Abstract
Previous studies have shown that multiple features of atherogenic dyslipidemia are improved by replacement of dietary carbohydrate with mixed sources of protein and that these lipid and lipoprotein changes are independent of dietary saturated fat content. Because epidemiological evidence suggests that red meat intake may adversely affect cardiovascular disease risk, we tested the effects of replacing dietary carbohydrate with beef protein in the context of high- vs. low-saturated fat intake in 40 healthy men. After a 3-wk baseline diet [50% daily energy (E) as carbohydrate, 13% E as protein, 15% E as saturated fat], participants consumed for 3 wk each in a randomized crossover design two high-beef diets in which protein replaced carbohydrate (31% E as carbohydrate, 31% E as protein, with 10% E as beef protein). The high-beef diets differed in saturated fat content (8% E vs. 15% E with exchange of saturated for monounsaturated fat). Two-week washout periods were included following the baseline diet period and between the randomized diets periods. Plasma TG concentrations were reduced after the 2 lower carbohydrate dietary periods relative to after the baseline diet period and these reductions were independent of saturated fat intake. Plasma total, LDL, and non-HDL cholesterol as well as apoB concentrations were lower after the low-carbohydrate, low-saturated fat diet period than after the low-carbohydrate, high-saturated fat diet period. Given our previous observations with mixed protein diets, the present findings raise the possibility that dietary protein source may modify the effects of saturated fat on atherogenic lipoproteins.
Introduction
The atherogenic dyslipidemia characterized by elevated TG, reduced HDL-C,8 and increased levels of small LDL particles is associated with increased CVD risk (1). Atherogenic dyslipidemia can be induced or amplified by increased dietary carbohydrate intake (2) and suppressed or reduced by replacing carbohydrate with protein (3). However, it is not known to what extent the benefit of protein may be influenced by food source. Observational studies suggest that white meat or dairy food consumption has neutral effects on CVD risk (4), whereas red meat intake may increase CVD risk (4–6). It has been suggested that the saturated fat content of red meat may contribute to its association with CVD risk (7). Although saturated fat intake has been reported to increase LDL-C and total cholesterol (8), it has not been found to have adverse effects on other measures of atherogenic dyslipidemia, including levels of small LDL particles (1, 9). Notably, we have demonstrated that increased saturated fat intake does not worsen features of atherogenic dyslipidemia when consumed in the context of a reduced-carbohydrate, high-mixed protein diet (3). These data are also consistent with the results of a meta-analysis of prospective observational cohort studies that could not demonstrate a rela-tionship between dietary saturated fat and incidence of CVD (8, 10). The present study was designed to test whether saturated fat intake affects components of atherogenic dyslipidemia when dietary carbohydrate is replaced with protein derived primarily from beef.
Methods
Study design and diets.
The study protocol was conducted in free-living participants through our outpatient clinic located in Berkeley, CA. All participants consumed the baseline diet for 3 wk and then consumed in random order two LC diets that were high in beef but differed in saturated fat content (Table 1): a LCHSF diet and a LCLSF diet. Differences in saturated fat intake were achieved primarily through the use of full-fat dairy products. The baseline diet contained no beef protein (13% daily E as protein from 5.2% E as vegetable, 4.5% E as white meat, 1.3% E as dairy, and <1.0% E as eggs or pork protein). Two 2-wk wash-out periods were included during which participants were instructed to consume their habitual daily diets: one following the baseline diet period and one between the randomized diet periods. Dietary control was achieved as previously described (11) with the provision of menus and 2 standardized prepared meals per day (lunch and dinner). Entrées and menus were designed by the Bionutrition Core of the University of California, San Francisco Clinical and Translational Sciences Institute (San Francisco, CA) and were based on a 4-d rotating cycle. The dietary composition of the entrées was validated by compositional analysis (Covance). All of the beef protein was provided within the entrées.
TABLE 1.
Macronutrient composition of diets1
| Baseline diet | LCHSF diet | LCLSF diet | |
| Carbohydrate, % E | 50 | 31 | 31 |
| Protein, % E | 13 | 31 | 32 |
| Beef protein, % E | 0 | 10 | 11 |
| Total fat, % E | 38 | 38 | 38 |
| Saturated fat, % E | 15 | 15 | 8 |
| MUFA, % E | 15 | 15 | 21 |
| PUFA, % E | 6 | 5 | 6 |
| Cholesterol, mg/d | 468 | 463 | 467 |
Diets were formulated to contain equivalent amounts of fiber (25 g/8400 kJ plus 2.5g/2100 kJ above this level), fat (1.5% daily E), cholesterol (465 mg/12,500 kJ), linoleic acid (5.5% E), and linolenic acid (0.5% E) and to have a similar ratio of sugar:starch (1:1). E, energy; LCHSF, lower carbohydrate, high-saturated fat diet; LCLSF, lower carbohydrate, low-saturated fat diet.
Blood samples were collected from participants following an overnight fast on 2 consecutive days after consumption of the baseline diet (20 and 21 d) and after consumption of each randomized diet (55 and 56 d; 90 and 91 d). On the first of these visits (20, 55, and 90 d), a post-heparin blood sample was also obtained. On the second of these visits (21, 56, and 91 d), participants also underwent a s.c. adipose tissue biopsy as previously described (32) and an oral fat tolerance test. For the latter test, blood samples were collected 0, 3, and 6 h after consuming a standard milkshake containing 49 g of fat and 292 mg cholesterol (5080 total kJ). Following each dietary intervention, body weight was measured and percentage body fat was determined by bioimpedance (TBF-551, Tanita). Blood samples were not collected following wash-out periods.
Study population.
This study was limited to adult males to increase the prevalence of atherogenic dyslipidemia, because its components represented the major outcome variables on which power calculations were based. Participants were screened to meet the following specifications: no history of CVD or other chronic diseases; not taking drugs known to affect lipid metabolism, blood thinning agents, or hormones; age ≥18 y; BMI ≥20 kg/m2 but ≤35 kg/m2; total cholesterol and LDL-C <the 95th percentile for age and sex (12); TG <5.7 mmol/L; blood pressure <150/90 mm Hg; fasting glucose ≤6.9 mmol/L; at least 3 mo at stable weight as defined by 3% change in body weight; not actively strength training with resistance weights for >4 h/wk; nonsmoker; and agreement to refrain from alcohol, recreational drugs, and dietary supplements during the study (Supplemental Table 1). The characteristics of the study population upon enrollment are presented in Supplemental Table 1. Participants were randomized to the LCLSF diet followed by the LCHSF diet or vice versa by permuting participant assignment within randomly determined size blocks of individuals. All participants gave informed consent under a protocol approved by and in accordance with the ethical standards of the Institutional Review Board of the Children’s Hospital and Research Center Oakland.
Laboratory measurements.
Total cholesterol, HDL-C, TG, glucose, insulin, apoB, and apoAI were measured in plasma samples obtained from fasting participants as previously described (3). LDL-C was calculated using the Friedewald formula (13). nonHDL-C was calculated by subtracting HDL-C from total cholesterol. Plasma apoB48 was measured by ELISA in samples collected at 0, 3, and 6 h following the oral fat tolerance test (Biovendor). Concentrations of LDL, VLDL, IDL, and subfractions of these classes were determined by ion mobility, which directly measures concentrations of lipoprotein particles as a function of their size (14).
Lipase activities were measured in post-heparin plasma by selective inhibition of LPL with protamine sulfate as previously described (15, 16). Total lipase activity was also measured in adipose tissue biopsies following homogenization in Tris-EDTA-sucrose buffer containing deoxycholate and protease inhibitor (Roche Diagnostics). Adipose tissue lipase activity was normalized to total protein as measured by the method of Lowry (17). Transcriptional expression of LPL was measured in adipose tissue biopsies using Taqman-based, real-time PCR as previously described (18).
Statistical analysis.
Fasting total cholesterol, LDL-C, nonHDL-C, TG, HDL-C, and lipoprotein particle concentrations following each diet period were calculated as the mean of two measurements and are reported as means ± SD across individuals. Data were analyzed for diet effects by ANOVA and post hoc Tukey’s test with random effect for participant. We did not adjust for diet randomization order, because there was no evidence for an association of diet order with any of the reported measurements. Time effects within the postprandial data were also analyzed by repeated-measures ANOVA followed by post hoc Tukey’s test. Those traits that were not normally distributed or did not have constant variance were log-transformed prior to analysis. Linear regression models were used to determine relations between variables. All statistical procedures were performed using JMP7.0 (SAS).
Results
Fasting plasma lipids and lipoproteins.
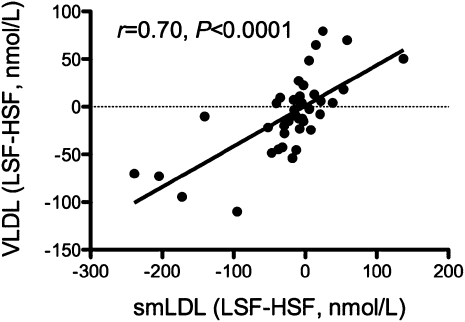
Plasma TG concentrations were significantly reduced following both LC diet periods compared to the baseline diet period (Table 2). Because changes in TG typically reflect changes in plasma VLDL, we also tested for differences in VLDL concentrations following the diet periods. Plasma total-, large-, and medium-VLDL concentrations were significantly reduced following intake of the LCLSF diet compared to the baseline diet. Similarly, HDL-C concentrations were significantly reduced by intake of the LCLSF diet compared to the baseline diet, with intermediate HDL-C concentrations following intake of the LCHSF diet. Interestingly, small LDL concentrations were significantly reduced following the LCLSF diet period compared to the LCHSF diet period. Differences in concentrations of small LDL particles between these two LC diet periods were positively correlated with differences in total VLDL particles (Fig. 1).
TABLE 2.
Fasting plasma lipid and lipoprotein responses to changes in carbohydrate and saturated fat intake in men1
| Baseline diet | LCHSF diet | LCLSF diet | P value for diet effect | |
| Weight, kg | 84.9 ± 11.1 | 85.2 ± 11.1 | 85.0 ± 11.1 | 0.69 |
| TC, mmol/L | 4.51 ± 0.83a | 4.44 ± 0.81a | 4.03 ± 0.72b | <0.0001 |
| LDL-C, mmol/L | 2.87 ± 0.75a | 2.86 ± 0.80a | 2.50 ± 0.69b | <0.0001 |
| nonHDL-C, mmol/L | 3.42 ± 0.87a | 3.37 ± 0.89a | 2.98 ± 0.76b | <0.0001 |
| ApoB, g/L | 0.74 ± 0.16a | 0.73 ± 0.14a | 0.68 ± 0.14b | <0.0001 |
| TG, mmol/L | 1.22 ± 0.61a | 1.10 ± 0.61b | 1.05 ± 0.49b | 0.0001 |
| HDL-C, mmol/L | 1.08 ± 0.27a | 1.07 ± 0.30ab | 1.04 ± 0.27b | 0.007 |
| ApoAI, g/L | 1.00 ± 0.12 | 0.99 ± 0.12 | 0.98 ± 0.12 | 0.39 |
| Total VLDL, nmol/L | 98.1 ± 47.9a | 92.5 ± 41.7ab | 86.0 ± 38.1b | 0.05 |
| Large VLDL, nmol/L | 15.7 ± 10.3a | 13.7 ± 7.5ab | 13.3 ± 8.4b | 0.01 |
| Medium VLDL, nmol/L | 40.4 ± 21.3a | 37.3 ± 18.6ab | 34.7 ± 17.5b | 0.02 |
| Small VLDL, nmol/L | 41.9 ± 17.7 | 41.6 ± 17.3 | 38.3 ± 14.7 | 0.15 |
| IDL, nmol/L | 133 ± 54 | 139 ± 52 | 126 ± 45 | 0.11 |
| Total LDL, nmol/L | 1400 ± 426a | 1440 ± 412a | 1220 ± 330b | <0.0001 |
| Large LDL, nmol/L | 698 ± 190 | 712 ± 218 | 655 ± 189 | 0.07 |
| Medium LDL, nmol/L | 284 ± 134a | 304 ± 133a | 214 ± 72.8b | <0.0001 |
| Small LDL, nmol/L | 207 ± 142ab | 222 ± 132a | 187 ± 108b | 0.01 |
| Very small LDL, nmol/L | 209 ± 97 | 205 ± 92 | 187 ± 62 | 0.23 |
| LDL peak diameter, nm | 22.0 ± 5.7 | 21.9 ± 5.4 | 22.0 ± 4.8 | 0.25 |
| LDL subclass phenotype B, n (%) | 10 (25) | 11 (32) | 9 (23) | 0.64 |
Values are mean ± SD, = 40. Means without a common letter differ, P < 0.05. The following traits were log-transformed prior to statistical analysis: TG, HDL-C, large VLDL, medium VLDL, small VLDL, total LDL, large LDL, medium LDL, small LDL, and very small LDL. HDL-C, HDL cholesterol; LCHSF, lower carbohydrate, high-saturated fat; LCLSF, lower carbohydrate, low-saturated fat; LDL-C, LDL cholesterol; nonHDL-C, non-HDL cholesterol; TC, total cholesterol.
FIGURE 1.
Associations between differences in plasma concentrations of small LDL particles and total VLDL particles in men following intake of two LC diets that differed in saturated fat content, n = 40. LC, lower carbohydrate.
Total cholesterol, LDL-C, nonHDL-C, apoB, and total LDL were reduced following the LCLSF diet period compared to both the baseline and LCHSF diet periods. Differences in total LDL particle concentrations between diet periods reflected changes in medium- and small-LDL. Significant differences between diet periods were not observed for large-LDL, very small- LDL, LDL peak diameter, or LDL subclass phenotype. TG and VLDL concentrations were reduced following consumption of the LC diets compared to the baseline diet independent of saturated fat intake.
Postprandial plasma lipids and lipoproteins.
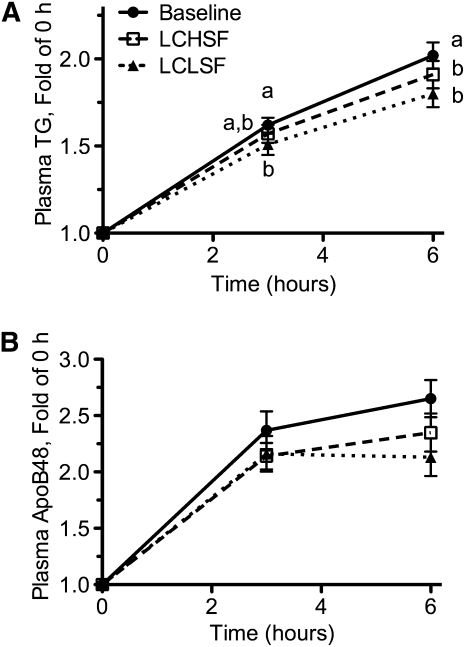
Plasma TG and apoB48 increased after a standard oral fat load following all three dietary periods (P < 0.0001). Plasma TG increased within 3 h of consumption (Fig. 2A) (P < 0.00001 vs. 0 h after each diet period) and continued to increase through the 6-h time point (P < 0.05 vs. 3 h for each diet period). Plasma apoB48 also increased within 3 h of meal consumption following all diet periods (Fig. 2B) (P < 0.05) but continued to increase between the 3- and 6-h time points only following the baseline diet period (P < 0.05). At the 3-h time point, the percent postprandial increase in plasma TG was significantly greater following intake of the baseline diet compared to the LCLSF diet, with an intermediate increase following the LCHSF diet period. At the 6-h time point, the percentage of postprandial increase in plasma TG was significantly greater following intake of the baseline diet compared to both LC diets. There were no significant differences in the percentage of postprandial increase in apoB48 at either the 3- or 6-h time point between any of the diet periods.
FIGURE 2.
Changes in plasma TG (A) and apoB48 (B) in men following administration of an oral fat tolerance test after diet periods that differed in carbohydrate and saturated fat content. Values are presented as the ratio of postprandial:fasting concentrations to adjust for differences in fasting measurements. Values are mean ± SEM, n = 40. Labeled means at a time without a common letter differ, P < 0.05. LCLSF, lower carbohydrate, low-saturated fat; LCHSF, lower carbohydrate, high-saturated fat.
Consistent with the above observations, the postprandial iAUC for plasma TG also differed across diet periods (P < 0.0001). The plasma TG iAUC was significantly reduced following both LC diet periods compared to the baseline diet period (Supplemental Table 2). The TG iAUC was also reduced following intake of the LCLSF diet compared to the LCHSF diet, suggesting that postprandial TG iAUC is influenced by both carbohydrate and saturated fat intake. ApoB48 iAUC was also different across diet periods (P = 0.0002) and was significantly lower following intake of the LCLSF diet compared to the baseline diet. Changes in TG iAUC and apoB48 iAUC between the LCHSF and LCLSF diet periods were correlated (r = 0.79; P < 0.0001). Changes in TG iAUC and apoB48 iAUC between the baseline and LCHSF diet periods were also correlated (r = 0.57; P = 0.007).
We also tested for postprandial effects on glucose and insulin iAUC. Glucose iAUC was significantly reduced following both LC diet periods compared to the baseline diet. Insulin iAUC did not differ across diet periods.
Lipase activities.
Post-heparin plasma HL and LPL activities also differed across diet periods (Table 3). Both HL and LPL activity were significantly different following the baseline diet compared to either LC diet period. Lipase activities did not differ between the LCLSF and LCHSF diet periods. Carbohydrate-induced changes in post-heparin LPL activity were inversely correlated with the changes in fasting TG (r = 0.39; P = 0.03) and postprandial insulin iAUC (r = 0.51; P = 0.004). Changes in HL activity were not correlated with any of the metabolic measurements. Lipase activity and LPL mRNA expression measured in adipose tissue did not differ between diet periods.
TABLE 3.
Lipase activities and expression in response to changes in carbohydrate and saturated fat intake in men1
| Baseline diet | LCHSF diet | LCLSF diet | P value for diet effect | |
| Plasma | ||||
| HL, μmol FFA/(h × L) | 0.0177 ± 0.0088a | 0.0152 ± 0.0078b | 0.0141 ± 0.0075b | <0.0001 |
| LPL, μmol FFA/(h × L) | 0.0120 ± 0.0081a | 0.0136 ± 0.0083b | 0.0140 ± 0.0083b | 0.0002 |
| HL:LPL | 3.6 ± 9.1a | 1.7 ± 2.1b | 1.5 ± 2.4b | <0.0001 |
| Adipose tissue | ||||
| Lipase, nmol FFA/(g·h) | 30.3 ± 25.6 | 38.0 ± 30.5 | 36.5 ± 27.6 | 0.08 |
| LPL mRNA, AU | 7370 ± 2720 | 8320 ± 3030 | 8630 ± 3490 | 0.14 |
Values are mean ± SD, = 40 (plasma measurements), 25 (lipase), or 28 (LPL). Means in a row with superscripts without a common letter differ, P < 0.05. All data were log-transformed prior to statistical analysis. HL, hepatic lipase; LCHSF, lower carbohydrate, high-saturated fat; LCLSF, lower carbohydrate, low-saturated fat; LPL, lipoprotein lipase.
Discussion
Epidemiological observations suggest that consumption of red meat is more strongly associated with increased risk of CVD events/mortality than other dietary sources of protein (4, 5) and that the major determinant of this association may be processed red meats (6). It has not been established to what extent saturated fat, and its effects on atherogenic lipoproteins, contributes to the CVD risk associated with red meat intake. We previously showed that isoenergetic substitution of protein and fat for carbohydrate, when protein is derived from mixed food sources, improves multiple features of atherogenic dyslipidemia and CVD risk factors, including apoB, total cholesterol:HDL-C ratio, and smaller LDL particles independent of saturated fat intake (3). In the present study, we tested whether lipids and lipoproteins are similarly affected by substitution of protein for carbohydrate when using red meat as the main protein source. We also tested whether these dietary effects were dependent on saturated fat intake.
We found that substituting protein for carbohydrate decreased plasma TG in a manner that was independent of saturated fat intake but that reductions in other lipoprotein-related risk factors, including apoB and small LDL, were greatest following consumption of a LCLSF diet. Because saturated fat intake was altered through substitution of monounsaturated for saturated fat, we cannot preclude the possibility that these changes were caused by higher monounsaturated fat intake rather than reduced saturated fat intake. However, a large body of evidence from clinical trials and epidemiological studies supports the hypothesis that changes in lipoprotein concentrations between the LC diets used in this study were due to changes in saturated fat intake (10, 19).
Substitution of dietary protein for carbohydrate did not improve lipid and lipoprotein measures of CVD risk when saturated fat consumption was high (baseline vs. LCHSF). In fact, this dietary substitution resulted in a trend toward increased LDL particle concentrations across all LDL subclasses. These results are in contrast to our previous findings that carbohydrate restriction improves atherogenic dyslipidemia independent of saturated fat intake (3). Although the difference in carbohydrate intake between the baseline and LCHSF diet period in the present study (−19% E) is less than for the comparable diets in our previous study (−28% E) (3), the changes in TG concentrations between diet periods were similar (−11 vs. −9%, respectively), indicating that the carbohydrate reduction in the present study was sufficient to achieve reduction in a key feature of atherogenic dyslipidemia.
The major difference between the diet composition in this study and our previous study was the use of beef as a major source of protein. Dietary saturated fat content was derived primarily from dairy foods in both studies. Beef fat was only a minor component of the saturated fat profiles of the LCHSF and LCLSF diets and there was only a 0.6% differential in saturated beef fat intake between these two diets. Hence, the present findings suggest an interaction between saturated fat and one or more nonfat components of beef on lipoprotein metabolism. Because there is little evidence for a major role of dietary protein composition on lipoprotein metabolism (20, 21), this interaction is not likely to be caused by specific amino acids within beef protein. However, saturated fat might be interacting with a micronutrient or other component that is more abundant in beef than in other food protein sources. For example, systemic iron stores have been associated with altered lipid metabolism (22–24) and there is evidence that heme iron absorption is substantially increased by saturated fat and, in particular, stearic acid (25–27), which is abundant in dairy fat.
The metabolic basis for the increases in small- and medium-LDL between the LCHSF and LCLSF diet periods in this study is not known. Although LDL receptor inhibition plays a major role in saturated fat-mediated increases in LDL-C (28), levels of smaller LDL subclasses are thought to be primarily influenced by pathways affecting metabolism of VLDL (29). Consistent with this, we observed that saturated fat-mediated changes in small LDL were correlated with changes in VLDL and that this relationship accounted for a major proportion of the variance in small LDL response (r2 = 0.54). We also tested for dietary effects on lipase activities, because both HL and LPL act to mediate production of LDL from VLDL precursors (30, 31) and we previously demonstrated that post-heparin plasma lipase activities are both responsive to diet and correlated with small LDL concentrations (15). In the present study, LPL activity was increased by carbohydrate restriction, but, in contrast to previous findings (15), HL activity was significantly decreased by this dietary change, consistent with other evidence that nutritional regulation of HL may be dependent on dietary context (32). Although the diet-induced changes in lipase activities were not found to be related to the changes in LDL subclass levels observed here, it is nevertheless possible that dietary effects on intravascular lipolysis and/or remodeling of lipoproteins may have played a role.
Although lipids and lipoproteins measured in the fasting state remain the standard for assessment of CVD risk, a number of studies have shown that postprandial lipemia independently predicts CVD risk (33–35). Both high-protein and low-carbohydrate intake have been suggested to improve the postprandial lipid response (36–38). In the current study, we found that exchange of protein for carbohydrate lowered postprandial lipemia and glycemia. The postprandial TG iAUC was lowest following the LCLSF diet period, suggesting that saturated fat intake may also influence postprandial lipoprotein metabolism. However, we did not capture the return to fasting concentrations of postprandial TG or apoB48, and our observations do not permit assessment as to whether saturated fat altered production or clearance of TG-rich lipoproteins.
Overall, we found that lipid and lipoprotein markers of CVD risk as well as components of atherogenic dyslipidemia measured in the fasting state were improved by replacement of carbohydrate with protein derived primarily from beef only in the context of lower saturated fat intake. These results suggest that the combined consumption of beef protein and saturated fat has a greater influence on components of atherogenic dyslipidemia than can be accounted for by either of these dietary components individually. Although our recent meta-analysis of prospective cohort studies did not demonstrate an association of saturated fat with CVD risk in the general population (8, 10), the present results raise the possibility that such an association may be present in subsets of the population who consume high amounts of saturated fat in the presence of red meat. These observations support the need for further studies to determine whether the dietary context in which saturated fat is consumed influences its relationship with CVD.
Supplementary Material
Acknowledgments
L.M.M. and R.M.K. designed research; L.M.M., S.C., K.W., and R.S.R conducted research; L.M.M., S.C., N.B., and R.M.K. analyzed data; L.M.M., S.C., N.B., and R.M.K. wrote the paper; and L.M.M. had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Supported by the Beef Checkoff through the National Cattlemen’s Beef Association and by NIH National Center for Resarch Resources, University of California, San Francisco Clinical and Translational Science Institute grant no. UL1 RR024131. The paper’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
This trial was registered at clinicaltrials.gov as NCT00852267.
Supplemental Figure 1 and Tables 1 and 2 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at jn.nutrition.org.
Abbreviations used: CVD, cardiovascular disease; E, energy; HDL-C, HDL cholesterol; HL, hepatic lipase; iAUC, incremental AUC; LC, lower carbohydrate; LCHSF, lower carbohydrate, high-saturated fat; LCLSF, lower carbohydrate, low-saturated fat; LDL-C, LDL cholesterol; LPL, lipoprotein lipase; nonHDL-C, non-HDL cholesterol.
Literature Cited
- 1.Austin MA, Breslow JL, Hennekens CH, Buring JE, Willett WC, Krauss RM. Low-density lipoprotein subclass patterns and risk of myocardial infarction. JAMA. 1988;260:1917–21 [PubMed] [Google Scholar]
- 2.Dreon DM, Fernstrom HA, Miller B, Krauss RM. Low-density lipoprotein subclass patterns and lipoprotein response to a reduced-fat diet in men. FASEB J. 1994;8:121–6 [PubMed] [Google Scholar]
- 3.Krauss RM, Blanche PJ, Rawlings RS, Fernstrom HS, Williams PT. Separate effects of reduced carbohydrate intake and weight loss on atherogenic dyslipidemia. Am J Clin Nutr. 2006;83:1025–31 [DOI] [PubMed] [Google Scholar]
- 4.Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC. Major dietary protein sources and risk of coronary heart disease in women. Circulation. 2010;122:876–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinha R, Cross AJ, Graubard BI, Leitzmann MF, Schatzkin A. Meat intake and mortality: a prospective study of over half a million people. Arch Intern Med. 2009;169:562–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation. 2010;121:2271–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu FB, Stampfer MJ, Manson JE, Ascherio A, Colditz GA, Speizer FE, Hennekens CH, Willett WC. Dietary saturated fats and their food sources in relation to the risk of coronary heart disease in women. Am J Clin Nutr. 1999;70:1001–8 [DOI] [PubMed] [Google Scholar]
- 8.Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Saturated fat, carbohydrate, and cardiovascular disease. Am J Clin Nutr. 2010;91:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dreon DM, Fernstrom HA, Campos H, Blanche P, Williams PT, Krauss RM. Change in dietary saturated fat intake is correlated with change in mass of large low-density-lipoprotein particles in men. Am J Clin Nutr. 1998;67:828–36 [DOI] [PubMed] [Google Scholar]
- 10.Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am J Clin Nutr. 2010;91:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siri-Tarino PW, Woods AC, Bray GA, Krauss RM. Reversal of small, dense LDL subclass phenotype by weight loss is associated with impaired fat oxidation. Obesity (Silver Spring). 2011;19:61–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rifkind BM, Segal P. Lipid Research Clinics Program reference values for hyperlipidemia and hypolipidemia. JAMA. 1983;250:1869–72 [PubMed] [Google Scholar]
- 13.Warnick GR, Nguyen T, Albers AA. Comparison of improved precipitation methods for quantification of high-density lipoprotein cholesterol. Clin Chem. 1985;31:217–22 [PubMed] [Google Scholar]
- 14.Caulfield MP, Li S, Lee G, Blanche PJ, Salameh WA, Benner WH, Reitz RE, Krauss RM. Direct determination of lipoprotein particle sizes and concentrations by ion mobility analysis. Clin Chem. 2008;54:1307–16 [DOI] [PubMed] [Google Scholar]
- 15.Campos H, Dreon DM, Krauss RM. Associations of hepatic and lipoprotein lipase activities with changes in dietary composition and low density lipoprotein subclasses. J Lipid Res. 1995;36:462–72 [PubMed] [Google Scholar]
- 16.Krauss RM, Windmueller HG, Levy RI, Fredrickson DS. Selective measurement of two different triglyceride lipase activities in rat postheparin plasma. J Lipid Res. 1973;14:286–95 [PubMed] [Google Scholar]
- 17.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75 [PubMed] [Google Scholar]
- 18.Mangravite LM, Dawon K, Davis RR, Gregg JP, Krauss RM. Fatty acid desaturase regulation in adipose tissue by dietary composition is independent of weight loss and correlated with plasma triacylglycerol response. Am J Clin Nutr. 2007;86:759–67 [DOI] [PubMed] [Google Scholar]
- 19.Mensink RP, Katan MB. Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler Thromb. 1992;12:911–9 [DOI] [PubMed] [Google Scholar]
- 20.Eklund A, Sjoblom L. Effects of the source of dietary protein on serum lower density lipoprotein (VLDL + LDL) and tocopherol levels in female rats. J Nutr. 1980;110:2321–35 [DOI] [PubMed] [Google Scholar]
- 21.Vega-López S, Matthan NR, Ausman LM, Harding SV, Rideout TC, Ai M, Otokozawa S, Freed A, Kuvin JT, et al. Altering dietary lysine:arginine ratio has little effect on cardiovascular risk factors and vascular reactivity in moderately hypercholesterolemic adults. Atherosclerosis. 2010;210:555–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozdemir A, Sevinc C, Selamet U, Turkmen F. The relationship between iron deficiency anemia and lipid metabolism in premenopausal women. Am J Med Sci. 2007;334:331–3 [DOI] [PubMed] [Google Scholar]
- 23.Silva M, Silva ME, de Paula H, Carneiro CM, Pedrosa ML. Iron overload alters glucose homeostasis, causes liver steatosis, and increases serum triacylglycerols in rats. Nutr Res. 2008;28:391–8 [DOI] [PubMed] [Google Scholar]
- 24.Mateo-Gallego R, Calmarza P, Jarauta E, Burillo E, Cenarro A, Civeira F. Serum ferritin is a major determinant of lipid phenotype in familial combined hyperlipidemia and familial hypertriglyceridemia. Metabolism. 2010;59:154–8 [DOI] [PubMed] [Google Scholar]
- 25.Boesch-Saadatmandi C, Most E, Weigand E. Influence of dietary fat and zinc supplementation on the iron utilization in growing rats. Ann Nutr Metab. 2007;51:395–401 [DOI] [PubMed] [Google Scholar]
- 26.Shotton AD, Droke EA. Iron utilization and liver mineral concentrations in rats fed safflower oil, flaxseed oil, olive oil, or beef tallow in combination with different concentrations of dietary iron. Biol Trace Elem Res. 2004;97:265–78 [DOI] [PubMed] [Google Scholar]
- 27.Kapsokefalou M, Miller DD. Lean beef and beef fat interact to enhance nonheme iron absorption in rats. J Nutr. 1993;123:1429–34 [DOI] [PubMed] [Google Scholar]
- 28.Spady DK, Dietschy JM. Dietary saturated triacylglycerols suppress hepatic low density lipoprotein receptor activity in the hamster. Proc Natl Acad Sci USA. 1985;82:4526–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res. 2002;43:1363–79 [DOI] [PubMed] [Google Scholar]
- 30.Eckel RH. Lipoprotein lipase. A multifunctional enzyme relevant to common metabolic diseases. N Engl J Med. 1989;320:1060–8 [DOI] [PubMed] [Google Scholar]
- 31.Auwerx JH, Marzetta CA, Hokanson JE, Brunzell JD. Large buoyant LDL-like particles in hepatic lipase deficiency. Arteriosclerosis. 1989;9:319–25 [DOI] [PubMed] [Google Scholar]
- 32.Gascon A, Jacques H, Moorjani S, Deshaies Y, Brun LD, Julien P. Plasma lipoprotein profile and lipolytic activities in response to the substitution of lean white fish for other animal protein sources in premenopausal women. Am J Clin Nutr. 1996;63:315–21 [DOI] [PubMed] [Google Scholar]
- 33.Zilversmit DB. Atherogenesis: a postprandial phenomenon. Circulation. 1979;60:473–85 [DOI] [PubMed] [Google Scholar]
- 34.Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298:309–16 [DOI] [PubMed] [Google Scholar]
- 35.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308 [DOI] [PubMed] [Google Scholar]
- 36.Sharman MJ, Gomez AL, Kraemer WJ, Volek JS. Very low-carbohydrate and low-fat diets affect fasting lipids and postprandial lipemia differently in overweight men. J Nutr. 2004;134:880–5 [DOI] [PubMed] [Google Scholar]
- 37.Volek JS, Sharman MJ, Gomez AL, Scheett TP, Kraemer WJ. An isoenergetic very low carbohydrate diet improves serum HDL cholesterol and triacylglycerol concentrations, the total cholesterol to HDL cholesterol ratio and postprandial lipemic responses compared with a low fat diet in normal weight, normolipidemic women. J Nutr. 2003;133:2756–61 [DOI] [PubMed] [Google Scholar]
- 38.Volek JS, Sharman MJ, Gomez AL, DiPasquale C, Roti M, Pumerantz A, Kraemer WJ. Comparison of a very low-carbohydrate and low-fat diet on fasting lipids, LDL subclasses, insulin resistance, and postprandial lipemic responses in overweight women. J Am Coll Nutr. 2004;23:177–84 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.