Abstract
Orogastric tube feeding, using either continuous or intermittent bolus delivery, is common in infants for whom normal feeding is contraindicated. To compare the impact of different feeding strategies on muscle protein synthesis, after withholding food overnight, neonatal pigs received a complete formula orally as a bolus feed every 4 h or were continuously fed. Protein synthesis rate and translational mechanisms in skeletal muscle were examined after 0, 24, and 25.5 h. Plasma amino acid and insulin concentrations increased minimally and remained constant in continuously fed compared to feed-deprived pigs; however, the pulsatile meal feeding pattern was mimicked in bolus-fed pigs. Muscle protein synthesis was stimulated by feeding and the greatest response occurred after a bolus meal. Bolus but not continuous feeds increased polysome aggregation, the phosphorylation of protein kinase B, tuberous sclerosis complex 2, proline-rich Akt substrate of 40 kDa, eukaryotic initiation factor (eIF) 4E binding protein (4EBP1), and rp S6 kinase and enhanced dissociation of the 4EBP1 ·eIF4E complex and formation of the eIF4E ·eIF4G complex compared to feed deprivation (P < 0.05). Activation of insulin receptor substrate-1, regulatory associated protein of mammalian target of rapamycin, AMP-activated protein kinase, eukaryotic elongation factor 2, and eIF2α phosphorylation were unaffected by either feeding modality. These results suggest that in neonates, intermittent bolus feeding enhances muscle protein synthesis to a greater extent than continuous feeding by eliciting a pulsatile pattern of amino acid- and insulin-induced translation initiation.
Introduction
Neonates grow at rapid rates and utilize nutrients from the diet with high efficiency (1, 2). Nevertheless, over 7% of all newborn babies born in the United States are of low birth weight (3). Because newborn pigs are metabolically similar to human infants, we have used neonatal pigs as a model to study the role of nutrition in the regulation of protein synthesis in neonates. These studies have shown that feeding stimulates protein synthesis in all tissues of neonates and the greatest increase occurs in skeletal muscle (4–6). The stimulation of muscle protein synthesis by a meal is rapid and is sustained for at least 2 h but then declines toward baseline, in parallel with the postprandial change in circulating insulin and amino acids (7). Indeed, our studies using the pancreatic-substrate clamp have demonstrated that the feeding-induced changes in muscle protein synthesis are mediated independently by the postprandial rise in insulin and amino acids (8, 9).
Feeding increases protein synthesis by stimulating translation initiation, through phosphorylation and activation of mTORC17, and is mediated by activation of insulin and amino acid signaling pathways (10–12). The stimulation of the insulin signaling cascade after feeding includes the activation of the insulin receptor, which elicits the activation of a series of signaling intermediates, including IRS-1/2, phosphatidylinositol 3-kinase, phosphoinositide-dependent kinase 1, and PKB. The activation of mTORC1 is induced by activation of PKB (13, 14). Studies have shown that PKB phosphorylates and inactivates an inhibitor of cell growth, TSC2, thereby allowing activation of mTORC1 (15). During conditions of energy depletion, when AMP levels within the cell rise, AMPK is activated (16), leading to phosphorylation of TSC2 and inactivation of mTORC1. Phosphorylation of the mTORC1 inhibitor, PRAS40, by PKB enhances mTORC1 activation.
Activation of mTORC1 by insulin or amino acids results in the phosphorylation of 4EBP1 and S6K1 (17, 18). Phosphorylation of 4EBP1 by mTORC1 permits the dissociation of eIF4E from 4EBP1, allowing eIF4E to bind to eIF4G. This active complex mediates the binding of mRNA to the 40S ribosomal complex and the activation of translation initiation (12, 19, 20). Translation initiation also requires an initiator, met-tRNAi, binding to the start codon, a step mediated by eIF2 (21). Phosphorylation of the α-subunit of eIF2 reduces initiator met-tRNAi binding to the ribosome. Translation is also dependent upon rates of elongation. It has been suggested that the eEF2 kinase is a substrate of S6K1 (22); however, rapamycin treatment to inhibit mTORC1 does not alter the phosphorylation of this factor (23).
The majority of the studies that have investigated the effect of feeding on skeletal muscle protein synthesis have done so at one time point after a bolus meal. To our knowledge, there have been no studies that compare the effects of different feeding strategies on muscle protein synthesis. Feeding by orogastric tube, using either continuous or intermittent bolus delivery, is common for infants unable to maintain oral feeding (24), but whether continuous or intermittent bolus feeding is more advantageous is controversial. There is evidence suggesting that bolus compared to continuous feeding promotes a more physiological surge of the intestinal hormones, improves weight gain, and stimulates small intestinal growth (25–27), but the effect of these feeding modalities on skeletal muscle anabolism in neonates is unknown. The purpose of the present study was to compare the impact of 24 h of intermittent bolus with continuous feeding on protein synthesis in skeletal muscle of neonatal pigs. We also aimed to identify the intracellular mechanisms that mediate the response to these modes of nutritional support. We hypothesized that intermittent bolus feeding increases muscle protein synthesis to a greater extent than continuous feeding by inducing a rapid and marked rise in amino acids and insulin that activates translation initiation.
Materials and Methods
Animals and design.
Three multiparous crossbred (Yorkshire × Landrace × Hampshire × Duroc) pregnant sows (Agricultural Headquarters of the Texas Department of Criminal Justice) were brought to the animal facility of the Children’s Nutrition Research Center prior to their due date. Sows and piglets were housed and managed as previously described (28). After birth, piglets resided with the sow and were not given supplemental creep feed. Piglets were studied at 5–7 d of age when they weighed 2.0 ± 0.4 kg. The study was approved by the Animal Care and Use Committee of Baylor College of Medicine and was conducted in accordance with the NRC’s Guide for the Care and Use of Laboratory Animals.
Three days prior to infusion, piglets underwent surgery to place catheters in the external jugular vein and common carotid artery. Surgical procedures were performed using sterile techniques under general anesthesia as previously described (4). Piglets were returned to their respective sows in ~1 h after they had recovered from anesthesia and achieved full mobility.
Treatments and infusion.
Overnight feed-deprived piglets were randomly assigned to 1 of 5 treatment groups (n = 5–7): 1) overnight feed deprived; 2) intermittently bolus fed for 24 h; 3) intermittently bolus fed for 25.5 h; 4) continuously fed for 24 h; and 5) continuously fed for 25.5 h. Briefly, piglets assigned to an intermittently bolus-fed group were fed by orogastric tube at 0 h and every 4 h (40 mL · kg body weight−1) with a complete enteral milk replacement (Table 1) administered over a 15-min period; they were killed either 24 h (4 h after the last meal and just before a new meal) or 25.5 h (90 min after the last meal) later. These time points were chosen based on the findings of our previous meal feeding study (7), which showed that the consumption of a meal that contains one-sixth of the daily requirements, similar to that in the current study, increased protein synthesis by 0.5 h and the increase in protein synthesis was sustained for at least 2 h but fell to baseline by 4 h after the meal. The continuously fed piglets were infused through an orogastric tube the same complete enteral milk replacement at a constant rate of 10 mL · kg body weight−1 · h−1 until the piglets were killed 24 or 25.5 h later. The overnight feed-deprived piglets were killed at 0 h. Thus, piglets were killed and data collected at time 0 for baseline, feed-deprived controls, and at 24 and 25.5 h in both the continuously fed and intermittently bolus-fed groups.
TABLE 1.
Ingredients and nutrient composition of the experimental diet
| Ingredient | g/kg as fed |
| Whey protein concentrate (80% CP)1 | 50.0 |
| Lactose | 10.2 |
| FatPak 802 | 65.0 |
| Water | 862.0 |
| Xanthan gum | 2.0 |
| Vitamin premix3 | 2.0 |
| Mineral premix3 | 9.0 |
Hilmar Ingredients; CP, crude protein.
Milk Specialties Global Animal Nutrition.
Dyets. Vitamin premix provided (g/kg): thiamine HCl, 0.1; riboflavin, 0.375; pyridoxine HCl, 0.1; niacin, 1; calcium pantothenate, 1.2; folic acid, 0.13; biotin, 0.02; cobalamin B-12, 1.5; retinyl palmitate, 0.8; cholecalciferol, 0.05; tocopheryl acetate, 8.8; menadione sodium bisulfite, 0.08. Trace mineral premix provided (g/kg): calcium phosphate, dibasic, 187; calcium carbonate, 279; sodium chloride, 85; potassium phosphate monobasic, 155; magnesium sulfate, anhydrous, 44; manganous carbonate, 0.93; ferric citrate, 10; zinc carbonate, 1.84; cupric carbonate, 0.193; potassium iodate, 0.005; sodium selenite, 0.007.
Hormone and substrate measurements.
Blood samples were collected every 30 min and immediately analyzed for plasma BCAA and serum glucose concentrations as previously described (9). Plasma samples were collected at 30-min or 1-h intervals, frozen, and later analyzed for radioimmunoreactive insulin concentrations using a porcine insulin RIA kit (Millipore) (5).
Tissue protein synthesis in vivo
The Ks was measured with a flooding dose of l-[4-3H] phenylalanine (4). Piglets received l-[4-3H] phenylalanine (1.5 mmol · kg body weight−1, 0.5 mCi · kg body weight−1, Amersham Bioscience) injected 30 min before they were killed. Muscle samples were obtained from the longissimus dorsi, immediately frozen in liquid nitrogen, and stored at −70°C until analyzed as previously described (4).
Polysome profiles.
Analysis of ribosome distribution between polysomal and nonpolysomal fractions was determined by sucrose density gradient centrifugation as previously described (29).
Protein immunoblot analysis.
Proteins from the tissue homogenates were separated by SDS-PAGE. For each assay, all samples were run at the same time on triple-wide gels (C.B.S. Scientific) to eliminate inter-assay variation. Proteins were electrophoretically transferred to polyvinylidene difluoride membranes (Pall), which subsequently were incubated with appropriate primary antibodies, washed, and exposed to an appropriate secondary antibody as previously described (30).
For normalization, immunoblots performed with antiphospho-specific antibodies were stripped in stripping buffer (Pierce Biotechnology) and reprobed with corresponding nonphospho-specific antibodies. Blots were developed using enhanced chemiluminescence (GE Health Sciences), visualized, and analyzed using a ChemiDoc-It Imaging System (UVP). Primary antibodies that were used for immunoblotting were IRS-1 (total, Santa Cruz Biotechnology, and Ser1101, Cell signaling), PKB (total and Ser473, Cell Signaling), AMPK (total and Thr172, Cell Signaling), TSC2 (total and Thr1462, Cell Signaling), Raptor (total and Ser792, Cell Signaling), PRAS40 (total and Thr246, Cell Signaling), 4EBP1 (total, Bethyl Laboratories, and Thr70, Cell Signaling), S6K1 (total and Thr389, Cell Signaling), eIF4G (total and Ser1180, Cell Signaling), eIF2α (total and Ser51, Cell Signaling), and eEF2 (total and Thr56, Cell Signaling).
Quantification of 4EBP1#x22C5eIF4E and eIF4G#x22C5eIF4E complexes.
The association of eIF4E with 4E-BP1 or eIF4G was determined from aliquots of fresh tissue homogenates following immunoprecipitation with an anti-eIF4E monoclonal antibody (gift of Dr. Leonard Jefferson, Penn State University College of Medicine) followed by immunoblotting analysis using anti-4EBP1 (Bethyl Laboratories) and anti-eIF4G (Novus Biologicals) antibodies, as previously described (31). The amounts of 4EBP1 and eIF4G were corrected by the eIF4E recovered from the immunoprecipitate.
Calculations and statistics.
The Ks (percentage of protein mass synthesized in a day) was calculated as Ks (%/d) = [(Sb/Sa) × (1440/t)] × 100, where Sb (in dpm · nmol−1) is the specific radioactivity of the protein-bound phenylalanine, Sa (in dpm · nmol−1) is the specific radioactivity of the tissue free phenylalanine at the time of tissue collection, corrected by the linear regression of the blood specific radioactivity of the animal against time, t is the time of labeling in minutes, and 1440 is the minutes-to-day conversion (4).
Statistical analysis was carried out in SPSS (version 17.0) using ANOVA to determine main statistical differences between groups. Within-group analysis was performed using a post hoc LSD t test. Analysis of glucose and insulin across time was carried out with SPSS general linear model using a repeated measures test for within-subject effects. P < 0.05 was considered significant for all comparisons and data are presented as means ± SEM.
Results
Plasma glucose, insulin, and BCAA concentrations.
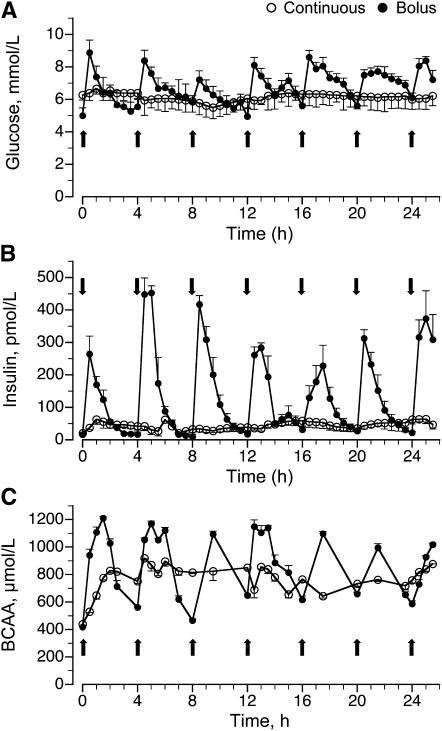
In the bolus-fed group, blood glucose was >80% greater than in the feed-deprived group (P < 0.05) 30 min after the feed, remained elevated for at least 2 h after each feeding, and decreased to baseline values before the next feed. In the continuously fed group, blood glucose levels increased by 30% compared to levels in the feed-deprived group (P > 0.05) and remained relatively constant throughout the feeding period (Fig. 1A). Plasma insulin levels also increased by 30 min after a bolus meal and remained elevated during the 2–3 h after the feeding (P < 0.05), returning to baseline before the next meal (Fig. 1B). In the continuously fed group, plasma insulin concentrations increased to ~100 pmol · L−1 (P > 0.05) and remained around this level throughout the infusion.
FIGURE 1.
Plasma glucose (A), insulin (B), and BCAA (C) concentrations in intermittently bolus-fed, continuously fed, and feed-deprived neonatal pigs. Values are means ± SEM, n = 5–7. Repeated-measures analysis showed an effect of time, treatment, and their interaction on plasma glucose, insulin, and BCAA concentrations, P < 0.05.
Plasma BCAA in the bolus-fed group increased 30 min after a meal compared to feed-deprived piglets (P < 0.05), remained elevated for at least 2 h, and decreased, although not to baseline, before the next meal (Fig. 1C). In the continuously fed group, BCAA increased to only ~800 μmol · L−1 (P < 0.05) and remained near these levels throughout the feeding period.
Protein synthesis and polysome profiles.
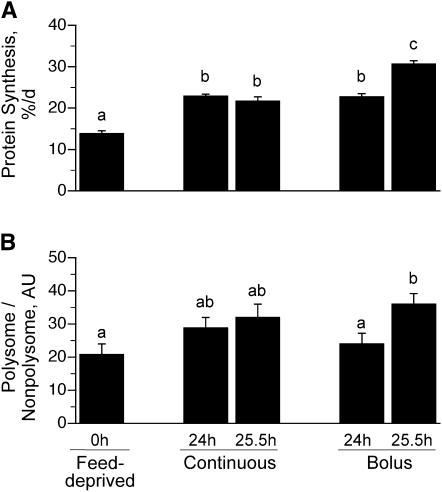
The Ks in the longissimus dorsi muscle of the continuously fed pigs were similar after 24 and 25.5 h (Fig. 2A) and were ~70% greater after 24 h (P < 0.0001) and 56% greater after 25.5 h (P < 0.0001) of continuous feeding compared to the overnight feed-deprived group. Ks were 64% greater in the 24 h bolus-fed group just before the meal (P < 0.0001) compared to feed-deprived values and similar to that in the continuously fed group. However, muscle protein synthesis was enhanced by 121% (P < 0.0001) after 25.5 h of bolus feeding (90 min after the last meal) compared to the feed-deprived group. The rate of muscle protein synthesis after 25.5 h of bolus feeding also was greater than that noted after 24 h of intermittent bolus feeding (P < 0.05) and 24 and 25.5 h of continuous feeding (P < 0.05).
FIGURE 2.
Ks (A) and the proportion of ribosomes in polysomes (B) in the longissimus dorsi muscle of intermittently bolus-fed, continuously fed, and feed-deprived neonatal pigs. Values are means ± SEM, n = 5–7. ANOVA showed an effect of treatment on Ks and the proportion of ribosomes in polysomes, P < 0.05. Means without a common letter differ, P < 0.05. Ks, fractional rate of protein synthesis.
Determination of the ribosomal fraction in the polysomes compared to nonpolysomes showed that the number of ribosomes associated with mRNA was greater in the 25.5 h bolus-fed group (90 min after the last feed) compared to the feed-deprived group (P < 0.05). The polysomal to nonpolysomal distribution did not differ in the bolus 24 h and continuously fed groups compared to the feed-deprived group (Fig. 2B).
Upstream mTOR signaling component activation.
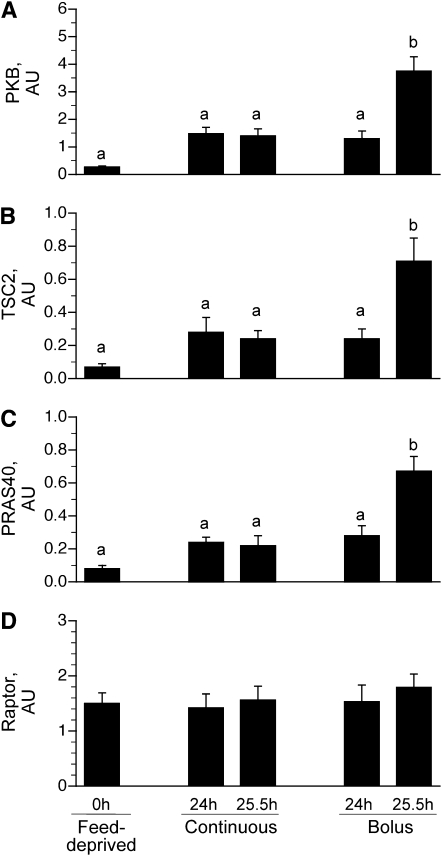
To examine the mechanism by which different feeding modalities increased protein synthesis in the longissimus dorsi muscle, markers of translation initiation signaling upstream of mTOR were measured. PKB mediates the insulin-associated activation of mTOR. Phosphorylation of PKB on Ser473 in the 25.5-h bolus group, 90 min after the last meal, was greater than in the feed-deprived (P < 0.05) and continuous groups (P < 0.05) (Fig. 3A). Differences in the phosphorylation of PKB between the 24- and 25.5-h continuous groups, the 24 h bolus-fed group, and the feed-deprived group were not detected. We found no effect of either continuous or intermittent bolus feeding on the phosphorylation of IRS-1 on Ser1101, AMPK on Thr172 (Table 2), or Raptor on Ser792 (Fig. 3D) compared to the feed-deprived group.
FIGURE 3.
PKB phosphorylation on Ser473 (A), TSC2 phosphorylation on Thr1462 (B), PRAS40 phosphorylation on Thr246 (C), and Raptor phosphorylation on Ser792 (D) in the longissimus dorsi muscle of intermittently bolus-fed, continuously fed, and feed-deprived neonatal pigs. All results are corrected for total protein. Values are means ± SEM, n = 5–7. ANOVA showed an effect of treatment on PKB, TSC2, and PRAS40 phosphorylation, P < 0.05. Means without a common letter differ, P < 0.05. PKB, protein kinase B; PRAS40, proline-rich Akt substrate of 40 kDa; TSC, tuberous sclerosis complex.
TABLE 2.
Phosphorylation of IRS-1, AMPK, eIF2α, and eEF2 in skeletal muscle of intermittently bolus-fed, continuously fed, and feed-deprived neonatal pigs1
| Feed-deprived | Continuous | Bolus | |||
| 0 h | 24 h | 25.5 h | 24 h | 25.5 h | |
| Arbitrary units | |||||
| IRS-1, Ser1101 | 1.24 ± 0.14 | 0.99 ± 0.19 | 1.24 ± 0.25 | 1.25 ± 0.26 | 1.17 ± 0.20 |
| AMPK, Thr172 | 0.81 ± 0.11 | 0.63 ± 0.12 | 0.64 ± 0.12 | 0.84 ± 0.15 | 0.76 ± 0.09 |
| eIF2-α, Ser51 | 0.57 ± 0.12 | 0.52 ± 0.10 | 0.64 ± 0.13 | 0.55 ± 0.09 | 0.55 ± 0.06 |
| eEF2, Thr56 | 0.85 ± 0.15 | 0.68 ± 0.12 | 0.71 ± 0.10 | 0.78 ± 0.09 | 0.86 ± 0.07 |
Values are means ± SEM, = 5–7. AMPK, AMP-activated protein kinase; eEF2, eukaryotic elongation factor 2; eIF, eukaryotic initiation factor; IRS-1, insulin receptor substrate-1.
Phosphorylation of TSC2 on Thr1462 (Fig. 3B) and PRAS40 on Thr246 (Fig. 3C) did not differ between the continuously fed and feed-deprived groups. In the bolus-fed group, just before a meal at 24 h, TSC2 Thr1462 (Fig. 3B) and PRAS40 phosphorylation (Fig. 3C) did not differ from the continuously fed and feed-deprived groups. However, in the bolus-fed group at 25.5 h, i.e., 90 min after the last meal, TSC2 and PRAS phosphorylation were higher (P < 0.0001) compared to continuously fed and feed-deprived groups.
Downstream mTOR signaling component activation.
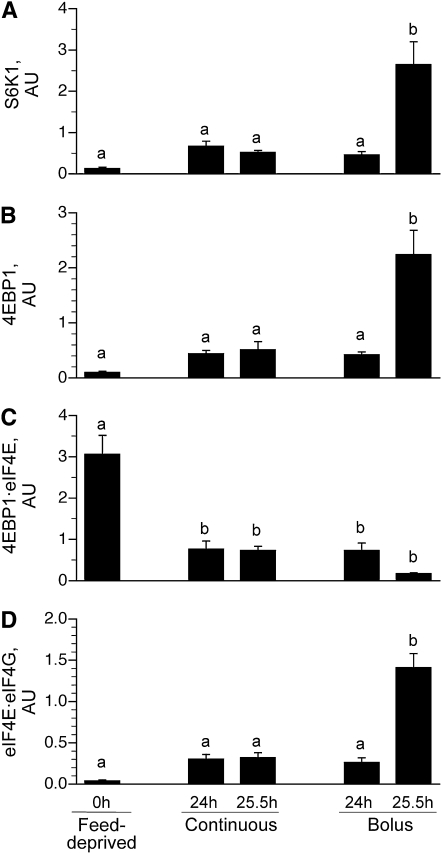
S6K1 (Fig. 4A) and 4EBP1 (Fig. 4B) phosphorylation did not differ between the continuously fed and feed-deprived groups. S6K1 (Fig. 4A) and 4EBP1 (Fig. 4B) phosphorylation also did not differ between the 24 h bolus-fed group (i.e., just before the last bolus meal) and the continuously fed and feed-deprived groups. However, 90 min after the last bolus meal (i.e., 25.5 h), their phosphorylation markedly increased (P < 0.05). The association of 4EBP1 · eIF4E (Fig. 4C) was lower in all feeding groups compared to the feed-deprived group (P < 0.05). The formation of the active eIF4E · eIF4G complex (Fig. 4D) in muscle was not significantly increased by continuous feeding compared to feed deprivation. However, in the bolus-fed group, the formation of the complex just before the meal at 24 h was similar to the continuously fed group but was markedly increased 90 min after the last meal (25.5 h) compared to other groups (P < 0.007).
FIGURE 4.
S6K1 phosphorylation on Thr389 (A), 4EBP1 phosphorylation on Thr70 (B), inactive 4EBP1·eIF4E complex abundance (C), and active eIF4E·eIF4G complex abundance (D) in the longissimus dorsi muscle of intermittently bolus-fed, continuously fed, and feed-deprived neonatal pigs. All results are corrected for total protein. Values are means ± SEM, n = 5–7. ANOVA showed an effect of treatment on S6K1 and 4EBP1 phosphorylation and 4EBP1·eIF4E and eIF4E·eIF4G abundance, P < 0.05. Means without a common letter differ, P < 0.05. eIF, eukaryotic initiation factor; S6K1, ribosomal protein S6 kinase 1.
Initiation and elongation signaling component activation.
Phosphorylation of eIF2α, which regulates initiator met-tRNAi binding to the ribosome, and eEF2 phosphorylation, which regulates elongation, did not differ among groups (Table 2).
Discussion
This is the first study to our knowledge to directly compare the effects of continuous and intermittent bolus formula feeding, delivered by orogastric tube, on the regulation of muscle protein synthesis. In so doing, we eliminated the possibility of differences in the type of food (elemental vs. formula), mode of nutritional support (parenteral vs. enteral), and other variables as confounding factors (32). We showed that although both continuous and intermittent bolus feeding stimulated muscle protein synthesis, the greatest increase in muscle protein synthesis occurred in the intermittently bolus-fed pigs after a meal. This greater increase in muscle protein synthesis in the intermittently bolus-fed pigs was associated with more rapid and profound changes in circulating amino acids and insulin that activate the translation initiation factors that regulate mRNA binding to the ribosomal complex.
Previously, we demonstrated that the consumption of a meal that contains one-sixth of the daily requirements, similar to that in the current study, increased protein synthesis after 0.5 h and the increase in protein synthesis was sustained for at least 2 h but fell to baseline by 4 h after the meal. These changes in protein synthesis paralleled the changes in circulating insulin and amino acids (7). In the current study, we found that circulating insulin and amino acid levels were minimally but constantly increased in the continuously fed group but rose rapidly and robustly in the intermittently bolus-fed group after each meal, falling to near baseline values just before the next feeding. Continuous feeding for 1 d increased muscle protein synthesis rates compared to feed deprivation, consistent with the demonstrated ability of continuous feeding, delivered enterally or parenterally, to promote growth (33, 34). However, the increase in protein synthesis was greatest after the bolus meal (25.5-h bolus group) compared to the feed-deprived group. Importantly, protein synthesis just before the meal (24-h bolus group) did not fall to levels observed for the baseline feed-deprived group and was similar to that in continuously fed pigs, likely because circulating amino acids did not fall completely to baseline just before the meal. Based on these results, we can infer that when amino acids and insulin are minimally increased and remain constant, as seen in the continuous group, protein synthesis is only modestly stimulated. Thus, it appears that the cyclic surge of amino acids and insulin is needed to maximally stimulate protein synthesis in skeletal muscle.
To further evaluate the effect of intermittent bolus and continuous feeding on protein synthesis, we evaluated the aggregation of ribosomes on mRNA by sucrose gradient density centrifugation. In our previous investigations (7), we found that the proportion of ribosomes in polysomes was elevated 30–120 min after a bolus meal. In the present study, we showed that, compared to feed-deprived pigs, the relative proportion of mRNA present in polysomes increased after the bolus meal (25.5-h bolus group). Because protein synthesis rates were also enhanced after the bolus meal, this suggests that the rate of translation initiation was upregulated compared to elongation in response to bolus feeding. From these findings, it is apparent that increased efficiency of translation initiation plays a role in the stimulation of muscle protein synthesis after an intermittent bolus meal. In the continuously fed and bolus-fed 24-h groups, polysome aggregation was similar to the feed-deprived group, although protein synthesis rates were elevated compared to feed deprivation. One possible explanation for these findings may be that the rate of elongation increased in proportion to initiation in the continuously fed and 24 h bolus-fed groups.
To better understand the mechanisms involved in the regulation of protein synthesis in skeletal muscle of neonatal pigs fed continuously or intermittently, we examined the activation of signaling proteins upstream and downstream of mTORC1. Previously, we showed that the feeding-induced increase in muscle protein synthesis is in part due to activation of the insulin signaling cascade and that the activation of mTOR is induced by activation of PKB (11, 13). In our present study, we showed that PKB phosphorylation on Ser473 increased only after a bolus meal and did not differ between feed-deprived and continuously fed groups. These findings may reflect a blunted activation of PKB due to the minimal increase in insulin levels during continuous feeding.
The pathway by which amino acids stimulate translation initiation is less well defined than that for insulin, and there is cross-talk between both pathways (35). Insulin-activated PKB phosphorylates TSC2 on Thr1462, resulting in inactivation of the inhibitory TCS1/TCS2 complex, followed by mTORC1 activation (36). We previously demonstrated that the insulin-induced increase in muscle protein synthesis involves reduced activation of TSC1/2 and enhanced activation of mTORC1 (37). In the current study, we showed that TSC2 phosphorylation on Thr1462 was not increased by continuous feeding but was markedly increased after an intermittent bolus meal, likely mediated by insulin-activated PKB.
PRAS40 is a negative regulator of mTORC1 when it binds to the mTOR complex. During nutrient deprivation, PRAS40 interacts with mTORC1 and, in response to insulin, PRAS40 dissociates from mTORC1 (38–40). Furthermore, mTOR and PKB can phosphorylate PRAS40 at Ser221 and Thr246, respectively, thereby inducing the dissociation of PRAS40 from mTORC1 (40). In this study, PRAS40 phosphorylation at Thr246 (a PKB phosphorylation site) increased after the meal in the bolus-fed group, consistent with our previous short-term studies (41), but there were no differences in the continuous, 24 h bolus-fed, and feed-deprived groups.
It has been reported that Raptor, a component of the mTORC1 complex, can be phosphorylated by AMPK at Ser792, resulting in inhibition of mTORC1 (42). Our results show no effect of either feeding modality on AMPK or Raptor phosphorylation, consistent with our previous studies (41), and support the hypothesis that AMPK is not involved in the regulation of mTOR under physiological feeding conditions (23, 43).
In our present study, the phosphorylation of S6K1 and 4EBP1 and the formation of the active eIF4E·eIF4G complex in muscle rose markedly after an intermittent bolus meal but did not increase in the continuously fed groups or just before the bolus meal compared to food deprivation. These results support our hypothesis that prolonged intermittent bolus feeding stimulates mTORC1-dependent translation initiation, likely due to the rapid pulse in insulin and amino acids levels after a meal. However, it appears that the modest elevation in circulating insulin and amino acids that occurs with prolonged continuous feeding is not sufficient to stimulate and/or sustain activation of signaling proteins downstream of mTORC1 in skeletal muscle of neonates.
Because prolonged continuous exposure to insulin and amino acids greater than feed-deprived levels can promote hyperphosphorylation of S6K1, leading to enhanced phosphorylation of IRS-1 on Ser/Thr residues and downregulation of IRS/phosphatidylinositol 3-kinase signaling cascade (35), we wished to determine whether similar effects may occur with continuous feeding in neonatal pigs. In our study, we found no effect of either feeding modality on IRS-1 Ser1101 phosphorylation, suggesting that continuous feeding, for a 24-h period, does not downregulate insulin signaling.
The finding that protein synthesis was elevated in pigs continuously fed compared to feed-deprived pigs appears to be inconsistent with the observation that biomarkers of mRNA translation (e.g., eIF4G association with eIF4E, and 4EBP1 and S6K1 phosphorylation) were at basal (i.e., feed-deprived) values. Although the mechanism involved is unknown, we speculate that reinitiation (i.e., the release of the ribosome from the mRNA at the stop codon and subsequent rebinding to the same mRNA at or near the start codon) may be upregulated. Although incompletely characterized, reinitiation may not be mediated by the eIF4E · eIF4G complex but instead may be facilitated by proteins such as poly (A) binding protein (PABP) and PABP-interacting protein-1 (PAIP-1), which are thought to stimulate translation by promoting mRNA circularization (44, 45).
We previously showed that feeding does not alter the phosphorylation of eIF2α that regulates tRNA-ribosome binding and eEF2 phosphorylation that regulates elongation (7). Consistent with our previous studies, in the present work, the activation of these factors was not altered by continuous or intermittent bolus feeding, suggesting that the feeding-induced increase in muscle protein synthesis in neonates primarily involves mTOR-dependent translation initiation.
The results of the present study suggest that the intermittent bolus pattern of feeding increases protein synthesis in skeletal muscle to a greater extent than continuous feeding. This greater increase in muscle protein synthesis in intermittently bolus-fed piglets is associated with more rapid and profound increases in circulating amino acids and insulin, which activate the intracellular signaling proteins that regulate mTOR-dependent translation initiation. Further studies are needed to evaluate the more prolonged effects of different feeding strategies on skeletal muscle protein accretion in the neonate. Nonetheless, the results suggest that the intermittent bolus pattern of feeding has the potential to enhance lean body mass and improve clinically important outcomes, such as weight gain, compared to continuous feeding, in neonates.
Acknowledgments
The authors thank Robert J. Shulman, M.D. for helpful advice, Rosemarie Almonaci for technical assistance, Jerome Stubblefield for care of animals, E. O’Brian Smith, Ph.D. for statistical assistance, Adam Gillum for graphics, and Linda F. Kemper for secretarial assistance. M.C.G., A.S., M.L.F., S.R.K., and T.A.D. designed the research; M.C.G., A.S., H.V.N., F.A.W., N.S., R.A.O., S.W.E., S.R.K., and R.M.T. conducted the research; M.C.G., A.S., S.R.K., S.W.E., R.M.T., and T.A.D. analyzed the data; M.C.G. and T.A.D. wrote the paper; and T.A.D. had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Supported in part by NIH grant AR-44474 (T.A.D.) and by the USDA/ARS under Cooperative Agreement 6250-510000-055 (T.A.D.). This research also was supported in part by NIH Training Grant T32-HL007937. This work is a publication of the USDA/Agricultural Research Service Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine. The contents of this publication do not necessarily reflect the views or politics of the USDA, nor does the mention of trade names, commercial products or organizations imply endorsement by the U.S. government.
Abbreviations used: AMPK, AMP-activated protein kinase; eEF2, eukaryotic elongation factor 2; eIF, eukaryotic initiation factor; IRS-1, insulin receptor substrate-1; Ks, fractional rate of protein synthesis; mTORC1, mammalian target of rapamycin complex 1; PKB, protein kinase B; PRAS40, proline-rich Akt substrate of 40 kDa; Raptor, regulatory associated protein of mammalian target of rapamycin; S6K1, ribosomal protein S6 kinase 1; TSC, tuberous sclerosis complex.
Literature Cited
- 1.Denne SC, Kalhan SC. Leucine metabolism in human newborns. Am J Physiol. 1987;253:E608–15 [DOI] [PubMed] [Google Scholar]
- 2.Denne SC, Rossi EM, Kalhan SC. Leucine kinetics during feeding in normal newborns. Pediatr Res. 1991;30:23–7 [DOI] [PubMed] [Google Scholar]
- 3.Saigal S, Stoskopf BL, Streiner DL, Burrows E. Physical growth and current health status of infants who were of extremely low birth weight and controls at adolescence. Pediatrics. 2001;108:407–15 [DOI] [PubMed] [Google Scholar]
- 4.Davis TA, Burrin DG, Fiorotto ML, Nguyen HV. Protein synthesis in skeletal muscle and jejunum is more responsive to feeding in 7-than in 26-day-old pigs. Am J Physiol. 1996;270:E802–9 [DOI] [PubMed] [Google Scholar]
- 5.Davis TA, Fiorotto ML, Nguyen HV, Reeds PJ. Enhanced response of muscle protein synthesis and plasma insulin to food intake in suckled rats. Am J Physiol. 1993;265:R334–40 [DOI] [PubMed] [Google Scholar]
- 6.Davis TA, Fiorotto ML. Regulation of muscle growth in neonates. Curr Opin Clin Nutr Metab Care. 2009;12:78–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson FA, Suryawan A, Orellana RA, Kimball SR, Gazzaneo MC, Nguyen HV, Fiorotto ML, Davis TA. Feeding rapidly stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing translation initiation. J Nutr. 2009;139:1873–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suryawan A, O'Connor PM, Bush JA, Nguyen HV, Davis TA. Differential regulation of protein synthesis by amino acids and insulin in peripheral and visceral tissues of neonatal pigs. Amino Acids. 2009;37:97–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Connor PMJ, Bush JA, Suryawan A, Nguyen HV, Davis TA. Insulin and amino acids independently stimulate skeletal muscle protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab. 2003;284:E110–9 [DOI] [PubMed] [Google Scholar]
- 10.Suryawan A, O'Connor PM, Kimball SR, Bush JA, Nguyen HV, Jefferson LS, Davis TA. Amino acids do not alter the insulin-induced activation of the insulin signaling pathway in neonatal pigs. J Nutr. 2004;134:24–30 [DOI] [PubMed] [Google Scholar]
- 11.O'Connor PM, Kimball SR, Suryawan A, Bush JA, Nguyen HV, Jefferson LS, Davis TA. Regulation of translation initiation by insulin and amino acids in skeletal muscle of neonatal pigs. Am J Physiol Endocrinol Metab. 2003;285:E40–53 [DOI] [PubMed] [Google Scholar]
- 12.Davis TA, Nguyen HV, Suryawan A, Bush JA, Jefferson LS, Kimball SR. Developmental changes in the feeding-induced stimulation of translation initiation in muscle of neonatal pigs. Am J Physiol Endocrinol Metab. 2000;279:E1226–34 [DOI] [PubMed] [Google Scholar]
- 13.Suryawan A, Escobar J, Frank JW, Nguyen HV, Davis TA. Developmental regulation of the activation of signaling components leading to translation initiation in skeletal muscle of neonatal pigs. Am J Physiol Endocrinol Metab. 2006;291:E849–59 [DOI] [PubMed] [Google Scholar]
- 14.Howell JJ, Manning BD. mTOR couples cellular nutrient sensing to organismal metabolic homeostasis. Trends Endocrinol Metab. 2011;22:94–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002;10:151–62 [DOI] [PubMed] [Google Scholar]
- 16.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–90 [DOI] [PubMed] [Google Scholar]
- 17.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–34 [DOI] [PubMed] [Google Scholar]
- 18.Proud CG. Cell signaling. mTOR, unleashed. Science. 2007;318:926–7 [DOI] [PubMed] [Google Scholar]
- 19.Kimball SR, Farrell PA, Nguyen HV, Jefferson LS, Davis TA. Developmental decline in components of signal transduction pathways regulating protein synthesis in pig muscle. Am J Physiol Endocrinol Metab. 2002;282:E585–92 [DOI] [PubMed] [Google Scholar]
- 20.Hands SL, Proud CG, Wyttenbach A. mTOR's role in ageing: protein synthesis or autophagy? Aging. 2009;1:586–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Proud CG. eIF2 and the control of cell physiology. Semin Cell Dev Biol. 2005;16:3–12 [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Proud CG. Methods for studying signal-dependent regulation of translation factor activity. Methods Enzymol. 2007;431:113–42 [DOI] [PubMed] [Google Scholar]
- 23.Suryawan A, Jeyapalan AS, Orellana RA, Wilson FA, Nguyen HV, Davis TA. Leucine stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing mTORC1 activation. Am J Physiol Endocrinol Metab. 2008;295:E868–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Academy of Pediatrics Enteral Nutrition Support. Pediatric nutrition handbook. Elk Grove Village, IL: American Academy of Pediatrics; 2004; pp. 391–403 [Google Scholar]
- 25.Mashako MN, Bernard C, Cezard JP, Chayvialle JA, Navarro J. Effect of total parenteral nutrition, constant rate enteral nutrition, and discontinuous oral feeding on plasma cholecystokinin immunoreactivity in children. J Pediatr Gastroenterol Nutr. 1987;6:948–52 [DOI] [PubMed] [Google Scholar]
- 26.Schanler RJ, Shulman RJ, Lau C, Smith EO, Heitkemper MM. Feeding strategies for premature infants: randomized trial of gastrointestinal priming and tube-feeding method. Pediatrics. 1999;103:434–9 [DOI] [PubMed] [Google Scholar]
- 27.Shulman RJ, Redel CA, Shathos TH. Bolus versus continuous feedings stimulate small-intestine growth and development in the newborn pig. J Pediatr Gastroenterol Nutr. 1994;18:350–4 [DOI] [PubMed] [Google Scholar]
- 28.Davis TA, Fiorotto ML, Beckett PR, Burrin DG, Reeds PJ, Wray-Cahen D, Nguyen HV. Differential effects of insulin on peripheral and visceral tissue protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab. 2001;280:E770–9 [DOI] [PubMed] [Google Scholar]
- 29.Kubica N, Bolster DR, Farrell PA, Kimball SR, Jefferson LS. Resistance exercise increases muscle protein synthesis and translation of eukaryotic initiation factor 2B {epsilon} mRNA in a mammalian target of rapamycin-dependent manner. J Biol Chem. 2005;280:7570–80 [DOI] [PubMed] [Google Scholar]
- 30.Frank JW, Escobar J, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Dietary protein and lactose increase translation initiation factor activation and tissue protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab. 2006;290:E225–33 [DOI] [PubMed] [Google Scholar]
- 31.Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Regulation of cardiac and skeletal muscle protein synthesis by individual branched-chain amino acids in neonatal pigs. Am J Physiol Endocrinol Metab. 2006;290:E612–21 [DOI] [PubMed] [Google Scholar]
- 32.Stoll B, Horst DA, Cui L, Chang X, Ellis KJ, Hadsell DL, Suryawan A, Kurundkar A, Maheshwari A, Davis TA, et al. Chronic parenteral nutrition induces hepatic inflammation, steatosis and insulin resistance in neonatal pigs. J Nutr. 2010;140:2193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dsilna A, Christensson K, Alfredsson L, Lagercrantz H, Blennow M. Continuous feeding promotes gastrointestinal tolerance and growth in very low birth weight infants. J Pediatr. 2005;147:43–9 [DOI] [PubMed] [Google Scholar]
- 34.Ibrahim HM, Jeroudi MA, Baier RJ, Dhanireddy R, Krouskop RW. Aggressive early total parental nutrition in low-birth-weight infants. J Perinatol. 2004;24:482–6 [DOI] [PubMed] [Google Scholar]
- 35.Hinault C, Mothe-Satney I, Gautier N, Lawrence JC, Jr, Van Obberghen E. Amino acids and leucine allow insulin activation of the PKB/mTOR pathway in normal adipocytes treated with wortmannin and in adipocytes from db/db mice. FASEB J. 2004;18:1894–6 [DOI] [PubMed] [Google Scholar]
- 36.Huang J, Manning BD. The TSC1–TSC2 complex: a molecular switchboard controlling cell growth. Biochem J. 2008;412:179–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suryawan A, Orellana RA, Nguyen HV, Jeyapalan AS, Fleming JR, Davis TA. Activation by insulin and amino acids of signaling components leading to translation initiation in skeletal muscle of neonatal pigs is developmentally regulated. Am J Physiol Endocrinol Metab. 2007;293:E1597–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fonseca BD, Smith EM, Lee VH, MacKintosh C, Proud CG. PRAS40 is a target for mammalian target of rapamycin complex1 and is required for signaling downstream of this complex. J Biol Chem. 2007;282:24514–24 [DOI] [PubMed] [Google Scholar]
- 39.Oshiro N, Yoshino K, Hidayat S, Tokunaga C, Hara K, Eguchi S, Avruch J, Yonezawa K. Dissociation of raptor from mTOR is a mechanism of rapamycin-induced inhibition of mTOR function. Genes Cells. 2004;9:359–66 [DOI] [PubMed] [Google Scholar]
- 40.McGhee NK, Jefferson LS, Kimball SR. Elevated corticosterone associated with food deprivation upregulates expression in rat skeletal muscle of the mTORC1 repressor, REDD1. J Nutr. 2009;139:828–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suryawan A, Davis TA. The abundance and activation of mTORC1 regulators in skeletal muscle of neonatal pigs are modulated by insulin, amino acids, and age. J Appl Physiol. 2010;109:1448–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeyapalan AS, Orellana RA, Suryawan A, O'Connor PM, Nguyen HV, Escobar J, Frank JW, Davis TA. Glucose stimulates protein synthesis in skeletal muscle of neonatal pigs through an AMPK- and mTOR-independent process. Am J Physiol Endocrinol Metab. 2007;293:E595–603 [DOI] [PubMed] [Google Scholar]
- 44.Martineau Y, Derry MC, Wang X, Yanagiya A, Berlanga JJ, Shyu AB, Imataka H, Gehring K, Sonenberg N. Poly(A)-binding protein-interacting protein 1 binds to eukaryotic translation initiation factor 3 to stimulate translation. Mol Cell Biol. 2008;28:6658–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Derry MC, Yanagiya A, Martineau Y, Sonenberg N. Regulation of poly(A)-binding protein through PABP-interacting proteins. Cold Spring Harb Symp Quant Biol. 2006;71:537–43 [DOI] [PubMed] [Google Scholar]