Abstract
The many challenges associated with lung transplantation provide a strong rationale for the development of cell- and tissue-based therapies for patients with respiratory failure caused by the loss of lung tissue that is associated with chronic pulmonary disease, injury, or resection. In this issue of the JCI, Chapman et al. take an important step forward in the development of regenerative medicine for the treatment of lung disease by identifying a novel integrin α6β4–expressing alveolar epithelial cell that serves as a multipotent progenitor during repair of the severely injured lung.
Lung function depends on the diversity of epithelial cells lining the respiratory tract
In the mammalian lung, conducting airways lead to an extensive alveolar surface on which epithelial cells and pulmonary capillaries come into close apposition to facilitate gas exchange required for cellular respiration. Pulmonary homeostasis requires the proliferation and differentiation of diverse epithelial cell types that vary along the proximal to peripheral axis of the lung as needed for mucociliary clearance, host defense, reduction of surface tension, and gas exchange. Lineage-tracing experiments in mice have demonstrated that epithelial cells lining both conducting airways and the alveoli of the mature lung are derived from lineage-restricted progenitor cells located within the embryonic foregut endoderm (1–3). Conducting airways, including the trachea, bronchi, and bronchioles, are lined primarily by pseudostratified or columnar epithelium consisting of ciliated, basal, and various secretory cell types including serous, Clara, and goblet cells. In contrast, the alveolar surface is thought to be lined by only two types of alveolar epithelial cells (AECs): squamous type I AECs, which facilitate oxygen and carbon dioxide transport to pulmonary capillaries, as required for respiration; and cuboidal type II AECs, which produce and secrete surfactant proteins and lipids, including surfactant protein C (SP-C), required to reduce surface tension at the air-liquid interface, thereby preventing atelectasis during the respiratory cycle (ref. 4 and Figure 1). Type II AECs have been long considered the sole progenitor cells mediating repair of the alveoli, but this view is now challenged by the findings of Chapman and colleagues in this issue of the JCI (5).
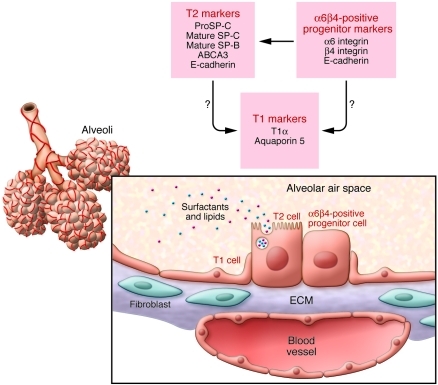
Figure 1. Location and markers of α6β4 epithelial progenitors in the lung.
Diagram shows the structure of the respiratory tree and alveolar region of the lung. Surfactant-producing cuboidal type II AECs (T2), squamous type I AECs (T1), and the α6β4-expressing epithelial progenitor cells described by Chapman and colleagues (5) are located in close proximity to blood vessels and interstitial tissue containing fibroblasts and ECM. Marker proteins expressed by T1, T2, and α6β4 cells are indicated.
Proliferative responses after lung injury
After birth, the respiratory tract is exposed to particles, toxicants, and infectious agents, all of which can cause severe damage, and the lung has elegantly evolved cellular and physiological repair mechanisms that maintain its function after injury. Whereas cell turnover occurs very slowly in the normal healthy lung, as indicated by low rates of cell proliferation, the lung is capable of rapid and extensive cell proliferation as required to repair damaged tissue and maintain respiratory function. Failure to regenerate functional lung tissue after acute or chronic injury contributes to the pathogenesis of many common lung disorders, including acute lung injury, pneumonia, pulmonary fibrosis, emphysema, cystic fibrosis, and lung cancer (6, 7). The limited capacity of the lung to regenerate after extensive injury or resection emphasizes the urgent need to develop cell- and tissue-based therapies for these life-threatening pulmonary disorders.
Integrin α6β4 defines a previously unrecognized alveolar progenitor cell
Given the remarkable diversity of the characteristics and functions of respiratory epithelial cells, the identification of lung progenitor cells, their differentiation, and the analysis of their regenerative capacities during lung formation and repair have been the focus of considerable research interest. Although the existence of multipotent progenitor cells capable of self-renewal and differentiation into distinct epithelial types has been demonstrated in the fetal lung (2, 8), the characterization of multipotent respiratory epithelial cell progenitors, or stem cells, that maintain lung homeostasis and mediate repair after injury in the mature lung is incomplete. The current view is that basal cells and secretory cells proliferate and differentiate into the distinct cell types of the conducting airways, whereas cuboidal type II AECs are the sole source of progenitor cells that proliferate and differentiate into squamous type I AECs during repair of the injured lung (9–11).
Chapman et al. have identified a subpopulation of AECs expressing the laminin receptor, α6β4, that do not express proSP-C, a protein considered a cell-specific marker for mature type II AECs (5). Purified α6β4-positive progenitor cells (which the authors refer to as β4+ cells) were found to proliferate and differentiate into both proSP-C–positive AECs and Clara cell secretory protein–positive (CCSP-positive) bronchiolar epithelial cells that contributed to highly organized bronchiolar and alveolar structures when implanted with fetal lung cells under the renal capsule of immunodeficient mice. Differentiated type II AECs expressing proSP-C did not have a similar proliferative capacity in vitro and did not contribute substantially to alveolar repair after severe bleomycin-induced injury. These α6β4-positive progenitor cells were found in both bronchiolar and alveolar regions of the normal mouse lung and lacked expression of proSP-C and the Clara cell marker CCSP, something that distinguishes them from the previously described dual proSP-C– and CCSP-positive bronchiolar-alveolar duct junction cells (12).
The α6β4-positive cells accounted for approximately 8%–10% of AECs in the quiescent lung, and therefore represent a pool of multipotent progenitors that are likely to play an important role in the repair of the lung. Lineage-tracing studies presented by Chapman et al. demonstrated that the regeneration of new type II AECs seen after severe lung injury in the mouse depended on proliferation of α6β4-positive progenitors, rather than expansion of preexisting type II AECs (5). It will be of considerable interest to determine the mechanisms by which α6β4-positive AECs self-renew and differentiate, whether they serve as sources of both type I and type II AECs, and whether they contribute to repair of conducting airways after clinically relevant injuries to the human lung. The identification and isolation of integrin α6β4–positive progenitor cells from human lung and from experimental models will be useful for identifying factors and conditions that optimize lung regeneration and repair.
Is there a hierarchy of lung progenitor cells?
The characteristics of the integrin α6β4–positive cells described by Chapman and colleagues (5) are consistent with a role for these cells as lineage-restricted multipotent progenitors. They are also consistent with a recent study by McQualter et al. (13), in which the authors concluded that there is a hierarchy of respiratory epithelial progenitor cells that includes both abundant, well-differentiated progenitors and less-abundant progenitor cell subsets. McQualter et al. further speculate that the latter are located in restricted cellular niches that may have increased capacity for proliferation and differentiation (13). While lineage-tracing studies support the endodermal derivation of all respiratory epithelial cells, recent observations regarding the remarkable ability of various cell types to be reprogrammed — epitomized in this context by the production of induced pluripotent stem cells and their differentiation into respiratory epithelial–like cells (14) — warrants rethinking of the nature of organ- and cell type–restricted progenitors. Indeed, recent studies by Kajstura et al. identified an extremely rare but pluripotent c-kit+ lung stem cell capable of regenerating lung tissue after injection into injured mouse lung in vivo (15). These c-kit+ lung cells served as progenitors of both bronchiolar and alveolar epithelial cells and had the capacity to differentiate into mesenchymal (vascular smooth muscle) components of lung tissue. Furthermore, the c-kit+ cells were capable of integrating into complex respiratory structures when directly injected into injured mouse lung tissue in vivo.
A lung organoid assay for identifying progenitor cells
To determine progenitor cell activity in vivo, Chapman et al. developed an in vivo embryonic lung organoid assay that represents a significant contribution to the field (5). Purified α6β4-positive progenitor cells self-organized into distinct airway-like saccular structures when mixed with fetal lung cells and transplanted under the kidney capsule of immunodeficient mice. This elegant assay will be useful in defining progenitor cell capabilities and will facilitate the discovery of factors regulating their growth, differentiation, and tissue-regenerating capacities.
Summary
The findings of Chapman et al. (5) challenge our conventional wisdom regarding the identity of alveolar progenitor cells. The authors have identified a previously unrecognized integrin α6β4–expressing AEC subset that proliferates and differentiates into multiple respiratory epithelial cell types in vitro and in vivo. The field can look forward to future studies identifying the roles of these α6β4-positive AECs in the pathogenesis of human lung disease. Elucidation of the mechanisms controlling their proliferation, differentiation, and ability to produce functional lung tissue will be highly relevant to the pathogenesis of acute and chronic lung disease. It will also provide a framework from which to develop new strategies to enhance lung regeneration for the treatment of life-threatening common pulmonary disorders.
Acknowledgments
The authors acknowledge funding from the NIH (grant HL090156 to J.A. Whitsett; grant HL084151 to V.V. Kalinichenko).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2011;121(7):2543–2545. doi:10.1172/JCI58704.
See the related article beginning on page 2855.
References
- 1.Hogan BL, Yingling JM. Epithelial/mesenchymal interactions and branching morphogenesis of the lung. Curr Opin Genet Dev. 1998;8(4):481–486. doi: 10.1016/S0959-437X(98)80121-4. [DOI] [PubMed] [Google Scholar]
- 2.Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell. 2010;18(1):8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardoso WV. Molecular regulation of lung development. Annu Rev Physiol. 2001;63:471–494. doi: 10.1146/annurev.physiol.63.1.471. [DOI] [PubMed] [Google Scholar]
- 4.Whitsett JA, Wert SE, Weaver TE. Alveolar surfactant homeostasis and the pathogenesis of pulmonary disease. Annu Rev Med. 2010;61:105–119. doi: 10.1146/annurev.med.60.041807.123500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman HA, et al. Integrin α6β4 identifies an adult distal lung epithelial population with regenerative potential in mice. J Clin Invest. 2011;121(7):2855–2862. doi: 10.1172/JCI57673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warburton D, et al. Lung organogenesis. Curr Top Dev Biol. 2010;90:73–158. doi: 10.1016/S0070-2153(10)90003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitsett JA, Wert SE, Trapnell BC. Genetic disorders influencing lung formation and function at birth. Hum Mol Genet. 2004;13(spec no 2):R207–R215. doi: 10.1093/hmg/ddh252. [DOI] [PubMed] [Google Scholar]
- 8.Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci U S A. 2002;99(16):10482–10487. doi: 10.1073/pnas.152238499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adamson IY, Bowden DH. The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab Invest. 1974;30(1):35–42. [PubMed] [Google Scholar]
- 10.Rawlins EL, et al. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4(6):525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rock JR, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A. 2009;106(31):12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim CF, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121(6):823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 13.McQualter JL, Yuen K, Williams B, Bertoncello I. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc Natl Acad Sci U S A. 2010;107(4):1414–1419. doi: 10.1073/pnas.0909207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green MD, et al. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nat Biotechnol. 2011;29(3):267–272. doi: 10.1038/nbt.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kajstura J, et al. Evidence for human lung stem cells. N Engl J Med. 2011;364(19):1795–1806. doi: 10.1056/NEJMoa1101324. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]