Summary
Head and Neck Cancer (HNC) has been studied in different regions of the world but little is known about its incidence patterns in the Middle East and Egypt.
In this study from Egypt’s only population-based registry, we analyzed data from 1999-2006, to estimate incidence, incidence rate ratios (IRRs) and 95% confidence intervals (CIs) categorized by age, district and subsites.
Overall urban incidence of HNC was twice or more that of rural incidence for both males (IRR = 2.59; 95% CI = 2.26, 2.97) and females (IRR = 2.00; 95% CI = 1.64, 2.43). Highest urban-rural difference for males was seen in 40-49 years (IRR = 2.79; 95% CI = 1.92, 4.05) and for females in 30-39 years (IRR = 2.94; 95% CI = 1.60, 5.40). Among subsites, highest incidence among males was for larynx (1.53/105) and among females for gum and mouth (0.48/105). Maximum urban-rural difference in males was for paranasal sinus (IRR = 4.66; 95% CI = 1.88, 11.54) and in females for lip (IRR = 8.91; 95% CI = 1.89, 41.98).
The study underscores the patterns of HNC incidence in Egypt while indicating the need for future analytical studies investigating specific risk factors of HNC in this population.
Keywords: Head and neck cancer, risk factors, descriptive epidemiology, urban-rural, Egypt, Africa, SEER
Introduction
Head and neck cancer (HNC) are a group of malignancies involving oral cavity, pharynx, ear/nose, and larynx. Among the 10 most common incident cancers in men worldwide, 90% of HNC is squamous cell carcinomas (SCC)1. Each year there are approximately 560,000 new cases of and 300,000 deaths due to HNC2. The highest incidences of HNC in the world are found in South Asia, and parts of central and southern Europe2. By far, the most common risk factors associated with HNC are tobacco and alcohol use with significant interaction observed between the two3. Other observed risk factors are poor oral hygiene4 and the human papillomavirus (HPV) 16 in tongue, tonsil and orpharyngeal HNC and, in particular, nonsmoking cases of HNC5. In South Asian countries the risk of HNC is further aggravated by smoking of bidis which increases the incidence of cancer of hypopharynx and larynx6, and chewing tobacco, betel quid and areca nut7.
Within the Middle East, rates of smoking are high8, 9 although alcohol consumption is limited. This is especially true for Egypt where smoking rates are increasing for both cigarettes and water-pipe9. However, there have been very few studies depicting the magnitude or etiologic factors of HNC in the Middle East and Egypt. Previous hospital-based studies from Egypt showed that HNC constitutes about 17-20% of all malignancies10, 11. The majority of cases are diagnosed at advanced stages12-14, and the suspected risk factors of subsites varied by tumor site and place of residence in small-scale hospital-based studies15-17. A report of the Middle-East Cancer Consortium (MECC) of the National Cancer Institute in Bethesda, USA, depicted that Egypt had one of the highest overall incidence rates of cancer of oral cavity and pharynx (5.5/105) among the MECC countries, equal to the rate seen in Israeli Jews18. As such, we conducted this study to investigate the incidence of different clinical subsites of HNC in Egypt’s only population-based cancer registry in the Province of Gharbiah. We also explored the demographic and geographical patterns of HNC to better understand the regional differences of the disease and generate hypotheses regarding the HNC etiology.
Patients and Methods
Study Population
The study population consisted of men and women diagnosed with HNC from 1999 through 2006 from the Gharbiah Population-Based Cancer Registry (GPCR) comprising the following sites: lip, tongue, gum, mouth (floor, palate, other), salivary glands, tonsils, pharynx (oro-, naso- and hypopharynx), and nose and ear. For each case, the following information from routinely-collected registry data was obtained: registry number, age at diagnosis, address, address code, smoking status, occupation, basis of diagnosis, tumor grade, stage, morphology, medical record number, and place of referral. Data were stripped of all personal identifiers and their analyses were approved by the University of Michigan Institutional Review Board and the Gharbiah Cancer Center Ethics Committee.
Gharbiah Population-Based Cancer Registry
The Gharbiah population-based cancer registry, founded in 1998 as a part of the Middle East Cancer Consortium (MECC) and funded by the U.S. National Cancer Institute (NCI), is located in Tanta, the capital of Gharbiah province9, 18. Through an active registration process, data on cancer cases are collected from various sources in the province. For this study, yearly HNC cases came from three locations; the Tanta Cancer Center (40-50%), Gharbiah Cancer Society (10-12%) and Tanta University Hospital (10-12%). Data obtained from these hospitals and centers were entered in a manner that ensured strict quality control checks and avoided repetition of cases using the International Agency for Research on Cancer (IARC) software CanReg4. Registrars were trained in data extraction and entry methods, and are periodically monitored by faculty of Emory School of Public Health, IARC, and the MECC18.
Gharbiah Province
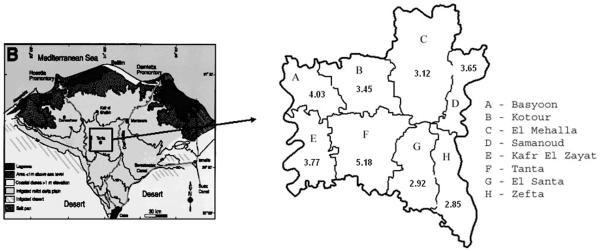
The Gharbiah province is an administrative region located 90 kilometers north of Cairo in the Nile delta region and has eight districts, each with a capital city (Figure 1). Tanta city also serves as the capital of the province. Gharbiah has a population of more than 4 million people, 49% of whom are women. Approximately 30% of the population resides in urban areas and almost 47% of the female population is below the age of 20, according to the 2006 Central Agency for Public Mobilization and Statistics (CAPMAS) national census of Egypt19.
Fig.1.
Census Data
The 1996 and 2006 CAPMAS censuses were used to obtain data on men and women residing in Gharbiah20, and linear regression was used to estimate the population during each study year. The linear growth rates of eight districts were applied to the urban and rural populations within those districts to determine urban and rural populations from 1999 through 2006. The census data consisted of 16 age categories at 5 year intervals. Six age categories were created from these by collapsing the age categories below 29 years followed by 10 year intervals. These population figures formed the denominators to calculate the overall, age-specific, and urban-rural incidence rates for HNC and its subsites.
Urban-Rural Classification
Urban and rural designations were made according to the CAPMAS definitions20. Urban areas consisted of all the capital cities of the eight districts of the province, while the remaining areas in the province were considered rural. Each case in the registry was assigned a residence code based on their residential address that follows the CAPMAS coding which was used to classify cases as urban or rural.
Statistical Analysis
Descriptive statistics and incidence rate analyses were completed using SAS (Version 9; SAS Institute, Cary, NC). Crude and age-adjusted incidence rates were calculated for Gharbiah province, each of the eight districts, and urban and rural areas for the province. Age-specific incidence rates for the entire population and for urban vs. rural areas for each of the six age categories. The six age categories were 0-29, 30-39, 40-49, 50-59, 60-69 and 70 or more. Direct age-adjusted incidence rates were calculated by using Gharbiah’s 2006 population as the standard. We then calculated urban-rural incidence rate ratios (IRRs) and 95% confidence intervals (CI) using Poisson regression. We also calculated incidence rates specific to HNC subsites and compared overall rates to US SEER, as well as compared urban and rural incidence rates.
Results
A total of 1140 cases of HNC were identified in Gharbiah, Egypt from 1999 to 2006 with 64.3% of all cases being male (Table 1). More than half of the cases were in the 50-69 year age-category (50.71%). Most cases belonged to the two largest districts of Tanta (33.07%) and El Mehalla (21.4%). Almost 40% of males were current or former smokers although more than half of the cases had missing information on smoking (52.02%). Most cases were detected at localized stage (28.42%) and had been detected by histopathological confirmation of the primary (91.84%) (Table 1).
Table 1.
Distribution of head and neck cancer patients in Gharbiah, Egypt 1999-2006
| Variables | Overall | Male | Female | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Total | 1140 | 100 | 733 | 64.30 | 407 | 35.70 |
| Age at Diagnosis | ||||||
| Age 0-29 | 81 | 7.11 | 47 | 6.41 | 34 | 8.35 |
| Age 30-39 | 93 | 8.16 | 51 | 6.96 | 42 | 10.32 |
| Age 40-49 | 201 | 17.63 | 111 | 15.14 | 90 | 22.11 |
| Age 50-59 | 291 | 25.53 | 192 | 26.19 | 99 | 24.32 |
| Age 60-69 | 287 | 25.18 | 202 | 27.56 | 85 | 20.88 |
| Age 70+ | 187 | 16.40 | 130 | 17.74 | 57 | 14.01 |
| Urban-Rural | ||||||
| Urban | 579 | 50.79 | 386 | 52.66 | 193 | 47.42 |
| Rural | 561 | 49.21 | 347 | 47.34 | 214 | 52.58 |
| District | ||||||
| Tanta | 377 | 33.07 | 251 | 34.24 | 126 | 30.96 |
| El Mehalla | 244 | 21.40 | 145 | 19.78 | 99 | 24.32 |
| Kafr El-Zayat | 109 | 9.56 | 66 | 9.00 | 43 | 10.57 |
| Zefta | 95 | 8.33 | 61 | 8.32 | 34 | 8.35 |
| Samanoud | 82 | 7.19 | 59 | 8.05 | 23 | 5.65 |
| El Santa | 82 | 7.19 | 56 | 7.64 | 26 | 6.39 |
| Kotoor | 75 | 6.58 | 48 | 6.55 | 27 | 6.63 |
| Basyoun | 76 | 6.67 | 47 | 6.41 | 29 | 7.13 |
| Smoking Status | ||||||
| Current Smokers | 151 | 13.25 | 150 | 20.46 | 1 | 0.25 |
| Non-smokers | 273 | 23.95 | 83 | 11.32 | 190 | 46.68 |
| Former smokers | 123 | 10.79 | 122 | 16.64 | 1 | 0.25 |
| Unknown | 593 | 52.02 | 378 | 51.57 | 215 | 52.83 |
| Stage at Diagnosis | ||||||
| In situ | 15 | 1.32 | 12 | 1.64 | 3 | 0.74 |
| Localized (stage I lymphoma) | 324 | 28.42 | 216 | 29.47 | 108 | 26.54 |
| Regional –direct extension | 190 | 16.67 | 117 | 15.96 | 73 | 17.94 |
| Regional-lymph nodes | 128 | 11.23 | 83 | 11.32 | 45 | 11.06 |
| Regional-direct extension & lymph nodes |
89 | 7.81 | 57 | 7.78 | 32 | 7.86 |
| Regional-stage II lymphoma | 85 | 7.46 | 33 | 4.50 | 52 | 12.78 |
| Distant-stage III & IV lymphomas |
120 | 10.53 | 87 | 11.87 | 33 | 8.11 |
| Unknown | 189 | 16.58 | 128 | 17.46 | 61 | 14.99 |
| Basis of Diagnosis | ||||||
| Death certificate | 11 | 0.96 | 9 | 1.23 | 2 | 0.49 |
| Clinical | 2 | 0.18 | 1 | 0.14 | 1 | 0.25 |
| Clinical/Radiographic | 9 | 0.79 | 2 | 0.27 | 7 | 1.72 |
| Cytology/Hematology | 18 | 1.58 | 10 | 1.36 | 8 | 1.97 |
| Histology of metastasis | 53 | 4.65 | 34 | 4.64 | 19 | 4.67 |
| Histology of Primary | 1047 | 91.84 | 677 | 92.36 | 370 | 90.91 |
Overall incidence of HNC was approximately twice among males (4.76/105) than in females (2.73/105) (Table 2). Overall age-specific incidence was highest in the 70+ age-group in both males (44.09/105) and females (18.39/105). Overall urban incidence of HNC was twice or more that of rural incidence for both males (IRR = 2.59; 95% CI = 2.26, 2.97) and females (IRR = 2.00; 95% CI = 1.64, 2.43). Highest urban-rural difference for males was seen in the 40-49 year age-category (IRR = 2.79; 95% CI = 1.92, 4.05) while for females it was in the 30-39 year age-category (IRR = 2.94; 95% CI = 1.60, 5.40) (Table 2).
Table 2.
Comparison of urban-rural age-specific incidencea of head and neck cancer by gender in Gharbiah, Egypt (1999-2006)
| Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|
| Age group | Overall | Urban | Rural | IRR# (95% CI) |
Overall | Urban | Rural | IRRb (95% CI) |
| Age 0-29 | 0.49 | 0.89 | 00.34 | 2.65 (1.50, 4.68) |
0.36 | 0.50 | 0.30 | 1.68 (0.85, 3.34) |
| Age 30-39 | 2.55 | 3.37 | 2.20 | 1.53 (0.87, 2.70) |
1.99 | 3.56 | 1.21 | 2.94 (1.60-5.40) |
| Age 40-49 | 6.33 | 11.14 | 3.99 | 2.79 (1.92, 4.05) |
5.89 | 8.10 | 4.63 | 1.75 (1.16, 2.64) |
| Age 50-59 | 16.28 | 22.37 | 12.86 | 1.74 (1.32, 2.29) |
11.01 | 12.68 | 10.14 | 1.25 (0.83, 1.89) |
| Age 60-69 | 31.47 | 46.69 | 22.39 | 2.09 (1.59-2.75) |
13.53 | 20.20 | 10.35 | 1.95 (1.27, 3.00) |
| Age 70+ | 44.09 | 69.43 | 30.63 | 2.27 (1.60, 3.23) |
18.39 | 31.80 | 12.80 | 2.48 (1.49, 4.13) |
| Total | 4.76 | 8.35 | 3.22 | 2.59 (2.26, 2.97) |
2.73 | 4.17 | 2.08 | 2.00 (1.64, 2.43) |
: All incidence is per 100,000;
: IRR = Incidence rate ratio, CI = Confidence interval
Highest overall incidence of HNC was in Tanta (6.79) followed by Samanoud (5.24) for males, and Tanta (3.57/105) followed by Basyoon (3.11/105) in females (Table 3). Highest urban-rural differences were observed in El Santa (IRR = 8.52; 95% CI = 5.02, 14.46) followed by Kotoor (IRR = 6.98; 95% CI = 3.88, 12.57) among males. Among females, highest urban-rural differences were present for Kotoor (IRR = 7.41; 95% CI = 3.45, 15.91) followed by Kafr El-Zayat (IRR = 5.92; 95% CI = 3.22, 10.87) (Table 3).
Table 3.
Comparison of urban-rural incidencea of head neck cancer for districts by gender in Gharbiah, Egypt (1999-2006)
| Districts | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall | Urban | Rural | IRRb (95% CI) |
Overall | Urban | Rural | IRRb (95% CI) |
|
| Tanta | 6.79 | 9.34 | 4.79 | 1.95 (1.51, 2.52) |
3.57 | 3.87 | 3.32 | 1.17 (0.82-1.66) |
| El Mehalla | 3.67 | 5.31 | 2.42 | 2.19 (1.57, 3.06) |
2.57 | 2.76 | 2.42 | 1.14 (0.77, 1.69) |
|
Kafr El-
Zayat |
4.51 | 11.10 | 2.95 | 3.76 (2.30, 6.14) |
3.03 | 8.93 | 1.51 | 5.92 (3.22,10.87) |
| Zefta | 3.58 | 7.70 | 2.46 | 3.13 (1.88, 5.21) |
2.12 | 3.70 | 1.68 | 2.20 (1.11, 4.37) |
| Samanoud | 5.24 | 8.86 | 4.39 | 2.02 (1.17, 3.50) |
2.06 | 3.75 | 1.66 | 2.26 (0.95, 5.35) |
| El Santa | 3.93 | 20.27 | 2.38 | 8.52 (5.02, 14.46) |
1.90 | 5.97 | 1.52 | 3.92 (1.65, 9.29) |
| Kotoor | 4.43 | 20.43 | 2.93 | 6.98 (3.88, 12.57) |
2.46 | 11.78 | 1.59 | 7.41 (3.45,15.91) |
| Basyoun | 4.95 | 10.00 | 3.51 | 2.85 (1.61, 5.03) |
3.11 | 8.57 | 1.52 | 5.62 (2.67,11.84) |
: All incidence is per 100,000;
: IRR = Incidence rate ratio, CI = Confidence interval
On analyzing incidence of HNC by subsite (Table 4) we discovered that the highest incidence among males was for larynx (1.53/105), nasopharynx (0.65/105), gum and mouth (except floor) (0.49/105), and tongue (0.42 /105). Among females, the highest incidence was observed for gum and mouth (except floor) (0.48/105), nasopharynx (0.40/105), hypopharynx (0.40/105), and salivary glands (0.34/105). Highest urban incidence of HNC subsites among males was present for larynx (3.09/105), nasopharynx (1.12/105), tongue (0.69/105), and gum and mouth (except floor) (0.67/105) with a similar order seen for rural males as well (Table 4). Among urban females highest incidence of HNC subsites was observed for gum and mouth (except floor) (0.76/105), nasopharynx (0.74/105), salivary glands (0.54/105), and hypopharynx (0.50/105). Among rural females the highest incidence of HNC subsites was in the following order: gum and mouth (except floor) (0.36/105), hypopharynx (0.36/105), tonsils and oropharynx (0.26/105), salivary glands (0.24/105), and nasopharynx (0.24/105). For males, maximum urban-rural difference was detectable for paranasal sinus (IRR = 4.66; 95% CI = 1.88, 11.54), larynx (IRR = 3.58; 95% CI = 2.76, 4.75), and lip (IRR = 3.01; 95% CI = 1.60, 5.67). Among females, maximum urban-rural differences were observed for lip (IRR = 8.91; 95% CI = 1.89, 41.98), floor of the mouth (IRR = 4.46; 95% CI = 49.15), and larynx (IRR = 3.90; 95% CI = 1.64, 9.30) (Table 4).
Table 4.
Comparison of incidencea of head neck cancer for subsites by urban-rural status within genders, and with SEER in Gharbiah, Egypt (1999-2006)
| Head and Neck Cancer Subsites |
Male | Female | ASWc | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Urban | Rural | IRRb (95% CI) |
Overall | Urban | Rural | IRR# (95% CI) |
Gharbiah Incidence |
SEER Incidence |
|
| Lip | 0.25 | 0.48 | 0.16 | 3.01 (1.60, 5.67) |
0.07 | 0.17 | 0.02 | 8.91 (1.89, 41.98) |
0.23 | 0.88 |
| Tongue | 0.42 | 0.69 | 0.30 | 2.33 (1.43, 3.80) |
0.27 | 0.37 | 0.22 | 1.65 (0.88, 3.08) |
0.49 | 2.80 |
|
Gum and
Mouth-Other |
0.49 | 0.67 | 0.41 | 1.64 (1.04, 2.60) |
0.48 | 0.76 | 0.36 | 2.11 (1.33, 3.35) |
0.67 | 1.64 |
| Mouth-Floor | 0.03 | 0.02 | 0.03 | 0.78 (0.08, 7.46) |
0.02 | 0.04 | 0.01 | 4.46 (0.40, 49.15) |
0.03 | 0.71 |
|
Salivary Glands |
0.37 | 0.63 | 0.26 | 2.41 (1.43, 4.05) |
0.34 | 0.54 | 0.24 | 2.23 (1.28, 3.88) |
0.51 | 1.25 |
|
Tonsils and
Oropharynx |
0.27 | 0.50 | 0.18 | 2.82 (1.54, 5.18) |
0.27 | 0.28 | 0.26 | 1.07 (0.55, 2.08) |
0.37 | 1.71 |
| Larynx | 1.53 | 3.09 | 0.86 | 3.58 (2.76, 4.65) |
0.15 | 0.30 | 0.08 | 3.90 (1.64, 9.30) |
1.32 | 3.61 |
| Nasopharynx | 0.65 | 1.12 | 0.45 | 2.52 (1.7, 3.73) |
0.40 | 0.74 | 0.24 | 3.03 (1.81, 5.08) |
0.70 | - |
| Piriform Sinus | 0.16 | 0.17 | 0.15 | 1.16 (0.5, 2.72) |
0.07 | 0.07 | 0.08 | 0.84 (0.22, 3.15) |
0.18 | - |
| Hypopharynx | 0.34 | 0.48 | 0.28 | 1.71 (0.98, 2.96) |
0.40 | 0.50 | 0.36 | 1.39 (0.82, 2.33) |
0.49 | - |
| Other | 0.06 | 0.11 | 0.04 | 2.91 (0.78, 10.84) |
0.07 | 0.06 | 0.07 | 0.96 (0.25, 3.69) |
0.08 | - |
| Ear/Nose | 0.06 | 0.09 | 0.05 | 1.86 (0.50, 6.94) |
0.12 | 0.22 | 0.08 | 2.79 (1.10, 7.06) |
0.10 | - |
|
Paranasal
sinus |
0.14 | 0.30 | 0.07 | 4.66 (1.88, 11.54) |
0.08 | 0.13 | 0.06 | 2.23 (0.72, 6.91) |
0.13 | - |
: All incidence is per 100,000;
: IRR = Incidence rate ratio, CI = Confidence interval;
: ASW = Age-standardized to the world population
On comparing age-standardized HNC incidence by subsite in Gharbiah with age-standardized incidence from SEER we discovered a lower incidence in Gharbiah for all subsites compared to SEER although incidence of laryngeal cancer was highest in both Gharbiah (1.32/105) and SEER (3.61/105) (Table 4).
Discussion
In this first study describing the epidemiology of HNC in Egypt using data from the only population-based cancer registry, we revealed higher incidence of HNC among males than females and higher incidence in urban than rural populations. Overall, the incidence of HNC was highest in the 70+ age group in both males and females. Highest urban-rural differences were observed in the 40-49 year age group in males and 30-39 year age group in females. Among districts, the highest overall HNC incidence was observed in Tanta and Samanoud districts among males, and Tanta and Basyoon districts among females. Highest urban-rural differences were found for El-Santa and Kotoor districts for males, and Kotoor and Kafr El-Zayat districts for females. The subsite analysis showed that males had the highest overall incidence for larynx and nasopharynx while females had the highest overall incidence for gum and mouth (except floor) and nasopharynx. The highest urban-rural incidence was observed for the subsites of paranasal sinus and larynx among males, and lip and floor of the mouth among females.
The overall highest incidence in the 70+ age group in both males and females might be related to previous extensive nutritional deficiencies and environmental exposures to nitrates a few decades ago in Egypt17, 21, 22. The highest urban-rural differences were observed in 40-49 year age group in males and 30-39 year age group in females could be due to the increasing and higher smoking rates in men in urban than rural areas and the possible viral etiology in younger women in urban compared to rural areas. The decline in age of incidence of HNC was also reported in the U.S.23, and is often attributed to increasing incidence of HPV-related HNC. Unfortunately, using registry data we have inadequate information on smoking history, which could indicate the frequency of non-smoking and likely HPV-related cases of HNC. The Surveillance Epidemiology and End-Results (SEER) analysis showed that since the mid-1990s, females in the U.S. between 10-40 years of age had an increase in the incidence of oral and pharyngeal cancers contrary to previous periods23. HPV and other sexually transmitted diseases were suspected in the decline of the age incidence in the U.S.23 and might be the reason for the age decline also in Egypt too. The highest rates observed in the 2 districts of Tanta and Samanoud among males and females in Tanta and Basyoon could be due to the higher smoking rates in urban areas (Tanta), and environmental pollution, infection and malnutrition in the 2 districts of Samanoud and Basyoon. The highest urban-rural differences seen for the districts of El-Santa and Kotoor for males, and the districts of Kotoor and Kafr El-Zayat for females might be related to differences in pollution risk factor information between urban and rural areas24-26 and higher smoking rates in urban than rural areas27-29. While previous studies highlighted the possible regional difference in pollution between urban and rural areas in those districts, our previous research in the region has also shown regional differences in incidence of liver, bladder, breast, and endometrial cancers in those particular districts30-33. The higher subsite incidence rate for larynx and nasopharynx and for gum and mouth (except floor) and nasopharynx for females might also be related to variable smoking rates, infection, and regional environmental pollution. The overall incidence of all subsites (except for larynx) was lower in Egypt than in SEER. This may indicate a difference in risk factors, including genetic susceptibility and diet between these two populations. However, the similarity between the Egyptian registry and SEER with respect to the incidence of laryngeal cancer underscores the importance of the association between smoking and laryngeal cancer in both countries.
Previous studies highlighted the importance of smoking in HNC in pooled international analysis of the HNC consortium34 and smoking and alcohol35. Meta-analysis of 62 studies showed the association between HPV in oral cancers and HNC36. In studies from India, coal was associated with hypopharyngeal/laryngeal cancer risk37, and cooking fumes exposure in childhood, tobacco and marijuana smoking, food contamination, and EBV in relation to nasopharyngeal cancer were reported from Algeria, Tunisia, and Morocco38-40. Previous small-scale hospital-based studies that were limited to one or a few subsites in Egypt suspected that nitrates, malnutrition, Herpes Simplex Virus and Candida infection as possible risk factors15-17.
Our study has several strengths. The population-based nature of the study on a relatively long time period is a major strength. The quality control measures of the data by IARC and NCI provides confidence about the data quality. These data clearly show a gradient of risk between different regions of Egypt. In the future, the heterogeneity of both traditional and novel risk factors in Egypt, including smoking, infection, environmental pollution, will provide a unique opportunity for understanding the etiology of the disease. It should be noted though that a usual limitation of all population-based registries is the limited number of variables available to allow for extensive investigation of the etiology of cancer. Future studies will build upon this work to investigate the epidemiologic and molecular characteristics of these cancers in this unique population.
This study is the first to quantify the magnitude of HNC from a population-based perspective from a long period of the registry. The study underscores the high-incidence age groups and the regional variations in subsites by urban-rural residence of patients. Future studies could benefit from the results of this study to tailor specific analytical studies to investigate specific viral etiology, such as HPV, environmental exposures to nitrates or other risk factors, and smoking in the etiology of this disease.
Acknowledgement
This work was supported in part by the Cancer Epidemiology Education in Special Populations (CEESP) Program of the University of Michigan (R25 CA112383).
Footnotes
Conflicts of Interest Statement None Declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Curado MP, Hashibe M. Recent changes in the epidemiology of head and neck cancer. Curr Opin Oncol. 2009;21(3):194–200. doi: 10.1097/CCO.0b013e32832a68ca. [DOI] [PubMed] [Google Scholar]
- 2.Boyle P, Levine B. World cancer report 2008. International Agency for Research on Cancer; Lyon, France: 2008. p. 330. [Google Scholar]
- 3.Blot WJ, McLaughlin JK, Winn DM, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48(11):3282–7. [PubMed] [Google Scholar]
- 4.Guha N, Boffetta P, Filho V Wunsch, et al. Oral health and risk of squamous cell carcinoma of the head and neck and esophagus: Results of two multicentric case-control studies. Am J Epidemiol. 2007;166(10):1159–73. doi: 10.1093/aje/kwm193. [DOI] [PubMed] [Google Scholar]
- 5.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: A systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14(2):467–75. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 6.Sapkota A, Gajalakshmi V, Jetly DH, et al. Smokeless tobacco and increased risk of hypopharyngeal and laryngeal cancers: A multicentric case-control study from India. Int J Cancer. 2007;121(8):1793–8. doi: 10.1002/ijc.22832. [DOI] [PubMed] [Google Scholar]
- 7.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Betel-quid and areca-nut chewing and some areca-nut derived nitrosamines. IARC Monogr Eval Carcinog Risks Hum. 2004;85:1–334. [PMC free article] [PubMed] [Google Scholar]
- 8.Mackay J, Eriksen M. The tobacco atlas. World Health Organization; Geneva (Switzerland): 2002. [Google Scholar]
- 9.El Awa F. Tobacco control in the eastern Mediterranean region: Overview and way forward. East Mediterr Health J. 2008;14(Suppl):S123–31. [PubMed] [Google Scholar]
- 10.Gad-El-Mawla N, Macdonald JS, Khaled H. Hexamethylmelamine in advanced head and neck cancer. A phase II study. Am J Clin Oncol. 1984;7(3):205–8. doi: 10.1097/00000421-198406000-00003. [DOI] [PubMed] [Google Scholar]
- 11.El-Bokainy MN. Head and Neck Cancer. In: El-Bokainy MN, National Cancer Institute, Cairo University, editor. Topographic Pathology of Cancer. Rhone-Poulenc Rorer-Egypt; 1998. pp. 7–18. [Google Scholar]
- 12.Nasr AL Aboul. Epidemiology of cancer of the gastrointestinal tract in Egyptians. Natl Cancer Inst Monogr. 1967;25:1–6. [PubMed] [Google Scholar]
- 13.Rifai M, Amer F, Abdel-Meguid H, Mebed H. Pharyngeal repair after laryngopharyngectomy: 4-year experience. Head Neck Surg. 1987;10(2):99–101. doi: 10.1002/hed.2890100207. [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim NK, Al Ashakar MS, Gad ZM, Warda MH, Ghanem H. An epidemiological study on survival of oropharyngeal cancer cases in Alexandria, Egypt. East Mediterr Health J. 2009;15(2):369–77. [PubMed] [Google Scholar]
- 15.Saleh EM, Abdullwahab AA, Kammal MM. Age and sex incidence of hypopharyngeal tumours in upper egypt: Assuit university experience. J Laryngol Otol. 1995;109(8):737–40. doi: 10.1017/s0022215100131184. [DOI] [PubMed] [Google Scholar]
- 16.el-Barrawy MA, Ismail KA, el-Barrawi SA, Sultan AA. Detection of herpes simplex virus and candida infection with special emphasis on their possible role in human squamous cell carcinoma and its variants. J Egypt Public Health Assoc. 1996;71(3-4):285–307. [PubMed] [Google Scholar]
- 17.Badawi AF, Hosny G, el-Hadary M, Mostafa MH. Salivary nitrate, nitrite and nitrate reductase activity in relation to risk of oral cancer in Egypt. Dis Markers. 1998;14(2):91–7. doi: 10.1155/1998/507653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freedman LS, Edwards BK, Ries LAG, Young JL. Cancer incidence in four member countries (Cyprus, Egypt, Israel, and Jordan) of the Middle East cancer consortium (MECC) compared with US SEER. 2006.
- 19.Central Agency for Public Mobilization and Statistics Census report of the central agency for public mobilization and statistics. Arab Republic of Egypt. 2009 url: http://www.capmas.gov.eg/eng_ver/homee.htm.
- 20.Ibrahim AS, Ismail K, Hablas A, Hussein H, Elhamzawy H, Ramadan M. Cancer in Egypt, Gharbiah. Trienniel report of 2000-2002. Gharbiah Population-Based Cancer Registry; 2007. [Google Scholar]
- 21.el-Ayoty SA, Abdelhamid AM. Effect of the presence of a urea fertilizer plant on the nitrate content of berseem and constituents of milk and blood of buffaloes. Arch Tierernahr. 1989;39(4-5):491–8. doi: 10.1080/17450398909428327. [DOI] [PubMed] [Google Scholar]
- 22.Mostafa MH, Helmi S, Badawi AF, Tricker AR, Spiegelhalder B, Preussmann R. Nitrate, nitrite and volatile N-nitroso compounds in the urine of schistosoma haematobium and schistosoma mansoni infected patients. Carcinogenesis. 1994;15(4):619–25. doi: 10.1093/carcin/15.4.619. [DOI] [PubMed] [Google Scholar]
- 23.Bleyer A. Cancer of the oral cavity and pharynx in young females: Increasing incidence, role of human papilloma virus, and lack of survival improvement. Semin Oncol. 2009;36(5):451–9. doi: 10.1053/j.seminoncol.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Soltan ME, Awadallah RM. Chemical survey of the river Nile water from Aswan into the outlet. J Environ Sci Health. 1995;A30(8):1647–58. [Google Scholar]
- 25.Awadallah RM, Soltan ME, Shabeb MSA, Moalla SMN. Bacterial removal of nitrate, nitrite and sulphate of wastewater. Water Res. 1998;32(10):3080–4. [Google Scholar]
- 26.Abdel-Gawad S, Abdel-Shafy M. Pollution control of industrial wastewater from soap and oil industries: A case study. Water Sci Technol. 2002;46(4-5):77–82. [PubMed] [Google Scholar]
- 27.Gadalla S, Aboul-Fotouh A, El-Setouhy M, et al. Prevalence of smoking among rural secondary school students in qualyobia governorate. J Egypt Soc Parasitol. 2003;33(3 Suppl):1031–50. [PubMed] [Google Scholar]
- 28.Islam SM, Johnson CA. Western media’s influence on Egyptian adolescents’ smoking behavior: The mediating role of positive beliefs about smoking. Nicotine Tob Res. 2007;9(1):57–64. doi: 10.1080/14622200601078343. [DOI] [PubMed] [Google Scholar]
- 29.Sabra AA. Smoking attitudes, behaviours and risk perceptions among primary health care personnel in urban family medicine centers in Alexandria. J Egypt Public Health Assoc. 2007;82(1-2):43–64. [PubMed] [Google Scholar]
- 30.Lehman EM, Soliman AS, Ismail K, et al. Patterns of hepatocellular carcinoma incidence in Egypt from a population-based cancer registry. Hepatol Res. 2008;38(5):465–73. doi: 10.1111/j.1872-034X.2007.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fedewa SA, Soliman AS, Ismail K, et al. Incidence analyses of bladder cancer in the Nile Delta region of Egypt. Cancer Epidemiol. 2009;33(3-4):176–81. doi: 10.1016/j.canep.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dey S, Hablas A, Seifeldin IA, et al. Urban-rural differences of gynaecological malignancies in Egypt (1999-2002) BJOG. 2010;117(3):348–55. doi: 10.1111/j.1471-0528.2009.02447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dey S, Soliman AS, Hablas A, et al. Urban-rural differences in breast cancer incidence by hormone receptor status across 6 years in Egypt. Breast Cancer Res Treat. 2010;120(1):149–60. doi: 10.1007/s10549-009-0427-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee YC, Boffetta P, Sturgis EM, et al. Involuntary smoking and head and neck cancer risk: Pooled analysis in the international head and neck cancer epidemiology consortium. Cancer Epidemiol Biomarkers Prev. 2008;17(8):1974–81. doi: 10.1158/1055-9965.EPI-08-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hashibe M, Brennan P, Chuang SC, et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: Pooled analysis in the international head and neck cancer epidemiology consortium. Cancer Epidemiol Biomarkers Prev. 2009;18(2):541–50. doi: 10.1158/1055-9965.EPI-08-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Termine N, Panzarella V, Falaschini S, et al. HPV in oral squamous cell carcinoma vs head and neck squamous cell carcinoma biopsies: A meta-analysis (1988-2007) Ann Oncol. 2008;19(10):1681–90. doi: 10.1093/annonc/mdn372. [DOI] [PubMed] [Google Scholar]
- 37.Sapkota A, Gajalakshmi V, Jetly DH, et al. Indoor air pollution from solid fuels and risk of hypopharyngeal/laryngeal and lung cancers: A multicentric case-control study from india. Int J Epidemiol. 2008;37(2):321–8. doi: 10.1093/ije/dym261. [DOI] [PubMed] [Google Scholar]
- 38.Laouamri S, Hamdi-Cherif M, Sekfali N, Mokhtari L, Kharchi R. Dietary risk factors of nasopharyngeal carcinoma in the setif area in algeria. Rev Epidemiol Sante Publique. 2001;49(2):145–56. [PubMed] [Google Scholar]
- 39.Feng BJ, Jalbout M, Ayoub WB, et al. Dietary risk factors for nasopharyngeal carcinoma in maghrebian countries. Int J Cancer. 2007;121(7):1550–5. doi: 10.1002/ijc.22813. [DOI] [PubMed] [Google Scholar]
- 40.Feng BJ, Khyatti M, Ben-Ayoub W, et al. Cannabis, tobacco and domestic fumes intake are associated with nasopharyngeal carcinoma in North Africa. Br J Cancer. 2009;101(7):1207–12. doi: 10.1038/sj.bjc.6605281. [DOI] [PMC free article] [PubMed] [Google Scholar]