Abstract
Chronic inflammation is considered to play a role in the development of cardiovascular disease. Various (n-3) fatty acids (FA) have been reported to have antiinflammatory effects, but there is a lack of consensus in this area, particularly in regard to optimal source(s) and dose(s). This study aimed to determine the effects of high and low doses of (n-3) FA from plant and marine sources on plasma inflammatory marker concentrations. One-hundred adults with metabolic syndrome were randomly assigned to a low or high dose of plant- (2.2 or 6.6 g/d α-linolenic acid) or marine- (1.2 or 3.6 g/d EPA and DHA) derived (n-3) FA or placebo for 8 wk, using a parallel arm design (n = 20/arm). Fasting blood samples collected at 0, 4, and 8 wk were analyzed for concentrations of monocyte chemotactic protein-1 (MCP-1), IL-6, and soluble intercellular adhesion molecule-1 (sICAM-1) and for cardiovascular risk factors. Baseline concentrations across all 5 groups combined were (mean ± SD) 103 ± 32 ng/L for MCP-1, 1.06 ± 0.56 ng/L for IL-6, and 0.197 ± 0.041 ng/L for sICAM-1. There were no significant differences in 8-wk changes in plasma inflammatory marker concentrations among the 5 groups. Plasma TG and blood pressure decreased significantly more and the LDL cholesterol concentration increased more in the high-dose fish oil group compared to the 8-wk changes in some of the other 4 groups (P ≤ 0.04). In conclusion, no beneficial effects were detected for any of the 3 inflammatory markers investigated in response to (n-3) FA in adults with metabolic syndrome regardless of dose or source.
Introduction
Chronic inflammation plays a major role in the development of atherosclerosis and CVD8 (1). Our increasing knowledge of essential FA metabolism has suggested a potential antiinflammatory role of 3 common dietary (n-3) FA: ALA (2), EPA, and DHA. Reduced inflammation has been recognized as one of the numerous mechanisms by which (n-3) FA may reduce the risk of CVD (3, 4).
In the last decade, an ever-increasing number of clinical trials have investigated the potential antiinflammatory effects of (n-3) FA both from supplements (5–23) and dietary sources (24–26), yielding encouraging but inconsistent results. The trials investigating higher doses of (n-3) FA (>1 g/d EPA and/or DHA) in populations at high risk for CVD have generally reported improvements (i.e. reduced concentrations) for selected inflammatory markers (13, 20, 21). Those studying the same range of dose but in healthy adults have had mixed results (6–10, 12, 13, 15–18, 20, 21, 23), whereas lower doses consistently have not shown a beneficial effect of (n-3) FA (5, 14). At least one study has reported that very high intakes (6.6 g/d), well beyond current recommendations (4), may raise the blood concentrations of some markers of inflammation (12).
In addition to the differences in dosage and study population characteristics, other variables, such as the source of (n-3) FA, study duration, and background diet may explain the inconsistencies in these findings. Notably, the majority of clinical trials have used marine sources of (n-3) FA (EPA and DHA from capsules or fish), whereas few have examined plant sources (ALA from flax oil) and only one compared the two in the same trial (5).
In summary, although (n-3) FA show potential for lowering inflammation, there is still a lack of consensus regarding the optimal source(s) and dose(s). To date, only a few studies have compared different doses (6, 7, 12, 22) or sources (5) of (n-3) FA and none to our knowledge has contrasted both different sources and doses in a single trial. In this study, we evaluated the effects of two sources of (n-3) FA, flaxseed oil (plant) and fish oil (marine), in two different doses (low vs. high) on three markers of inflammation in adults with metabolic syndrome.
Methods
Participants
Participants were recruited from the local community through radio and newspaper advertisements and completed an online screening and a clinic visit between May 2007 and September 2008. The primary inclusion criterion was meeting Adult Treatment Panel III/National Cholesterol Education Program Expert Panel guidelines for metabolic syndrome (27). Other inclusion criteria included age ≥ 18 y and general good health. Participants were excluded if they had a BMI ≥ 40, diabetes, renal disease, significant liver enzyme abnormality, were pregnant or lactating, were smokers, had a history of CVD, inflammatory disease, malignant neoplasm, clotting disorder, or were taking antiinflammatory, lipid-lowering, or antihypertensive drugs. Of 1350 individuals assessed for eligibility, 100 participants were randomized to 1 of the 5 treatment groups (Supplemental Fig. 1). Two dropped out of the study (98% retention), one participant in the high-dose fish oil group was not able to accommodate the study visits in her schedule, and one participant in the high-dose flaxseed oil group found it too difficult to take 12 pills/d. Participants were middle-aged adults (age 50 ± 10 y [mean ± SD]), with a mean BMI of 30 ± 4 kg/m2, predominantly men (64%) and Caucasian (68%), and with a relatively high mean education level (17 ± 3 y) (Table 1). The study was approved annually by the Stanford University Human Subjects Committee.
TABLE 1.
Characteristics of the participants at baseline1
| Characteristics | P2 | LFx | HFx | LFO | HFO |
| Demographics | |||||
| Sex, women, n (%) | 8 (40) | 7 (35) | 8 (40) | 4 (20) | 9 (45) |
| Age, y | 48 ± 7 | 50 ± 12 | 50 ± 12 | 51 ± 8 | 52 ± 10 |
| Education, y | 18 ± 2 | 17 ± 3 | 17 ± 3 | 17 ± 3 | 17 ± 3 |
| Race/ethnicity, n (%) white | 10 (50) | 16 (80) | 14 (70) | 14 (70) | 14 (70) |
| Asian | 7 (35) | 1 (5) | 5 (25) | 2 (10) | 2 (10) |
| Hispanic | 2 (10) | 1 (5) | 1 (5) | 3 (15) | 3 (15) |
| Black | 0 | 1 (5) | 0 | 0 | 1 (5) |
| Other | 1 (5) | 1 (5) | 0 | 1 (5) | 0 |
| Anthropometrics | |||||
| BMI, kg/m2 | 29 ± 5 | 30 ± 3 | 30 ± 5 | 30 ± 4 | 31 ± 4 |
Data are means ± SD, = 20, or n (%). HFO, high-dose fish oil; HFx, high-dose flaxseed oil; LFO, low-dose fish oil; LFx, low-dose flaxseed oil; P, placebo.
Intervention
Prior to randomization, participants completed a 4-wk run-in phase during which they were instructed to abstain from any fish or flaxseed oil supplements, any foods rich in or supplemented with (n-3) FA, and supplements containing antioxidants. They were then randomly assigned to 1 of 5 groups (n = 20 each) for 8 wk: 1) low-dose flaxseed oil (LFx) (Barlean’s Organic Oils) (2.2 g ALA/d, 4 capsules/d); 2) high-dose flaxseed oil (HFx) (6.6 g ALA/d, 12 capsules/d); 3) low-dose fish oil (LFO) (Nordic Naturals) [1.2 g EPA+DHA (700 mg EPA and 500 mg DHA)/d, 2 capsules/d)]; 4) high-dose fish oil (HFO) [3.6 g EPA+DHA (2.1 g EPA and 1.5 g DHA)/d, 6 capsules/d)]; or 5) placebo (P) (4 or 6 g soybean oil/d, 4 or 6 capsules/d). Two (instead of 4) different doses of placebo were chosen as a compromise in an effort to keep staff unaware of the flaxseed vs. fish oil treatment assignment (i.e. the 4 capsules/d P dose matched the 4 capsules/d for LFx, and the 6 capsules/d P dose matched the 6 capsules/d for the HFO). Participants were instructed to maintain their usual diet [with continued avoidance of (n-3) FA as indicated above] and usual level of physical activity for the duration of the study.
Data collection
Participants completed 3 on-study clinic visits: at baseline and 4 and 8 wk after randomization. At baseline and 4 wk, participants received a 4-wk supply of study capsules and at 4 and 8 wk they returned used bottles for leftover capsule counting. At each visit, anthropometric variables, blood pressure, and a fasting blood sample were collected. Blood pressure was measured 3 times at 2-min intervals after resting for 5 min. The first measurement was discarded and the last 2 averaged. Blood samples were analyzed for 3 inflammatory markers (primary outcomes): MCP-1, IL-6, and sICAM-1. These were chosen based on the existing literature at the time the study was conducted and the expertise of the investigative team (28). Secondary outcomes included plasma lipids (total cholesterol, LDL cholesterol, HDL cholesterol, and TG) insulin, and glucose. RBC FA were also analyzed. Participants completed 3-d food records and 7-d physical activity record (29) prior to randomization and at the end of the study. Dietary intake data were analyzed using Nutrition Data System for Research software version 2007, developed by the Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN. Participants and data collectors were unaware of the treatment assignments.
Biochemical analyses
Inflammatory markers.
Plasma concentrations of MCP-1, IL-6, and sICAM-1 were measured with high-sensitivity ELISA kits from R&D Systems in duplicate, with a minimal detectable concentration of 5.0 ng/L, 0.04 ng/L, and 96 fg/L, respectively. All samples from each person were analyzed on the same day and in the same assay. Inter- and intra-assay variation (expressed as CV %) were 7.4/7.8, 5.8/5.7, and 4.6/5.5, for IL-6, MCP-1, and sICAM-1, respectively.
RBC FA.
FA analysis was performed as previously described (30). Briefly, an RBC aliquot was thawed and heated at 100°C for 10 min with methanol containing 14% boron trifluoride. The FAME thus generated were extracted with hexane and water and were analyzed via GC using a GC2010 (Shimadzu) equipped with a 100-m capillary column (SP-2560; Supelco). FA were identified through comparison with a standard FAME mixture (GLC-727; Nuchek Prep). FAME composition is reported as the percentage by weight of total identified FA. The CV for FA present at <0.5% was 33%; from 0.5% to 5%, 3.5%; and > 5%, 2.3%.
Lipids.
Plasma lipids were measured with standard enzymatic methods (31–33). LDL cholesterol was calculated according to the method of Friedewald et al. (34). Lipid assays were monitored by the Lipid Standardization Program of the CDC and were consistently within specified limits. The laboratory staff conducting these analyses were unaware of the treatment assignments.
Statistics.
Sample size calculations for the study were made using projections for baseline concentrations and SD of the primary outcome variables: the selected inflammatory markers. Given that there are no established guidelines for clinically relevant reductions in the specific inflammatory markers assessed in this study, projected percent reductions from baseline were used to guide these decisions. Using an α = 0.05 and 1-β= 0.80, it was projected that 20 participants/treatment arm would be adequate to detect differences between pairs of treatment arms in 8-wk changes of ~12% for sICAM-1, ~25% for MCP-1, and ~40% for IL-6.
All analyses were conducted using SAS 9.1.3 with Service Pack 3 (SAS Institute). Descriptive statistics and graphs (PROC UNIVARIATE and PROC MEANS) were used to summarize the characteristics of the study population. When variable distributions violated basic testing assumptions, appropriate transformations of the data were used (e.g. log TG). For the primary outcomes (inflammatory marker concentrations) and secondary outcomes (i.e. blood lipids), 1-way ANOVA with repeated measures using PROC GLM were conducted; participant was used as a repeated measure. The changes in RBC ALA, EPA, DHA, and EPA+DHA (the omega-3 index) in each group from baseline to the 8-wk endpoint were compared by ANOVA. When the ANOVA for the main effect of treatment group was significant, the contrasts comparing pairs of treatments were examined using 2 sample t tests. Values in the text are mean ± SD or SE. All statistical tests were 2-tailed using α < 0.05.
Results
Capsule adherence and RBC (n-3) FA concentrations.
Study capsule adherence was ≥88% in all 5 groups: 89 ± 5, 90 ± 6, 88 ± 8, 88 ± 10, and 88 ± 8% in the P, LFx, HFx, LFO, and HFO groups, respectively (mean ± SD). The number of participants with <80% adherence was 1, 1, 2, 3, and 3, respectively. After 8 wk, the RBC ALA level was greater in both Fx groups than in the other 3 groups and those of EPA and DHA were greater in both FO groups than in the others (P ≤ 0.01), reflecting the FA compositions of the oils (Table 2). The increase in the omega-3 index from baseline to 8 wk was greater in the HFO group (6.3 ± 1.0%, mean ± SE) than in the LFO group (2.8 ± 0.3%) and both were greater than in the HFx (0.1 ± 0.1%) and LFx (−0.1 ± 0.3%,) groups, which did not differ from one another.
TABLE 2.
Plasma RBC FA in adults with metabolic syndrome at baseline and 8-wk changes after treatment with low high doses of fish or flax oil or placebo1
| RBC FA | P | LFx | HFx | LFO | HFO | P value |
| ALA, 18:3 (n-3) | % | |||||
| Baseline | 0.43 ± 0.12 | 0.43 ± 0.14 | 0.40 ± 0.12 | 0.54 ± 0.14 | 0.34 ± 0.13 | |
| 8-wk change | 0.01 ± 0.02a | 0.38 ± 0.14b | 0.90 ± 0.31b | −0.02 ± 0.06a | −0.09 ± 0.05a | 0.0003 |
| EPA, 20:5 (n-3) | ||||||
| Baseline | 1.02 ± 0.20 | 0.68 ± 0.12 | 0.69 ± 0.19 | 0.98 ± 0.29 | 1.31 ± 0.38 | |
| 8-wk change | −0.01 ± 0.06a | 0.21 ± 0.17a,b | 0.45 ± 0.10b | 1.26 ± 0.26c | 4.50 ± 1.23c | <0.0001 |
| DHA, 22:6 (n-3) | ||||||
| Baseline | 4.76 ± 0.42 | 4.30 ± 0.24 | 4.13 ± 0.43 | 3.94 ± 0.39 | 5.46 ± 0.40 | |
| 8-wk change | −0.41 ± 0.12a | −0.34 ± 0.13a | −0.58 ± 0.10a | 1.56 ± 0.26b | 2.23 ± 0.30b | <0.0001 |
Data are means ± SE, = 15–17. Sample sizes are smaller than total number of participants randomized to each group because RBC were not collected for the first 20 participants. Means in a row with superscripts without a common letter differ, P < 0.05. ALA, EPA, and DHA at baseline did not differ among groups. ALA, α-linolenic acid; FA, fatty acid; HFO, high-dose fish oil; HFx, high-dose flaxseed oil; LFO, low-dose fish oil; LFx, low-dose flaxseed oil; P, placebo.
Based on 3-d food records and the 7-d physical activity record, energy, percent energy from macronutrients, fiber, (n-3) FA, and energy expenditure did not change during the study in any of the groups and there were no significant differences in changes among the groups (data not shown).
Primary outcomes.
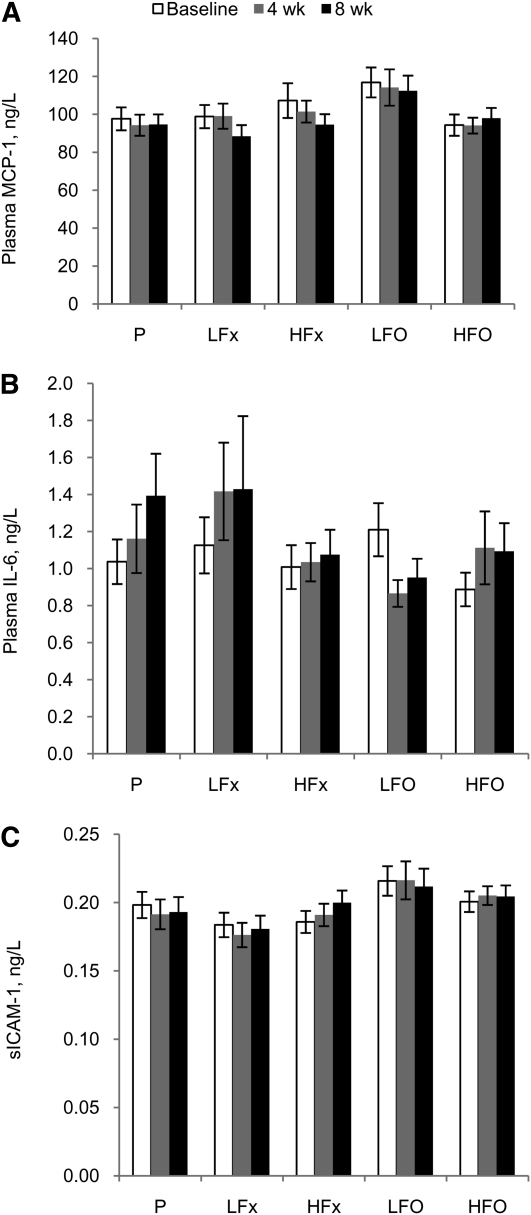
Baseline inflammatory marker concentrations across all 5 groups combined were 103 ± 32 ng/L for MCP-1, 1.06 ± 0.56 ng/L for IL-6, and 0.197 ± 0.041 ng/L for sICAM-1 (mean ± SD). There were no significant differences among groups at baseline. After 8 wk of treatment, there were no significant differences among the groups in changes in plasma MCP-1 (Fig. 1A), IL-6 (Fig. 1B), or sICAM-1 (Fig. 1C). Overall changes in concentrations from baseline to 8 wk were: −5.5 ± 22.3 ng/L for MCP-1, 0.13 ± 0.94 ng/L for IL-6, and 0.001 ± 0.027 ng/L for s-ICAM-1 (mean ± SD).
FIGURE 1.
Plasma MCP-1 (A), IL-6 (B), and sICAM-1 (C) in adults with metabolic syndrome treated with placebo or low or high doses of fish or flax oil for 8 wk. Values are mean ± SE, n = 17–20. HFO, high-dose fish oil; HFx, high-dose flaxseed oil; LFO, low-dose fish oil; LFx, low-dose flaxseed oil; MCP-1, monocyte chemotactic protein-1; sICAM-1, soluble intercellular adhesion molecule-1; P, placebo.
At post hoc analysis, participants were divided into those with inflammatory marker concentrations below and above the median at baseline (99.4 ng/L for MCP-1, 0.94 ng/L for IL-6, and 0.192 ng/L for sICAM-1) to explore the possibility of a significant effect among those with more elevated concentrations. There were no significant differences in changes in plasma inflammatory markers among groups in the subset with concentrations above the median (Supplemental Table 2), as tested by ANCOVA, including the baseline concentration as a covariate in the model.
Secondary outcomes.
The pairwise significant differences among the groups in 8-wk changes in cardiovascular risk factors (Supplemental Table 1) were as follows: LDL-cholesterol increased in both fish oil groups compared to both flaxseed oil groups (P ≤ 0.04); TG decreased in the HFO group compared to both flaxseed oil groups (P ≤ 0.01); systolic blood pressure decreased in the HFO group compared to the HFx and P groups (P ≤ 0.01); and diastolic blood pressure decreased in the HFO group compared to all other groups (P ≤ 0.02).
Discussion
In this study we sought to determine whether (n-3) FA from either plant (flaxseed oil) or marine (fish oil) sources, at either a low or high dose, lowered blood concentrations of selected markers of inflammation (MCP-1, IL-6, and sICAM-1) in adults with metabolic syndrome after 8 wk of treatment. There were no significant changes in any of the 3 inflammatory markers investigated regardless of dose or source of (n-3) FA supplementation. Findings for secondary outcomes, cardiovascular risk factors, were generally in agreement with the current literature (4): LDL-cholesterol concentrations were increased and TG concentrations and blood pressure levels were reduced by fish oil consumption.
Among the more rigorous and recent investigations of potential antiinflammatory effects of (n-3) FA, several observed beneficial outcomes of (n-3) FA supplementation. In one trial, supplementation with 2.4 g/d of marine (n-3) FA or a prescribed diet high in (n-3) FA lowered concentrations of sICAM-1, soluble thrombomodulin, and IL-18 but not other inflammatory markers (IL-6, MCP-1, or TNFα) in elderly men with hyperlipidemia (13, 19). In another trial examining the antiinflammatory effects of ALA, investigators reported that taking 8.1 g of ALA daily for 12 wk decreased circulating concentrations of C-reactive protein, IL-6, serum amyloid A, sVCAM-1, and macrophage colony-stimulating factor in dyslipidemic men who habitually ate a diet high in SFA and poor in MUFA; and lowered concentrations of macrophage colony-stimulating factor and sVCAM-1 in dyslipidemic men who habitually ate a diet high in MUFA (11). In a trial comparing the effects of 1 g EPA+ DHA or 2 g ALA/d, decreased concentrations of some (sVCAM-1 and soluble E-selectin) but not all (sICAM-1) markers of endothelial activation were observed (5). However, the participants in that study were generally healthy, which may have limited the potential opportunity to observe antiinflammatory effects of (n-3) FA consumption. It has been reported that individuals at high risk for CVD, such as persons with metabolic syndrome, have chronically elevated levels of inflammation (35, and may be most likely to benefit from this type of intervention.
Both the dose and population appear to be important factors in assessing potential antiinflammatory effects of (n-3) FA. Trials utilizing lower doses and/or healthy populations have been mostly unsuccessful in decreasing concentrations of the inflammatory markers studied as a result of (n-3) FA supplementation. In a recent randomized controlled trial that examined 19 different inflammatory markers, no beneficial effects of supplementation were reported using 1.5g/d (n-3) FA for 12 wk in healthy, middle-aged adults (18). Using a similar dose of EPA+DHA, another study failed to observe any antiinflammatory effects of fish oil with or without α-tocopherol after 12 wk of treatment in generally healthy adults (9). Finally, in a recent crossover study, no detectable changes were observed in blood concentrations of IL-1β, IL-6, or TNFα in healthy participants with mildly elevated TG after 8 wk of supplementation with either a 0.85- or 3.4-g/d dose of EPA+DHA (22).
Baseline concentrations of inflammatory markers may also be important in eliciting detectable effects. One study that used very high doses of ALA (~11 g/d) in obese but otherwise healthy individuals did not observe an effect on inflammatory marker concentrations but noted that the initial concentrations in their study were ~30% lower than those in the trials reporting successful lowering effects. Our findings are in agreement with this hypothesis, because the baseline IL-6 concentrations (the only marker investigated in both studies) were even lower (~50–100%) than those in the study described above (23).
Finally, the specific markers of inflammation selected as study outcomes are also an important variable of these study designs. Growing awareness that inflammation may be a crucial factor in the development of atherosclerosis and CVD has spurred the investigation of numerous biomarkers of inflammation as predictors of cardiovascular risk (36). This large and growing number of potential and plausible biomarkers can generally be classified into several broad categories, including adhesion molecules, cytokines, proteases, platelet products, adipokines, and acute phase reactants. However, the clinical utility of this plethora of biomarkers has been difficult to establish. More research is needed to elucidate the markers that are the most etiologically relevant to CVD risk and can be most reliably and accurately measured using standardized assays (36, 37).
Cross-sectional studies, in contrast to intervention studies, have frequently reported inverse relationships between blood (n-3) FA biomarkers and circulating inflammatory markers (38–42). It is possible that the typical intervention study of several weeks or months is not of sufficient duration to fundamentally alter inflammatory processes. The success of the 3-y DOIT study in observing effects of supplementation on at least some markers is consistent with this view (13). It will be informative to learn what is discovered from major interventional, multi-year trials with clinically relevant endpoints that will be reporting effects on inflammatory markers (e.g. JELIS, GISSI) (43, 44).
There were several strengths in the design and conduct of the current study. These included enrolling participants with metabolic syndrome (i.e. increased likelihood of elevated inflammation) and comparing 2 different doses, one that may be attained through dietary intake alone and one that was 3 times as high and would likely be achievable only with supplementation. The sample size was also larger than most of the studies that have been cited in this discussion. In addition, the retention rate and adherence to the study protocol were excellent and adherence was documented by analysis of RBC (n-3) FA, which is an assessment that is often not conducted in this type of study (5, 8, 9, 11, 16, 18). Finally, the assessment of diet and physical activity levels indicated that these potentially confounding factors remained stable from baseline to the end of the trial.
The trial also had several limitations. For example, the participants were not screened for concentrations of inflammatory markers to determine eligibility but rather were enrolled on the basis of meeting the criteria for metabolic syndrome, a condition associated with elevated concentrations of inflammatory markers (35). This resulted in enrolling some participants that had relatively low concentrations of inflammatory markers to begin with, with little room for improvement. This was partially addressed by a secondary analysis among those above the median concentrations at baseline, and still no effect was observed. A second limitation is that the 3 inflammatory markers investigated in this trial may not have been the most suitable to capture the effects of (n-3) FA, because numerous inflammatory markers have been documented in the literature (36). As described above, however, the endpoints used were carefully selected and were similar to those used in many other recent studies in this field. Finally, these results can only be generalized to the specific study population enrolled in this study and may not be extended to other individuals that may have different underlying levels of chronic inflammation, other race/ethnic or groups, etc.
In conclusion, adults with metabolic syndrome taking (n-3) FA from either plant or marine sources for 8 wk, at doses that were either moderate and obtainable through dietary intake or high and would require supplementation, did not show a reduction in the blood concentrations of selected inflammatory markers. Although the relationship between (n-3) FA supplementation or consumption from fish and decreased cardiovascular events is generally accepted, the mechanism for this effect is not fully understood. The potential health benefits of (n-3) FA may be mediated by a mechanism that has an insignificant effect on specific markers of inflammation in the blood, such as the prevention of fatal arrhythmias (45). Further research is warranted to better elucidate the mechanism of action and ideal consumption of (n-3) FA for potential health benefits.
Supplementary Material
Acknowledgments
The authors thank Gretchen George for assessing dietary composition, David Ahn for statistical analyses, and Ben Varasteh of the Stanford Clinical and Translational Research Unit (CTRU) for blood drawing and processing. C.D.G. designed the research; A.D. conducted the research; A.D., C.D.G., W.S.H., F.F.M., and P.T. wrote the paper; W.S.H. conducted the RBC analyses; P.T. conducted the inflammatory markers analyses; and A.D. had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Supported by NIH grant R21AT003465-01 (C.D.G.) and the Clinical and Translational Science Award 1UL1 RR025744 for the Stanford Center for Clinical and Translational Education and Research (Spectrum) from the National Center for Research Resources, NIH.
This trial was registered at clinicaltrials.gov as NCT-01129050.
Supplemental Figure 1 and Supplemental Tables 1 and 2 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at jn.nutrition.org.
Abbreviations used: ALA, α-linolenic acid; CVD, cardiovascular disease; FA, fatty acid; HFO, high-dose fish oil; HFx, high-dose flaxseed oil; LFO, low-dose fish oil; LFx, low-dose flaxseed oil; MCP-1, monocyte chemotactic protein-1; P, placebo; sICAM-1, soluble intercellular adhesion molecule-1; sVCAM-1, soluble vascular cell-adhesion molecule-1.
Literature Cited
- 1.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–26 [DOI] [PubMed] [Google Scholar]
- 2.James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000;71:S343–8 [DOI] [PubMed] [Google Scholar]
- 3.Connor WE. Importance of n-3 fatty acids in health and disease. Am J Clin Nutr. 2000;71:S171–5 [DOI] [PubMed] [Google Scholar]
- 4.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–57 [DOI] [PubMed] [Google Scholar]
- 5.Thies F, Miles EA, Nebe-von-Caron G, Powell JR, Hurst TL, Newsholme EA, Calder PC. Influence of dietary supplementation with long-chain n-3 or n-6 polyunsaturated fatty acids on blood inflammatory cell populations and functions and on plasma soluble adhesion molecules in healthy adults. Lipids. 2001;36:1183–93 [DOI] [PubMed] [Google Scholar]
- 6.Madsen T, Christensen JH, Blom M, Schmidt EB. The effect of dietary n-3 fatty acids on serum concentrations of C-reactive protein: a dose-response study. Br J Nutr. 2003;89:517–22 [DOI] [PubMed] [Google Scholar]
- 7.Trebble T, Arden NK, Stroud MA, Wootton SA, Burdge GC, Miles EA, Ballinger AB, Thompson RL, Calder PC. Inhibition of tumour necrosis factor-alpha and interleukin 6 production by mononuclear cells following dietary fish-oil supplementation in healthy men and response to antioxidant co-supplementation. Br J Nutr. 2003;90:405–12 [DOI] [PubMed] [Google Scholar]
- 8.Ciubotaru I, Lee YS, Wander RC. Dietary fish oil decreases C-reactive protein, interleukin-6, and triacylglycerol to HDL-cholesterol ratio in postmenopausal women on HRT. J Nutr Biochem. 2003;14:513–21 [DOI] [PubMed] [Google Scholar]
- 9.Vega-López S, Kaul N, Devaraj S, Cai RY, German B, Jialal I. Supplementation with omega3 polyunsaturated fatty acids and all-rac alpha-tocopherol alone and in combination failed to exert an anti-inflammatory effect in human volunteers. Metabolism. 2004;53:236–40 [DOI] [PubMed] [Google Scholar]
- 10.Geelen A, Brouwer IA, Schouten EG, Kluft C, Katan MB, Zock PL. Intake of n-3 fatty acids from fish does not lower serum concentrations of C-reactive protein in healthy subjects. Eur J Clin Nutr. 2004;58:1440–2 [DOI] [PubMed] [Google Scholar]
- 11.Paschos GK, Rallidis LS, Liakos GK, Panagiotakos D, Anastasiadis G, Votteas V, Zampelas A. Background diet influences the anti-inflammatory effect of alpha-linolenic acid in dyslipidaemic subjects. Br J Nutr. 2004;92:649–55 [DOI] [PubMed] [Google Scholar]
- 12.Eschen O, Christensen JH, Toft E, Schmidt EB. Soluble adhesion molecules and marine n-3 fatty acids in patients referred for coronary angiography. Atherosclerosis. 2005;180:327–31 [DOI] [PubMed] [Google Scholar]
- 13.Hjerkinn EM, Seljeflot I, Ellingsen I, Berstad P, Hjermann I, Sandvik L, Arnesen H. Influence of long-term intervention with dietary counseling, long-chain n-3 fatty acid supplements, or both on circulating markers of endothelial activation in men with long-standing hyperlipidemia. Am J Clin Nutr. 2005;81:583–9 [DOI] [PubMed] [Google Scholar]
- 14.Fujioka S, Hamazaki K, Itomura M, Huan M, Nishizawa H, Sawazaki S, Kitajima I, Hamazaki T. The effects of eicosapentaenoic acid-fortified food on inflammatory markers in healthy subjects: a randomized, placebo-controlled, double-blind study. J Nutr Sci Vitaminol (Tokyo). 2006;52:261–5 [DOI] [PubMed] [Google Scholar]
- 15.Browning LM, Krebs JD, Moore CS, Mishra GD, O'Connell MA, Jebb SA. The impact of long chain n-3 polyunsaturated fatty acid supplementation on inflammation, insulin sensitivity and CVD risk in a group of overweight women with an inflammatory phenotype. Diabetes Obes Metab. 2007;9:70–80 [DOI] [PubMed] [Google Scholar]
- 16.Damsgaard CT, Frokiaer H, Andersen AD, Lauritzen L. Fish oil in combination with high or low intakes of linoleic acid lowers plasma triacylglycerols but does not affect other cardiovascular risk markers in healthy men. J Nutr. 2008;138:1061–6 [DOI] [PubMed] [Google Scholar]
- 17.Yusof HM, Miles EA, Calder P. Influence of very long-chain n-3 fatty acids on plasma markers of inflammation in middle-aged men. Prostaglandins Leukot Essent Fatty Acids. 2008;78:219–28 [DOI] [PubMed] [Google Scholar]
- 18.Pot GK, Brouwer IA, Enneman A, Rijkers GT, Kampman E, Geelen A. No effect of fish oil supplementation on serum inflammatory markers and their interrelationships: a randomized controlled trial in healthy, middle-aged individuals. Eur J Clin Nutr. 2009;63:1353–9 [DOI] [PubMed] [Google Scholar]
- 19.Trøseid M, Arnesen H, Hjerkinn EM, Seljeflot I. Serum levels of interleukin-18 are reduced by diet and n-3 fatty acid intervention in elderly high-risk men. Metabolism. 2009;58:1543–9 [DOI] [PubMed] [Google Scholar]
- 20.Micallef MA, Garg ML. Anti-inflammatory and cardioprotective effects of n-3 polyunsaturated fatty acids and plant sterols in hyperlipidemic individuals. Atherosclerosis. 2009;204:476–82 [DOI] [PubMed] [Google Scholar]
- 21.Kelley DS, Siegel D, Fedor DM, Adkins Y, Mackey BE. DHA supplementation decreases serum C-reactive protein and other markers of inflammation in hypertriglyceridemic men. J Nutr. 2009;139:495–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skulas-Ray AC, Kris-Etherton PM, Harris WS, Vanden Heuvel JP, Wagner PR, West SG. Dose-response effects of omega-3 fatty acids on triglycerides, inflammation, and endothelial function in healthy persons with moderate hypertriglyceridemia. Am J Clin Nutr. 2011;93:243–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson TL, Stevens JR, Hickey MS. Inflammatory markers are not altered by an eight week dietary alpha-linolenic acid intervention in healthy abdominally obese adult males and females. Cytokine. 2007;38:101–6 [DOI] [PubMed] [Google Scholar]
- 24.Zhao G, Etherton TD, Martin KR, West SG, Gillies PJ, Kris-Etherton PM. Dietary alpha-linolenic acid reduces inflammatory and lipid cardiovascular risk factors in hypercholesterolemic men and women. J Nutr. 2004;134:2991–7 [DOI] [PubMed] [Google Scholar]
- 25.Bloedon LT, Balikai S, Chittams J, Cunnane SC, Berlin JA, Rader DJ, Szapary PO. Flaxseed and cardiovascular risk factors: results from a double blind, randomized, controlled clinical trial. J Am Coll Nutr. 2008;27:65–74 [DOI] [PubMed] [Google Scholar]
- 26.Pot GK, Geelen A, Majsak-Newman G, Harvey LJ, Nagengast FM, Witteman BJ, van de Meeberg PC, Hart AR, Schaafsma G, Lund EK, et al. Increased consumption of fatty and lean fish reduces serum C-reactive protein concentrations but not inflammation markers in feces and in colonic biopsies. J Nutr. 2010;140:371–6 [DOI] [PubMed] [Google Scholar]
- 27.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421 [PubMed] [Google Scholar]
- 28.McLaughlin T, Deng A, Gonzales O, Aillaud M, Yee G, Lamendola C, Abbasi F, Connolly AJ, Sherman A, Cushman SW, et al. Insulin resistance is associated with a modest increase in inflammation in subcutaneous adipose tissue of moderately obese women. Diabetologia. 2008;51:2303–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blair SN, Haskell WL, Ho P, Paffenbarger RS, Jr, Vranizan KM, Farquhar JW, Wood PD. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol. 1985;122:794–804 [DOI] [PubMed] [Google Scholar]
- 30.Harris WS, Von Schacky C. The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39:212–20 [DOI] [PubMed] [Google Scholar]
- 31.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–5 [PubMed] [Google Scholar]
- 32.Sampson EJ, Demers LM, Krieg AF. Faster enzymatic procedure for serum triglycerides. Clin Chem. 1975;21:1983–5 [PubMed] [Google Scholar]
- 33.Warnick GR, Albers JJ. A comprehensive evaluation of the heparin-manganese precipitation procedure for estimating high density lipoprotein cholesterol. J Lipid Res. 1978;19:65–76 [PubMed] [Google Scholar]
- 34.Friedewald WT, Levy R, Fredrickson D. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, with use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502 [PubMed] [Google Scholar]
- 35.Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111:1448–54 [DOI] [PubMed] [Google Scholar]
- 36.Packard RR, Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clin Chem. 2008;54:24–38 [DOI] [PubMed] [Google Scholar]
- 37.Koenig W. Update on integrated biomarkers for assessment of long-term risk of cardiovascular complications in initially healthy subjects and patients with manifest atherosclerosis. Ann Med. 2009;41:332–43 [DOI] [PubMed] [Google Scholar]
- 38.Baggott JE. Serum folate and homocysteine concentrations in large population samples of US ethnic and racial groups. Am J Clin Nutr. 1999;70:937. [DOI] [PubMed] [Google Scholar]
- 39.Farzaneh-Far R, Harris WS, Garg S, Na B, Whooley MA. Inverse association of erythrocyte n-3 fatty acid levels with inflammatory biomarkers in patients with stable coronary artery disease: The Heart and Soul Study. Atherosclerosis. 2009;205:538–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harris WS, Miller M, Tighe AP, Davidson MH, Schaefer EJ. Omega-3 fatty acids and coronary heart disease risk: clinical and mechanistic perspectives. Atherosclerosis. 2008;197:12–24 [DOI] [PubMed] [Google Scholar]
- 41.Murakami K, Sasaki S, Takahashi Y, Uenishi K, Yamasaki M, Hayabuchi H, Goda T, Oka J, Baba K, Ohki K, et al. Total n-3 polyunsaturated fatty acid intake is inversely associated with serum C-reactive protein in young Japanese women. Nutr Res. 2008;28:309–14 [DOI] [PubMed] [Google Scholar]
- 42.Baghai TC, Varallo-Bedarida G, Born C, Hafner S, Schule C, Eser D, Rupprecht R, Bondy B, von Schacky C. Major depressive disorder is associated with cardiovascular risk factors and low Omega-3 index. J Clin Psychiatry. 2011;72:1242–7 [DOI] [PubMed] [Google Scholar]
- 43.Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet. 1999;354:447–55 [PubMed] [Google Scholar]
- 44.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–8 [DOI] [PubMed] [Google Scholar]
- 45.Breslow JL. n-3 fatty acids and cardiovascular disease. Am J Clin Nutr. 2006;83:S1477–82 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.