Abstract
Acetaminophen (N-acetyl-p-aminophenol, paracetamol [APAP])-induced acute liver failure is the most common cause of acute liver failure in adults. In children, APAP accounts for 25% of all cases of acute liver failure. The high mortality rate associated with this preventable condition makes it vital that paediatricians are aware of the potential adverse effects associated with this widely used drug. While APAP is generally considered to be safe when used as directed, its inclusion in multiple over-the-counter medications, as well as in prescription drugs, mandates that physicians promote and educate the general public about the proper use of acetaminophen in children.
Keywords: Adducts, Children, Hepatitis, Liver, Safety
Abstract
Chez les adultes, l’insuffisance hépatique aiguë est induite principalement par l’acétaminophène (N-acétyl-p-aminophénol, paracétamol [APAP]). Chez les enfants, l’APAP est responsable de 25 % de tous les cas d’insuffisance hépatique aiguë. En raison du fort taux de mortalité associé à cette maladie évitable, il est essentiel que les pédiatres connaissent les effets indésirables qui peuvent s’associer à ce médicament largement utilisé. Bien que l’APAP soit généralement considéré comme sécuritaire lorsqu’il est utilisé conformément aux directives, son inclusion dans de multiples médicaments en vente libre et sur ordonnance oblige les médecins à informer et éduquer le grand public quant au bon usage de l’acétaminophène chez les enfants.
Acetaminophen (N-acetyl-p-aminophenol, paracetamol [APAP]) is the most widely used drug for the treatment of pain and fever experienced by children around the world. While APAP is generally considered to be safe and effective in doses recommended by the manufacturer, concerns have arisen over the past decade as APAP has been increasingly recognized as a major cause of acute liver failure in adults in the United States (US) (1). While APAP is also an important cause of acute liver failure in children, it plays a relatively smaller role in the etiology of acute liver failure from a global standpoint (2). Recent reports have revealed that a significant number of adults develop elevations in hepatic transaminase levels while receiving recommended doses of APAP in a controlled research setting (3). Similar data are not available in children. The present review addresses data that have contributed to the growing concern about APAP use in children, and highlights knowledge gaps in our understanding of APAP use and safety in children.
APAP AVAILABILITY AND THE US FOOD AND DRUG ADMINISTRATION
APAP is a major component of the paediatric formulary. It is widely used in inpatient and outpatient settings for the treatment of mild pain and fever. Its widespread use developed, in large part, after the reported development of Reye’s syndrome following the use of acetylsalicylic acid in children in the 1970s (4). APAP first became available in the US in 1955 and in the United Kingdom in 1956 (5). It is available in multiple formulations including tablets, suspensions and rectal suppositories. Intravenous (IV) paracetamol (Ofirmev, Cadence Pharmaceuticals Inc, USA) was approved by the US Food and Drug Administration in January 2011 for short-term use in relieving mild to moderate pain. An approved use of IV APAP is in children two years of age and older for the treatment of pain at a dose of 15 mg/kg every 6 h, not to exceed 60 mg/kg/day. The drug represents an attractive alternative to IV opioids and nonsteroidal anti-inflammatory drugs.
With widespread availability of any drug, misuse of the drug is more likely. The clinical significance of availability and safety is a function of the therapeutic index (also known as the therapeutic ratio), which is defined as the ratio of the amount of drug that causes a therapeutic effect to the amount that causes death. While this issue may have limited clinical significance or safety implications for drugs in which the therapeutic index is high, for drugs that have widespread availability and a low therapeutic index such as APAP, possible dosing errors present greater safety concerns (6). A toxic dose for single acute ingestions of APAP in adults is traditionally defined as 150 mg/kg or 10 g (7,8). This definition is generally used in children as well, although more recent data based on case reports and case series in children and pharmacokinetic modelling (9,10) suggest that a higher dose definition of toxicity for children with acute overdoses may be appropriate. The recommended daily APAP dose for children is generally accepted to be 75 mg/kg/day (15 mg/kg/dose, not to exceed five doses in 24 h). A dose of 90 mg/kg/day is considered by many authorities to be the definition of ‘supra-therapeutic dosing’ (11,12). The restriction of IV APAP to hospital settings will likely result in relatively lower overall liability for this drug compared with oral APAP. Recognition and prevention of 10-fold dose errors with the IV formulation – a recognized and common scenario for paediatric-related hospital medication errors – can help assure that the product has a favourable safety profile in the hospital setting.
Many over-the-counter cough and cold remedies containing antihistamines and decongestants also include APAP. Failure to appreciate that APAP is included in these over-the-counter remedies may result in excessive APAP dosing and inadvertent toxicity when single-ingredient APAP is used with combination formulations containing APAP. A US Food and Drug Administration advisory meeting, held in June 2009, reviewed issues related to the increased regulation of over-the-counter combination APAP products, prescription combination (APAP-opioid) products, limitations to the amount of APAP contained in prescription combination products, and product confusion among paediatric liquid formulations of APAP (13). As a result of this meeting, new US regulations will include restrictions on the amount of APAP contained in APAP-opioid combination products, and standardization of paediatric liquid APAP formulations to a single concentration (160 mg/5 mL).
APAP OVERDOSE: CHILDREN VERSUS ADULTS
Historically, children have been considered to be at lower risk for the development of toxicity following acute overdoses of APAP compared with adults. Data from US poison control centres support the association of greater morbidity and mortality rates with APAP poisoning cases in adults than in children (14). In the acute overdose setting, it is believed that paediatric APAP poisonings are less severe than adult APAP poisonings. Presumed reasons for this relative paediatric resistance to APAP toxicity include a higher likelihood of emesis following ingestion, higher glutathione turnover rates in children than in adults, different rates of oxidative metabolism and relatively higher sulfation capacity in children than in adults. However, little data support these assumptions, and one of the greatest factors associated with this observed relative protection may be related to earlier recognition and treatment in children than in adults. Ingestion of liquid APAP formulations in children are common and, in general, single overdose ingestions with liquid formulations are not associated with toxicity (4,9).
The standard treatment for APAP overdose is dependent on recognition of the overdose by history and clinical laboratory data. Elevation of APAP levels in blood, plotted as a function of the time lapse since the APAP ingestion (ie, the Rumack nomogram), is the standard toxicity assessment tool used in hospital emergency departments. Antidotal treatment with N-acetylcysteine (NAC), using either the oral or the IV formulation, is initiated for patients deemed to be at risk according to the Rumack nomogram. While no controlled prospective clinical trial has compared the efficacy of the two NAC formulations, a recent publication compared the efficacy of the 20 h IV protocol in a Canadian database with the 72 h oral protocol in a US database (15). Overall, the efficacy of the two formulations/protocols was comparable, but differences in the relative risk of toxicity were related to the time of initiation of treatment with NAC. The relative risk of hepatotoxicity was lower in patients who received IV NAC when the treatment was started within 12 h of the ingestion. In contrast, the relative risk of hepatotoxicity was lower in patients who received oral NAC when treatment was started 18 h after the overdose.
In the acute liver failure literature, APAP accounts for approximately 51% of all acute cases in adults (1), and 14% of cases in children (2). The results of a population-based study representing 3.3 million patients in metropolitan Atlanta, Georgia (USA), suggest that APAP-related acute liver failure in children (three days to 14 years of age) may be responsible for 25% of all cases, and all cases in this age group were deemed to be unintentional in nature (16). In contrast, data generated in Canadian hospitals suggest that APAP-related hospitalizations decreased over the period between 1995 and 2004 (17). APAP-induced acute liver failure has been reported to have a mortality rate ranging from 20% to 50% (8,18). Approximately one-half of APAP-induced acute liver failure in adults is related to unintentional overdoses. Concurrent use of two APAP formulations and the use of prescription APAP-opioid combination products (eg, Vicodin [Abbott Laboratories, USA], APAP/hydrocodone), are common covariates in the subset of unintentional APAP overdoses (1). Covariates of APAP toxicity, such as alcoholism and concurrent use of APAP with opioids, are less significant issues in children than in adults (1). The predominance of unintentional ingestions in APAP-related cases of acute liver failure emphasizes the ongoing need to educate the community about the potential danger of misuse of APAP through misdosing and use of multiple APAP-containing drugs.
SAFETY OF APAP AT RECOMMENDED DOSES
Recent reports from the adult literature indicate that doses of APAP recommended for use by the manufacturer are associated with elevation of hepatic transaminase levels in a significant proportion of the population. Watkins et al (3) reported that 39% of healthy adult volunteers who received APAP at 4 g/day for 14 days had elevations of alanine aminotransferase (ALT) that were threefold above the upper limit of normal. Similar studies have not been conducted in children. A recent survey by Lavonas et al (11) summarized the available literature examining the use of APAP at recommended doses in children. In this survey, the authors reviewed published studies of children who received APAP as repeated therapeutic dosing (<75 mg/kg/day) for a minimum of 24 h. From this review of 62 studies involving 32,414 children, the authors reported that none of the children exhibited signs or symptoms of liver disease, required treatment with the antidote NAC or liver transplantation, or died. Limitations of the study design include the lack of standardized assessment of hepatic transaminases in the majority of the studies covered in this review. It is well known that elevation of hepatic transaminase levels may occur without the development of overt clinical signs and symptoms of liver injury. An additional review of published case reports by Lavonas et al (11) identified 22 cases of children who developed liver injury in association with the use of therapeutic doses of APAP; for nine of these cases, the association between APAP and the liver injury was deemed ‘probable’ according to the Naranjo scale.
METABOLISM AND TOXICITY OF APAP
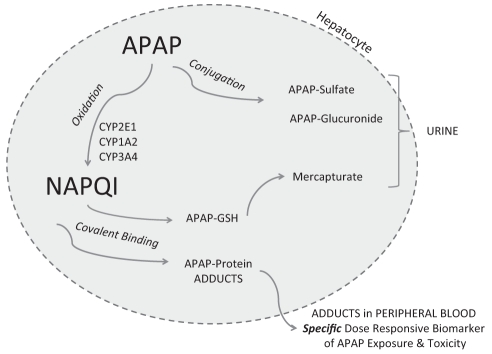
APAP is the demethylated derivative of the analgesic phenacetin, which was withdrawn from the pharmaceutical market in 1983 because of carcinogenic and nephrotoxicity concerns (19). Mechanistic studies to understand the hepatotoxicity of APAP were initiated by the US National Institutes of Health in the 1970s after the publication of case reports of hepatotoxicity occurring in adults following intentional APAP poisonings (5). The mechanism of APAP toxicity is a consequence of drug metabolism and has been the subject of numerous comprehensive reviews (20,21). Mitchell et al (22) and Gillette et al (23) demonstrated that toxicity was associated with the depletion of hepatic glutathione. Following a toxic dose of APAP, the conjugation pathways of metabolism become saturated and an increased proportion of the parent compound is oxidized by the cytochrome (CYP) P450 system, forming the toxic reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI; Figure 1). From a clinical standpoint, the most important CYP P450 isoform is CYP2E1 and, to a lesser degree, CYP1A2 and CYP3A4. While NAPQI is normally detoxified by glutathione, in APAP overdose, glutathione is depleted and NAPQI binds to the amino acid cysteine on hepatic proteins as 3-(cystein-S-yl) APAP. Subsequent to their formation in the liver, APAP protein adducts are released into the blood when hepatocytes rupture as a result of necrosis. Multiple studies conducted in a mouse model of APAP toxicity demonstrated the dose dependency of this covalent binding of NAPQI to hepatic proteins, and the relationship between adduct formation and subsequent hepatocyte lysis (24–26).
Figure 1).
Role of metabolism in acetaminophen toxicity. APAP N-acetyl-p-aminophenol, paracetamol (acetaminophen); GSH Glutathione; NAPQI N-acetyl-p-benzoquinone imine
Clinical studies using a highly sensitive and specific high-performance liquid chromatography with electrochemical detection (HPLC-EC) assay for the quantitation of APAP-cysteine (APAP-CYS) derived from APAP protein adducts showed that high levels of adducts were present in the blood of patients from well-characterized cases of APAP toxicity resulting in acute liver failure (27,28). In addition, determination of APAP-protein adducts using HPLC-EC accurately distinguished between known APAP cases and cases of acute liver failure of other known etiologies (27,29). Correlation analysis between peak adduct levels and determinations of peak ALT demonstrated a correlation between these two parameters (30). Adducts were found to have a long elimination half-life and can be detected in peripheral blood for up to 10 days following APAP overdose. In addition, high adduct levels (>1.0 nmol APAP-CYS/mL sera) were detected in 19% of adults with acute liver failure of indeterminate etiology, indicating probable APAP etiology (27,31). In acute poisoning cases involving children, it was shown that the time to treatment with NAC influenced the magnitude of adduct levels in peripheral blood (32). Adduct levels were higher in children who received delayed treatment with NAC, compared with children who were treated within 10 h of APAP overdose. Thus, these clinical and translational studies demonstrated that APAP protein adducts, which are mechanistic biomarkers of APAP toxicity, can identify cases of APAP toxicity.
Additional studies have been conducted to determine the possible presence of APAP protein adducts in patients receiving standard therapeutic doses of APAP. Low adduct levels (ie, <0.6 nmol APAP-CYS/mL sera) were detected in adults receiving APAP 1 g every 6 h; no adducts were detected in placebo-treated patients (33). Similar adduct levels were detected in alcoholic individuals who received standard doses of APAP over a 10-day period (31). The clinical significance of low adduct levels in patients receiving standard doses of APAP is uncertain, but one interpretation of the data is that the presence of APAP protein adducts in peripheral blood is similar to the presence of elevated hepatic transaminase levels – an indication of the release of cellular contents from dying hepatocytes. In this context, APAP adducts can be considered to be an APAP-specific indicator of liver toxicity, in contrast to the non-specific indicator ALT. This interpretation is consistent with the time course and appearance of adducts in peripheral blood in relation to hepatocellular lysis (25). While the correlation between adducts and ALT levels in the low-dose exposure studies is relatively low (31), the data may reflect different kinetic profiles between ALT and adduct release at low-dose (ie, therapeutic) exposures. Alternatively, the data may reflect differences in the sensitivity and precision of the analytical HPLC-EC assay (29), which measures a specific compound, and the standard ALT assay performed by hospital clinical laboratories, which measures enzyme activity. Another factor is that changes in ALT levels are superimposed on baseline levels, whereas APAP-protein adducts are toxicity specific and, thus, are absent before APAP exposure.
NEW APPROACHES TO STUDY DRUG TOXICITY
The role of glutathione depletion in the development of APAP toxicity has been well characterized in a mouse model of APAP toxicity (34). Until recently, relatively noninvasive analytical approaches were not available to enable an accurate assessment of glutathione status in patients. With the development of new analytical approaches, such as sensitive online nuclear magnetic resonance spectroscopy and ultra-pressure liquid chromatography-mass spectrometry, it is now possible to study the relationship of glutathione synthesis and turnover (35) to identify potential mechanisms of susceptibility for individual patients and ‘at-risk’ groups. ‘Pharmacometabolomics’, defined as the contribution of the individual’s metabolic capacity to the subsequent drug response (36), represents a new science that has emerged as a result of recent advances in analytical technology. Ongoing studies of APAP in both preclinical models and human subjects will generate new data regarding the relationship between antioxidant reserves and other pathways of endogenous metabolism that may influence the development of toxicity (37,38).
New data generated in experimental models of APAP toxicity have highlighted future potential avenues for clinical and translational research that may help to clarify our understanding regarding the overall safety of APAP in the general population, including in children. Potential risk factors that are frequently cited, but have not been addressed in clinical studies, include the potential impact of nutrition, fasting states, pharmacogenetics and glutathione depletion on possible susceptibility to APAP toxicity (39). In addition, data generated in the mouse model of APAP toxicity have emphasized the contribution of reactive oxygen and nitrogen species, mitochondrial injury and the inflammatory response as mechanisms important in the development of toxicity. (40,41). Few studies have systematically examined the role of these mechanisms as determinants of toxicity in humans.
USE OF APAP AND THE ROLE OF THE PAEDIATRICIAN
Paediatricians and practitioners caring for children must continue to educate parents and caregivers about the safe and proper use of APAP. Education should emphasize the responsibility of parents and caregivers to monitor over-the-counter and prescription drugs for APAP content. Adherence to paediatric-specific formulations and dosing devices is another key point for education. The available data suggest that short-term use of APAP for pain and fever is safe for the vast majority of patients. Additional data are required to delineate the pharmacokinetics of APAP in certain subpopulations of children. For example, little research has examined the disposition of APAP in children with pre-existing liver disease in which the use of nonsteroidal anti-inflammatory drugs may be contraindicated for the treatment of pain and fever (eg, bleeding). Similarly, little data are available to guide dosing of APAP in premature infants (<32 weeks’ gestation) who may benefit from the use of APAP as a nonopioid approach to the treatment of pain.
In addition, hospital pharmacies and medication safety programs must respond to the widespread availability of APAP by directed monitoring of APAP prescribing and administration. For physicians, a review of safe APAP dose and drug administration practices should be a component of each fever-related ‘sick visit’ involving a child. Ongoing education of parents, caregivers and educators about the beneficial aspects of fever as a component of the body’s response to infection is also critical (12). Responsible advertising and clear product labelling by pharmaceutical companies may also help to combat the issue of ‘fever phobia’ that persists in the lay community. Education of families about the potential for overdosing with the use of multiple APAP-containing medications is another important component of the treatment plan for children with febrile illnesses.
Acknowledgments
Grant support from the National Institutes of Health (DK 81406; DK79387) is gratefully acknowledged.
Footnotes
DISCLOSURE: Drs James and Roberts have a patent application pending for the measurement of APAP protein adducts in human blood samples.
REFERENCES
- 1.Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: Results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–72. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 2.Squires RH, Jr, Shneider BL, Bucuvalas J, et al. Acute liver failure in children: The first 348 patients in the pediatric acute liver failure study group. J Pediatr. 2006;148:652–8. doi: 10.1016/j.jpeds.2005.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watkins PB, Kaplowitz N, Slattery JT, et al. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily: A randomized controlled trial. JAMA. 2006;296:87–93. doi: 10.1001/jama.296.1.87. [DOI] [PubMed] [Google Scholar]
- 4.Rumack BH, Matthew H. Acetaminophen poisoning and toxicity. Pediatrics. 1975;55:871–6. [PubMed] [Google Scholar]
- 5.Davidson DG, Eastham WN. Acute liver necrosis following overdose of paracetamol. Br Med J. 1966;2:497–9. doi: 10.1136/bmj.2.5512.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heubi JE, Barbacci MB, Zimmerman HJ. Therapeutic misadventures with acetaminophen: Hepatoxicity after multiple doses in children. J Pediatr. 1998;132:22–7. doi: 10.1016/s0022-3476(98)70479-2. [DOI] [PubMed] [Google Scholar]
- 7.Jackson CH, MacDonald NC, Cornett JW. Acetaminophen: A practical pharmacologic overview. CMAJ. 1984;131:25–32. 37. [PMC free article] [PubMed] [Google Scholar]
- 8.Heard KJ. Acetylcysteine for acetaminophen poisoning. N Engl J Med. 2008;359:285–92. doi: 10.1056/NEJMct0708278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tenenbein M. Acetaminophen: The 150 mg/kg myth. J Toxicol Clin Toxicol. 2004;42:145–8. doi: 10.1081/clt-120030939. [DOI] [PubMed] [Google Scholar]
- 10.Anderson BJ, Holford NH, Armishaw JC, Aicken R. Predicting concentrations in children presenting with acetaminophen overdose. J Pediatr. 1999;135:290–5. doi: 10.1016/s0022-3476(99)70122-8. [DOI] [PubMed] [Google Scholar]
- 11.Lavonas EJ, Reynolds KM, Dart RC. Therapeutic acetaminophen is not associated with liver injury in children: A systematic review. Pediatrics. 2010;126:e1430–44. doi: 10.1542/peds.2009-3352. [DOI] [PubMed] [Google Scholar]
- 12.Section on Clinical Pharmacology and Therapeutics. Committee on Drugs. Sullivan JE, Farrar HC. Fever and antipyretic use in children. Pediatrics. 2011;127:580–7. doi: 10.1542/peds.2010-3852. [DOI] [PubMed] [Google Scholar]
- 13.Woodcock J. A difficult balance – pain management, drug safety, and the FDA. N Engl J Med. 2009;361:2105–7. doi: 10.1056/NEJMp0908913. [DOI] [PubMed] [Google Scholar]
- 14.Litovitz TL, Klein-Schwartz W, Rodgers GC, Jr, et al. 2001 Annual report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med. 2002;20:391–452. doi: 10.1053/ajem.2002.34955. [DOI] [PubMed] [Google Scholar]
- 15.Yarema MC, Johnson DW, Berlin RJ, et al. Comparison of the 20-hour intravenous and 72-hour oral acetylcysteine protocols for the treatment of acute acetaminophen poisoning. Ann Emerg Med. 2009;54:606–14. doi: 10.1016/j.annemergmed.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Bower WA, Johns M, Margolis HS, Williams IT, Bell BP. Population-based surveillance for acute liver failure. Am J Gastroenterol. 2007;102:2459–63. doi: 10.1111/j.1572-0241.2007.01388.x. [DOI] [PubMed] [Google Scholar]
- 17.Myers RP, Li B, Fong A, Shaheen AA, Quan H. Hospitalizations for acetaminophen overdose: A Canadian population-based study from 1995 to 2004. BMC Public Health. 2007;7:143. doi: 10.1186/1471-2458-7-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ostapowicz G, Fontana RJ, Schiodt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–54. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 19.Buckalew VM., Jr Habitual use of acetaminophen as a risk factor for chronic renal failure: A comparison with phenacetin. Am J Kidney Dis. 1996;28(1 Suppl 1):S7–13. doi: 10.1016/s0272-6386(96)90562-4. [DOI] [PubMed] [Google Scholar]
- 20.James LP, Mayeux PR, Hinson JA. Acetaminophen-induced hepatotoxicity. Drug metabolism and disposition: The biological fate of chemicals. 2003;31:1499–506. doi: 10.1124/dmd.31.12.1499. [DOI] [PubMed] [Google Scholar]
- 21.Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci. 2006;89:31–41. doi: 10.1093/toxsci/kfi336. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther. 1973;187:211–7. [PubMed] [Google Scholar]
- 23.Gillette JR, Nelson SD, Mulder GJ, et al. Formation of chemically reactive metabolites of phenacetin and acetaminophen. Adv Exp Med Biol. 1981;136:931–50. [PubMed] [Google Scholar]
- 24.Hinson JA, Pumford NR, Roberts DW. Mechanisms of acetaminophen toxicity: Immunochemical detection of drug-protein adducts. Drug Metab Rev. 1995;27:73–92. doi: 10.3109/03602539509029816. [DOI] [PubMed] [Google Scholar]
- 25.Pumford NR, Hinson JA, Potter DW, Rowland KL, Benson RW, Roberts DW. Immunochemical quantitation of 3-(cystein-S-yl) acetaminophen adducts in serum and liver proteins of acetaminophen-treated mice. J Pharmacol Exp Ther. 1989;248:190–6. [PubMed] [Google Scholar]
- 26.Roberts DW, Bucci TJ, Benson RW, et al. Immunohistochemical localization and quantification of the 3-(cystein-S-yl)-acetaminophen protein adduct in acetaminophen hepatotoxicity. Am J Pathol. 1991;138:359–71. [PMC free article] [PubMed] [Google Scholar]
- 27.Davern TJ, Jr, James LP, Hinson JA, et al. Measurement of serum acetaminophen-protein adducts in patients with acute liver failure. Gastroenterology. 2006;130:687–94. doi: 10.1053/j.gastro.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 28.James LP, Alonso EM, Hynan LS, et al. Detection of acetaminophen protein adducts in children with acute liver failure of indeterminate cause. Pediatrics. 2006;118:e676–81. doi: 10.1542/peds.2006-0069. [DOI] [PubMed] [Google Scholar]
- 29.Muldrew KL, James LP, Coop L, et al. Determination of acetaminophen-protein adducts in mouse liver and serum and human serum after hepatotoxic doses of acetaminophen using high-performance liquid chromatography with electrochemical detection. Drug Metab Dispos. 2002;30:446–51. doi: 10.1124/dmd.30.4.446. [DOI] [PubMed] [Google Scholar]
- 30.James LP, Letzig L, Simpson PM, et al. Pharmacokinetics of acetaminophen-protein adducts in adults with acetaminophen overdose and acute liver failure. Drug Metab Dispos. 2009;37:1779–84. doi: 10.1124/dmd.108.026195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khandelwal N, James LP, Sanders C, Larson AM, Lee WM. Unrecognized acetaminophen toxicity as a cause of indeterminate acute liver failure. Hepatology. 2011;53:567–76. doi: 10.1002/hep.24060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.James LP, Farrar HC, Sullivan JE, et al. Measurement of acetaminophen-protein adducts in children and adolescents with acetaminophen overdoses. Pediatric Pharmacology Research Unit Network, NICHD. J Clin Pharmacol. 2001;41:846–51. doi: 10.1177/00912700122010744. [DOI] [PubMed] [Google Scholar]
- 33.James LP, Simpson P, Russo M, Watkins PB. Detection of acetaminophen protein adducts in serum during therapeutic exposure to acetaminophen in healthy volunteers. The Liver Meeting 2007; Boston. November 2 to 6, 2007; (Abst) [Google Scholar]
- 34.Mitchell JR, Thorgeirsson SS, Potter WZ, Jollow DJ, Keiser H. Acetaminophen-induced hepatic injury: Protective role of glutathione in man and rationale for therapy. Clin Pharmacol Therapeut. 1974;16:676–84. doi: 10.1002/cpt1974164676. [DOI] [PubMed] [Google Scholar]
- 35.Soga T, Baran R, Suematsu M, et al. Differential metabolomics reveals ophthalmic acid as an oxidative stress biomarker indicating hepatic glutathione consumption. J Biol Chem. 2006;281:16768–76. doi: 10.1074/jbc.M601876200. [DOI] [PubMed] [Google Scholar]
- 36.Clayton TA, Lindon JC, Cloarec O, et al. Pharmaco-metabonomic phenotyping and personalized drug treatment. Nature. 2006;440:1073–7. doi: 10.1038/nature04648. [DOI] [PubMed] [Google Scholar]
- 37.Fannin RD, Russo M, O’Connell TM, et al. Acetaminophen dosing of humans results in blood transcriptome and metabolome changes consistent with impaired oxidative phosphorylation. Hepatology. 2010;51:227–36. doi: 10.1002/hep.23330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun J, Schnackenberg LK, Holland RD, et al. Metabonomics evaluation of urine from rats given acute and chronic doses of acetaminophen using NMR and UPLC/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;871:328–40. doi: 10.1016/j.jchromb.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 39.Moyer AM, Fridley BL, Jenkins GD, et al. Acetaminophen-NAPQI hepatotoxicity: A cell line model system genome-wide association study. Toxicol Sci. 2011;120:33–41. doi: 10.1093/toxsci/kfq375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaeschke H. Role of inflammation in the mechanism of acetaminophen-induced hepatotoxicity. Expert Opin Drug Metab Toxicol. 2005;1:389–97. doi: 10.1517/17425255.1.3.389. [DOI] [PubMed] [Google Scholar]
- 41.Hinson JA, Roberts DW, James LP. Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol. 2010:369–405. doi: 10.1007/978-3-642-00663-0_12. [DOI] [PMC free article] [PubMed] [Google Scholar]