Abstract
l-Arginine, as a precursor of NO synthesis, has attracted much scientific attention in recent years. Experimental mouse models suggest that l-arginine supplementation can retard, halt, or even reverse atherogenesis. In human studies, supplementation with l-arginine improved endothelium-dependent vasodilation. However, l-arginine levels are best interpreted in the context of levels of asymmetric dimethylarginine (ADMA), a competitive inhibitor of NO synthase. Thus, reference limits for circulating l-arginine and the l-arginine:ADMA ratio may help to determine the nutritional state of individuals at high cardiovascular risk in light of increased ADMA levels. We defined reference limits for plasma l-arginine in 1141 people and for the l-arginine:ADMA ratio in 1138 relatively healthy individuals from the Framingham Offspring Cohort. Plasma l-arginine and ADMA concentrations were determined by using a stable isotope-based LC-MS/MS method. The reference limits (2.5th and 97.5th percentiles) for plasma l-arginine were 41.0 μmol/L (95% CI = 39.5–42.5 μmol/L) and 114 μmol/L (95% CI = 112–115 μmol/L), whereas corresponding reference limits (2.5th and 97.5th percentiles) for the l-arginine:ADMA ratio were 74.3 μmol/L (95% CI = 71.1–77.3 μmol/L) and 225 μmol/L (95% CI = 222–228 μmol/L). Plasma l-arginine was positively associated with the estimated glomerular filtration rate (eGFR) and blood glucose levels, whereas the l-arginine:ADMA ratio was positively associated with eGFR and diastolic blood pressure but inversely associated with homocysteine and (log)C-reactive protein. We report reference levels for plasma l-arginine and for the l-arginine:ADMA ratio that may be helpful for evaluation of the effects of l-arginine supplementation in participants with an impaired l-arginine/NO pathway.
Introduction
l-Arginine (2-amino-5-guanidino-pentanoic acid) is a conditionally essential amino acid that is a natural constituent of dietary proteins, with the relative amount of l-arginine ranging from 3 to 15% in various proteins. In addition to its role in protein anabolism, l-arginine is involved in various metabolic pathways, such as the synthesis of creatine, l-ornithine, l-glutamate, and polyamines (1), and it is the substrate for synthesis of NO, one of the most potent endogenous vasodilators (2). NO is mainly released by the endothelium to regulate vascular tone and modulate the interaction of circulating blood cells with the vascular wall. The synthesis of NO by the endothelial NO synthase results in multiple vasoprotective effects that have been summarized as antiatherogenic (3).
Intracellular l-arginine levels have been demonstrated to be considerably higher than those in the extracellular fluid or in plasma (3, 4). However, plasma l-arginine can be rapidly taken up by endothelial cells via the cellular y+ transporter for cationic amino acids and can directly contribute to NO production (5), suggesting that plasma l-arginine concentrations may influence endothelial NO production. Although a lack of l-arginine–derived NO formation may result in endothelial dysfunction, a pathophysiological finding that is common in patients with cardiovascular risk factors, reduced l-arginine concentrations in plasma have rarely been described (6). Mouse and human studies that used 15N-labeled l-arginine as a precursor demonstrate that the major part of dietary l-arginine is metabolized in the liver and utilized in the hepatic urea cycle (7). Only a small portion of dietary l-arginine is converted to NO (8).
Additionally, besides its function as a substrate for NO synthase, l-arginine plays an important role in glucose metabolism. In adipocytes, l-arginine enhances glycogen synthesis in response to insulin, an effect that is independent of NO generation (9). Oral l-arginine supplementation during 6 mo in a referral sample has been reported to improve glucose tolerance and enhance insulin sensitivity (10).
One common cause of impaired l-arginine/NO metabolism is the presence of elevated levels of ADMA11. ADMA displaces l-arginine from the substrate binding site of the NO synthase and thereby competitively inhibits this enzyme. ADMA is elevated in patients with renal failure and CVD and in advanced stages of diabetes mellitus (11–14).
In the present study, we determined reference limits for plasma l-arginine and the l-arginine:ADMA ratio, because these data may be of value for determining the nutritional state of participants at high cardiovascular risk due to their elevated ADMA levels. Such reference limits may also be useful for guiding nutraceutical interventions with l-arginine supplements. Accordingly, we measured plasma l-arginine and plasma ADMA concentrations in the Framingham Offspring Cohort and subsequently calculated the l-arginine:ADMA ratio. Plasma l-arginine and ADMA measurements were performed with a LC-MS/MS method that is characterized by high accuracy and precision (15, 16). Plasma ADMA reference levels from this cohort have been reported elsewhere (17).
Methods
Study sample.
The design and sampling strategy of the Framingham Offspring Study were previously described (18). In brief, in 1971 the Framingham Offspring Study began with the enrolment of 5124 participants who were the children of the original cohort or the spouses of these children. Of 3532 participants who attended the 6th examination cycle (1995 through 1998), we excluded participants with a serum creatinine level > 1.8 × 10−4 mol/L, those with missing l-arginine, and attendees with missing covariate data. From this sample consisting of 3320 participants, smokers and individuals with CVD, obesity, hypertension, and diabetes were excluded in a hierarchical fashion (Supplemental Fig. 1). After the exclusions, 1165 participants comprised the reference sample for the present investigation. To create reference limits, outliers [defined as values <Q1–1.5 × IQR or >Q3+1.5 × IQR according to Solberg and Lahti (19)] were removed so that the final reference sample size comprised 1141 participants for analysis of plasma l-arginine and 1138 for analysis of the l-arginine:ADMA ratio, respectively. Hypertension was defined as increased BP (SBP >140 mm Hg or DBP >90 mm Hg) or use of antihypertensive medication. All participants provided written informed consent and the study protocol was approved by the Institutional Review Board of the Boston University Medical Center and by the Ethics Committee at the Hamburg Board of Physicians.
Blood sample collection and analysis.
Laboratory assessment of several biomarkers was conducted on samples from fasting participants drawn at the 6th examination cycle; plasma/serum samples used for the present investigation were stored for ~8 y at –80°C without freeze–thaw cycles.
Phlebotomy was performed (typically between 0800 and 0900 h) on fasting participants. All participants were supine for ~5–10 min. The blood was immediately centrifuged and plasma/serum was separated and stored at −80°C until analysis. Serum high-sensitivity CRP was measured with an immunoprecipitation assay (20). Plasma homocysteine was analyzed by using HPLC with fluorometric detection (21). Serum blood lipids and creatinine were analyzed in the Framingham Heart Study laboratory with automated enzymatic assays (22).
Mass spectrometric determination of plasma l-arginine and ADMA was performed by using a fully validated, high throughput LC-MS/MS assay. The method details and stability of l-arginine and ADMA were previously described (15, 16, 23). In brief, plasma samples were analyzed using 96-well, 0.20-μm microfiltration plates precoated with 40 pmol of [2H6]-ADMA and 800 pmol of l-[2H7]- l-arginine (internal standards). After conversion to their butyl ester derivatives, analytes were analyzed on a Varian 1200L Triple Quadrupole MS (Varian) in the positive electrospray ionization mode. The sample run time was 1.6 min, with an intra- and inter-assay precision of 2.2 and 4.8% for l-arginine and 3.2% and 4.4% for ADMA, respectively.
Statistical analysis.
The SAS statistical software (SAS Institute) was used for statistical analyses. A 2-sided P value of <0.05 was considered significant. Data in the text are mean ± SD unless otherwise indicated. Associations between variables were assessed by Pearson correlation coefficients.
The calculation of the reference limits was done according to the recommendations of the International Federation of Clinical Chemistry as described elsewhere (24). Plasma l-arginine and ADMA levels were normally distributed in our sample and we analyzed these values as continuous dependent variables (without transformation). We constructed multivariable linear regression models with stepwise forward selection (significance criterion for entry of independent variables into the model, P < 0.1) using the following eligible covariates: age, sex, BMI, SBP, DBP total-:HDL cholesterol ratio, TG, eGFR, homocysteine, alcohol consumption, glucose, and (log)CRP. To calculate the eGFR, we used the Modification of Diet in Renal Disease equation [186.3 × (serum creatinine)−1.154 × age−0.203 × (0.742 for women)] (25).
Results
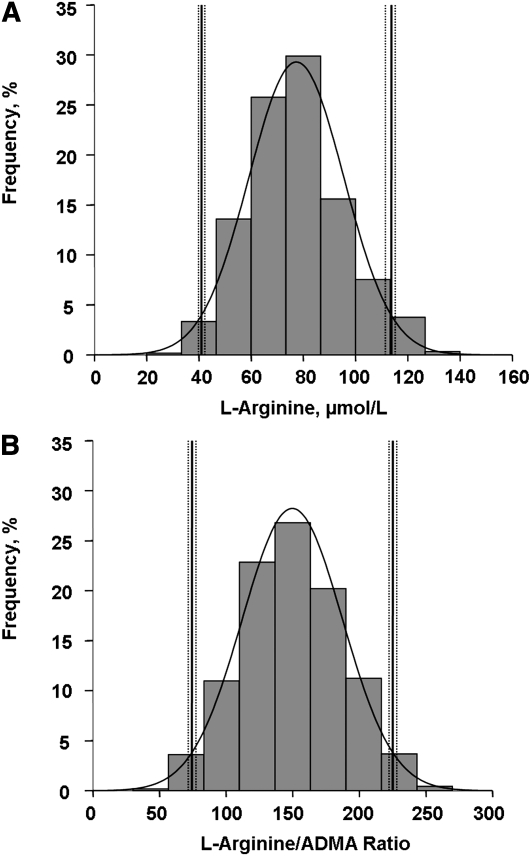
At baseline, the plasma l-arginine concentrations in the reference sample used for l-arginine analysis were 77.4 ± 18.2 μmol/L and the plasma ADMA concentrations were 0.53 ± 0.12 μmol/L (Table 1) . The l-arginine:ADMA ratio in the reference sample used for the l-arginine:ADMA ratio analysis was 150 ± 38. The reference limits (2.5th and 97.5th percentiles) for plasma l-arginine were 41.0 μmol/L (95% CI = 39.5–42.5 μmol/L) and 114 μmol/L (95% CI = 112–115 μmol/L) (Fig. 1A), whereas corresponding reference limits (2.5th and 97.5th percentiles) for the l-arginine:ADMA ratio were 74.3 μmol/L (95% CI = 71.1–77.3 μmol/L) and 225 μmol/L (95% CI = 222–228 μmol/L) (Fig. 1B).
TABLE 1.
Clinical characteristics of the reference samples used for calculating the reference levels of l-arginine and l-arginine:ADMA ratio in the Framingham Offspring Cohort1
| Reference sample for l-arginine | Reference sample for l-arginine:ADMA ratio | |
| n | 1141 | 1138 |
| Age, y | 56 ± 9 | 56 ± 9 |
| BMI, kg/m2 | 25.1 ± 2.7 | 25.1 ± 2.7 |
| SBP, mm Hg | 118 ± 12 | 118 ± 12 |
| DBP, mm Hg | 72 ± 8 | 72 ± 8 |
| Fasting glucose, mmol/L | 5.2 ± 0.4 | 5.2 ± 0.4 |
| LDL cholesterol, mmol/L | 3.3 ± 0.9 | 3.3 ± 0.9 |
| Total-:HDL cholesterol | 3.92 ± 1.29 | 3.93 ± 1.30 |
| TG, mmol/L | 1.3 ± 0.7 | 1.3 ± 0.7 |
| eGFR, mL/min | 88.3 ± 21.4 | 88.6 ± 25.4 |
| High sensitive CRP, mg/L | 1.20 (0.59–2.79) | 1.20 (0.60–2.80) |
| Total homocysteine, μmol/L | 8.96 ± 3.05 | 8.98 ± 3.06 |
Values are means ± SD or median (Q1–Q3). All analytes were measured in serum. ADMA, asymmetric dimethylarginine; CRP, C-reactive protein; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure.
FIGURE 1.
Distribution of plasma l-arginine concentration (A; n = 1141) and the l-arginine:ADMA ratio (B; n = 1138) from participants of the Framingham Offspring Cohort with reference intervals and integrated normal distribution lines. ADMA, asymmetric dimethylarginine.
The sex-specific 2.5th and 97.5th percentile reference limits for l-arginine were 42.0 μmol/L (95% CI = 39.5–44.2 μmol/L) and 113 μmol/L (95% CI = 111–116 μmol/L) in men and 40.8 μmol/L (95% CI = 38.8–42.6 μmol/L) and 113 μmol/L (95% CI = 111–115 μmol/L) in women. The sex-specific 2.5th and 97.5th percentile reference limits for the l-arginine:ADMA ratio were 72.0 (95% CI = 66.6–76.7 μmol/L) and 228 μmol/L (95% CI = 224–234 μmol/L) in men and 75.6 μmol/L (95% CI = 71.5–79.4 μmol/L) and 224 μmol/L (95% CI = 220–228 μmol/L) in women.
ADMA plasma concentrations were positively correlated with l-arginine (age- and sex-adjusted r = 0.31; P < 0.0001). A correlation between l-arginine and blood glucose was observed in the reference sample (unadjusted Pearson correlation r = 0.09; P = 0.002), Also, a correlation between l-arginine and eGFR was observed in the reference sample (unadjusted Pearson correlation r = 0.06; P < 0.05).
In the final multivariable regression model for l-arginine, glucose and eGFR were positively associated with l-arginine (Table 2), with other variables not meeting the P value of 0.05 required for denoting significance. The l-arginine:ADMA ratio was positively associated with eGFR and diastolic BP and inversely associated with homocysteine and (log)CRP (Table 3).
TABLE 2.
Multivariable regression analysis of plasma l-arginine concentrations in 1141 healthy individuals from the Framingham Offspring Cohort
| Variable1 | β ± SE2 | P value |
| Glomerular filtration rate | 0.05 ± 0.03 | 0.044 |
| Fasting glucose | 0.20 ± 0.06 | 0.002 |
Candidate variables that were considered for model entry were age, sex, BMI, SBP, DBP, the total-:HDL cholesterol ratio, alcohol consumption, TG, glomerular filtration rate, homocysteine, fasting glucose, and CRP. CRP, C-reactive protein; DBP, diastolic blood pressure; SBP, systolic blood pressure.
All regression coefficients (β estimates) represent the estimated mean change in l-arginine ( mol/L) per 1-unit increase in the corresponding covariate.
TABLE 3.
Multivariable regression analysis of the l-arginine:ADMA ratio in 1138 healthy individuals of the Framingham Offspring Cohort1
| Variable2 | β ± SE3 | P value |
| Homocysteine | −1.21 ± 0.37 | 0.001 |
| log CRP | −2.35 ± 1.02 | 0.022 |
| Glomerular filtration rate | 0.09 ± 0.04 | 0.035 |
| DBP | 0.31 ± 0.15 | 0.037 |
ADMA, asymmetric dimethylarginine; CRP, C-reactive protein; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Candidate variables that were considered for model entry were age, sex, BMI, SBP, DBP, the total:HDL ratio, alcohol consumption, TG, glomerular filtration rate, homocysteine, fasting glucose, and CRP.
All regression coefficients (β estimates) represent the estimated mean change in l-arginine:ADMA ratio per 1-unit increase in the corresponding covariate.
Discussion
The amino acid l-arginine has gained major scientific interest in recent years due to its multi-faceted roles in metabolism, among which the most prominent is its role as a precursor of NO synthesis. In this respect, not only l-arginine plasma concentrations per se but also its levels relative to its competitive antagonist, ADMA, are important; thus, the l-arginine:ADMA ratio has been inversely associated with all-cause mortality in a prospective cohort study (26). We report here reference limits for plasma l-arginine and for the l-arginine:ADMA ratio from a healthy reference sample of participants from the Framingham Offspring cohort. These reference limits were similar for men and women.
In most clinical conditions associated with endothelial dysfunction, circulating l-arginine concentrations have been observed to be within the usual normal range. Yet, it is intriguing that short-term dietary supplementation with oral l-arginine restores vascular function and improves clinical symptoms of diseases associated with vascular dysfunction [examples include angina pectoris (27), hypertension (28) erectile dysfunction (29, 30), congestive heart failure (31), and pulmonary arterial hypertension (32)]. Many of these conditions are associated with elevated circulating ADMA levels and also a lower l-arginine ADMA ratio (28, 33). Thus, an imbalance between l-arginine and ADMA can result in relative l-arginine deficiency. Our data therefore extend previous data on circulating l-arginine reference levels (34), because we also report reference intervals for the l-arginine:ADMA ratio in a healthy, community-based sample. Our data may help to better understand the roles of l-arginine and ADMA in the pathophysiology of various cardiovascular and metabolic conditions and also facilitate the interpretation of some of the inconsistent results of recent studies evaluating dietary supplementation with l-arginine.
Our reference sample was chosen to be free of CVD, hypertension, diabetes, obesity, and smoking, and participants with higher serum creatinine concentrations were also excluded. Therefore, we were unable to assess the influence of variables such as BP, serum lipids, BMI, and renal function on plasma l-arginine levels across their broad range of values.
We observed a mean decrease in the l-arginine plasma concentration of 0.05 μmol/L per 1 mL/min decline of eGFR. This may not necessarily point toward an association of l-arginine plasma concentration with glomerular filtration per se, because it is well known from experimental studies that the kidney releases more l-arginine into plasma than it extracts (35, 36), suggesting that renal l-arginine synthesis outweighs l-arginine consumption. Thus, renal diseases leading to reductions in eGFR may result in diminished l-arginine production, independent of the age-related decline in eGFR itself. These data are in line with the observation in clinical studies that patients with end-stage renal disease undergoing chronic hemodialysis treatment have significantly lower plasma l-arginine concentrations than participants without renal disease (37).
We also observed a mean increase of l-arginine plasma concentration of 0.20 μmol/L per 0.6 mmol/L increase of fasting blood glucose. There is growing evidence that dietary supplementation with l-arginine reduces plasma levels of glucose, fatty acids, and TG and improves insulin sensitivity in various mouse and rat models of diabetes and obesity (38). Similar results have been reported for obese humans with type II diabetes receiving l-arginine supplementation (10, 39).
The findings of a positive association of the l-arginine:ADMA ratio with DBP and the inverse association with total homocysteine are consistent with our findings for plasma ADMA in the same study population and may thus be mainly driven by the associations of ADMA with these variables (17).
The inverse relation of the l-arginine:ADMA ratio and plasma homocysteine may be partly explained by a redox-mediated inhibition of DDAH, the enzyme degrading ADMA, by homocysteine (40). DDAH is known to be redox sensitive and homocysteine might modify redox status and the protein stability of DDAH, resulting in decreased DDAH activity (41). Böger et al. (41) also reported a metabolic link between the homocysteine and ADMA pathways at the level of protein arginine methylation, which might also contribute to this inverse association.
l-Arginine has been studied in numerous clinical trials involving various patient groups with respect to its ability to improve endothelium-dependent, NO-mediated vasodilation. Whereas some of the early, smaller studies showed improved endothelial function after l-arginine supplementation (42, 43), 2 larger and more recent studies showed presumed deleterious effects of l-arginine on vascular function (44, 45). It is noteworthy, however, that in both of these studies patients were not selected for relative or absolute l-arginine deficiency. The results of these studies have generated considerable debate. Whether l-arginine may have more pronounced beneficial effects in patients with a low l-arginine:ADMA ratio is the focus of ongoing clinical trials. The normal ranges presented here may help to define patient selection in future clinical trials and may facilitate interpretation of data from such trials.
Strengths and limitations.
The large, community-based reference sample and the quantification of l-arginine plasma levels by a validated LC-MS/MS method are strengths of our investigation. However, we acknowledge several limitations. We evaluated a community-based reference sample of white, middle-aged individuals of European descent. Caution must be exercised in extrapolating these results to other populations of a different ethnicity or with a different age range. Also, no causal inferences can be drawn from the associations observed in our cross-sectional epidemiological investigation.
In conclusion, we report reference ranges for both plasma l-arginine and the l-arginine:ADMA ratio, which may reflect NOS substrate availability more closely than plasma l-arginine concentrations alone. These reference limits may facilitate the design of better clinical trials of l-arginine supplementation in those with relative deficiency of this amino acid as reflected by the l-arginine:ADMA ratio.
Supplementary Material
Acknowledgments
The authors thank Mariola Kastner and Anna Steenpaß for their excellent technical assistance. L.M.S., R.S.V., R.H.B., E.S., and R.M. designed research; E.S., N.L., U.R., N.L.G., and M.A. conducted research; V.X. and N.L. analyzed data; N.L. and R.H.B. wrote the paper; and N.L., R.H.B., and R.S.V. had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Supported by the NIH/National Heart, Lung, and Blood Institute contract N01-HC-25195 (R.S.V.), and by the Deutsche Forschungsgemeinschaft grant Bo 1431/4-1 (R.H.B.).
Supplemental Figure 1 is available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at jn.nutrition.org.
Abbreviations used: ADMA, asymmetric dimethylarginine; BP, blood pressure; CRP, C-reactive protein; CVD, cardiovascular disease; DBP, diastolic blood pressure; DDAH, dimethylarginine dimethylaminohydrolase; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure.
Literature Cited
- 1.Wu G, Morris SM., Jr Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmer RM, Rees DD, Ashton DS, Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988;153:1251–6 [DOI] [PubMed] [Google Scholar]
- 3.Napoli C, Ignarro LJ. Nitric oxide and atherosclerosis. Nitric Oxide. 2001;5:88–97 [DOI] [PubMed] [Google Scholar]
- 4.Böger RH, Sydow K, Borlak J, Thum T, Lenzen H, Schubert B, Tsikas D, Bode-Böger SM. LDL cholesterol upregulates synthesis of asymmetrical dimethylarginine in human endothelial cells: involvement of S-adenosylmethionine-dependent methyltransferases. Circ Res. 2000;87:99–105 [DOI] [PubMed] [Google Scholar]
- 5.Schmidt HH, Nau H, Wittfoht W, Gerlach J, Prescher KE, Klein MM, Niroomand F, Bohme E. Arginine is a physiological precursor of endothelium-derived nitric oxide. Eur J Pharmacol. 1988;154:213–6 [DOI] [PubMed] [Google Scholar]
- 6.Jeserich M, Münzel T, Just H, Drexler H. Reduced plasma L-arginine in hypercholesterolaemia. Lancet. 1992;339:561. [DOI] [PubMed] [Google Scholar]
- 7.Maas R, Schwedhelm E, Kahl L, Li H, Benndorf R, Lüneburg N, Förstermann U, Böger RH. Simultaneous assessment of endothelial function, nitric oxide synthase activity, nitric oxide-mediated signaling, and oxidative stress in individuals with and without hypercholesterolemia. Clin Chem. 2008;54:292–300 [DOI] [PubMed] [Google Scholar]
- 8.Böger RH, Tsikas D, Bode-Böger SM, Phivthong-Ngam L, Schwedhelm E, Frölich JC. Hypercholesterolemia impairs basal nitric oxide synthase turnover rate: a study investigating the conversion of L-[guanidino-(15)N(2)]-arginine to (15)N-labeled nitrate by gas chromatography-mass spectrometry. Nitric Oxide. 2004;11:1–8 [DOI] [PubMed] [Google Scholar]
- 9.Egan JM, Henderson TE, Bernier M. Arginine enhances glycogen synthesis in response to insulin in 3T3–L1 adipocytes. Am J Physiol. 1995;269:E61–6 [DOI] [PubMed] [Google Scholar]
- 10.Lucotti P, Monti L, Setola E, La Canna G, Castiglioni A, Rossodivita A, Pala MG, Formica F, Paolini G, Catapano AL, et al. Oral l-arginine supplementation improves endothelial function and ameliorates insulin sensitivity and inflammation in cardiopathic nondiabetic patients after an aortocoronary bypass. Metabolism. 2009;58:1270–6 [DOI] [PubMed] [Google Scholar]
- 11.Böger RH. The emerging role of asymmetric dimethylarginine as a novel cardiovascular risk factor. Cardiovasc Res. 2003;59:824–33 [DOI] [PubMed] [Google Scholar]
- 12.Böger RH, Cooke JP, Vallance P. ADMA: an emerging cardiovascular risk factor. Vasc Med. 2005;10 Suppl 1:S1–2 [DOI] [PubMed] [Google Scholar]
- 13.Siroen MP, Teerlink T, Nijveldt RJ, Prins HA, Richir MC, Leeuwen PA. The clinical significance of asymmetric dimethylarginine. Annu Rev Nutr. 2006;26:203–28 [DOI] [PubMed] [Google Scholar]
- 14.Vallance P, Leone A, Calver A, Collier J, Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339:572–5 [DOI] [PubMed] [Google Scholar]
- 15.Schwedhelm E, Maas R, Tan-Andresen J, Schulze F, Riederer U, Boger RH. High-throughput liquid chromatographic-tandem mass spectrometric determination of arginine and dimethylated arginine derivatives in human and mouse plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;851:211–9 [DOI] [PubMed] [Google Scholar]
- 16.Schwedhelm E, Tan-Andresen J, Maas R, Riederer U, Schulze F, Boger RH. Liquid chromatography-tandem mass spectrometry method for the analysis of asymmetric dimethylarginine in human plasma. Clin Chem. 2005;51:1268–71 [DOI] [PubMed] [Google Scholar]
- 17.Schwedhelm E, Xanthakis V, Maas R, Sullivan LM, Schulze F, Riederer U, Benndorf RA, Boger RH, Vasan RS. Asymmetric dimethylarginine reference intervals determined with liquid chromatography-tandem mass spectrometry: results from the Framingham Offspring Cohort. Clin Chem. 2009;55:1539–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–90 [DOI] [PubMed] [Google Scholar]
- 19.Solberg HE, Lahti A. Detection of outliers in reference distributions: performance of Horn's algorithm. Clin Chem. 2005;51:2326–32 [DOI] [PubMed] [Google Scholar]
- 20.Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, Sawyer DB, Levy D, Wilson PWF, D’Agostino RB. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction. Circulation. 2003;107:1486–91 [DOI] [PubMed] [Google Scholar]
- 21.Vasan RS, Beiser A, D'Agostino RB, Levy D, Selhub J, Jacques PF, Rosenberg IH, Wilson PWF. Plasma homocysteine and risk for congestive heart failure in adults without prior myocardial infarction. JAMA. 2003;289:1251–7 [DOI] [PubMed] [Google Scholar]
- 22.Wilson PWF, Christiansen JC, Anderson KM, Kannel WB. Impact of national guidelines for cholesterol risk factor screening. JAMA. 1989;262:41–4 [PubMed] [Google Scholar]
- 23.Shin S, Fung S-M, Mohan S, Fung H-L. Simultaneous bioanalysis of l-arginine, l-citrulline, and dimethylarginines by LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:467–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fallang A, Larsen S, Horsberg TE. Upper susceptibility threshold limits with confidence intervals: a method to identify normal and abnormal population values for laboratory toxicological parameters, based on acetylcholinesterase activities in sea lice. Pest Manag Sci. 2006;62:208–13 [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Greene T, Schluchter M, Cleary P, Teschan P, Lorenz R, Molitch M, Mitch W, Siebert C, Hall P. Glomerular filtration rate measurements in clinical trials. Modification of Diet in Renal Disease Study Group and the Diabetes Control and Complications Trial Research Group. J Am Soc Nephrol. 1993;4:1159–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Böger RH, Sullivan LM, Schwedhelm E, Wang TJ, Maas R, Benjamin EJ, Schulze F, Xanthakis V, Benndorf RA, Vasan RS. Plasma asymmetric dimethylarginine and incidence of cardiovascular disease and death in the community. Circulation. 2009;119:1592–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker HA, McGing E, Fisher I, Boger RH, Bode-Boger SM, Jackson G, Ritter JM, Chowienczyk PJ. Endothelium-dependent vasodilation is independent of the plasma L-arginine/ADMA ratio in men with stable angina: lack of effect of oral L-arginine on endothelial function, oxidative stress and exercise performance. J Am Coll Cardiol. 2001;38:499–505 [DOI] [PubMed] [Google Scholar]
- 28.Maas R. Pharmacotherapies and their influence on asymmetric dimethylargine (ADMA). Vasc Med. 2005;10 Suppl 1:S49–57 [DOI] [PubMed] [Google Scholar]
- 29.Maas R, Schwedhelm E, Albsmeier J, Boger RH. The pathophysiology of erectile dysfunction related to endothelial dysfunction and mediators of vascular function. Vasc Med. 2002;7:213–25 [DOI] [PubMed] [Google Scholar]
- 30.Maas R, Wenske S, Zabel M, Ventura R, Schwedhelm E, Steenpass A, Klemm H, Noldus J, Boger RH. Elevation of asymmetrical dimethylarginine (ADMA) and coronary artery disease in men with erectile dysfunction. Eur Urol. 2005;48:1004–11, discussion 1011–2 [DOI] [PubMed] [Google Scholar]
- 31.Doutreleau S, Mettauer B, Piquard F, Rouyer O, Schaefer A, Lonsdorfer J, Geny B. Chronic L-arginine supplementation enhances endurance exercise tolerance in heart failure patients. Int J Sports Med. 2006;27:567–72 [DOI] [PubMed] [Google Scholar]
- 32.Howell K, Costello CM, Sands M, Dooley I, McLoughlin P. L-Arginine promotes angiogenesis in the chronically hypoxic lung: a novel mechanism ameliorating pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2009;296:L1042–50 [DOI] [PubMed] [Google Scholar]
- 33.Böger RH, Maas R, Schulze F, Schwedhelm E. Asymmetric dimethylarginine (ADMA) as a prospective marker of cardiovascular disease and mortality: an update on patient populations with a wide range of cardiovascular risk. Pharmacol Res. 2009;60:481. [DOI] [PubMed] [Google Scholar]
- 34.Meinitzer A, Seelhorst U, Wellnitz B, Halwachs-Baumann G, Boehm BO, Winkelmann BR, Marz W. Asymmetrical dimethylarginine independently predicts total and cardiovascular mortality in individuals with angiographic coronary artery disease (the Ludwigshafen Risk and Cardiovascular Health study). Clin Chem. 2007;53:273–83 [DOI] [PubMed] [Google Scholar]
- 35.Böger RH. The pharmacodynamics of L-arginine. J Nutr. 2007;137:S1650–5 [DOI] [PubMed] [Google Scholar]
- 36.Böger RH. L-Arginine therapy in cardiovascular pathologies: beneficial or dangerous? Curr Opin Clin Nutr Metab Care. 2008;11:55–61 [DOI] [PubMed] [Google Scholar]
- 37.Kalousova M, Kielstein JT, Hodkova M, Zima T, Dusilova-Sulkova S, Martens-Lobenhoffer J, Bode-Boger SM. No benefit of hemodiafiltration over hemodialysis in lowering elevated levels of asymmetric dimethylarginine in ESRD patients. Blood Purif. 2006;24:439–44 [DOI] [PubMed] [Google Scholar]
- 38.Wu G, Bazer F, Davis T, Kim S, Li P, Marc Rhoads J, Carey Satterfield M, Smith S, Spencer T, Yin Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids. 2009;37:153–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wascher TC, Graier WF, Dittrich P, Hussain MA, Bahadori B, Wallner S, Toplak H. Effects of low-dose L-arginine on insulin-mediated vasodilatation and insulin sensitivity. Eur J Clin Invest. 1997;27:690–5 [DOI] [PubMed] [Google Scholar]
- 40.Stühlinger MC, Tsao PS, Her JH, Kimoto M, Balint RF, Cooke JP. Homocysteine impairs the nitric oxide synthase pathway: role of asymmetric dimethylarginine. Circulation. 2001;104:2569–75 [DOI] [PubMed] [Google Scholar]
- 41.Böger RH, Bode-Böger SM, Sydow K, Heistad DD, Lentz SR. Plasma concentration of asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, is elevated in monkeys with hyperhomocyst(e)inemia or hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2000;20:1557–64 [DOI] [PubMed] [Google Scholar]
- 42.Böger RH, Ron ES. L-Arginine improves vascular function by overcoming deleterious effects of ADMA, a novel cardiovascular risk factor. Altern Med Rev. 2005;10:14–23 [PubMed] [Google Scholar]
- 43.Schwedhelm E, Maas R, Freese R, Jung D, Lukacs Z, Jambrecina A, Spickler W, Schulze F, Boger RH. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: impact on nitric oxide metabolism. Br J Clin Pharmacol. 2008;65:51–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schulman SP, Becker LC, Kass DA, Champion HC, Terrin ML, Forman S, Ernst KV, Kelemen MD, Townsend SN, Capriotti A, et al. l-Arginine therapy in acute myocardial infarction. JAMA. 2006;295:58–64 [DOI] [PubMed] [Google Scholar]
- 45.Wilson AM, Harada R, Nair N, Balasubramanian N, Cooke JP. L-Arginine supplementation in peripheral arterial disease: no benefit and possible harm. Circulation. 2007;116:188–95 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.