk-Tg mice, is indicative of seizure suppression via a mechanism involving A1Rs. To test this further, we injected a nonconvulsive dose of 8-cyclopentyl-1,3-dipropylxanthine (DPCPX; 1 mg/kg), an A1R-selective antagonist. In Adk-Tg and A1R+/– mice, DPCPX restored seizure frequency to control levels, whereas it had no significant effect in WT or A1R–/– mice (Figure 1 and Table 1). DPCPX in KD-fed Adk-Tg mice increased seizure duration significantly compared with both CD and KD conditions; however, it did not change seizure duration in A1R+/– mice, which suggests that its effect on duration depended on the relative abundance of A1Rs. As expected, DPCPX had no effect on seizures in A1R–/– mice. These data, coupled with the lack of KD efficacy in A1R–/– mice, demonstrated that A1Rs are a molecular target whereby KD reduces ictogenesis in vivo.
WT and A1R–/– mice have similar metabolic responses.
To confirm that A1R–/– mice experienced a similar metabolic response to KD, we measured the plasma level of β-hydroxybutyrate (βHB) in A1R–/– compared with WT mice. There was no difference in βHB levels between genotypes when mice were fed CD (WT, 0.48 ± 0.09 mM; A1R–/–, 0.60 ± 0.09 mM; n = 5 per group, P > 0.05), and there was no evidence for inadequate or decreased ketones in A1R–/– mice fed KD for 3 weeks. βHB levels were elevated similarly and significantly in both genotypes in KD- versus CD-fed mice (WT, P < 0.001; A1R–/–, P < 0.002); overall, KD-fed A1R–/– mice demonstrated a nonsignificant trend toward higher βHB levels compared with KD-fed WT mice (WT, 1.38 ± 0.15 mM; A1R–/–, 1.96 ± 0.29 mM; n = 5 per group, P > 0.05).
KD reduces ADK expression.
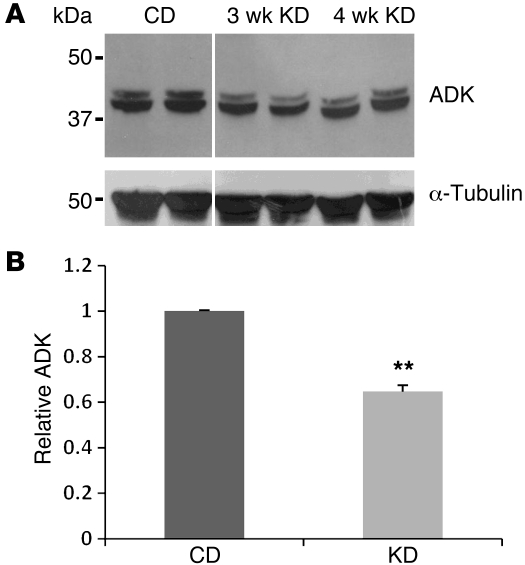
Ambient adenosine in brain is regulated largely by ADK (10). Whereas overexpression of ADK (causing adenosine deficiency) has been linked to seizures (6), reduced expression of ADK (thus increasing adenosine) renders the brain less susceptible to seizures (6) and might be involved in an endogenous protective mechanism of the brain in response to stress or injury (10). We therefore hypothesized that metabolic stress imposed by KD might likewise lead to reduction in brain ADK. KD- or CD-fed WT mice were sacrificed at 3 and 4 weeks of feeding, corresponding to the time span of seizure analysis above. Western blot showed that this key adenosine-regulating enzyme was downregulated significantly in the KD group (Figure 2), which suggests a possible mechanism for KD’s seizure suppression.
Figure 2. KD leads to downregulation of ADK.
(A) Representative Western blot from brain extracts of WT mice fed CD or KD for 3 and 4 weeks. Note the 2 different splice variants of ADK in the ADK-reactive bands. Anti-tubulin immunoreactivity was used to normalize for equal loading. Lanes were run on the same gel but were noncontiguous (white line). (B) Brain ADK from mice fed CD or KD for 3–4 weeks, expressed relative to CD (n = 4 per group). Data are mean ± SEM. **P < 0.01 vs. CD.
ADK overexpression in human epilepsy.
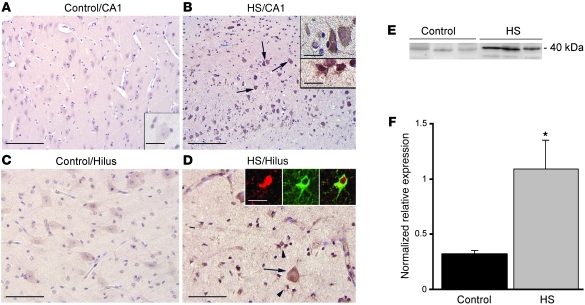
We have demonstrated previously that astrogliosis, ADK overexpression, and resulting adenosine deficiency are implicated in seizure generation in rodent models of epilepsy (6, 10). Therefore, reconstitution of normal adenosine signaling by KD might be of therapeutic value for epileptic patients with refractory temporal lobe epilepsy (TLE). To investigate whether adenosine dysfunction is likewise implicated in the pathology of human epilepsy, we studied the expression of ADK in hippocampus of patients with hippocampal sclerosis (HS) and histologically normal human hippocampus. ADK was upregulated within specific hippocampal regions in HS specimens compared with control hippocampus (Figure 3, A–D). Abundant ADK-immunopositive cells with typical astroglial morphology were observed in areas with prominent gliosis in all HS specimens; double-labeling confirmed ADK expression in glial fibrillary acidic protein–positive (GFAP-positive) reactive astrocytes (Figure 3, B and D). Western blot analysis confirmed a greater than 3-fold increase in ADK levels in hippocampus of TLE patients (Figure 3, E and F), which suggests that adenosine deficiency could contribute to the epileptic phenotype in those patients.
Figure 3. ADK immunoreactivity in hippocampus of control and TLE patients with medial temporal HS.
(A–D) Sections were counterstained with hematoxylin. Shown are representative CA1 (A and B) and hilus (C and D) from the same sample. (A and C) Control hippocampus showed weak ADK immunoreactivity. Histologically normal surgical hippocampus displayed an immunoreactivity pattern similar to that in control autopsy hippocampus (not shown). (B and D) The HS specimen demonstrated increased ADK expression in both residual pyramidal and hilar neurons (arrows and B, top inset) and in reactive astrocytes (arrowheads and B, bottom inset). Insets in D show expression of ADK (red) in a reactive astrocyte (GFAP, green). Scale bars: 160 μm (A and B); 80 μm (C and D); 40 μm (A, inset, and B, top inset); 15 μm (B, bottom inset, and D, insets). (E and F) Western blot analysis of ADK of total homogenates from control autopsy hippocampus and HS specimens. (E) Representative immunoblots. (F) Densitometric data, expressed relative to optical density of β-actin (n = 5 per group). Data are mean ± SEM. *P < 0.05 vs. control.
Using transgenic mice and complementary pharmacology, we demonstrated that KD-induced seizure control was dependent on A1Rs: KD virtually abolished seizures in Adk-Tg mice (with reduced endogenous adenosine and intact A1Rs), reduced seizures partially but significantly in A1R+/– mice, and had no effects in A1R–/– mice or in the presence of A1R blockade. We showed overexpression of ADK (which should reduce A1R activation) in human epilepsy and KD-induced reduction of ADK in mouse brain, thus providing a mechanism for KD-induced increase in adenosine and anticonvulsant effects of KD via A1Rs. Whereas altered adenosinergic signaling might not be the underlying cause for seizures in all epilepsies, adenosine has stopped seizures in every seizure model tested to date, including models of pharmacoresistant epilepsy. Thus, no matter the cause of the seizure, adenosine can help to resolve it, provided — as shown here — that A1Rs are present. Subclinical, brief electrographic seizure events (undetectable with scalp electrodes), as exhibited in all 3 strains of transgenic mice in the present study, are now increasingly recognized and likely play an important role in early epileptogenesis (11).
Acute reversal of seizure suppression by glucose confirmed the specificity of KD’s metabolic effects and comported with clinical observations and animal studies highlighting the importance of low glucose to the effects of KD (12). Indeed, we recently demonstrated in vitro that metabolic consequences of KD — reduced extracellular glucose and increased intracellular ATP — produce A1R-dependent inhibition of cornu ammonis region 3 (CA3) pyramidal cells (3). Of interest, glucose-induced seizure reversal in Adk-Tg mice was not complete (which indicates that the specific enzymatic activity of ADK might have been increased based on rapid glucose-dependent restoration of the energy charge), although ADK levels were still quantitatively reduced (Figure 2). Additional mechanisms might also contribute to KD-induced seizure suppression: in vitro studies demonstrated direct acute effects of ketones (13, 14), although the relative importance of these mechanisms to the chronic in vivo effects of KD administration in humans remains to be determined.
When relating these findings to human epilepsy, we found that brain tissue resected from humans with intractable epilepsy showed increased ADK — and therefore likely a relative adenosine deficiency. Previously, adenosine deficiency has been demonstrated directly in microdialysis samples from epileptogenic hippocampus in human patients with TLE (15). These results and our current data indicate that alteration of adenosine signaling is relevant to human epilepsy. Specifically, at least some forms of human epilepsy replicate key features of our adenosine-deficient model, increasing the translational potential of these results. Together, these data delineate a clinically relevant relationship among KD, adenosine, and epilepsy, which could lead to less-restrictive diets, alternate pharmaceutical approaches, and broader applications of metabolic strategies to different medical conditions.
Methods
Animals.
All animal procedures were approved by the Legacy Institutional Animal Care and Use Committee. Adk-Tg mice are based on expression of a ubiquitously expressed Adk transgene on an Adk-null background, leading to brain-wide ADK overexpression (6). A1R+/– and A1R–/– mice lack 1 or both A1R alleles (9). All animals were maintained on a C57BL/6 background. Male mice were fed either CD or KD (product no. F3666; Bioserv) ad libitum for 4 weeks (n = 10 per genotype). To reverse KD effects, animals received i.p. injection of either glucose in water (30% w/v; 2 g/kg) or DPCPX (1 mg/kg; 2 ml/kg in 20% DMSO [w/v]).
EEG studies.
Bipolar, coated stainless-steel electrodes were implanted into the right CA3, and monopolar reference and ground electrodes were affixed above cortex and cerebellum, as described previously (6). 24 hours after implantation, we recorded 12 hours of continuous EEG from each animal using a Nervus EEG recording system (6). Seizures were defined as high-amplitude rhythmic discharges (repetitive spikes, spike-and-wave discharges, slow waves) lasting at least 5 seconds. After 12 hours, each animal received glucose or DPCPX, and EEG monitoring resumed for another 12 hours. All EEG recordings were performed at 3–4 weeks of diet treatment and within 48 hours of electrode implantation to exclude chronic reactions to the electrodes.
βHB measurement.
Mice were deeply anesthetized and blood collected via cardiac puncture into EDTA-containing syringes. After centrifugation, plasma was assayed for βHB with a Precision Xtra monitor and ketone test strips (Abbott Laboratories).
Human material.
Human specimens were a gift from J.C. Baajen (University of Amsterdam Academic Medical Center and VU University Medical Center, Amsterdam, The Netherlands). Written informed consent was obtained from patients or their guardians, and brain tissue was handled in a manner compliant with the guidelines and approval of AMC’s Medical Ethics Committee (MEC; University of Amsterdam, Amsterdam, The Netherlands). Patients underwent resection for refractory epilepsy and had predominantly medically intractable complex partial seizures. We examined 11 surgical epilepsy specimens: 6 with HS (mean age, 26.4 years; range, 18–42 years) and 5 without HS (mean age, 29.5 years; range, 18–41 years; with a focal lesion not involving hippocampus proper and no appreciable neuronal loss or reactive gliosis observed in hippocampus). Normal-appearing control hippocampus was obtained at autopsy from adults (mean age, 31 years; range, 18–35 years) without a history of seizures or other neurological diseases within 12 hours of death. Tissue was fixed, paraffin embedded, sectioned (6 μm), and processed for hematoxylin and eosin and immunocytochemistry for GFAP (monoclonal mouse, 1:4,000; DAKO) and ADK (polyclonal rabbit, 1:500) (6). Single- and double-label immunocytochemistry was performed as described previously (16).
Western blotting.
Freshly frozen histologically normal autopsy hippocampus and HS samples (n = 5 per group) were homogenized. 30 μg protein was separated by standard gel electrophoresis, transferred, and incubated overnight in buffer (20 mM Tris, 150 mM NaCl, 0.1% Tween, 5% nonfat dry milk, pH 7.5) containing the primary ADK antibody (1:5,000). Preparation and analysis of Western blots were performed as described previously (16). Mouse brain was processed similarly using 50 μg protein derived from WT whole brain extracts (n = 4; CD and KD). ADK levels were normalized to internal standards and reported relative to control. Densitometry was performed using ImageJ (NIH).
Statistics.
Seizure data were analyzed using StatView for Windows (version 5.0.1; SAS Institute Inc.), reported as mean ± SD from 5 hours of blinded recording from each animal and condition, and analyzed using 1-way ANOVA followed by Bonferroni/Dunn multiple test for individual means. Data for ADK and ketone quantification were reported as mean ± SEM and analyzed using 2-tailed Student’s t test. A P value of 0.05 or less was considered significant.
Acknowledgments
This study was supported by the NIH (NS058780, NS061844, NS065957, NS066932, 2P20RR00017699), National Science Foundation (IOS-0843585), Swedish Research Council, CHDI Foundation, National Epilepsy Fund (09-05), EU FP7 (NeuroGlia), Grant Agreement 202167, Trinity College, and the Legacy Foundations. We thank Cory Szybala and Jenny Nord for assistance with mouse breeding.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2011;121(7):2679–2683. doi:10.1172/JCI57813.
References
- 1.Kossoff EH, Rho JM. Ketogenic diets: evidence for short- and long-term efficacy. Neurotherapeutics. 2009;6(2):406–414. doi: 10.1016/j.nurt.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neal EG, et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7(6):500–506. doi: 10.1016/S1474-4422(08)70092-9. [DOI] [PubMed] [Google Scholar]
- 3.Kawamura M, Jr, Ruskin DN, Masino SA. Metabolic autocrine regulation of neurons involves cooperation among pannexin hemichannels, adenosine receptors, and KATP channels. . J Neurosci. 2010;30(11):3886–3895. doi: 10.1523/JNEUROSCI.0055-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunwiddie TV, Worth T. Sedative and anticonvulsant effects of adenosine analogs in mouse and rat. J Pharmacol Exp Ther. 1982;220(1):70–76. [PubMed] [Google Scholar]
- 5.Etherington LA, Frenguelli BG. Endogenous adenosine modulates epileptiform activity in rat hippocampus in a receptor subtype-dependent manner. Eur J Neurosci. 2004;19(9):2539–2550. doi: 10.1111/j.0953-816X.2004.03355.x. [DOI] [PubMed] [Google Scholar]
- 6.Li T, et al. Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J Clin Invest. 2008;118(2):571–582. doi: 10.1172/JCI33737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pignataro G, Simon RP, Boison D. Transgenic overexpression of adenosine kinase aggravates cell death in ischemia. J Cereb Blood Flow Metab. 2007;27(1):1–5. doi: 10.1038/sj.jcbfm.9600334. [DOI] [PubMed] [Google Scholar]
- 8.Wilz A, Pritchard EM, Li T, Lan JQ, Kaplan DL, Boison D. Silk polymer-based adenosine release: Therapeutic potential for epilepsy. Biomaterials. 2008;29(26):3609–3616. doi: 10.1016/j.biomaterials.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johansson B, et al. Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. . Proc Natl Acad Sci U S A. 2001;98(16):9407–9412. doi: 10.1073/pnas.161292398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boison D. The adenosine kinase hypothesis of epileptogenesis. Prog Neurobiol. 2008;84(3):249–262. doi: 10.1016/j.pneurobio.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Ambrosio R, et al. Functional definition of seizure provides new insight into post-traumatic epileptogenesis. Brain. 2009;132(pt 10):2805–2821. doi: 10.1093/brain/awp217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eagles DA. Design of dietary treatment: humans versus rodents. Epilepsia. 2008;49 suppl 8:61–63. doi: 10.1111/j.1528-1167.2008.01837.x. [DOI] [PubMed] [Google Scholar]
- 13.Juge N, et al. Metabolic control of vesicular glutamate transport and release. Neuron. 2010;68(1):99–112. doi: 10.1016/j.neuron.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma W, Berg J, Yellen G. Ketogenic diet metabolites reduce firing in central neurons by opening KATP channels. . J Neurosci. 2007;27(14):3618–3625. doi: 10.1523/JNEUROSCI.0132-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.During MJ, Spencer DD. Adenosine: a potential mediator of seizure arrest and postictal refractoriness. Ann Neurol. 1992;32(5):618–624. doi: 10.1002/ana.410320504. [DOI] [PubMed] [Google Scholar]
- 16.Aronica E, et al. Expression and localization of voltage dependent potassium channel Kv4.2 in epilepsy associated focal lesions. Neurobiol Dis. 2009;36(1):81–95. doi: 10.1016/j.nbd.2009.06.016. [DOI] [PubMed] [Google Scholar]