Abstract
Inward eutrophic remodeling is a common structural change found in small resistance arteries that has been associated with an increased risk for life threatening cardiovascular events, the number one cause of death in industrialized societies. Because inward eutrophic remodeling is the most prevalent small artery structural change found in hypertension, hypertensive animals are the most common in vivo models used to study this particular remodeling process. In vitro, the isolated artery, pressure myograph has also been used as a model to study the mechanisms responsible for the development of small artery remodeling. Compelling recent evidence indicates that the matrix metalloproteinases (MMPs), a family of endopeptidases whose primary function is the cleavage and degradation of extracellular matrix components, are involved in vasoconstriction and the pathogenesis of hypertension. In this review we provide an overview of the known and potential roles that MMPs have on vascular remodeling, paying particular attention to their role on the inward eutrophic remodeling process of small resistance arteries that occurs in hypertension.
Introduction
The remodeling of a tissue or organ is a dynamic process that entails the reorganization, resorption, or renovation of an exiting structure. At the vascular level, it involves changes in the structural characteristics of the blood vessel wall including its cellular and extracellular components. Functionally, vascular remodeling is an ongoing physiological phenomenon that allows the circulatory system to adapt to injury or changes in growth, hemodynamics, and metabolic demands. However, under certain circumstances vascular remodeling is counterproductive and becomes an important contributor to cardiovascular disease, the number one cause of death in industrialized societies.
Inward eutrophic remodeling of the small resistance arteries and arterioles is a specific type of vascular remodeling that has been associated with an increased risk for life threatening cardiovascular events including myocardial infarction and stroke [1,2]. This specific type of vascular remodeling is characterized by a reduction in the passive luminal diameter of the blood vessel without a significant change in the amount of wall material [3]. Although it is well established that inward eutrophic remodeling of small arteries is associated with cardiovascular risk, the mechanisms that control the remodeling process have not been fully elucidated.
During typical tissue remodeling processes there is a continuous turnover and deposition of extracellular matrix (ECM) materials. In the vascular wall, ECM turnover is in part performed by the matrix metalloproteinases (MMPs), a family of zinc-dependent endopeptidases produced by all cell types present within the blood vessel wall [4]. The traditional function associated with the activity of MMPs is the degradation of ECM components. However, evidence indicates that MMPs participate in additional actions including the shedding of vasoactive factors that are attached to the ECM [5], the cleavage of cellular receptors that possess vasoactive functions [6], the disruption of intercellular adhesions [7], and potentially, the remodeling of intracellular cytoskeletal and nuclear structures [8,9]. Most of the 23 members of the MMP family are secreted by cells in an inactive form and later become activated in response to a variety of stimuli. A number of MMPs, however, have transmembrane domains and remain membrane bound while they perform their enzymatic activities. Because the stimuli associated with the development of inward eutrophic remodeling in small arteries also induce the secretion and/or activation of MMPs, it has been hypothesized that MMPs are involved in the remodeling process [10]. However, direct evidence linking MMP-activity with inward remodeling at the level of the resistance vasculature is limited.
In this review we will discuss a number of models used to study the inward eutrophic remodeling process and the data implicating the activity of MMPs in this process (See Table 1). Due to the limited information on MMP involvement in small artery inward eutrophic remodeling, we will extrapolate information from larger vessels and from other types of vascular remodeling with the caveat that the information extrapolated needs experimental corroboration.
Table I.
Representative Studies Implicating MMPs in Vascular Remodeling
| Model | Stimulus | MMP Blockade | Remodeling | MMPs Investigated | References |
|---|---|---|---|---|---|
| SHR | Spontaneous Hypertension | GM6001, EDTA, Doxycycline | Rarefaction | 2,9,7 | [13] |
| 2K1C Hypertensive Rat | Renovascular hypertension | Tempol | Inward | 2 | [36] |
| 2K1C Hypertensive Rat | Renovascular Hypertension | Doxycycline | Inward | 2,9,14 | [25,35] |
| Rabbit Aorta | In vivo Balloon Angioplasty | Doxycycline | Inward | 2 | [57] |
| Rat Carotid Artery | In vivo Balloon Angioplasty | GM6001 | Neointima | Gelatinases | [54] |
| Rat Carotid Artery | In vivo Balloon Angioplasty | Neointima | 1,2,9 | [67] | |
| Rat Carotid Artery | In vivo Balloon Angioplasty | Batimastat, RGD peptide | Inward | 2 | [56] |
| Rat Carotid Artery | In vivo Balloon Angioplasty | Doxycycline | Neointima | 2,9 | [55] |
| Rat Carotid Artery | In vivo Balloon Angioplasty | GM-6001 | Neointima | Not specified | [68] |
| Pig iliac Artery | In vivo Balloon Angioplasty | Batimastat | Inward | Not specified | [69] |
| Pig iliac/femoral Artery | In vivo Balloon Angioplasty | Marimastat | Inward | Not specified | [70,71] |
| L-NAME Hypertensive Rat | In vivo L-NAME | Doxycycline | Inward | 2 | [72] |
| Rat Mesenteric Artery Ligation | In vivo High Flow | L-NAME, Doxycycline | Outward | 9 | [73] |
| Mouse Carotid Artery | In vitro Ang-II | MMP-9 KO Mouse | Outward | 2,9 | [26] |
| Mouse Carotid Artery | In vitro High Flow | Doxycycline, SB-3CT, MMP-9 and MMP-12 KO Mouse | Outward | 2,9,12 | [32] |
| Mouse Carotid Artery | In vitro Transmural Pressure | FN-439 | Outward | 2,9 | [27] |
SHR, spontaneous hypertensive rats; Ang-II, angiotensin-II; 2K1C, two-kidneys one-clip; L-NAME, NG-nitro-L-arginine-methyl-ester; KO, knockout; SB-3CT and FN-439 are selective MMP inhibitors.
Models of hypertension and inward eutrophic remodeling of resistance vessels
Essential Hypertension, Inward Remodeling and MMPs
Vascular remodeling has been long associated with essential hypertension. At the level of the resistance vasculature, arterioles isolated from subcutaneous biopsies or the mesentery of essential hypertensive humans, as well as, arterioles obtained from diverse vascular beds of spontaneously hypertensive rats (SHRs) show inward eutrophic remodeling [10,11]. In both, hypertensive humans and SHRs, plasma MMP activity is elevated [12–14], and experimental inhibition of MMP activity in the SHR ameliorates hypertension [15].
At the vascular level, diverse mechanisms attributed to the activity of MMPs increase blood pressure by either augmenting vasoconstriction or reducing vasodilation. MMP-dependent mechanisms that augment vasoconstriction include the cleavage of big endothelin that produces smaller vasoactive fragments of this potent vasoconstrictor, and the transactivation of epidermal growth factor receptors that subsequently activate constrictive pathways in vascular smooth muscle cells [5,16]. With regard to vasodilation, MMPs have been shown to cleave calcitonin gene-related peptide (CGRP), as well as beta2 adrenergic receptors [5,6,16,17]. Cleavage of CGRP reduces the vasodilatory effects of this peptide, while cleavage of beta2 adrenergic receptors diminishes catecholamine-induced vasodilation. Experimental hypertension induced by either increased vasoconstriction or reduced vasodilation is associated with inward eutrophic remodeling of small arteries. Therefore, MMPs could be involved in the remodeling process through their vasomotor effects. However, no analyses have been performed to determine whether amelioration of the hypertensive state by MMP-inhibition is associated with a reduction of the remodeling process in small arteries from essential hypertensive patients or SHRs. Results obtained from individuals or animal models treated for hypertension indicate that a mere reduction in blood pressure is not sufficient to eliminate inward remodeling in resistance vessels. Treatments that lower blood pressure by reducing cardiac output do not eliminate the inward remodeling process [11]. In comparison interventions that lower blood pressure by promoting vasodilation or reducing vasoconstriction successfully prevent or stop the inward remodeling process [18]. Therefore, although the drop in blood pressure achieved by MMP-inhibition in the SHR is likely caused by a reduction in vasoconstriction, it remains to be determined whether MMP-inhibition also prevents the development of inward eutrophic remodeling in the resistance arteries.
Vasoconstriction-Induced Hypertension, Inward Remodeling and MMPs
Numerous animal models of nongenetic secondary hypertension that induce vasoconstriction develop small artery remodeling. For example, prolonged in vivo infusion of the nitric oxide (NO) synthase inhibitor NG-nitro-L-arginine methyl ester (L-NAME), or the vasoconstrictor agonists norepinephrine or angiotensin-II induces hypertension and inward remodeling of resistance vessels [19–21]. In these models of hypertension, as in the SHR, MMP-activity is increased, and broad-based MMP-inhibition prevents the blood pressure augmentation induced by the hypertensive agents [15]. The aforementioned results indicate that up-regulation of MMP expression and activity is a common feature in both essential and vasoconstriction-induced hypertension. However, with 23 members in the MMP family, an important question pertains to the specific involvement of each MMP member on increasing blood pressure. In this regard, evidence indicates that different MMPs are responsible for specific features of the hypertensive state, while others oppose hypertension. For example, in mice, specific inhibition of the matrilysin, MMP-7, abolished the pressor effect induced by the infusion of norepinephrine or angiotensin-II, and significantly diminished the hypertension caused by L-NAME [15]. In a different study, MMP-2-inhibition also prevented the pressor response induced by angiotensin-II, but did not prevent the cardiac fibrotic effect that was blocked when MMP-7 was inhibited [22]. This suggests that, during stimulation with angiotensin-II, MMP-2 is more closely responsible for the activation of vasoconstrictor pathways, while MMP-7 is responsible for the fibrotic and hypertrophic effects induced by the agonist. A relationship between MMP-7 and MMP-2 was found in those studies that indicated the up-regulation of MMP-2 was dependent on the activity of MMP-7 and the metalloproteinase desintegrin ADAM-12 [22].
The activity of MMP-2 has also been associated with MMP-14, a membrane bound member of the MMP family that is up-regulated in hypertension and also mediates the expression of MMP-9 in keratinocytes [23–25]. In human patients with essential hypertension plasma-levels of MMP-9 are elevated [14], while a paradoxical reduction on MMP-9 expression in the hearts and aortae of mice infused with angiotensin-II has been reported [22]. In comparison to the hypotensive effect of MMP-7- or MMP-2-inhibition on angiotensin-II-induced hypertension, the pressor effect of angiotensin-II is enhanced in MMP-9 knock out mice [26]. These results suggest that the activation of MMP-9 may serve to ameliorate hypertension, while MMP-7 and MMP-2 may participate in what are considered the detrimental effects of the hypertensive state (i.e., vasoconstriction and tissue fibrosis).
In the vasculature, the activity of MMP-9 is commonly associated with the development of outward remodeling. For example, the carotid arteries of mice with hypertension have greater internal diameters and greater levels of MMP-9 activity than those of control mice [26,27]. In vitro, intraluminal pressure augmentation in carotid arteries also increases the activity of MMP-9 and causes outward remodeling [27]. This up-regulation of MMP-9 and the subsequent outward remodeling of conduit arteries are considered to be initial compensatory mechanisms that ameliorate the augmented intraluminal pressure by increasing vascular compliance. However, a chronic up-regulation of MMP-9 may become detrimental and cause the formation of vascular aneurysms [28–30]. A direct association between MMP-activity and pathological vascular distension was well established in a study showing that MMP-9 knockout mice are resistant to aneurysm formation in response to topical application of CaCl2 [31]. Reinfusion of wild type competent macrophages to MMP-9 knockout mice restored their capacity to develop aneurysms, which indicated that macrophage-derived MMP-9 was required for formation of the vascular distension. Recent studies on flow-induced remodeling also indicate that the main source of MMP-9 associated with outward vascular remodeling comes from inflammatory cells that infiltrate the vascular wall [32,33]. Importantly, flow-induced outward remodeling was inhibited in MMP-9 mice, but not in MMP-2 knockouts [32]. These data depicting the role of MMP-9 on outward remodeling and aneurysm formation suggests that MMP-9 up-regulation may be beneficial at the onset of hypertension, but may eventually become deleterious if chronic inflammation develops within the vascular wall. Opposing beneficial and detrimental effects of MMP up-regulation have also been found in cancer, where MMP-activity can both enhance and inhibit tumor progression [34]. Therefore, although MMP up-regulation represents a common feature in hypertension, it remains to be fully determined which specific MMPs participate in vasoconstriction and which in vascular remodeling, which ones ameliorate the hypertensive state and which ones increase it. In addition, the timeframe and specific cellular expression of MMPs warrants further investigation as different MMPs may be up-regulated at specific times in different cells during the progression of hypertension having specific beneficial or detrimental effects on vascular remodeling and disease.
Renovascular Hypertension, Inward Remodeling and MMPs
The role of MMPs in hypertension and vascular remodeling has been further elucidated in a series of studies performed in a renovascular model of hypertension [25,35]. Evidence from those studies revealed that in the two-kidneys, one-clip rat model of hypertension, the aortic expression and activity of MMP-2, -9 and -14 were increased [25,35]. Broad-based MMP-inhibition reduced the augmented deposition of extracellular matrix in the aortic wall, and reduced the level of hypertension [25,35]. Antioxidant treatment also reduced the increased blood pressure, the remodeling of aortic tissue, and the activity of MMP-2 [36]. This suggested that the increased expression and activity of MMPs were associated with the production of reactive oxygen species (ROS) in renovascular hypertension, which is particularly relevant when considering that vast evidence indicates an increased production of ROS is a common feature of essential hypertension [37]. In hypertension, ROS may participate in the vascular remodeling process in different fashions. For example, ROS reduce the bioavailability of NO, and inhibition of NO-dependent signaling has been shown to induce inward eutrophic remodeling in resistance vessels [38]. ROS have also been shown to induce the synthesis and activation of a number of MMPs [8,39,40], which in turn can promote vasoconstriction and ECM turnover. Furthermore, it has been recently shown that a ROS-dependent activation of MMPs is required for the development of inward remodeling in isolated arterioles (see below) [41]. Additional studies are needed to identify the specific cellular sources of ROS and determine which specific ROS participate in the activation of MMPs and the inward remodeling process.
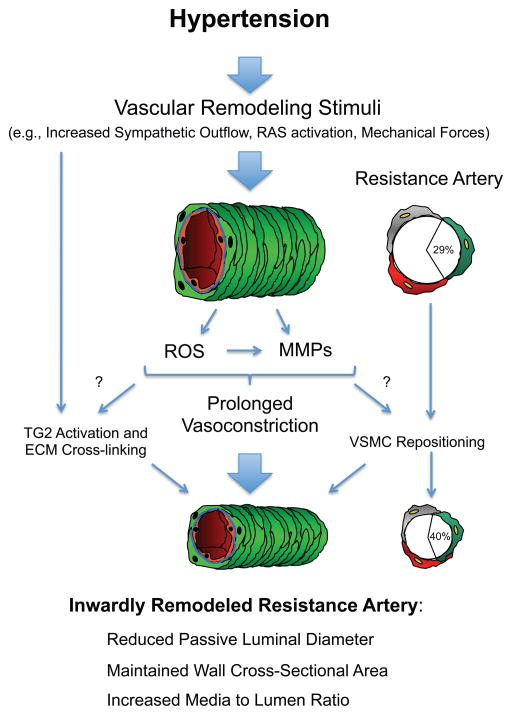
In hypertension, mechanical forces affecting the vascular wall also up-regulate MMPs expression and activity [42–44]. Therefore it is tempting to speculate that the increased circumferential stress experienced by the vascular wall in the hypertensive state is responsible for the activation of MMPs and the development of vascular remodeling. However, results obtained from hypertensive individuals and animal models of hypertension suggest that a mere increase in intravascular pressure is not sufficient to induce inward remodeling in resistance vessels. In addition, it has been argued that small arteries are not exposed to an increased wall stress in hypertension because they are constricted [45]. This suggests that the vasoconstrictor agents associated with hypertension are the main stimuli responsible for the induction of inward eutrophic remodeling in resistance vessels. Nevertheless, it is likely that mechanical forces and vasoconstrictor agonists have additive or synergistic effects on the expression and activity of MMPs in hypertension at different levels of the vascular tree. In Figure 1, we outline the pathways we propose involve MMPs on the inward eutrophic remodeling that occurs in hypertension.
Figure 1. Proposed pathway for the involvement of the matrix metalloproteinases (MMPs) on the inward eutrophic remodeling that occurs in hypertension.
In hypertension, small resistance arteries are exposed to stimuli, such as, an increased level of sympathetic stimulation, an increased exposure to angiotensin-II and other components of the renin-angiotensin system (RAS), and/or an augmented level of intraluminal pressure. These stimuli induce vasoconstriction though processes that involve the synthesis and activation of MMPs, the production of reactive oxygen species (ROS), and a ROS-dependent activation of MMPs. Prolonged vasoconstriction in turn induces inward eutrophic remodeling via mechanisms that involve the activation of tissue-type transglutaminase (TG2) and the repositioning of smooth muscle cells. It remains to be determined whether a prolonged activation of MMPs and ROS is associated with the activation of TG2 and whether it participates in allowing smooth muscle cells to move within the vascular wall to increase their overlap and each occupy a greater proportion of the wall circumference.
Ex vivo models of arteriolar inward eutrophic remodeling
Using the pressure myograph, Bakker et al. first documented that prolonged isobaric vasoconstriction of isolated arterioles results in inward eutrophic remodeling [46]. The pressure myograph is a system that consists of two micropipettes on which a small artery is mounted. The pipettes are filled with physiological saline, and water columns or pumps are used to apply intraluminal pressure with or without flow. Further studies using the pressure myograph have shown that inward eutrophic remodeling requires the activation of multiple kinases and signaling pathways including cSRC, ERK, tissue-type transglutaminase (TG-2), ROS, and MMPs [41,47–49].
Cellular Mechanisms for Vasoconstriction-Induced Inward Remodeling
It is well established that at the onset of agonist-induced vasoconstriction, contractile activation is contingent on the calcium-dependent activation of acto-myosin cross-bridge cycling. During prolonged vasoconstriction a series of mechanisms different from the initial increase in intracellular calcium but triggered by common stimuli initiate a cascade of events leading to maintenance of the constricted state and inward eutrophic remodeling [10]. Thus far, the kinases reported as needed in the inward remodeling process including cSRC and ERK are in part associated with the capacity of the artery to maintain active vasoconstriction during prolonged exposure to vasoactive agonists [47,48]. On the other hand, the involvement of TG2 is believed to be associated with collagen crosslinking and the stiffening and contraction of the ECM within the vessel wall.
On the involvement of ROS and MMPs, we recently reported that a ROS-dependent activation of MMPs is needed for a prolonged exposure to norepinephrine and angiotensin-II to induce inward remodeling in isolated cremaster arterioles [41]. Prolonged exposure to the agonists also induced an increased expression of MMP-2. ROS promote the activation of MMPs in part through the modulation of the thiol interaction between the prodomain and catalytic domain of the enzymes [8]. In addition, an NAD(P)H oxidase-dependent up-regulated expression of MMP-2 has been shown to occur in response to stretch or exposure to angiotensin-II in cultured vascular smooth muscle cells [39,50]. In isolated arterioles constricted under isobaric conditions, smooth muscle cells are not exposed to stretch or an augmented circumferential stress, therefore it is likely that the increased expression in MMP-2 observed after prolonged vasoconstriction is dependent on the angiotensin-II activation of NAD(P)H oxidase and the subsequent production of superoxide. Similarly, in hypertension the increased activity of ROS and MMPs is in part the result of an augmented activity of vasoconstrictor agonists, as angiotensin-II type 1 receptor blockade reduces the elevated plasma levels of MMP-9 in patients with essential hypertension [51]. Whether a cellular stretch-dependent production of ROS and ensuing synthesis and activation of MMPs participate in the development of inward eutrophic remodeling in hypertension remains to be fully elucidated, but a number of evidences argue against it. First, resistance vessels undergo vasoconstriction in response to intraluminal pressure augmentation through a process known as the myogenic response. Myogenic vasoconstriction has been shown to normalize wall stress in arterioles as vascular diameter becomes smaller under greater intraluminal pressure [52]. Thus, a stretch-dependent activation of NAD(P)H oxidase and subsequent activation of MMPs may partake in the initial constriction induced with the vascular distention caused by the elevated pressure, but once the vessel reduces its diameter, circumferential stress is reduced and the cellular stretch abolished. In addition, as mentioned above, it has been shown that in hypertension a mere reduction in intravascular pressure is not sufficient to prevent the inward eutrophic remodeling of resistance vessels. It is rather the inhibition of vasoconstrictor pathways and the promotion of vasodilation that prevents the remodeling process.
Because we have previously shown that prolonged arteriolar vasoconstriction is associated with the repositioning of vascular smooth muscle cells within the vessel wall [53], it is possible that the up-regulated activity of MMPs during prolonged exposure to agonists serves to disrupt intercellular and cell-ECM attachments allowing smooth muscle cells to move within the vascular wall. In support of this hypothesis, MMPs have been associated with the migration and proliferation of vascular smooth muscle cells in injured conduit arteries. For example, balloon angioplasty, used as a model of intimal injury, has been associated with an increased expression of MMP-2 and MMP-9 [54]. Additional studies show that inhibition of MMPs or their respective activation pathways attenuate the inward constrictive remodeling associated smooth muscle cell migration and neointimal formation [55–57]. Overall, It is well characterized that remodeling processes include continual resorption, deposition and crosslinking of ECM and cellular components. Therefore, it is likely that the inward remodeling process in small arteries of hypertensive individuals requires concomitant destruction and formation of ECM proteins as well as the repositioning of cells within the vascular wall and that MMPs are active participants in this processes.
Ultimately, an important query that is being gradually elucidated relates to the relationship between the mechanisms that induce prolonged vasoconstriction and develop inward eutrophic remodeling. Numerous reports indicate that multiple kinases, including those reported as needed in the maintenance of prolonged vasoconstriction and the development of inward remodeling are associated with the production of ROS and the up-regulation of MMPs in vascular cells [58–60]. In addition, a number of studies suggests that ROS may, in some instances, participate in the activation of TG2 [61–63], and TG2 in turn may contribute to the remodeling process through multiple processes, from the activation of Rho kinase signaling pathways and the formation of cytoskeletal stress fibers to the crosslinking and contraction of collagen fibers [49,64]. Furthermore, TG2 has been associated with the activity of integrins. It has the capacity to cluster these cellular receptors for ECM and mediate stable ternary interactions between fibronectin and the beta1 and beta3 integrin subunits [65,66]. Interestingly alphaV-beta3 and alpha5-beta1 integrins are up-regulated in hypertension, and alphaV-beta3 blockade has been shown to inhibit the inward eutrophic remodeling observed in arterioles of the Ren2 rat model of hypertension [10]. Additional studies are needed to determine the association between ROS, MMPs, TG2, and integrins within the vascular wall during the inward remodeling process. Moreover, as the production of ROS and the activation of MMPs and TG2 may be temporally based, it is necessary to determine the time frame of ROS-production, MMP-up-regulation, and TG2-activation evoked by exposure of vascular cells to remodeling stimuli.
Conclusion
Evidence indicates that to reduce the risk for cardiovascular events related to hypertension, it is important to prevent, stop, or revert the inward eutrophic remodeling process of the small arteries that is associated with the hypertensive state. Recent studies suggest that an up-regulation of MMP expression and activity is a common feature of hypertension. Moreover, experimental inhibition of MMP-activity in animal models of hypertension successfully lowers blood pressure and reduces vascular remodeling. However, whether MMP-inhibition specifically reverts the inward remodeling process in small arteries and reduces the risk for life threatening cardiovascular events associated with essential hypertension remains to be determined. Because a number of studies suggest that up-regulation of specific MMPs may help in reducing blood pressure, there is a need to determine the specific effect of individual MMPs on the vascular remodeling process and hypertension. For this purpose a combination of in vivo and in vitro models to study the remodeling process would be highly beneficial. Experiments using MMP knockout animals and tissue specific down regulation of MMP-expression may shed light on the remodeling mechanisms dependent on individual MMPs. These experiments should shed evidence to stimulate the development of novel inhibitors of specific MMPs, which in turn may prove beneficial not only for the treatment of vasculopaties, vascular remodeling, and hypertension, but also for the treatment of cancer and other pathological conditions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mathiassen ON, et al. Small artery structure is an independent predictor of cardiovascular events in essential hypertension. J Hypertens. 2007;25 (5):1021–1026. doi: 10.1097/HJH.0b013e32805bf8ed. [DOI] [PubMed] [Google Scholar]
- 2.Rizzoni D, et al. Prognostic significance of small-artery structure in hypertension. Circulation. 2003;108 (18):2230–2235. doi: 10.1161/01.CIR.0000095031.51492.C5. [DOI] [PubMed] [Google Scholar]
- 3.Mulvany MJ, et al. Vascular remodeling. Hypertension. 1996;28 (3):505–506. [PubMed] [Google Scholar]
- 4.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008;75 (2):346–359. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hao L, et al. Agonist-induced activation of matrix metalloproteinase-7 promotes vasoconstriction through the epidermal growth factor-receptor pathway. Circ Res. 2004;94 (1):68–76. doi: 10.1161/01.RES.0000109413.57726.91. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigues SF, et al. Matrix metalloproteinases cleave the beta2-adrenergic receptor in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2010;299 (1):H25–35. doi: 10.1152/ajpheart.00620.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Y, et al. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27 (4):697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- 8.Kandasamy AD, et al. Matrix metalloproteinase-2 and myocardial oxidative stress injury: beyond the matrix. Cardiovasc Res. 2010;85 (3):413–423. doi: 10.1093/cvr/cvp268. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y, et al. Increased intranuclear matrix metalloproteinase activity in neurons interferes with oxidative DNA repair in focal cerebral ischemia. J Neurochem. 2010;112 (1):134–149. doi: 10.1111/j.1471-4159.2009.06433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez-Lemus LA, et al. The plastic nature of the vascular wall: a continuum of remodeling events contributing to control of arteriolar diameter and structure. Physiology (Bethesda) 2009;24:45–57. doi: 10.1152/physiol.00029.2008. [DOI] [PubMed] [Google Scholar]
- 11.Schiffrin EL. Remodeling of resistance arteries in essential hypertension and effects of antihypertensive treatment. Am J Hypertens. 2004;17 (12 Pt 1):1192–1200. doi: 10.1016/j.amjhyper.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 12.DeLano FA, Schmid-Schonbein GW. Proteinase activity and receptor cleavage: mechanism for insulin resistance in the spontaneously hypertensive rat. Hypertension. 2008;52 (2):415–423. doi: 10.1161/HYPERTENSIONAHA.107.104356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tran ED, et al. Enhanced Matrix Metalloproteinase Activity in the Spontaneously Hypertensive Rat: VEGFR-2 Cleavage, Endothelial Apoptosis, and Capillary Rarefaction. J Vasc Res. 2010;47 (5):423–431. doi: 10.1159/000281582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friese RS, et al. Matrix metalloproteinases: discrete elevations in essential hypertension and hypertensive end-stage renal disease. Clin Exp Hypertens. 2009;31 (7):521–533. doi: 10.3109/10641960802668730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, et al. Matrix metalloproteinase-7 and ADAM-12 (a disintegrin and metalloproteinase-12) define a signaling axis in agonist-induced hypertension and cardiac hypertrophy. Circulation. 2009;119 (18):2480–2489. doi: 10.1161/CIRCULATIONAHA.108.835488. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Patron C, et al. Vascular matrix metalloproteinase-2 cleaves big endothelin-1 yielding a novel vasoconstrictor. Circ Res. 1999;85 (10):906–911. doi: 10.1161/01.res.85.10.906. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Patron C, et al. Vascular matrix metalloproteinase-2-dependent cleavage of calcitonin gene-related peptide promotes vasoconstriction. Circ Res. 2000;87 (8):670–676. doi: 10.1161/01.res.87.8.670. [DOI] [PubMed] [Google Scholar]
- 18.Christensen KL, Mulvany MJ. Vasodilatation, not hypotension, improves resistance vessel design during treatment of essential hypertension: a literature survey. J Hypertens. 2001;19 (6):1001–1006. doi: 10.1097/00004872-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Zhou MS, et al. Vascular but not cardiac remodeling is associated with superoxide production in angiotensin II hypertension. J Hypertens. 2005;23 (9):1737–1743. doi: 10.1097/01.hjh.0000179513.71018.09. [DOI] [PubMed] [Google Scholar]
- 20.Dao HH, et al. Transient involvement of endothelin in hypertrophic remodeling of small arteries. J Hypertens. 2001;19 (10):1801–1812. doi: 10.1097/00004872-200110000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Pistea A, et al. Small artery remodeling and erythrocyte deformability in L-NAME-induced hypertension: role of transglutaminases. J Vasc Res. 2008;45 (1):10–18. doi: 10.1159/000109073. [DOI] [PubMed] [Google Scholar]
- 22.Odenbach J, et al. MMP-2 Mediates Angiotensin II-Induced Hypertension Under the Transcriptional Control of MMP-7 and TACE. Hypertension. 2011;57 (1):123–130. doi: 10.1161/HYPERTENSIONAHA.110.159525. [DOI] [PubMed] [Google Scholar]
- 23.Seomun Y, et al. MMP-14 mediated MMP-9 expression is involved in TGF-beta1-induced keratinocyte migration. J Cell Biochem. 2008;104 (3):934–941. doi: 10.1002/jcb.21675. [DOI] [PubMed] [Google Scholar]
- 24.Chang DI, et al. Activation systems for latent matrix metalloproteinase-2 are upregulated immediately after focal cerebral ischemia. J Cereb Blood Flow Metab. 2003;23 (12):1408–1419. doi: 10.1097/01.WCB.0000091765.61714.30. [DOI] [PubMed] [Google Scholar]
- 25.Castro MM, et al. Imbalance between matrix metalloproteinases and tissue inhibitor of metalloproteinases in hypertensive vascular remodeling. Matrix Biol. 2010;29 (3):194–201. doi: 10.1016/j.matbio.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Flamant M, et al. Role of matrix metalloproteinases in early hypertensive vascular remodeling. Hypertension. 2007;50 (1):212–218. doi: 10.1161/HYPERTENSIONAHA.107.089631. [DOI] [PubMed] [Google Scholar]
- 27.Lehoux S, et al. Pressure-induced matrix metalloproteinase-9 contributes to early hypertensive remodeling. Circulation. 2004;109 (8):1041–1047. doi: 10.1161/01.CIR.0000115521.95662.7A. [DOI] [PubMed] [Google Scholar]
- 28.Abdul-Hussien H, et al. Doxycycline therapy for abdominal aneurysm: Improved proteolytic balance through reduced neutrophil content. J Vasc Surg. 2009;49 (3):741–749. doi: 10.1016/j.jvs.2008.09.055. [DOI] [PubMed] [Google Scholar]
- 29.Keeling WB, et al. An overview of matrix metalloproteinases in the pathogenesis and treatment of abdominal aortic aneurysms. Vasc Endovascular Surg. 2005;39 (6):457–464. doi: 10.1177/153857440503900601. [DOI] [PubMed] [Google Scholar]
- 30.Krishna SM, et al. Genetic and epigenetic mechanisms and their possible role in abdominal aortic aneurysm. Atherosclerosis. 2010;212 (1):16–29. doi: 10.1016/j.atherosclerosis.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Longo GM, et al. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110 (5):625–632. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ota R, et al. Roles of matrix metalloproteinases in flow-induced outward vascular remodeling. J Cereb Blood Flow Metab. 2009;29 (9):1547–1558. doi: 10.1038/jcbfm.2009.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nuki Y, et al. Roles of macrophages in flow-induced outward vascular remodeling. J Cereb Blood Flow Metab. 2009;29 (3):495–503. doi: 10.1038/jcbfm.2008.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coussens LM, et al. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295 (5564):2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 35.Castro MM, et al. Metalloproteinase inhibition ameliorates hypertension and prevents vascular dysfunction and remodeling in renovascular hypertensive rats. Atherosclerosis. 2008;198 (2):320–331. doi: 10.1016/j.atherosclerosis.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 36.Castro MM, et al. Antioxidant treatment reduces matrix metalloproteinase-2-induced vascular changes in renovascular hypertension. Free Radic Biol Med. 2009;46 (9):1298–1307. doi: 10.1016/j.freeradbiomed.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 37.Touyz RM, Briones AM. Reactive oxygen species and vascular biology: implications in human hypertension. Hypertens Res. 2011;34 (1):5–14. doi: 10.1038/hr.2010.201. [DOI] [PubMed] [Google Scholar]
- 38.Pistea A, et al. Flow inhibits inward remodeling in cannulated porcine small coronary arteries. Am J Physiol Heart Circ Physiol. 2005;289 (6):H2632–2640. doi: 10.1152/ajpheart.00205.2005. [DOI] [PubMed] [Google Scholar]
- 39.Grote K, et al. Mechanical stretch enhances mRNA expression and proenzyme release of matrix metalloproteinase-2 (MMP-2) via NAD(P)H oxidase-derived reactive oxygen species. Circ Res. 2003;92 (11):e80–86. doi: 10.1161/01.RES.0000077044.60138.7C. [DOI] [PubMed] [Google Scholar]
- 40.Siwik DA, Colucci WS. Regulation of matrix metalloproteinases by cytokines and reactive oxygen/nitrogen species in the myocardium. Heart Fail Rev. 2004;9 (1):43–51. doi: 10.1023/B:HREV.0000011393.40674.13. [DOI] [PubMed] [Google Scholar]
- 41.Martinez-Lemus LA, et al. Inward remodeling of resistance arteries requires reactive oxygen species-dependent activation of matrix metalloproteinases. American journal of physiology. Heart and circulatory physiology. 2011;300 (6):H2005–2015. doi: 10.1152/ajpheart.01066.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chesler NC, et al. Transmural pressure induces matrix-degrading activity in porcine arteries ex vivo. Am J Physiol. 1999;277 (5 Pt 2):H2002–2009. doi: 10.1152/ajpheart.1999.277.5.H2002. [DOI] [PubMed] [Google Scholar]
- 43.Lucchesi PA, et al. Involvement of metalloproteinases 2/9 in epidermal growth factor receptor transactivation in pressure-induced myogenic tone in mouse mesenteric resistance arteries. Circulation. 2004;110 (23):3587–3593. doi: 10.1161/01.CIR.0000148780.36121.47. [DOI] [PubMed] [Google Scholar]
- 44.Asanuma K, et al. Uniaxial strain upregulates matrix-degrading enzymes produced by human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2003;284 (5):H1778–1784. doi: 10.1152/ajpheart.00494.2002. [DOI] [PubMed] [Google Scholar]
- 45.Girardot D, et al. ERK1/2-mediated vasoconstriction normalizes wall stress in small mesenteric arteries during NOS inhibition in vivo. J Cardiovasc Pharmacol. 2003;42 (3):339–347. doi: 10.1097/00005344-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 46.Bakker EN, et al. Inward remodeling follows chronic vasoconstriction in isolated resistance arteries. J Vasc Res. 2002;39 (1):12–20. doi: 10.1159/000048989. [DOI] [PubMed] [Google Scholar]
- 47.Hill MA, et al. Delayed arteriolar relaxation after prolonged agonist exposure: functional remodeling involving tyrosine phosphorylation. Am J Physiol Heart Circ Physiol. 2003;285 (2):H849–856. doi: 10.1152/ajpheart.00986.2002. [DOI] [PubMed] [Google Scholar]
- 48.Martinez-Lemus LA. Persistent Agonist-Induced Vasoconstriction Is Not Required for Angiotensin II to Mediate Inward Remodeling of Isolated Arterioles with Myogenic Tone. J Vasc Res. 2008;45 (3):211–221. doi: 10.1159/000112513. [DOI] [PubMed] [Google Scholar]
- 49.Bakker EN, et al. Small artery remodeling depends on tissue-type transglutaminase. Circ Res. 2005;96 (1):119–126. doi: 10.1161/01.RES.0000151333.56089.66. [DOI] [PubMed] [Google Scholar]
- 50.Luchtefeld M, et al. Angiotensin II induces MMP-2 in a p47phox-dependent manner. Biochemical and biophysical research communications. 2005;328 (1):183–188. doi: 10.1016/j.bbrc.2004.12.152. [DOI] [PubMed] [Google Scholar]
- 51.Fortuno A, et al. Losartan metabolite EXP3179 blocks NADPH oxidase-mediated superoxide production by inhibiting protein kinase C: potential clinical implications in hypertension. Hypertension. 2009;54 (4):744–750. doi: 10.1161/HYPERTENSIONAHA.109.129353. [DOI] [PubMed] [Google Scholar]
- 52.Carlson BE, Secomb TW. A theoretical model for the myogenic response based on the length-tension characteristics of vascular smooth muscle. Microcirculation. 2005;12 (4):327–338. doi: 10.1080/10739680590934745. [DOI] [PubMed] [Google Scholar]
- 53.Martinez-Lemus LA, et al. Acute mechanoadaptation of vascular smooth muscle cells in response to continuous arteriolar vasoconstriction: implications for functional remodeling. Faseb J. 2004;18 (6):708–710. doi: 10.1096/fj.03-0634fje. [DOI] [PubMed] [Google Scholar]
- 54.Bendeck MP, et al. Smooth muscle cell migration and matrix metalloproteinase expression after arterial injury in the rat. Circ Res. 1994;75 (3):539–545. doi: 10.1161/01.res.75.3.539. [DOI] [PubMed] [Google Scholar]
- 55.Bendeck MP, et al. Doxycycline modulates smooth muscle cell growth, migration, and matrix remodeling after arterial injury. Am J Pathol. 2002;160 (3):1089–1095. doi: 10.1016/S0002-9440(10)64929-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Margolin L, et al. Metalloproteinase inhibitor attenuates neointima formation and constrictive remodeling after angioplasty in rats: augmentative effect of alpha(v)beta(3) receptor blockade. Atherosclerosis. 2002;163 (2):269–277. doi: 10.1016/s0021-9150(02)00035-7. [DOI] [PubMed] [Google Scholar]
- 57.Courtman DW, et al. Inward remodeling of the rabbit aorta is blocked by the matrix metalloproteinase inhibitor doxycycline. J Vasc Res. 2004;41 (2):157–165. doi: 10.1159/000077145. [DOI] [PubMed] [Google Scholar]
- 58.Furmaniak-Kazmierczak E, et al. Formation of extracellular matrix-digesting invadopodia by primary aortic smooth muscle cells. Circ Res. 2007;100 (9):1328–1336. doi: 10.1161/CIRCRESAHA.106.147744. [DOI] [PubMed] [Google Scholar]
- 59.Jin YJ, et al. Fibronectin and vitronectin induce AP-1-mediated matrix metalloproteinase-9 expression through integrin alpha(5)beta(1)/alpha(v)beta(3)-dependent Akt, ERK and JNK signaling pathways in human umbilical vein endothelial cells. Cell Signal. 2011;23 (1):125–134. doi: 10.1016/j.cellsig.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 60.Meng D, et al. Insulin-like growth factor-I (IGF-I) induces epidermal growth factor receptor transactivation and cell proliferation through reactive oxygen species. Free Radic Biol Med. 2007;42 (11):1651–1660. doi: 10.1016/j.freeradbiomed.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 61.Hur GY, et al. Tissue transglutaminase can be involved in airway inflammation of toluene diisocyanate-induced occupational asthma. J Clin Immunol. 2009;29 (6):786–794. doi: 10.1007/s10875-009-9314-8. [DOI] [PubMed] [Google Scholar]
- 62.Lee ZW, et al. Activation of in situ tissue transglutaminase by intracellular reactive oxygen species. Biochem Biophys Res Commun. 2003;305 (3):633–640. doi: 10.1016/s0006-291x(03)00835-0. [DOI] [PubMed] [Google Scholar]
- 63.Yi SJ, et al. [Ca(2+)]-dependent generation of intracellular reactive oxygen species mediates maitotoxin-induced cellular responses in human umbilical vein endothelial cells. Mol Cells. 2006;21 (1):121–128. [PubMed] [Google Scholar]
- 64.Langille BL, Dajnowiec D. Cross-linking vasomotor tone and vascular remodeling: a novel function for tissue transglutaminase? Circ Res. 2005;96 (1):9–11. doi: 10.1161/01.RES.0000153883.55971.81. [DOI] [PubMed] [Google Scholar]
- 65.Janiak A, et al. Cell surface transglutaminase promotes RhoA activation via integrin clustering and suppression of the Src-p190RhoGAP signaling pathway. Mol Biol Cell. 2006;17 (4):1606–1619. doi: 10.1091/mbc.E05-06-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Akimov SS, et al. Tissue transglutaminase is an integrin-binding adhesion coreceptor for fibronectin. J Cell Biol. 2000;148 (4):825–838. doi: 10.1083/jcb.148.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bendeck MP, et al. Smooth muscle cell matrix metalloproteinase production is stimulated via alpha(v)beta(3) integrin. Arterioscler Thromb Vasc Biol. 2000;20 (6):1467–1472. doi: 10.1161/01.atv.20.6.1467. [DOI] [PubMed] [Google Scholar]
- 68.Bendeck MP, et al. Inhibition of matrix metalloproteinase activity inhibits smooth muscle cell migration but not neointimal thickening after arterial injury. Circ Res. 1996;78 (1):38–43. doi: 10.1161/01.res.78.1.38. [DOI] [PubMed] [Google Scholar]
- 69.de Smet BJ, et al. Metalloproteinase inhibition reduces constrictive arterial remodeling after balloon angioplasty: a study in the atherosclerotic Yucatan micropig. Circulation. 2000;101 (25):2962–2967. doi: 10.1161/01.cir.101.25.2962. [DOI] [PubMed] [Google Scholar]
- 70.Sierevogel MJ, et al. Oral Matrix Metalloproteinase Inhibition and Arterial Remodeling After Balloon Dilation: An Intravascular Ultrasound Study in the Pig. Circulation. 2001;103 (2):302–307. doi: 10.1161/01.cir.103.2.302. [DOI] [PubMed] [Google Scholar]
- 71.Sierevogel MJ, et al. Matrix metalloproteinase inhibition reduces adventitial thickening and collagen accumulation following balloon dilation. Cardiovasc Res. 2002;55 (4):864–869. doi: 10.1016/s0008-6363(02)00467-4. [DOI] [PubMed] [Google Scholar]
- 72.Bouvet C, et al. Different involvement of extracellular matrix components in small and large arteries during chronic NO synthase inhibition. Hypertension. 2005;45 (3):432–437. doi: 10.1161/01.HYP.0000154680.44184.01. [DOI] [PubMed] [Google Scholar]
- 73.Dumont O, et al. Key role of the NO-pathway and matrix metalloprotease-9 in high blood flow-induced remodeling of rat resistance arteries. Arterioscler Thromb Vasc Biol. 2007;27 (2):317–324. doi: 10.1161/01.ATV.0000254684.80662.44. [DOI] [PMC free article] [PubMed] [Google Scholar]