Abstract
Proteinases play a key role during angiogenesis and have been implicated in vascular morphogenesis, stabilization and regression. Major advances have identified specific proteinases and their inhibitors that separately control these processes. Relevant proteinases include cell surface or soluble metalloproteinases, serine proteinases and cathepsins that affect these events and a critical issue concerns how these proteinases are balanced by their inhibitors to affect tissue vascularization. Importantly, heterotypic communication of endothelial cells with vessel supporting cells such as pericytes controls proteinase and inhibitor expression to regulate these processes.
1. Introduction
Proteinases that are secreted or expressed on cell surfaces are major regulators of biochemical and biological processes such as coagulation, complement activation and extracellular matrix (ECM) degradation. Together, these molecules and pathways play critical roles in major cellular events such as inflammation, platelet activation, angiogenesis, wound repair and tumor invasion and metastasis (Apte, 2009; Arroyo and Iruela-Arispe, 2010; Binder et al., 2007; Blobel, 2005; Castellino and Ploplis, 2005; Coughlin, 2005; Davis and Saunders, 2006; Porter et al., 2005; Sabeh et al., 2009; van Hinsbergh and Koolwijk, 2008). Major functions of proteinases include degradation of proteins, activation of proteins including proteinase zymogens, cell surface receptors, latent growth factors and, creation of new biologically active cryptic sites within other proteins (Arroyo and Iruela-Arispe, 2010; Davis, 2009) (Figure 1). They also participate as critical cell signaling regulators by cell surface shedding events and direct ability to control signal transduction.
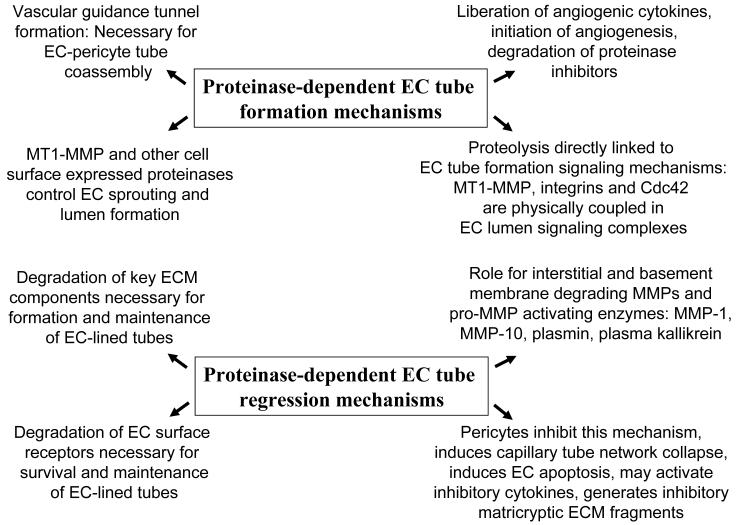
Figure 1. Schematic diagrams describing mechanisms and influences of proteinases on the separate processes of vascular tube morphogenesis versus vascular tube regression in 3D extracellular matrix environments.
Key events in proteinase-dependent tube morphogenesis are; i) the formation of vascular guidance tunnels and the role of MT1-MMP during this process, ii) the intriguing coupling of EC cell surface proteolysis with downstream signaling molecules controlling tube formation and, iii) the ability of proteinases to liberate cytokines and other signaling molecules from the ECM to control angiogenesis. Key events in proteinase-dependent tube regression are; i) the ability of proteinases to degrade the ECM in which tubes are suspended and to generate pro-regressive matricryptic ECM fragments, ii) to degrade EC receptors that control survival as well as cell-cell contacts necessary to maintain tube networks, iii) the ability of particular proteinases to control vascular regression such as MMP-1 and MMP-10 that lead to capillary network collapse and EC apoptosis, and iv) the ability of pericytes to inhibit this proteinase-dependent process by delivery of proteinase inhibitors that are induced as a result of EC-pericyte interactions.
The molecular control of angiogenesis and developmental vasculogenesis is a major topic of investigation and very significant progress has been made over the past several decades (Adams and Alitalo, 2007; Arroyo and Iruela-Arispe, 2010; Davis et al., 2011; Senger and Davis, 2010). Key controls for these events are interactions of vascular cells such as endothelial cells (ECs) with extracellular matrices which is a fundamental regulator of how new blood vessels form and mature (Davis et al., 2011; Senger and Davis, 2010). In fact, many studies indicate that EC-matrix interactions represent the primary regulator of vascular morphogenesis while growth factors such as VEGF controls other steps including cell proliferation and survival (Davis and Senger, 2005; Senger and Davis, 2010). The interface of growth factor and ECM-mediated signaling is a topic of active investigation which is elucidating how these different molecules control various steps in vascular morphogenesis and stabilization (Hynes, 2009; Senger and Davis, 2010). Clearly, ECM and growth factor signaling is affected by proteinases (Arroyo and Iruela-Arispe, 2010; Davis, 2009; Senger and Davis, 2010) and thus, proteinases represent an important mediator of how cells such as ECs interact with their environment during these processes (Figure 1).
2. Proteinases control vascular tube morphogenesis and angiogenic sprouting
There is considerable evidence that proteinases are fundamental regulators of vascular morphogenesis during either vasculogenic tube assembly or angiogenic sprouting (Davis et al., 2007; Davis et al., 2011). A key point is that their role appears to be particularly critical in 3D extracellular matrix environments where cells need to degrade ECM in order to migrate, invade and undergo morphologic changes. For example, vascular basement membrane degradation is an important step for the initiation of angiogenic sprouting (Senger and Davis, 2010). Interestingly, ECs must invade from a 2D relationship with ECM where they are part of the vessel wall lumenal surface (and in contact with vascular basement membrane) and transition into a 3D relationship with ECM as a leading EC tip cell invades into ECM (i.e. interstitial matrix rich in collagen type I) to control the sprouting response. This EC invasion response is a proteinase-dependent event and leads to a change in the interaction of ECs with different ECM components (and interactions with degraded ECM also) (Senger and Davis, 2010). Another important issue is the ability of proteinases to liberate molecules that are anchored to the ECM such as cytokines, and possibly also, biologically active lipids and peptides. Proteinases may participate in regulating the activity of other molecules by inactivating inhibitors or activating stimulatory molecules for a pro-morphogenic event, while controlling the opposite during a pro-regression event. Proteinases can activate other proteinase zymogens, activate growth factors, release membrane-bound growth factors, and inactivate proteinase inhibitors. All of these activities are highly relevant to the mechanisms underlying how vessels form, stabilize and regress (Figure 1). Interestingly, proteinases and their inhibitors have also been reported to have biologic functions independent of their proteinase and inhibitory activities (Gonzalo et al., 2010; Seo et al., 2003; Stetler-Stevenson and Seo, 2005).
MT1-MMP controls vascular tube morphogenesis and sprouting in 3D extracellular matrices
Matrix metalloproteinases have been reported to have important effects on vascular morphogenic events. Two major activities appear critical which are; i) direct effects of membrane type matrix metalloproteinases (MT-MMPs) to stimulate EC sprouting as well as vascular lumen and tube formation in 3D extracellular matrices (Chun et al., 2004; Davis et al., 2011; Sacharidou et al., 2010; Saunders et al., 2006; Stratman et al., 2009b), and ii) release of angiogenic cytokines from the ECM to modulate vascular morphogenic responses (Bergers et al., 2000) (Figure 1). Importantly, MT1-MMP has been recently reported to directly participate in critical signaling transduction events such as modulating the activities of Rho GTPases (Gonzalo et al., 2010; Sacharidou et al., 2010), which regulate cell shape, motility and morphogenesis. Thus, in addition to their ability to degrade the ECM to affect EC invasion and morphogenesis, they directly participate in cell signaling cascades that control these processes (Sacharidou et al., 2010).
MT1-MMP is a cell surface expressed MMP that has been implicated in many processes, most notably in tumor cell invasion and angiogenesis (Davis et al., 2007; Davis et al., 2011; Haas and Madri, 1999; Sabeh et al., 2009; Senger and Davis, 2010; van Hinsbergh and Koolwijk, 2008). In the latter process, it has been directly demonstrated to be required for angiogenic sprouting into 3D collagen or fibrin matrices and more recently, it has been shown to be fundamentally involved in how ECs form lumens and tubes in 3D collagen matrices (Davis et al., 2007; Davis et al., 2011). The mouse knockout of MT1-MMP (which causes early postnatal lethality) revealed abnormal vascular morphogenesis within developing bone (a matrix dense tissue) and importantly, angiogenic responses do not occur in vivo in the postnatal mice (Zhou et al., 2000). Thus, MT1-MMP is a required molecule for EC tubulogenesis (Sacharidou et al., 2010; Saunders et al., 2006; Stratman et al., 2009b) and it does so as a critical component of an EC lumen signaling complex that is necessary for the human EC lumen and tube formation process in 3D collagen matrices (Sacharidou et al., 2010). In fact, it has been shown that MT1-MMP and the Rho GTPase, Cdc42, are interdependent signaling molecules that are physically and functionally coupled during these events. MT1-MMP activity is necessary for Cdc42 activation (a fundamental control step in EC lumen formation) in 3D collagen matrices and likewise, Cdc42 activity is necessary for MT1-MMP to perform its proteolytic functions during tube formation (Sacharidou et al., 2010). A key step in vascular morphogenesis is to create networks of EC-lined tubes, but also to carve out networks of physical tunnel spaces within the 3D ECM (termed vascular guidance tunnels- a process dependent on MT1-MMP) (Stratman et al., 2009b). Importantly, blockade of EC tubulogenesis using a variety of inhibitors leads to complete interference of MT1-MMP-dependent vascular guidance tunnel formation (Stratman et al., 2009b). TIMP-2 and TIMP-3 addition strongly abrogates EC tube and tunnel formation while TIMP-1 does not due to its inability to block MT1-MMP (Saunders et al., 2006). Furthermore, EC tubes reside within these vascular guidance tunnel networks and can utilize them to freely migrate to remodel tube structures (Stratman et al., 2009b), but also to stimulate pericyte recruitment to the ablumenal tube surface within tunnel spaces (Stratman et al., 2009a). Vascular guidance tunnels represent matrix conduits that control EC tube remodeling events (via EC motility or applied external stimuli such as flow), mural cell recruitment, and possibly cell sorting events that distinguish arterial versus venous EC tubes (Davis et al., 2011). Also, dramatic EC and pericyte motility events occur within vascular guidance tunnels and these heterotypic cell-cell interactions lead to vascular basement membrane matrix assembly (Stratman et al., 2009a), a fundamental step in vascular maturation and stabilization (Senger and Davis, 2010) (Figure 2). Thus, MT1-MMP represents a major regulator of vascular tube morphogenesis and maturation due its ability to control signaling events necessary to form tube structures but also to play a fundamental role in the creation of vascular guidance tunnels in 3D matrices through proteolysis.
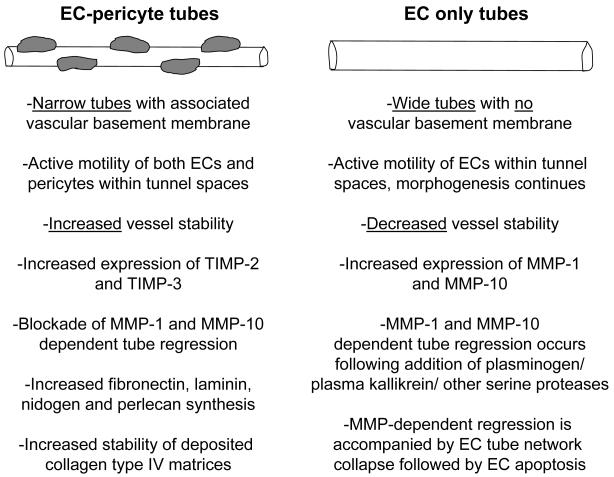
Figure 2. Molecular and functional distinctions that characterize EC tubes which are associated with pericytes compared to those without pericytes in 3D matrices.
Major distinctions between pericyte-lined EC tubes versus those without pericytes are listed. EC-pericytes tubes are characterized by being narrow with deposited vascular basement membrane matrices due to the co-contribution of basement membrane components by ECs and pericytes. Furthermore, EC-pericyte interactions lead to upregulated TIMP-2 and TIMP-3 expression which facilitates vessel stability and, also, resistance to MMP-dependent tube regression stimuli. In contrast, EC only tubes are much wider and do not deposit basement membrane matrix. These tubes continue to undergo morphogenesis, are more unstable and are susceptible to MMP-1 and MMP-10 dependent tube regression mechanisms when these pro-enzymes are activated by serine proteases such as plasmin or plasma kallikrein.
Modulation of angiogenic responses by proteinase-induced modulation of cytokine release and activity
Proteinases, such as soluble MMPs and plasmin, have been reported to liberate angiogenic cytokines from either the ECM or cell surfaces (Bergers et al., 2000; Lee et al., 2005). A variety of recent studies show that neutrophil-derived MMP-9 (which does not have associated TIMP-1) can strongly stimulate angiogenic events (Ardi et al., 2007; Heissig et al., 2010). This is one mechanism whereby acute inflammation and angiogenesis are strongly linked processes (Arroyo and Iruela-Arispe, 2010). Thus, both neutrophils as well as monocyte/macrophages produce proteinases and cytokines that stimulate angiogenic events as a part of the coupled inflammation and wound repair processes (Arroyo and Iruela-Arispe, 2010; Heissig et al., 2010). Both MMP-3 and plasmin have been reported to cleave VEGF such that it releases from ECM and mimics the activity of the VEGF121 isoform which shows little to no affinity for ECM (Lee et al., 2005). Interestingly, expression of mutated forms of VEGF that cannot be cleaved by MMPs or plasmin, markedly alter angiogenic responses in vivo compared to cleavable VEGF forms (Lee et al., 2005). Interestingly, other proteinases such as Adamts1 have been reported to bind VEGF and interfere with angiogenic responses (Luque et al., 2003). One interesting possibility is that proteinase release of VEGF may alter its functional capacity due to the ability of ECM-bound VEGF to induce unique signals distinct from that of soluble VEGF (Chen et al., 2010). ECM binding may also modulate the susceptibility of VEGF to blockade from soluble molecules such as released VEGFR1 isoforms or Adamts1.
Mechanisms underlying proteinase-dependent modulation of angiogenesis: Influence on cell surface molecule shedding, initiation of angiogenic sprouting and inactivation of proteinase inhibitors
A variety of other proteinases have been reported to modulate angiogenic responses and these include serine proteinases such as plasminogen activators, plasmin, thrombin, Adam metalloproteinases, and cathepsins. Adam-15 and Adam-17 have been reported to affect angiogenesis in mouse knockout animals (Horiuchi et al., 2003; Weskamp et al., 2010). The ability of Adam-17 to shed a variety of molecules including growth factors of the EGF family is likely to play a role in its influence (Swendeman et al., 2008). Also, Adam-10 is known to play a critical role in Notch activation (Hartmann et al., 2002), a major regulator of vascular development as well as angiogenesis (Sainson et al., 2005). Notch signaling which is strongly activated in the arterial system controls signaling in both ECs and mural cells during these events (Adams and Alitalo, 2007). Interestingly, cathepsin S, which is expressed on the EC cell surface has been reported to affect vascular development (Shi et al., 2003). Other cathepsins and their specific inhibitors have been shown to be involved in the initiation of VEGF-dependent angiogenic responses (Chang et al., 2009; Joyce et al., 2004).
One mechanism by which some of these enzymes may influence angiogenic responses is by inactivation of proteinase inhibitors. For example, plasmin and cathepsins have both been reported to inactivate TIMP-1, along with oxidants (Frears et al., 1996; Itoh and Nagase, 1995). It is important to consider if these proteinase or chemical inhibitors might also inactivate TIMP-2 or TIMP-3, which then could affect MT1-MMP activity to regulate vascular morphogenesis. Also, plasmin and other serine proteinases activate pro-MMPs which can facilitate degradation of ECM including interstitial and basement membrane matrices (Davis and Saunders, 2006; Saunders et al., 2005; Saunders et al., 2006). Thrombin has been reported to stimulate angiogenesis but interestingly, it is also known to cause EC junction disassembly as well as EC tube collapse in 3D matrices (Bayless and Davis, 2004; Coughlin, 2005; Garcia et al., 1996). Perhaps these latter events could actually initiate angiogenic signaling by converting stable EC tube networks into activated ones that remodel and then initiate new vascular morphogenic events. Interestingly, PAI-1 can also be inactivated by proteinase cleavage, but this also leads to a PAI-1 fragment that possesses anti-angiogenic activity (Drinane et al., 2006). Similar approaches to those described for PAI-1 should be performed for TIMPs to address whether similar cryptic activities might exist for them following their cleavage. Interestingly, TIMP-2 has been reported to affect angiogenic responses in a manner that does not necessarily depend on its ability to inhibit MMPs. As discussed above, TIMP-2 can markedly block EC tube morphogenesis and sprouting through blockade of MT-MMP (Saunders et al., 2006). However, using a recombinant TIMP-2 that is mutated to inactivate its N-terminal proteinase inhibitory domain, it has been shown that it can also block angiogenesis by interfering with VEGFR2 phosphorylation events (Seo et al., 2003; Stetler-Stevenson and Seo, 2005).
3. Proteinases that regulate vascular tube regression
It is clear that angiogenic and vasculogenic responses are balanced by positive and negative signals. Proteinases play major roles in these balanced vascular responses which are necessary for the development and maintenance of large vessels as well as the functional microcirculation. Interestingly, major diseases such as aortic aneurysm, aortic dissection and atherosclerosis involve proteinase-degradation of vascular ECM (Raffetto and Khalil, 2008). Recent data suggests that mural cell interactions with EC tubes reduced proteolysis and this is a major mechanism underlying why these interactions lead to vessel stabilization (Saunders et al., 2006; Stratman et al., 2009a). Interestingly, EC-pericyte interactions in 3D matrices leads to increased production of EC-derived TIMP-2 and pericyte-derived TIMP-3 which can block pro-regression stimuli mediated by MMP-1, MMP-10, plasmin, and plasma kallikrein (Saunders et al., 2006).
MMP-1 and MMP-10 control vascular tube regression in 3D collagen matrices
Using models of EC tube morphogenesis and regression in 3D collagen matrices, the roles of specific MMPs and inhibitors have been addressed by several laboratories (Davis et al., 2001; Saunders et al., 2005; Saunders et al., 2006; Zhu et al., 2000). It is clear that particular soluble MMPs, such as MMP-1 and MMP-10, play a specific role in vascular tube regression responses. siRNA suppression of these genes led to selective blockade of vascular tube regression responses but does not affect the ability of human ECs to form tubes in 3D collagen matrices (Saunders et al., 2005). In this model under defined serum-free conditions, the addition of plasminogen (which is converted to plasmin by EC-derived plasminogen activators) or other serine proteases such as plasma kallikrein and neutrophil elastase leads to activation of pro-MMP-1 and pro-MMP-10 which leads to vascular tube regression responses (Saunders et al., 2005). Increased expression of either pro-MMP-1 or pro-MMP-10 accelerates tube regression responses but has no influence on tube formation. Thus, reducing MMP-1 and MMP-10 expression interferes with tube regression, while increasing expression of these MMPs stimulates tube regression. In contrast, these expression changes have no influence on tube formation in 3D matrices (Davis and Saunders, 2006; Saunders et al., 2005). Also, it was observed that siRNA suppression of Adam-15 acted in conjunction with MMP-1 and MMP-10 to affect vascular tube regression (Davis and Saunders, 2006; Saunders et al., 2006). Overall, this work strongly supports the concept that MMP-1 and MMP-10 primarily control tube regression responses and do not directly influence EC tube formation in 3D collagen matrices under defined serum-free conditions. In strong support for these concepts are data showing that mouse knockouts of TIMP-1 and PAI-1 tend to reduce angiogenic responses (Noel et al., 2004) and lead to decreased vascular density in tissues (i.e. likely mediated by increased MMP-dependent tube regression) such as the retina (Yamada et al., 2001). In further support of these concepts is work showing that mouse knockout of histone deacetylase (HDAC)7 leads to a developmental vascular lethal phenotype with severe vessel breakdown and vascular hemorrhage (Chang et al., 2006). siRNA suppression of HDAC7 was shown to markedly increase the expression of MMP-10 while at the same time causing reduced expression of TIMP-1 (Chang et al., 2006). This combination of genetic changes led to increased susceptibility of EC tubes during vascular development in vivo toward regression mechanisms leading to tube disruption and hemorrhage. Thus, work performed in vitro demonstrating a role for MMP-10 and MMP-1 in controlling vascular tube regression (Davis et al., 2001; Davis and Saunders, 2006; Saunders et al., 2005; Saunders et al., 2006) are strongly supported by in vivo developmental studies showing the ability of MMP-10 to mediate vascular regression leading hemorrhage and embryonic lethality (Chang et al., 2006). Furthermore, there is also considerable evidence for related MMP-dependent tube regression phenomena during the reproductive cycle and mammary duct regression following the cessation of lactation (Khokha and Werb, 2010; Zhang and Nothnick, 2005).
Also, very recent work suggests that proteinase-dependent shedding of important signaling receptors such as VEGFR2 and insulin receptors within the microcirculation may play a pathogenetic role in vascular diseases such as hypertension (DeLano and Schmid-Schonbein, 2008; Tran et al., 2010). It is of particular interest that many factors that promote vascular regression tend to have the ability to promote vasoconstriction and activate platelets which in conjunction with activation of coagulation mechanisms leads to thrombotic diseases (Coughlin, 2005). Of great interest is the ability of platelet-derived thrombospondin to promote vasoconstriction responses by inhibiting nitric oxide-dependent vascular smooth muscle signaling (Isenberg et al., 2008; Isenberg et al., 2007). This signaling effect appears to occur primarily in a CD47-dependent manner which inhibits guanylate cyclase. Thrombospondins 1 and 2 have been reported to have anti-angiogenic activity (Isenberg et al., 2009) and this may primarily occur due to this very interesting ability of thrombospondin to block nitric oxide signaling. Many stimuli are known to activate platelets to stimulate release of thrombospondin-1 including thrombin which can acutely disassemble EC-EC junctions (and expose sub-endothelial ECM) to further activate platelets (Coughlin, 2005).
Overall, proteinases affect tube regression through a number of important mechanisms including; i) activation of proenzymes to facilitate proteolytic cascades that stimulate vessel regression, ii) degradation of critical ECM components necessary for EC adhesive interactions necessary to maintain tube structure; iii) degradation of proteinase inhibitors to accelerate the activity of proteinases that catalyze this process, iv) generation of cryptic fragments of ECM, proteinases or inhibitors that regulate tube regression, and v) shedding or secretion of growth factor receptor traps that sequester growth factors such as VEGF; and vi) degradation or shedding of growth factor receptors (e.g. VEGFR2 and Tie-2) or cell-cell adhesion molecules (e.g. VE-cadherin) (Guaiquil et al., 2009) that are necessary to maintain vessel tube signaling and shape (Figure 1). Thus, there are multiple regulatory steps in blood vessel assembly and maintenance and many of these are directly regulated by proteinase activities during development and postnatal life.
4. Proteinase and proteinase inhibitor balances control vascular tube maturation and stabilization
There is considerable evidence that mural cell recruitment to developing EC-lined tubes plays a major role during vessel maturation and stabilization (Benjamin et al., 1998; Saunders et al., 2006; Stratman et al., 2009a) (Figure 2). In fact, pericyte recruitment to EC tubes has recently been shown to stimulate vascular basement membrane matrix assembly, a critical step that is necessary for this process (Stratman et al., 2009a). Also, recent data has shown that pericyte recruitment to EC-lined tubes also is accompanied by the increased production of the MMP inhibitors, TIMP-2 and TIMP-3 (Saunders et al., 2006), and these play a key role in the tube stabilization process. Interestingly, ECs appear to be the major source of TIMP-2, while pericytes contribute TIMP-3, an ECM-binding TIMP (Saunders et al., 2006). These two TIMPs are capable of inhibiting a broad spectrum of MMPs including soluble MMPs, MT-MMPs and in the case of TIMP-3, also Adam proteinases such as Adam-17 (Brew and Nagase, 2010). Thus, it appears to make considerable sense that these two TIMPs could facilitate the transition of ECs from a pro-morphogenic process to a pro-stabilization process following pericyte recruitment to these assembling tubes.
Endothelial cell-derived TIMP-2 and pericyte-derived TIMP-3 play key roles in vascular tube maturation and stabilization
TIMP-2 and TIMP-3 can block MT1-MMP activity to interfere with further tube morphogenesis (by inhibiting sprouting and lumen formation) and also inhibit tube regression mediated by MMP-1 and MMP-10 (Saunders et al., 2006). Furthermore, TIMP-2 and TIMP-3 can block VEGFR2 signaling (Qi et al., 2003; Stetler-Stevenson and Seo, 2005) to also decrease further EC tube morphogenic events. Another very interesting finding is that pericyte-derived TIMP-3 plays a major role in regulating basement membrane deposition (and/or stability) around developing tubes. siRNA suppression of pericyte TIMP-3 leads to markedly decreased collagen type IV deposition around EC tubes during EC-pericyte tube coassembly (Stratman et al., 2009a). A consequence of this decreased collagen type IV deposition is increased vascular tube width, a response that occurs when basement membranes are not deposited in vitro and in vivo (Stratman et al., 2009a; Stratman et al., 2010) (Figure 2). A similar response occurs (i.e. increased EC tube width) when fibronectin knockout mice are generated (Hynes, 2007). The deposition of collagen type IV, fibronectin and laminins (as well as nidogens and perlecan) strongly occurs when ECs and pericytes are co-cultured in 3D collagen matrices, but are minimally deposited in cultures with ECs alone (Stratman et al., 2009a) (Figure 2). Thus, EC-pericyte interactions play a major functional role in ECM remodeling events such as the process of vascular basement membrane assembly.
These interactions are not only necessary for the appropriate production of basement membrane matrix components from both cell types for deposition, but also for the production of TIMP-3 which contributes to the stabilization of this newly deposited ECM (Stratman et al., 2009a). Furthermore, the process of vascular basement membrane assembly occurs within vascular guidance tunnels where both ECs and pericytes can be shown to migrate across each other. Pericytes exclusively migrate along the EC tube ablumenal surface in real-time movies where these events have been observed (Stratman et al., 2009a). Interestingly, once the EC tube networks and vascular guidance tunnels have formed (in an MT1-MMP-dependent manner) (Stratman et al., 2009b), pericytes are recruited to these tubes and this is followed by elevated expression of EC TIMP-2 and pericyte TIMP-3 through EC and pericyte interactions (Saunders et al., 2006) (Figure 2). The EC-pericyte interactions and motility responses within tunnels appear to be critical for the basement membrane assembly process. Once both cell types are enclosed within these tunnel spaces, both can migrate in an MT1-MMP-independent manner since cell motility on 2D matrix surfaces does not depend on MT1-MMP activity (Stratman et al., 2009a; Stratman et al., 2009b). However, the increased expression of TIMP-2 and TIMP-3 that occurs as a result of EC-pericyte interactions will prevent either cell type from leaving these pre-formed matrix spaces (since the matrix invasion mechanism is blocked by these TIMPs). This facilitates the key event that needs to occur which is to form the basement membrane matrix (i.e. for maturation and stabilization) rather than continue with invasive events from either ECs or pericytes (i.e. for continued tube morphogenesis and assembly).
In addition, under defined serum-free conditions, the EC-derived growth factors, PDGF-BB and HB-EGF, have been shown to be responsible for this recruitment process as well as the ability of pericytes to proliferate in 3D collagen matrices (Stratman et al., 2010). Without pericyte recruitment, EC-lined tubes are physically wider and they do not properly deposit basement membranes (Figure 2). Interestingly, pericyte motility and invasion in 3D matrices was completely dependent on the co-presence of ECs and this was secondary to their production of PDGF-BB and HB-EGF (Stratman et al., 2010). Also, blockade of PDGF-BB and HB-EGF or their receptors in vivo leads to inhibition of vascular basement membrane matrix in conjunction with the lack of pericyte recruitment (Stratman et al., 2010). Adam proteinases are known to control HB-EGF shedding from ECs and other cells (Blobel, 2005), so it is likely that the action of these proteinases will be important for this process. Another possibility is that other isoforms of PDGF could play a role during these events including PDGF-CC and PDGF-DD. Interestingly, both of these isoforms are secreted as pro-forms which need to be activated by proteolysis (in particular through plasminogen activators) to be able to activate PDGF receptors (Bergsten et al., 2001). An isoform of VEGF, VEGF-D, is also activated by proteolysis (i.e. plasmin) and has been shown to control lymphangiogenic responses (McColl et al., 2003), and TGF-beta isoforms, which control cardiovascular development, are activated by integrins as well as multiple proteases through cleavage of the latency associated peptide (Hynes, 2009).
5. Conclusions
Considerable progress has been made on elucidating the role of proteinases and their inhibitors during angiogenesis in both developmental and pathologic contexts. A major advance in the field has been to identify particular proteinases that regulate distinct biologic events such as vessel formation, regression or stabilization. Furthermore, key advances include the development of systems such as defined in vitro models of EC-pericyte tube coassembly to understand how proteinases and proteinase inhibitors control these events in 3D matrix environments. Future studies need to utilize combined in vitro and in vivo approaches to address the role of specific proteinases and inhibitors that affect key steps such as EC sprouting, lumen formation, pericyte recruitment and vascular tube stability mechanisms, and finally, how vessel sprouting initiates from stabilized vessel walls.
Acknowledgements
I would like to thank the members of the Davis laboratory from the past and present for their contributions to our collective work and understanding of the role of proteinases and proteinase inhibitors in vascular morphogenesis versus regression. This work was supported by NIH grants HL79460 and HL 87308 to GED.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–78. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- Apte SS. A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms. J Biol Chem. 2009;284:31493–7. doi: 10.1074/jbc.R109.052340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardi VC, Kupriyanova TA, Deryugina EI, Quigley JP. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc Natl Acad Sci U S A. 2007;104:20262–7. doi: 10.1073/pnas.0706438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo AG, Iruela-Arispe ML. Extracellular matrix, inflammation, and the angiogenic response. Cardiovasc Res. 2010;86:226–35. doi: 10.1093/cvr/cvq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayless KJ, Davis GE. Microtubule depolymerization rapidly collapses capillary tube networks in vitro and angiogenic vessels in vivo through the small GTPase Rho. J Biol Chem. 2004;279:11686–95. doi: 10.1074/jbc.M308373200. [DOI] [PubMed] [Google Scholar]
- Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125:1591–8. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–44. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsten E, Uutela M, Li X, Pietras K, Ostman A, Heldin CH, Alitalo K, Eriksson U. PDGF-D is a specific, protease-activated ligand for the PDGF beta-receptor. Nat Cell Biol. 2001;3:512–6. doi: 10.1038/35074588. [DOI] [PubMed] [Google Scholar]
- Binder BR, Mihaly J, Prager GW. uPAR-uPA-PAI-1 interactions and signaling: a vascular biologist’s view. Thromb Haemost. 2007;97:336–42. [PubMed] [Google Scholar]
- Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta. 2010;1803:55–71. doi: 10.1016/j.bbamcr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellino FJ, Ploplis VA. Structure and function of the plasminogen/plasmin system. Thromb Haemost. 2005;93:647–54. doi: 10.1160/TH04-12-0842. [DOI] [PubMed] [Google Scholar]
- Chang S, Young BD, Li S, Qi X, Richardson JA, Olson EN. Histone deacetylase 7 maintains vascular integrity by repressing matrix metalloproteinase 10. Cell. 2006;126:321–34. doi: 10.1016/j.cell.2006.05.040. [DOI] [PubMed] [Google Scholar]
- Chang SH, Kanasaki K, Gocheva V, Blum G, Harper J, Moses MA, Shih SC, Nagy JA, Joyce J, Bogyo M, Kalluri R, Dvorak HF. VEGF-A induces angiogenesis by perturbing the cathepsin-cysteine protease inhibitor balance in venules, causing basement membrane degradation and mother vessel formation. Cancer Res. 2009;69:4537–44. doi: 10.1158/0008-5472.CAN-08-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TT, Luque A, Lee S, Anderson SM, Segura T, Iruela-Arispe ML. Anchorage of VEGF to the extracellular matrix conveys differential signaling responses to endothelial cells. J Cell Biol. 2010;188:595–609. doi: 10.1083/jcb.200906044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun TH, Sabeh F, Ota I, Murphy H, McDonagh KT, Holmbeck K, Birkedal-Hansen H, Allen ED, Weiss SJ. MT1-MMP-dependent neovessel formation within the confines of the three-dimensional extracellular matrix. J Cell Biol. 2004;167:757–67. doi: 10.1083/jcb.200405001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost. 2005;3:1800–14. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- Davis GE. Matricryptic sites control tissue injury responses in the cardiovascular system: Relationships to pattern recognition receptor regulated events. J Mol Cell Cardiol. 2009 doi: 10.1016/j.yjmcc.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GE, Koh W, Stratman AN. Mechanisms controlling human endothelial lumen formation and tube assembly in three-dimensional extracellular matrices. Birth Defects Res C Embryo Today. 2007;81:270–85. doi: 10.1002/bdrc.20107. [DOI] [PubMed] [Google Scholar]
- Davis GE, Pintar Allen KA, Salazar R, Maxwell SA. Matrix metalloproteinase-1 and -9 activation by plasmin regulates a novel endothelial cell-mediated mechanism of collagen gel contraction and capillary tube regression in three-dimensional collagen matrices. J Cell Sci. 2001;114:917–30. doi: 10.1242/jcs.114.5.917. [DOI] [PubMed] [Google Scholar]
- Davis GE, Saunders WB. Molecular balance of capillary tube formation versus regression in wound repair: role of matrix metalloproteinases and their inhibitors. J Investig Dermatol Symp Proc. 2006;11:44–56. doi: 10.1038/sj.jidsymp.5650008. [DOI] [PubMed] [Google Scholar]
- Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97:1093–107. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- Davis GE, Stratman AN, Sacharidou A, Koh W. Molecular basis for endothelial lumen formation and tubulogenesis during vasculogenesis and angiogenic sprouting. Int Rev Cell Mol Biol. 2011 doi: 10.1016/B978-0-12-386041-5.00003-0. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLano FA, Schmid-Schonbein GW. Proteinase activity and receptor cleavage: mechanism for insulin resistance in the spontaneously hypertensive rat. Hypertension. 2008;52:415–23. doi: 10.1161/HYPERTENSIONAHA.107.104356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drinane M, Walsh J, Mollmark J, Simons M, Mulligan-Kehoe MJ. The anti-angiogenic activity of rPAI-1(23) inhibits fibroblast growth factor-2 functions. J Biol Chem. 2006;281:33336–44. doi: 10.1074/jbc.M607097200. [DOI] [PubMed] [Google Scholar]
- Frears ER, Zhang Z, Blake DR, O’Connell JP, Winyard PG. Inactivation of tissue inhibitor of metalloproteinase-1 by peroxynitrite. FEBS Lett. 1996;381:21–4. doi: 10.1016/0014-5793(96)00065-8. [DOI] [PubMed] [Google Scholar]
- Garcia JG, Verin AD, Schaphorst KL. Regulation of thrombin-mediated endothelial cell contraction and permeability. Semin Thromb Hemost. 1996;22:309–15. doi: 10.1055/s-2007-999025. [DOI] [PubMed] [Google Scholar]
- Gonzalo P, Guadamillas MC, Hernandez-Riquer MV, Pollan A, Grande-Garcia A, Bartolome RA, Vasanji A, Ambrogio C, Chiarle R, Teixido J, Risteli J, Apte SS, del Pozo MA, Arroyo AG. MT1-MMP is required for myeloid cell fusion via regulation of Rac1 signaling. Dev Cell. 2010;18:77–89. doi: 10.1016/j.devcel.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guaiquil V, Swendeman S, Yoshida T, Chavala S, Campochiaro PA, Blobel CP. ADAM9 is involved in pathological retinal neovascularization. Mol Cell Biol. 2009;29:2694–703. doi: 10.1128/MCB.01460-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas TL, Madri JA. Extracellular matrix-driven matrix metalloproteinase production in endothelial cells: implications for angiogenesis. Trends Cardiovasc Med. 1999;9:70–7. doi: 10.1016/s1050-1738(99)00014-6. [DOI] [PubMed] [Google Scholar]
- Hartmann D, de Strooper B, Serneels L, Craessaerts K, Herreman A, Annaert W, Umans L, Lubke T, Lena Illert A, von Figura K, Saftig P. The disintegrin/metalloprotease ADAM 10 is essential for Notch signalling but not for alpha-secretase activity in fibroblasts. Hum Mol Genet. 2002;11:2615–24. doi: 10.1093/hmg/11.21.2615. [DOI] [PubMed] [Google Scholar]
- Heissig B, Nishida C, Tashiro Y, Sato Y, Ishihara M, Ohki M, Gritli I, Rosenkvist J, Hattori K. Role of neutrophil-derived matrix metalloproteinase-9 in tissue regeneration. Histol Histopathol. 2010;25:765–70. doi: 10.14670/HH-25.765. [DOI] [PubMed] [Google Scholar]
- Horiuchi K, Weskamp G, Lum L, Hammes HP, Cai H, Brodie TA, Ludwig T, Chiusaroli R, Baron R, Preissner KT, Manova K, Blobel CP. Potential role for ADAM15 in pathological neovascularization in mice. Mol Cell Biol. 2003;23:5614–24. doi: 10.1128/MCB.23.16.5614-5624.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Cell-matrix adhesion in vascular development. J Thromb Haemost. 2007;5(Suppl 1):32–40. doi: 10.1111/j.1538-7836.2007.02569.x. [DOI] [PubMed] [Google Scholar]
- Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–9. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg JS, Frazier WA, Roberts DD. Thrombospondin-1: a physiological regulator of nitric oxide signaling. Cell Mol Life Sci. 2008;65:728–42. doi: 10.1007/s00018-007-7488-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg JS, Hyodo F, Matsumoto K, Romeo MJ, Abu-Asab M, Tsokos M, Kuppusamy P, Wink DA, Krishna MC, Roberts DD. Thrombospondin-1 limits ischemic tissue survival by inhibiting nitric oxide-mediated vascular smooth muscle relaxation. Blood. 2007;109:1945–52. doi: 10.1182/blood-2006-08-041368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg JS, Martin-Manso G, Maxhimer JB, Roberts DD. Regulation of nitric oxide signalling by thrombospondin 1: implications for anti-angiogenic therapies. Nat Rev Cancer. 2009;9:182–94. doi: 10.1038/nrc2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y, Nagase H. Preferential inactivation of tissue inhibitor of metalloproteinases-1 that is bound to the precursor of matrix metalloproteinase 9 (progelatinase B) by human neutrophil elastase. J Biol Chem. 1995;270:16518–21. doi: 10.1074/jbc.270.28.16518. [DOI] [PubMed] [Google Scholar]
- Joyce JA, Baruch A, Chehade K, Meyer-Morse N, Giraudo E, Tsai FY, Greenbaum DC, Hager JH, Bogyo M, Hanahan D. Cathepsin cysteine proteases are effectors of invasive growth and angiogenesis during multistage tumorigenesis. Cancer Cell. 2004;5:443–53. doi: 10.1016/s1535-6108(04)00111-4. [DOI] [PubMed] [Google Scholar]
- Khokha R, Werb Z. Mammary Gland Reprogramming: Metalloproteinases Couple Form with Function. Cold Spring Harb Perspect Biol. 2010 doi: 10.1101/cshperspect.a004333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169:681–91. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque A, Carpizo DR, Iruela-Arispe ML. ADAMTS1/METH1 inhibits endothelial cell proliferation by direct binding and sequestration of VEGF165. J Biol Chem. 2003;278:23656–65. doi: 10.1074/jbc.M212964200. [DOI] [PubMed] [Google Scholar]
- McColl BK, Baldwin ME, Roufail S, Freeman C, Moritz RL, Simpson RJ, Alitalo K, Stacker SA, Achen MG. Plasmin activates the lymphangiogenic growth factors VEGF-C and VEGF-D. J Exp Med. 2003;198:863–8. doi: 10.1084/jem.20030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel A, Maillard C, Rocks N, Jost M, Chabottaux V, Sounni NE, Maquoi E, Cataldo D, Foidart JM. Membrane associated proteases and their inhibitors in tumour angiogenesis. J Clin Pathol. 2004;57:577–84. doi: 10.1136/jcp.2003.014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. Biochem J. 2005;386:15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi JH, Ebrahem Q, Moore N, Murphy G, Claesson-Welsh L, Bond M, Baker A, Anand-Apte B. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat Med. 2003;9:407–15. doi: 10.1038/nm846. [DOI] [PubMed] [Google Scholar]
- Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008;75:346–59. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeh F, Shimizu-Hirota R, Weiss SJ. Protease-dependent versus - independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. J Cell Biol. 2009;185:11–9. doi: 10.1083/jcb.200807195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacharidou A, Koh W, Stratman AN, Mayo AM, Fisher KE, Davis GE. Endothelial lumen signaling complexes control 3D matrix-specific tubulogenesis through interdependent Cdc42- and MT1-MMP-mediated events. Blood. 2010 doi: 10.1182/blood-2009-11-252692. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainson RC, Aoto J, Nakatsu MN, Holderfield M, Conn E, Koller E, Hughes CC. Cell-autonomous notch signaling regulates endothelial cell branching and proliferation during vascular tubulogenesis. Faseb J. 2005;19:1027–9. doi: 10.1096/fj.04-3172fje. [DOI] [PubMed] [Google Scholar]
- Saunders WB, Bayless KJ, Davis GE. MMP-1 activation by serine proteases and MMP-10 induces human capillary tubular network collapse and regression in 3D collagen matrices. J Cell Sci. 2005;118:2325–40. doi: 10.1242/jcs.02360. [DOI] [PubMed] [Google Scholar]
- Saunders WB, Bohnsack BL, Faske JB, Anthis NJ, Bayless KJ, Hirschi KK, Davis GE. Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J Cell Biol. 2006;175:179–91. doi: 10.1083/jcb.200603176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger DR, Davis GE. Angiogenesis. Cold Spring Harb. Perspect. Biol. 2010 doi: 10.1101/cshperspect.a005090. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo DW, Li H, Guedez L, Wingfield PT, Diaz T, Salloum R, Wei BY, Stetler-Stevenson WG. TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell. 2003;114:171–80. doi: 10.1016/s0092-8674(03)00551-8. [DOI] [PubMed] [Google Scholar]
- Shi GP, Sukhova GK, Kuzuya M, Ye Q, Du J, Zhang Y, Pan JH, Lu ML, Cheng XW, Iguchi A, Perrey S, Lee AM, Chapman HA, Libby P. Deficiency of the cysteine protease cathepsin S impairs microvessel growth. Circ Res. 2003;92:493–500. doi: 10.1161/01.RES.0000060485.20318.96. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson WG, Seo DW. TIMP-2: an endogenous inhibitor of angiogenesis. Trends Mol Med. 2005;11:97–103. doi: 10.1016/j.molmed.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood. 2009a;114:5091–101. doi: 10.1182/blood-2009-05-222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratman AN, Saunders WB, Sacharidou A, Koh W, Fisher KE, Zawieja DC, Davis MJ, Davis GE. Endothelial cell lumen and vascular guidance tunnel formation requires MT1-MMP-dependent proteolysis in 3-dimensional collagen matrices. Blood. 2009b;114:237–47. doi: 10.1182/blood-2008-12-196451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratman AN, Schwindt AE, Malotte KM, Davis GE. Endothelial-derived PDGF-BB and HB-EGF coordinately regulate pericyte recruitment during vasculogenic tube assembly and stabilization. Blood. 2010;116:4720–30. doi: 10.1182/blood-2010-05-286872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swendeman S, Mendelson K, Weskamp G, Horiuchi K, Deutsch U, Scherle P, Hooper A, Rafii S, Blobel CP. VEGF-A stimulates ADAM17-dependent shedding of VEGFR2 and crosstalk between VEGFR2 and ERK signaling. Circ Res. 2008;103:916–8. doi: 10.1161/CIRCRESAHA.108.184416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran ED, DeLano FA, Schmid-Schonbein GW. Enhanced matrix metalloproteinase activity in the spontaneously hypertensive rat: VEGFR-2 cleavage, endothelial apoptosis, and capillary rarefaction. J Vasc Res. 2010;47:423–31. doi: 10.1159/000281582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hinsbergh VW, Koolwijk P. Endothelial sprouting and angiogenesis: matrix metalloproteinases in the lead. Cardiovasc Res. 2008;78:203–12. doi: 10.1093/cvr/cvm102. [DOI] [PubMed] [Google Scholar]
- Weskamp G, Mendelson K, Swendeman S, Le Gall S, Ma Y, Lyman S, Hinoki A, Eguchi S, Guaiquil V, Horiuchi K, Blobel CP. Pathological neovascularization is reduced by inactivation of ADAM17 in endothelial cells but not in pericytes. Circ Res. 2010;106:932–40. doi: 10.1161/CIRCRESAHA.109.207415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada E, Tobe T, Yamada H, Okamoto N, Zack DJ, Werb Z, Soloway PD, Campochiaro PA. TIMP-1 promotes VEGF-induced neovascularization in the retina. Histol Histopathol. 2001;16:87–97. doi: 10.14670/HH-16.87. [DOI] [PubMed] [Google Scholar]
- Zhang X, Nothnick WB. The role and regulation of the uterine matrix metalloproteinase system in menstruating and non-menstruating species. Front Biosci. 2005;10:353–66. doi: 10.2741/1533. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Apte SS, Soininen R, Cao R, Baaklini GY, Rauser RW, Wang J, Cao Y, Tryggvason K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci U S A. 2000;97:4052–7. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu WH, Guo X, Villaschi S, Francesco Nicosia R. Regulation of vascular growth and regression by matrix metalloproteinases in the rat aorta model of angiogenesis. Lab Invest. 2000;80:545–55. doi: 10.1038/labinvest.3780060. [DOI] [PubMed] [Google Scholar]