Abstract
Alginate is a biomaterial that has found numerous applications in biomedical science and engineering due to its favorable properties, including biocompatibility and ease of gelation. Alginate hydrogels have been particularly attractive in wound healing, drug delivery, and tissue engineering applications to date, as these gels retain structural similarity to the extracellular matrices in tissues and can be manipulated to play several critical roles. This review will provide a comprehensive overview of general properties of alginate and its hydrogels, their biomedical applications, and suggest new perspectives for future studies with these polymers.
Keywords: biomaterial, hydrogel, wound dressing, drug delivery, tissue engineering
1. Introduction
Biomaterials have traditionally been designed to be inert and not to interact with biological systems in the host. Materials derived from natural sources (e.g., wood) have a long history as biomaterials, and have often been used to replace tissues lost to disease or trauma (e.g., prosthetics). Since the early twentieth century, however, these materials began to be replaced by synthetic polymers, ceramics, and metal alloys, due to their better performance and more reproducible properties, as compared to naturally derived materials [1, 2]. The more recent evolution in this field has now led to the definition of a biomaterial as a material intended to interface with biological systems to evaluate, treat, augment or replace any tissue, organ or function of the body [3], and boundaries for the use of biomaterials are still expanding. The design of new biomaterials is now focused on mimicking many functions of the extracellular matrices of body tissues, as these can regulate host responses in a well-defined manner, and naturally-derived materials have recently been regaining much attention owing to their inherent biocompatibility.
Alginate is a naturally occurring anionic polymer typically obtained from brown seaweed, and has been extensively investigated and used for many biomedical applications, due to its biocompatibility, low toxicity, relatively low cost, and mild gelation by addition of divalent cations such as Ca2+ [4]. Alginate hydrogels can be prepared by various cross-linking methods, and their structural similarity to extracellular matrices of living tissues allows wide applications in wound healing, delivery of bioactive agents such as small chemical drugs and proteins, and cell transplantation. Alginate wound dressings maintain a physiologically moist microenvironment, minimize bacterial infection at the wound site, and facilitate wound healing. Drug molecules, from small chemical drugs to macromolecular proteins, can be released from alginate gels in a controlled manner, depending on the cross-linker types and cross-linking methods. In addition, alginate gels can be orally administrated or injected into the body in a minimally invasive manner, which allows extensive applications in the pharmaceutical arena. Alginate gels are also promising for cell transplantation in tissue engineering. Tissue engineering aims to provide man-made tissue and organ replacements to patients who suffer the loss or failure of an organ or tissue [5]. In this approach, hydrogels are used to deliver cells to the desired site, provide a space for new tissue formation, and control the structure and function of the engineered tissue [6]. In this review, general properties of alginate and its current and potential applications in biomedical science and engineering will be discussed.
2. Alginate: general properties
Commercially available alginate is typically extracted from brown algae (Phaeophyceae), including Laminaria hyperborea, Laminaria digitata, Laminaria japonica, Ascophyllum nodosum, and Macrocystis pyrifera [7] by treatment with aqueous alkali solutions, typically with NaOH [8]. The extract is filtered, and either sodium or calcium chloride is added to the filtrate in order to precipitate alginate. This alginate salt can be transformed into alginic acid by treatment with dilute HCl. After further purification and conversion, water-soluble sodium alginate power is produced [9]. On a dry weight basis, the alginate contents are 22–30% for Ascophyllum nodosum and 25–44% for Laminaria digitata [10].
Bacterial biosynthesis may provide alginate with more defined chemical structures and physical properties than can be obtained from seaweed-derived alginate. Bacterial alginate can be produced from Azotobacter and Pseudomonas. The pathway of alginate biosynthesis is generally divided into (i) synthesis of precursor substrate, (ii) polymerization and cytoplasmic membrane transfer, (iii) periplasmic transfer and modification, and (iv) export through the outer membrane [11]. Recent progress in regulation of alginate biosynthesis in bacteria, and the relative ease of bacteria modification may enable production of alginate with tailor-made features and wide applications in biomedical applications.
2.1. Structure and characterization
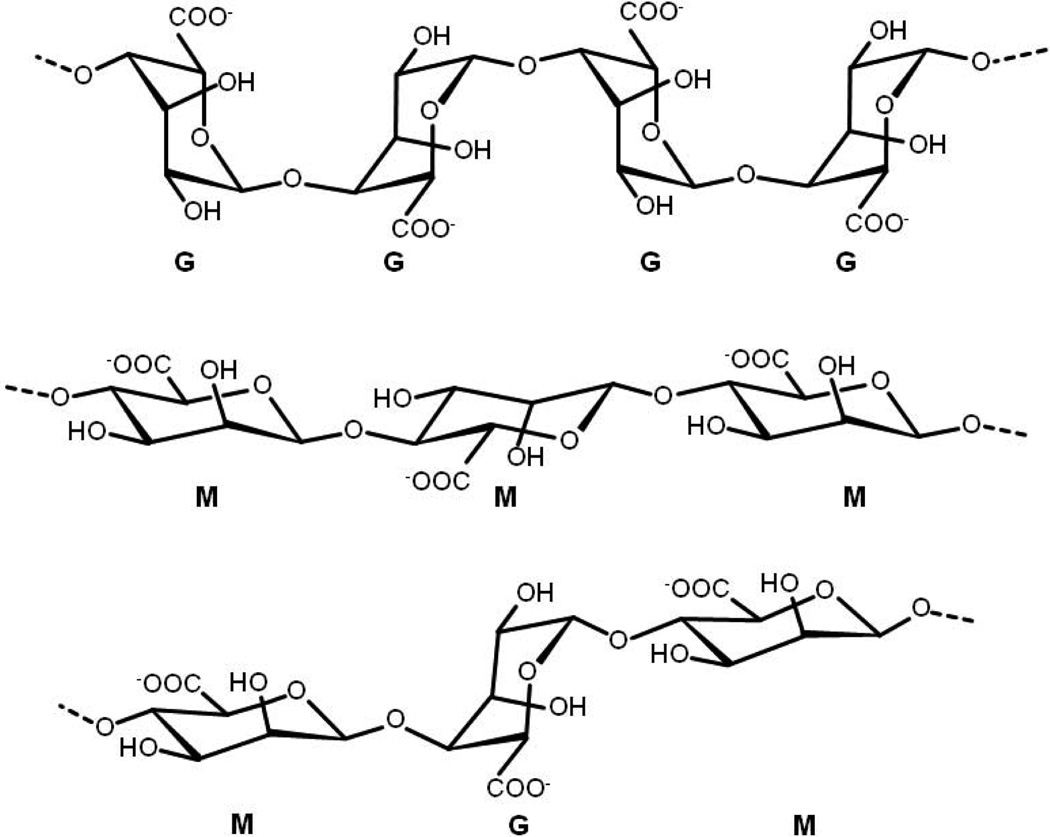
Until Fischer and Dörfel identified the L-guluronate residue [12], D-mannuronate was regarded as the major component of alginate. Fractional precipitation with manganese and calcium salts demonstrated later that alginates are actually block copolymers, and that the ratio of guluronate to mannuronate varies depending on the natural source [13]. Alginate is now known to be a whole family of linear copolymers containing blocks of (1,4)-linked β-D-mannuronate (M) and α-L-guluronate (G) residues. The blocks are composed of consecutive G residues (GGGGGG), consecutive M residues (MMMMMM), and alternating M and G residues (GMGMGM) (Fig. 1). Alginates extracted from different sources differ in M and G contents as well as the length of each block, and more than 200 different alginates are currently being manufactured [14]. The G-block content of Laminaria hyperborean stems is 60%, and for other commercially available alginates is in the range of 14.0–31.0% [10].
Fig. 1.
Chemical structures of G-block, M-block, and alternating block in alginate.
Only the G-blocks of alginate are believed to participate in intermolecular cross-linking with divalent cations (e.g., Ca2+) to form hydrogels. The composition (i.e., M/G ratio), sequence, G-block length, and molecular weight are thus critical factors affecting the physical properties of alginate and its resultant hydrogels [15]; the mechanism of gelation will be addressed in more detail in a later section. The mechanical properties of alginate gels typically are enhanced by increasing the length of G-block and molecular weight. It is important to note that different alginate sources provide polymers with a range of chemical structures (e.g., bacterial alginate produced from Azotobacter has a high concentration of G-blocks and its gels have a relatively high stiffness [16]). The physical properties significantly control the stability of the gels, the rate of drug release from gels, and the phenotype and function of cells encapsulated in alginate gels.
2.2. Molecular weight and solubility
The molecular weight of commercially available sodium alginates range between 32,000 and 400,000 g/mol. The parameters of the Mark-Houwink relationship ([η] = KMva) for sodium alginate in 0.1 M NaCl solution at 25°C are K = 2×10−3 and a = 0.97, where [η] is intrinsic viscosity (mL/g) and Mv is the viscosity-average molecular weight (g/mol) [17]. The viscosity of alginate solutions increase as pH decreases, and reach a maximum around pH = 3–3.5, as carboxylate groups in the alginate backbone become protonated and form hydrogen bonds.
Increasing the molecular weight of alginate can improve the physical properties of resultant gels. However, an alginate solution formed from high molecular weight polymer becomes greatly viscous, which is often undesirable in processing [18]. For example, proteins or cells mixed with an alginate solution of high viscosity risk damage from the high shear forces generated during mixing and injection into the body [19]. Manipulation of the molecular weight and its distribution can independently control the pre-gel solution viscosity and post-gelling stiffness. The elastic modulus of gels can be increased significantly, while the viscosity of the solution minimally raises, by using a combination of high and low molecular weight alginate polymers [20].
2.3. Biocompatibility
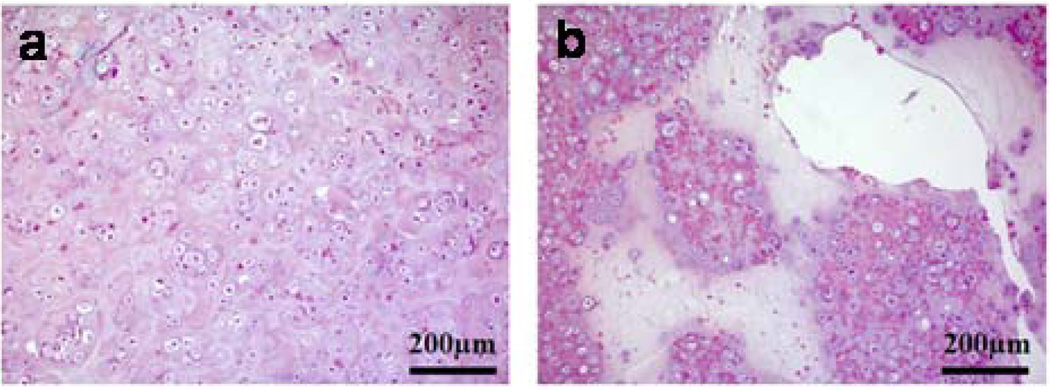
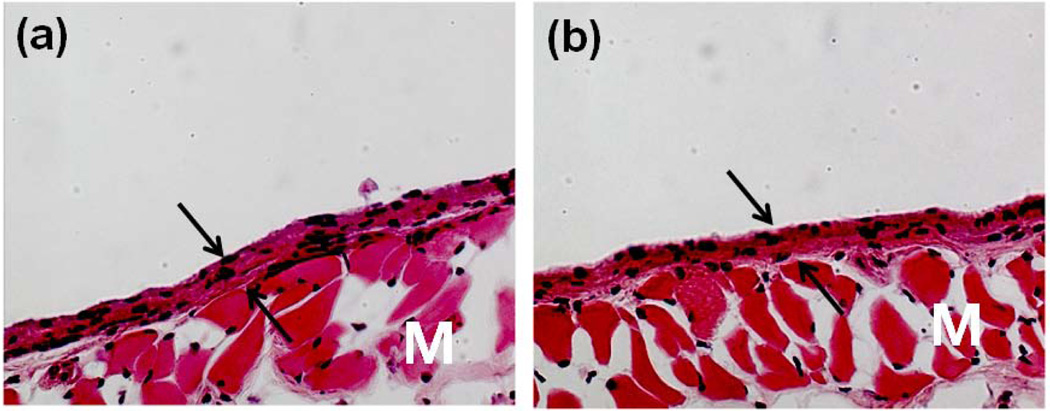
Although the biocompatibility of alginate has been extensively evaluated in vitro as well as in vivo, there is still debate regarding the impact of the alginate composition. Much of this confusion, though, likely relates to varying levels of purity in the alginate studied in various reports. For example, it has been reported that high M content alginates were immunogenic and approximately 10 times more potent in inducing cytokine production compared with high G alginates [21], but others found little or no immunoresponse around alginate implants [22]. The immunogenic response at the injection or implantation sites might be attributed to impurities remaining in the alginate. Since alginate is obtained from natural sources, various impurities such as heavy metals, endotoxins, proteins, and polyphenolic compounds could potentially be present. Importantly, alginate purified by a multi-step extraction procedure to a very high purity did not induce any significant foreign body reaction when implanted into animals [23]. Similarly, no significant inflammatory response was observed when gels formed from commercially available, highly purified alginate have been subcutaneously injected into mice (Fig. 2) [24].
Fig. 2.
Photomicrographs of tissue sections three weeks post-injection with (a) PBS and (b) alginate hydrogels. Original pictures were taken at 100× magnification. Photomicrographs have labels for the muscle layer (M). Arrows indicate the newly formed granulation tissue adjacent to the site of injection: this is a connective tissue typically formed following biomaterial implantation [24]. Copyright 2009, Springer, New York, USA.
2.4. Derivatives
2.4.1. Amphiphilic alginate
Various alginate derivatives used in a range of biomedical applications have been reported to date. Amphiphilic alginate derivatives have been synthesized by introducing hydrophobic moieties (e.g., alkyl chains, hydrophobic polymers) to the alginate backbone. These derivatives can form self-assembled structures such as particles and gels in aqueous media, and have potential in many drug delivery applications.
Amphiphilic derivatives of sodium alginate have been prepared by conjugation of long alkyl chains (i.e., dodecyl, octadecyl) to the alginate backbone via ester bond formation. Aqueous solutions of these alginate derivatives exhibited the typical rheological properties of physically cross-linked, gel-like networks in the semidilute regime, which could be useful for cartilage repair and regeneration [25]. Microparticles were prepared from these derivatives by dispersion in a sodium chloride solution, as this technique can allow encapsulation of proteins and their subsequent release by the addition of either surfactants that disrupt intermolecular hydrophobic junctions or esterases that hydrolyze the ester bond between alkyl chains and the alginate backbone [26]. Dodecylamine was also conjugated to the alginate backbone via amide linkage formation using 2-chloro-1-methylpyridinium iodide as a coupling reagent. Hydrogels prepared from this alginate derivative exhibited long-term stability in aqueous media, compared to those prepared from alginate derivatives with dodecyl ester, which are labile to hydrolysis [27]. Water soluble, amphiphilic alginate derivatives grafted with cholesteryl groups were also synthesized using N,N'-dicyclohexylcarbodiimide as a coupling agent and 4-(N,N'-dimethylamino)pyridine as a catalyst at room temperature. The derivatives formed self-aggregates with a mean diameter of 136 nm in an aqueous sodium chloride solution [28]. The associative behavior of alginate grafted with poly(ε-caprolactone) (PCL) in an aqueous sodium chloride solution was strongly dependent on the length of PCL chains, unlike the behavior of derivatives in water [29]. Sodium alginate has also been hydrophobically modified with poly(butyl methacrylate), leading to prolonged release of model drugs as compared with unmodified alginate gels [30].
2.4.2. Cell-interactive alginate
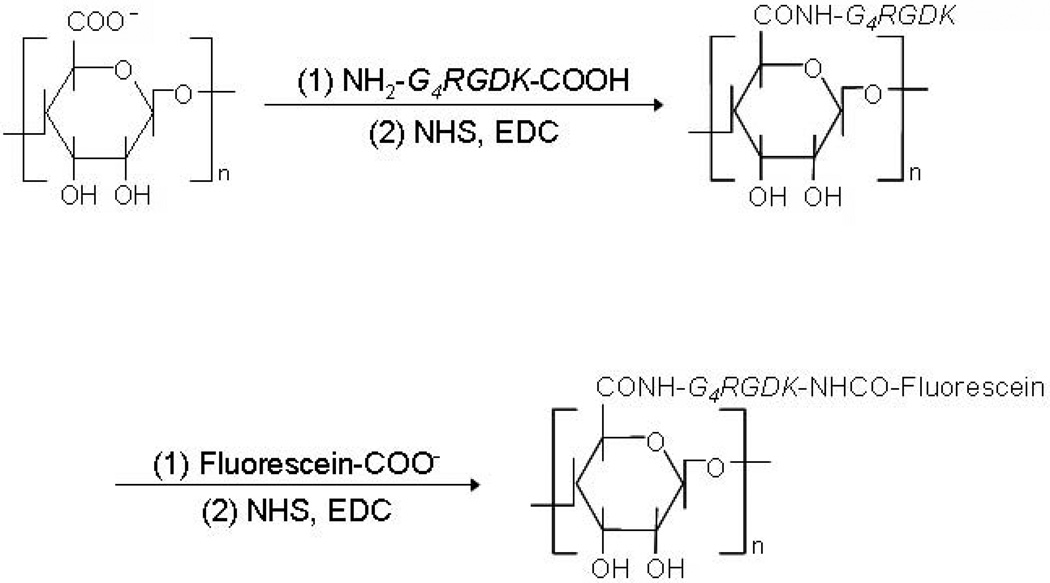
Alginate derivatives containing cell-adhesive peptides have been gaining significant attraction recently. These derivatives are typically prepared by chemically introducing peptides as side-chains, using carbodiimide chemistry to couple via the carboxylic groups of the sugar residues. Since alginate inherently lacks mammalian cell-adhesivity, appropriate ligands are crucial to promote and regulate cellular interactions, especially for cell culture and tissue engineering applications. Peptides including the sequence arginine-glycine-aspartic acid (RGD) have been extensively used as model adhesion ligands, due to the wide-spread presence of integrin receptors (e.g., αvβ3, α5β1) for this ligand on various cell types [31, 32]. RGD containing peptides can be chemically coupled to the alginate backbone using water-soluble carbodiimide chemistry (Fig. 3) [33]. A minimum concentration of RGD peptides in alginate gels is needed for the adhesion and growth of cells, and this minimum is likely cell type specific. For example, minimal concentrations for substantial adhesion of MC3T3-E1 and C2C12 cells to alginate gels in vitro have been reported as 12.5 [34, 100] and 10.0 [98] µg/mg alginate, respectively. The affinity of the RGD peptide will also play an important role, and cyclic RGD peptides have been demonstrated to be more potent and are needed at lower concentrations than linear RGD peptides [105]. Various peptides containing the DGEA (Asp–Gly–Glu–Ala) [34] and YIGSR (Tyr-Ile-Gly-Ser-Arg) [35] sequences derived from other extracellular matrix proteins have also been exploited to modify alginate gels and enhance the adhesive interactions with various cell types. For example, alginate has been modified with YIGSR peptides using water-soluble carbodiimide chemistry to promote neural cell adhesion [35]. However, the majority of work to date has been with RGD-alginate. Cell-interactive alginates have been widely utilized as cell culture substrates both in 2-D and 3-D culture, and as scaffolds in tissue engineering applications, as described in detail in a later section.
Fig. 3.
Synthetic scheme of RGD-modified alginate using water-soluble carbodiimide chemistry (NHS, N-hydroxysulfosuccinimide; EDC, 1-ethyl-3-(dimethylaminopropyl) carbodiimide). For further analysis, RGD peptide can be labeled with fluorescein [33], as demonstrated in this scheme. Copyright 2008, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany.
3. Hydrogel formation and its properties
3.1. Methods of gelling
Alginate is typically used in the form of a hydrogel in biomedicine, including wound healing, drug delivery and tissue engineering applications. Hydrogels are three-dimensionally cross-linked networks composed of hydrophilic polymers with high water content. Hydrogels are often biocompatible, as they are structurally similar to the macromolecular-based components in the body, and can often be delivered into the body via minimally invasive administration [36]. Chemical and/or physical cross-linking of hydrophilic polymers are typical approaches to form hydrogels, and their physicochemical properties are highly dependent on the cross-linking type and cross-linking density, in addition to the molecular weight and chemical composition of the polymers [37, 38]. Here, we summarize various approaches to cross-link alginate chains to prepare gels, and how these approaches alter the hydrogel properties relevant to biomedical applications.
3.1.1. Ionic cross-linking
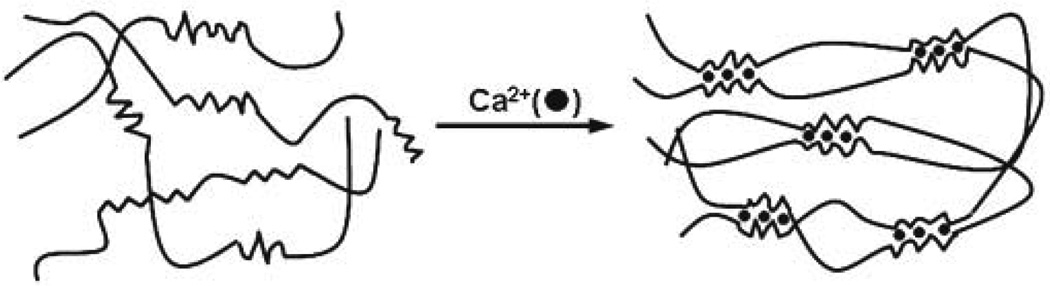
The most common method to prepare hydrogels from an aqueous alginate solution is to combine the solution with ionic cross-linking agents, such as divalent cations (i.e., Ca2+). The divalent cations are believed to bind solely to guluronate blocks of the alginate chains, as the structure of the guluronate blocks allows a high degree of coordination of the divalent ions. The guluronate blocks of one polymer then form junctions with the guluronate blocks of adjacent polymer chains in what is termed the egg-box model of cross-linking, resulting in a gel structure (Fig. 4) [39]. Calcium chloride (CaCl2) is one of the most frequently used agents to ionically cross-link alginate. However, it typically leads to rapid and poorly controlled gelation due to its high solubility in aqueous solutions. One approach to slow and control gelation is to utilize a buffer containing phosphate (e.g., sodium hexametaphosphate), as phosphate groups in the buffer compete with carboxylate groups of alginate in the reaction with calcium ions, and retard gelation. Calcium sulfate (CaSO4) and calcium carbonate (CaCO3), due to their lower solubilities, can also slow the gelation rate and widen the working time for alginate gels. For example, an alginate solution can be mixed with CaCO3, which is not soluble in water at neutral pH. Glucono-δ-lactone is then added to the alginate/CaCO3 mixture in order to dissociate Ca2+ from the CaCO3 by lowering the pH. The released Ca2+ subsequently initiates the gelation of the alginate solution in a more gradual manner [40].
Fig. 4.
Alginate hydrogels prepared by ionic cross-linking (egg-box model) [38]. Only guluronate blocks participate in the formation of a corrugated egg-box-like structure with interstices in which calcium ions are placed. Copyright 2007, Elsevier Science Ltd., Oxford, UK.
The gelation rate is a critical factor in controlling gel uniformity and strength when using divalent cations, and slower gelation produces more uniform structures and greater mechanical integrity [41]. The gelation temperature also influences gelation rate, and the resultant mechanical properties of the gels. At lower temperatures, the reactivity of ionic cross-linkers (e.g., Ca2+) is reduced, and cross-linking becomes slower. The resulting cross-linked network structure has greater order, leading to enhanced mechanical properties [42]. In addition, the mechanical properties of ionically cross-linked alginate gels can vary significantly depending on the chemical structure of alginate. For example, gels prepared from alginate with a high content of G residues exhibit higher stiffness than those with a low amount of G residues [43].
One critical drawback of ionically cross-linked alginate gels is the limited long-term stability in physiological conditions, because these gels can be dissolved due to release of divalent ions into the surrounding media due to exchange reactions with monovalent cations. In addition, the calcium ions released from the gel may promote hemostasis, while the gel serves as a matrix for aggregation of platelets and erythrocytes [44]. These features may be beneficial or negative, depending on the situation, but a desire to avoid these biological reactions, along with more general limitations of ionically cross-linked gels has led to interest in covalently cross-linked alginate hydrogels.
3.1.2. Covalent cross-linking
Covalent cross-linking has been widely investigated in an effort to improve the physical properties of gels for many applications, including tissue engineering. The stress applied to an ionically cross-linked alginate gel relaxes as the cross-links dissociate and reform elsewhere, and water is lost from the gel, leading to plastic deformation. While water migration also occurs in covalently cross-linked gels, leading to stress relaxation, the inability to dissociate and reform bonds leads to significant elastic deformation [45]. However, covalent cross-linking reagents may be toxic, and the unreacted chemicals may need to be removed thoroughly from gels.
Covalent cross-linking of alginate with poly(ethylene glycol)-diamines of various molecular weights was first investigated in order to prepare gels with a wide range of the mechanical properties. While the elastic modulus initially increased gradually with an increase in the cross-linking density or weight fraction of poly(ethylene glycol) (PEG) in the gel, it subsequently decreased when the molecular weight between cross-links (Mc) became less than the molecular weight of the softer PEG [46]. It was subsequently demonstrated that the mechanical properties and swelling of alginate hydrogels can be tightly regulated by using different kinds of cross-linking molecules, and by controlling the cross-linking densities. The chemistry of the cross-linking molecules also significantly influences hydrogel swelling, as would be expected. The introduction of hydrophilic cross-linking molecules as a second macromolecule (e.g. PEG) can compensate for the loss of hydrophilic character of the hydrogel resulting from the cross-linking reaction [47].
The use of multi-functional cross-linking molecules to form hydrogels provides a wider range and tighter control over degradation rates and mechanical stiffness than bi-functional cross-linking molecules. For example, the physical properties and degradation behavior of poly(aldehyde guluronate) (PAG) gels prepared with either poly(acrylamide-co-hydrazide) (PAH) as a multi-functional cross-linker or adipic acid dihydrazide (AAD) as a bi-functional cross-linker were monitored in vitro. PAG/PAH gels showed higher mechanical stiffness before degradation and degraded more slowly than PAG/AAD gels. The enhanced mechanical stiffness and prolonged degradation behavior could be attributed to the multiple attachment points of PAH in the gel even at the same concentration of overall functional groups [48].
Photo cross-linking is an exciting approach to in situ gelation that exploits covalent cross-linking. Photo cross-linking can be carried out in mild reaction conditions, even in direct contact with drugs and cells, with the appropriate chemical initiators. Alginate, modified with methacrylate and cross-linked by exposure to a laser (argon ion laser, 514 nm) for 30 s in the presence of eosin and triethanol amine, forms clear and flexible hydrogels. The gels were useful for sealing corneal perforation in vivo, indicating a potential clinical use in sutureless surgery [49]. Photo cross-linking reactions typically involve the use of a light sensitizer or the release of acid, which may be harmful to the body. An alternative photo cross-linking approach uses polyallylamine partially modified with α-phenoxycinnamyldiene acetylchloride, which dimerizes on light exposure at about 330 nm, and releases no toxic byproducts during the cross-linking reaction [50]. The mechanical properties of the gels formed from this photosensitive polyallylamine and alginate were significantly improved by light irradiation, and the gels were freely permeable to cytochrome c and myoglobin [51].
3.1.3. Thermal gelation
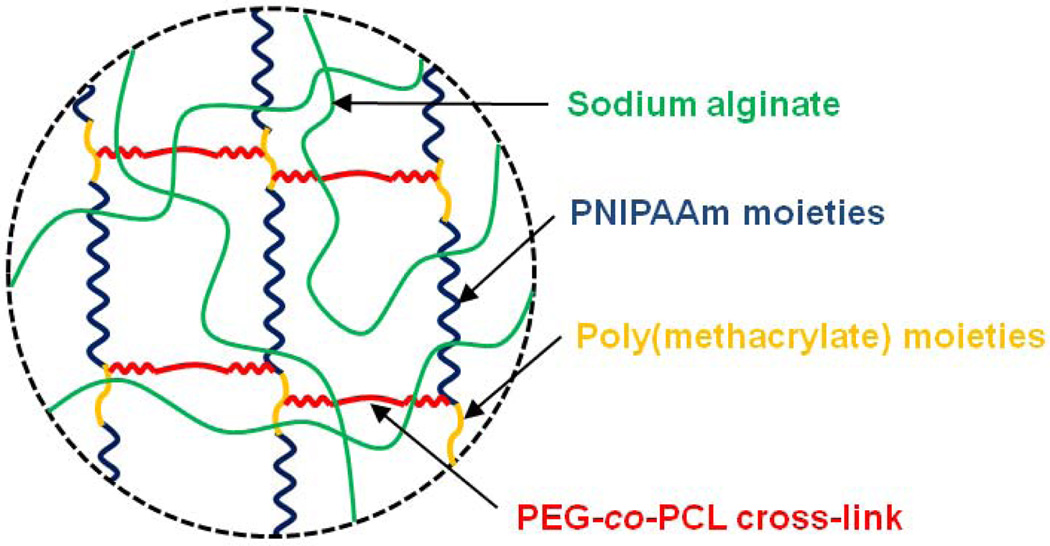
Thermo-sensitive hydrogels have been widely investigated to date in many drug delivery applications, due to their adjustable swelling properties in response to temperature changes, leading to on-demand modulation of drug release from the gels [52]. Poly(N-isopropylacrylamide) (PNIPAAm) hydrogels are the most extensively exploited thermo-sensitive gels, and these undergo a reversible phase transition near body temperature in aqueous media (lower critical solution temperature near 32°C). The transition temperature can be altered by copolymerization with hydrophilic monomers such as acrylic acid and acrylamide [53]. Despite the potential importance of thermo-sensitive hydrogels in biomedical applications, few systems using alginate have been reported yet, as alginate is not inherently thermo-sensitive. However, semi-interpenetrating polymer network (semi-IPN) structures were prepared via in situ copolymerization of N-isopropylacrylamide (NIPAAm) with poly(ethylene glycol)-co-poly(ε-caprolactone) (PEG-co-PCL) macromer in the presence of sodium alginate by UV irradiation (Fig. 5). The swelling ratio of the gels increased with the concentration of sodium alginate at a constant temperature, and decreased with an increase in temperature. The use of sodium alginate in semi-IPN structures also improved the mechanical strength and the cumulative release of BSA from the gels, indicating potential in drug delivery applications [54]. Graft copolymerization of NIPAAm onto the alginate backbone after reaction with ceric ions also provided a useful means to prepare temperature-responsive alginate gels, with sensitivity near body temperature (Lee and Mooney, unpublished data) (Fig. 6).
Fig. 5.
Schematic description of thermo-sensitive semi-IPN alginate hydrogels [54].
Fig. 6.
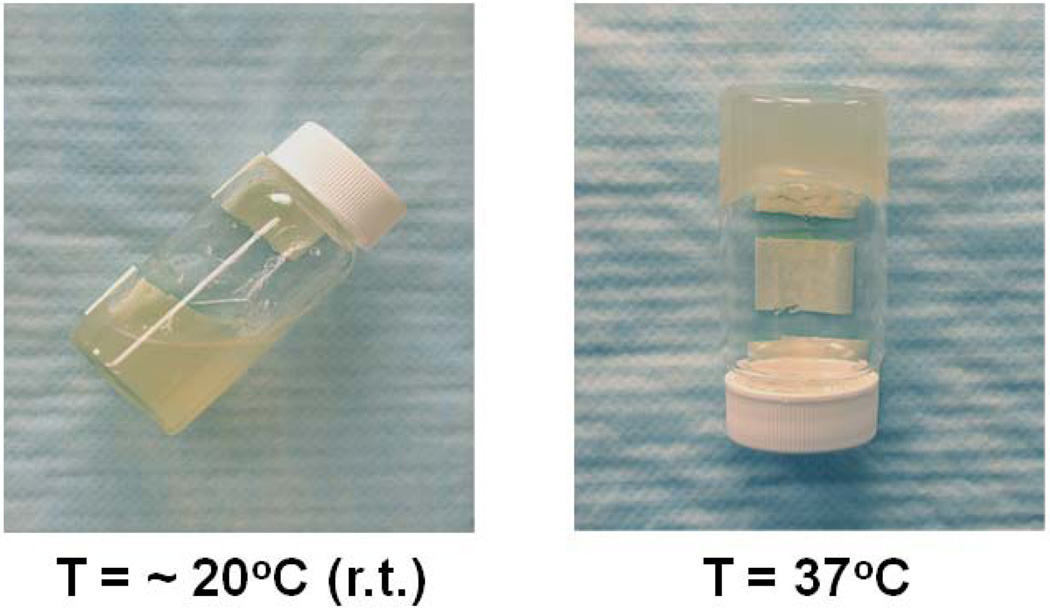
Thermal gelation of an aqueous alginate-g-NIPAAm solution at 37°C.
3.1.4. Cell cross-linking
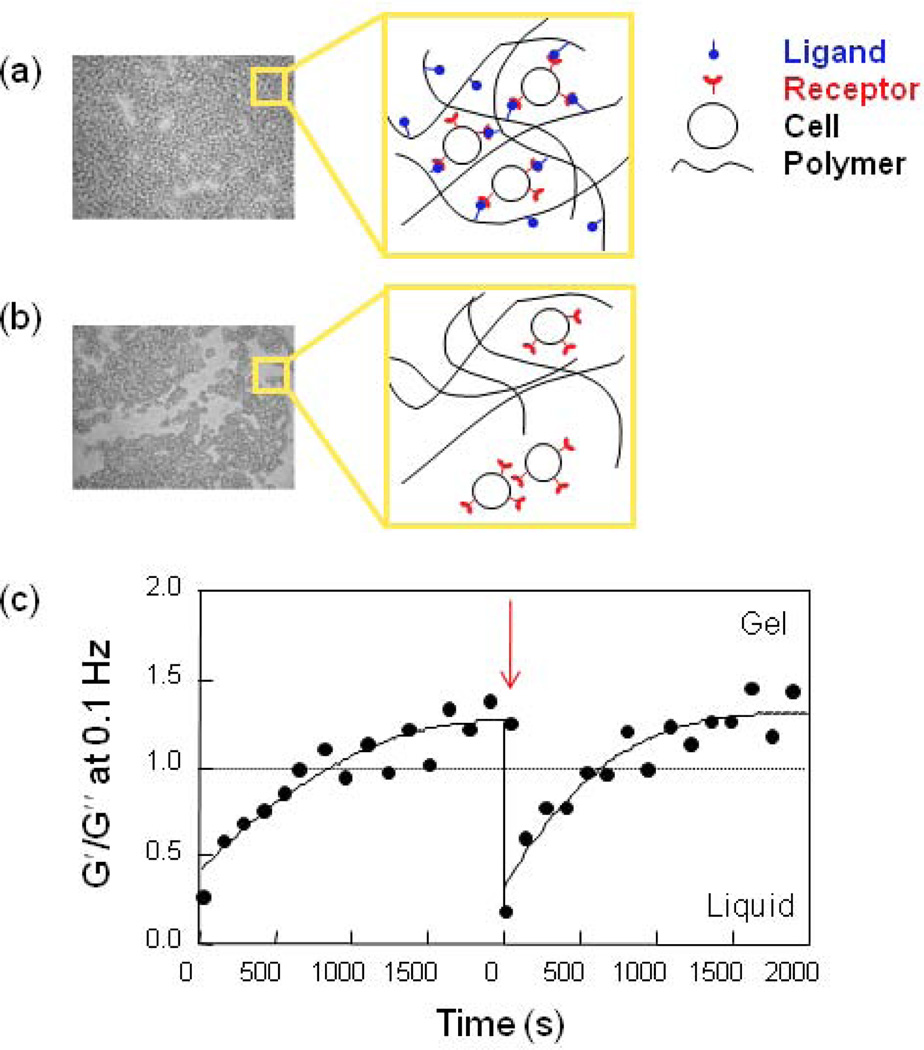
While a number of chemical and physical methods have been reported to form alginate gels, the ability of cells to contribute to gel formation has been largely ignored. When alginate is modified with cell adhesion ligands, the ability of cells to bind multiple polymer chains can lead to long-distance, reversible network formation even in the absence of chemical cross-linking agents. Cells added to an RGD-modified alginate solution form a uniform dispersion within the solution, and this system subsequently generates the cross-linked network structure via specific receptor-ligand interactions without using any additional cross-linking molecules (Fig.7) [55]. In contrast, cells added to non-modified alginate solutions aggregate and form a non-uniform structure, due to the dominance of cell-cell interactions in that system. This gelation behavior is shear reversible and can be repeated multiple times. Once the gel structure is broken down by applying shear forces, cross-linked structures are recovered within a few minute. This behavior is governed by the weak and reversible ligand-receptor interactions in the system. This system might be ideal for cell delivery in tissue engineering because a gel can flow like a liquid during injection into the body, but solidify once it is placed in the body. Further, it was reported that cells can provide additional mechanical integrity to RGD-alginate gels that are ionically cross-linked with calcium ions, again via binding interactions between cells and the adhesion ligands coupled to the alginate chains [56].
Fig. 7.
Photomicrographs and schematics of cell-cross-linked network structures when cells are mixed with (a) RGD-modified alginate and (b) non-modified alginate. (c) Shear-reversible gelation of cell-cross-linked RGD-alginate hydrogels. The arrow indicates disruption of the gel structure with intentional shear force. The cross-over point where G′ (storage shear modulus) = G″ (loss shear modulus) provides a measure of the gelation threshold (dotted line) [55]. Copyright 2003, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany.
3.2. Biodegradation of alginate and its hydrogels
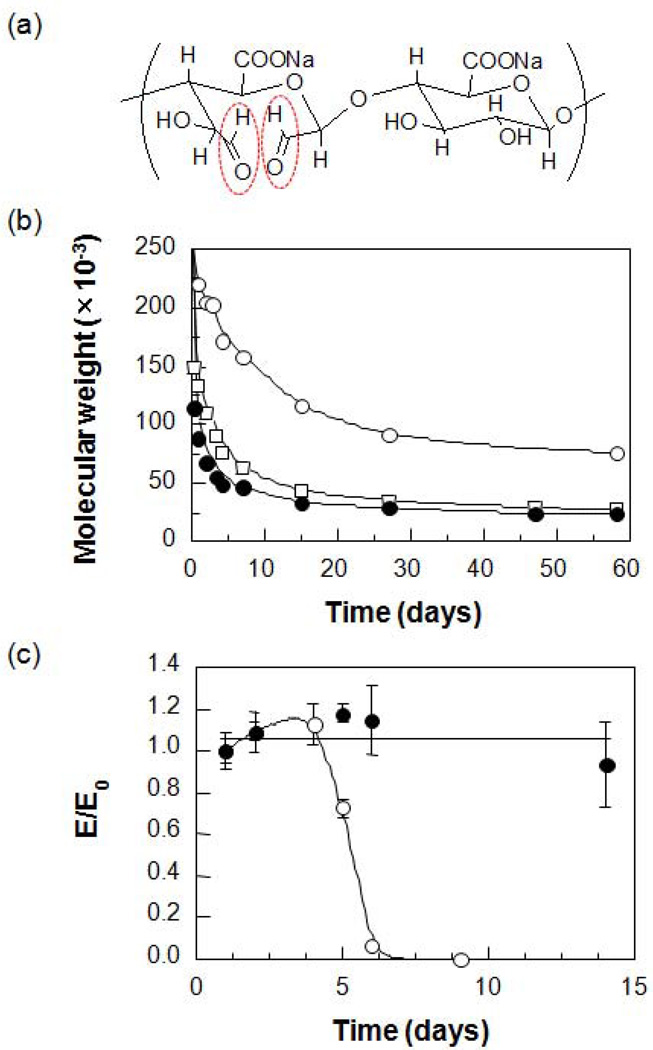
Alginate is inherently non-degradable in mammals, as they lack the enzyme (i.e., alginase) which can cleave the polymer chains, but ionically cross-linked alginate gels can be dissolved by release of the divalent ions cross-linking the gel into the surrounding media due to exchange reactions with monovalent cations such as sodium ions. Even if the gel dissolves, though, the average molecular weights of many commercially available alginates are higher than the renal clearance threshold of the kidneys, and likely will not be completely removed from the body [57]. An attractive approach to make alginate degradable in physiological conditions includes partial oxidation of alginate chains. Slightly oxidized alginate can degrade in aqueous media, and these materials have demonstrated potential as a delivery vehicle of drugs and cells for various applications. Alginate is typically oxidized with sodium periodate (Fig. 8a). The periodate oxidation cleaves the carbon-carbon bond of the cis-diol group in the uronate residue and alters the chair conformation to an open-chain adduct, which enables degradation of the alginate backbone. A slight reduction in the molecular weight during oxidation is expected. However, partial oxidation of alginate does not significantly interfere with its gel forming capability in the presence of divalent cations. The resulting degradation rate of the gels is strongly dependent on the degree of oxidation, as well as on the pH and temperature of the media (Figs. 8b & 8c) [58].
Fig. 8.
(a) Chemical structure of partially oxidized alginate (aldehyde groups generated after oxidation reaction highlighted in red) and (b) its degradation behavior at various pHs (○, pH 4.5; □, pH 7.4; ●, pH 9.2). (c) Changes in the compressive modulus of hydrogels prepared from alginate (●) and partially oxidized alginate (○) over time (pH 7.2, 37°C) [58]. Copyright 2001, John Wiley & Sons, New York, USA.
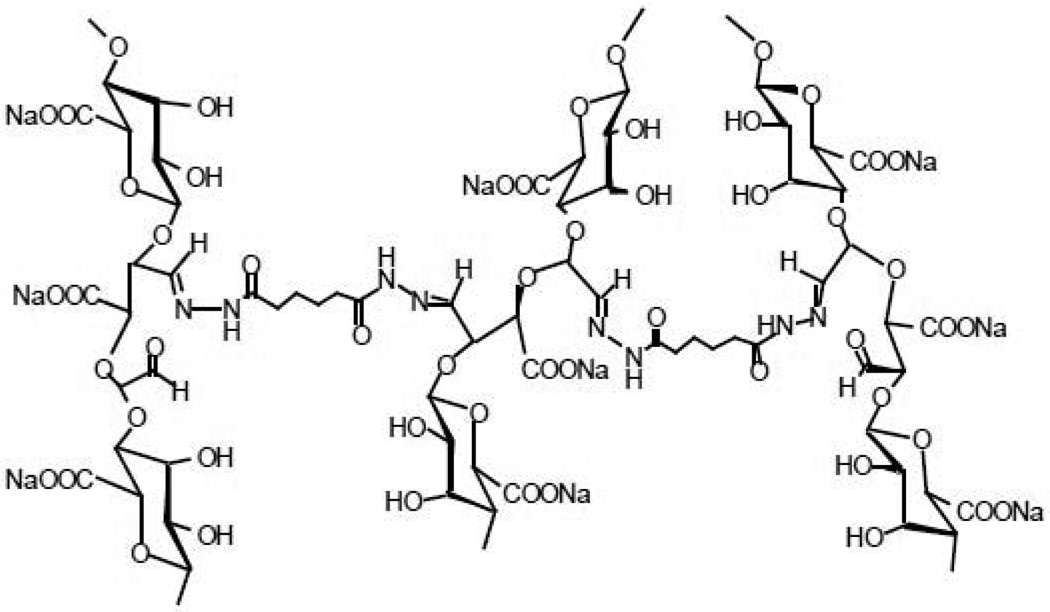
One can create gels solely from G-blocks isolated from alginate; partial oxidation of the G-blocks enables degradable gel formation. For example, polyguluronate (PG) was isolated from alginate at pH 2.85, and then oxidized with sodium periodate to prepare poly(aldehyde guluronate) (PAG). PAG can be covalently cross-linked with adipic acid dihydrazide (AAD) to form gels (Fig. 9), in place of ionic cross-linking. The reaction between aldehydes and hydrazides is very fast and the resulting hydrazone bonds will be labile to hydrolysis, which leads to gels that degrade in aqueous media. The higher the AAD concentration used to form gels, the slower the degradation rate. The degradation rate and mechanical properties have been regarded as critical factors in new tissue formation in tissue engineering, but are typically coupled, making it difficult to decouple their effects. Surprisingly, PAG gels with a high content of dangling single-end AAD molecules showed retarded degradation behavior, irrespective of the low cross-linking density, because this large number of single-end AAD molecules allows recross-linking of PAG chains following hydrolysis of the initial hydrazone bond [59]. This finding clearly indicates that one can make soft gels that degrade slowly over time, unlike conventional gels.
Fig. 9.
Chemical structure of poly(aldehyde guluronate) gels cross-linked with adipic acid dihydrazide [59]. Copyright 2000, American Chemical Society, Washington DC, USA.
In addition, the degradation rate and mechanical properties of alginate gels can be decoupled by adjusting the molecular weight distribution of alginate. Binary alginate gels have been formed from partially oxidized alginates with low and high molecular weights by either ionic or covalent cross-linking. The molecular weight (MW) of alginate was varied via γ-irradiation; the length of G-blocks was nearly unchanged by this treatment. Increasing the fraction of low MW alginate to 0.50 maintained the mechanical stiffness of the gels compared with the high MW alginate gels, but led to faster degradation, irrespective of the cross-linking method [60]. Alternatively, gels prepared from two types of alginate with a size mismatch in G-block length exhibited a more rapid ion exchange, and resultant gel dissolution [61]. These various approaches may be useful alone or in combination for manipulating the physical properties of various hydrogels in the development of drug delivery and cell transplantation vehicles.
4. Biomedical applications
4.1. Pharmaceutical applications
The conventional role of alginate in pharmaceutics includes serving as thickening, gel forming, and stabilizing agents, as alginate can play a significant role in controlled-release drug products. Oral dosage forms are currently the most frequent use of alginate in pharmaceutical applications, but the use of alginate hydrogels as depots for tissue localized drug delivery is growing. Here, we briefly describe recent progress in controlled drug delivery using alginate and/or its derivatives.
4.1.1. Delivery of small chemical drugs
Alginate gels have been investigated for the delivery of a variety of low molecular weight drugs, and are likely most useful when a primary or secondary bond between the drug and the alginate can be exploited to regulate the kinetics of drug release. Alginate gels are typically nanoporous (pore size ~ 5 nm) [62], leading to rapid diffusion of small molecules through the gel. For example, the release of flurbiprofen from ionically cross-linked, partially oxidized alginate gels is almost complete in 1.5 hr. However, incorporation into beads formed from partially oxidized alginate in the presence of both calcium ions and adipic acid dihydrazide (combination of ionic and covalent cross-linking) led to a prolonged release due to the increased number of cross-links and resultant reduced swelling [63]. The controlled and localized delivery of antineoplastic agents has also been achieved using partially oxidized alginate gels. Multiple drugs can be loaded into alginate-based gels for simultaneous or sequential delivery, as the chemical structure of the drug and mode of incorporation will dramatically alter the release kinetics. For example, methotrexate (non-interactive with alginate) was rapidly released by diffusion, while doxorubicin, covalently attached to the alginate, was released via chemical hydrolysis of the cross-linker. Mitoxantrone, ionically complexed to alginate, was only released after the dissociation of the gel [64].
Amphiphilic gel beads have also prepared to modulate the release of hydrophobic drugs. Alginate grafted with poly(ε-caprolactone) (PCL) has been cross-linked with calcium ions for controlled delivery of theophylline, a model drug with poor water-solubility. The length of the hydrophobic PCL chains controlled the swelling behavior of the gel beads, and the PCL slowed the release of theophylline. The drug release was complete within 2 hr for alginate-g-PCL/Ca2+ beads, but in 1 hr for alginate/Ca2+ beads [65]. The sustained release of theophylline was also achieved from carbon nanotube (CNT)-incorporated alginate microspheres. The addition of CNT enhanced the mechanical stability of gels, without affecting the structure and morphology of the microspheres and no significant cytotoxicity was observed, indicating potential application as a delivery carrier to the intestine and colon [66].
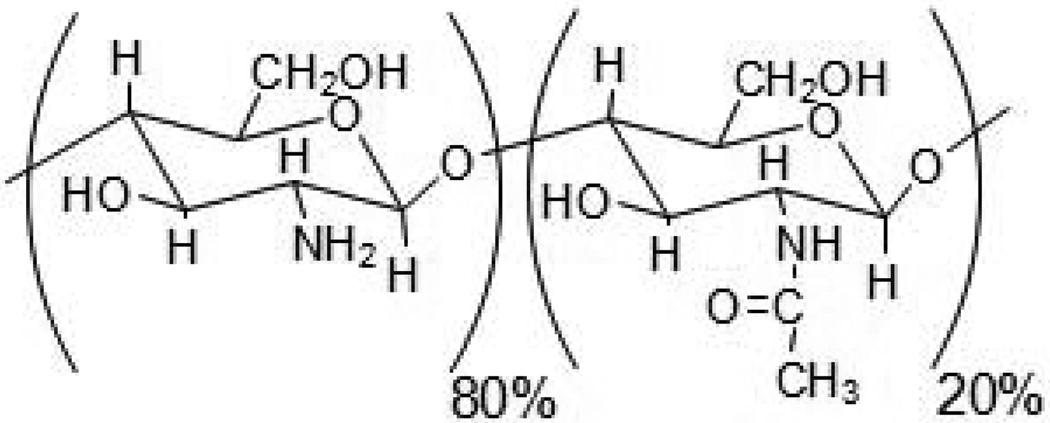
Alginate has also been widely exploited in many drug delivery applications in combination with chitosan, as the combination forms ionic complexes. Chitosan is a derivative of chitin, the second most abundant natural polymer in the world, and has a repeating structure of (1,4) linked β-D-glucosamine, with an apparent pK of 6.5. Traditionally, commercial products are composed of 80% β-D-glucosamine and 20% N-acetyl-β-D-glucosamine (Fig. 10) [67]. Chitosan is a cationic polymer and has been widely used in the areas of food, cosmetics, biomedical and pharmaceutical applications, [68], due to its biocompatibility and other favorable properties. Multiparticulate systems of alginate and chitosan containing triamcinolone were prepared by a complex coacervation/ionotropic gelation method for colonic drug delivery. A higher swelling degree and faster drug release were observed from the particulate systems in a simulated enteric environment (pH 7.5), as compared to a simulated gastric environment (pH 1.2) [69]. Magnetic alginate-chitosan beads loaded with albendazole (ABZ) were also prepared for passive targeting to the gastrointestinal tract using physical capture mechanisms (e.g., magnetic field, pH). The beads showed unique pH-dependent swelling behaviors and a continuous release of ABZ [70]. Chitosan-treated alginate microparticles containing all-trans retinoic acid (ATRA) have also been shown to enhance dermal localization and achieved the sustained release of ATRA into the skin [71]. Metronidazole was also entrapped into chitosan-treated alginate beads by an ionotropic gelation method, and the beads were effective in eradication of Helicobacter pylori when orally administrated into mice [72]. Alginate gels have also been utilized to form a matrix in which depots releasing small drugs can be incorporated; Amoxicillin-loaded chitosan/poly(γ-glutamic acid) nanoparticles have been incorporated into alginate/Ca2+ hydrogels for effective treatment of H. pylori infection. The alginate gel outer layer protected the amoxicillin-loaded nanoparticles in the gastric environment, and facilitated amoxicillin interactions specifically with intercellular spaces, which is the infection site of H. pylori [73].
Fig. 10.
Chemical structure of chitosan.
4.1.2. Protein delivery
The protein drug market is rapidly growing, and various protein drugs are now available owing to the development of recombinant DNA technology. Alginate is an excellent candidate for delivery of protein drugs, since proteins can be incorporated into alginate-based formulations under relatively mild conditions that minimize their denaturation, and the gels can protect them from degradation until their release. A variety of strategies have been investigated to control the rate of protein release from alginate gels.
In general, the release rate of proteins from alginate gels is rapid, due to the inherent porosity and hydrophilic nature of the gels. However, heparin binding growth factors such as vascular endothelial growth factor (VEGF) or basic fibroblast growth factor (bFGF) exhibit similar, reversible binding to alginate hydrogels, enabling a sustained and localized release [74,75]. The release in this scenario can be readily manipulated by altering the degradation rate of the gels (e.g., use of partially oxidized alginate), in order to make protein release at least partially dependent on the degradation reaction [75]. Many attempts have been made to further control the release of angiogenic molecules from alginate gels, particularly for factors that are not heparin binding.
Ionically cross-linked alginate microspheres efficiently encapsulated high pI proteins such as lysozyme and chymotrypsin; these proteins appear to physically cross-link the sodium alginate, allowing for more sustained release [76]. Amino group-terminated poly((2-dimethylamino) ethyl methacrylate) has also been reacted with oxidized alginate without using a catalyst, and gel beads were prepared by dropping the aqueous solution of the alginate derivative into an aqueous CaCl2 solution to form particles for oral delivery of proteins [77]. Alginate was also used as a building block in the synthesis of a tetra-functional acetal-linked polymer network for stimuli-responsive gels with adjustable pore sizes. The gels protected acid-labile proteins such as insulin from denaturation in the gastric environment (pH 1.2), while releasing the loaded protein at near zero-order kinetics in neutral pH [78].
The low encapsulation efficiency and fast release from alginate gels exhibited by many proteins can also be addressed with various cross-linking or encapsulation techniques, and/or by enhancing protein-hydrogel interactions [15]. For example, insulin-loaded alginate microspheres were prepared by blending alginate with anionic polymers (e.g., cellulose acetate phthalate, polyphosphate, dextran sulfate), followed by chitosan-coating in order to protect insulin at gastric pH and obtain its sustained release at intestinal pH [79]. Alginate microspheres have also been coated with Bombyx mori silk fibroin using layer-by-layer deposition techniques, which provided mechanically stable shells as well as a diffusion barrier to the encapsulated proteins [80]. A combination of microspheres that serve as a depot for proteins and alginate hydrogels also enables sustained protein release. Hydrogels loaded with microspheres were prepared by encapsulation of a suspension of poly(d,l-lactide-co-glycolide) (PLGA) microspheres in alginate prior to ionic cross-linking. A homogeneous dispersion of PLGA microspheres was observed by SEM (Fig. 11), and the release of bovine serum albumin (BSA), a model protein, from this combination delivery system was primarily controlled by the mixing ratio between PLGA microspheres and alginate hydrogels, independent of total BSA content and the size of PLGA microspheres used [81]. The release behavior of TAT-HSP27 (heat shock protein 27 fused with transcriptional activator) was also regulated by varying the mixing ratio between microspheres and gels (Fig. 12) [82]. Alginate gels releasing proteins are being widely explored in tissue engineering and regeneration, as described in the following sections on blood vessel, bone, and muscle regeneration.
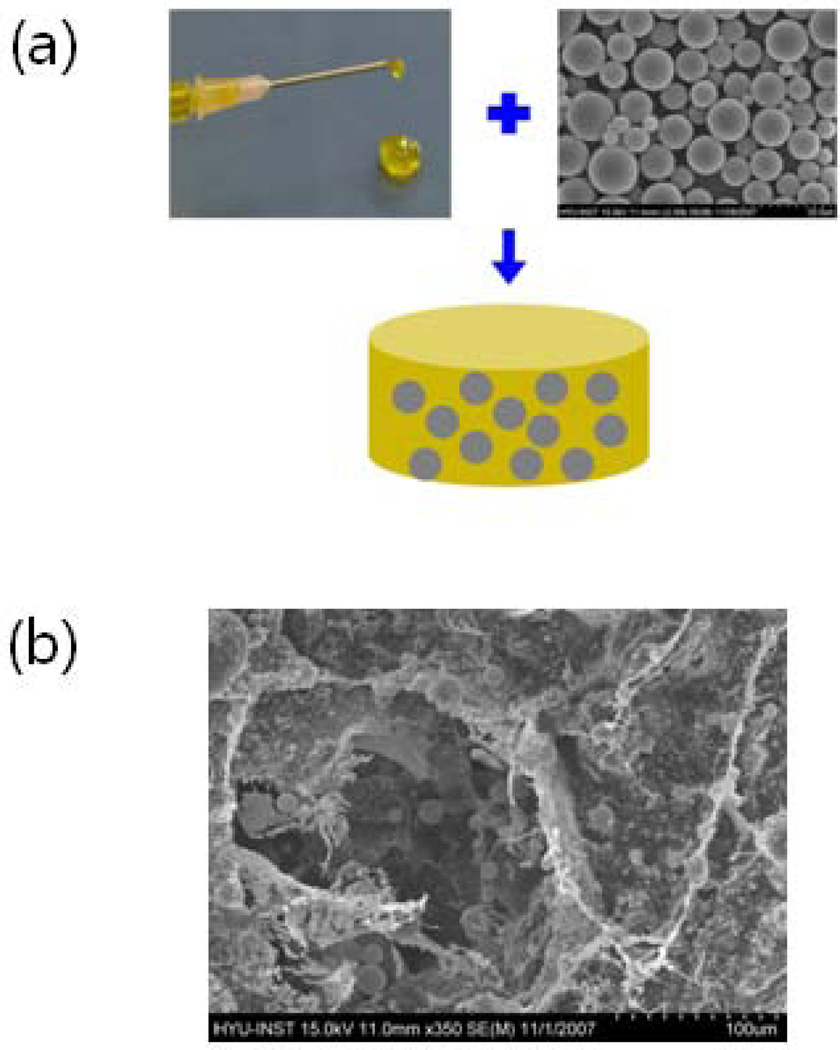
Fig. 11.
(a) A microsphere/hydrogel combination system can be prepared by ionic cross-linking of a suspension of poly(d,l-lactide-co-glycolide) (PLGA) microspheres containing protein drugs in an alginate solution. (b) Scanning electron microscopic image clearly shows even dispersion of PLGA microspheres in an alginate hydrogel [81]. Copyright 2009, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany.
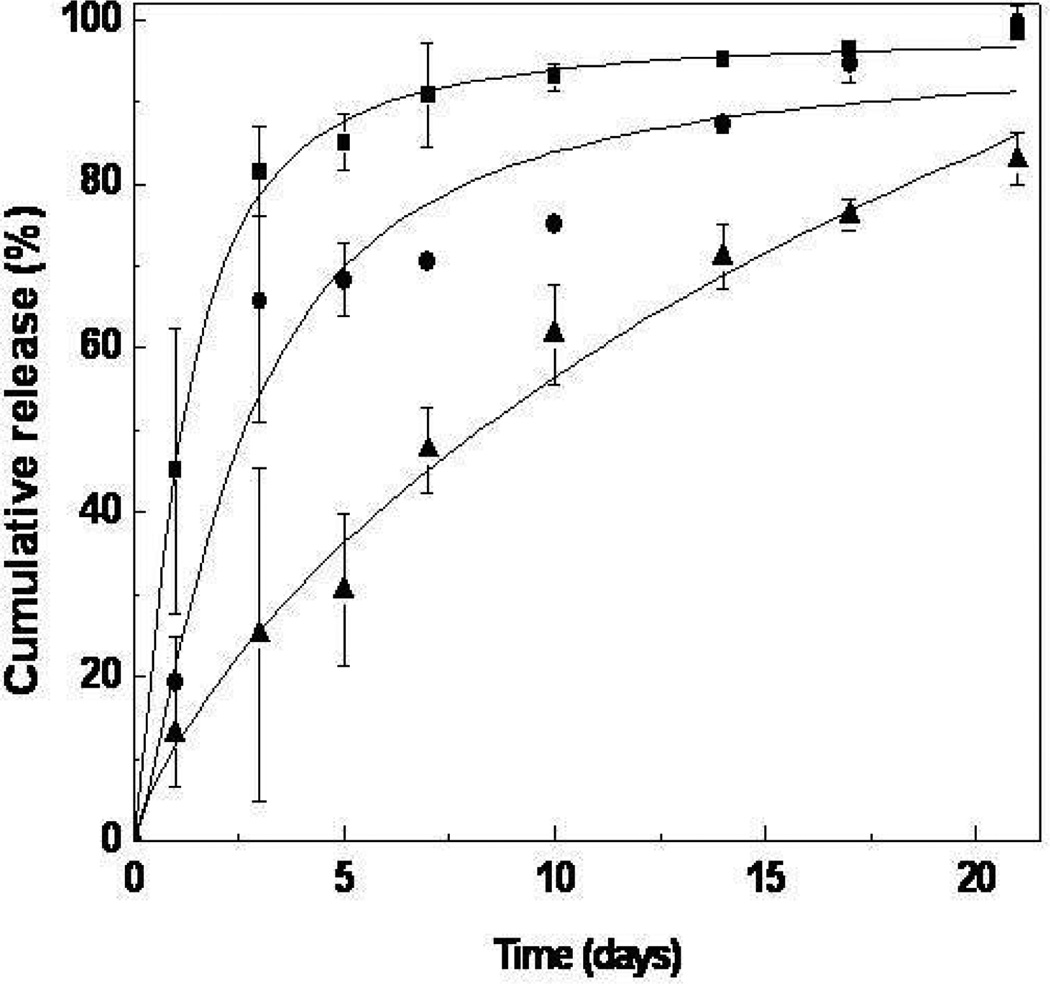
Fig. 12.
In vitro TAT-HSP27 release from microsphere/hydrogel combination delivery systems prepared at different mixing ratios (■, 0; ●, 1.0; ▲, 1.5; PLGA/alginate = w/w) [82]. Copyright 2009, Elsevier Science Ltd., Oxford, UK.
4.2. Wound dressings
The treatment of acute and chronic wounds is a pressing need in many facets of medicine, and alginate-based wound dressings offer many advantageous features. Traditional wound dressings (e.g., gauze) have provided mainly a barrier function – keeping the wound dry by allowing evaporation of wound exudates while preventing entry of pathogen into the wound [83]. In contrast, modern dressings (e.g., alginate dressings) provide a moist wound environment and facilitate wound healing [84]. Alginate dressings are typically produced by ionic cross-linking of an alginate solution with calcium ions to form a gel, followed by processing to form freeze-dried porous sheets (i.e., foam), and fibrous non-woven dressings. Alginate dressings in the dry form absorb wound fluid to re-gel, and the gels then can supply water to a dry wound, maintaining a physiologically moist microenvironment and minimizing bacterial infection at the wound site. These functions can also promote granulation tissue formation, rapid epithelialization, and healing. Various alginate dressings including Algicell™ (Derma Sciences) AlgiSite M™ (Smith & Nephew), Comfeel Plus™ (Coloplast), Kaltostat™ (ConvaTec), Sorbsan™ (UDL Laboratories), and Tegagen™ (3M Healthcare) are commercially available.
A variety of more functional and bioactive alginate-based wound dressings have also been studied to date. The sustained release of dibutyryl cyclic adenosine monophosphate, a regulator of human keratinocyte proliferation, from partially oxidized alginate gels accelerated wound healing, leading to complete re-epithelialization of full-thickness wounds within 10 days in a rat model [85]. Alginate gels releasing stromal cell-derived factor-1 were also effective in accelerating wound closure rates and reducing scar formation in pigs with acute surgical wounds [86]. Incorporation of silver into alginate dressings increased antimicrobial activity and improved the binding affinity for elastase, matrix metalloproteases-2 (MMP-2), and proinflammatory cytokines (e.g., TNF-α, IL-8). The addition of silver into alginate dressings also enhanced the antioxidant capacity [87]. Alginate fibers cross-linked with zinc ions have also been proposed for wound dressings, as zinc ions may generate immunomodulatory and antimicrobial effects, as well as enhanced keratinocyte migration and increased levels of endogenous growth factors [88]. Blends of alginate, chitin/chitosan, and fucoidan gels have been reported to provide a moist healing environment in rats, with an ease of application and removal [89].
4.3. Cell culture
Alginate gels are increasingly being utilized as a model system for mammalian cell culture in biomedical studies. These gels can be readily adapted to serve as either 2-D or more physiologically relevant 3-D culture systems. The lack of mammalian cell receptors for alginate, combined with the low protein adsorption to alginate gels allows these materials to serve in many ways as an ideal blank slate, upon which highly specific and quantitative modes for cell adhesion can be incorporated (e.g., coupling of synthetic peptides specific for cellular adhesion receptors). Further, basic findings uncovered with in vitro studies can be readily translated in vivo, due to the biocompatibility and easy introduction of alginate into the body.
RGD-modified alginate gels have been most frequently used as in vitro cell culture substrates to date. The presence of RGD peptides in alginate gels allows one to control the phenotype of interacting myoblasts [90], chondrocytes [91, 92], osteoblasts [93], ovarian follicle [94], as well as bone marrow stromal cells [95–97]. For example, the adhesion and proliferation of myoblasts cultured on alginate gels were dramatically enhanced by chemical conjugation of RGD peptides to the alginate backbone, compared with non-modified alginate gels (Fig. 13) [98]. Further, the number of cells adherent to the gels, as well as the growth rate, were strongly dependent on the bulk RGD density in the gels. The length of the spacer arm between the RGD peptide and the alginate chain is a key parameter in regulation of cellular responses. The adhesion and growth of primary human fibroblasts cultured on alginate gels modified with a peptide with the sequence of (glycine)n-arginine-glycine-aspartic acid-serine-proline (GnRGDSP) was dramatically influenced by the spacer arm length, irrespective of the same total concentration of the peptides in the gels (Fig. 14). At least four glycine units as a spacer arm allowed proper binding to the cellular receptors, but using more than 12 glycine units led to no further improvement in cell adhesion and growth [99]. The number of RGD peptides per alginate chain, and the spacing between clusters of RGD peptides, even independently of the overall density of RGD ligands, dramatically impact the response of cells to RGD-modified alginate gels [100, 101], likely due to the ability of these variables to affect the clustering of integrin receptors [102]. While the presence of the RGD ligands typically enhances cell adhesion and differentiation, chondrogenic gene expression and matrix accumulation of BMSCs encapsulated in RGD-alginate gels (3-D) was inhibited with an increase of the RGD density in vitro [103]. Interestingly, alginate gels have recently been formed in a microfluidic device through light-triggered release of caged calcium using DM-nitrophen™ compounds, and used as a 3-D cell culture substrate. Preosteoblasts (MC3T3-E1) and human umbilical vein endothelial cells were co-cultured in the microfluidic device using photo-patterning of alginate hydrogels, and this system may provide a useful means for integrating 3-D culture microenvironments into microfluidic systems [104].
Fig. 13.
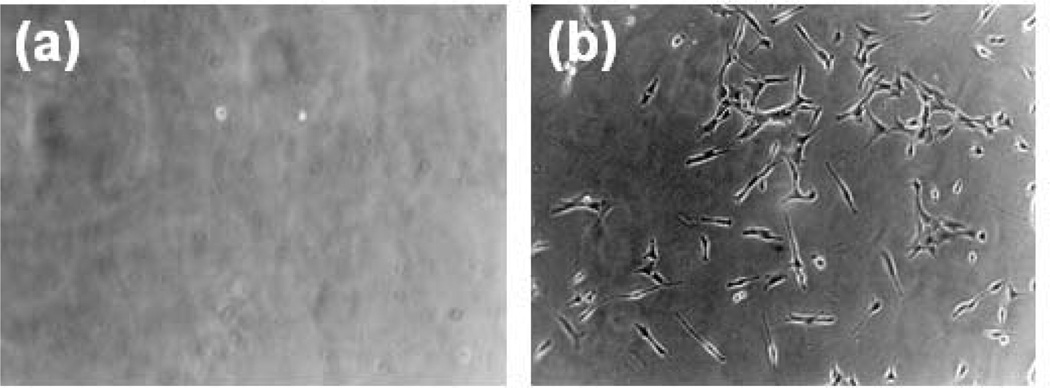
Optical microscope images of C2C12 myoblasts adhered to the surface of (a) non-peptide-modified alginate gels and (b) RGD-modified alginate gels. No cells attach and spread on the unmodified gels, while large numbers of well spread cells are found on the RGD-modified alginate. Images were taken after 24 hr culture at 100× magnification [98]. Copyright 1999, Elsevier Science Ltd., Oxford, UK.
Fig. 14.
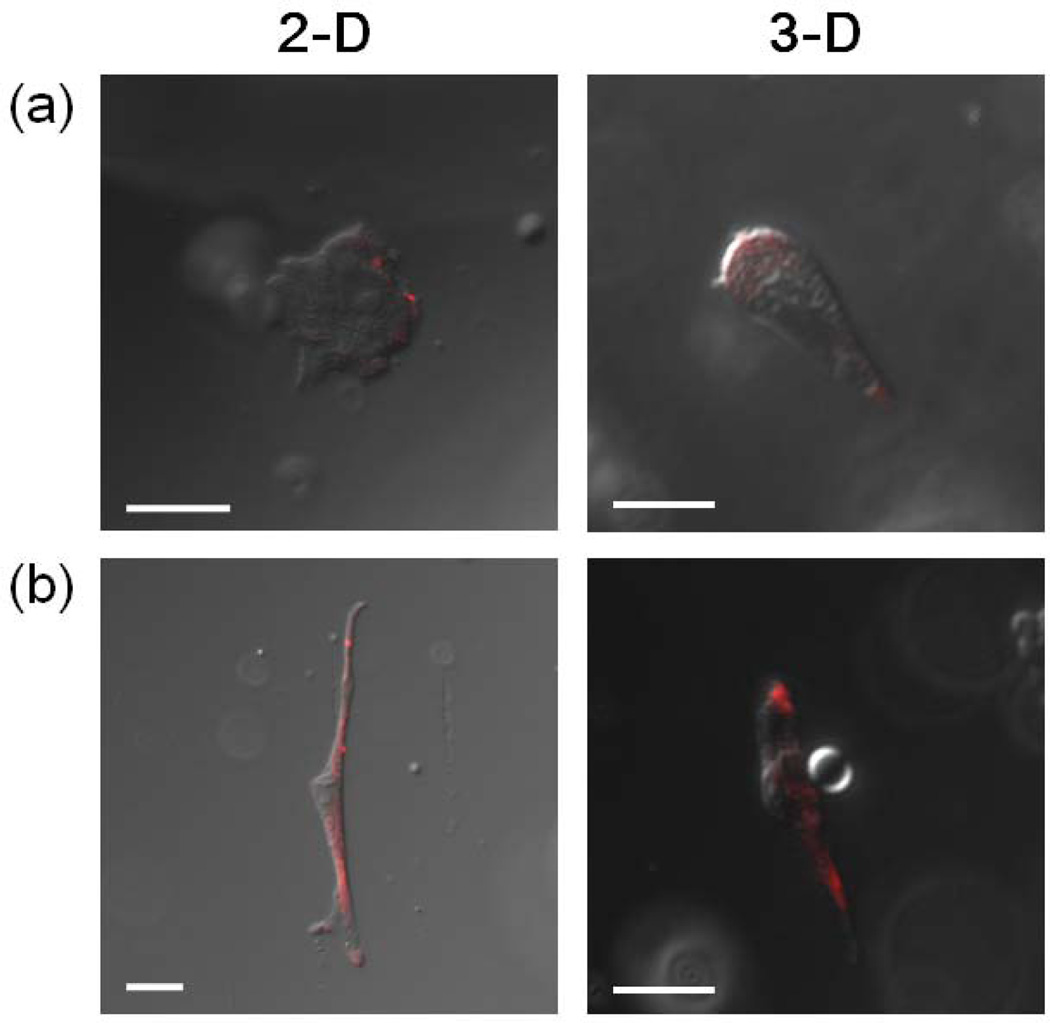
Confocal microscopic images of primary human fibroblasts cultured on alginate gels (2-D) modified with either (a) RGDSP or (b) G12RGDSP, and cells encapsulated within the same two types of gels (3-D). Images were taken after cells were treated with anti-vinculin antibody, followed by visualization with rhodamine-conjugated donkey anti-mouse IgG (scale bar, 20 µm) [99]. Cells cultured with G12RGDSP-alginate gels, both in 2-D and 3-D, clearly display focal contact formation, which is a sign of strong cell adhesion and regulates cell spreading and migration, as demonstrated by positive immunostaining for vinculin. Copyright 2010, Elsevier Science Ltd., Oxford, UK.
The affinity of the cell adhesion peptides for cellular receptors is also important to the cell response, as demonstrated with studies using high affinity cyclic RGD-containing peptides. Alginate gels presenting cyclic RGD peptides (glycine4-cystine-arginine-glycine-aspartate-serine-proline-cystine; G4CRGDSPC) enhanced osteogenic differentiation of stem cells (primary human bone marrow stromal cells and mouse bone marrow stromal D1 cell line) better than gels modified with linear RGD peptides [105]. Cyclic RGD peptides are more resistant to proteolysis [106], and have higher binding affinity and selectivity than linear RGD peptides [107, 108]. Synthesis of alginate derivatives presenting appropriate cyclic RGD peptides capable of promoting stem cell differentiation may enhance tissue regeneration by reducing the need for exogenous soluble factors.
Recent studies utilizing alginate gels as 3-D cell culture substrates have revealed key insights regarding stem cell and cancer biology. The fate of mesenchymal stem cells was demonstrated to be controlled by the elastic modulus of the RGD-alginate gels in which they were encapsulated, as differentiation down fat and bone pathways was promoted at different values of gel stiffness. Strikingly, and in contrast to 2-D culture systems used in previous mechanotransduction studies, the control over stem cell fate was related to the number of adhesive bonds formed between the gel and the cells, as well as alterations in the receptors cells utilized to adhere to the RGD peptides in 3-D versus 2-D culture. The cells actively reorganized on the nanoscale the adhesion ligands presented from the gels [109]. Alginate gels have also been used to examine how a 3-D culture microenvironment influences cancer cell signaling and tumor vascularization. Integrin engagement within a 3-D tumor microenvironment (i.e., encapsulation in RGD-alginate gels) dramatically altered how cancer cells signal to recruit blood vessels, and this finding may lead to the development of new anti-angiogenic cancer therapies [110].
A crucial limitation of most 3-D cell culture systems is the difficulty in analyzing and quantifying cell-matrix interactions, particularly in a non-invasive, real time manner. However the development of several FRET techniques has recently enabled a previously unprecedented ability to quantitatively probe the relation between cell adhesion and decision-making. In one FRET technique, cell membranes are pre-stained with florescent molecules (i.e., acceptor), and a different fluorophore (i.e., donor) can be coupled to the cell adhesion peptides conjugated to the polymer chains (Fig. 3) [33]. This FRET technique allows one to quantify cell receptor-ligand binding, and a similar FRET technique provides information on cell-mediated rearrangements, at the nanometer size scale, of the adhesion ligands attached to gels [111, 112]. The relationship between cell behavior and the number of receptor–ligand bonds was investigated by encapsulating either preosteoblasts (MC3T3-E1) or myoblasts (C2C12) in alginate gels presenting RGD peptides using a FRET technique [113]. The adhesive interactions can be directly visualized, as the green emission of fluorescein in the cell membrane was greatly decreased and the red emission of rhodamine at the interface between cells and gels was increased when the cells were encapsulated in rhodamine-G4RGDASSKY-alginate gels, due to FRET (Fig. 15). The proliferation and differentiation of both cell types were significantly dependent on the number of receptor–ligand bonds calculated using the FRET signal. This type of analysis may allow one to predict cell behavior, particularly in 3-D culture, and to design proper 3-D cell culture substrates for many applications.
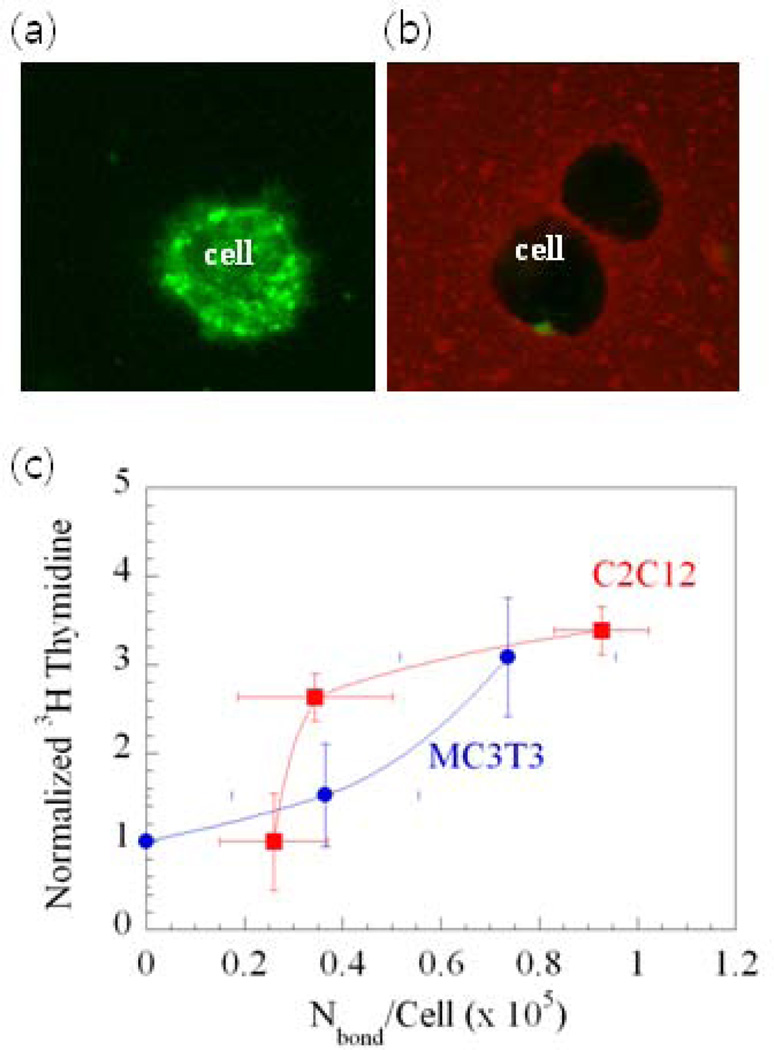
Fig. 15.
Directly visualizing and quantifying cell-gel adhesions, and their relation to cell phenotype. (a) A strong green emission of fluorescein in the cell membrane within unmodified gels was observed. (b) The color intensity of fluorescein in the cell membrane was greatly decreased and the color intensity of rhodamine at the interface between cells and gels was increased when cells were encapsulated in alginate gels presenting rhodamine-G4RGDASSKY, due to fluorescence resonance energy transfer (FRET). (c) The relationship between the amount of [3H]thymidine uptake by cells (indication of cell multiplication) and Nbond/cell for two cell types: muscle cells (C2C12) and bone cells (MC3T3-E1). The number of cell receptor–ligand bonds (Nbond) was determined using the FRET measurements [113]. Copyright 2006, National Academy of Sciences, Washington DC, USA.
4.4. Tissue Regeneration with protein and cell delivery
Alginate gels have been widely explored over the past several decades as a vehicle to deliver proteins or cell populations that can direct the regeneration or engineering of various tissues and organs in the body. The various applications of alginate gels have exploited the wide range of gelling approaches, physical properties, cell adhesion, and degradation behavior of this family of materials. There are limits to the size of a regenerative agent that can be released from alginate hydrogels with diffusion, due to the pore size of ~5 nm. Most proteins readily diffuse out from alginate gels, even in the absence of gel degradation, although degradation can speed release [75]. Molecules too large to have significant diffusion-based release can still be delivered if the gel degrades. For example, condensed plasmid DNA (size ~ 100 nm) [177] can be released from degrading alginate gels [171], and antibody might be released from alginate gels by the same mechanism. Cells must migrate out of alginate hydrogels, and/or be released as the gel degrades. There have been a number of qualitative studies of cell migration in various nanoporous alginate gels, in which migration occurred but was not quantitatively analyzed [178]. The number of cells migrating outward as a function of the porosity and RGD presentation in macroporous alginate gels has been quantified [124,150], as has the migration speed of cells both within a macroporous RGD-alginate gel and in the surrounding ECM gel [179].
4.4.1. Blood vessels
Networks of blood vessels are critical for transport of oxygen and nutrients to all tissues, removal of metabolic waste products, and trafficking of stem and progenitor cells, which are critical for organ growth in the embryo and wound repair in the adult [114]. New blood vessel formation is crucial for tissue engineering, as cells more than a few hundred microns from blood vessels will suffer from hypoxia and limited supply of nutrients. In addition, new blood vessel formation (neovascularization) is also a promising alternative to treat patients suffering from restricted or obstructed blood flow caused by coronary and peripheral arterial diseases. Neovascularization can be achieved by transplantation of various cell types into the body, delivery of angiogenic molecules such as recombinant proteins or genes, or a combination of both. Spatiotemporal control over the delivery of therapeutic molecules has been especially attractive for neovascularization [115], and alginate gels have been widely exploited as a delivery vehicle of various angiogenic molecules.
The most widely examined application of alginate gels to promote blood vessel formation has exploited their ability to provide a sustained and localized release of heparin binding growth factors such as vascular endothelial growth factor (VEGF) [73, 116, 117]. Injection of alginate gels directly into ischemic muscle tissue has been demonstrated to lead to long-term (> 14 days) release of VEGF in the ischemic tissue, and formation of VEGF gradients in the surrounding tissue capable of guiding new capillary formation into the ischemic tissue and relieving tissue ischemia [74]. The differential binding of various growth factors to alginate has also been exploited to provide sequential delivery of factors involved in early and late stages of angiogenesis, in order to promote maturation of the new networks of vessels, and increased functionality. Sequential delivery of VEGF followed by platelet-derived growth factor-BB (PDGF-BB) using alginate gels resulted in enhanced blood vessel formation, maturation and function when injected into ischemic hindlimbs of mice [118] and sites of myocardial infarction [119]. In general, VEGF plays an important role in initiating angiogenesis and forming new capillaries, while PDGF promotes the maturation of the resulting capillaries into larger, more functional vessels. Two strategies have been used to achieve sequential release of these factors from alginate gels. In the first, the PDGF was pre-encapsulated into poly(lactide-co-glycolide) PLG microspheres that were then encapsulated into gels with free VEGF [118]. In the second approach, the greater heparin binding of PDGF was exploited to slow down its release, relative to VEGF, by simply encapsulating both factors in free form into the gels [119]. In both situations, VEGF was released faster than PDGF. Similarly, a sequential release of IGF-1 followed by HGF from alginate-sulfate gels preserved scar thickness, attenuated infarct expansion, and reduced scar fibrosis after 4 weeks, as well as enhanced mature blood vessel formation at the infarct site [120]. The release of VEGF can also be slowed by first encapsulating it into PLG microspheres, and including these microspheres in alginate gels. This approach to VEGF delivery enhanced the number of newly formed blood vessels in ischemic hindlimbs of mice (Fig. 16) [121].
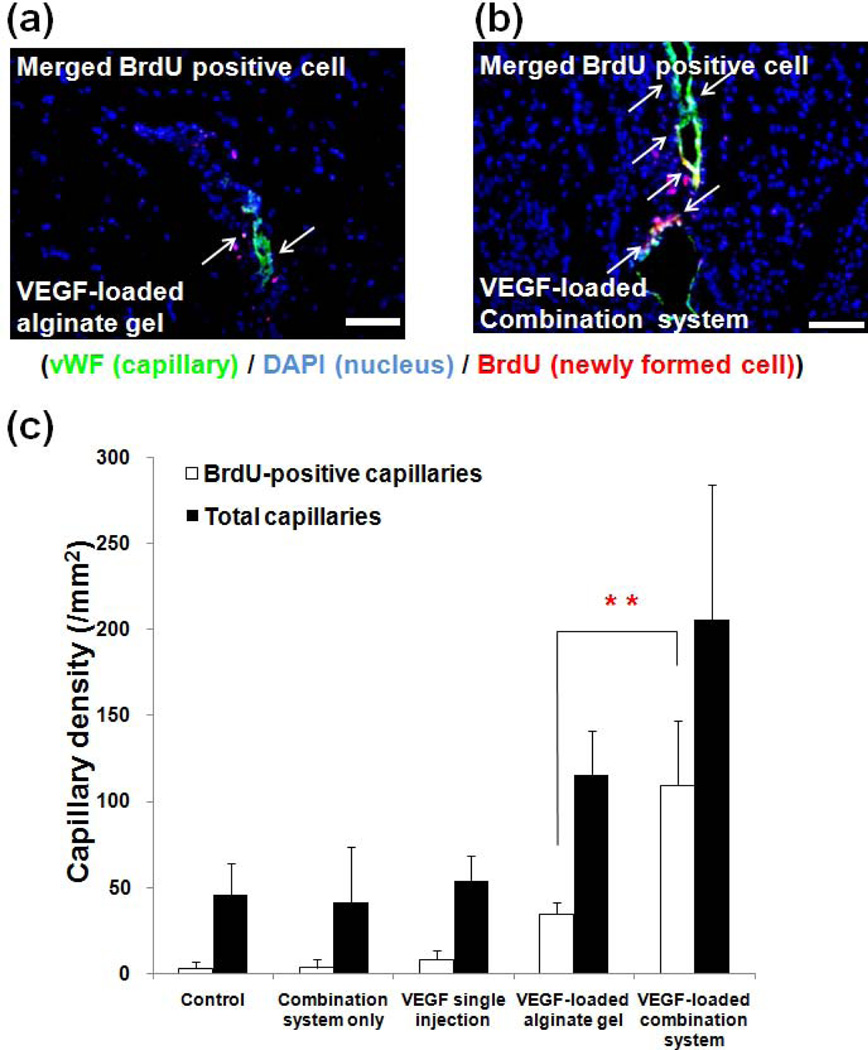
Fig. 16.
Tissues from mouse ischemic hindlimbs stained with anti-von Willebrand factor (vWF) (indicator of endothelial cells lining blood vessels) 4 weeks post-treatment with either (a) VEGF-loaded alginate hydrogel or (b) VEGF-loaded microsphere/hydrogel combination system. Blue, green and red colors represent DAPI (cell nuclei), vWF, and BrdU (indicating cells actively multiplying), respectively. Arrows indicate newly formed capillaries (scale bar, 20µm). (c) Quantification of the capillary density in tissue [121]. Copyright 2010, Springer, New York, USA.
The transplantation of cells is an attractive approach to promote new blood vessel formation, particularly when host cells capable of responding to delivered growth factors are lacking or dysfunctional. However, transplantation of endothelial cells or endothelial progenitor cells has not been effective in clinical trials, likely due to massive death of transplanted cells, insufficient integration of the transplanted cells with the host vascular network, and poor recruitment of host smooth muscle cells to promote mature blood vessel formation. Delivery of VEGF from alginate-PLG vehicles in concert with endothelial cell transplantation has been demonstrated to significantly increase the number of blood vessels formed by transplanted endothelial cells [122]. Further, transplantation of endothelial cells in combination with dual delivery of VEGF and monocyte chemotactic protein-1 (MCP-1) using alginate microparticles enhanced functional vessel formation and increased the number of smooth muscle cell-invested, mature vessels in mice [123]. Recently, alginate delivery vehicles capable of actively promoting the outward migration of transplanted endothelial progenitor cells and their dispersion throughout ischemic tissues have been developed [124]. Endothelial progenitor cells were transplanted on vehicles formed from RGD-alginate to allow for cell adhesion and migration, and the gels also released VEGF to promote cell movement. Mice that would otherwise suffer from complete autoamputation of an ischemic hindlimb demonstrated normal limb structure and function with this new delivery approach [124].
4.4.2. Bone
Despite recent progress, treatment of bone injuries is still often limited due to poor healing, and alginate gels have found potential in bone regeneration by delivery of osteoinductive factors, bone-forming cells, or combination of both. Alginate gels have advantages for bone and cartilage regeneration, as compared to other materials, due to their ability to be introduced into the body in a minimally invasive manner, their ability to fill irregularly shaped defects, and the ease of chemical modification with adhesion ligands (e.g., RGD) and controlled release of tissue induction factors (e.g., BMP, TGF-β). However, alginate gels do not have sufficient mechanical properties to allow load bearing in the initial stages of regeneration without fixation. They are also not inherently degradable in physiological conditions, as reviewed earlier, highlighting the need to control their degradation in order that residual gels do not interfere with regeneration. Alginate gels have proven useful in animal models for the delivery of growth factors that can effectively drive bone regeneration (e.g., bone morphogenetic proteins). The use of RGD-alginate gels allows complete regeneration of critical-sized femoral defects in rodents with a low dose of BMP [125]. Alginate gels that deliver DNA encoding bone morphogenetic proteins (BMPs) have also demonstrated significant bone tissue can be regenerated [126,127]. The delivery of multiple factors, either in combination or sequence, is also being explored, in a similar manner as described for angiogenesis. Sequential delivery of BMP-2 and BMP-7 using alginate gels enhanced osteogenic differentiation of bone marrow derived stem cells in vitro [128], and co-delivery of BMP-2 and VEGF releasing from alginate gels enhanced the repair and regeneration of critical sized bone defects [129].
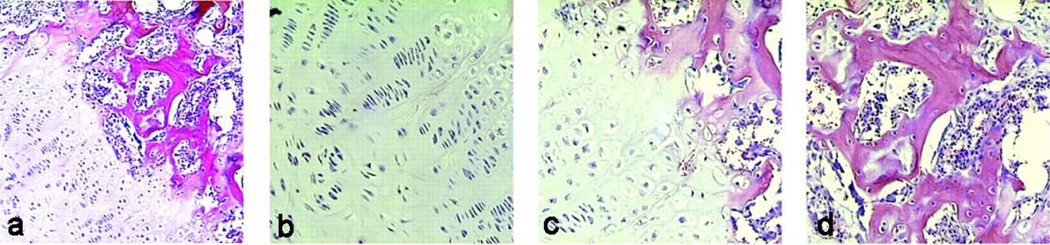
When host cells capable of responding to morphogens are lacking, or one desires to accelerate tissue formation, alginate-based devices may also be used to transplant cell populations that directly participate in bone formation. Transplantation of primary rat calvarial osteoblasts into mice using RGD-alginate gels enhanced in vivo bone formation [34], as compared to control alginate gels. In addition, co-transplantation of primary chondrocytes and osteoblasts into mice using RGD-alginate gels enabled the formation of growth-plate-like structures, which may be potentially used to replace dysfunctional epiphyses (Fig. 17) [130]. Degradable and injectable alginate-derived gels, composed of PAG and AAD, have also been mixed with rat primary calvarial osteoblasts, and subcutaneously injected into the backs of mice. Mineralized bone tissues were observed after 9 weeks [131].
Fig. 17.
(a) Growth-plate-like structures formed by co-transplantation of chondrocytes and osteoblasts, similar to that observed in developing long bones (×100). Magnified images of the (b) cartilage, (c) transition, and (d) bone and marrow space regions (200× magnification) [130]. Copyright 2002, National Academy of Sciences, Washington DC, USA.
The transplantation of stem cells using alginate hydrogels has been widely explored in bone tissue engineering. The thickness of calcium cross-linked alginate gels was demonstrated to alter the behavior of rat bone marrow cells; however, different geometries did not influence cell differentiation [132]. Bone marrow stromal cells, after being induced down the osteoblast pathway in vitro and mixed with calcium cross-linked alginate gels, repaired horizontal alveolar bone defects in dogs [133]. Alginate/chitosan gels entrapping mesenchymal stem cells and bone morphogenetic protein-2 also showed potential for trabecular bone formation in mice [134].
Alginate has also been combined with inorganic materials to enhance bone tissue formation. Alginate/hydroxyapatite (HAP) composite scaffolds with interconnected porous structures were prepared by a phase separation method, which enhanced the adhesion of osteosarcoma cells [135]. Cell-encapsulating alginate gel beads were introduced into calcium phosphate cement, and demonstrated potential for bone tissue engineering under moderate stress-bearing conditions [136]. In addition, alginate gels containing collagen type I and β-tricalcium phosphate enhanced adhesion and proliferation of human bone marrow stromal cells that do not readily attach or proliferate on pure alginate gels [137].
4.4.3. Cartilage
Repair of damaged or degraded cartilage is still one of the major challenges facing the orthopedics field, but tissue engineering approaches have recently shown potential in cartilage regeneration. Alginate gels have proved to be useful for transplanting chondrogenic cells to restore damaged cartilage in animal models. Early studies utilized a suspension of chondrocytes in an alginate solution mixed with calcium sulfate, and injected into molds of facial implants in order to produce pre-shaped cartilage. These constructs formed cartilage with three-dimensional shape retention after 30 weeks of subcutaneous implantation into mice and sheep, and the contents of proteoglycan and collagen as well as the elastic modulus of the engineered cartilage reached about 80% of those found in native cartilage [138, 139]. Shape-memory alginate gels were subsequently developed to engineer cartilage with desired shape and size in vivo following minimally-invasive delivery. In brief, macroporous alginate gels with predefined geometries were compressed into a significantly smaller form (dry state) and introduced into mice through a small catheter. The gels were then rehydrated in situ with a suspension of primary bovine articular chondrocytes, and recovered their original shape and size within 1 hr, which allowed cartilage formation in mice with the desired geometry [140].
The use of stem cells in cartilage regeneration is very attractive, due to the invasive and destructive processes required to obtain primary chondrocytes from tissues. Encapsulation in alginate can regulate differentiation of stem cells, and in particular chondrogenesis may be enhanced. It has been demonstrated that chondrogenic lineage of adult stem cells could be regulated via the introduction of soluble factors and biophysical cues in 3D cell culture systems [180]. In addition, it has been hypothesized that chondrogenesis of stem cells relates to the morphology of the encapsulated cells (i.e., rounded cell shape) [181], and alginate gels promote a rounded morphogology that may promote the cellular differentiation process [182].
Human mesenchymal stem cells (MSCs) encapsulated in alginate gel beads have been cultured in serum-free medium with the addition of transforming growth factor (TGF)-β1, dexamethasone, and ascorbate 2-phosphate for more than one week, and demonstrated to form cartilage in large osteochondral defects [141]. Rabbit bone marrow stromal cells (BMSCs) cultured in alginate gels have also been injected into osteochondral defects in rabbit knees without the use of a periosteal patch, which significantly enhanced the cellular proliferation and chondrogenic differentiation of BMSCs. This resulted in histologically and mechanically improved tissues in osteochondral defects [142]. The chondrogenic potential of human adipose derived stem cells (hASCs) suggests these cells as a possible cell source for cartilage regeneration, and chondrogenic differentiation of hASCs seeded in alginate gels was greatly improved in the presence of TGF-β1 [143]. Pre-differentiated hASCs induced by transduction with an adenovirus carrying a TGF-β2 plasmid maintained the chondrogenic phenotype in vivo and led to new cartilage formation when the cells were encapsulated into alginate gel beads and subcutaneously transplanted into mice [144].
Coupling of RGD-containing peptides to alginate can greatly enhance adhesive interactions with chondrocytes, allowing control of cell phenotype. Shear-reversibly cross-linked gels, which can recover their gel structure after shear-induced breakdown, were formed using an RGD-alginate solution and primary rabbit chondrocytes. These gels were injectable into mice in a minimally invasive manner, and were effective in engineering cartilage in vivo (Fig. 18) [145].
Fig. 18.
Images of H&E-stained tissues after 6 weeks following transplantation of primary chondrocytes into mice using either (a) cell cross-linked RGD-alginate gels or (b) media only [145]. Chondrocytes in lacunae (typically found in natural cartilage) were clearly observed for cell cross-linked RGD-alginate gels. Copyright 2009, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany.
4.4.4. Muscle, nerve, pancreas, and liver
Alginate gels are also being actively investigated for their ability to mediate the regeneration and engineering of a variety of other tissues and organs, including skeletal muscle, nerve, pancreas, and liver. Current strategies for skeletal muscle regeneration include cell transplantation, growth factor delivery, or a combination of both approaches [146, 147], and alginate gels have found potential in these strategies. A combined delivery of VEGF and insulin-like growth factor-1 (IGF-1) from alginate gels was used to modulate both angiogenesis and myogenesis. The localized and sustained delivery of both growth factors led to significant muscle regeneration and functional muscle formation, due to satellite cell activation and proliferation, and cellular protection from apoptosis by the released factors [148]. Long-term survival and outward migration of primary myoblasts into damaged muscle tissue in vivo from RGD-alginate gels were dramatically enhanced by the sustained delivery of hepatocyte growth factor (HGF) and fibroblast growth factor 2 (FGF 2) from the gels [149]. This led to extensive repopulation of host muscle tissues and increased the regeneration of muscle fibers at the wound site [150].
Alginate gels have also been investigated for the repair of the central and peripheral nerve systems. Alginate-based highly anisotropic capillary gels, introduced into acute cervical spinal cord lesions in adult rats, were integrated into the spinal cord parenchyma without major inflammatory responses and directed axonal regrowth [151]. Alginate gels, covalently cross-linked with ethylenediamine, were useful to restore a 50-mm gap in cat sciatic nerves [152], and promoted the outgrowth of regenerating axons and astrocyte reactions at the stump of transected spinal cords in young rats [153]. Alginate gels were also used as glue for repair of peripheral nerve gaps that could not be sutured [154]. Alginate gels may be useful for cell-based neural therapies, as mouse-derived neural stem cells cultured in calcium alginate beads maintained their capacity for multilineage differentiation into neurons and glial cells [155]. Alginate gels modified with a peptide containing the YIGSR (Tyr-Ile-Gly-Ser-Arg) sequence promoted adhesion of NB2a neuroblastoma cells and neurite outgrowth from the cells, depending on the peptide density in the gels [35].
Tissue engineering is a potential approach to provide hepatic tissues for replacement of a failing liver, and alginate gels encapsulating hepatocytes may offer a suitable platform for developing a bioartificial liver as they are easily manipulated and can be cryopreserved [156, 157]. The hydrophilic nature of alginate gels processed to exhibit an interconnected porous structure allows efficient seeding of hepatocytes into the gels, while maintaining high hepatocellular functions [158, 159]. Primary rat hepatocytes maintained viability in alginate gels and appeared to synthesize fibronectin, which was deposited on the spheroids and promoted their functional expression [160]. Hepatocyte engraftment was improved when hepatocytes were transplanted into the liver lobe of Lewis rats using VEGF-releasing porous alginate gels [161].
One of the first applications of alginate gels in tissue engineering involved the transplantation of encapsulated pancreatic islet allografts and xenografts in an effort to cure Type I diabetes. In this approach, the gel was used to provide protection from the host immune system, in order to avoid the use of immunosuppressive drugs that would otherwise be required to prevent graft rejection. This approach has been successfully used to treat animal models of Type I diabetes without the use of immunosuppressive drugs [162–164]. These alginate beads encapsulating islets are generally coated with poly(amino acids), such as poly-L-lysine, to decrease the outer pore size, while keeping a liquid core structure [165]. The transplant volume of microencapsulated islet cells can be reduced by choosing an appropriate alginate composition and purity [166]. However, the mechanical and chemical instability of alginate beads is believed to be a limiting factor for long term survival of the transplanted islets in vivo, prompting continued investigations into the use of different poly(amino acids) as a coating material and the use of various microfabrication methods [167].
5. Conclusions and future perspectives
Alginate has demonstrated great utility and potential as a biomaterial for many biomedical applications, particularly in the areas of wound healing, drug delivery, in vitro cell culture, and tissue engineering. The most attractive features of alginate for these applications include biocompatibility, mild gelation conditions, and simple modifications to prepare alginate derivatives with new properties. Alginate has a track record of safe clinical uses as a wound healing dressing material and pharmaceutical component, and has been safely implanted in a variety of applications, including islet transplantation for treatment of type 1 diabetes [168] and chondrocyte transplantation for treatment of urinary incontinence and vesicoureteral reflux [169,170]. A chemically modified alginate has also been widely used as a carrier to promote periodontal regeneration [176]. Like other hydrogels, however, alginate gels have very limited mechanical stiffness, and more generally physical properties. A continuing challenge is matching the physical properties of alginate gels to the need in a particular application. Consideration of the range of different available cross-linking strategies, using molecules with various chemical structures, molecular weights, and cross-linking functionality will often yield gels suitable for each application. Although not a focus here, many of the concepts reviewed in this article are directly relevant to cell encapsulation strategies. However, covalent cross-linking reactions can cause toxicity to the cells to be encapsulated, and an appropriate choice of cell-compatible chemical reagents (e.g., initiator), and thorough removal of unreacted reagents and by-products will likely be needed in those applications [49].
As one looks to the future, the alginate-based materials used in medicine are likely to evolve considerably. While alginate gels are already used clinically in wound healing applications, they play a fairly passive role. Future dressings will likely play a much more active role. One or more bioactive agents that facilitate wound healing can be incorporated into alginate dressings, as these gels have demonstrated utility in maintaining local concentrations of biological factors, such as proteins, for extended time periods. In wound healing, and more generally drug delivery applications, precise control over the delivery of single vs. multiple drugs, or sustained vs. sequential release in response to external environmental changes is highly desirable.
Dynamical control over delivery can potentially improve the safety and effectiveness of drugs, and provide new therapies. On-demand drug release from alginate gels in response to external cues such as mechanical signals [172] and magnetic fields [173] can be used to design active depots of many drugs, including therapeutic cells. The introduction of appropriate cell-interactive features to alginate will also be crucial in many tissue engineering applications. The type of adhesion ligands and their spatial organization in gels are key variables, as they can regulate cell phenotype and the resultant function of regenerated tissues. While RGD peptides have been extensively exploited to date as a cell adhesion ligand, multiple ligands and/or a combination of ligands and soluble factors may be required to properly produce replacement tissues and organs.
Furthering our current understating of fundamental properties of alginate, and developing new types of cell and tissue-interactive alginate gels may enable future advances in biomedical science and engineering. The design and creation of new alginate polymers with better or distinct properties can potentially be achieved using genetic engineering techniques to control bacterial synthesis. Various polypeptides and proteins with improved structural properties and novel functions have already been prepared by this approach and explored for biomedical applications [174, 175]. The ability to engineer new classes of alginates with precisely controlled chemical and physical characteristics, unlike the limited repertoire available from natural sources, designed for a specific application could revolutionize the use of these materials.
Acknowledgements
The authors acknowledge financial support from the Defense Advanced Research Projects Agency (W911NF-10-0113), the NIH (R01 DE019917, R01 DE013349, R01 HL069957, R37 DE013033), the National Research Foundation of Korea (2010-0002086, 2010-0012608), and the LG Yonam Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huebsch N, Mooney DJ. Inspiration and application in the evolution of biomaterials. Nature. 2009;462:426–432. doi: 10.1038/nature08601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ratner BD, Bryant SJ. Biomaterials: where we have been and where we are going? Ann Rev Biomed Eng. 2004;6:41–75. doi: 10.1146/annurev.bioeng.6.040803.140027. [DOI] [PubMed] [Google Scholar]
- 3.Williams DF. On the nature of biomaterials. Biomaterials. 2009;30:5897–5909. doi: 10.1016/j.biomaterials.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 4.Gombotz WR, Wee SF. Protein release from alginate matrices. Adv Drug Delivery Rev. 1998;31:267–285. doi: 10.1016/s0169-409x(97)00124-5. [DOI] [PubMed] [Google Scholar]
- 5.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 6.Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101:1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 7.Smidsrod O, Skjak-Bræk G. Alginate as immobilization matrix for cells. Trend Biotechnol. 1990;8:71–78. doi: 10.1016/0167-7799(90)90139-o. [DOI] [PubMed] [Google Scholar]
- 8.Clark DE, Green HC. Alginic acid and process of making same. 2036922 US Patent. 1936
- 9.Rinaudo M. Main properties and current applications of some polysaccharides as biomaterials. Polym Int. 2008;57:397–430. [Google Scholar]
- 10.Qin Y. Alginate fibres: an overview of the production processes and applications in wound management. Polym Int. 2008;57:171–180. [Google Scholar]
- 11.Remminghorst U, Rehm BHA. Bacterial alginates: from biosynthesis to applications. Biotechnol Lett. 2006;28:1701–1712. doi: 10.1007/s10529-006-9156-x. [DOI] [PubMed] [Google Scholar]
- 12.Fischer FG, Dörfel H. Die polyuronsauren der braunalgen-(kohlenhydrate der algen-I) Z Physiol Chem. 1955;302:186–203. [PubMed] [Google Scholar]
- 13.Haug A. Fractionation of alginic acid. Acta Chem Scand. 1959;13:601–603. [Google Scholar]
- 14.Tonnesen HH, Karlsen J. Alginate in drug delivery systems. Drug Dev Ind Pharm. 2002;28:621–630. doi: 10.1081/ddc-120003853. [DOI] [PubMed] [Google Scholar]
- 15.George M, Abraham TE. Polyionic hydrocolloids for the intestinal delivery of protein drugs. J Controlled Release. 2006;114:1–14. doi: 10.1016/j.jconrel.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 16.Hay ID, Rehman ZU, Ghafoor A, Rehm BHA. Bacterial biosynthesis of alginates. J Chem Technol Biotechnol. 2010;85:752–759. [Google Scholar]
- 17.Rinaudo M. On the abnormal exponents aη and aD in Mark-Houwink type equations for wormlike chain polysaccharides. Polym Bull. 1992;27:585–589. [Google Scholar]
- 18.LeRoux MA, Guilak F, Setton LA. Compressive and shear properties of alginate gel: effects of sodium ions and alginate concentration. J Biomed Mater Res. 1999;47:46–53. doi: 10.1002/(sici)1097-4636(199910)47:1<46::aid-jbm6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 19.Kong HJ, Smith MK, Mooney DJ. Designing alginate hydrogels to maintain viability of immobilized cells. Biomaterials. 2003;24:4023–4029. doi: 10.1016/s0142-9612(03)00295-3. [DOI] [PubMed] [Google Scholar]
- 20.Kong HJ, Lee KY, Mooney DJ. Decoupling the dependence of rheological/mechanical properties of hydrogels from solids concentration. Polymer. 2002;43:6239–6246. [Google Scholar]
- 21.Otterlei M, Ostgaard K, Skjakbraek G, Smidsrod O, Soonshiong P, Espevik T. Induction of cytokine production from human monocytes stimulated with alginate. J Immunother. 1991;10:286–291. doi: 10.1097/00002371-199108000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Zimmermann U, Klock G, Federlin K, Haning K, Kowaslski M, Bretzel RG, Horcher A, Entenmann H, Siebers U, Zekorn T. Production of mitogen contamination free alginates with variable ratios of mannuronic to guluronic acid by free flow electrophoresis. Electrophoresis. 1992;13:269–274. doi: 10.1002/elps.1150130156. [DOI] [PubMed] [Google Scholar]
- 23.Orive G, Ponce S, Hernandez RM, Gascon AR, Igartua M, Pedraz JL. Biocompatibility of microcapsules for cell immobilization elaborated with different type of alginates. Biomaterials. 2002;23:3825–3831. doi: 10.1016/s0142-9612(02)00118-7. [DOI] [PubMed] [Google Scholar]
- 24.Lee J, Lee KY. Local and sustained vascular endothelial growth factor delivery for angiogenesis using an injectable system. Pharm Res. 2009;26:1739–1744. doi: 10.1007/s11095-009-9884-4. [DOI] [PubMed] [Google Scholar]
- 25.Pelletier S, Hubert P, Payan E, Marchal P, Choplin L, Dellacherie E. Amphiphilic derivatives of sodium alginate and hyaluronate for cartilage repair: rheological properties. J Biomed Mater Res. 2001;54:102–108. doi: 10.1002/1097-4636(200101)54:1<102::aid-jbm12>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 26.Leonard M, De Boisseson AR, Hubert P, Dalencon F, Dellacherie E. Hydrophobically modified alginate hydrogels as protein carriers with specific controlled release properties. J Controlled Release. 2004;98:395–405. doi: 10.1016/j.jconrel.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Vallee F, Muller C, Durand A, Schimchowitsch S, Dellacherie E, Kelche C, Cassel JC, Leonard M. Synthesis and rheological properties of hydrogels based on amphiphilic alginate-amide derivatives. Carbohydr Res. 2009;344:223–228. doi: 10.1016/j.carres.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 28.Yang LQ, Zhang BF, Wen LQ, Liang QY, Zhang LM. Amphiphilic cholesteryl grafted sodium alginate derivative: Synthesis and self-assembly in aqueous solution. Carbohydr Polym. 2007;68:218–225. [Google Scholar]
- 29.Colinet I, Dulong V, Hamaide T, Le Cerf D, Picton L. New amphiphilic modified polysaccharides with original solution behaviour in salt media. Carbohydr Polym. 2009;75:454–462. [Google Scholar]
- 30.Yao BL, Ni CH, Xiong C, Zhu CP, Huang B. Hydrophobic modification of sodium alginate and its application in drug controlled release. Bioprocess Biosyst Eng. 2010;33:457–463. doi: 10.1007/s00449-009-0349-2. [DOI] [PubMed] [Google Scholar]
- 31.Lehenkari PP, Horton MA. Single integrin molecule adhesion forces in intact cells measured by atomic force microscopy. Biochem Biophys Res Commun. 1999;259:645–650. doi: 10.1006/bbrc.1999.0827. [DOI] [PubMed] [Google Scholar]
- 32.Koo LY, Irvine DJ, Mayes AM, Lauffenburger DA, Griffith LG. Co-regulation of cell adhesion by nanoscale RGD organization and mechanical stimulus. J Cell Sci. 2002;115:1423–1433. doi: 10.1242/jcs.115.7.1423. [DOI] [PubMed] [Google Scholar]
- 33.Lee KY, Kong HJ, Mooney DJ. Quantifying interactions between cell receptors and adhesion ligand-modified polymers in solution. Macromol Biosci. 2008;8:140–145. doi: 10.1002/mabi.200700169. [DOI] [PubMed] [Google Scholar]
- 34.Alsberg E, Anderson KW, Albeiruti A, Franceschi RT, Mooney DJ. Cell-interactive alginate hydrogels for bone tissue engineering. J Dental Res. 2001;80:2025–2029. doi: 10.1177/00220345010800111501. [DOI] [PubMed] [Google Scholar]
- 35.Dhoot NO, Tobias CA, Fischer I, Wheatley MA. Peptide-modified alginate surfaces as a growth permissive substrate for neurite outgrowth. J Biomed Mater Res Part A. 2004;71:191–200. doi: 10.1002/jbm.a.30103. [DOI] [PubMed] [Google Scholar]
- 36.Sakiyama-Elbert SE, Hubbell JA. Functional biomaterials: design of novel biomaterials. Ann Rev Mater Res. 2001;31:183–201. [Google Scholar]
- 37.Varghese S, Elisseeff JH. Hydrogels for musculoskeletal tissue engineering. Adv Polym Sci. 2006;203:95–144. [Google Scholar]
- 38.Lee KY, Yuk SH. Polymeric protein delivery systems. Progr Polym Sci. 2007;32:669–697. [Google Scholar]
- 39.Grant GT, Morris ER, Rees DA, Smith PJC, Thom D. Biological interactions between polysaccharides and divalent cations - egg-box model. FEBS Lett. 1973;32:195–198. [Google Scholar]
- 40.Crow BB, Nelson KD. Release of bovine serum albumin from a hydrogel-cored biodegradable polymer fiber. Biopolymers. 2006;81:419–427. doi: 10.1002/bip.20442. [DOI] [PubMed] [Google Scholar]
- 41.Kuo CK, Ma PX. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: part 1. structure, gelation rate and mechanical properties. Biomaterials. 2001;22:511–521. doi: 10.1016/s0142-9612(00)00201-5. [DOI] [PubMed] [Google Scholar]
- 42.Augst AD, Kong HJ, Mooney DJ. Alginate hydrogels as biomaterials. Macromol Biosci. 2006;6:623–633. doi: 10.1002/mabi.200600069. [DOI] [PubMed] [Google Scholar]
- 43.Drury JL, Dennis RG, Mooney DJ. The tensile properties of alginate hydrogels. Biomaterials. 2004;25:3187–3199. doi: 10.1016/j.biomaterials.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki Y, Nishimura Y, Tanihara M, Suzuki K, Nakamura T, Shimizu Y, Yamawaki Y, Kakimaru Y. Evaluation of a novel alginate gel dressing. J Biomed Mater Res. 1998;39:317–322. doi: 10.1002/(sici)1097-4636(199802)39:2<317::aid-jbm20>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 45.Zhao XH, Huebsch N, Mooney DJ, Suo ZG. Stress-relaxation behavior in gels with ionic and covalent crosslinks. J Appl Phys. 2010;107 doi: 10.1063/1.3343265. 063509/1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eiselt P, Lee KY, Mooney DJ. Rigidity of two-component hydrogels prepared from alginate and poly(ethylene glycol)-diamines. Macromolecules. 1999;32:5561–5566. [Google Scholar]
- 47.Lee KY, Rowley JA, Eiselt P, Moy EM, Bouhadir KH, Mooney DJ. Controlling mechanical and swelling properties of alginate hydrogels independently by cross-linker type and crosslinking density. Macromolecules. 2000;33:4291–4294. [Google Scholar]
- 48.Lee KY, Bouhadir KH, Mooney DJ. Controlled degradation of hydrogels using multifunctional cross-linking molecules. Biomaterials. 2004;25:2461–2466. doi: 10.1016/j.biomaterials.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 49.Smeds KA, Grinstaff MW. Photocrosslinkable polysaccharides for in situ hydrogel formation. J Biomed Mater Res. 2001;54:115–121. doi: 10.1002/1097-4636(200101)54:1<115::aid-jbm14>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 50.Tanaka H, Sato Y. Photosensitivity of polyvinylesters of substituted cinnamylideneacetic acids. J Polym Sci. 1972;10:3279–3287. [Google Scholar]
- 51.Lu MZ, Lan HL, Wang FF, Chang SJ, Wang YJ. Cell encapsulation with alginate and α-phenoxycinnamylidene-acetylated poly(allylamine) Biotechnol Bioeng. 2000;70:479–483. doi: 10.1002/1097-0290(20001205)70:5<479::aid-bit1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 52.Roy D, Cambre JN, Sumerlin BS. Future perspectives and recent advances in stimuliresponsive materials. Progr Polym Sci. 2010;35:278–301. [Google Scholar]
- 53.Rzaev ZMO, Dincer S, Piskin E. Functional copolymers of N-isopropylacrylamide for bioengineering applications. Progr Polym Sci. 2007;32:534–595. [Google Scholar]
- 54.Zhao SP, Cao MJ, Li H, Li LY, Xu WL. Synthesis and characterization of thermo-sensitive semi-IPN hydrogels based on poly(ethylene glycol)-co-poly(epsilon-caprolactone) macromer, N-isopropylacrylamide, and sodium alginate. Carbohydr Res. 2010;345:425–431. doi: 10.1016/j.carres.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 55.Lee KY, Kong HJ, Larson RG, Mooney DJ. Hydrogel formation via cell cross-linking. Adv Mater. 2003;15:1828–1832. [Google Scholar]
- 56.Drury JL, Boontheekul T, Mooney DJ. Cellular cross-linking of peptide modified hydrogels. J Biomech Eng-Trans ASME. 2005;127:220–228. doi: 10.1115/1.1865194. [DOI] [PubMed] [Google Scholar]
- 57.Al-Shamkhani A, Duncan R. Radioiodination of alginate via covalently-bound tyrosinamide allows monitoring of its fate in vivo. J Bioact Compat Polym. 1995;10:4–13. [Google Scholar]
- 58.Bouhadir KH, Lee KY, Alsberg E, Damm KL, Anderson KW, Mooney DJ. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol Prog. 2001;17:945–950. doi: 10.1021/bp010070p. [DOI] [PubMed] [Google Scholar]
- 59.Lee KY, Bouhadir KH, Mooney DJ. Degradation behavior of covalently cross-inked poly(aldehyde guluronate) hydrogels. Macromolecules. 2000;33:97–101. [Google Scholar]
- 60.Kong HJ, Kaigler D, Kim K, Mooney DJ. Controlling rigidity and degradation of alginate hydrogels via molecular weight distribution. Biomacromolecules. 2004;5:1720–1727. doi: 10.1021/bm049879r. [DOI] [PubMed] [Google Scholar]
- 61.Kong HJ, Alsberg E, Kaigler D, Lee KY, Mooney DJ. Controlling degradation of hydrogels via the size of cross-linked junctions. Adv Mater. 2004;16:1917–1921. doi: 10.1002/adma.200400014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boontheekul T, Kong HJ, Mooney DJ. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials. 2005;26:2455–2465. doi: 10.1016/j.biomaterials.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 63.Maiti S, Singha K, Ray S, Dey P, Sa B. Adipic acid dihydrazide treated partially oxidized alginate beads for sustained oral delivery of flurbiprofen. Pharm Develop Technol. 2009;14:461–470. doi: 10.1080/10837450802712658. [DOI] [PubMed] [Google Scholar]
- 64.Bouhadir KH, Alsberg E, Mooney DJ. Hydrogels for combination delivery of antineoplastic agents. Biomaterials. 2001;22:2625–2633. doi: 10.1016/s0142-9612(01)00003-5. [DOI] [PubMed] [Google Scholar]
- 65.Colinet I, Dulong V, Mocanu G, Picton L, Le Cerf D. New amphiphilic and pH-sensitive hydrogel for controlled release of a model poorly water-soluble drug. Eur J Pharm Biopharm. 2009;73:345–350. doi: 10.1016/j.ejpb.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 66.Zhang XL, Hui ZY, Wan DX, Huang HT, Huang J, Yuan H, Yu JH. Alginate microsphere filled with carbon nanotube as drug carrier. Int J Biol Macromol. 2010;47:389–395. doi: 10.1016/j.ijbiomac.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 67.Sandford PA, Steinnes A. Biomedical application of high-purity chitosan. In: Shalaby SW, McCormick CL, Butler GB, editors. Water-soluble polymers: synthesis, solution properties, and applications. vol. 467. Washington DC: Am Chem Soc; 1991. pp. 430–445. [Google Scholar]
- 68.Rinaudo M. Chitin and chitosan: properties and applications. Progr Polym Sci. 2006;31:603–632. [Google Scholar]
- 69.Lucinda-Silva RM, Salgado HRN, Evangelista RC. Alginate-chitosan systems: in vitro controlled release of triamcinolone and in vivo gastrointestinal transit. Carbohydr Polym. 2010;81:260–268. [Google Scholar]
- 70.Wang FQ, Li P, Zhang JP, Wang AQ, Wei Q. A novel pH-sensitive magnetic alginate74 chitosan beads for albendazole delivery. Drug Dev Ind Pharm. 2010;36:867–877. doi: 10.3109/03639040903567117. [DOI] [PubMed] [Google Scholar]
- 71.Lira AAM, Rossetti FC, Nanclares DMA, Neto AF, Bentley MVLB, Marchetti JM. Preparation and characterization of chitosan-treated alginate microparticles incorporating all-trans retinoic acid. J Microencapsulation. 2009;26:243–250. doi: 10.1080/02652040802305105. [DOI] [PubMed] [Google Scholar]
- 72.Ishak RAH, Awad GAS, Mortada ND, Nour SAK. Preparation, in vitro and in vivo evaluation of stomach-specific metronidazole-loaded alginate beads as local anti-Helicobacter pylori therapy. J Controlled Release. 2007;119:207–214. doi: 10.1016/j.jconrel.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 73.Chang CH, Lin YH, Yeh CL, Chen YC, Chiou SF, Hsu YM, Chen YS, Wang CC. Nanoparticles incorporated in pH-sensitive hydrogels as amoxicillin delivery for eradication of Helicobacter pylori. Biomacromolecules. 2010;11:133–142. doi: 10.1021/bm900985h. [DOI] [PubMed] [Google Scholar]
- 74.Lee KY, Peters MC, Mooney DJ. Comparison of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in SCID mice. J Controlled Release. 2003;87:49–56. doi: 10.1016/s0168-3659(02)00349-8. [DOI] [PubMed] [Google Scholar]
- 75.Silva EA, Mooney DJ. Effects of VEGF temporal and spatial presentation on angiogenesis. Biomaterials. 2010;31:1235–1241. doi: 10.1016/j.biomaterials.2009.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wells LA, Sheardown H. Extended release of high pI proteins from alginate microspheres via a novel encapsulation technique. Eur J Pharm Biopharm. 2007;65:329–335. doi: 10.1016/j.ejpb.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 77.Gao CM, Liu MZ, Chen SL, Jin SP, Chen J. Preparation of oxidized sodium alginate-graftpoly((2-dimethylamino) ethyl methacrylate) gel beads and in vitro controlled release behavior of BSA. Int J Pharm. 2009;371:16–24. doi: 10.1016/j.ijpharm.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 78.Chan AW, Neufeld RJ. Tuneable semi-synthetic network alginate for absorptive encapsulation and controlled release of protein therapeutics. Biomaterials. 2010;31:9040–9047. doi: 10.1016/j.biomaterials.2010.07.111. [DOI] [PubMed] [Google Scholar]
- 79.Silva CM, Ribeiro AJ, Ferreira D, Veiga F. Insulin encapsulation in reinforced alginate microspheres prepared by internal gelation. Eur J Pharm Sci. 2006;29:148–159. doi: 10.1016/j.ejps.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 80.Wang X, Wenk E, Hu X, Castro GR, Meinel L, Wang X, Li C, Merkle H, Kaplan DL. Silk coatings on PLGA and alginate microspheres for protein delivery. Biomaterials. 2007;28:4161–4169. doi: 10.1016/j.biomaterials.2007.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee J, Lee KY. Injectable microsphere/hydrogel combination systems for localized protein delivery. Macromol Biosci. 2009;9:671–676. doi: 10.1002/mabi.200800317. [DOI] [PubMed] [Google Scholar]
- 82.Lee J, Tan CY, Lee SK, Kim YH, Lee KY. Controlled delivery of heat shock protein using an injectable microsphere/hydrogel combination system for the treatment of myocardial infarction. J Controlled Release. 2009;137:196–202. doi: 10.1016/j.jconrel.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 83.Boateng JS, Matthews KH, Stevens HNE, Eccleston GM. Wound healing dressings and drug delivery systems: a review. J Pharm Sci. 2008;97:2892–2923. doi: 10.1002/jps.21210. [DOI] [PubMed] [Google Scholar]
- 84.Queen D, Orsted H, Sanada H, Sussman G. A dressing history. Int Wound J. 2004;1:59–77. doi: 10.1111/j.1742-4801.2004.0009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Balakrishnan B, Mohanty M, Fernandez AC, Mohanan PV, Jayakrishnan A. Evaluation of the effect of incorporation of dibutyryl cyclic adenosine monophosphate in an in situforming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials. 2006;27:1355–1361. doi: 10.1016/j.biomaterials.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 86.Rabbany SY, Pastore J, Yamamoto M, Miller T, Rafii S, Aras R, Penn M. Continuous delivery of stromal cell-derived factor-1 from alginate scaffolds accelerates wound healing. Cell Transplantation. 2010;19:399–408. doi: 10.3727/096368909X481782. [DOI] [PubMed] [Google Scholar]
- 87.Wiegand C, Heinze T, Hipler UC. Comparative in vitro study on cytotoxicity, antimicrobial activity, and binding capacity for pathophysiological factors in chronic wounds of alginate and silver-containing alginate. Wound Repair Regener. 2009;17:511–521. doi: 10.1111/j.1524-475X.2009.00503.x. [DOI] [PubMed] [Google Scholar]
- 88.Agren MS. Zinc in wound repair. Arch Dermatol. 1999;135:1273–1274. doi: 10.1001/archderm.135.10.1273-a. [DOI] [PubMed] [Google Scholar]
- 89.Murakami K, Aoki H, Nakamura S, Nakamura S, Takikawa M, Hanzawa M, Kishimoto S, Hattori H, Tanaka Y, Kiyosawa T, Sato Y, Ishihara M. Hydrogel blends of chitin/chitosan, fucoidan and alginate as healing-impaired wound dressings. Biomaterials. 2010;31:83–90. doi: 10.1016/j.biomaterials.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 90.Rowley JA, Sun ZX, Goldman D, Mooney DJ. Biomaterials to spatially regulate cell fate. Adv Mater. 2002;14:886–889. [Google Scholar]
- 91.Jeon O, Powell C, Ahmed SM, Alsberg E. Biodegradable, photocrosslinked alginate hydrogels with independently tailorable physical properties and cell adhesivity. Tissue Eng Part A. 2010;16:2915–2925. doi: 10.1089/ten.TEA.2010.0096. [DOI] [PubMed] [Google Scholar]
- 92.Degala S, Zipfel WR, Bonassar LJ. Chondrocyte calcium signaling in response to fluid flow is regulated by matrix adhesion in 3-D alginate scaffolds. Arch Biochem Biophys. 2011;505:112–117. doi: 10.1016/j.abb.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 93.Evangelista MB, Hsiong SX, Fernandes R, Sampaio P, Kong HJ, Barrias CC, Salema R, Barbosa MA, Mooney DJ, Granja PL. Upregulation of bone cell differentiation through immobilization within a synthetic extracellular matrix. Biomaterials. 2007;28:3644–3655. doi: 10.1016/j.biomaterials.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 94.Kreeger PK, Deck JW, Woodruff TK, Shea LD. The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials. 2006;27:714–723. doi: 10.1016/j.biomaterials.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hsiong SX, Carampin P, Kong HJ, Lee KY, Mooney DJ. Differentiation stage alters matrix control of stem cells. J Biomed Mater Res Part A. 2008;85:145–156. doi: 10.1002/jbm.a.31521. [DOI] [PubMed] [Google Scholar]
- 96.Wang L, Shelton RM, Cooper PR, Lawson M, Triffitt JT, Barralet JE. Evaluation of sodium alginate for bone marrow cell tissue engineering. Biomaterials. 2003;24:3475–3481. doi: 10.1016/s0142-9612(03)00167-4. [DOI] [PubMed] [Google Scholar]
- 97.Bidarra SJ, Barrias CC, Barbosa MA, Soares R, Granja PL. Immobilization of human mesenchymal stem cells within RGD-grafted alginate microspheres and assessment of their angiogenic potential. Biomacromolecules. 2010;11:1956–1964. doi: 10.1021/bm100264a. [DOI] [PubMed] [Google Scholar]
- 98.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20:45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 99.Lee JW, Lee SJ, Lee SK, Lee KY. The effect of spacer arm length of an adhesion ligand coupled to an alginate gel on the control of fibroblast phenotype. Biomaterials. 2010;31:5545–5551. doi: 10.1016/j.biomaterials.2010.03.063. [DOI] [PubMed] [Google Scholar]
- 100.Lee KY, Alsberg E, Hsiong S, Comisar W, Linderman J, Ziff R, Mooney D. Nanoscale adhesion ligand organization regulates osteoblast proliferation and differentiation. Nano Letters. 2004;4:1501–1506. doi: 10.1021/nl0493592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Comisar WA, Hsiong SX, Kong HJ, Mooney DJ, Linderman JJ. Multi-scale modeling to predict ligand presentation within RGD nanopatterned hydrogels. Biomaterials. 2006;27:2322–2329. doi: 10.1016/j.biomaterials.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 102.Comisar WA, Kazmers NH, Mooney DJ, Linderman JJ. Engineering RGD nanopatterned hydrogels to control preosteoblast behavior: A combined computational and experimental approach. Biomaterials. 2007;28:4409–4417. doi: 10.1016/j.biomaterials.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 103.Connelly JT, Garcia AJ, Levenston ME. Inhibition of in vitro chondrogenesis in RGDmodified three-dimensional alginate gels. Biomaterials. 2007;28:1071–1083. doi: 10.1016/j.biomaterials.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 104.Chueh BH, Zheng Y, Torisawa YS, Hsiao AY, Ge CX, Hsiong S, Huebsch N, Franceschi R, Mooney DJ, Takayama S. Patterning alginate hydrogels using light-directed release of caged calcium in a microfluidic device. Biomed Microdevices. 2010;12:145–151. doi: 10.1007/s10544-009-9369-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hsiong SX, Boontheekul T, Huebsch N, Mooney DJ. Cyclic arginine-glycine-aspartate peptides enhance three-dimensional stem cell osteogenic differentiation. Tissue Eng Part A. 2009;15:263–272. doi: 10.1089/ten.tea.2007.0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bogdanowich-Knipp SJ, Chakrabarti S, Williams TD, Dillman RK, Siahaan TJ. Solution stability of linear vs. cyclic RGD peptides. J Pept Res. 1999;53:530–541. doi: 10.1034/j.1399-3011.1999.00052.x. [DOI] [PubMed] [Google Scholar]
- 107.Kumagai H, Tajima M, Ueno Y, Giga-Hama Y, Ohba M. Effect of cyclic RGD peptide on cell adhesion and tumor metastasis. Biochem Biophys Res Commun. 1991;177:74–82. doi: 10.1016/0006-291x(91)91950-h. [DOI] [PubMed] [Google Scholar]
- 108.Liu S. Radiolabeled multimeric cyclic RGD peptides as integrin αvβ3 targeted radiotracers for tumor imaging. Mol Pharm. 2006;3:472–487. doi: 10.1021/mp060049x. [DOI] [PubMed] [Google Scholar]
- 109.Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 2010;9:518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fischbach C, Kong HJ, Hsiong SX, Evangelista MB, Yuen W, Mooney DJ. Cancer cell angiogenic capability is regulated by 3D culture and integrin engagement. Proc Natl Acad Sci USA. 2009;106:399–404. doi: 10.1073/pnas.0808932106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kong HJ, Polte TR, Alsberg E, Mooney DJ. FRET measurements of cell-traction forces and nano-scale clustering of adhesion ligands varied by substrate stiffness. Proc Natl Acad Sci USA. 2005;102:4300–4305. doi: 10.1073/pnas.0405873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kong HJ, Liu JD, Riddle K, Matsumoto T, Leach K, Mooney DJ. Non-viral gene delivery regulated by stiffness of cell adhesion substrates. Nat Mater. 2005;4:460–464. doi: 10.1038/nmat1392. [DOI] [PubMed] [Google Scholar]
- 113.Kong HJ, Boontheekul T, Mooney DJ. Quantifying the relation between adhesion ligandreceptor bond formation and cell phenotype. Proc Natl Acad Sci USA. 2006;103:18534–18539. doi: 10.1073/pnas.0605960103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 115.Cao L, Mooney DJ. Spatiotemporal control over growth factor signaling for therapeutic neovascularization. Adv Drug Delivery Rev. 2007;59:1340–1350. doi: 10.1016/j.addr.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gu F, Amsden B, Neufeld R. Sustained delivery of vascular endothelial growth factor with alginate beads. J Controlled Release. 2004;96:463–472. doi: 10.1016/j.jconrel.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 117.Jay SM, Saltzman WM. Controlled delivery of VEGF via modulation of alginate microparticle ionic cross-linking. J Controlled Release. 2009;134:26–34. doi: 10.1016/j.jconrel.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sun QH, Silva EA, Wang AX, Fritton JC, Mooney DJ, Schaffler MB, Grossman PM, Rajagopalan S. Sustained release of multiple growth factors from injectable polymeric system as a novel therapeutic approach towards angiogenesis. Pharm Res. 2010;27:264–271. doi: 10.1007/s11095-009-0014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hao XJ, Silva EA, Mansson-Broberg A, Grinnemo KH, Siddiqui AJ, Dellgren G, Wardell E, Brodin LA, Mooney DJ, Sylven C. Angiogenic effects of sequential release of VEGFA(165) and PDGF-BB with alginate hydrogels after myocardial infarction. Cardiovasc Res. 2007;75:178–185. doi: 10.1016/j.cardiores.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 120.Ruvinov E, Leor J, Cohen S. The promotion of myocardial repair by the sequential delivery of IGF-1 and HGF from an injectable alginate biomaterial in a model of acute myocardial infarction. Biomaterials. 2011;32:565–578. doi: 10.1016/j.biomaterials.2010.08.097. [DOI] [PubMed] [Google Scholar]
- 121.Lee J, Bhang SH, Park H, Kim BS, Lee KY. Active blood vessel formation in the ischemic hindlimb mouse model using a microsphere/hydrogel combination system. Pharm Res. 2010;27:767–774. doi: 10.1007/s11095-010-0067-0. [DOI] [PubMed] [Google Scholar]
- 122.Peters MC, Polverini PJ, Mooney DJ. Engineering vascular networks in porous polymer matrices. J Biomed Mater Res. 2002;60:668–678. doi: 10.1002/jbm.10134. [DOI] [PubMed] [Google Scholar]
- 123.Jay SM, Shepherd BR, Andrejecsk JW, Kyriakides TR, Pober JS, Saltzman WM. Dual delivery of VEGF and MCP-1 to support endothelial cell transplantation for therapeutic vascularization. Biomaterials. 2010;31:3054–3062. doi: 10.1016/j.biomaterials.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Silva EA, Kim ES, Kong HJ, Mooney DJ. Material-based deployment enhances efficacy of endothelial progenitor cells. Proc Natl Acad Sci USA. 2008;105:14347–14352. doi: 10.1073/pnas.0803873105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kolambkar YM, Dupont KM, Boerckel JD, Huebsch N, Mooney DJ, Hutmacher DW, Guldberg RE. An alginate-based hybrid system for growth factor delivery in the functional repair of large bone defects. Biomaterials. 2011;32:65–74. doi: 10.1016/j.biomaterials.2010.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lopiz-Morales Y, Abarrategi A, Ramos V, Moreno-Vicente C, Lopez-Duran L, Lopez-Lacomba JL, Marco F. In vivo comparison of the effects of rhBMP-2 and rhBMP-4 in osteochondral tissue regeneration. Eur Cells Mater. 2010;20:367–378. doi: 10.22203/ecm.v020a30. [DOI] [PubMed] [Google Scholar]
- 127.Krebs MD, Salter E, Chen E, Sutter KA, Alsberg E. Calcium alginate phosphate-DNA nanoparticle gene delivery from hydrogels induces in vivo osteogenesis. J Biomed Mater Res Part A. 2010;92:1131–1138. doi: 10.1002/jbm.a.32441. [DOI] [PubMed] [Google Scholar]
- 128.Basmanav FB, Kose GT, Hasirci V. Sequential growth factor delivery from complexed microspheres for bone tissue engineering. Biomaterials. 2008;29:4195–4204. doi: 10.1016/j.biomaterials.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 129.Kanczler JM, Ginty PJ, White L, Clarke NMP, Howdle SM, Shakesheff KM, Oreffo ROC. The effect of the delivery of vascular endothelial growth factor and bone morphogenic protein-2 to osteoprogenitor cell populations on bone formation. Biomaterials. 2010;31:1242–1250. doi: 10.1016/j.biomaterials.2009.10.059. [DOI] [PubMed] [Google Scholar]
- 130.Alsberg E, Anderson KW, Albeiruti A, Rowley JA, Mooney DJ. Engineering growing tissues. Proc Natl Acad Sci USA. 2002;99:12025–12030. doi: 10.1073/pnas.192291499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lee KY, Alsberg E, Mooney DJ. Degradable and injectable poly(aldehyde guluronate) hydrogels for bone tissue engineering. J Biomed Mater Res. 2001;56:228–233. doi: 10.1002/1097-4636(200108)56:2<228::aid-jbm1089>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 132.Barralet JE, Wang L, Lriffitt JT, Cooper PR, Shelton RM. Comparison of bone marrow cell growth on 2D and 3D alginate hydrogels. J Mater Sci Mater Med. 2005;16:515–519. doi: 10.1007/s10856-005-0526-z. [DOI] [PubMed] [Google Scholar]
- 133.Weng YL, Wang M, Liu W, Hu XJ, Chai G, Yan QM, Zhu L, Cui L, Cao YL. Repair of experimental alveolar bone defects by tissue-engineered bone. Tissue Eng. 2006;12:1503–1513. doi: 10.1089/ten.2006.12.1503. [DOI] [PubMed] [Google Scholar]
- 134.Park DJ, Choi BH, Zhu SJ, Huh JY, Kim BY, Lee SH. Injectable bone using chitosanalginate gel/mesenchymal stem cells/BMP-2 composites. J Cranio Maxill Surg. 2005;33:50–54. doi: 10.1016/j.jcms.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 135.Lin HR, Yeh YJ. Porous alginate/hydroxyapatite composite scaffolds for bone tissue engineering: preparation, characterization, and in vitro studies. J Biomed Mater Res Part B. 2004;71:52–65. doi: 10.1002/jbm.b.30065. [DOI] [PubMed] [Google Scholar]
- 136.Weir MD, Xu HHK, Simon CG. Strong calcium phosphate cement-chitosan-mesh construct containing cell-encapsulating hydrogel beads for bone tissue engineering. J Biomed Mater Res Part A. 2006;77:487–496. doi: 10.1002/jbm.a.30626. [DOI] [PubMed] [Google Scholar]
- 137.Lawson MA, Barralet JE, Wang L, Shelton RM, Triffitt JT. Adhesion and growth of bone marrow stromal cells on modified alginate hydrogels. Tissue Eng. 2004;10:1480–1491. doi: 10.1089/ten.2004.10.1480. [DOI] [PubMed] [Google Scholar]
- 138.Chang SCN, Rowley JA, Tobias G, Genes NG, Roy AK, Mooney DJ, Vacanti CA, Bonassar LJ. Injection molding of chondrocyte/alginate constructs in the shape of facial implants. J Biomed Mater Res. 2001;55:503–511. doi: 10.1002/1097-4636(20010615)55:4<503::aid-jbm1043>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 139.Chang SCN, Tobias G, Roy AK, Vacanti CA, Bonassar LJ. Tissue engineering of autologous cartilage for craniofacial reconstruction by injection molding. Plast Reconstr Surg. 2003;112:793–799. doi: 10.1097/01.PRS.0000069711.31021.94. [DOI] [PubMed] [Google Scholar]
- 140.Thornton AJ, Alsberg E, Albertelli M, Mooney DJ. Shape-defining scaffolds for minimally invasive tissue engineering. Transplantation. 2004;77:1798–1803. doi: 10.1097/01.tp.0000131152.71117.0e. [DOI] [PubMed] [Google Scholar]
- 141.Ma HL, Hung SC, Lin SY, Chen YL, Lo WH. Chondrogenesis of human mesenchymal stem cells encapsulated in alginate beads. J Biomed Mater Res Part A. 2003;64:273–281. doi: 10.1002/jbm.a.10370. [DOI] [PubMed] [Google Scholar]
- 142.Igarashi T, Iwasaki N, Kasahara Y, Minami A. A cellular implantation system using an injectable ultra-purified alginate gel for repair of osteochondral defects in a rabbit model. J Biomed Mater Res Part A. 2010;94:844–855. doi: 10.1002/jbm.a.32762. [DOI] [PubMed] [Google Scholar]
- 143.Awad HA, Wickham MQ, Leddy HA, Gimble JM, Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004;25:3211–3222. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 144.Jin XB, Sun YS, Zhang K, Wang J, Shi TP, Ju XD, Lou SQ. Ectopic neocartilage formation from predifferentiated human adipose derived stem cells induced by adenoviral-mediated transfer of hTGF-beta2. Biomaterials. 2007;28:2994–3003. doi: 10.1016/j.biomaterials.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 145.Park H, Kang SW, Kim BS, Mooney DJ, Lee KY. Shear-reversibly cross-linked alginate hydrogels for tissue engineering. Macromol Biosci. 2009;9:895–901. doi: 10.1002/mabi.200800376. [DOI] [PubMed] [Google Scholar]
- 146.Saxena AK, Marler J, Benvenuto M, Willital GH, Vacanti JP. Skeletal muscle tissue engineering using isolated myoblasts on synthetic biodegradable polymers: preliminary studies. Tissue Eng. 1999;5:525–532. doi: 10.1089/ten.1999.5.525. [DOI] [PubMed] [Google Scholar]
- 147.Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, Darland DC, Marini R, van Blitterswijk CA, Mulligan RC, D'Amore PA, Langer R. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879–884. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 148.Borselli C, Storrie H, Benesch-Lee F, Shvartsman D, Cezar C, Lichtman JW, Vandenburgh HH, Mooney DJ. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc Natl Acad Sci USA. 2010;107:3287–3292. doi: 10.1073/pnas.0903875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hill E, Boontheekul T, Mooney DJ. Designing scaffolds to enhance transplanted myoblast survival and migration. Tissue Eng. 2006;12:1295–1304. doi: 10.1089/ten.2006.12.1295. [DOI] [PubMed] [Google Scholar]
- 150.Hill E, Boontheekul T, Mooney DJ. Regulating activation of transplanted cells controls tissue regeneration. Proc Natl Acad Sci USA. 2006;103:2494–2499. doi: 10.1073/pnas.0506004103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Prang P, Muller R, Eljaouhari A, Heckmann K, Kunz W, Weber T, Faber C, Vroemen M, Bogdahn U, Weidner N. The promotion of oriented axonal regrowth in the injured spinal cord by alginate-based anisotropic capillary hydrogels. Biomaterials. 2006;27:3560–3569. doi: 10.1016/j.biomaterials.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 152.Hashimoto T, Suzuki Y, Suzuki K, Nakashima T, Tanihara M, Ide C. Peripheral nerve regeneration using non-tubular alginate gel crosslinked with covalent bonds. J Mater Sci-Mater Med. 2005;16:503–509. doi: 10.1007/s10856-005-0524-1. [DOI] [PubMed] [Google Scholar]
- 153.Kataoka K, Suzuki Y, Kitada M, Hashimoto T, Chou H, Bai HL, Ohta M, Wu S, Suzuki K, Ide C. Alginate enhances elongation of early regenerating axons in spinal cord of young rats. Tissue Eng. 2004;10:493–504. doi: 10.1089/107632704323061852. [DOI] [PubMed] [Google Scholar]
- 154.Suzuki K, Suzuki Y, Tanihara M, Ohnishi K, Hashimoto T, Endo K, Nishimura Y. Reconstruction of rat peripheral nerve gap without sutures using freeze-dried alginate gel. J Biomed Mater Res. 2000;49:528–533. doi: 10.1002/(sici)1097-4636(20000315)49:4<528::aid-jbm11>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 155.Li XQ, Liu TQ, Song KD, Yao LS, Ge D, Bao CY, Ma XH, Cui ZF. Culture of neural stem cells in calcium alginate beads. Biotechnol Prog. 2006;22:1683–1689. doi: 10.1021/bp060185z. [DOI] [PubMed] [Google Scholar]
- 156.Selden C, Hodgson H. Cellular therapies for liver replacement. Transpl Immunol. 2004;12:273–288. doi: 10.1016/j.trim.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 157.Koizumi T, Aoki T, Kobayashi Y, Yasuda D, Izumida Y, Jin ZH, Nishino N, Shimizu Y, Kato H, Murai N, Niiya T, Enami Y, Mitamura K, Yamamoto T, Kusano M. Long-term maintenance of the drug transport activity in cryopreservation of microencapsulated rat hepatocytes. Cell Transplantation. 2007;16:67–73. doi: 10.3727/000000007783464489. [DOI] [PubMed] [Google Scholar]
- 158.Zmora S, Glicklis R, Cohen S. Tailoring the pore architecture in 3-D alginate scaffolds by controlling the freezing regime during fabrication. Biomaterials. 2002;23:4087–4094. doi: 10.1016/s0142-9612(02)00146-1. [DOI] [PubMed] [Google Scholar]
- 159.Dvir-Ginzberg M, Gamlieli-Bonshtein I, Agbaria R, Cohen S. Liver tissue engineering within alginate scaffolds: effects of cell-seeding density on hepatocyte viability, morphology, and function. Tissue Eng. 2003;9:757–766. doi: 10.1089/107632703768247430. [DOI] [PubMed] [Google Scholar]
- 160.Glicklis R, Shapiro L, Agbaria R, Merchuk JC, Cohen S. Hepatocyte behavior within threedimensional porous alginate scaffolds. Biotechnol Bioeng. 2000;67:344–353. doi: 10.1002/(sici)1097-0290(20000205)67:3<344::aid-bit11>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 161.Kedem A, Perets A, Gamlieli-Bonshtein I, Dvir-Ginzberg M, Mizrahi S, Cohen S. Vascular endothelial growth factor-releasing scaffolds enhance vascularization and engraftment of hepatocytes transplanted on liver lobes. Tissue Eng. 2005;11:715–722. doi: 10.1089/ten.2005.11.715. [DOI] [PubMed] [Google Scholar]
- 162.Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210:908–910. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- 163.Lacy PE, Hegre OD, Gerasimidivazeou A, Gentile FT, Dionne KE. Maintenance of normoglycemia in diabetic mice by subcutaneous xenografts of encapsulated islets. Science. 1991;254:1782–1784. doi: 10.1126/science.1763328. [DOI] [PubMed] [Google Scholar]
- 164.Calafiore R. Alginate microcapsules for pancreatic islet cell graft immunoprotection: struggle and progress towards the final cure for type 1 diabetes mellitus. Exp Opin Biol Ther. 2003;3:201–205. doi: 10.1517/14712598.3.2.201. [DOI] [PubMed] [Google Scholar]
- 165.Sakai S, Ono T, Ijima H, Kawakami K. In vitro and in vivo evaluation of alginate/sol-gel synthesized aminopropyl-silicate/alginate membrane for bioartificial pancreas. Biomaterials. 2002;23:4177–4183. doi: 10.1016/s0142-9612(02)00159-x. [DOI] [PubMed] [Google Scholar]
- 166.Kendall WF, Darrabie MD, El-Shewy HM, Opara EC. Effect of alginate composition and purity on alginate microspheres. J Microencapsulation. 2004;21:821–828. doi: 10.1080/02652040400015452. [DOI] [PubMed] [Google Scholar]
- 167.Liu K, Ding HJ, Liu J, Chen Y, Zhao XZ. Shape-controlled production of biodegradable calcium alginate gel microparticles using a novel microfluidic device. Langmuir. 2006;22:9453–9457. doi: 10.1021/la061729+. [DOI] [PubMed] [Google Scholar]
- 168.Soon-Shiong P, Heintz RE. Insulin independence in a type 1 diabetic patient after encapsulated islet transplantation. Lancet. 1994;343:950. doi: 10.1016/s0140-6736(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 169.Diamond DA, Caldamone AA. Endoscopic treatment of vesicoureteric reflux in children using autologous chondrocytes - preliminary results. Pediatrics. 1998;102:107A. doi: 10.1016/S0022-5347(01)68124-2. [DOI] [PubMed] [Google Scholar]
- 170.Kershen RT, Fefer SD, Atala A. Tissue-engineered therapies for the treatment of urinary incontinence and vesicoureteral reflux. World J Urol. 2000;18:51–55. doi: 10.1007/pl00007072. [DOI] [PubMed] [Google Scholar]
- 171.Kong HJ, Kim ES, Huang YC, Mooney DJ. Design of biodegradable hydrogel for the local and sustained delivery of angiogenic plasmid DNA. Pharm Res. 2009;25:1230–1238. doi: 10.1007/s11095-007-9526-7. [DOI] [PubMed] [Google Scholar]
- 172.Lee KY, Peters MC, Anderson KW, Mooney DJ. Controlled growth factor release from synthetic extracellular matrices. Nature. 2000;408:998–1000. doi: 10.1038/35050141. [DOI] [PubMed] [Google Scholar]
- 173.Zhao XH, Kim J, Cezar CA, Huebsch N, Lee K, Bouhadir K, Mooney DJ. Active scaffolds for on-demand drug and cell delivery. Proc Natl Acad Sci USA. 2011;108:67–72. doi: 10.1073/pnas.1007862108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Petka WA, Harden JL, McGrath KP, Wirtz D, Tirrell DA. Reversible hydrogels from selfassembling artificial proteins. Science. 1998;281:389–392. doi: 10.1126/science.281.5375.389. [DOI] [PubMed] [Google Scholar]
- 175.MacKay JA, Chen MN, McDaniel JR, Liu WG, Simnick AJ, Chilkoti A. Self-assembling chimeric polypeptide-doxorubicin conjugate nanoparticles that abolish tumours after a single injection. Nat Mater. 2009;8:993–999. doi: 10.1038/nmat2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sculean A, Auschill TM, Donos N, Brecx M, Arweiler NB. Effect of an enamel matrix protein (Emdogain®) on ex vivo dental plaque vitality. J Clin Periodontology. 2001;28:1074–1078. doi: 10.1111/j.1600-051X.2001.281113.x. [DOI] [PubMed] [Google Scholar]
- 177.Ali OA, Mooney DJ. Sustained GM-CSF and PEI condensed pDNA presentation increases the level and duration of gene expression in dendritic cells. J Controlled Release. 2008;132:273–278. doi: 10.1016/j.jconrel.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 178.Hashimoto T, Suzuki Y, Kitada M, Kataoka K, Wu S, Suzuki K, Endo K, Nishimura Y, Ide C. Peripheral nerve regeneration through alginate gel: analysis of early outgrowth and late increase in diameter of regenerating axons. Exp Brain Res. 2002;146:356–368. doi: 10.1007/s00221-002-1173-y. [DOI] [PubMed] [Google Scholar]
- 179.Vacharathit V, Silva EA, Mooney DJ. Viability and functionality of cells delivered from peptide conjugated scaffolds. Biomaterials. 2011;32:3721–3728. doi: 10.1016/j.biomaterials.2010.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Guilak F, Cohen D, Estes B, Gimble JM, Liedtke W, Chen CS. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5:17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dashtdar H, Rothan HA, Tay T, Ahmad RE, Ali R, Tay LX, Chong PP, Kamarul T. A preliminary study comparing the use of allogenic chondrogenic pre-differentiated and undifferentiated mesenchymal stem cells for the repair of full thickness articular cartilage defects in rabbits. J Orthop Res. 2011 doi: 10.1002/jor.21413. [DOI] [PubMed] [Google Scholar]
- 182.Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther. 2003;51:32–45. doi: 10.1186/ar614. [DOI] [PMC free article] [PubMed] [Google Scholar]