Abstract
Spiral ganglion neurons are the first neural element of the auditory system. They receive precise synaptic signals which represent features of sound stimuli encoded by hair cell receptors and they deliver a digital representation of this information to the central nervous system. It is well known that spiral ganglion neurons are selectively responsive to specific sound frequencies, and that numerous structural and physiological specializations in the inner ear increase the quality of this tuning, beyond what could be accomplished by the passive properties of the basilar membrane. Further, consistent with what we know about other sensory systems, it is becoming clear that the parallel divergent innervation pattern of type I spiral ganglion neurons has the potential to encode additional features of sound stimuli. To date, we understand the most about the sub-modalities of frequency and intensity coding in the peripheral auditory system. Work reviewed herein will address the issue of how intrinsic electrophysiological features of the neurons themselves have the potential to contribute to the precision of coding and transmitting information about these two parameters to higher auditory centers for further processing.
Keywords: Spiral ganglion neurons, primary afferents, firing patterns, ion channels
1. Introduction
A defining feature of the peripheral auditory system is its graded specializations utilized to separate complex sounds into their component frequencies (Raphael and Altschuler, 2003; Rubel and Fritzsch, 2002). The systematic variation in width and stiffness of the basilar membrane is the first step in setting up the tonotopic map that represents the primary sensory parameter coded within the auditory neuronal pathway (von Békésy, 1970). Subsequently, many other cellular specializations have been discovered that appear to be necessary to achieve greater precision in frequency discrimination. Analogous to the strings of a harp, the collagen fibers in the low frequency region of the basilar membrane are longer and more flexible than those in the high frequency region (Slepecky, 1996). Much like the basilar membrane, the tectorial membrane also displays a stiffness gradient along the tonotopic contour (Richter et al., 2007). Further, the receptor cells are specifically tailored in much the same manner. The stereocilia of the inner and outer hair cells in the mammalian cochlea vary systematically with cochlear location. Stereociliary length is maximal in the apex where relatively slow vibrations occur; the stereociliary length decreases along the extent of the cochlea such they are shortest in the base to most efficiently transduce high frequency vibration (Corwin and Warchol, 1991). Particularly pronounced in the outer hair cells, but also evident in the inner hair cells, is a variation in soma area, such that the largest cells are present in the apex compared to those in the base (Ashmore and Gale, 2000). Furthermore, electrical resonance (Art and Fettiplace, 1987) and transduction channel conductance and adaptation rate (Ricci et al., 2003) increase with increasing frequency in turtle hair cells. Thus, the multiple mechanical and electrical cochlear specializations that are graded along the cochlear contour to sharpen frequency tuning illustrate the importance of this parameter in auditory perception. Further evidence for this can be found in the large number of frequency maps found throughout the auditory neuronal pathways, which are refined during development (Kandler et al., 2009). Therefore, the peripheral auditory system utilizes layers of increasingly refined mechanical and electrical specializations to enhance frequency-specific coding.
The name “spiral ganglion” beautifully conveys the shape of this peripheral cluster of neurons within the confines of the snail-shaped cochlea. The longitudinal organization of the ganglion also suggests that the neurons themselves may display tonotopic features that typify the peripheral end organ. Because this ganglion consists almost entirely (~95%) of a single cell class, the type I neurons, this question is reasonably straightforward to address. Type I spiral ganglion neurons constitute a highly divergent pathway from the inner hair cells into the CNS. Each neuron receives synaptic input from only a single inner hair cell, yet each inner hair cell forms synapses onto 10–30 type I neurons (Keithley and Schreiber, 1987; Liberman et al., 1990). This innervation pattern is quite different from that seen for the type II neurons that compose the remaining ~5% of the ganglion. Instead of being divergent, type II neurons receive synaptic input from multiple (15–20) outer hair cells (Spoendlin, 1972), forming a convergent neural pathway. The type I and type II neurons with their distinctly different innervation patterns, have been shown to synapse in separate regions within the cochlear nucleus (Benson and Brown, 2004). Although there are many speculations (Brown, 1994; Reid et al., 2004; Robertson et al., 1999; Spoendlin, 1975; Weisz et al., 2009), very little is known about the functional significance of the type II neurons. Therefore, we will focus our attention on the type I neurons that not only constitute the majority of the nerve but are also known to comprise the primary pathway for auditory perception.
The question considered in this review is whether type I spiral ganglion neurons show electrophysiological specializations for conduction of auditory signals into the CNS. Once detached from their peripheral receptor cells and central targets, are these neurons uniform in their properties such that they faithfully convey input from their peripheral synaptic partners, or do they augment detection of specific auditory sub-modalities by shaping the signal that they transmit?
2. Diversity of type I spiral ganglion neurons
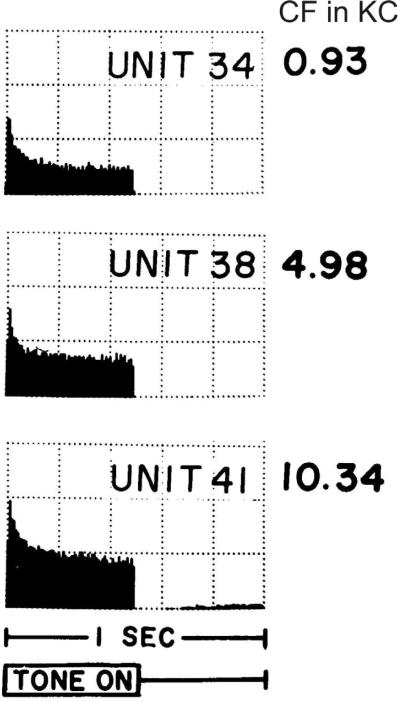
Electrophysiological recordings from central neurons separated from their synaptic targets have revealed a wide array of endogenous firing patterns that undoubtedly contribute to their signaling capabilities (Bean, 2007). In the same regard, investigators have found that although it is clear that the fundamental parameters extracted from sensory stimuli are determined in large part by the characteristics of sensory receptors, intrinsic properties of primary afferent neurons also contribute (Loewenstein and Mendelson, 1965; Masland and Raviola, 2000; Scroggs and Fox, 1992). This evolution in our view of sensory processing has occurred for the auditory system as well. Before we and others began the examination of spiral ganglion neurons with intracellular recordings in order to characterize their intrinsic electrophysiological features (Chen, 1997; Chen and Davis, 2006; Davis, 1996; Garcia-Diaz, 1999; Hisashi et al., 1995; Jagger and Housley, 2002; Jimenez et al., 1997; Lin, 1997; Lin and Chen, 2000; Mo et al., 2002; Mo and Davis, 1997a; Moore et al., 1996; Santos-Sacchi, 1993; Sheppard et al., 1992; Szabo et al., 2002; Yamaguchi and Ohmori, 1990), the general view of the field was that all type I neurons that composed the ganglion were identical. This was based upon the wealth of in vivo recordings revealing that neurons fire in a similar fashion to pure tone stimuli (Kiang et al., 1965; Kiang et al., 1984; Sachs et al., 1974). The data shown in Figure 1 exemplifies these observations. Extracellular auditory neuron activity in response to pure tone stimuli as characterized by post-stimulus time (PST) histograms clearly display patterns of activity that are almost identical (Fig. 1). However, one could interpret this finding differently and wonder why each PST histogram pattern is similar despite the clear differences in the sensory stimulus used to evoke the neuronal responses.
Fig. 1.
Post-stimulus time histograms from three units with low to high characteristic frequencies (CF) showed similar response patterns to tone bursts. Recordings were made from cat auditory nerve fibers. Each histogram averages 2 minutes of data. Y-scale: 200 spikes/per increment. Burst levels used: −50 dB for unit 38; −70 dB for unit 34 and 41. Adapted from Kiang et al., 1965
Insight into this question was obtained with whole-cell patch clamp recordings from spiral ganglion neurons of known cochlear location. Using this technique, we discovered that spiral ganglion neurons do not display identical firing features along the frequency contour of the cochlea or even within each frequency region (Adamson et al., 2002b; Mo et al., 2002; Mo and Davis, 1997a). In retrospect, these findings were foreshadowed by early observations of the organization of basic spiral ganglion morphology. Similar to the diverse frequency-specific specializations noted throughout the organ of Corti (Raphael and Altschuler, 2003), numerous investigators have shown that the soma size of putative type I neurons varied tonotopically, with the largest neurons situated toward the basal regions (Echteler and Nofsinger, 2000; Liberman and Oliver, 1984; Nadol et al., 1990; Rosbe et al., 1996; Ryugo, 1992). Unlike most other neurons, spiral ganglion neurons are notable in that the soma is part of the action potential conduction pathway, meaning that, in addition to reflecting structural specializations such as axonal diameter or extent of the axonal arbor, differences in cell body size could regulate signaling parameters as well. In addition to which, the cell bodies of the auditory primary afferents are surrounded by a unique type of myelin, referred to as loose myelin. Distinct from compact myelin in both its anatomical structure and molecular composition (Rosenbluth, 1962), loose myelin is thought to surround electrically excitable spiral ganglion soma membrane (Robertson, 1976; Toesca, 1996) In sum, the spiral ganglion neuronal somata clearly shows distinctive variation along the tonotopic axis of the cochlea.
To our knowledge this was the first demonstration that spiral ganglion neurons possess tonotopic specializations. Soma size differences noted for spiral ganglion neurons varied inversely with hair cell soma size, stereocilia length and basilar membrane width. This relationship is not particularly surprising since the structural requirements for specialized cellular elements within the organ of Corti is driven by their role in sound transduction, where mechanical specializations are required; clearly this is not relevant to how spiral ganglion neurons perform their function. Instead, it is action potential generation and conduction that must be considered, in which the soma size and axon diameter can have a powerful impact. In the case of soma size, one would predict that the increased surface area and the consequent increase in membrane capacitance could delay signal transmission through the cell body and could also filter high frequency action potential bursts (Robertson, 1976). Enlarged axonal diameter, on the other hand, would increase action potential conduction velocity due to the decreased internal resistance. Whether the transmission rates for each of these features compensate for one another to produce uniform transmission in high and low frequency neurons has yet to be explored.
Peripheral axon diameter of the spiral ganglion neurons display subtle tonotopic gradation, but also show clear local variation (Liberman, 1982a; Ryugo, 1992). This feature varies systematically around the circumference of each individual inner hair cell (Liberman, 1982a), the organization of which was largely maintained in the ganglion (Kawase and Liberman, 1992; Leake et al., 1992). Larger diameter axons, possessing greater numbers of mitochondria, physiologically associated with cells that fire at high spontaneous rates and have low thresholds to sound stimuli, were located along the pillar side of the inner hair cell. In contrast, small and medium diameter axons of less mitochondria content, which fire at low spontaneous rates and have high thresholds to auditory stimuli, were located on the opposite side of the inner hair cells. This precise local variation, compared to the obvious type I-type II divergent-convergent pathways and tonotopic layout, strengthens the idea that there is an additional organizational principle at work. Because the threshold for firing leads to a clear shift in the rate/level function for these neurons (Kiang et al., 1965; Liberman, 1982a; Schmiedt, 1989; Winter et al., 1990), it appears that this is a separate neural pathway for processing intensity information.
These characterizations of soma area and axon diameter are revealing because they indicate that spiral ganglion neurons possess specializations that vary in tandem with two well-characterized sub-modalities. Like frequency coding, spiral ganglion neuron soma area is graded along the cochlear axis, while, similar to threshold differences in intensity coding, spiral ganglion axon diameter varies around the inner hair cell circumference. This begs the question: are there other specializations within the spiral ganglion that further modulate signals that are sent to the CNS? Early work using single unit extracellular recording revealed cells with unique characteristics that support this possibility (Kiang, 1990). Moreover, there have been numerous observations of variations in diverse protein levels between putative type I neurons (Anniko et al., 1995; Lopez et al., 1995; Romand et al., 1990; Salih et al., 1999), which also hint at additional complexity. The next step is to clearly define how these various phenotypes are distributed within the ganglion in order to examine their functional significance.
3. Tonotopic distribution of firing features, voltage-gated ion channels, and synaptic proteins
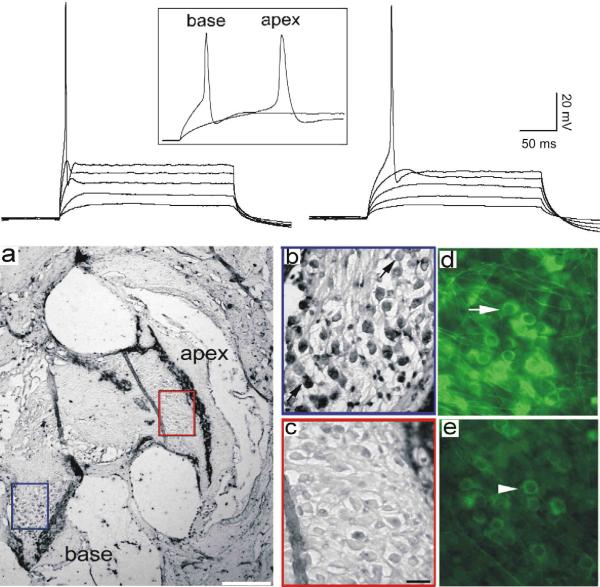
Complementing the tonotopic variation in morphological features of type I spiral ganglion neurons described above, previous studies from our laboratory have shown that aspects of spiral ganglion neuron firing features are graded as well. For example, the differences in accommodation that were originally observed from in vitro recordings of postnatal spiral ganglion neurons (Mo and Davis, 1997a) were found in subsequent investigations to be distributed according to area of innervation. Neurons isolated from apical, low frequency regions showed greater levels of slow accommodation compared to those isolated from basal, high frequency regions (Adamson et al., 2002b). In addition to this very obvious feature, more subtle characteristics such as action potential latency, onset time course at threshold, and duration were also related to the area from which neurons were originally isolated. Neurons that showed prolonged latency, slow onset time course, and relatively long action potential duration were typically observed from neurons isolated from apical regions of the cochlea. By comparison, neurons that showed abbreviated latency, rapid onset time course, and brief action potential duration were characteristic of neurons isolated from basal cochlear regions. An example of whole-cell current clamp recordings at threshold from spiral ganglion neurons isolated from the base and apex of the cochlea are shown in Figure 2 (upper left and right panels, respectively). These observations were augmented with immunocytochemical labeling of ion channels known via pharmacological studies to be likely regulators of the electrophysiological features of spiral ganglion neurons: large conductance calcium-activated potassium channels (BK), Kv1.1, Kv3.1, and Kv4.2 (Adamson et al., 2002b). In that study, immunocytochemical analysis showed that ion channel types which could contribute to abbreviated firing features (BK, Kv1.1 and Kv3.1) were enriched in basal neurons, both in vitro and in vivo (Fig. 2. panels a–e). Correspondingly, an antibody against the ion channel type that could contribute to the slowing of neuronal responses (Kv4.2) was found to be enriched in apical spiral ganglion neurons (Adamson et al., 2002b).
Fig 2.
Endogenous electrophysiological firing patterns and voltage-dependent ion channel composition differ between apical and basal spiral ganglion neurons. Top Panel. Series of stacked whole-cell current clamp traces from a basal (left) and apical (right) spiral ganglion neuron highlight differences in response speed. The onset time course and difference in latency are evident from the series of sweeps; the differences in action potential duration can be observed from the inset traces (box). a–e, Anti-BK antibody labeling was enriched in basal compared to apical spiral ganglion neurons in adult cochlea (a–c) and in vitro (d, e). a, Section taken from an adult CBA/CaJ mouse cochlea stained with anti-BK antibody (Alomone Labs, APC-02) showing that the neurons in the base of the cochlea (blue box) were considerably darker than those in the apex (red box). The calibration bar, lower right, represents 200 μm. b, high-magnification view of the basal neurons. c, High-magnification view of the apical neurons. d, postnatal spiral ganglion neurons isolated from the base and maintained in vitro for 7 days also showed intense anti-BK antibody labeling. The arrow indicates a darkly stained neuron. e, Sister cultures of apical neurons treated identically to those in panel d showed only low staining levels. The arrowhead indicates a lightly stained neuron. The scale bar in panel c = 20 μm and applies to panels b–e. Adapted from Adamson et al., 2002a.
Firing characteristics and voltage-gated ion channel composition were not the only electrophysiologically-relevant features that changed from apical to basal regions of the spiral ganglion. In a systematic examination of the distribution of presynaptic and postsynaptic proteins, a distinct pattern emerged. Two postsynaptic AMPA receptor subunits, GluR2 and GluR3, showed the highest antibody labeling levels within the neurons innervating the basal cochlear region (Flores-Otero et al., 2007). Conversely, two presynaptic proteins, synaptophysin and SNAP-25, were analyzed and showed a significant enhancement in apical neurons. Interestingly, within any given region of the ganglion, levels of AMPA receptors were relatively uniform whereas levels of presynaptic proteins in the apical region of the ganglion showed an obvious heterogeneity. The presynaptic protein distribution in apical neurons could reflect the heterogeneous accommodation displayed by apical spiral ganglion neurons and may furthermore be associated with the enhanced complexity of the end bulbs of Held within the low frequency regions (Cant, 1992; Rouiller et al., 1986; Ryugo, 1992). Increased AMPA receptor density in basal neurons could serve to enhance neuronal responses to synaptic input, thus overcoming the low input resistance that results from the large numbers of low-voltage activated ion channels found within these cells (Adamson et al., 2002b).
The distribution of endogenous electrophysiological features and synaptic proteins within the spiral ganglion was subsequently shown to be regulated in a highly predictable manner by two neurotrophins. Brain derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) are produced by hair cells, satellite cells, and neurons within the organ of Corti (Ernfors et al., 1992; Farinas et al., 2001; Pirvola et al., 1994; Pirvola et al., 1997), thus potentially acting via their respective high affinity trkB and trkC receptors (Barbacid, 1994; Patapoutian and Reichardt, 2001), which are present in spiral ganglion neurons (Cochran et al., 1999; Farinas et al., 2001; Knipper et al., 1996; Mou et al., 1997; Pirvola et al., 1994; Pirvola et al., 1992; Ylikoski et al., 1993). NT-3, expressed in the highest amounts in the low frequency region late in development and throughout adulthood (Fritzsch et al., 1997; Pirvola et al., 1992; Sugawara et al., 2007; Ylikoski et al., 1993), up regulates synaptophysin, SNAP-25, and Kv4.2, which is consistent with our finding that these molecules are elevated in apical neurons.
With regard to BDNF, there is evidence that it is present in spiral ganglion neurons from early development throughout adulthood (Ruttiger et al., 2007; Schecterson and Bothwell, 1994; Schimmang et al., 2003; Singer et al., 2008). Moreover, the distribution is graded, with higher levels in the base than in the apex (Ruttiger et al., 2007; Schimmang et al., 2003; Singer et al., 2008). It is also clear that BDNF can be found in the cochlear hair cell receptors during embryonic development, but whether it is present postnatally and during adulthood is controversial. Research utilizing postnatal tissues show that BDNF is either absent (Wheeler et al., 1994) or present at lower levels than detected embryonically (Pirvola et al., 1992; Ylikoski et al., 1993). Descriptions of BDNF tonotopic distribution range from observations of levels that are higher in the apical cochlea during early development (Farinas et al., 2001; Pirvola et al., 1992) to studies utilizing cochlear microisolates which demonstrate that BDNF is selectively secreted from basal postnatal cochleae (Flores-Otero et al., 2007). Investigations of BDNF distributions in the adult cochlea report that it is either not expressed (Wheeler et al., 1994; Ylikoski et al., 1993), or is present (Breuskin et al., 2010) and elevated within the basal regions (Flores-Otero and Davis, in press; Tan and Shepherd, 2006). Moreover, BDNF up regulates Kv1.1, Kv3.1, BK, GluR2 and GluR3, which are all elevated in basal neurons. Nevertheless, it is evident that additional work needs to be done to clarify this important issue.
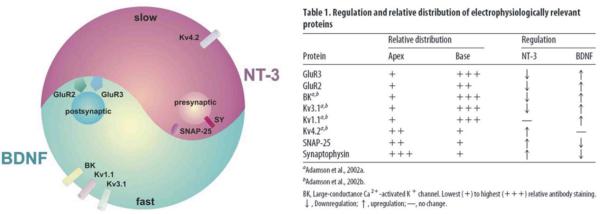
Interestingly, we observed that BDNF and NT-3 generally have `yin-yang' effects on these electrophysiologically-relevant proteins (Fig. 3). Most of the voltage-gated ion channels and synaptic proteins that are up regulated by NT-3 are down regulated by BDNF. The mirror image is true: most of the voltage-gated ion channels and synaptic proteins that are up regulated by BDNF are down regulated by NT-3. This indicates that the phenotypes produced by the two oppositely-oriented neurotrophin distributions are amplified by this subtractive effect such that they form a steeper overall gradient than what would ensue from the action of either neurotrophin alone. Furthermore, the down regulation by neurotrophins indicates that the phenotype can be regulated below baseline levels. Observations of the yin-yang regulation of electrophysiologically-relevant proteins strongly indicate that BDNF and NT-3 activate distinctly different elements of trk-based signaling pathways. The spiral ganglion is one of very limited neuronal systems that can be studied to assess this important distinction (Huang and Reichardt, 2003).
Fig. 3.
The neurotrophins BDNF and NT-3 have `yin-yang' regulatory effects on voltage-gated ion channel and synaptic protein composition in spiral ganglion neurons. In general, when one of the electrophysiologically-relevant proteins (described in the text) was up regulated by one neurotrophin, it was down regulated by the other. Kv1.1 and Kv4.2 were exceptions; only up regulation by the respective neurotrophins, BDNF and NT-3, was noted. Table from Flores-Otero et al., 2007.
4. Timing-specific endogenous neuronal properties are graded within the spiral ganglion
Initial experiments on spiral ganglion neurons concentrated on differences between cells isolated separately from high and low frequency regions of the ganglion (Adamson et al., 2002a; Adamson et al., 2002b; Davis, 1996; Flores-Otero and Davis, in press; Flores-Otero et al., 2007; Reid et al., 2004; Zhou et al., 2005). To take the next step in relating form to function, the properties of neurons along the entire extent of the spiral ganglion have begun to be characterized. Immunolocalization of anti-GluR2/3 and anti-synaptophysin antibodies along the tonotopic contour of cochlear sections show unequivocally that each of these proteins is distributed in a systematic linear gradient but in opposite cochlear orientations (Flores-Otero and Davis, in press). Furthermore, we directly assessed the mid-frequency distribution of electrophysiological parameters by recording from and labeling neurons in a novel “gangliotopic” preparation that consists of neurons obtained from the entire length of the ganglion with their relative locations still intact (Fig. 4a).
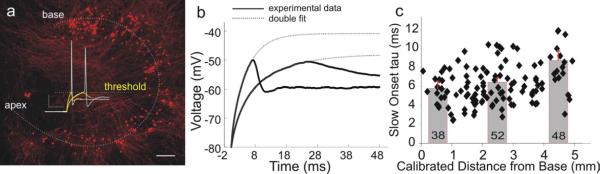
Fig. 4.
Spiral ganglion neurons plated with their relative locations intact showed graded differences in membrane kinetics that become progressively slower from base to apex. a, A gangliotopic culture, stained with anti-β-tubulin antibody (red), was used to correlate neuronal firing properties with their gangliotopic position. A dotted line was drawn by eye to approximate the apex to base tonotopic axis. Scale bar: 200 μm. Inset current clamp recordings showed differences in onset time constant and latency at threshold with matched holding potential (−80 mV) and voltage threshold from one apical and one basal neuron. b, double-exponential functions (dotted line) fitted to threshold onset (thick black) from the same two recordings in panel a (yellow traces). The long-latency apical neuron (right) showed slower onset time constant than the basal neuron (left). c, slow onset tau (time constant measured from slow exponential component) plotted as a function of location in gangliotopic and neuronal cultures. For this and subsequent figures, scatter plot and bar graph represents gangliotopic culture and neuronal culture data respectively. Number of recordings shown in each bar applies to this and subsequent figures. Panels b and c were adapted from Liu and Davis 2007.
To examine the distribution of timing-related features along the length of the spiral ganglion, the onset time course of the membrane potential in response to sub-threshold constant current injection was quantified (see sub-threshold traces in Fig. 2 top panel, Fig. 4a insert, Fig. 4b). It is obvious that the change in membrane potential is more rapid in basal neurons than in apical ones, and consequently the latency to action potential firing is shorter in the base than in the apex. When onset time course was fitted with double exponentials as shown in Figure 4b and evaluated along with the gangliotopic location of each neuron from which the recording was made (Fig. 4c, black diamonds) a clear gradient was observed. As a control, additional data averaged from neurons separated into apical, middle and basal fifths of the ganglion and plated into separate culture dishes showed a similar distribution (Fig. 4c, gray bars). An additional result obtained from this study was the degree of local heterogeneity observed at each cochlear location. As we discuss in the next section, this heterogeneity may be an important feature of information coding in the spiral ganglion.
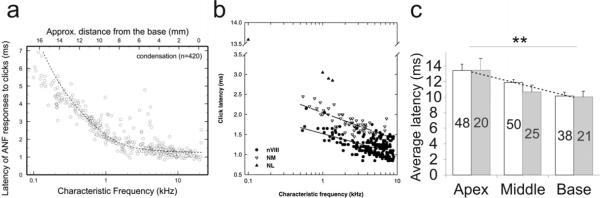
Slow onset time course could effectively introduce delays in the responses of spiral ganglion neurons to auditory stimuli, particularly for low frequency sounds. Interestingly, when extracellular single unit recordings were made from auditory nerve fibers in adult chinchilla (Temchin et al., 2005), the longest latency delays to click stimuli were found in the lowest characteristic frequency regions, revealing a parameter with a clear tonotopic distribution (Fig. 5a). The systematic changes in signal delay have been commonly attributed to mechanics of the basilar membrane traveling wave as it moves from base to apex (Ruggero, 1992). However, systematic tonotopic latency responses to clicks were not significantly altered in species having a prominent auditory fovea where spatial occupancy of the basilar membrane over-represents the best frequencies of hearing (3–10 kHz; Fig. 5b). This indicates strongly that basilar membrane mechanics cannot be the sole explanation for the delay in apical neuron responses and that subsequent stage(s) of peripheral auditory processing might contribute to this phenomenon (Koppl, 1997b). Latency differences observed from our in vitro studies (Fig. 5c) could indicate that endogenous properties of spiral ganglion neurons along with potential contributions from synaptic input may insert different delays in high and low frequency regions that decrease from apex to base (Nagel, 1974; Miller et al., 1993). Furthermore, similar specializations have been noted in higher auditory centers (Brew and Forsythe, 2005; Li et al., 2001) and may also contribute. Taken together these data strengthen the idea that intrinsic delay by endogenous neuronal properties is an important regulator of signal timing in addition to synaptic delays and signal transmission along the axons. What remains to be elucidated is the relative timing relationship contributed by each stage of signal processing.
Fig. 5.
Tonotopic comparison of response latencies in vivo and in vitro. a, chinchilla auditory nerve latency responses to intense condensation clicks with custom fit to group data. b, Barn owl auditory nerve latencies as a function of characteristic frequency at different recording sites with logarithmic fits. Filled circles represent responses from the primary afferents, upward and downward triangles show responses from the nucleus laminaris (NL) and nucleus magnocellularis (NM), respectively. c, Average action potential latency at threshold from apex to base. White and gray bar represents data from neuronal and gangliotopic culture respectively. Line fits to combined means. **, P < 0.01, one-way ANOVA with post hoc Tukey-Kramer pairwise comparison. Panels a–c adapted from Temchin et al., 2005, (Koppl, 1997a; Liu and Davis, 2007; Temchin et al., 2005), respectively.
5. Sensitivity-related endogenous neuronal properties are distributed non-monotonically within the spiral ganglion
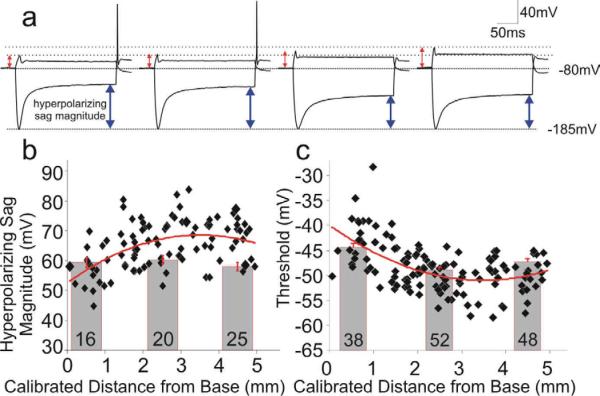
The in vitro gangliotopic preparation that we developed was also instrumental in revealing electrophysiological parameters that do not shift monotonically with cochlear location, thus identifying features that could contribute to the coding of sub-modalities other than frequency, such as intensity. We noted from current clamp recordings that the prominent hyperpolarizing sag that typifies the hyperpolarization-activated cationic current (Ih) found in spiral ganglion neurons (Chen, 1997; Mo and Davis, 1997b) was one of these features. Rather than displaying a linear tonotopic gradient, we found that the magnitude of this inward rectification peaked in the mid-apical cochlear region and was heterogeneous throughout (Fig. 6b). Assuming that this measurement from current clamp recordings is indicative of the magnitude of the Ih current, one would predict that the resting membrane potential would be more depolarized in mid-frequency neurons, thus rendering neurons in this region more excitable. Utilizing the Ih blocker CsCl, we and others have found that the Ih current does indeed contribute to setting the resting membrane potential of spiral ganglion neurons (Liu and Davis, 2008; Yi et al., 2010). Moreover, direct measurement of resting membrane potentials showed that neurons isolated from the mid-apical regions were more depolarized than those isolated from the apical or basal ends, further supporting the idea that this parameter is distributed non-monotonically (Liu et al., in preparation).
Fig. 6.
Intrinsic threshold and inward rectification of spiral ganglion neurons showed a non-monotonic distribution pattern, with elevated sensitivity and inward rectification positioned in the mid-apical spiral ganglion. a, Current clamp traces illustrate an inverse relationship between threshold voltage (red arrowheads) and hyperpolarizing sag magnitude (blue arrowheads): the higher the threshold (left to right), the lower the sag magnitude. Dashed lines indicate their highest (−45.8mV) and lowest (−57.6mV) threshold levels. Holding potential: −80mV; hyperpolarization peak: −185mV. b & c: hyperpolarizing sag magnitude (b) and threshold (c) plotted as a function of location in gangliotopic and neuronal cultures. Adapted from Liu and Davis, 2007.
Experiments of this type also showed that the threshold voltage for firing an action potential varied systematically with the magnitude of hyperpolarizing inward rectification (Fig. 6a). Threshold voltage was heterogeneous, yet showed a clear trend such that neurons with the lowest thresholds were located toward the mid-apical regions of the gangliotopic cultures (Fig. 6c). Interestingly, these two findings regarding resting membrane potential and action potential threshold are complementary, and they correlate well with in vivo observations that neurons in the mid-ganglion region are typically the most excitable (Fay and Popper, 2000; Liberman, 1982b; Rosowski, 1991; Wever, 1974). What is remarkable about the distribution pattern of spiral ganglion neuronal excitability is that it appears to overlap with the best frequency region. Although the known mid-frequency sensitivity can be explained almost exclusively by middle ear mechanics (Rosowski, 2003), it has been reported that cochlear and/or neuronal response properties may also contribute to the augmented sensitivity in the mid-frequency range (Ruggero and Temchin, 2002). The spiral ganglion neurons with their dual intrinsic specializations of low voltage threshold and elevated resting membrane potential in the mid-apical cochlear region may fulfill this role. An investigation into additional voltage-gated current types that contribute to these notable features is currently underway.
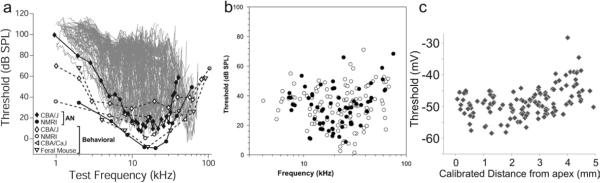
Another interesting aspect of our observations is that despite rigorous controls for data assessment we note a marked overall heterogeneity in many electrophysiological parameters measured from spiral ganglion neurons (Adamson et al., 2002a; Liu and Davis, 2007; Mo et al., 2002). This is especially true for threshold voltage levels and hyperpolarizing sag magnitude. One possible interpretation is that the variation is utilized to construct a range of threshold sensitivities among the spiral ganglion neurons innervating each inner hair cell, much like what one observes from in vivo recordings in which tuning curves are plotted against the test frequency (Fig. 7a). The tip of the tuning curve (Fig. 7b) can be best compared to our measures of threshold voltage (Fig. 7c). Despite the parameters of the stimulus being necessarily different for the in vivo and in vitro recordings, there is a remarkable similarity between the overall distributions obtained from the two methods, although we find that our measure of threshold voltage appears to be less heterogeneous than that seen in the in vivo recordings (Fig. 7b vs. 7c).
Fig. 7.
Comparison of murine auditory thresholds and neuronal intrinsic thresholds. a, The lowest threshold envelope of superimposed tuning curves of auditory nerve fibers in response to tone bursts matched behavioral threshold data. Filled diamonds and circles were auditory nerve data from CBA/J or NMRI mice. Open shapes of diamond, circle, apex-leftward and apex-downward triangles were behavior data from the indicated species. b, Best thresholds (tips of tuning curves) plotted as a function of nerve fiber characteristic frequency. Close/open circles indicate the success/failure in the use of HRP to label the recording site. c, Gangliotopic data from figure 6c re-plotted from apex to base. Panels a–c adapted from (Müller and Smolders, 2005; Taberner and Liberman, 2005) and Liu and Davis, 2007, respectively.
Overall our findings that neurons in the mid-apical cochlear regions show the greatest sensitivity are strengthened by observations that two separate intrinsic features of the neurons co-vary. Neurons within the region of highest sensitivity show both reduced absolute voltage thresholds and increased resting potential levels. Either of these features alone would enhance the excitability of spiral ganglion neurons, yet when expressed together predicts an even more robust neuronal phenotype. Therefore, these two parameters, threshold and resting membrane potential, can enhance neuronal sensitivity and may also contribute to the heterogeneity that is observed prominently in the most sensitive frequency region. In placing these findings into a functional context, one must consider the possibility that in addition to synaptic specializations that correlate with spontaneous rate (Merchan-Perez and Liberman, 1996), the endogenous properties of the neurons may also contribute to a feature which is likely to underlie intensity coding in the auditory system.
6. Concluding remarks
The tonotopic distribution of timing-related parameters and the non-monotonic distribution of excitability within the spiral ganglion correspond well with the known functional organization of the peripheral auditory system. Because these parameters are controlled by numerous kinds of ion channels, sophisticated regulatory mechanisms must be in place to orchestrate their cell-specific expression. The opposite actions of BDNF and NT-3 in this regard have provided a great deal of information, but there is much that remains to be done, particularly with reconciling the gradients of the two neurotrophins with the distribution of ion channels that determine resting membrane potential and the threshold voltage for action potential firing.
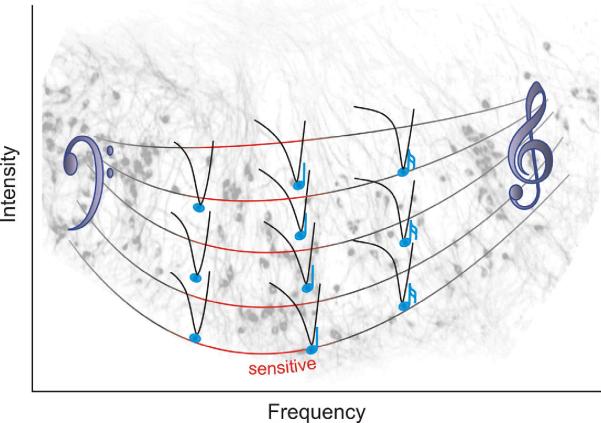
It is clear, however, that the question posed at the beginning of this review has a functionally-relevant answer - neurons of the spiral ganglion possess electrophysiological specializations that may contribute to encoding of auditory information in vivo. Multiple approaches have been utilized to show convincingly that the kinetic features change systematically along the tonotopic axis whereas neuronal excitability is distributed such that the most sensitive neurons are located in the mid-cochlear region associated with greatest sensitivity. The duality of endogenous timing and sensitivity of spiral ganglion neurons and their unique distributions along the tonotopic contour are summarized in Figure 8. Superimposed over a cluster of β-tubulin stained spiral ganglion neurons (gray profiles) is the heterogeneous and enhanced sensitivity in the mid-apical frequency region depicted by the elongation of the scale bars toward a lower intensity level and the greater number of tuning curves. Simultaneously, there is a smooth progression from `slow' to `fast' firing features that are graded smoothly from the low frequency region (left) to the high frequency region (right) denoted by the musical note symbols that demark the tips of the tuning curves. The sophisticated phenotypic tuning of spiral ganglion neurons undoubtedly plays a functional role in auditory signal encoding and should be carefully considered when designing new clinical approaches.
FIG. 8.
There are two parameters of spiral ganglion neuron phenotypic specializations that vary along the tonotopic map. The first represented by the timing of the musical notes at the tips of the neuronal tuning curves is the kinetic features of the neurons at thresholds that vary in a linear gradient. The second represented by the shape of the neuronal cluster shown in the background (gray, stained with anti-β-tubulin antibody) and the U-shaped scale bar is the non-monotonic neuronal sensitivity. The most sensitive neurons (red within the mid-apical region) have elevated resting membrane potentials and enhanced excitability due to reduced threshold voltages.
Acknowledgement
We thank Dr. Mark R. Plummer for discussions and critical reading of the manuscript. Supported by NIH NIDCD RO1 DC01856.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Adamson CL, Reid MA, Davis RL. Opposite actions of brain-derived neurotrophic factor and neurotrophin- 3 on firing features and ion channel composition of murine spiral ganglion neurons. J Neurosci. 2002a;22:1385–96. doi: 10.1523/JNEUROSCI.22-04-01385.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson CL, Reid MA, Mo ZL, Bowne-English J, Davis RL. Firing features and potassium channel content of murine spiral ganglion neurons vary with cochlear location. J Comp Neurol. 2002b;447:331–50. doi: 10.1002/cne.10244. [DOI] [PubMed] [Google Scholar]
- Anniko M, Arnold W, Stigbrand T, Strom A. The human spiral ganglion. ORL J Otorhinolaryngol Relat Spec. 1995;57:68–77. doi: 10.1159/000276714. [DOI] [PubMed] [Google Scholar]
- Art JJ, Fettiplace R. Variation of membrane properties in hair cells isolated from the turtle cochlea. J Physiol (Lond) 1987;385:207–42. doi: 10.1113/jphysiol.1987.sp016492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore J, Gale J. The cochlea. Curr Biol. 2000;10:R325–7. doi: 10.1016/s0960-9822(00)00457-7. [DOI] [PubMed] [Google Scholar]
- Barbacid M. The Trk family of neurotrophin receptors. J Neurobiol. 1994;25:1386–403. doi: 10.1002/neu.480251107. [DOI] [PubMed] [Google Scholar]
- Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci. 2007;8:451–65. doi: 10.1038/nrn2148. [DOI] [PubMed] [Google Scholar]
- Benson TE, Brown MC. Postsynaptic targets of type II auditory nerve fibers in the cochlear nucleus. J Assoc Res Otolaryngol. 2004;5:111–25. doi: 10.1007/s10162-003-4012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuskin I, Bodson M, Thelen N, Thiry M, Borgs L, Nguyen L, Stolt C, Wegner M, Lefebvre PP, Malgrange B. Glial but not neuronal development in the cochleo-vestibular ganglion requires Sox10. J Neurochem. 2010;114:1827–39. doi: 10.1111/j.1471-4159.2010.06897.x. [DOI] [PubMed] [Google Scholar]
- Brew HM, Forsythe ID. Systematic variation of potassium current amplitudes across the tonotopic axis of the rat medial nucleus of the trapezoid body. Hear Res. 2005;206:116–32. doi: 10.1016/j.heares.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Brown MC. Antidromic responses of single units from the spiral ganglion. J Neurophysiol. 1994;71:1835–47. doi: 10.1152/jn.1994.71.5.1835. [DOI] [PubMed] [Google Scholar]
- Cant NB. The Cochlear Nucleus: Neuronal types and their synaptic organization. In: Webster DB, Popper AN, Fay RR, editors. The Mammalian Auditory Pathway: Neuroanatomy. Springer-Verlag; New York: 1992. pp. 66–116. [Google Scholar]
- Chen C. Hyperpolarization-activated current (Ih) in primary auditory neurons. Hear Res. 1997;110:179–90. doi: 10.1016/s0378-5955(97)00078-6. [DOI] [PubMed] [Google Scholar]
- Chen WC, Davis RL. Voltage-gated and two-pore-domain potassium channels in murine spiral ganglion neurons. Hear Res. 2006;222:89–99. doi: 10.1016/j.heares.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Cochran SL, Stone JS, Bermingham-McDonogh O, Akers SR, Lefcort F, Rubel EW. Ontogenetic expression of trk neurotrophin receptors in the chick auditory system. J Comp Neurol. 1999;413:271–88. doi: 10.1002/(sici)1096-9861(19991018)413:2<271::aid-cne8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Corwin JT, Warchol ME. Auditory hair cells: structure, function, development, and regeneration. Annu Rev Neurosci. 1991;14:301–33. doi: 10.1146/annurev.ne.14.030191.001505. [DOI] [PubMed] [Google Scholar]
- Davis RL. Differential distribution of potassium channels in acutely demyelinated, primary-auditory neurons in vitro. J Neurophysiol. 1996;76:438–47. doi: 10.1152/jn.1996.76.1.438. [DOI] [PubMed] [Google Scholar]
- Echteler SM, Nofsinger YC. Development of ganglion cell topography in the postnatal cochlea. J Comp Neurol. 2000;425:436–46. [PubMed] [Google Scholar]
- Ernfors P, Merlio J-P, Persson H. Cells expressing mRNA for neurotrophins and their receptors during embryonic rat development. Eur. J. Neurosci. 1992;4:1140–1158. doi: 10.1111/j.1460-9568.1992.tb00141.x. [DOI] [PubMed] [Google Scholar]
- Farinas I, Jones KR, Tessarollo L, Vigers AJ, Huang E, Kirstein M, de Caprona DC, Coppola V, Backus C, Reichardt LF, Fritzsch B. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. J Neurosci. 2001;21:6170–6180. doi: 10.1523/JNEUROSCI.21-16-06170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay RR, Popper AN. Evolution of hearing in vertebrates: the inner ears and processing. Hear Res. 2000;149:1–10. doi: 10.1016/s0378-5955(00)00168-4. [DOI] [PubMed] [Google Scholar]
- Flores-Otero J, Davis RL. Synaptic proteins are tonotopically graded in postnatal and adult type I and type II spiral ganglion neurons. J Comp Neurol. doi: 10.1002/cne.22576. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Otero J, Xue HZ, Davis RL. Reciprocal regulation of presynaptic and postsynaptic proteins in bipolar spiral ganglion neurons by neurotrophins. J Neurosci. 2007;27:14023–34. doi: 10.1523/JNEUROSCI.3219-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Farinas I, Reichardt LF. Lack of neurotrophin 3 causes losses of both classes of spiral ganglion neurons in the cochlea in a region-specific fashion. J Neurosci. 1997;17:6213–25. doi: 10.1523/JNEUROSCI.17-16-06213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Diaz JF. Development of a fast transient potassium current in chick cochlear ganglion neurons. Hear Res. 1999;135:124–34. doi: 10.1016/s0378-5955(99)00099-4. [DOI] [PubMed] [Google Scholar]
- Hisashi K, Nakagawa T, Yasuda T, Kimitsuki T, Komune S, Komiyama S. Voltage-dependent Ca2+ channels in the spiral ganglion cells of guinea pig cochlea. Hear Res. 1995;91:196–201. doi: 10.1016/0378-5955(95)00191-3. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–42. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Jagger DJ, Housley GD. A-type potassium currents dominate repolarisation of neonatal rat primary auditory neurones in situ. Neuroscience. 2002;109:169–82. doi: 10.1016/s0306-4522(01)00454-7. [DOI] [PubMed] [Google Scholar]
- Jimenez C, Gireldez F, Represa J, Garcia-Diaz JF. Calcium currents in dissociated cochlear neurons from the chick embryo and their modification by neurotrophin-3. Neuroscience. 1997;77:673–82. doi: 10.1016/s0306-4522(96)00505-2. [DOI] [PubMed] [Google Scholar]
- Kandler K, Clause A, Noh J. Tonotopic reorganization of developing auditory brainstem circuits. Nat Neurosci. 2009;12:711–7. doi: 10.1038/nn.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawase T, Liberman MC. Spatial organization of the auditory nerve according to spontaneous discharge rate. J Comp Neurol. 1992;319:312–8. doi: 10.1002/cne.903190210. [DOI] [PubMed] [Google Scholar]
- Keithley EM, Schreiber RC. Frequency map of the spiral ganglion in the cat. J Acoust Soc Am. 1987;81:1036–42. doi: 10.1121/1.394675. [DOI] [PubMed] [Google Scholar]
- Kiang NY. Curious oddments of auditory-nerve studies. Hear Res. 1990;49:1–16. doi: 10.1016/0378-5955(90)90091-3. [DOI] [PubMed] [Google Scholar]
- Kiang NY, Watanabe T, Thomas EC, Clark LF. Discharge Patterns of single fibers in the cat's auditory nerve. Vol. 35. The M.I.T. Press; Cambridege, Massachusetts: 1965. [Google Scholar]
- Kiang YNS, Liberman MC, Gage JS, Northrop CC, Dodds LW, Oliver ME. Comparative Physiology of Sensory Systems. Cambridge University Press; 1984. Afferent innervation of the mammalian cochlea; pp. 143–161. [Google Scholar]
- Knipper M, Zimmermann U, Rohbock K, Kopschall I, Zenner HP. Expression of neurotrophin receptor trkB in rat cochlear hair cells at time of rearrangement of innervation. Cell Tissue Res. 1996;283:339–53. doi: 10.1007/s004410050545. [DOI] [PubMed] [Google Scholar]
- Koppl C. Frequency tuning and spontaneous activity in the auditory nerve and cochlear nucleus magnocellularis of the barn owl Tyto alba. J Neurophysiol. 1997a;77:364–77. doi: 10.1152/jn.1997.77.1.364. [DOI] [PubMed] [Google Scholar]
- Koppl C. Phase locking to high frequencies in the auditory nerve and cochlear nucleus magnocellularis of the barn owl, Tyto alba. J Neurosci. 1997b;17:3312–21. doi: 10.1523/JNEUROSCI.17-09-03312.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake PA, Snyder RL, Merzenich MM. Topographic organization of the cochlear spiral ganglion demonstrated by restricted lesions of the anteroventral cochlear nucleus. J Comp Neurol. 1992;320:468–78. doi: 10.1002/cne.903200405. [DOI] [PubMed] [Google Scholar]
- Li W, Kaczmarek LK, Perney TM. Localization of two high-threshold potassium channel subunits in the rat central auditory system. J Comp Neurol. 2001;437:196–218. doi: 10.1002/cne.1279. [DOI] [PubMed] [Google Scholar]
- Liberman MC. Single-neuron labeling in the cat auditory nerve. Science. 1982a;216:1239–41. doi: 10.1126/science.7079757. [DOI] [PubMed] [Google Scholar]
- Liberman MC. The cochlear frequency map for the cat: labeling auditory-nerve fibers of known characteristic frequency. J Acoust Soc Am. 1982b;72:1441–9. doi: 10.1121/1.388677. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Dodds LW, Pierce S. Afferent and efferent innervation of the cat cochlea: quantitative analysis with light and electron microscopy. J Comp Neurol. 1990;301:443–60. doi: 10.1002/cne.903010309. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Oliver ME. Morphometry of intracellularly labeled neurons of the auditory nerve: correlations with functional properties. J Comp Neurol. 1984;223:163–76. doi: 10.1002/cne.902230203. [DOI] [PubMed] [Google Scholar]
- Lin X. Action potentials and underlying voltage-dependent currents studied in cultured spiral ganglion neurons of the postnatal gerbil. Hear Res. 1997;108:157–79. doi: 10.1016/s0378-5955(97)00050-6. [DOI] [PubMed] [Google Scholar]
- Lin X, Chen S. Endogenously generated spontaneous spiking activities recorded from postnatal spiral ganglion neurons in vitro. Brain Res Dev Brain Res. 2000;119:297–305. doi: 10.1016/s0165-3806(99)00169-8. [DOI] [PubMed] [Google Scholar]
- Liu Q, Davis RL. Regional specification of threshold sensitivity and response time in CBA/CaJ mouse spiral ganglion neurons. J Neurophysiol. 2007;98:2215–22. doi: 10.1152/jn.00284.2007. [DOI] [PubMed] [Google Scholar]
- Liu Q, Davis RL. Regional determinants of neuronal excitability in the murine spiral ganglion neurons. Abstracts Assoc. Res. Otolaryngol. 2008;31 [Google Scholar]
- Loewenstein WR, Mendelson M. Components of receptor adaptation in a Pacinian corpuscle. Journal of Physiology (London) 1965;177:377–397. doi: 10.1113/jphysiol.1965.sp007598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez CA, Olson ES, Adams JC, Mou K, Denhardt DT, Davis RL. Osteopontin expression detected in adult cochleae and inner ear fluids. Hear Res. 1995;85:210–22. doi: 10.1016/0378-5955(95)00046-7. [DOI] [PubMed] [Google Scholar]
- Masland RH, Raviola E. Confronting complexity: strategies for understanding the microcircuitry of the retina. Annu Rev Neurosci. 2000;23:249–84. doi: 10.1146/annurev.neuro.23.1.249. [DOI] [PubMed] [Google Scholar]
- Merchan-Perez A, Liberman MC. Ultrastructural differences among afferent synapses on cochlear hair cells: correlations with spontaneous discharge rate. J Comp Neurol. 1996;371:208–21. doi: 10.1002/(SICI)1096-9861(19960722)371:2<208::AID-CNE2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Mo ZL, Adamson CL, Davis RL. Dendrotoxin-sensitive K(+) currents contribute to accommodation in murine spiral ganglion neurons. J Physiol. 2002;542:763–78. doi: 10.1113/jphysiol.2002.017202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo ZL, Davis RL. Endogenous firing patterns of murine spiral ganglion neurons. J Neurophysiol. 1997a;77:1294–305. doi: 10.1152/jn.1997.77.3.1294. [DOI] [PubMed] [Google Scholar]
- Mo Z-L, Davis RL. Heterogeneous voltage dependence of inward rectifier currents in spiral ganglion neurons. J Neurophysiol. 1997b;78:3019–27. doi: 10.1152/jn.1997.78.6.3019. [DOI] [PubMed] [Google Scholar]
- Moore EJ, Hall DB, Narahashi T. Sodium and potassium currents of type I spiral ganglion cells from rat. Acta Otolaryngol. 1996;116:552–60. doi: 10.3109/00016489609137888. [DOI] [PubMed] [Google Scholar]
- Mou K, Hunsberger CL, Cleary JM, Davis RL. Synergistic effects of BDNF and NT-3 on postnatal spiral ganglion neurons. J Comp Neurol. 1997;386:529–39. [PubMed] [Google Scholar]
- Müller M, Smolders JW. Shift in the cochlear place-frequency map after noise damage in the mouse. Neuroreport. 2005;16:1183–7. doi: 10.1097/00001756-200508010-00010. [DOI] [PubMed] [Google Scholar]
- Nadol JB, Jr, Burgess BJ, Reisser C. Morphometric analysis of normal human spiral ganglion cells. Ann Otol Rhinol Laryngol. 1990;99:340–8. doi: 10.1177/000348949009900505. [DOI] [PubMed] [Google Scholar]
- Patapoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol. 2001;11:272–80. doi: 10.1016/s0959-4388(00)00208-7. [DOI] [PubMed] [Google Scholar]
- Pirvola U, Arumae U, Moshnyakov M, Palgi J, Saarma M, Ylikoski J. Coordinated expression and function of neurotrophins and their receptors in the rat inner ear during target innervation. Hear Res. 1994;75:131–44. doi: 10.1016/0378-5955(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Pirvola U, Hallbook F, Xing-Qun L, Virkkala J, Saarma M, Ylikoski J. Expression of neurotrophins and Trk receptors in the developing, adult, and regenerating avian cochlea. J Neurobiol. 1997;33:1019–33. doi: 10.1002/(sici)1097-4695(199712)33:7<1019::aid-neu11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Pirvola U, Ylikoski J, Palgi J, Lehtonen E, Arumae U, Saarma M. Brain-derived neurotrophic factor and neurotrophin 3 mRNAs in the peripheral target fields of developing inner ear ganglia. Proc Natl Acad Sci U S A. 1992;89:9915–9. doi: 10.1073/pnas.89.20.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael Y, Altschuler RA. Structure and innervation of the cochlea. Brain Res Bull. 2003;60:397–422. doi: 10.1016/s0361-9230(03)00047-9. [DOI] [PubMed] [Google Scholar]
- Reid MA, Flores-Otero J, Davis RL. Firing patterns of type II spiral ganglion neurons in vitro. J Neurosci. 2004;24:733–42. doi: 10.1523/JNEUROSCI.3923-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci AJ, Crawford AC, Fettiplace R. Tonotopic variation in the conductance of the hair cell mechanotransducer channel. Neuron. 2003;40:983–90. doi: 10.1016/s0896-6273(03)00721-9. [DOI] [PubMed] [Google Scholar]
- Richter CP, Emadi G, Getnick G, Quesnel A, Dallos P. Tectorial membrane stiffness gradients. Biophys J. 2007;93:2265–76. doi: 10.1529/biophysj.106.094474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. Possible relation between structure and spike shapes of neurones in guinea pig cochlear ganglion. Brain Res. 1976;109:487–96. doi: 10.1016/0006-8993(76)90029-9. [DOI] [PubMed] [Google Scholar]
- Robertson D, Sellick PM, Patuzzi R. The continuing search for outer hair cell afferents in the guinea pig spiral ganglion. Hear Res. 1999;136:151–8. doi: 10.1016/s0378-5955(99)00120-3. [DOI] [PubMed] [Google Scholar]
- Romand R, Sobkowicz H, Emmerling M, Whitlon D, Dahl D. Patterns of neurofilament stain in the spiral ganglion of the developing and adult mouse. Hear Res. 1990;49:119–25. doi: 10.1016/0378-5955(90)90099-b. [DOI] [PubMed] [Google Scholar]
- Rosbe KW, Burgess BJ, Glynn RJ, Nadol JB., Jr Morphologic evidence for three cell types in the human spiral ganglion. Hear Res. 1996;93:120–7. doi: 10.1016/0378-5955(95)00208-1. [DOI] [PubMed] [Google Scholar]
- Rosenbluth J. The fine structure of acoustic ganglia in the rat. J Cell Biol. 1962;12:329–59. doi: 10.1083/jcb.12.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosowski JJ. The effects of external- and middle-ear filtering on auditory threshold and noise-induced hearing loss. J Acoust Soc Am. 1991;90:124–35. doi: 10.1121/1.401306. [DOI] [PubMed] [Google Scholar]
- Rosowski JJ. Sensors and Sensing in Biology and Engineering. Springer-Verlag; New York: 2003. The Middle and External Ears of Terrestrial Vertebrates as Mechanical and Acoustic Transducers; pp. 59–69. [Google Scholar]
- Rouiller EM, Cronin-Schreiber R, Fekete DM, Ryugo DK. The central projections of intracellularly labeled auditory nerve fibers in cats: an analysis of terminal morphology. J Comp Neurol. 1986;249:261–78. doi: 10.1002/cne.902490210. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory System Development: Primary Auditory Neurons and Their Targets. Annu Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Ruggero MA, Temchin AN. The roles of the external, middle, and inner ears in determining the bandwidth of hearing. Proc Natl Acad Sci U S A. 2002;99:13206–10. doi: 10.1073/pnas.202492699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttiger L, Panford-Walsh R, Schimmang T, Tan J, Zimmermann U, Rohbock K, Kopschall I, Limberger A, Muller M, Fraenzer JT, Cimerman J, Knipper M. BDNF mRNA expression and protein localization are changed in age-related hearing loss. Neurobiol Aging. 2007;28:586–601. doi: 10.1016/j.neurobiolaging.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Ryugo DK. The auditory nerve: Peripheral innervation cell body morphology, and central projections. In: Webster DB, Popper AN, Fay RR, editors. The Mammalian Auditory Pathway: Neuroanatomy. Springer-Verlag; New York: 1992. pp. 23–65. [Google Scholar]
- Sachs MB, Young ED, Lewis RH. Discharge patterns of single fibers in the pigeon auditory nerve. Brain Res. 1974;70:431–47. doi: 10.1016/0006-8993(74)90253-4. [DOI] [PubMed] [Google Scholar]
- Salih SG, Housley GD, Raybould NP, Thorne PR. ATP-gated ion channel expression in primary auditory neurones. Neuroreport. 1999;10:2579–86. doi: 10.1097/00001756-199908200-00026. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J. Voltage-dependent ionic conductances of type I spiral ganglion cells from the guinea pig inner ear. J Neurosci. 1993;13:3599–611. doi: 10.1523/JNEUROSCI.13-08-03599.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecterson LC, Bothwell M. Neurotrophin and neurotrophin receptor mRNA expression in developing inner ear. Hear Res. 1994;73:92–100. doi: 10.1016/0378-5955(94)90286-0. [DOI] [PubMed] [Google Scholar]
- Schimmang T, Tan J, Müller M, Zimmermann U, Rohbock K, Kopschall I, Limberger A, Minichiello L, Knipper M. Lack of Bdnf and TrkB signalling in the postnatal cochlea leads to a spatial reshaping of innervation along the tonotopic axis and hearing loss. Development. 2003;130:4741–50. doi: 10.1242/dev.00676. [DOI] [PubMed] [Google Scholar]
- Schmiedt RA. Spontaneous rates, thresholds and tuning of auditory-nerve fibers in the gerbil: comparisons to cat data. Hear Res. 1989;42:23–35. doi: 10.1016/0378-5955(89)90115-9. [DOI] [PubMed] [Google Scholar]
- Scroggs RS, Fox AP. Calcium current variation between acutely isolated adult rat dorsal root ganglion neurons. Journal of Physiology (London) 1992;445:639–658. doi: 10.1113/jphysiol.1992.sp018944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard DN, Valverde MA, Represa J, Giraldez F. Transient outward currents in cochlear ganglion neurons of the chick embryo. Neuroscience. 1992;51:631–9. doi: 10.1016/0306-4522(92)90302-i. [DOI] [PubMed] [Google Scholar]
- Singer W, Panford-Walsh R, Watermann D, Hendrich O, Zimmermann U, Kopschall I, Rohbock K, Knipper M. Salicylate alters the expression of calcium response transcription factor 1 in the cochlea: implications for brain-derived neurotrophic factor transcriptional regulation. Mol Pharmacol. 2008;73:1085–91. doi: 10.1124/mol.107.041814. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Innervation densities of the cochlea. Acta Otolaryngol. 1972;73:235–48. doi: 10.3109/00016487209138937. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Neuroanatomical basis of cochlear coding mechanisms. Audiology. 1975;14:383–407. doi: 10.3109/00206097509071752. [DOI] [PubMed] [Google Scholar]
- Sugawara M, Murtie JC, Stankovic KM, Liberman MC, Corfas G. Dynamic patterns of neurotrophin 3 expression in the postnatal mouse inner ear. J Comp Neurol. 2007;501:30–7. doi: 10.1002/cne.21227. [DOI] [PubMed] [Google Scholar]
- Szabo ZS, Harasztosi CS, Sziklai I, Szucs G, Rusznak Z. Ionic currents determining the membrane characteristics of type I spiral ganglion neurons of the guinea pig. Eur J Neurosci. 2002;16:1887–95. doi: 10.1046/j.1460-9568.2002.02258.x. [DOI] [PubMed] [Google Scholar]
- Taberner AM, Liberman MC. Response properties of single auditory nerve fibers in the mouse. J Neurophysiol. 2005;93:557–69. doi: 10.1152/jn.00574.2004. [DOI] [PubMed] [Google Scholar]
- Tan J, Shepherd RK. Aminoglycoside-induced degeneration of adult spiral ganglion neurons involves differential modulation of tyrosine kinase B and p75 neurotrophin receptor signaling. Am J Pathol. 2006;169:528–43. doi: 10.2353/ajpath.2006.060122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temchin AN, Recio-Spinoso A, van Dijk P, Ruggero MA. Wiener kernels of chinchilla auditory-nerve fibers: verification using responses to tones, clicks, and noise and comparison with basilar-membrane vibrations. J Neurophysiol. 2005;93:3635–48. doi: 10.1152/jn.00885.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toesca A. Central and peripheral myelin in the rat cochlear and vestibular nerves. Neurosci Lett. 1996;221:21–4. doi: 10.1016/s0304-3940(96)13273-0. [DOI] [PubMed] [Google Scholar]
- von Békésy G. Travelling waves as frequency analysers in the cochlea. Nature. 1970;225:1207–9. doi: 10.1038/2251207a0. [DOI] [PubMed] [Google Scholar]
- Weisz C, Glowatzki E, Fuchs P. The postsynaptic function of type II cochlear afferents. Nature. 2009;461:1126–9. doi: 10.1038/nature08487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wever EG. Handbook of Sensory Physiology. Vol. I. Springer-Verlag; New York: 1974. The Evolution of Vertebrate Hearing; pp. 423–454. [Google Scholar]
- Wheeler EF, Bothwell M, Schecterson LC, von Bartheld CS. Expression of BDNF and NT-3 mRNA in hair cells of the organ of Corti: quantitative analysis in developing rats. Hear Res. 1994;73:46–56. doi: 10.1016/0378-5955(94)90281-x. [DOI] [PubMed] [Google Scholar]
- Winter IM, Robertson D, Yates GK. Diversity of characteristic frequency rate-intensity functions in guinea pig auditory nerve fibres. Hear Res. 1990;45:191–202. doi: 10.1016/0378-5955(90)90120-e. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Ohmori H. Voltage-gated and chemically gated ionic channels in the cultured cochlear ganglion neurone of the chick. J Physiol (Lond) 1990;420:185–206. doi: 10.1113/jphysiol.1990.sp017907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi E, Roux I, Glowatzki E. Dendritic HCN channels shape excitatory postsynaptic potentials at the inner hair cell afferent synapse in the mammalian cochlea. J Neurophysiol. 2010;103:2532–43. doi: 10.1152/jn.00506.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylikoski J, Pirvola U, Moshnyakov M, Palgi J, Arumae U, Saarma M. Expression patterns of neurotrophin and their receptor mRNAs in the rat inner ear. Hear Res. 1993;65:69–78. doi: 10.1016/0378-5955(93)90202-c. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Liu Q, Davis RL. Complex regulation of spiral ganglion neuron firing patterns by neurotrophin-3. J Neurosci. 2005;25:7558–66. doi: 10.1523/JNEUROSCI.1735-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]