Abstract
Rapid and accurate retrospective dosimetry is of critical importance and strategic value for the emergency medical response to a large-scale radiological/nuclear event. One technique that has the potential for rapid and accurate dosimetry measurements is electron paramagnetic resonance (EPR) spectroscopy of relatively stable radiation-induced signals (RIS) in fingernails and toenails. Two approaches are being developed for EPR nail dosimetry. In the approach using ex vivo measurements on nail clippings, accurate estimation of the dose-dependent amplitude of the RIS is complicated by the presence of mechanically-induced signals (MIS) that are generated during the nail clipping. Recent developments in ex vivo nail dosimetry, including a thorough characterization of the MIS and an appreciation of the role of hydration and the development of effective analytic techniques, have led to improvements in the accuracy and precision of this approach. An in vivo nail dosimetry approach is also very promising, as it eliminates the problems of MIS from the clipping and it has the potential to be an effective and efficient approach for field deployment. Two types of EPR resonators are being developed for in vivo measurements of fingernails and toenails.
Keywords: EPR dosimetry, nail clippings, in vivo nail dosimetry, ex vivo nail dosimetry
1 Introduction
A catastrophic radiological event, such as the denotation of a nuclear device in a major U.S. city, would cause mass casualties with potentially millions affected (Howe, 2004). Since the medical response effort could not possibly treat everyone who was potentially exposed, it is imperative to rapidly measure individual doses with sufficient accuracy to enable effective triage (Flood et al., this issue; Grace et al., 2010; Swartz et al., this issue). This paper describes the development of a physically-based dosimetry method that uses EPR spectroscopy of human nails to determine the magnitude of exposure to ionizing radiation for the emergency medical response to a large-scale radiological/nuclear event.
Human fingernails and toenails are composed largely of α-keratin, which consists of α-helical peptide chains that are twisted into a left-handed coil and strengthened by disulphide cross links (Chandra and Symons, 1987). Ionizing radiation generates free radicals in the keratin matrix, and these radicals are relatively stable. While there is no significant signal decay for more than a week, this stability is affected by the temperature and the water content of the nail. Most importantly, the number of radicals is proportional to the magnitude of the dose over a wide dose range (0–30Gy) (Black and Swarts, 2010; Reyes et al., 2007; 2008; Romanyuhka et al., 2010; Swartz et al., 2007; Swartz et al., 2010; Trompier et al., 2007; Wilcox et al., 2010). These properties, together with the ability to obtain EPR measurements on nails easily and non-invasively, makes them excellent materials for EPR dosimetry.
Nail clippings could be collected and evaluated to determine the radiation dose based on the magnitude of the radiation-induced EPR signal (RIS). Unfortunately, ex vivo measurements of nail clippings necessarily involve a harvesting cut. Since the mechanical cutting of nails also generates EPR-detectable free radicals (mechanically induced signals, denoted MIS), a major challenge to using these samples for EPR dosimetry is the ability to accurately determine the RIS in samples that also possess MIS. Several approaches have been developed to remove the MIS from the nail and quantify the remaining RIS signal (Romanyuhka et al., 2010). These approaches require soaking the nail in water for a short time (5–10 min) to remove the MIS and measure the residual RIS signal (Reyes et al. 2007; Reyes et al. 2008; Romanyukha et al. 2010). This approach requires that the RIS signal is stable during the water soak treatment. However, there is evidence to suggest a decay in the RIS signal as a function of the soak time (Black and Swarts, 2010). Given this uncertainty in the stability of the RIS signal, we have developed several other approaches to overcome the interference of MIS in order to accurately measure the RIS.
One approach is based on more traditional methods of spectral decomposition using a set of MIS spectral components to separate the MIS from the RIS in ex vivo EPR measurements of nail clippings. Another approach for nail dosimetry involves making EPR measurements in situ, thereby avoiding the MIS from the harvesting cut. For this in vivo EPR nail dosimetry, the critical development involves achieving a sufficiently localized field from the resonator so that the microwaves interact only with the keratinized fingernail and not the underlying lossy, soft tissue. Novel resonator designs that limit the effects on nearby tissue are under development and described briefly here.
EPR nail dosimetry has some significant advantages for dose assessment. The dose can be estimated at four different locations on the human body, providing information on the homogeneity of the radiation exposure. Also, as a physically-based dosimetric approach, it should not be confounded by time-dependent changes to the radiation and other factors that negatively impact biologically-based methods (Swartz et. al., this issue). The results from EPR nail dosimetry are immediately available and there is no latent period before the measurements can be made. Further, the changes persist for a considerable time with a predictable pattern of decay.
2 Materials and methods
2.1 Ex vivo nail dosimetry
2.1.1 Sample preparation
Nail clippings were obtained from a large number of individuals, with the major source being the annual Prouty fundraiser for the Norris Cotton Cancer Center at the Dartmouth-Hitchcock Medical Center. Nails were collected from individuals with a broad demographic distribution and a variety of conditions of their nails, simulating the expected diversity of the population to be sampled in a real event.
Fingernail and toenail clippings were collected in separate bags for each donor. All nail clippings were stored in a −20 °C freezer immediately after harvesting. For each set of experiments, nails were chosen to achieve relatively uniform size and thickness and thereby reduce the number of variables in the development of a method for dose assessment. Each sample is one nail (approximately 15 mg), cut into 2–3 pieces. In order to simulate the irradiation of nails in vivo, the MIS from the pre-irradiation harvesting cut was significantly reduced by water treatment of the nail clippings. Each sample was weighed once following nail irradiation or sham irradiation.
Two large datasets, Sets A and B, were collected and prepared separately in order to test the effects of the water treatment step. Set A includes 5 fingernail samples from each of 20 individuals and Set B includes 5 samples from each of 10 individuals. The MIS from the harvest cut of each sample was reduced and the nail restored to its original state by water treatment and then gentle drying prior to irradiation. Set A was dried for 20 minutes, while Set B was a dried for 50 minutes, to investigate the impact of hydration level on the dose response.
2.1.2 Sample irradiation
The nail samples were placed in micro centrifuge tubes and irradiated with a well-calibrated Cs-137 source. The five fingernail samples from each individual were irradiated to doses of 0, 1, 2, 4 or 8 Gy.
2.1.3 EPR measurement
EPR measurements were performed with a Bruker EMX X-band continuous wave EPR spectrometer equipped with a high sensitivity ER 4119HS-W1 cavity. Nail pieces were placed into a high purity quartz tube (Suprasil) with 4 mm inner diameter, which was positioned so that the sample was in the region of maximum sensitivity. Measurements were typically performed at an incident microwave power of 3 mW (development of reference spectra also included the use of 12 mW), a microwave frequency of 9.8 GHz, a modulation frequency of 100 kHz, a modulation amplitude of 5 G, a time constant of 10.24 ms, a sweep width of 150 G, and a sweep time of 20.97 s.
2.2 In vivo nail dosimetry
Two different types of resonators have been developed for potential use for in vivo measurements: an aperture resonator and surface resonator array. The resonator designs were simulated using Ansoft High Frequency Structure Simulator (HFSS) (Canonsburg, PA, USA), a commercial finite-element modeling program. Ansoft HFSS is a full-wave solver of Maxwell’s equations. By using a three-dimensional full wave solver, the entire resonator, including the losses associated with the sample, can be simulated. From these models, EPR signal characteristics can be calculated and used to obtain optimal designs for the fabrication of EPR resonators.
3 Results and discussion
3.1 Ex vivo nail dosimetry
3.1.1 Spectral decomposition approach
To resolve RIS in the presence of both MIS and RIS, it is necessary to have a thorough understanding of these signals. Based on differences in signal shape and g-value, power saturation, and signal decay behavior, we have identified three distinct components that form the overall MIS signal (Black and Swarts, 2010; Wilcox et al., 2010). One major MIS feature is a symmetric singlet located at g = 2.005. It has long term stability at ambient temperature and relative humidity. A second feature is a broad signal with multiple peaks at higher g-values (2.056 and 2.012) that is consistent with the spectrum from a perthiyl or sulfuranyl radical (Chandra and Symons, 1987; Black and Swarts, 2010). It has a lower stability than the singlet but is prominent for several hours up to days, depending on nail hydration, and does not power saturate below an incident microwave power of 20 mW. A third spectral component is an 18 G doublet centered at g = 2.002. This signal is transient but prominent when spectra are obtained shortly after cutting. Like the MIS singlet, the doublet begins to power saturates above 3 mW. The RIS has only one spectral feature at doses below 50 Gy, which is a symmetrical singlet at g = 2.005. The RIS has very long term stability and is spectroscopically indistinguishable from the MIS singlet using our current X-band EPR analytical methods.
Based on experimental data, we have developed a set of reference spectra for each of the MIS components, as shown in Figure 1. The doublet signal decays rapidly (>50% in about 10 min), while the MIS singlet and broad component are more stable. Therefore, the doublet spectrum can be obtained by spectral subtraction of earlier time points from later time points. After the disappearance of the doublet, the residual EPR spectrum is collected at 3 mW and 12 mW. The MIS singlet saturates such that its signal amplitude is roughly the same at 3 mW and 12 mW; since the broad component signal does not saturate, its amplitude doubles from 3 mW to 12 mW. Based on their different power saturation behavior, the MIS singlet and broad component spectra can be separated. This method of signal separation is similar to the technique applied by Reyes and Romanyukha et al. (Reyes et al., 2008; Reyes et al., 2009; Romanyukha, 2010).
Figure 1.
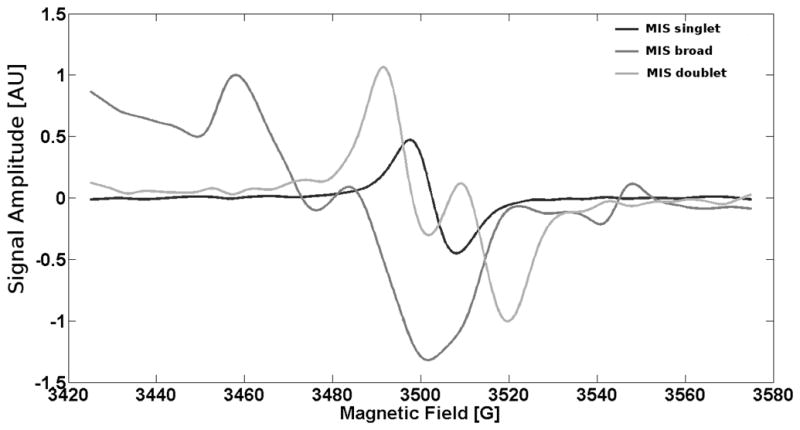
MIS singlet, MIS broad component, and MIS doublet component spectra that are used in spectral decomposition.
One analytic approach for identifying RIS in clipped fingernail spectra is spectral decomposition, where the reference spectrum of each MIS component is used as part of a linear system to obtain the signal amplitudes of the individual components. Current data suggest that the ratios of the amplitudes of the MIS singlet and the broad component do not change significantly over time at least for the first 10 minutes after cutting. However, there is a significant variability in the amplitude ratios of these two signals among samples and this contributes to variability in RIS estimates obtained with the decomposition model (Section 3.1.2); the source of this variability will be addressed in future studies described in Sections 3.1.3 and 3.1.4. Even so, a preliminary set of correlation factors have been established where the broad component amplitude is used to estimate the MIS singlet amplitude. Using this approach, the calculated MIS singlet can be subtracted from the total singlet amplitude of irradiated samples to estimate the amplitude of the RIS.
3.1.2 Dose response from two datasets
Two large spectral datasets were acquired from fingernail samples irradiated with different doses as a test of this approach, Sets A and B, as described above. Each set was analyzed by applying the spectral decomposition approach. The data analysis procedure for the spectral decomposition is as follows:
-
Each spectrum was fit using the model:
where MISsinglet, MISbroad, and MISdoublet are the reference spectra shown in Figure 1, Asinglet, Abroad and Adoublet are their corresponding amplitudes, ARIS and RIS are the amplitude and reference spectrum of the radiation-induced signal respectively, and S is the raw signal.
-
Since the RIS is assumed to have a spectral shape indistinguishable from MISsinglet, the equation can be rewritten as:
where .
The signal amplitudes of each component can then be found using matrix decomposition, where the spectra from each set are collected in a matrix: where S is a npts × nspectra matrix, MIS is a npts × 3 matrix, and A is a 3 × nspectra matrix. Note that the vectors MISsinglet, MISbroad, and MISdoublet are linearly independent and have distinct non-overlapping features. The fact that the amplitudes of the components, Asinglet*, Abroad, and Adoublet, are correlated does not make the linear system under-determined.
The mean ratio C = Asinglet/Abroad was calculated across all of the 0 Gy spectra.
ARIS for each spectrum can then be calculated as .
The ARIS values for each sample are averaged together and then the estimated RIS is divided by the sample mass.
A linear fit is used to determine the slope and intercept of the dose response.
A linear dose-response relationship with a standard error of prediction (SEP) of less than 0.5 Gy was observed after averaging across fingernails within each dose group. The SEP is defined as follows:
where n is the number of measurements, x is the true dose, y is the mass-corrected RIS amplitude, and a and b are the offset and slope of the linear calibration curve.
The Set A test results are shown in Figure 2. The MISsinglet/MISbroad ratio obtained from Set A was then used for the analysis of Set B. The set B test results are shown in Figure 3. The linear relationship in Set B suggests that the correlation between MISsinglet and MISbroad is valid for samples irradiated at the two hydration levels that were tested.
Figure 2.
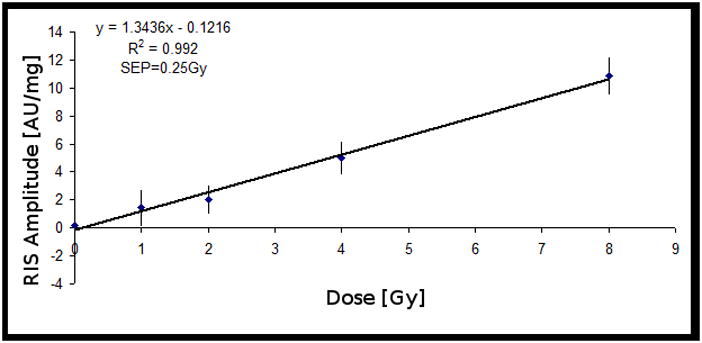
Dose-response from Set A: 20 sets of 5 clipped fingernails irradiated at different dose levels. The RIS was estimated based on the correlation between the MIS singlet and the MIS broad component, shown in Figure 1. The signal intensities were normalized to sample mass. Error bars represent the standard error of the mean (SEM). SEP is the standard error of prediction.
Figure 3.
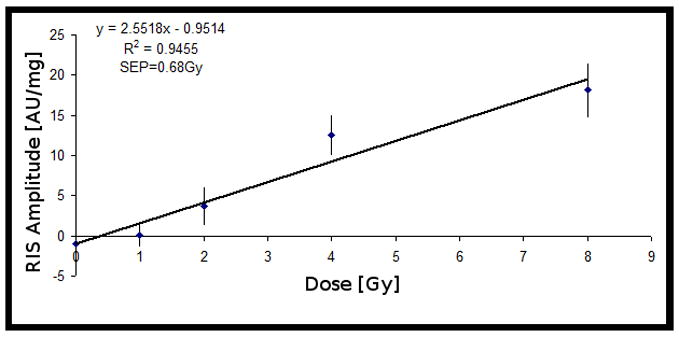
Dose-response from Set B (10 sets), where the value of C from Set A (20 sets) was used to relate the MIS singlet and the MIS broad component. The signal intensities were normalized to sample mass. Error bars represent SEM.
The nail samples in Set B were dried 30 min longer than the samples in Set A. The lower hydration level for the nails in Set B results in a larger slope of the calibration curve in Figure 3.
These results demonstrate the potential effectiveness of this approach, and additional studies are now underway into sample handling and possible hydration dependencies of the MISsinglet/MISbroad ratio to reduce the variation observed across individual measurements. These studies will also assess the potential dependency of the ratio on demographic factors and nail diseases that may affect the composition and/or conformation states of the keratin fibers within the nail.
3.1.3 Variability of dose response and its source
While the linearity of the dose response plots indicates the potential of ex vivo nail dosimetry, the variability among individual samples was too large to use the dose-response relationships to determine the dose for individual samples. More work is needed to understand the sources of the variability in individual samples.
The physical properties of fingernails, such as water content, rigidity and thickness, differ from person to person, which may cause the variability in the dose response among individuals. Preliminary data suggest that different levels of hydration may be a key source of variability. The discrepancy between the dose-response results from Set A and Set B, Figures 2 and 3, respectively, demonstrates the impact of hydration on the amount of RIS that is estimated for the two data sets. It would be desirable to determine the hydration level of each sample and establish a relationship between the dose-response and the hydration level.
A procedure to decrease the MIS in cut nails is to soak the nail in water for a short interval (10–15 min). Although this reduces the MIS, it reduces the RIS as well (Black and Swarts, 2010). Also, the MIS can rebound if the nails are kept dry for a long period (> 60 min) after water treatment (Reyes et al., 2008), a phenomenon we also observe. Due to the similarity between the RIS and MIS singlets, the MIS rebound would affect the estimation of the RIS amplitude and could be another source of variability in the RIS estimation from our model and other approaches that rely on eliminating the MIS by soaking the nail in water.
Studies of MIS and RIS decay kinetics under controlled temperature and humidity conditions are underway to understand the impact of hydration on RIS and MIS stability (Black and Swarts, 2010; Wilcox et al., 2010). Preliminary results suggest that the decay rates for the MIS components and the RIS depend on both the hydration of the nail and the ambient temperature. These results could potentially explain a major component of the variability of RIS estimates in this model and will be a major focus of efforts to further characterize the MIS and RIS in nails.
3.1.4 Future work on nails irradiated during TBIs
Nail dosimetry studies in the laboratory involve the irradiation of donated nail clippings. To emulate a real situation, the ex vivo irradiated nails are then clipped to simulate the harvesting cut of nails irradiated in vivo. Because both irradiation and mechanical clipping generate radicals in nails, the sequence and context of these processes may affect the EPR spectral properties that are the basis of the ex vivo fingernail dosimetry. To determine if nail clippings irradiated ex vivo are a valid model for nails irradiated in vivo, we are collaborating with the Dana Farber Cancer Institute (DFCI) and Dartmouth’s Norris Cotton Cancer Center to investigate the nail clippings from patients undergoing total body irradiation (TBI) as part of stem cell therapy. These individuals receive doses in the 2–14 Gy range, which is the dose range of interest for triage that guides medical treatment in field settings. Comparison of results from in vivo irradiated nails and ex vivo irradiated nails (harvested prior to TBI) from the same individual can provide very useful results.
3.2. In vivo nail dosimetry
In parallel to characterization of the MIS signals, our group is working on resonant structures to measure absorbed dose in vivo. A potential major benefit of an in vivo method is that it will eliminate the challenge of characterizing the MIS found in clipped samples. We show here two ongoing projects with the overall goal to obtain sensitivity comparable to that of nail clippings in high Q resonators. Both projects utilize Ansoft HFSS as a method to characterize and optimize the resonant structure in order to guide fabrication. Three surface resonators in two categories are described here.
3.2.1 Aperture resonator
An aperture resonator is a surface resonator where the magnetic fields are supplied to the sample by a hole in an X-band TE102 (or similar) resonant cavity. The hole in the wall creates a potential in which microwaves are coupled out and absorbed into the surrounding region, illustrated in Figure 4A. An aperture resonator-based system for in vivo measurement of radiation dose of a human tooth without extraction is described first by Ikeya and Ishii in 1989 (Ikeya and Ishii, 1989). A second kind of aperture resonator is a sub-wavelength-slot aperture resonator (Ishii and Ikeya, 1990), which has an array of several apertures (slots) whose height is below the cut-off wavelength of the incident field excited by the X-band cavity. Using the aperture as a surface coil, the depth sensitivity can be adjusted by varying the aperture size and geometry, illustrated in Figure 4B. Both of these geometries rely on a microwave cavity, such as the TE102, to create the standing waves on the wall of the resonator to excite the apertures.
Figure 4.
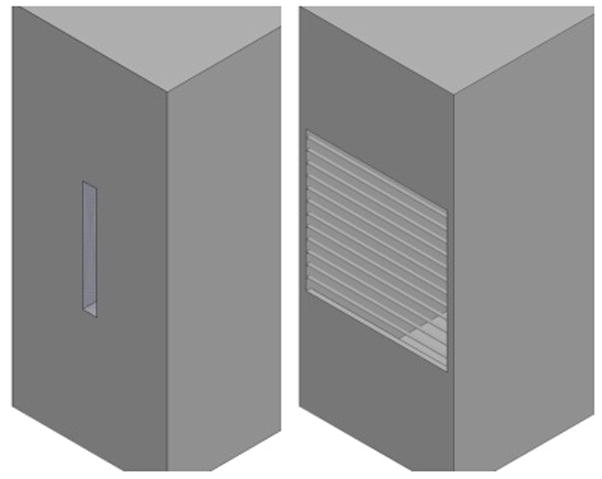
Aperture geometry for the A) hole (left) and B) sub-wavelength surface coils (right).
Both designs use diffused waves, limited to the near field, as the primary magnetic field excitation for EPR spectroscopy. However, the aperture resonator has a tendency to couple more strongly to the surrounding environment, such as lossy tissue under the fingernail. Therefore, a sub-wavelength aperture resonator could be less sensitive to lossy tissue for in vivo measurements. It is proposed, that using Ansoft HFSS to optimize the sub-wavelength aperture geometries, a surface resonator can be fabricated which maximizes its filling factor to the fingernail while minimizing losses to the nail bed.
EPR measurements of freshly clipped nails placed at the aperture of a resonator, made of a regular TE102 Bruker cavity with a hole of 4.5 mm in diameter, were performed. EPR spectra of clipped fingernails were collected using an incident RF power of 23.4 mW, a modulation amplitude of 5 G, a scan width of 150 G, time constant of 16 ms, an RF gain of 2 × 105, and a total scan time of 90 s. The spectra were recorded on a Varian E4 EPR Spectrometer; an example spectrum is shown in Figure 5.
Figure 5.
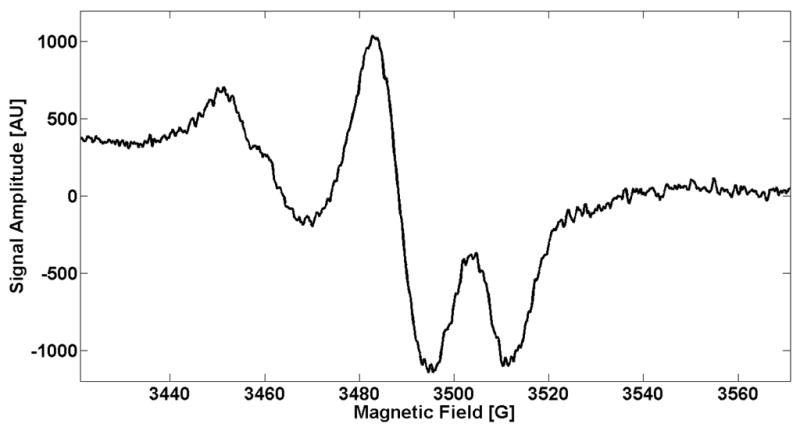
Sample EPR spectrum taken using the aperture resonator, developed from a TE102 Bruker cavity with a hole diameter of 4.5 mm. The spectrum was acquired with a modulation amplitude of 5 G, a scan width of 150 G, a time constant of 16 ms, an incident RF power of 23.4 mW, and a total scan time of 90 s.
3.2.2 Surface resonator array
The surface resonator array (SRA) structure is designed to reduce depth sensitivity by creating an array of anti-parallel transmission line modes so that the losses associated with tissues beneath the nail are reduced. These anti-parallel transmission lines create a near field magnetic field that is canceled in the far field. This approach was first developed by placing two magnetic resonance imaging (MRI) coils “edge-on” and spacing them some distance apart (Froncisz et al., 1986). Hyde et al. reported a magnetic field profile that penetrated approximately half of the spacing of the two coils (Hyde et al., 1987). Similarly, Bohning et al. used surface coil meander-line and parallel array technology to study 31P on surface tissues using magnetic resonance spectroscopy (MRS)(Bohning et al., 1998). Illustrated in Figure 6 is the geometry, simulated and optimized in Ansoft HFSS, for a SRA structure at X-band.
Figure 6.
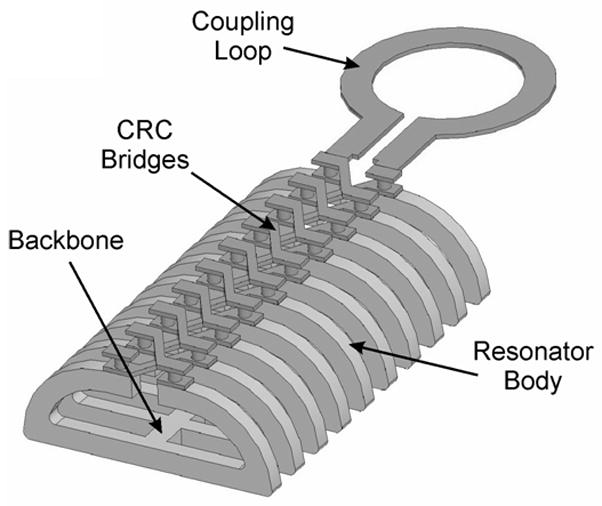
Design of surface resonator array showing coupling and mode suppression bridges.
By using an array of near field surface coils, the SRA builds an area of potential EPR excitation. The SRA geometry used in the first design had a 10 mm × 5 mm cross-section with an element spacing of 1 mm. After bench tests, this design was found to be too conservative, since an element spacing of 1 mm yields only ~0.5 mm of depth. Newer designs have expanded the spacing to 1.75 mm, as well as curved the resonator to match the fingernail sample, and are calculated to yield a 30% gain in EPR sensitivity.
4 Conclusions
While the potential for these techniques has been demonstrated, several essential steps remain to be accomplished:
Understand and reduce the variability of dose response among individuals for the ex vivo approach and the MISsinglet/MISbroad ratio in the spectral decomposition model
Demonstrate that the in vivo nail approach can provide sufficient sensitivity to be utilized for practical measurements in situ
Develop procedures and devices that can be operated reliably and effectively by non-expert personnel under field conditions
We anticipate that because of the almost unique ability of EPR nail dosimetry to provide direct, physical information on the homogeneity of the exposure, it is likely to be an important part of the response system for triage after the potential exposure of large numbers of people to clinically significant levels of ionizing radiation.
Acknowledgments
This research was supported by NIH (U19AI067733 and U19AI091173)and DARPA HR0011-08-C-0022.
Abbreviations
- RIS
Radiation-induced signal
- MIS
Mechanically-induced signal
- SEP
Standard error of prediction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Black PJ, Swarts SG. Ex Vivo Analysis of Irradiated Fingernails: Chemical Yields and Properties of Radiation-Induced and Mechanically-Induced Radicals. Health Phys. 2010;98:301–308. doi: 10.1097/HP.0b013e3181b0c045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohning DE, Pecheny AP, Wright AC, Spicer KM. Magnetic resonance coil for 31P spectroscopy of skin over curved body surfaces. Skin Res Tech. 1998;4:63–70. doi: 10.1111/j.1600-0846.1998.tb00088.x. [DOI] [PubMed] [Google Scholar]
- Chandra H, Symons MCR. Sulfur Radicals Formed by Cutting Alpha-Keratin. Nature. 1987;328:833–834. doi: 10.1038/328833a0. [DOI] [PubMed] [Google Scholar]
- Flood AB, Nicolalde RJ, Demidenko E, Williams BB, Shapiro A, Wiley AL, Swartz HM. A Framework for Comparative Evaluation of Dosimetryic Methods to Triage a Large Population Following a Radiological Event. Radiat Measur. doi: 10.1016/j.radmeas.2011.02.019. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froncisz W, Jesmanowicz A, Kneel JB, Hyde JS. Counter rotating current local coils for high-resolution magnetic resonance imaging. Magn Reson Med. 1986;3:590–603. doi: 10.1002/mrm.1910030412. [DOI] [PubMed] [Google Scholar]
- Fuchs J, Groth N, Herrling T. Applications of in vivo EPR and imaging for skin in biological magnetic resonance. In: Berliner LJ, editor. In vivo EPR Theory and Applications. Vol. 18. 2003. pp. 483–513. [Google Scholar]
- Furusawa M, Ikey M. Electron spin resonance imaging utilizing localized microwave magnetic field. Jpn J Appl Phys. 1990;29:270–276. [Google Scholar]
- Grace MB, Moyer BR, Prasher J, Cliffer KD, Ramakrishnan N, Kaminski J, Coleman N, Manning RG, Maidment BW, Hatchett R. Rapid Radiation Dose Assessment for Radiological Public Health Emergencies: Roles of NIAID and BARDA. Health Phys. 2010;98:172–178. doi: 10.1097/01.HP.0000348001.60905.c0. [DOI] [PubMed] [Google Scholar]
- Howe D Council, T.H.S. 2004. National planning scenarios executive summaries [Google Scholar]
- Hyde JS, Jesmanowicz A, Kneeland JB. Surface coil for MR imaging of the skin. Magn Reson Med. 1987;5:456–461. doi: 10.1002/mrm.1910050507. [DOI] [PubMed] [Google Scholar]
- Ikeya M, Ishii H. Atomic bomb and accident dosimetry with ESR: natural rocks and human tooth in-vivo spectrometer. Int J Rad Appl Instrum A. 1989;40:1021–7. doi: 10.1016/0883-2889(89)90035-x. [DOI] [PubMed] [Google Scholar]
- Ishii H, Ikeya M. An electron spin resonance system for in-vivo human tooth dosimetry. Jpn J Appl Phys. 1990;29:871–5. [Google Scholar]
- Romanyukha A, Reyes RA, Trompier F, Benevides LA. Fingernail dosimetry: current status and perspectives. Health Phys. 2010;98:296–300. doi: 10.1097/01.HP.0000347999.01948.74. [DOI] [PubMed] [Google Scholar]
- Reyes RA, Romanyukha A, Trompier F, Mitchell CA, Clairand I, De T, Benevides LA, Swartz HM. Electron paramagnetic resonance in human fingernails: the sponge model implication. Radiat Environ Biophys. 2008;47:515–526. doi: 10.1007/s00411-008-0178-8. [DOI] [PubMed] [Google Scholar]
- Reyes RA, Romanyukja A, Olsen C, Trompier F, Benevides LA. Electron paramagnetic resonance in irradiated fingernails: variability of dose dependence and possibilities of initial dose assessment. Radiat Environ Biophys. 2009;48:295–310. doi: 10.1007/s00411-009-0232-1. [DOI] [PubMed] [Google Scholar]
- Swartz HM, Burke G, Coey M, Demidenko E, Dong R, Grinberg O, Hilton J, Iwasaki A, Lesniewski P, Kmiec M, Lo K, Nicolalde RJ, Ruuge A, Sakata Y, Sucheta A, Walczak T, Williams BB, Mitchell CA, Romanyukha A, Schauer DA. In vivo EPR for dosimetry. Radiat Measur. 2007;42:1075–1084. doi: 10.1016/j.radmeas.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz HM, Flood AB, Gougelet RM, Rea ME, Nicolalde RJ, Williams BB. A Critical Assessment of Biodosimetry Methods for Large-Scale Incidents. Health Phys. 2010;98:95–108. doi: 10.1097/HP.0b013e3181b8cffd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz HM, Williams BB, Nicolalde RJ, Demidenko E, Flood AB. Overview of Biodosimetry for Management of Unplanned Exposures to Ionizing Radiation. Radiat Measur this issue. [Google Scholar]
- Trompier F, Kornak L, Calas C, Romanyukha A, LeBlanc B, Mitchell CA, Swartz HM, Clairand I. Protocol for emergency EPR dosimetry in fingernails. Radiat Measur. 2007;42:1085–1088. doi: 10.1016/j.radmeas.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox DE, He X, Gui J, Ruuge AE, Li H, Williams BB, Swartz HM. Dosimetry Based on Epr Spectral Analysis of Fingernail Clippings. Health Phys. 2010;98:309–317. doi: 10.1097/HP.0b013e3181b27502. [DOI] [PMC free article] [PubMed] [Google Scholar]


