Abstract
AtCBR, a cDNA encoding NADH-cytochrome (Cyt) b5 reductase, and AtB5-A and AtB5-B, two cDNAs encoding Cyt b5, were isolated from Arabidopsis. The primary structure deduced from the AtCBR cDNA was 40% identical to those of the NADH-Cyt b5 reductases of yeast and mammals. A recombinant AtCBR protein prepared using a baculovirus system exhibited typical spectral properties of NADH-Cyt b5 reductase and was used to study its electron-transfer activity. The recombinant NADH-Cyt b5 reductase was functionally active and displayed strict specificity to NADH for the reduction of a recombinant Cyt b5 (AtB5-A), whereas no Cyt b5 reduction was observed when NADPH was used as the electron donor. Conversely, a recombinant NADPH-Cyt P450 reductase of Arabidopsis was able to reduce Cyt b5 with NADPH but not with NADH. To our knowledge, this is the first evidence in higher plants that both NADH-Cyt b5 reductase and NADPH-Cyt P450 reductase can reduce Cyt b5 and have clear specificities in terms of the electron donor, NADH or NADPH, respectively. This substrate specificity of the two reductases is discussed in relation to the NADH- and NADPH-dependent activities of microsomal fatty acid desaturases.
The ER membrane of eukaryotic cells contains two electron-transfer systems: one is the NADH-dependent system containing NADH-Cyt b5 reductase and Cyt b5, and the other is the NADPH-dependent system containing NADPH-Cyt P450 reductase.
NADH-Cyt b5 reductase is a membrane-bound flavoprotein containing a single FAD as a prosthetic group. It transfers electrons from NADH to Cyt b5, which is another membrane protein containing a single heme group. In higher plants Cyt b5 has been shown to function as an intermediate electron donor in the desaturation of fatty acids of the microsomal membranes from developing safflower cotyledons (Smith et al., 1990; Kearns et al., 1991), in the C5(6) desaturation of sterol precursors in maize (Rahier et al., 1997), and in the hydroxylation of oleate in castor bean seeds (Smith et al., 1992). It is therefore generally accepted that NADH-Cyt b5 reductase in higher plants is the major electron-transfer component involved in these lipid-modification reactions and that it transfers reducing equivalents from NADH to Cyt b5. The cDNAs encoding Cyt b5 have been isolated from several plant species (Kearns et al., 1992; Smith et al., 1994b; Napier et al., 1995), whereas a cDNA encoding NADH-Cyt b5 reductase has not yet been isolated from higher plants. Thus, no direct evidence has been presented so far from reconstitution assay systems containing NADH-Cyt b5 reductase, Cyt b5, and the fatty acid desaturases.
Slack et al. (1976) showed in pea and maize leaves that both NADH and NADPH could stimulate microsomal oleate desaturation. Smith et al. (1990) demonstrated with the microsomes of developing safflower cotyledons that Cyt b5 reduction was observed by the addition of NADH (and also NADPH, but to a lesser extent). Furthermore, they showed that microsomal fatty acid desaturation activity was supported by both NADH and NADPH. Taton and Rahier (1996) also reported that both NADH and NADPH served as the electron donors for the C5(6) desaturation of sterol precursors in maize microsomes. These results suggested the involvement of a NADPH-dependent electron transfer as well as the NADH-dependent transfer in the desaturation reactions. Thus, one possibility is that NADH-Cyt b5 reductase in higher plants may accept the electrons from both NADH and NADPH to reduce Cyt b5. The other possibility is that, as reported in mammalian systems (Enoch and Strittmatter, 1979), NADH and NADPH may be used for the reduction of Cyt b5 through two distinct reductases, NADH-Cyt b5 reductase and NADPH-Cyt P450 reductase, respectively.
To address these questions, we characterized these two microsomal electron-transfer chains in higher plants by reconstituting in vitro the NADH- and NADPH-dependent electron-transfer chains. First we isolated cDNAs encoding NADH-Cyt b5 reductase and two Cyt b5 isoforms of Arabidopsis and demonstrated, using the recombinantly expressed NADH-Cyt b5 reductase and Cyt b5 proteins, that NADH but not NADPH was specifically utilized for the reduction of Cyt b5 by the Arabidopsis NADH-Cyt b5 reductase. We also confirmed that the recombinant Arabidopsis NADPH-Cyt P450 reductase prepared previously (Mizutani and Ohta, 1998) has a clear specificity toward NADPH in the reduction of Cyt b5. To our knowledge, this is the first reconstitution study to show that both NADH-Cyt b5 reductase and NADPH-Cyt P450 reductase are able to reduce Cyt b5 with strict specificity in the utilization of NADH and NADPH, respectively. We discuss the possible physiological significance of the distinction and functional overlap between the NADH- and NADPH-dependent microsomal electron-transfer systems in higher plants.
MATERIALS AND METHODS
Plant Materials
Arabidopsis ecotype Columbia (Col-0; Lehle Seeds, Tucson, AZ) seedlings were grown under the conditions described previously (Mizutani et al., 1997).
Isolation of the NADH-Cyt b5 Reductase cDNA
A keyword search looking into the Arabidopsis EST database of the Institute for Genomic Research (TIGR, http://www.tigr.org/tdb/at/at.html) led to the identification of an Arabidopsis EST clone, H10B3T7 (accession no. AA042434), which contained an open reading frame homologous to mammalian NADH-Cyt b5 reductases. PCR was performed using a set of primers derived from the DNA sequence of the EST clone: 5′-CGACATTCTCTTGAAGGA-3′ and 5′-ACAACTCTCGTAGTTGGG-3′. pBluescript II phagemids were excised en masse from a λZapII cDNA library constructed from 7-d-old Arabidopsis seedlings (Mizutani et al., 1997) and used as the template for the PCR. A 270-bp fragment amplified by the PCR was labeled with digoxigenin-UTP (Boehringer Mannheim) and used as a probe to screen the Arabidopsis cDNA library, according to the manufacturer's instructions. Approximately 30 positive clones were obtained out of a total of 2 × 105 plaques, and the clone containing the longest insert, AtCBR, was completely sequenced. DNA sequencing was performed and analyzed as described previously (Fukuchi-Mizutani et al., 1998). The accession number for this sequence is AB007799.
Isolation of Cyt b5 cDNAs
The keyword search of the Arabidopsis EST database provided two different EST assemblies (TC10161 and TC9046) that were highly homologous to a cauliflower Cyt b5 cDNA (Kearns et al., 1992). PCR was performed as described above using a set of oligonucleotide primers: 5′-TCATCGGAGATGGGCGGAGA-3′ and 5′-AGGCACAAACTTAGCTTT-3′ for TC10161; 5′-GTGAAGATGTCTTCAGATCG-3′ and 5′-GGTGCCTTGCCTTGGTTG-3′ for TC9046. Each of the amplified fragments was used as a probe to screen the Arabidopsis cDNA library as described above. Approximately 100 positive clones were hybridized out of a total of 2 × 105 plaques, and the clones containing the longest insert for the respective probes were completely sequenced. The accession numbers of the two Cyt b5 isoforms (AtB5-A and AtB5-B) are AB007801 and AB007802, respectively.
Isolation of the AtCBR Gene
Genomic DNA was isolated from shoots of 3-week-old Arabidopsis seedlings and purified by ethidium bromide-CsCl density gradient centrifugation as described by Ausubel et al. (1987). PCR was performed with the genomic DNA as a template using a set of primers synthesized according to the AtCBR cDNA sequence: 5′-CCAATCCCCATTTTTTCCCTTTTAC-3′ for the 5′ end; 5′-CGTAAACCAATCAATGGAAACTTTC-3′ for 3′ end. The amplified PCR fragments were cloned into a pCRII vector using a TA cloning kit (Invitrogen, San Diego, CA). The DNA sequence of the AtCBR gene was deposited in the databank (accession no. AB007800).
DNA and RNA Analysis
One microgram of genomic DNA was digested with EcoRI, BamHI, or XhoI, and used for Southern analysis. Hybridization was performed with the full length of AtCBR cDNA labeled with [α-32P]dCTP as a probe. Hybridization and washing conditions were essentially the same as described previously (Fukuchi-Mizutani et al., 1995). The membranes were rehybridized under low-stringency conditions: 5× Denhardt's reagent, 30% formamide, 5× SSC, and 0.5% SDS, followed by washing for 60 min at 65°C in 5× SSC with 1% SDS.
Total RNA was isolated as described by Lagrimini et al. (1987), and 5 μg of total RNA was analyzed by northern hybridization with the full length of cDNAs of AtCBR, AtB5-A, and AtB5-B labeled with [α-32P]dCTP. The hybridization signals were detected using an imaging analyzer (BAS2000, Fuji Film, Tokyo, Japan).
Heterologous Expression of the AtCBR Protein in Insect Cells
The entire coding region of the AtCBR cDNA was expressed using a baculovirus expression vector system according to the method described previously (Summers and Smith, 1987; Mizutani et al., 1997), using the baculovirus transfer vector pFASTBAC (Invitrogen) and Sf21 (Spodoptera furugiperda 21) cells (Invitrogen). Preparation of the recombinant baculovirus DNA containing the AtCBR cDNA and transfection of the insect cells were carried out according to the manufacturer's instructions (Invitrogen).
The expressed AtCBR protein was purified from the infected Sf21 cells. The infected cells were sonicated and centrifuged at 100,000g for 1 h. The pellet was homogenized with buffer A containing 20 mm potassium phosphate, pH 7.25, 20% glycerol, and 1 mm DTT, and proteins were solubilized in buffer B containing the same constituents as buffer A plus 1% Emulgen 913 (Kao Atlas, Tokyo, Japan). After centrifugation at 100,000g for 1 h, the supernatant was applied to a 5′-AMP Sepharose column (1 × 7 cm) equilibrated with buffer B, and the protein was eluted from the column with 10 mm potassium phosphate buffer, pH 7.25, containing 20% glycerol, 1 mm EDTA, 0.1 mm DTT, and 0.5 mm NAD.
Heterologous Expression of the AtB5-A Protein in Escherichia coli
The entire coding region of the AtB5-A cDNA was expressed in E. coli using the QIAexpress system (Qiagen, Chatsworth, CA). The expression of the AtB5-A cDNA was induced by adding 2 mm (final concentration) isopropyl β-d-thiogalactoside. The E. coli cells expressing the AtB5-A cDNA were disrupted by sonication and treated with 1% Triton X-100 at 4°C overnight for protein solubilization. After the sample was centrifuged at 100,000g for 30 min, the supernatant was collected. The recombinant AtB5-A protein tagged with the six His residues at its N terminus was purified using a Ni-nitrilotriacetic acid agarose column according to the manufacturer's instructions (Qiagen).
Assay Methods
The protein content was assayed by the procedure of Bradford (1976) using BSA as a standard. The Cyt b5 content was determined from the Soret absorbance maximum (A413) of the oxidized Cyt b5, using an extinction coefficient (ε) of 117 mm−1 cm−1 (Estabrook and Werringloer, 1978). The concentration of NADH-Cyt b5 reductase was determined from the absorbance maximum (A461) of the oxidized form using an ε of 11.3 mm−1 cm−1 (Mihara and Sato, 1972). Cyt b5 reduction was assayed at 25°C in a reaction mixture (100 μL) containing 10 mm potassium phosphate buffer, pH 7.25, 1 to 5 μm Cyt b5, 0.1 mm NADH or NADPH, and catalytic amounts of NADH-Cyt b5 reductase or NADPH-Cyt P450 reductase. The Cyt b5 reduction was measured by monitoring the increase in A424 for the reduced Cyt b5 (Tamura et al., 1983).
Km values of the recombinant NADH-Cyt b5 reductase or NADPH-Cyt P450 reductase were determined for the electron-donor substrate NADH or NADPH, respectively. For the measurement of the Km values, the concentration of NADH or NADPH was varied from 0.1 to 100 μm in the reaction mixture, and the Cyt b5 reduction was measured as described above. Data were transformed and plotted as Lineweaver-Burk graphs to allow calculation of Km values.
RESULTS
Cloning of Arabidopsis NADH-Cyt b5 Reductase cDNA
We isolated an Arabidopsis cDNA, AtCBR, encoding a protein homologous to mammalian NADH-Cyt b5 reductases, with the aid of an EST. The AtCBR cDNA consists of a 846-bp open reading frame, and an in-frame termination codon was found 50 bp upstream of the first ATG codon in the AtCBR cDNA sequence, indicating that the AtCBR cDNA contains a full-length open reading frame. The AtCBR cDNA encodes a polypeptide of 281 amino acid residues with a calculated molecular mass of 31,489 D, which is comparable to the apparent molecular mass (33,000 D) of the NADH-Cyt b5 reductase protein purified from the microsomal fractions of Catharanthus roseus (Madyashta et al., 1993).
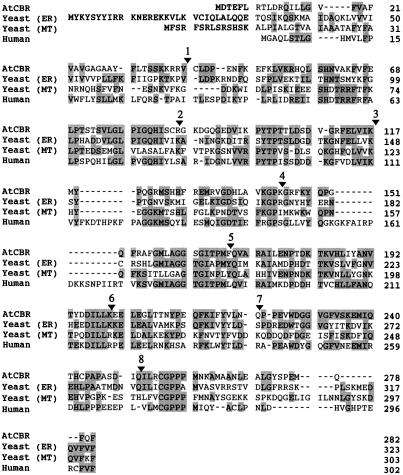
Figure 1 shows the alignment of the amino acid sequence deduced from the AtCBR cDNA and those of the NADH-Cyt b5 reductases from yeast and human. The deduced primary structure of the AtCBR protein is homologous to the NADH-Cyt b5 reductases of mammals and yeast: 40% identical to human (Yubisui et al., 1984) and yeast microsomal NADH-Cyt b5 reductases (Csukai et al., 1994) and 38% identical to yeast mitochondrial NADH-Cyt b5 reductase (Hahne et al., 1994). The regions highly homologous to those of yeast and mammalian NADH-Cyt b5 reductases were found principally in the presumed FAD- and NADH-binding regions (Nishida et al., 1995). The AtCBR protein sequence also contained a region significantly similar to the flavin-binding domain of the Arabidopsis nitrate reductase (Crawford et al., 1988; data not shown). These observations suggested that the AtCBR cDNA encodes a NADH-Cyt b5 reductase of Arabidopsis.
Figure 1.
The multiple alignment of amino acid sequences of AtCBR with those of NADH-Cyt b5 reductase from human (Yubisui et al., 1984) and ER (Csukai et al., 1994) and mitochondria (MT) (Hahne et al., 1994) of yeast. Shading indicates the conserved amino acid residues among the aligned sequences. The arrowheads and numbers over the AtCBR sequence indicate positions where introns are inserted in the AtCBR gene. The sequence of the AtCBR cDNA and the AtCBR gene were deposited in the databank under accession nos. AB007799 and AB007800, respectively.
The AtCBR protein does not have a typical ER retention signal (KXKXX or KKXX) at the C terminus (Jackson et al., 1990). The AtCBR protein, however, contained an N-terminal hydrophobic stretch with approximately 30 amino acids and a few charged residues flanking the hydrophobic stretch, Asp-2, Glu-4, and continuous Lys-16 to Arg-19. These structural properties are similar to those observed in the signal-anchor sequences of microsomal Cyt P450s, which are suggested to be major determinants of targeting to the ER and transmembrane orientation on the ER surface of newly synthesized Cyt P450s (Beltzer et al., 1991).
In mammalian tissues NADH-Cyt b5 reductase is expressed as N-myristoylated and non-myristoylated forms encoded by a single gene (Meldolesi et al., 1980; Pietrini et al., 1988; Borgese et al., 1990, 1993). N-myristoylation is the cotranslational attachment of myristic acid to the Nterminal Gly of target proteins. The first five amino acid residues conform to a loose consensus sequence including the essential second Gly residue (Johnson et al., 1994; Casey, 1995). The predicted amino acid sequence in the AtCBR, however, contains no N-terminal consensus sequences for N-myristoylation, which is responsible for the targeting of the mammalian NADH-Cyt b5 reductase protein to mitochondrial outer membranes (Borgese et al., 1996). Thus, together with its N-terminal properties, which are similar to the microsomal Cyt P450s described above, it is more likely that the AtCBR protein is localized at the ER membrane, as reported for the non-myristoylated NADH-Cyt b5 reductase isoform in mammalian cells (Borgese et al., 1996).
Characterization of AtCBR Gene Organization
To characterize the genomic organization of the AtCBR gene, a 2186-bp DNA fragment was amplified by PCR based on sequences at the 5′ and 3′ ends of the AtCBR cDNA. Sequencing analysis revealed that the 2186-bp fragment covered the entire open reading frame of the AtCBR gene, consisting of nine exons and eight introns. The sequences of the exons found in the AtCBR gene were completely identical to the AtCBR cDNA sequence (Figs. 1 and 2A). The sequences found at all the exon-intron boundaries were “gt… ag,” which is consistent with the proposed sequence rule for an exon-intron junction (Hanley and Schuler, 1988). The three-dimensional structure of the NADH-Cyt b5 reductase from pig-liver microsomes consists of the hydrophobic membrane anchor domain and the FAD- and NADH-binding domains connected through an insertion region (Nishida et al., 1995). Assuming that the Arabidopsis NADH-Cyt b5 reductase has a structure homologous to that from the pig, the AtCBR gene consists of an interesting exon/intron organization. The introns are apparently located at positions that separate the sequences corresponding to each of the functional domains (Figs. 1 and 2A); exon 1 corresponded to the first 39 amino acids of the putative hydrophobic membrane anchor region, exons 2, 3, and 4 encoded the FAD-binding domain (residues spanning Cys-40 to Lys-142), and exons 5 to 9 appeared to encode the insertion and the NADH-binding domain (from Gly-143 to Phe-282).
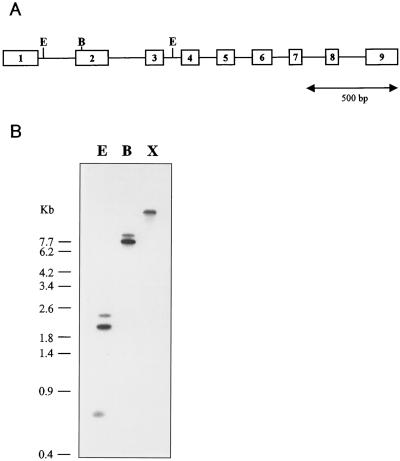
Figure 2.
Gene organization of the AtCBR gene. A, AtCBR gene organization. Open boxes with numbers show the exons, and bars between open boxes show introns. B, Southern analysis of the AtCBR gene. One microgram of genomic DNA was digested with the indicated restriction enzymes and probed with [α-32P]dCTP-labeled AtCBR cDNA. E, EcoRI; B, BamHI; X, XhoI.
Southern analysis was performed to estimate the copy number of the AtCBR gene in the Arabidopsis genome (Fig. 2B). Genomic DNA was digested with each of the three restriction enzymes, EcoRI, BamHI, and XhoI, and probed with the full length of AtCBR cDNA. The AtCBR gene contained two EcoRI recognition sites, one in the first intron and the other in the third intron, and one BamHI site, whereas XhoI had no recognition site in the AtCBR gene. EcoRI digestion produced three hybridization signals, including a band at 0.6 kb, which was consistent with the size expected for the region between the two EcoRI sites in the AtCBR gene. Two hybridization signals were observed in the digestion with BamHI, whereas a single band was detected in the digestion with XhoI. Hybridization under low-stringency conditions gave the same results as those observed under high-stringency conditions. These hybridization patterns were consistent with the restriction map of the AtCBR gene (Fig. 2A), indicating that AtCBR exists as a single-copy gene in the Arabidopsis genome.
As described above, mammalian tissues contain both mitochondrial and ER forms of the NADH-Cyt b5 reductases, which are encoded by a single gene (Pietrini et al., 1988), and cotranslational N-myristoylation of a NADH-Cyt b5 reductase precursor protein is necessary for targeting of the NADH-Cyt b5 reductase to the mitochondrial outer membrane (Borgese et al., 1996). In contrast to mammals, yeast contains two independent genes for NADH-Cyt b5 reductase isoforms targeted to either the ER or the mitochondrial outer membrane (Csukai et al., 1994; Hahne et al., 1994). Although no additional NADH-Cyt b5 reductase genes in Arabidopsis were revealed by the genomic Southern hybridization analysis, we cannot rule out the possibility that, as in the yeast system, Arabidopsis contains another NADH-Cyt b5 reductase gene encoding a mitochondrial isoform.
Isolation of Two Cyt b5 cDNAs
AtB5-A and AtB5-B, two cDNAs encoding Cyt b5 isoforms, were isolated from Arabidopsis using the DNA sequences of two EST assemblies homologous to the Cyt b5 from cauliflower (Kearns et al., 1992). The AtB5-A and AtB5-B cDNAs encode polypeptides of 140 and 134 amino acids, respectively, and contain most of the conserved residues characteristic to the “Cyt b5 fold,” including two His residues as the axial ligand for the heme binding (Fig. 3; Mathews, 1985). The amino acid sequences deduced from the cDNAs were compared with those of plant Cyt b5 proteins so far reported (Fig. 3). The AtB5-A and AtB5-B proteins shared only 57% identity, whereas individually each of them showed relatively high identities to the Cyt b5 proteins from the other plant species. AtB5-B showed the highest identity (90%) to cauliflower Cyt b5 purified from the microsomal fraction (Kearns et al., 1992), and AtB5-A showed 70% identity to two tobacco Cyt b5 proteins (Smith et al., 1994b; Napier et al., 1995). This observation suggests that the two Arabidopsis Cyt b5 proteins may have a distinct role(s) and/or have spatial or temporal distinction.
Figure 3.
Multiple alignment of the amino acid sequences of AtB5-A and AtB5-B with those of Cyt b5 from cauliflower (Brassica) (Kearns et al., 1992), tobacco (Smith et al., 1994a, 1994b), tobacco seeds (Napier et al., 1995), and rice (Smith et al., 1994a, 1994b). Shading indicates the conserved amino acid sequences among the aligned sequences.
Expression Patterns of the AtCBR and AtB5 Genes in Arabidopsis
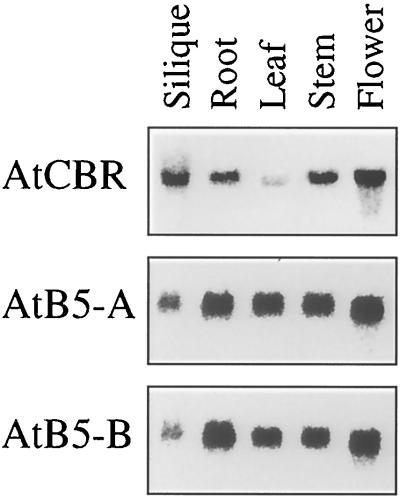
Steady-state levels of the AtCBR, AtB5-A, and AtB5-B mRNAs were analyzed by Northern hybridization using total RNA. The transcripts of the AtCBR, AtB5-A, and AtB5-B genes were detected in all of the organs analyzed (Fig. 4). The amount of transcript from the AtCBR gene was relatively higher in the flower and in the silique containing immature seeds, whereas it was lower in the leaf than in the other organs. On the other hand, the transcripts of both of the AtB5 genes accumulated to lower levels in the silique than in the other organs.
Figure 4.
Tissue-specific expression of the AtCBR, AtB5-A, and AtB5-B genes. Total RNA was isolated from the roots and the leaves of 3-week-old plants, from the inflorescence stems and flowers of 4-week-old plants, and from the siliques of 5-week-old plants. Plants were grown under continuous light.
Tobacco contains two isoforms of Cyt b5: one is specifically expressed in the developing seed and the other is expressed in the whole plant (Smith et al., 1994b; Napier et al., 1995). Considering the essential roles of NADH-Cyt b5 reductase and Cyt b5 in fatty acid biosynthesis, the low expression levels of the AtB5 genes in the silique containing developing seeds suggests that Arabidopsis may have an additional seed-specific Cyt b5 isoform that is predominantly involved in the biosynthesis of storage lipids.
Characterization of Recombinant NADH-Cyt b5 Reductase Proteins
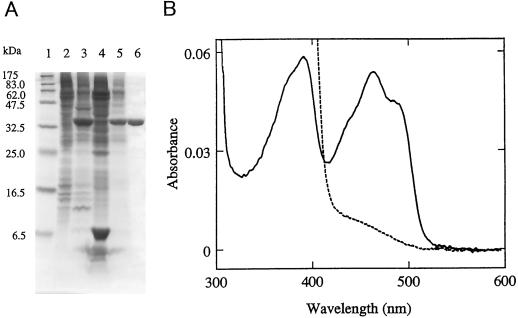
The entire coding region of the AtCBR cDNA was expressed in insect cells using a baculovirus expression vector system. SDS-PAGE analysis (Fig. 5) showed that a new, intense band of 33 kD appeared in the microsomal fraction of the insect cells upon infection with the virus containing the recombinant AtCBR cDNA. The apparent molecular mass of the expressed protein was nearly identical to that calculated from the primary structure of the AtCBR protein (31,489 D). Most of the recombinant AtCBR protein was recovered in the membrane fraction (the 100,000g precipitate), indicating the membrane association of the AtCBR protein. The recombinant AtCBR protein was solubilized in 1% Emulgen 913 and purified to homogeneity by single-step affinity-column chromatography of 5′-AMP Sepharose (Fig. 5A). The recombinant AtCBR protein showed the absolute absorption spectra characteristic of flavoproteins (Fig. 5B). The oxidized form showed prominent peaks at 463 and 380 nm, typical of a flavoprotein, and the 463-nm peak disappeared when reduced by 100 μm NADH. These spectral properties of the recombinant NADH-Cyt b5 reductase protein suggested that the AtCBR cDNA encodes a functionally active NADH-Cyt b5 reductase of Arabidopsis.
Figure 5.
Heterologous expression of the recombinant AtCBR protein in insect cells. A, SDS-PAGE was performed using a 16% polyacrylamide slab gel, and proteins were visualized by staining with Coomassie brilliant blue R-250. Lane 1, Molecular mass marker proteins; lane 2, 100,000g precipitate of mock-infected Sf21 cells; lane 3, 100,000g precipitate of the Sf21 cells infected with the recombinant virus containing the full-length AtCBR cDNA; lane 4, 100,000g supernatant of the AtCBR-expressing Sf21 cells; lane 5, solubilized fractions of 100,000g precipitate of the AtCBR-expressing Sf21 cells; lane 6, the purified recombinant AtCBR. B, Absolute absorption spectrum of the purified recombinant AtCBR in its oxidized form (solid line) and reduced by the addition of 100 μm NADH (dashed line).
Reduction of Cyt b5
We expressed the entire coding region of the AtB5-A cDNA in E. coli and used the recombinant Cyt b5 (AtB5-A) as the electron acceptor in the reconstitution system, focusing on the electron transfer from NAD(P)H to Cyt b5.
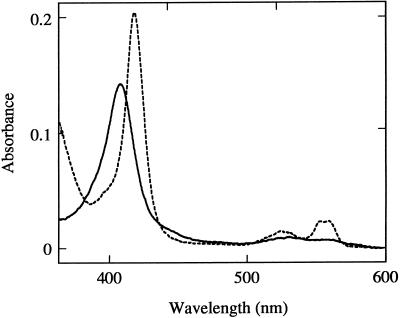
Most of the recombinant Cyt b5 protein was recovered in the membrane fraction in E. coli lysate, implying that the recombinant Cyt b5 could be interacting with the bacterial membrane, probably via the C-terminal hydrophobic anchor sequence. This recombinantly expressed Cyt b5 was solubilized in 1% Triton X-100 and affinity purified with the aid of the N-terminal His tag using a Ni-nitrilotriacetic acid agarose column. The recombinant Cyt b5 showed an absorption maximum at 413 nm (Fig. 6), and the dithionite-reduced form exhibited prominent peaks at 424, 526, and 557 nm (data not shown). These spectral characteristics are typical of the native Cyt b5 proteins purified from other organisms (Bonnerot et al., 1985).
Figure 6.
Absolute absorption spectra of the recombinant AtB5-A protein. Solid line, Oxidized AtB5-A protein; dashed line, the AtB5-A protein reduced by the recombinant AtCBR with 100 μm NADH.
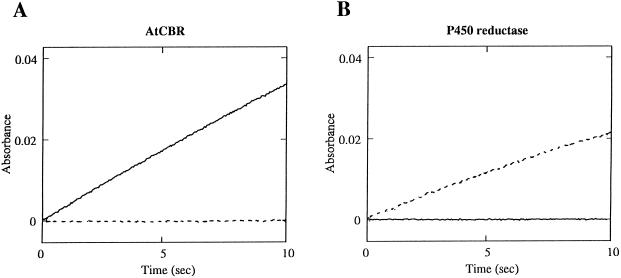
When the oxidized form of the recombinant Cyt b5 was incubated with the recombinant AtCBR protein and 100 μm NADH, it was rapidly reduced and showed an absolute absorption spectrum similar to that of the dithionite-reduced form (Fig. 6). Thus, the recombinant AtCBR protein was functionally active as a NADH-Cyt b5 reductase. The reduction of the recombinant Cyt b5 by AtCBR was NADH dependent, with the Km value for NADH of 1.5 μm (data not shown). On the other hand, no reduction of Cyt b5 was observed in the presence of the AtCBR protein and NADPH, demonstrating that the AtCBR did not transfer the reducing equivalents from NADPH to Cyt b5 (Fig. 7A).
Figure 7.
NADH- and NADPH-dependent reduction of Cyt b5. Recombinant Cyt b5 (AtB5-A) was reduced at 25°C in a reaction mixture containing 10 mm potassium phosphate buffer, pH 7.25, 1.5 μm Cyt b5, either NADH or NADPH (100 μm), and a catalytic amount of AtCBR (A) or NADPH-Cyt P450 reductase (B). The reaction was initiated by addition of either NADH (solid line, 100 μm) or NADPH (dashed line, 100 μm) as an electron donor.
It has been reported that in the microsomal fractions of higher plants Cyt b5 is reduced by the addition of not only NADH but also NADPH (Slack et al., 1976; Smith et al., 1990), suggesting, as reported in a mammalian system (Enoch and Strittmatter, 1979), the involvement of NADPH-Cyt P450 reductase in the NADPH-dependent reduction of Cyt b5. We previously isolated two Arabidopsis cDNAs encoding NADPH-Cyt P450 reductase and obtained the recombinantly expressed NADPH-Cyt P450 reductase proteins (Mizutani and Ohta, 1998). The NADPH-Cyt P450 reductases were able to reduce the recombinant Cyt b5 protein with NADPH (the Km value for NADPH = 2 μm, data not shown) but not with NADH (Fig. 7B). The two NADPH-Cyt P450 reductase isoforms in Arabidopsis (Mizutani and Ohta, 1998) showed the same properties in the reduction of the Arabidopsis Cyt b5 encoded in AtB5-A cDNA (data not shown).
Even after a longer incubation (up to 30 min) or incubation with a higher concentration of pyridine nucleotide (1 mm), no significant reduction of Cyt b5 by NADH-Cyt b5 reductase with NADPH or by NADPH-Cyt P450 reductase with NADH was observed (data not shown).
DISCUSSION
We isolated a cDNA encoding NADH-Cyt b5 reductase (AtCBR) and two cDNAs for Cyt b5 isoforms (AtB5-A and AtB5-B) from Arabidopsis and recombinantly expressed the NADH-Cyt b5 reductase protein and the Cyt b5 protein encoded in AtB5-A. The recombinant NADH-Cyt b5 reductase and Cyt b5 were used to study the microsomal NADH-dependent electron transfer of higher plants, which is involved in the desaturation and hydroxylation of fatty acids and in the desaturation of sterol precursors (Smith et al., 1990, 1992; Kearns et al., 1991; Rahier et al., 1997).
It is widely accepted from studies of the mammalian microsomal electron-transfer system that Cyt b5, which is usually reduced by NADH-Cyt b5 reductase in the presence of NADH, can also be reduced by NADPH-Cyt P450 reductase, with NADPH as the electron donor (Enoch and Strittmatter, 1979). Cyt b5 reduced by NADH-Cyt b5 reductase or by NADPH-Cyt P450 reductase can donate its reducing equivalents to a series of lipid-modification reactions such as desaturation and hydroxylation. In addition, the reduced Cyt b5 can provide the second electron for some of the Cyt P450-dependent monooxygenase reactions (Enoch and Strittmatter, 1979; Imai, 1981; Ruckpaul et al., 1989). Thus, both NADH and NADPH can serve as the electron donors via the two different reductases for the various microsomal terminal electron acceptors, such as the fatty acid desaturases and Cyt P450 monooxygenases.
Several studies of the electron-transfer system in higher plants at the microsomal level have also been reported. In a microsomal preparation from developing safflower cotyledons, Cyt b5 was fully reduced by the addition of NADH and partially reduced by the addition of NADPH (13% of the reduction by NADH; Smith et al., 1990). On the other hand, the Δ12 desaturase activity in the microsomal preparation was observed in the presence of either NADH or NADPH as the electron donor (Smith et al., 1990). It has also been reported that with the microsomal fraction of maize either NADH or NADPH can support the C5(6) desaturation of sterol precursors (Taton and Rahier, 1996; Rahier et al., 1997). These observations suggested, as reported in a mammalian system (Enoch and Strittmatter, 1979), the involvement of NADPH-Cyt P450 reductase in the NADPH-dependent reduction of Cyt b5, which is implicated in the NADPH-dependent activities of the desaturation of fatty acids and sterol precursors. However, it has not been elucidated whether plant NADPH-Cyt P450 reductase can directly reduce Cyt b5, or what specificities, if any, exist between NADH-Cyt b5 reductase and NADPH-Cyt P450 reductase in terms of the utilization of NAD(P)H for the reduction of Cyt b5.
This ambiguity was solved with our in vitro reconstitution studies using the recombinantly expressed proteins involved in microsomal electron-transfer systems (Fig. 7). The Arabidopsis NADH-Cyt b5 reductase was able to reduce Cyt b5 in the presence of NADH, but not in the presence of NADPH. Arabidopsis NADPH-Cyt P450 reductase reduced Cyt b5 with NADPH, but NADH was not accepted as the electron donor by the NADPH-Cyt P450 reductase. These results indicate that NADH-Cyt b5 reductase and NADPH-Cyt P450 reductase have strict substrate specificities toward NADH and NADPH, respectively. Nonetheless, both reductases are capable of reducing Cyt b5, which is also involved in a wide range of microsomal enzymatic activities.
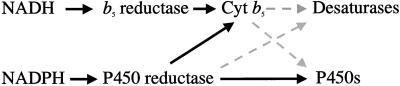
Our results demonstrated that both reductases have the ability to reduce Cyt b5 in vitro. The next question is how the reducing equivalents from NAD(P)H are transferred through these two reductases to the microsomal terminal electron acceptors in vivo. It is possible that the fatty acid and sterol desaturases of the ER may accept their reducing equivalents from both NADH and NADPH in vivo (Fig. 8). Although it has been suggested that only NADH-Cyt b5 reductase and Cyt b5 are predominantly involved in the desaturation of microsomal fatty acids and sterol precursors, the NADPH-dependent electron transfer through NADPH-Cyt P450 reductase could also participate in these reactions in microsomal membranes of higher plants. We also cannot rule out the possibility that the desaturases may bypass Cyt b5 and accept electrons directly from NADPH-Cyt P450 reductase.
Figure 8.
Microsomal electron-transfer systems in higher plants. Solid black line, Electron transfers confirmed by in vitro assay; dashed gray line, possible electron transfers to be investigated further.
As described above, NADPH can be utilized via NADPH-Cyt P450 reductase as the electron donor for those reactions, which are generally NADH dependent. The reducing equivalents from NADH apparently also participate in some Cyt P450-dependent reactions in higher plants; Cyt P450-dependent hydroxylations of lauric acid and monoterpenes were stimulated by the addition of NADH (Benveniste et al., 1982; Karp et al., 1990). Therefore, together with the possible involvement of NADPH and NADH in some microsomal desaturase reactions and Cyt P450-dependent reactions, it is conceivable that the NADH- and NADPH-dependent microsomal electron-transfer chains in higher plants are not completely independent but occasionally cross over and complement each other in a wide variety of reactions, such as the desaturations of fatty acids and sterols and Cyt P450-dependent hydroxylations (Fig. 8). It has been reported that a mutant in yeast deficient in either NADH-Cyt b5 reductase or NADPH-Cyt P450 reductase was able to grow under normal conditions (Sutter and Loper, 1989; Csukai et al., 1994; Truan et al., 1994), but the disruption of both of the reductases was lethal (Csukai et al., 1994). These observations provide further evidence for the possible complementation of the NADH- and NADPH-dependent electron-transfer chains in vivo.
Another important question remains to be answered regarding the physiological significance of the NADPH-dependent lipid-desaturation activities. This will be clarified through studies with reconstitution systems containing the fatty acid desaturases with other NADH- and NADPH-dependent electron-transfer components. Reconstitution studies with these electron-transfer components should also focus on the characterization of the microsomal fatty acid desaturases in higher plants, including the fatty acid desaturase homologs with unknown enzymatic activities from rose and Arabidopsis (Fukuchi-Mizutani et al., 1995, 1998). These biochemical studies, along with molecular-genetics studies such as mutant and transgenic analyses, will constitute important advances toward understanding microsomal lipid metabolism in higher plants.
ACKNOWLEDGMENTS
The authors thank Mmes. Yoriyo Takeuchi and Matsuyo Okamoto for their technical support.
Abbreviations:
- EST
expressed sequence tag
LITERATURE CITED
- Ausubel FM, Brent R, Kingston RE, Moore DD, Siedman JG, Smith JA, Stuhl K (1987) Current Protocols in Molecular Biology. John Wiley, New York
- Beltzer JP, Fiedler K, Fuhrer C, Geffen I, Handschin C, Wessels HP, Spiess M. Charged residues are major determinants of the transmembrane orientation of a signal-anchor sequence. J Biol Chem. 1991;266:973–978. [PubMed] [Google Scholar]
- Benveniste I, Salaun JP, Simon A, Reichhart D, Durst F. Cytochrome P-450-dependent ω-hydroxylation of lauric acid by microsomes from pea seedlings. Plant Physiol. 1982;70:122–126. doi: 10.1104/pp.70.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnerot C, Galle AM, Jolliot A, Kader JC. Purification and properties of plant cytochrome b5. Biochem J. 1985;226:331–334. doi: 10.1042/bj2260331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgese N, Aggujaro D, Carreca P, Pietrini G, Bassetti M. A role for N-myristoylation in protein targeting: NADH-cytochrome b5 reductase requires myristic acid for association with outer mitochondrial but not ER membranes. J Cell Biol. 1996;135:1501–1513. doi: 10.1083/jcb.135.6.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgese N, D'Arrigo A, Silvestris MD, Pietrini G. NADH-cytochrome b5 reductase and cytochrome b5 isoforms as models for study of post-translational targeting to the endoplasmic reticulum. FEBS Lett. 1993;325:70–75. doi: 10.1016/0014-5793(93)81416-w. [DOI] [PubMed] [Google Scholar]
- Borgese N, Longhi R. Both the outer mitochondrial membrane and the microsomal forms of cytochrome b5 reductase contain covalently bound myrisitic acid. Quantitative analysis on the polyvinylidene difluoride-immobilized proteins. Biochem J. 1990;266:341–347. doi: 10.1042/bj2660341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Casey PJ. Protein lipidation in cell signaling. Science. 1995;268:221–225. doi: 10.1126/science.7716512. [DOI] [PubMed] [Google Scholar]
- Crawford NM, Smith M, Bellissimo D, Davis RW. Sequence and nitrate regulation of the Arabidopsis thaliana mRNA encoding nitrate reductase, a metalloflavoprotein with three functional domains. Proc Natl Acad Sci USA. 1988;85:5006–5010. doi: 10.1073/pnas.85.14.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csukai M, Murray M, Orr E. Isolation and complete sequence of CBR, a gene encoding a putative cytochrome b reductase in Saccharomyces cerevisiae. Eur J Biochem. 1994;219:441–448. doi: 10.1111/j.1432-1033.1994.tb19957.x. [DOI] [PubMed] [Google Scholar]
- Enoch HG, Strittmatter P. Cytochrome b5 reduction by NADPH-cytochrome P450 reductase. J Biol Chem. 1979;254:8976–8981. [PubMed] [Google Scholar]
- Estabrook RW, Werringloer J. The measurement of difference spectra: application to the cytochromes of microsomes. Methods Enzymol. 1978;52:212–220. doi: 10.1016/s0076-6879(78)52024-7. [DOI] [PubMed] [Google Scholar]
- Fukuchi-Mizutani M, Savin K, Cornish E, Tanaka Y, Ashikari T, Kusumi T, Murata N. Senescence-induced expression of a homologue of Δ9 desaturase in rose petals. Plant Mol Biol. 1995;42:627–635. doi: 10.1007/BF00041154. [DOI] [PubMed] [Google Scholar]
- Fukuchi-Mizutani M, Tasaka Y, Tanaka Y, Ashikari T, Kusumi T, Murata N. Characterization of Δ9 acyl-lipid desaturase homologues from Arabidopsis thaliana. Plant Cell Physiol. 1998;39:247–253. doi: 10.1093/oxfordjournals.pcp.a029364. [DOI] [PubMed] [Google Scholar]
- Hahne K, Haucke V, Ramage L, Schatz G. Incomplete arrest in the outer membrane sorts NADH-cytochrome b5 reductase to two different submitochondrial compartments. Cell. 1994;79:829–839. doi: 10.1016/0092-8674(94)90072-8. [DOI] [PubMed] [Google Scholar]
- Hanley BA, Schuler MA. Plant intron sequences: evidence for distinct group of introns. Nucleic Acids Res. 1988;16:7159–7174. doi: 10.1093/nar/16.14.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y. The roles of cytochrome b5 in reconstituted monooxygenase systems containing various forms of hepatic microsomal cytochrome P450. J Biochem. 1981;89:351–362. doi: 10.1093/oxfordjournals.jbchem.a133209. [DOI] [PubMed] [Google Scholar]
- Jackson MR, Nilsson T, Peterson P. Identification of a consensus motif for retention of transmembrane proteins in the endoplasmic reticulum. EMBO J. 1990;9:3153–3162. doi: 10.1002/j.1460-2075.1990.tb07513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DR, Bhatnager RS, Knoll LJ, Gordon JI. Genetic and biochemical studies of protein N-myristoylation. Annu Rev Biochem. 1994;63:869–914. doi: 10.1146/annurev.bi.63.070194.004253. [DOI] [PubMed] [Google Scholar]
- Karp F, Mihaliak CA, Harris JL, Croteau R. Monoterpene biosynthesis: specificity of the hydroxylations of (−)-limonene by enzyme preparations from peppermint (Mentha piperita), spearmint (Mentha spicata), and perilla (Perilla frutescence) leaves. Arch Biochem Biophys. 1990;276:219–226. doi: 10.1016/0003-9861(90)90029-x. [DOI] [PubMed] [Google Scholar]
- Kearns EV, Hugly S, Somerville CR. The role of cytochrome b5 in Δ12 desaturation of oleic acid by microsomes of safflower (Carthamus tinctorius L.) Arch Biochem Biophys. 1991;284:431–436. doi: 10.1016/0003-9861(91)90319-e. [DOI] [PubMed] [Google Scholar]
- Kearns EV, Keck P, Somerville CR. Primary structure of cytochrome b5 from cauliflower (Brassica oleracea L.) deduced from peptide and cDNA sequences. Plant Physiol. 1992;99:1254–1257. doi: 10.1104/pp.99.3.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrimini LM, Burkhart W, Moyer M, Rothstein S. Molecular cloning of complementary DNA encoding the lignin-forming peroxidase from tobacco: molecular analysis and tissue-specific expression. Proc Natl Acad Sci USA. 1987;84:7542–7546. doi: 10.1073/pnas.84.21.7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madyastha NK, Chary NK, Holla R, Karegowdar TB. Purification and partial characterization of microsomal NADH:cytochrome b5 reductase from higher plant Catharanthus roseus. Biochem Biophys Res Commun. 1993;197:518–522. doi: 10.1006/bbrc.1993.2509. [DOI] [PubMed] [Google Scholar]
- Mathews FS. The structure, function and evolution of cytochromes. Progr Biophys Mol Biol. 1985;45:1–56. doi: 10.1016/0079-6107(85)90004-5. [DOI] [PubMed] [Google Scholar]
- Meldolesi J, Gorte G, Pietrini G, Borgese N. Localization and biosynthesis of NADH-cytochrome b5 reductase, an integral membrane protein, in rat liver cells. II. Evidence that a single enzyme accounts for the activity in its various subcellular locations. J Cell Biol. 1980;85:516–526. doi: 10.1083/jcb.85.3.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara K, Sato R. Partial purification of NADH-cytochrome b5 reductase from rabbit liver microsomes with detergent and its properties. J Biochem. 1972;71:725–735. [PubMed] [Google Scholar]
- Mizutani M, Ohta D. Two isoforms of NADH:cytochrome P450 reductase in Arabidopsis thaliana. Plant Physiol. 1998;116:357–367. doi: 10.1104/pp.116.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani M, Ohta D, Sato R. Isolation of a cDNA and a genomic clone encoding cinnamate 4-hydroxylase from Arabidopsis and its expression manner in planta. Plant Physiol. 1997;113:755–763. doi: 10.1104/pp.113.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napier JA, Smith MA, Stobart AK, Shewry PR. Isolation of a cDNA encoding a cytochrome b5 specifically expressed in developing tobacco seeds. Planta. 1995;197:200–202. doi: 10.1007/BF00239957. [DOI] [PubMed] [Google Scholar]
- Nishida H, Inaka K, Yamanaka M, Kaida S, Kobayashi K, Miki K. Crystal structure of NADH:cytochrome b5 reductase from pig liver at 2.4 Å resolution. Biochemistry. 1995;34:2763–2767. doi: 10.1021/bi00009a004. [DOI] [PubMed] [Google Scholar]
- Pietrini G, Carrera P, Borgese N. Two transcripts encode rat cytochrome b5 reductase. Proc Natl Acad Sci USA. 1988;85:7246–7250. doi: 10.1073/pnas.85.19.7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahier A, Smith M, Taton M. The role of cytochrome b5 in 4α-methyl-oxidation and C5(6) desaturation of plant sterol precursors. Biochem Biophys Res Commun. 1997;236:434–437. doi: 10.1006/bbrc.1997.6974. [DOI] [PubMed] [Google Scholar]
- Ruckpaul K, Rein H, Black J (1989) Regulation mechanisms of the activity of the hepatic endoplasmic cytochrome P-450. In K Ruckpaul, H Rein, eds, Basis and Mechanisms of Regulation of Cytochrome P-450, Vol 1. Taylor & Francis, London, pp 1–65
- Slack CR, Roughan PG, Terpstra J. Some properties of a microsomal oleate desaturase from leaves. Biochem J. 1976;155:71–80. doi: 10.1042/bj1550071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Cross AR, Jones OTG, Griffiths WT, Stymne S, Stobart K. Electron-transport components of the 1acyl-2-oleoyl-sn-glycero-3-phosphocholine Δ12-desaturase (Δ12-desaturase) in microsomal preparations from developing safflower (Carthamus tinctorius L.) cotyledons. Biochem J. 1990;272:23–29. doi: 10.1042/bj2720023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Jonsson L, Stymne S, Stobart K. Evidence for cytochrome b5 as an electron donor in ricinoleic acid biosynthesis in microsomal preparations from developing castor bean (Ricinus communis L.) Biochem J. 1992;287:141–144. doi: 10.1042/bj2870141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Napier JA, Stymne S, Tatham AS, Shewry PR, Stobart AK. Expression of a biologically active plant cytochrome b5 in Escherichia coli. Biochem J. 1994a;303:73–79. doi: 10.1042/bj3030073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Stobart AK, Shewry PR, Napier JA. Tobacco cytochrome b5: cDNA isolation, expression analysis and in vitro protein targeting. Plant Mol Biol. 1994b;25:527–537. doi: 10.1007/BF00043880. [DOI] [PubMed] [Google Scholar]
- Summers MD, Smith GE (1987) A manual of methods for baculovirus vectors and insect cell culture procedures, bulletin no. 1555. Texas Agricultural Experiment Station and Texas A&M University, College Station
- Sutter TR, Loper JC. Disruption of the Saccharomyces cerevisiae gene for NADPH:cytochrome P450 reductase causes increased sensitivity to ketoconazole. Biochem Biophys Res Commun. 1989;160:1257–1266. doi: 10.1016/s0006-291x(89)80139-1. [DOI] [PubMed] [Google Scholar]
- Tamura M, Yubisui T, Takeshita M. Microsomal NADH-cytochrome b5 reductase of bovine brain: purification and properties. J Biochem. 1983;94:1547–1555. [PubMed] [Google Scholar]
- Taton M, Rahier A. Plant sterol biosynthesis: identification and characterization of higher plant Δ7-sterol C5(6)-desaturase. Arch Biochem Biophys. 1996;325:279–288. doi: 10.1006/abbi.1996.0035. [DOI] [PubMed] [Google Scholar]
- Truan G, Epinat JC, Rougeulle C, Cullin C, Pompon D. Cloning and characterization of a yeast cytochrome b5-encoding gene which suppresses ketoconazole hypersensitivity in a NADPH-P450 reductase-deficient strain. Gene. 1994;142:123–127. doi: 10.1016/0378-1119(94)90366-2. [DOI] [PubMed] [Google Scholar]
- Yubisui T, Miyata T, Iwanaga S, Tamura M, Yoshida S, Takeshita M, Nakajima H. Amino acid sequence of NADH-cytochrome b5 reductase of human erythrocytes. J Biochem. 1984;96:579–582. doi: 10.1093/oxfordjournals.jbchem.a134871. [DOI] [PubMed] [Google Scholar]