Abstract
Inhalation of ambient particulate matter causes morbidity and mortality in humans. One hypothesized mechanism of toxicity is the particle-induced formation of reactive oxygen species (ROS) – including the highly damaging hydroxyl radical (·OH) – followed by inflammation and a variety of diseases. While past studies have found correlations between ROS formation and a variety of metals, there are no quantitative measurements of ·OH formation from transition metals at concentrations relevant to 24-hour ambient particulate exposure. This research reports specific and quantitative measurements of ·OH formation from 10 individual transition metals (and several mixtures) in a cell-free surrogate lung fluid (SLF) with four antioxidants: ascorbate, citrate, glutathione, and uric acid. We find that Fe and Cu can produce ·OH under all antioxidant conditions as long as ascorbate is present and that mixtures of the two metals synergistically increase ·OH production. Manganese and vanadium can also produce ·OH under some conditions, but given that their ambient levels are typically very low, these metals are not likely to chemically produce significant levels of ·OH in the lung fluid. Cobalt, chromium, nickel, zinc, lead, and cadmium do not produce ·OH under any of our experimental conditions. The antioxidant composition of our SLF significantly affects ·OH production from Fe and Cu: ascorbate is required for ·OH formation, citrate increases ·OH production from Fe, and both citrate and glutathione suppress ·OH production from Cu. MINTEQ ligand speciation modeling indicates that citrate and glutathione affect ·OH production by changing metal speciation, altering the reactivity of the metals. In the most realistic SLF (i.e., with all four antioxidants), Fe generates approximately six times more ·OH than does the equivalent amount of Cu. Since levels of soluble Fe in PM are typically higher than those of Cu, our results suggest that Fe dominates the chemical generation of ·OH from deposited particles in the lungs.
Keywords: Reactive oxygen species (ROS), particulate matter (PM), oxidative stress, iron, copper, Fenton reaction
1. Introduction
Abundant epidemiological evidence links exposure to ambient particulate matter (PM) with adverse health effects (Daniels et al., 2000; Brunekreef and Holgate, 2002; Schwartz et al., 2002; Pope and Dockery, 2006). Although the toxic mechanism(s) responsible are not well understood, one likely pathway is the formation of reactive oxygen species (ROS) after PM deposition in the lungs, followed by an oxidant/antioxidant imbalance (i.e., oxidative stress), which has been linked to inflammation and several disease states (Halliwell and Cross, 1994; Rahman et al., 1996; Ayres et al., 2008).
ROS as a class includes superoxide radical anion (O2·-), hydrogen peroxide (H2O2), and hydroxyl radical (·OH) (Halliwell and Cross, 1994). ·OH is the most destructive ROS species in vivo (Halliwell, 1995), damaging DNA and initiating a catalytic cycle of cell membrane lipid peroxidation (Halliwell and Cross, 1994). ROS occur naturally in cells and extracellular fluids, but are not damaging at low levels due to protective antioxidants (AOs) and enzymes; adverse effects only occur at higher oxidant levels, or when AOs are depleted (Halliwell and Whiteman, 2004). PM can form ROS in two ways after deposition into the lungs: 1) through chemical reactions of redox-active particle components and 2) through biological responses to the PM. Our research aims to understand oxidant production from the first mechanism.
ROS production from ambient particles has been correlated with the soluble transition metal (TM) content of PM, which has also been correlated with particle-mediated cardiopulmonary toxicity (Carter et al., 1997; Costa and Dreher, 1997; Dreher et al., 1997; Gavett et al., 1997; DiStefano et al., 2009; Vidrio et al., 2009; Zhong et al., 2010). Dreher et al. (1997) found that after washing PM in saline, the PM no longer produced adverse effects in rats, while the leachate containing soluble iron, nickel, and vanadium produced an inflammatory response that was similar to that from the unwashed PM.
In many previous studies of oxidant production from PM, correlations between non-specific ROS measurements and chemical components in PM are used to elucidate the components that may be responsible for oxidant generation. While this method can identify chemicals of interest, correlation does not indicate causation; indeed, these correlations often identify chemical species that are not redox active and that are not likely to directly produce ROS. For example, studies have found correlations between ROS production and water-soluble compounds that are not redox active such as boron, magnesium, potassium, and ammonium (Zhang et al., 2008; Verma et al., 2009). While the non-redox-active species may be associated with PM components that can produce ROS, their correlations with ROS production could also be specious. In fact, even when redox-active species are identified by correlations, it is unclear whether these species contribute significantly to the measured ROS response.
To better understand the importance of particulate components as sources of ROS, we need quantitative and specific measurements of ROS formation by these components under physiologically relevant conditions. Valavanidis et al. (2005) were one of the first groups to measure ·OH from a large number of TMs; however, their metal concentration (40 mM) was many orders of magnitude higher than ambient PM could produce in lung fluid (e.g., Vidrio et al., 2009). Vidrio et al. (2008) quantified ·OH from Fe and Cu, but their metal concentrations (20 µM) were also higher than would be produced in lungs from ambient PM; in addition they did not examine other TMs.
The role of complex mixtures of lung AOs on the production of specific ROS from different particle components in a surrogate lung fluid (SLF) has not been well explored. It is well known that ascorbate (Asc) is important in oxidant production from metals (Ayres et al., 2008; DiStefano et al., 2009): reduced forms of TMs can reduce dissolved oxygen to, sequentially, ·O2-, H2O2, and ·OH (R1 – R3), and ascorbate can recycle the oxidized metal back to its reduced form (R4), allowing a small amount of TM to potentially produce a large amount of ROS.
| (R1) |
| (R2) |
| (R3) |
| (R4) |
Though Asc has a vital role in oxidant production from iron and copper, there have been no fully quantitative investigations into interactions of multiple AOs on ·OH production from many of the 10 TMs studied here. Citrate can chelate iron making it more bioavailable and able to increase oxidative stress (Zhong et al., 2010), while GSH has been observed to increase or decrease ·OH production from metals (Hanna and Mason, 1992; Kachur et al., 1998). Research that uses relatively simple lung fluid surrogates which contain no AOs or contain only Asc or GSH may miss important effects from other AOs.
The purpose of this research is to quantify ·OH production from TMs in the presence of different mixtures of lung AOs. To do this we measured ·OH formation from atmospherically relevant concentrations of 10 common TMs – both individually and in mixtures. We also examined the effects of four endogenous lung AOs on ·OH production: Asc, citrate (Cit), reduced L-glutathione (GSH), and uric acid (UA).
2. Methods
2.1 Chemicals and Surrogate Lung Fluid
Information about the supplier and purity for each chemical is in section S.1 of the supplementary information. All solutions were prepared in purified water from a Milli-Q Plus system (Millipore, ≥ 18.2 MΩ-cm). ·OH measurements were performed in a cell-free SLF containing AOs in phosphate-buffered saline (PBS) (114 mM NaCl, 7.8 mM sodium phosphate dibasic and 2.2 mM potassium phosphate monobasic, pH 7.2–7.4), and 10.0 mM sodium benzoate (as an ·OH probe). 500 mL of this solution was allowed to drip slowly through a chromatography column containing chelex-100 to remove trace metals and was then refrigerated and used within a week.
2.2 Quantification of Hydroxyl Radical
24-hour total ·OH generation was quantified using a benzoate (BA) probe technique (Jung et al., 2006; Vidrio et al., 2008; Vidrio et al., 2009) where BA scavenges ·OH and forms para-hydroxybenzoic acid (p-HBA), which is quantified using high performance liquid chromatography (HPLC). Because BA is simply a probe, and is not involved in ROS production, this method is able to quantify ·OH from any chemical species, including transition metals and organics. Note that we are measuring the production of ·OH, which is the key to understanding the likely lung burden of ·OH resulting from PM deposition. We are not quantifying the sinks of ·OH, which in vivo include AOs, mucin, and cellular components.
The total concentration of ·OH formed over the 24-hr reaction period is:
| (1) |
where [p-HBA] is the concentration measured by HPLC, Yp-HBA is the molar yield of p-HBA under our conditions (0.215±0.018) (Jung et al., 2006), and fBA is the fraction of ·OH that reacts with BA (as opposed to other solution components) in the SLF (Vidrio et al., 2008). fBA is calculated as the rate of ·OH reaction with BA divided by the sum of the rates of reaction of ·OH with all solution components (A, B…N):
| (2) |
Note that [·OH] in this equation cancels and thus fBA depends only on the concentration of each solution component ([BA], [A], etc.) and their second-order rate constants with ·OH (kBA, kA, etc.), which are published (Walling et al., 1974; Buxton et al., 1988; Zepp et al., 1992). Values of fBA ranged from 0.96 to 0.99 to 0.96 (section S1).
The HPLC consisted of either a manual injection Shimadzu LC-10AT pump and SPD-10AV UV-Vis detector or a Shimadzu LC-10ATVP pump, SPD-10AV UV-Vis detector and SIL-10AF autosampler with CMB-20A controller. Other HPLC parameters were identical to Vidrio et al. (2009). A calibration curve was run daily using p-HBA standards in SLF.
2.3 Sample Preparation
We examined the ability of dissolved metals to produce ·OH in SLFs containing different combinations of Asc, Cit, GSH and UA. For each reaction solution, 10.0 mL of PBS was added to a 125-mL, acid-washed FEP bottle wrapped in aluminum foil to exclude light. The desired metal(s) and AO(s) were added to each reaction bottle and the solution was shaken on a wrist-action shake table in the dark for 24 hours. AO concentrations were 200 µM Asc, 300 µM Cit, 100 µM GSH, and 100 µM UA in all experiments, which are similar to AO concentrations observed in human lung fluid (Cross et al., 1994; van der Vliet et al., 1999). While Cit has apparently not been examined in lung fluid, it is commonly used as a surrogate for protein, e.g., in Gamble’s solution (Moss, 1979; Colombo et al., 2008), and it has been measured in blood plasma (Walser, 1961), cerebrospinal fluid, kidneys, and liver (Mycielska et al., 2009).
While past work has shown that the pro-oxidant versus anti-oxidant nature of AOs can depend upon the metal/AO ratio (Reed and Douglas, 1991), these studies typically use high concentrations of metals (which we do not) and they are not focused on the production of ·OH (which we are). In this work we use physiologically realistic concentrations of antioxidants and environmentally realistic metal concentrations, resulting in metal/AO ratios that are on the order of 0.01. In this regime neither metal speciation nor ·OH production should change appreciably with realistic perturbations of AO or metal concentration. The exception is ascorbate: ·OH production does vary with the concentration of Asc (data not shown), which we believe is the limiting reagent in our system. While this effect is interesting, it is outside the scope of the current work.
After 24 hours of shaking, ·OH generation was quenched using 100 µM desferoxamine (DSF) and 50 µM sodium bisulfite. Solutions were then acidified to pH 2 using 120 µL of 1.0 M H2SO4 and injected on the HPLC within 18 hours; we have found that p-HBA in quenched solutions is stable (< 4% change) for up to 18 hours at room temperature in the dark. Sample results on a given day are blank corrected by subtracting the average blank (run on the same day) containing the same composition of AOs but without any metals; uncertainties in the resulting blank-corrected data are ± 1σ propagated from the blank and sample data (Taylor, 1997). Background concentrations of ·OH are due to trace metal contamination that could not be removed even with our rigorous cleaning. Average blank ·OH concentrations were 31 ± 6 nmol (n=32) for Asc + Cit and 26 ± 3 nmol (n=24) for All AOs. We ran regular positive controls of either 10 nmol Fe(II) or 10 nmol Cu(II) in SLF containing either Asc + Cit or All AOs. With Asc + Cit, the average blank-corrected Fe(II) and Cu(II) positive controls were 183 ± 4 nmol (n=13) and 66 ± 3 nmol (n=8), respectively. Corresponding values in SLF with All AOs were 188 ± 4 nmol (n=11) and 27 ± 1 nmol (n=9) for iron and copper, respectively.
2.4 Ligand Speciation Modeling
MINTEQ A2 Version 4.03 (US EPA; http://www.epa.gov/ceampubl/mmedia/minteq) was used to determine metal speciation. Formation constants from the MINTEQ database were supplemented with data from the literature for Asc and GSH (Martin and Edsall, 1959; Faust and Zepp, 1993; Martell et al., 2004) (see section S.2). We could not find formation constants for any of the TMs with UA, or for Fe(II) with GSH. Because abundant data for GSH with other TMs were available, we used the Irving-Williams series (Irving and Williams, 1953) to estimate that the likely upper bound for the Fe[HGSH] stability constant is equal to that of Co[HGSH] (log K = 3.3). Kretzel and Bal (1999) indicate that Fe-GSH bonding is generally weak, in agreement with these results. UA was not included in any model runs, as no data were available; however, UA does not appear to be a significant ligand since the addition of UA had no effect on ·OH generation (see below).
2.5 Statistics
All experiments on a given day were run in triplicate. Data are reported as the average ± standard deviation. Outliers of repeated experiments (n>5) were identified using Chauvenet’s Criteria with a cutoff of 0.5 (Taylor, 1997). Chauvenet’s Criteria could not identify outliers for samples run on a single day due to the small sample size (n=3). Outliers in these samples were identified if they were outside three times the relative standard deviation (RSD) of measurements repeated many times, whether for blanks (RSD = 21.2%, n = 64) or samples (RSD = 6.4%, n = 41). A total of 12 out of 736 data points were identified as outliers using these methods. Statistical significance between two means was determined using the students t test for P<0.05 and P<0.01.
3. Results and Discussion
3.1 Individual Transition Metals
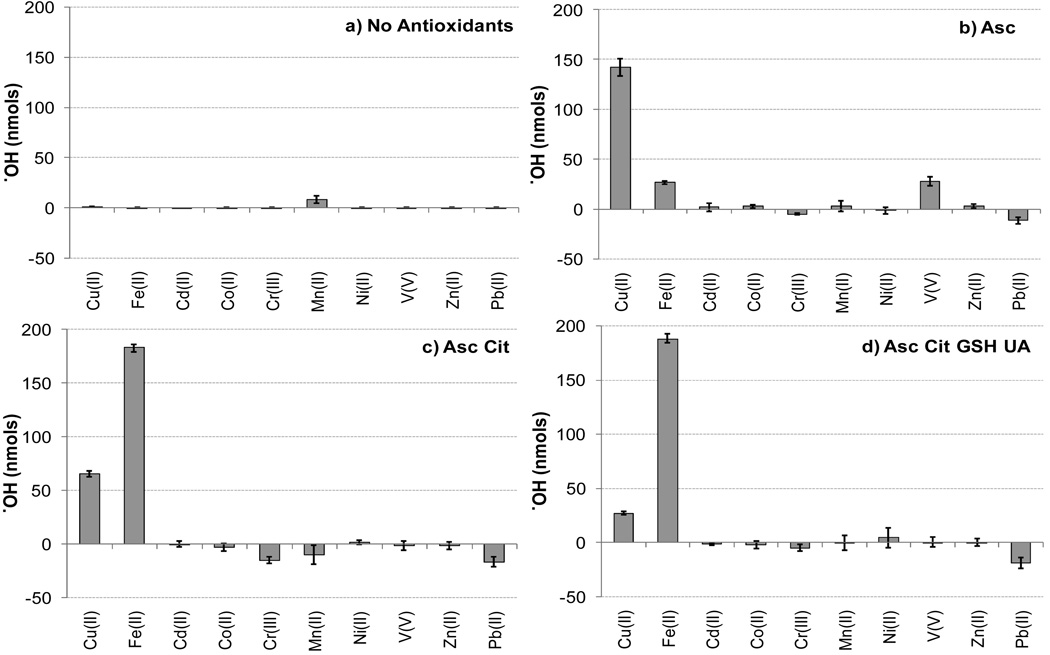
As an initial screening, we quantified the ability of 10 nmol (i.e., 1.0 µM) of 10 individual dissolved TMs to produce ·OH in surrogate lung fluid (SLF) with four different antioxidant (AO) compositions: no AOs; Ascorbate (Asc) only; Asc + citrate (Cit); and Asc + Cit + glutathione (GSH) + uric acid (UA) (i.e., “All AOs”) (Fig. 1).
Figure 1.
·OH production from 1.0 µM (10 nmol) of individual transition metals in four antioxidant solutions: a) no antioxidants added, b) Asc only, c) Asc + Cit, d) Asc + Cit + GSH + UA (i.e., All AOs). Each bar is the mean ± σ (n ≥ 3).
Without any AOs, only Mn(II) produces ·OH, but only a small amount (8 ± 4 nmol) (Fig. 1a). This is the only case of ·OH formation without Asc. We do not have an explanation for this production or why it disappears in the presence of AOs (Figs. 1b – 1d), but the result is reproducible, consisting of six data points from two experimental days. MINTEQ modeling results indicate that 100% of Mn(II) precipitates in the presence of phosphate buffer. To test whether precipitation was precluding ·OH formation from Mn(II) we measured ·OH production in a bicarbonate buffering system without phosphate (Table S4). There was no ·OH formation in the bicarbonate buffering system confirming our result that Mn(II) cannot produce ·OH in the presence of AOs (See Section S3).
In SLF with only Asc, three metals – Cu(II), Fe(II) and V(V) – produce ·OH, with Cu showing the most reactivity (Fig. 1b). In the final two AO combinations, Asc + Cit (Fig. 1c) and all AOs (Fig. 1d), Fe(II) and Cu(II) are the only metals that produce ·OH. In every SLF containing Asc, Pb(II) is consistently inhibitory, reducing ·OH levels by an average of 16 ± 4 nmol compared to the corresponding blank solution without any metal. Cr(III) was also inhibitory in some cases. We do not have an explanation for these effects.
Of the metals we examined, Fe(II) and Cu(II) are the most important sources of ·OH. Valavanidis et al. (2005) observed ·OH production from Fe(II), V(IV), Cr(III), Cu(I), Co(II), Ni(II), Pb(II), and Cd(II) in solutions containing 0.04 M metal and 0.04 M hydrogen peroxide. The difference between our results and theirs are likely due to the enormous difference in metal concentration and the difference in added hydrogen peroxide. While we only tested a single oxidation state for each TM, the effect of oxidation state is likely to be small, as found previously for iron (Vidrio et al., 2008). This is because, based on redox couple data (Lide, 1999; Merkofer et al., 2006), Asc can reduce most of the metals studied here (although we found no data for Ni, Cd or Pb, and Zn does not redox cycle; Table S1).
3.2 Effect of Antioxidants
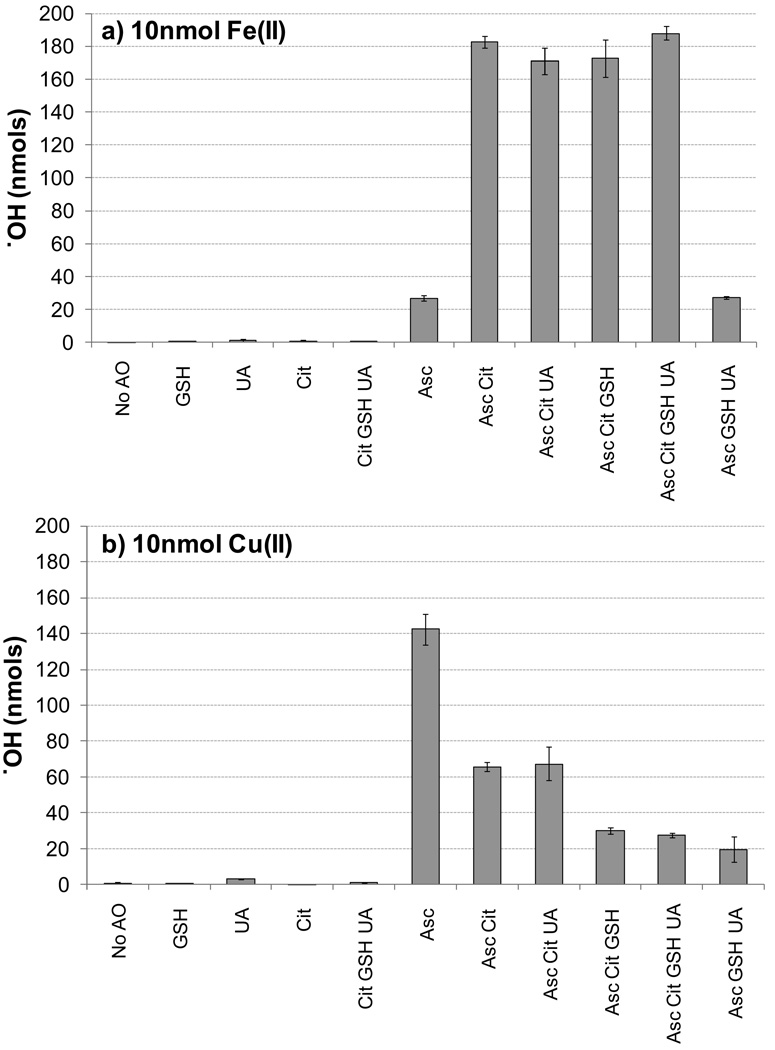
Results from Fig. 1 indicate that AO composition can impact ·OH production. To further investigate this, we tested a variety of AO combinations with Fe(II) and Cu(II) using 10.0 nmol (1.0 µM) of metal. As shown in Fig. 2, neither GSH, UA, or Cit (individually or in combination) leads to ·OH production from Fe(II) or Cu(II). In contrast, Asc alone leads to ·OH production from Fe(II) and, especially, Cu(II). Adding Cit to Asc increases ·OH production from Fe(II) by a factor of seven, but there is no effect from adding GSH and/or UA (Fig. 2a). Cu(II) shows different behavior: adding Cit to an Asc solution causes more than a two-fold decrease in ·OH (Fig. 2b). Adding UA to this solution (i.e., Asc + Cit + UA) has no effect on ·OH production from Cu(II), but the addition of GSH suppresses ·OH production – by five-fold compared to Asc only – regardless of whether the SLF contains Cit and/or UA. This indicates that – compared to solutions with only Asc – both Cit and, especially, GSH inhibit ·OH production by Cu(II), while UA has no effect. These different, and sometimes dramatic, effects of endogenous AOs on ·OH production by Fe and Cu highlight the need to use physiologically relevant AO mixtures in the extraction fluids used for ROS measurements from PM.
Figure 2.
·OH production under various antioxidant compositions from 1.0 µM (10 nmol) of a) Fe(II) or b) Cu(II). Each bar is the mean ± σ (n ≥ 3).
We can compare our ·OH production results from Fe and Cu to previously reported data obtained under similar experimental conditions but with higher levels of metal (200 nmol) (Vidrio et al., 2008). Though the metal concentrations in Vidrio et al. are 20 times higher than those we have used, ·OH concentrations are only higher by factors of 1.3 to 6.2 compared to our results. This is because ·OH production is not linearly related to metal concentration, but rather plateaus at approximately 40 nmol Fe(II) and 1 nmol Cu(II) (Supplement Fig. S1), probably because Asc becomes the limiting reagent.
To understand this behavior it is useful to calculate the reaction efficiency (RE) of each metal for producing ·OH:
Note that the denominator here is the total amount of metal and not individual metal species (Supplement Table S4). Because each metal-ligand species can have a different reactivity, the RE values cannot be confidently extrapolated to other solution compositions. Since three electrons are necessary to produce ·OH from dissolved oxygen (see R1 – R3), multiplying the RE by 3 gives an estimate of the number of times each metal atom was recycled by Asc. Metal reaction efficiencies are large at low metal concentrations and decrease with increasing metal content (Fig. S2). The maximum observed RE values for Cu(II) and Fe(II) (found at the lowest metal concentrations tested) were 500 and 33, indicating that each atom of Cu(II) and Fe(II) can be recycled up to approximately 1,500 and 100 times, respectively. These extremely high recycling efficiencies indicate that very low concentrations of metals can still produce significant amounts of oxidants in the lungs. The fact that RE values do not qualitatively match the (thermodynamic) metal-Asc redox couples (Table S1) suggests either that ·OH production in our system is determined by kinetics rather than thermodynamics or that ligand speciation changes the metal-Asc redox couple.
Our results in Fig. 2 can also help explain why different studies find that different particle components are most responsible for ·OH production in cell-free solutions. For example, using methods similar to our current work, DiStephano et al. (2009) found that soluble Cu was the most important indicator of ·OH production by PM0.18 from Southern California, while Vidrio et al. (2009) found that soluble Fe was the most important predictor of ·OH from PM2.5 from Northern California. While differences in particle composition may contribute to the observed difference in the relative importance of Fe and Cu between these two studies, the AOs used in each study is another likely explanation. DiStephano et al. extracted their particles into a solution containing 500 µM Asc – conditions where Cu will likely be more than five times as efficient as Fe at producing ·OH (Fig. 2) – consistent with their observation of a strong correlation between ·OH and soluble Cu. In contrast, Vidrio et al. extracted their particles in a solution containing 200 µM Asc and 300 µM Cit – conditions where Fe is nearly three times as efficient as Cu at producing ·OH – consistent with their observation that soluble Fe could account for the majority of ·OH production. While the concentration of Asc is different in these two studies, our preliminary data (not shown) suggests that this will not change the relative importance of Fe and Cu, although it will change the absolute amounts of ·OH formed.
3.3 Ligand Speciation Modeling
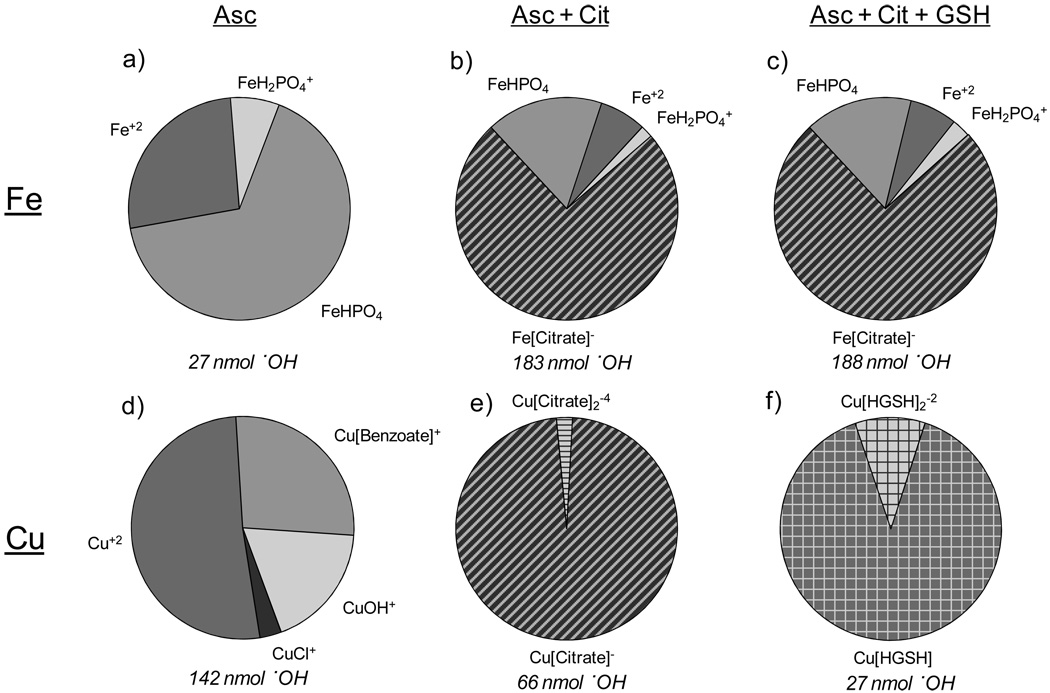
The effect of AO composition on ·OH production observed in section 3.2 may have two explanations: 1) redox interactions between the AOs and metals are affecting ·OH production (as is likely for Asc), or 2) the AOs affect the metal speciation, which alters metal reactivity (Halliwell, 1995). To examine this second possibility, we modeled metal speciation for three different SLF AO compositions: Asc, Asc + Cit, and Asc + Cit + GSH. (UA was not included because no data were available, but it appears to have no effect on ·OH production (Fig. 2)). Fig. 3 shows speciation results for Fe(II) and Cu(II) in these three AO mixtures; results for other metals are in Table S4 of the supplementary data. With Asc only, Fe is primarily bound to phosphate or is present as free Fe2+ (Fig. 3a). When Cit is added to the Asc solution, the speciation changes significantly, with the Fe[Cit]− complex becoming dominant (Fig. 3b). The enhancement in ·OH observed with the addition of Cit (Fig. 2a) suggests that Fe[Cit]− is more reactive than FeHPO4. Adding GSH causes no apparent change in Fe speciation (Fig. 3c), consistent with the fact that adding GSH does not affect ·OH production from Fe(II) (Fig. 2a).
Figure 3.
MINTEQ speciation modeling results for 10 nmol Fe(II) or Cu(II) for three antioxidant compositions: a,d) Asc, b,e) Asc + Cit and c,f) Asc + Cit + GSH. Numbers under the graphs represent the amount of ·OH produced for each experimental condition.
Modeling results with Cu(II) show that Cu2+ dominates in SLF with Asc, with the remainder of Cu(II) bound to benzoate, OH−, or Cl− (Fig. 3d). The addition of Cit to the SLF with Asc causes a dramatic shift in Cu speciation, with 97% of Cu(II) now bound as Cu[Citrate]− (Fig. 3e). Vidrio et al.(2008) found a similar result using MINEQL+ for 20 µM Cu(II) solutions with Asc and Cit, but with a different Cit species, Cu2[Citrate]+, dominating. When GSH is added to the SLF with Asc and Cit, the Cu(II) speciation shifts dramatically again, now to Cu[HGSH] and Cu[HGSH]2−2 (Fig. 3f). These results are consistent with a previous in vivo study, which observed that a majority of Cu is bound to GSH and postulated that this complex is used to store and transport Cu (Aliaga et al., 2010).
In conjunction with data in Fig. 2b, these speciation data indicate that copper complexes have the following relative reactivities for OH generation: Cu+2 > Cu-Cit > Cu-GSH. This suggests that GSH has two protective mechanisms in vivo: as a sacrificial AO (i.e., as a sink for ·OH and other ROS) and as a strong ligand for Cu to form Cu-GSH complexes that are less efficient at generating ROS. Oxidant suppression from Cu-GSH mixtures has been observed previously (Hanna and Mason, 1992; Maestre et al., 1992).
Based on chemical speciation modeling (Table S4), BA does not significantly bind to most of the metals tested here. One exception is the BA-Mn(II) complex, which accounts for about 25% of Mn, but this should be unimportant since Mn(II) is a negligible source of ·OH.
3.4 Binary Mixtures of Transition Metals
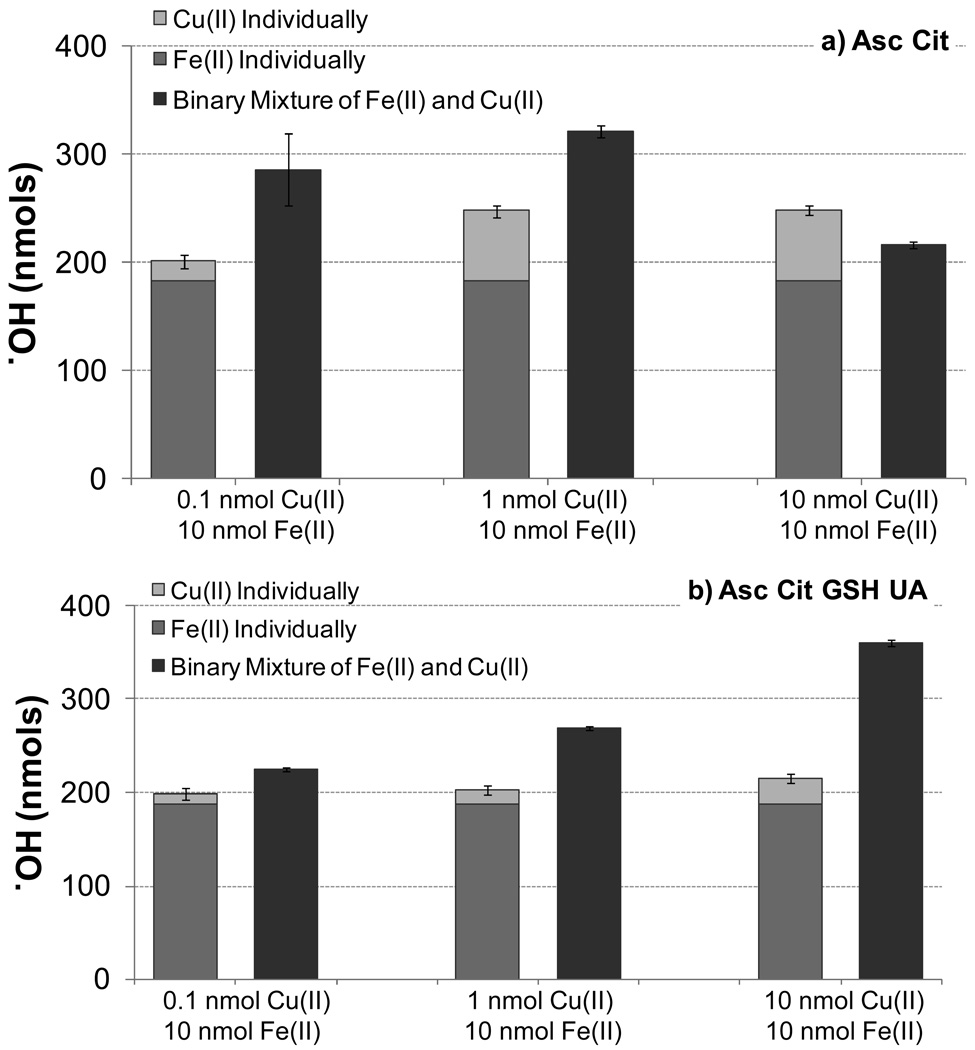
Ambient PM contains a wide variety of TMs and the reactivity of these mixtures can be different than what is expected from a simple addition of individual metal effects (Maestre et al., 1992; Sedlak et al., 1997; Vidrio et al., 2008). To explore the impacts of metal mixtures on ·OH production we first examined binary mixtures of Fe(II) (10.0 nmol) and Cu(II) (0.10, 1.0 or 10.0 nmol) under two AO conditions: Asc + Cit and All AOs (Fig. 4). The stacked bars in Fig. 4 represent the sum of ·OH production from Fe(II) and Cu(II) measured individually, while the dark grey bars show ·OH production from Fe(II) and Cu(II) mixed in the same bottle. The sum of ·OH from the individual metals is statistically different (P<0.01) than ·OH production in the metal mixtures for all six cases. For Asc + Cit, the addition of small amounts of Cu(II) enhances ·OH production from Fe(II) (by 49% and 34% for Fe/Cu ratios of 10:0.1 and 10:1, respectively), but a larger amount of Cu suppresses ·OH (by 10% for a 1:1 Fe/Cu mixture; Fig. 4a). Similar behavior of ·OH from mixtures of higher concentrations of Fe(II) and Cu(II) have been observed in the literature, with enhancement or suppression depending on the ratio of Fe(II) to Cu(II) and greatest suppression at the 1:1 Cu(II):Fe(II) ratio (Maestre et al., 1992; Vidrio et al., 2008). However, in SLF with all four AOs, greater amounts of Cu(II) lead to greater enhancements in ·OH production by Fe(II), ranging from 13% to 67% (Fig. 4b). This difference in Cu(II) reactivity may be due to the change in ligand speciation from Cu-Cit to Cu-GSH forms (Fig. 3).
Figure 4.
·OH from binary mixtures of Fe(II) + Cu(II) compared to the sum of ·OH production from the corresponding individual solutions of Fe and Cu. The error in the sum of the individual Fe(II) and Cu(II) results (stacked bars) is the propagated standard deviation. For each pair, the sum of the individual solutions is significantly different (P<0.01) from the value in the binary mixture.
There are two explanations for the observed synergistic ·OH production from mixtures of Fe(II) and Cu(II): 1) Cu(II) produces hydrogen peroxide more efficiently than Fe(II) (Shen et al., 2010) which will increase the rate of the Fenton reaction and 2) redox reactions between Fe and Cu contribute to metal recycling (Sedlak et al., 1997).
We performed similar experiments for binary mixtures of Fe(II) or Cu(II) with Pb(II), Mn(II), or V(V) (Fig. S4). Mixtures of Fe(II) with Mn(II) or V(V) and Cu(II) with V(V) enhanced ·OH production, but only at high levels of soluble Mn(II) or V(V) (i.e., 10 nmol), which are probably greater than experienced due to ambient PM inhalation. For example, we estimate average (± 1σ) amounts of dissolved Fe, Cu, Mn and V in lung fluid after 24 hours of PM2.5 exposure in Davis, CA to be 5.3 ± 4.7, 0.9 ± 1.2, 0.6 ± 0.4, and 0.2 ± 0.2 nmol, respectively, based on data from Vidro et al. (2009).
3.5 Complex Transition Metal Mixtures
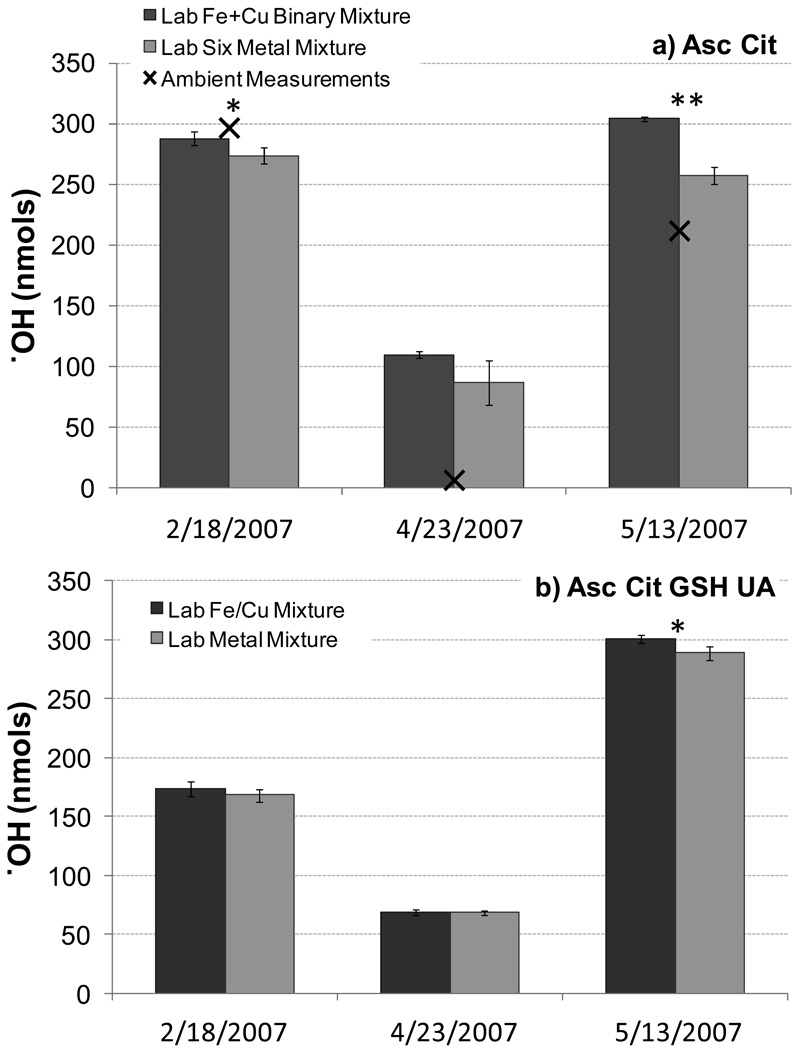
Since binary mixtures of metals – especially Fe with Cu – led to synergistic production of ·OH, we also examined more complex mixtures of environmentally relevant concentrations of six TMs: Fe(II), Cu(II), Co(II), Mn(II), V(V), and Zn(II). ·OH production and SLF-soluble TMs from extracts of ambient 24-hr PM2.5 samples were measured in a previous study in a SLF containing the same concentrations of Asc and Cit used here (Vidrio et al., 2009). We reproduced the TM mixtures observed in three of these 24-hr PM2.5 extracts and tested these lab mixtures for two AO conditions: Asc + Cit and All AOs (Fig. 5). In parallel, we tested binary mixtures containing only Fe(II) and Cu(II) at the concentrations measured in each of the three samples.
Figure 5.
·OH production from SLF extracts of ambient PM2.5 samples (×; only available for the Asc + Cit conditions; data from Vidrio et al. 2009) and from laboratory solutions containing either a binary mixture of Fe and Cu (dark bars) or a mixture of six TMs (light bars), all at the concentrations measured in the corresponding ambient sample. SLF-soluble Fe, Cu, Mn, V, Co, and Zn levels in each sample were as follows: 2/18/2007 – 2.8, 0.05, 0.43, 0.06, 0.04 and 0.06 nmol, respectively; 4/23/2007 – 6.1, 0.67, 0.56, 0.02, 0.02, and 1.1 nmol, respectively; 5/13/2007 – 16.4, 2.0, 0.75, 0.02, 0.02, and 1.2 nmol, respectively. Asterisks (*, **) indicate laboratory solution means (binary and six-metal mixtures) that are significantly different at P<0.05 and P<0.01, respectively.
In all cases, ·OH production from the binary mixture of Fe(II) and Cu(II) is greater than or equal to ·OH production from the mixture of six TMs (Fig. 5), indicating that the other four TMs – Co(II), Mn(II), V(V), and Zn(II) – do not add to ·OH production but can suppress ·OH slightly (by 3 – 15% here). In the SLF with Asc + Cit, ·OH measured in the ambient PM extract from 2/18/2007 is reproduced well by the mixture of Fe(II) and Cu(II) (Fig. 5a), suggesting that the Fe + Cu synergistic behavior seen in laboratory solutions (Fig. 4) also occurs in inhaled ambient PM. However, in the other two samples, ·OH in the ambient PM extracts is lower than both the laboratory Fe(II) + Cu(II) mixture and the laboratory six-TM mixture; this suppression of ·OH is especially large (98% reduction compared to the TM mixture) for the 4/23/2007 sample. One possible reason for the suppression is that the ambient PM extracts might contain organic ligands that alter the speciation of TMs and thereby reduce their ability to generate ·OH.
3.6 Implications
We can use our results to estimate which TM components are most important for ·OH production in ambient PM. Vidrio et al. (2009) measured the range of SLF-soluble Fe and Cu in 4-mL extracts of 24-hr PM2.5 samples to be 0.99 – 22 and 0.05 – 4.4 nmol respectively (n=38). Considering individual metals under the most realistic AO composition – i.e., with all AOs – this amount of Fe can produce between 25 and 300 nmol of ·OH, while this range of Cu can produce 1 – 30 nmol of ·OH. This suggests that Fe typically dominates ·OH production from particles deposited in the lung, as observed by Vidrio et al. (2009) for a cell-free SLF. Indeed, in SLF with all AOs, the larger contribution of copper is its synergism with Fe to enhance ·OH production (Fig. 4). While a larger number of Fe and Cu mixtures need to be investigated to predict the effect of metal synergism on ·OH production, based on Fig. 4 it appears that Fe will be responsible for the majority of ·OH production at ambient metal concentrations.
4. Conclusions
We have quantified ·OH production from 10 dissolved TMs in a surrogate lung fluid containing physiological levels of four endogenous lung antioxidants. Only Fe, Cu, and sometimes V and Mn, are able to produce ·OH in our solutions; the other TMs (Co, Ni, Zn, Cr, Cd, Pb) do not produce ·OH at the low metal concentrations expected from ambient PM in the lungs. As seen in previous studies, Asc acts as a pro-oxidant in the presence of Fe, Cu, and V and is a necessary component for ·OH production from these metals. Cit and/or GSH can dramatically affect ·OH production from Fe(II) and Cu(II), likely due to metal-ligand interactions as indicated by speciation modeling, while UA has no effect. Our work shows that it is important to consider antioxidant composition when measuring oxidants in a cell-free SLF; we recommend that the most realistic condition should be used – i.e., the combination of all four AOs considered here, each at a physiologically relevant concentration.
Mixtures of Fe(II) and Cu(II) act synergistically and can substantially increase ·OH production. Mixtures of V(V) and Mn(II) with either Cu(II) or Fe(II) cause ·OH enhancement only at relatively high (and generally unrealistic) concentrations of V and Mn. In more complex TM mixtures, Fe(II) and Cu(II) together can still explain the majority of ·OH production, while Mn, V, Co, Pb and Zn have minor, often inhibitory, effects on ·OH production. There is also evidence that other particle components – possibly organic ligands – can reduce the ability of ambient PM components to generate ·OH compared to what is expected from dissolved Fe(II) and Cu(II).
Research Highlights
Of 10 metals tested, Fe and Cu produce the most ·OH in a surrogate lung fluid
Antioxidant composition significantly affects ·OH production from metals
Glutathione and citrate alter ·OH production by changing metal speciation
Mixtures of Fe and Cu synergistically produce ·OH
Supplementary Material
Acknowledgements
We thank Dr. Robert Rice for providing transition metal salts. This project was supported by Award Number P42ES004699 from the National Institute of Environmental Health Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health. Additional support was provided by the California Agricultural Experiment Station (Project CA-D*-LAW-6403-RR) and the University of California Toxic Substances Research and Teaching Program (TSR&TP) through the Atmospheric Aerosols and Health Lead Campus Program (aah.ucdavis.edu).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aliaga ME, Carrasco-Pozo C, Lopez-Alarcon C, Speisky H. The Cu(I)-glutathione complex: factors affecting its formation and capacity to generate reactive oxygen species. Transition Metal Chemistry. 2010;35:321–329. [Google Scholar]
- Ayres JG, Borm P, Cassee FR, Castranova V, Donaldson K, Ghio A, Harrison RM, Hider R, Kelly F, Kooter IM, Marano F, Maynard RL, Mudway I, Nel A, Sioutas C, Smith S, Baeza-Squiban A, Cho A, Duggan S, Froines J. Evaluating the toxicity of airborne particulate matter and nanoparticles by measuring oxidative stress potential - a workshop report and consensus statement. Inhalation Toxicology. 2008;20:75–99. doi: 10.1080/08958370701665517. [DOI] [PubMed] [Google Scholar]
- Brunekreef B, Holgate ST. Air pollution and health. Lancet. 2002;360:1233–1242. doi: 10.1016/S0140-6736(02)11274-8. [DOI] [PubMed] [Google Scholar]
- Buxton GV, Greenstock CL, Helman WP, Ross AB. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/O-) in aqueous solution. Journal of Physical and Chemical Reference Data. 1988;17:513–886. [Google Scholar]
- Carter JD, Ghio AJ, Samet JM, Devlin RB. Cytokine production by human airway epithelial cells after exposure to an air pollution particle is metal-dependent. Toxicology and Applied Pharmacology. 1997;146:180–188. doi: 10.1006/taap.1997.8254. [DOI] [PubMed] [Google Scholar]
- Colombo C, Monhemius AJ, Plant JA. Platinum, palladium and rhodium release from vehicle exhaust catalysts and road dust exposed to simulated lung fluids. Ecotoxicology and Environmental Safety. 2008;71:722–730. doi: 10.1016/j.ecoenv.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Costa DL, Dreher KL. Bioavailable transition metals in particulate matter mediate cardiopulmonary injury in healthy and compromised animal models. Environmental Health Perspectives. 1997;105:1053–1060. doi: 10.1289/ehp.97105s51053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross CE, Vandervliet A, Oneill CA, Louie S, Halliwell B. Oxidants, antioxidants, and respiratory-tract lining fluids. Environmental Health Perspectives. 1994;102:185–191. doi: 10.1289/ehp.94102s10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels MJ, Dominici F, Samet JM, Zeger SL. Estimating particulate matter-mortality dose-response curves and threshold levels: an analysis of daily time-series for the 20 largest US cities. American Journal of Epidemiology. 2000;152:397–406. doi: 10.1093/aje/152.5.397. [DOI] [PubMed] [Google Scholar]
- DiStefano E, Eiguren-Fernandez A, Delfino RJ, Sioutas C, Froines JR, Cho AK. Determination of metal-based hydroxyl radical generating capacity of ambient and diesel exhaust particles. Inhalation Toxicology. 2009;21:731–738. doi: 10.1080/08958370802491433. [DOI] [PubMed] [Google Scholar]
- Dreher KL, Jaskot RH, Lehmann JR, Richards JH, McGee JK, Ghio AJ, Costa DL. Soluble transition metals mediate residual oil fly ash induced acute lung injury. Journal of Toxicology and Environmental Health. 1997;50:285–305. [PubMed] [Google Scholar]
- Faust BC, Zepp RG. Photochemistry of aqueous iron(III) polycarboxylate complexes - roles in the chemistry of atmospheric and surface waters. Environmental Science & Technology. 1993;27:2517–2522. [Google Scholar]
- Gavett SH, Madison SL, Dreher KL, Winsett DW, McGee JK, Costa DL. Metal and sulfate composition of residual oil fly ash determines airway hyperreactivity and lung injury in rats. Environmental Research. 1997;72:162–172. doi: 10.1006/enrs.1997.3732. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Antioxidant characterization - methodology and mechanism. Biochemical Pharmacology. 1995;49:1341–1348. doi: 10.1016/0006-2952(95)00088-h. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Cross CE. Oxygen-derived species - their relation to human-disease and environmental stress. Environmental Health Perspectives. 1994;102:5–12. doi: 10.1289/ehp.94102s105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? British Journal of Pharmacology. 2004;142:231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna PM, Mason RP. Direct evidence for inhibition of free-radical formation from Cu(I) and hydrogen-peroxide by glutathione and other potential ligands using the EPR spin-trapping technique. Archives of Biochemistry and Biophysics. 1992;295:205–213. doi: 10.1016/0003-9861(92)90507-s. [DOI] [PubMed] [Google Scholar]
- Irving H, Williams RJP. The stability of transition-metal complexes. Journal of the Chemical Society. 1953:3192–3210. [Google Scholar]
- Jung H, Guo B, Anastasio C, Kennedy IM. Quantitative measurements of the generation of hydroxyl radicals by soot particles in a surrogate lung fluid. Atmospheric Environment. 2006;40:1043–1052. [Google Scholar]
- Kachur AV, Koch CJ, Biaglow JE. Mechanism of copper-catalyzed oxidation of glutathione. Free Radical Research. 1998;28:259–269. doi: 10.3109/10715769809069278. [DOI] [PubMed] [Google Scholar]
- Krezel A, Bal W. Coordination chemistry of glutathione. Acta Biochimica Polonica. 1999;46:567–580. [PubMed] [Google Scholar]
- Lide DR. CRC Handbook of Chemistry and Physics. Boca Raton FL: CRC Press; 1999. [Google Scholar]
- Maestre P, Lambs L, Thouvenot JP, Berthon G. Copper-ligand interactions and physiological free-radical processes - pH-dependent influence of Cu2+ ions on Fe2+-driven OH generation. Free Radical Research Communications. 1992;15:305–317. doi: 10.3109/10715769209049146. [DOI] [PubMed] [Google Scholar]
- Martell AE, Smith RM, Motekaitis RJ. NIST critically selected stability constants of metal complexes. 2004 NIST Standard Reference Data: http://www.nist.gov/srd/nist46.htm.
- Martin RB, Edsall JT. The association of divalent cations with glutathione. Journal of the American Chemical Society. 1959;81:4044–4047. [Google Scholar]
- Merkofer M, Kissner R, Hider RC, Brunk UT, Koppenol WH. Fenton chemistry and iron chelation under physiologically relevant conditions: electrochemistry and kinetics. Chemical Research in Toxicology. 2006;19:1263–1269. doi: 10.1021/tx060101w. [DOI] [PubMed] [Google Scholar]
- Moss OR. Simulants of lung interstitial fluid. Health Physics. 1979;36:447–448. [PubMed] [Google Scholar]
- Mycielska ME, Patel A, Rizaner N, Mazurek MP, Keun H, Ganapathy V, Djamgoz MBA. Citrate transport and metabolism in mammalian cells prostate epithelial cells and prostate cancer. Bioessays. 2009;31:10–20. doi: 10.1002/bies.080137. [DOI] [PubMed] [Google Scholar]
- Pope CA, Dockery DW. Health effects of fine particulate air pollution: lines that connect. Journal of the Air and Waste Management Association. 2006;56:709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- Rahman I, Morrison D, Donaldson K, MacNee W. Systemic oxidative stress in asthma, COPD, and smokers. American Journal of Respiratory and Critical Care Medicine. 1996;154:1055–1060. doi: 10.1164/ajrccm.154.4.8887607. [DOI] [PubMed] [Google Scholar]
- Reed CJ, Douglas T. Chemical cleavage of plasmid DNA by glutathione in the presence of Cu(II) ions. Biochemical Journal. 1991;275:601–608. doi: 10.1042/bj2750601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J, Laden F, Zanobetti A. The concentration-response relation between PM2.5 and daily deaths. Environmental Health Perspectives. 2002;110:1025–1029. doi: 10.1289/ehp.021101025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlak DL, Hoigné J, David MM, Colvile RN, Seyffer E, Acker K, Wiepercht W, Lind JA, Fuzzi S. The cloudwater chemistry of iron and copper at Great Dun Fell, UK. Atmospheric Environmen. 1997;31:2515–2526. [Google Scholar]
- Shen H, Barakat AI, Anastasio C. Generation of hydrogen peroxide from San Joaquin Valley particles in a cell-free solution. Atmospheric Chemistry and Physics. 2010 doi: 10.5194/acp-11-9671-2011. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR. An Introduction to Error Analysis. Sausalito, CA: University Science Books; 1997. [Google Scholar]
- Valavanidis A, Vlahoyianni T, Fiotakis K. Comparative study of the formation of oxidative damage marker 8-hydroxy-2 '-deoxyguanosine (8-OHdG) adduct from the nucleoside 2 '-deoxyguanosine by transition metals and suspensions of particulate matter in relation to metal content and redox reactivity. Free Radical Research. 2005;39:1071–1081. doi: 10.1080/10715760500188671. [DOI] [PubMed] [Google Scholar]
- van der Vliet A, O'Neill CA, Cross CE, Koostra JM, Volz WG, Halliwell B, Louie S. Determination of low-molecular-mass antioxidant concentrations in human respiratory tract lining fluids. American Journal of Physiology-Lung Cellular and Molecular Physiology. 1999;276:L289–L296. doi: 10.1152/ajplung.1999.276.2.L289. [DOI] [PubMed] [Google Scholar]
- Verma V, Ning Z, Cho AK, Schauer JJ, Shafer MM, Sioutas C. Redox activity of urban quasi-ultrafine particles from primary and secondary sources. Atmospheric Environment. 2009;43:6360–6368. [Google Scholar]
- Vidrio E, Jung H, Anastasio C. Generation of hydroxyl radicals from dissolved transition metals in surrogate lung fluid solutions. Atmospheric Environment. 2008;42:4369–4379. doi: 10.1016/j.atmosenv.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidrio E, Phuah CH, Dillner AM, Anastasio C. Generation of hydroxyl radicals from ambient fine particles in a surrogate lung fluid solution. Environmental Science & Technology. 2009;43:922–927. doi: 10.1021/es801653u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walling C, El-Taliawi GM, Johnson RA. Fenton's reagent. IV. structure and reactivity relations in the reactions of hydroxyl radicals and the redox reactions of radicals. Journal of the American Chemical Society. 1974;96:133–139. [Google Scholar]
- Walser M. Ion association 6. interactions between calcium, magnesium, inorganic phosphate, citrate, and protein in normal human plasma. Journal of Clinical Investigation. 1961;40:723–730. doi: 10.1172/JCI104306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zepp RG, Faust BC, Hoigné J. Hydroxyl radical formation in aqueous reactions (pH 3–8) of iron(II) with hydrogen peroxide - the photo-Fenton reaction. Environmental Science & Technology. 1992;26:313–319. [Google Scholar]
- Zhang YX, Schauer JJ, Shafer MM, Hannigan MP, Dutton SJ. Source apportionment of in vitro reactive oxygen species bioassay activity from atmospheric particulate matter. Environmental Science & Technology. 2008;42:7502–7509. doi: 10.1021/es800126y. [DOI] [PubMed] [Google Scholar]
- Zhong CY, Zhou YM, Smith KR, Kennedy IM, Chen CY, Aust AE, Pinkerton KE. Oxidative injury in the lungs of neonatal rats following short-term exposure to ultrafine iron and soot particles. Journal of Toxicology and Environmental Health-Part a-Current Issues. 2010;73:837–847. doi: 10.1080/15287391003689366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.